Abstract
Dementia represents an umbrella term encompassing many distinct syndromes leading to cognitive and functional impairment. Symptom burden throughout the course of dementia is high and can escalate as the disease progresses. These symptoms are often difficult to assess in the patient with dementia and effective treatment options are often limited. Palliative care interventions, whether through a primary provider or a palliative specialist, should include a multifaceted approach, which evolves based on disease stage and individual need. Early identification of surrogate decision makers and advance care planning, anticipating care needs based on stage of disease, identifying and managing symptoms, caregiver support, and timely identification of hospice eligible patients are the cornerstones of effective treatment. While research continues to search for treatment options that alter disease course and effectively target cognitive and behavioral symptoms, other gaps in our understanding of disease also need attention. Prognostic uncertainty contributes to the aggressive and burdensome care that many patients experience at end-of-life, as well as low hospice utilization. The accuracy of the current Medicare guidelines in predicting 6-month mortality is limited and there is a need for more exploration and adoption of better predictive tools for prognostication.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dementia
- Palliative care
- Dementia with Lewy body
- Alzheimer’s disease
- Behavioral and psychological symptoms of dementia
Mrs. Sebor is an 81 year old woman with Alzheimer’s Dementia who until recently resided in an assisted living facility. She was diagnosed with Alzheimer’s seven years ago. At that time, she was still living in the community with help from her children. Over time, she had increasing difficulty maintaining the home, preparing meals, and often forgot her medications, at which point she transitioned to an assisted living community. Over the next 2 years, her disease slowly advanced with bladder incontinence and difficulty ambulating. Two weeks ago, she was hospitalized for pneumonia, after which it became clear she needed more support and she was transitioned to a nursing home. She continues to struggle with progressive difficulty in swallowing, even with hand feeding, and has been losing weight; her family asks about a feeding tube. Mrs. Sebor is also becoming increasingly confused and agitated at night, resulting in several falls. She has been aggressive and combative with care and was recently started on quetiapine as needed for this agitation behavior. Her family wants to know what stage of dementia their loved one is at and what her prognosis is. They ask when hospice would be appropriate and if anything more can be done for her confusion and agitation.
Dementia is an umbrella term encompassing many distinct syndromes and diseases associated with cognitive and functional impairment. These syndromes can be static, as is the case with traumatic brain injury, progressive like Alzheimer’s disease (AD), or potentially reversible, as may occur with vitamin B12 deficiency. Progressive dementia syndromes, which is the focus of this chapter, can be caused by primary neurodegenerative conditions, such as AD and related dementias, or secondary to other neurologic conditions, such as multiple sclerosis. This decline in cognitive function occurs over one or more domains of cognition, most commonly memory, but also executive function, language and visuospatial abilities [1]. All of the progressive neurodegenerative conditions lead to functional dependence, debility, progressive symptom burden, and ultimately death.
Dementia is quite common with global estimates suggesting that 35.6 million adults suffer from dementia, a number which is expected to double by 2030 [2]. In a prevalence study of adults 71 and older in the United States, 13.9% had dementia with Alzheimer’s disease accounting for 70% of cases [3]. All told, one out of every three older adults will die from or with dementia [4]. This also represents a major burden on the healthcare system. While some are able to live at home with most care provided by family and friends, many need additional support. More than half of all nursing home residents have dementia, and 2/3 of patients who die of dementia do so in a nursing home. Aggregate healthcare cost for those with dementia is estimated at 226 billion in 2017. Most of these costs are paid for by Medicare and Medicaid, but significant out of pocket costs are also incurred [5].
Common Types of Dementia
AD remains the most common dementia, accounting for an estimated 60–80% of all cases [6,7,8] and is currently estimated to affect 5.3 million Americans [9]. The incidence rapidly increases with age. While one in ten people over the age 65 have the disease, one in three over the age of 85 are afflicted [9]. With 93,541 deaths in 2014, AD ranks as the 6th overall cause of death in the United States [10]. It is marked by prominent difficulty with memory, particularly short term memory, and the processing of new information [11]. Cognitive decline tends to begin later in life, in the seventh decade and beyond, and progresses slowly over the course of years to more significant impairment in all areas of cognition. The time course varies by age at symptom onset but tends to lead to death within 3–10 years [12,13,14].
Vascular dementia occurs as a result of clinical or subclinical ischemia in the setting of cerebrovascular disease. It shares the same risk factors for cardiovascular disease including hypertension, diabetes, and hypercholesterolemia. Vascular dementia can be secondary to large vessel strokes or pervasive small vessel damage, and accounts for an estimated 10–20% of all dementias, but frequently contributes to other dementia subtypes, creating a mixed pattern of pathology [6, 7, 15]. Patterns of cognitive loss tend to associate with the area of damage and often occur with focal neurologic deficits, particularly with larger vessel involvement. Small vessel disease often presents with memory retrieval deficits, slowed information processing, and subtle neuropsychiatric changes and executive dysfunction [15]. Vascular dementia has been described as having a “step-wise” pattern of cognitive and functional loss as opposed to the slow and progressive nature of AD. This step-wise pattern has periods of steep decline followed by more quiescent periods in which cognitive function may plateau, followed again by an acute worsening in function, presumably due to new areas of evolving ischemia. Vascular dementia may have a shorter overall survival, generally 3–5 years, with death often occurring due to underlying cardiovascular disease [14, 15].
Dementia with Lewy bodies (DLB) is estimated to account for 5–20% of cases, but estimates vary greatly between studies and some experts assert the prevalence may actually be much higher and under recognized [16, 17]. It should be suspected in patients with prominent visual hallucinations, especially if it is early in the disease course and out of proportion to other cognitive deficits. Parkinsonism and fluctuating levels of attention and alertness, similar to that seen in delirium are also hallmarks of the disease. Phenotypically similar, Parkinson’s disease dementia (PDD) is defined by cognitive deficits which begin to evolve at least 1 year after onset of the typical motor symptoms of Parkinson’s disease. Both DLB and PDD also tend to have prominent psychiatric disturbance, sleep disorders, autonomic instability, and marked sensitivity to antipsychotics in terms of motor side effects (see Chap. 5 “Parkinson’s Disease and Related Disorders”) [18]. DLB tends to have more rapid cognitive decline and shorter time from diagnosis to death than AD, with average survival from symptom onset of DLB being 5–7 years [16].
Frontotemporal dementia (FTD), which is the underlying etiology of 5–10% of cases results in prominent decline in executive function and language abilities, usually beginning at a younger age than other dementia syndromes [6]. The prevalence is higher in those with early onset dementia and approximately 60% of cases of FTD occur between the ages of 45 and 64 [19]. This most often manifests as behavioral issues, loss of social graces, and personality changes in patients with relatively intact memory. This can often delay diagnosis as it may be initially misdiagnosed as a primary psychiatric disorder. Other forms of FTD include primary progressive aphasia and semantic dementia, which are defined by gradually progressive expressive or receptive aphasia, respectively. Survival varies by subtype, but is generally 6–10 years after symptom onset [19].
The common dementias have distinct early stages, which facilitates clinical diagnosis, and may provide helpful insight into expected progression and prognosis. As disease progresses into more moderate and severe phases, resulting in significant functional and cognitive debility, the clinical distinction is less, and determining the underlying cause becomes difficult. At autopsy there are often mixed patterns of disease with multiple underlying pathologic patterns of disease present, with concomitant AD and vascular dementia being particularly common [7].
Alzheimer’s Disease Phenotype: Early Stages
The early stages of AD are marked by changes in memory and this remains the most frequent presenting symptom (Table 6.1). This usually begins with subtle difficulty with short-term memory and retaining of newly learned information, which often begins months or years prior to formal diagnosis. At this point, many patients are able to compensate for these cognitive deficits with the use of reminders or aides and these deficits are often attributed to normal aging. During this phase, patients often begin to have noticeable deficits in other cognitive domains as well as changes in mood and personality, which often manifests as social withdrawal, apathy, or loss of interest, often times appearing very much like depressive type symptoms.
These changes generally become evident to others as the patient begins to have difficulty with more challenging tasks, such as failure to maintain work performance and difficulty with complex tasks and instrumental activities of daily living (e.g. managing finances, cooking, and medication management). At this point, long-term memory and verbal fluency are generally intact.
Alzheimer’s Disease Phenotype: Advanced Stages
As the disease advances, everyday tasks (basic activities of daily living) become increasingly difficult and patients may require assistance with bathing, dressing, and eating. Incontinence and failure to maintain personal hygiene are also common. Declines also begin to occur in language abilities; maintaining a conversation is difficult. By now, the symptoms are generally obvious even to casual acquaintances. Throughout this process, memory continues to decline and long-term memory may begin to erode. Mobility decreases, falling is common, and parkisonism may arise as the dependence on others for basic care increases. In the final stages, sufferers are bed bound, unable to communicate verbally, and are totally dependent on others for all care needs. At this stage patients may need to be hand-fed to maintain oral intake.
Throughout the course of disease, but especially common in later stages, are changes in personality and behavioral patterns. These behavioral and psychological symptoms of dementia (BPSD) are challenging to treat and frequently a source of distress for caregivers. Early changes of loss of interest and depressed mood often give way to angry outbursts, restlessness, and frank agitation. As long as mobility is maintained, wandering is a potential issue, especially for those still living in the home. Sexual disinhibition combines with poor judgment and impulsivity, often leading to social situations that are distressing to caregivers and families. Insight, both to the cognitive decline and personality changes is generally minimal, especially in later stages.
Estimating Prognosis
Trajectories of Death
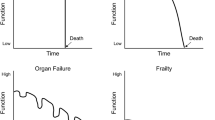
The timeline and trajectory of functional decline and, ultimately death, generally occurs over many years (See Chap. 1 “Neuropalliative Care: Introduction”, illness trajectories). The pervasive pattern is that of gradual and inexorable decline in function punctuated by periods of rapid deterioration. These rapid declines may occur as part of the primary disease process (new infarcts in vascular dementia), or secondary to other acute events, such as a hospitalization for hip fracture or pneumonia. Function after these acute declines may recover slightly but generally does not reach prior baseline, and may not occur at all. Dementia in general tends to follow a less predictable course than other terminal conditions, such as malignancy, with some prolonged phases of very gradual decline, or plateauing of function, which may confer a longer life span than expected. Conversely, acute illnesses and hospitalizations often trigger a downward spiral of functional decline, leading to death in a much quicker fashion than would be predicted from stage of dementia and baseline function alone. To further complicate matters, prognosis may be altered by treatment decisions and care planning, depending on a family’s goals of care. While some aggressive interventions, such as feeding tube placement, have not been shown to extend life [20], other interventions may impact survival such as the decision to not treat pneumonia with antibiotic agents [21].
Prognostication
In the final stages of all dementia, most afflicted are completely dependent and bedbound. With diminished appetite and difficulty feeding, malnutrition sets in. This is often accompanied by the development of skin breakdown and chronic wound formation. Common recurrent infections are urinary tract infections, wound infections, and pneumonia; aspiration is particularly common and often ultimately leads to death. Multiple staging systems and criterion exist to help identify those patients at high risk of mortality and disease related complications, as well to assist with timely referral to hospice services.
The Functional Assessment Staging (FAST) (Table 6.2) is a seven-stage framework for standardizing the categorization of the stage of dementia for an individual, and is often used in the determination for hospice eligibility (See Chap. 16 “Hospice and End of Life Care in Neurologic Disease”) [22]. It focuses more on an individual’s level of functioning and activities of daily living versus cognitive decline. The progression of functional and cognitive impairment in this staging model is primarily based on AD, but the staging also is used in other dementia subtypes, particularly in the late stages of disease. FAST scale 7 and subsequent substages (7A-7F) represent severe dementia, characterized by urinary and fecal incontinence, limited or no intelligible speech, inability to walk (or even to sit up unsupported in later phases), and ultimately inability to smile or hold up head.
Once this degree of debility exists, the prognosis is quite poor. In an 18-month longitudinal study of nursing home residents with advanced dementia, 25% of residents had died within 6 months, with a median survival of 1.3 years. Disease-related complications also became common; in the same study, the majority of patients developed nutritional problems and nearly half developed pneumonia or febrile episodes. Once these complications developed, 6-month mortality substantially increased, to nearly 50% [23].
Under the current Medicare guidelines, patients are eligible for hospice services when they have signs of both severe functional impairment (FAST stage 7) as well as disease-related complications including aspiration pneumonia, sepsis, multiple advanced stage (stage III/IV) pressure ulcers, recurrent fevers, or significant malnutrition and weight loss. There is evidence that the current guidelines are relativity poor at accurately predicting 6-month mortality, which may contribute to under referral to hospice services [24]. Other scoring systems can also be useful in predicting mortality and guiding hospice referral. Based on patient demographics in conjunction with functional status, presence of disease-related complications, and serious medical comorbidity, the ADEPT criterion were developed to help better guide prognostication for nursing home residents with dementia [25]. This has demonstrated improved, albeit modest accuracy in prognostication and can also be used to guide hospice referral [24].
The Palliative Care Approach Across Dementia Stages
Being a progressive and terminal disease, without effective disease-modifying treatments available, it is important to recognize the need for ongoing symptom management and support, as well as the transition ultimately towards end-of-life care. Early palliative care intervention, whether it be through a specialist or primary provider, is essential in high quality care for both the patient and family. The ideal palliative care services for these patients encompass a multifaceted approach; anticipating care needs and exploring goals of care, identifying and managing symptoms, and providing support to the patient and their family and caregivers. While a palliative care approach is certainly helpful throughout the course of the disease, symptom burden tends to increase with progressive decline and each major decrement in cognition or function should trigger revisiting of all the critical components of palliative care. The nature of dementia, with both functional and cognitive decline, presents unique challenges at all of these stages as the patient’s needs are changing. Table 6.3 provides triggers for serious conversation in dementia based on disease stage.
Mild Dementia
As significant cognitive changes begin in mild dementia, advance care planning should be addressed promptly. It is critical to have open discussion regarding anticipated decline and lack of curative therapy when the patient’s own decision-making is intact. Basic questions to establish who they trust most to manage their finances or to make medical decisions also function to identify critical caregivers and partners in care. This is the ideal time to open goals of care discussion. Establishing an advance directive that is in line with the patient goals while they are able to participate offloads burden from family and helps to avoid unwanted medical interventions down the line. Encouraging early involvement of surrogate decision makers in this discussion is also crucial. While a patient with dementia may want aggressive medical treatment early in the course of their disease, many wish to forgo life prolonging measures in the advanced stages [26], at a time they will depend on others, usually family or caregivers, to voice those wishes. Given the estimated prognosis of 3–10 years from diagnosis (based on dementia type, age, and comorbidities) it is also appropriate to discuss cessation of medical interventions requiring a prolonged time horizon to benefit, such as screening mammography and colonoscopy [27].
Moderate Dementia
The progression from mild to moderate dementia (FAST scale 5–6) is characterized by increasing care needs, development of new symptoms, particularly behavioral and psychological symptoms of dementia (BPSD), and more difficulty with decision-making (often leading to both loss of medical decision making capacity and safety concerns). Patients and their families should be carefully screened for difficulty in all of these areas.
Directly inquiring about behavioral and psychological symptoms, particularly wandering, agitation, mood changes, and aggression, can guide further evaluation and treatment. If not already done, progressive cognitive decline can also trigger evaluation for trial of pharmacologic therapy, such as an acetylcholinesterase inhibitor. Similarly, if not addressed previously, advance care planning and goals of care should be explored as the patient is still likely able to provide some guidance and input into their wishes, even if they are unable to fully understand the terminal nature of their disease.
Safety becomes a paramount concern as disease progresses, and counseling patients and caregivers in an attempt to maintain maximal safe level of independence is key. While many patients with mild dementia continue to drive safely, as the disease progresses driving ability becomes significantly impaired, with lower scores on road-testing and more motor vehicle collisions. Patients with dementia are also more likely to continue driving despite prior accidents [28]. Clinicians should directly inquire about accidents, or near accidents, and discuss safety concerns with family and caregivers. If there remains a question regarding driving safety, or if the patient is hesitant to stop driving, it can be helpful to refer them to a local resource for a formal driving evaluation. Clinician reporting of suspected unsafe driving practices varies by state and should be reviewed through the state department of motor vehicles.
Older adults with dementia are also at elevated risk of abuse; psychological, physical, financial, and neglect, with psychological abuse and financial exploitation being the most common [29, 30]. Most perpetrators are caregivers, either an adult child or spouse, which may impact self-reporting but patient physical, verbal or sexual abuse of their caregiver is not uncommon [30,31,32]. Multiple screening tools and questionnaires exist, but there is not an evidence-based consensus on a preferred screening method [33, 34]. Medical providers are morally obligated (and legally mandated in most states) to report suspected abuse. Information about local resources and contact information for Adult Protective Services can be found through the National Center for Elder Abuse.
As care needs and caregiver burden are increasing, the transition to moderate dementia should trigger referral to social work or local agencies if not already done earlier in the disease. This can help in identifying patients whose needs cannot be met in the current setting and who need more care in the home or a more supportive environment such as assisted living or nursing home placement. They can also function to connect patients and their families to local resources for both physical assistance and emotional support.
Severe (Advanced) Dementia
The transition to severe dementia (FAST scale 7) is characterized by markedly impaired cognition, functional dependence, as well as the development of disease-associated secondary complications, such as pneumonia and pressure ulcer development. Focus should continue on identifying and managing symptoms that are present, with particular emphasis on evaluation of pain as the ability to localize symptoms becomes severely impaired. Ongoing caregiver evaluation and support should also continue, as most patients with severe dementia require intensive 24-hour supervision.
As disease progresses and life expectancy becomes limited, how best to approach medical care to meet a patient’s needs and goals shifts. While continuing attempts at life prolonging and preventive measures, such as a statin medications for hypercholesterolemia and bisphosphonates for osteoporosis, is reasonable initially, this should be revisited over time. As disease progresses, burdens of continuing treatments mount; patients are often more resistive to taking medications, frequently have concomitant dysphagia, have difficulty remembering to take medications, may take them incorrectly, and are more sensitive to side effects of polypharmacy. It is important at this point to revisit the time horizon for benefit for any therapeutic interventions, including the sum total of medications prescribed. A patient with severe dementia whose prognosis is months to 1–2 years are unlikely to benefit from medications requiring prolonged periods of time to see positive clinical outcomes. Given this, prime considerations for “de-prescribing” efforts in advanced dementia such as the lipid-lowering agents, antihypertensive, bisphosphonates, and acetylcholinesterase inhibitors. Nonetheless, prevalence of prescribing these medications near end of life remains high, nearly 50% in one study of Medicare beneficiaries [35].
Throughout the course of disease, revisiting goals of care is critical, shifting focus from prolonging life to maintaining quality of life and personal dignity is appropriate, especially in the terminal stages of disease. Those patients with severe functional impairment (FAST stage 7) and evidence of secondary complications related to their disease are eligible for hospice services.
Capacity Evaluation
The changes which begin in mild cognitive impairment, even prior to the development of frank clinical dementia impact the patient’s ability to understand and process complex medical decisions, particularly if deficits in verbal fluency and executive function are present [36, 37]. While most patients with mild dementia retain decision-making capacity [38] as disease progresses this is lost, making the identification of a surrogate decision maker and establishment of advance directives early in the disease a clinical imperative. Capacity should be evaluated on initial diagnosis and also with progression of disease. Demonstrating capacity requires that a patient show understanding of the information they have been given, are able to apply that understanding to their own health, manipulate this in a logical fashion consistent with their values, and express a choice. This should be decision specific; while a patient may be able to name a health care agent more complex decisions such as forgoing hospitalization for acute illness may need to be made by a surrogate decision maker. Even when a patient does lack capacity they should be involved in discussions as much as is plausible and still may be able to provide insight into their values, hopefully taking some of decision-making burden off of their surrogate decision maker.
Symptom Assessment and Management
Cognitive Treatment
Symptoms can be challenging to assess in a patient with dementia and also difficult to target with safe, effective treatment. Effective symptom management is important throughout the spectrum of disease but becomes especially critical as patients enter the final stages of disease. Treatment of cognitive symptoms of disease is both complex and patient specific. While several pharmacologic treatment options for dementia exist, their efficacy is limited and true benefit is likely small for most patients. The first class of medications, acetylcholinesterase inhibitors (donepezil, rivastigmine, galantamine) are associated with small improvements in cognition and functional status for some patients. Unfortunately, this does not translate to demonstrable effects on disease progression, entry into the nursing home setting, or overall prognosis [39, 40]. There is no clear consensus as to how long to continue these medications and at what point to discontinue, but there is little evidence supporting the continuation of these agents once disease is severely advanced. Side effects upon initiation of medications are common with acetylcholinesterase inhibitors (nausea or diarrhea), but longer-term side effects can be potentially life threatening such as bradycardia or complete heart block. For those with moderate to severe disease, memantine can also be used for treatment of cognitive symptoms. Similar to the acetylcholinesterase inhibitors, there is some small improvement in scores on testing of cognition and function, but the margin of benefit is small and may not be overly clinically significant [39]. In both cases, it is reasonable to reassess patients for cognitive improvement after a therapeutic trial, with taper and discontinuation if no significant clinical improvement is noted.
Whether or not a patient is started on pharmacologic treatment, ongoing non-pharmacologic management of cognitive symptoms is needed. Early in the disease course, reminders and notes may be helpful to allow patients to cope with cognitive deficits and declining memory. As the disease progresses, working with the patient and family (or care facility) to provide the highest level of independence without adding undo risk of harm is critical.
Dysphagia and Weight Loss
Particularly in advanced disease, weight loss and dysphagia become apparent. This is often distressing to family and caregivers; addressing this as an anticipated disease complication early on can be helpful in managing expectations going forward. The dysphagia that develops is not reversible; dietary consistency modifications help to some degree but do not prevent aspiration and often the change in texture may not be palatable to some patients and contributes to decreased nutritional intake at meals. Similarly, feeding tubes do not decrease or prevent aspiration pneumonia or pressure ulcers in advanced dementia and are not indicated for the treatment of dysphagia and weight loss in this setting [20, 41]. Progressive weight loss also occurs from a combination of dysphagia, functional decline, difficulty with feeding, and cognitive decline with decreased appetite and drive to eat. Careful hand feeding remains the standard treatment to maintain nutritional status and prevent aspiration for as long as possible. Oral nutritional supplements are also frequently recommended for caloric supplementation, and do lead to weight gain [42], although it is unclear what impact this has on clinical outcomes, such as prognosis or pressure ulcer formation.
Behavioral and Psychological Symptoms
Particularly early in the disease or soon following the diagnosis of dementia, a patient may experience grief, frustration, guilt, boredom, and other difficult mood or behavioral symptoms, which may be normal emotional reactions to a diagnosis of dementia. Depression and suicide risk screening as well as ongoing psychosocial support for the patient and caregiver are critical elements. Perhaps the most troubling type of symptom, particularly with moderate-severe dementia, is the behavioral and psychological symptoms of dementia (BPSD). These symptoms can be especially problematic for caregivers and can be a trigger for entry into long term care. BPSD will affect the vast majority of those with dementia at some point in their disease. The most common symptoms are apathy, depression, agitation, and wandering. While less common, frank psychosis with hallucinations and delusions, as well as violent or aggressive behavior, are especially problematic [43, 44].
The first step in evaluation of BPSD is screening for a source of distress. Often an unmet physical need: pain, hunger, thirst, the need to urinate or defecate, triggers behavioral symptoms as the patient is unable to make this known in any other way [45]. Psychological distress or the need for emotional connection can also be a trigger. A patient with a history of past trauma, such as physical or sexual abuse is likely to feel threatened with personal care and responds in the only way they can; with agitation and violence. Patterns of behavior tend to manifest over time and with careful observation and history, and modifications to environment can often be sufficient to manage behavioral symptoms.
Clinical evaluation of patients with BPSD is also critical, particularly if this is a new or progressively worsening symptom. In addition to unmet physical or emotional needs, injury or acute illness is another common trigger. Pain is an especially important factor to evaluate for, but can be difficult to assess and localize in dementia. While self-report is considered the standard for pain assessment, many patients with dementia do not reliably report pain when compared to objective pain measurements [46]. For most patients, pain evaluation includes a history with screening questions for pain, as well as for recent falls, medical procedures, or changes in condition. For patients with moderate-severe dementia, direct observational tools should be employed to screen for pain. While no single gold standard test exists, multiple scales such as the PAINAD (Table 6.4), Abbey score, and the CNPI scoring system can be used [47]. While there have been very few high quality studies regarding the blanket use of analgesics in agitation, what evidence there is does support their use. In a study of nursing home residents the addition of analgesics in a stepwise approach, based on the American Geriatric Society guidelines (generally acetaminophen followed by a low dose opioid) resulted in significant decreases in pain scores, behavioral disturbances, and agitation, particularly verbal agitation [48, 49]. Most of these patients (70%) were treated with acetaminophen alone and did not require the addition of opioid agents to achieve this response. Once therapy has been initiated, close follow-up for improvement in behavioral symptoms and subjective or objective signs of pain is warranted.
Behavioral management and environmental modifications should always be the first line intervention when addressing troubling symptoms. When no medical trigger or other reversible cause is identified and non-pharmacologic interventions have failed, medical management of behavioral or psychological symptoms can be considered. Although the effect is modest, acetylcholinesterase inhibitors and memantine can be helpful in some cases to treat BPSD [50, 51]. Citalopram or sertraline have also been used to reduce agitation and treat BPSD [52] and should also be strongly considered, especially when underlying anxiety or depression is suspected. Trazodone has also been shown to increase nocturnal sleep time in patients with dementia and insomnia [53]. Other medications are often used but with less evidence, such as are mirtazapine and divalproic acid [53]. Benzodiazepines and anticholinergics should generally be avoided in the management of behavioral or psychological symptoms due to their potential for precipitating delirium and worsening cognitive symptoms.
As antipsychotics have only modest efficacy in the treatment of agitation and aggression, and significant risk of adverse events, their use should be restricted to those with evidence of significant psychosis (often manifested by delusions or hallucinations) causing distress or danger to the patient or caregiver [54, 55]. The efficacy of antipsychotics even in the treatment of delirium has been called into question, with a recent study investigating their use in patients receiving palliative care services revealed no reduction in delirium severity or symptom burden [56]. These medications, both typical and atypical, should be initiated at low doses and to target a particular symptom, such as paranoid or persecutory delusions causing distress to the patient. They should be tapered and discontinued if there is no improvement, or the patient has remained stable for several months. Doses are typically much lower than for primary psychiatric disorders. Antipsychotics are associated with serious side effects and increased risk of death in patients with dementia, although their causal role in hastened death remains controversial. Important caveats include patients with underlying severe mental illness, such as schizophrenia or bipolar disorder with mania, for whom coordination with psychiatric providers is helpful.
DLB is associated with high rates of BPSD with psychotic symptoms, particularly visual hallucinations. It also confers high risk of severe neuroleptic sensitivity; parkinsonism, increased confusion, autonomic dysfunction, neuroleptic malignant syndrome, and even death. Most typical and atypical antipsychotics should be avoided in patients with a known or suspected history of DLB. For these patients, when possible, reduction of any psychoactive medications should be a first step for managing psychotic symptoms, starting by eliminating dopamine agonists, anticholinergics, and MAO-inhibitors. Then reducing or eliminating carbidopa/levodopa if the patient is receiving, especially considering that classically the motor symptoms related to DLB is less responsive to the dopaminergic medications compared to idiopathic Parkinson’s disease. Quetiapine or clozapine are considered first line antipsychotics for patients with DLB, as they have a significantly lower antidopaminergic profile than other typical and atypical antipsychotics and may be used if symptoms are refractory to all other interventions or motor symptoms worsen with reductions in dopaminergic therapy. Pimavanserin is a newer serotonergic atypical antipsychotic that may also be considered in this population but has been associated with a worsening of psychotic symptoms in approximately 10% of patients and high mortality [57].
Caregiver Support
Dementia is also very distressing for family members and caregivers. Nearly half of the estimated 6.5 million Americans who are providing substantial assistance to an older adult are faced with caring for a loved one with dementia [58]. Caregivers often feel the burden of progressive cognitive and functional loss more than the patient themselves, which is associated with poor health outcomes for the caregiver (both psychological and physical) as well as early nursing home placement for the patient. Caregiver burden generally increases as disease progresses and is particularly high in patients experiencing the BPSD including wandering, agitation, or aggression [59]. Assessing caregiver burden is critical in providing good patient and family centered care.
Multiple screening tools exist to assess caregiver burden, including the Zarit Burden Interview and Caregiver Strain Index, which attempt to quantify the amount of financial strain, emotional distress, and the impact of caregiving on their social and family life [59,60,61]. During medical evaluations of the patient, questions targeting the hopes and fears of not only the patient, but also the caregiver are helpful. The progression from mild to moderate dementia is characterized by the loss of independence and an emergence of new symptoms. Care needs in this transition point also increase, so realistic expectations and frank discussion with family and caregivers is critical in identifying patients whose needs cannot be met in the current setting and who need more care in the home or a more supportive environment such as assisted living or nursing home placement. Questions can be as specific and identify practical concerns for the future, such as: “How will you be able to care for your mother when she is no longer is able to use the toilet on her own?” “What if your father falls at home and is unable to contact anyone for help?” Providing anticipatory guidance for the future and encouraging proactive planning can allay caregiver anxiety and burden. An in-office social worker or outside referral to community resources, such as the Alzheimer’s Association, can be extraordinarily helpful for patients and their families. These services can provide educational resources and help caregivers become connected with support groups, respite, or day care services. They can also help with assessment of the patient and their caregivers’ financial situation and guidance for the future. Referral to home care and physical/occupational therapy can also help make environmental changes, such as the addition of a commode, which can foster safety and decrease burden of providing care. These steps to support caregivers are necessary to keep patients in the home as long as is feasible (see Chap. 20 “Caregiver Assessment and Support”).
End-of-Life Care
Patients with dementia are frequently subjected to aggressive treatment at the end of life, with 40% being subjected to burdensome treatments, such as hospital evaluation or tube feeding in the last 3 months of life [23]. This can be mitigated by ongoing evaluation and referral to hospice for those with severe dementia and evidence of disease related complications. For those patients whose goals align with their philosophy, hospice provides the most robust support services to augment care and keep patients at home when possible. Whether end of life occurs in the home, a nursing home, or hospital, attention should be paid to identifying and treating underlying symptoms. As discussed above, pain should be evaluated and treated based on observational tools, not just patient or caregiver report. As pneumonia and cardiovascular disease are very common causes of death, dyspnea should be monitored and treated if bothersome to the patient, generally with opioids in addition to any appropriate adjuvant agents (such as diuretics in the treatment of congestive heart failure). Dehydration is also common, and may be the underlying cause of death, so attention to oral care with liberal use of oral lubricants is indicated. As opposed to earlier in the disease, in the imminently dying patient, it is reasonable to trial benzodiazepines for refractory anxiety or dyspnea, and anticholinergic agents, such as scopolamine, for secretions (see Chap. 16 “Hospice and End of Life Care in Neurologic Disease”).
Education and Research Agenda
Given the extremely high prevalence of neurodegenerative dementias in older adults and the common occurrence of cognitive deficits and dementia due to other neurologic disorders (e.g. multiple sclerosis, stroke, glioblastoma) there is a great need for primary palliative care education in neurology to address the effects of dementia on symptom assessment, caregiver distress, goals of care discussions, advance care planning and end-of-life care. Fellowships in behavioral neurology, neuropsychiatry, geriatric medicine, and geriatric psychiatry should also plan to cover these issues in depth.
There is a pressing need for better tools to assist in prognostication in advanced dementia which could facilitate earlier hospice referral and be utilized to coordinate home, community, and specialty-based services to meet patient needs. More attention needs to focus upon the level of the patient and caregiver, to develop evidence-based interventions to decrease caregiver stress/burden and maintain and enhance care at home in order to avoid or delay the need for institutional based care. Improved pharmacologic options for BPSD would be helpful for management of distressing symptoms, however the nonpharmacologic interventions and identification of a patient’s unmet needs will remain as a gold standard for quality person-centered care. Given the considerable prevalence of these conditions, there is need for cost-effectiveness, implementation, dissemination and healthcare policy research to develop best practices for caring for patients with dementia and care coordination to ensure the best outcomes for these vulnerable patients. These approaches must consider workforce issues, as palliative care specialists, and even general neurologists, will not likely fill all the gaps in the current system.
Take Home Messages
-
Alzheimer’s disease and other dementias are leading causes of disability, institutionalization and death in older adults.
-
Severe limitations of functional abilities, recurrent infections, weight loss and impaired mobility and communication abilities should suggest consideration for hospice referral.
-
Safety must be assessed when developing plans of care including driving safety, wandering, falls, and potential for physical, verbal/emotional and financial abuse.
-
Goals of care and advance care planning should be completed as early as feasible while patients have decision-making capacity and can meaningfully contribute to discussions regarding goals of care.
-
For patients with behavioral issues, such as agitation, treatable causes (e.g. pain) and environmental triggers should be considered before utilizing pharmacologic treatments.
References
Association AP. Diagnostic and statistical manual of mental disorders. 5th ed. Alrington: American Psychiatric Association; 2013. p. 591–644.
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s & dementia. J Alzheimers Assoc. 2013;9(1):63–75.e2.
Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–32.
Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer’s disease (2010–2050). Alzheimers Dement: J Alzheimers Assoc. 2014;10(2):e40–6.
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2017;13(4):325–73.
Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203–12.
Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204.
Katzman R. The prevalence and malignancy of Alzheimer disease: a major killer. Alzheimers Dement: J Alzheimers Assoc. 2008;4(6):378–80.
Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83.
Kochanek KDMS, Xu JQ, Tejada-Vera B. Deaths: final data for 2014. Hyattsville: National Center for Health Statistics; 2016. Contract No.: 4.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939.
Zanetti O, Solerte SB, Cantoni F. Life expectancy in Alzheimer’s Disease (AD). Arch Gerontol Geriatr. 2009;49:237–43.
Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140(7):501–9.
Kua EH, Ho E, Tan HH, Tsoi C, Thng C, Mahendran R. The natural history of dementia. Psychogeriatr: Off J Japan Psychogeriatr Soc. 2014;14(3):196–201.
O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–706.
Mueller C, Ballard C, Corbett A, Aarsland D. The prognosis of dementia with Lewy bodies. Lancet Neurol. 2017;16(5):390–8.
Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44(4):673–83.
Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet. 2015;386(10004):1683–97.
Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet (London, England). 2015;386(10004):1672–82.
Sampson EL, Candy B, Jones L. Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst Rev. 2009;(2):CD007209.
Givens JL, Jones RN, Shaffer ML, Kiely DK, Mitchell SL. Survival and comfort after treatment of pneumonia in advanced dementia. Arch Intern Med. 2010;170(13):1102–7.
Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull. 1988;24(4):653–9.
Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–38.
Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929–35.
Mitchell SL, Miller SC, Teno JM, Davis RB, Shaffer ML. The advanced dementia prognostic tool: a risk score to estimate survival in nursing home residents with advanced dementia. J Pain Symptom Manag. 2010;40(5):639–51.
Black BS, Fogarty LA, Phillips H, Finucane T, Loreck DJ, Baker A, et al. Surrogate decision makers’ understanding of dementia patients’ prior wishes for end-of-life care. J Aging Health. 2009;21(4):627–50.
Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ: Br Med J. 2013;346:e8441.
Breen DA, Breen DP, Moore JW, Breen PA, O’Neill D. Driving and dementia. BMJ. 2007;334(7608):1365–9.
Lichtenberg P. The intersection of financial exploitation and financial capacity. Am Psychol. 2016;71(4):312–20.
Boye F, Yan E. Abuse of older persons with dementia. Trauma Violence Abuse.0(0):1524838016650185.
Thomson MJ, Lietzau LK, Doty MM, Cieslik L, Williams R, Meurer LN. An analysis of elder abuse rates in Milwaukee county. WMJ: Off Publ State Med Soc Wis. 2011;110(6):271–6.
Bruno V, Mancini D, Ghoche R, Arshinoff R, Miyasaki JM. High prevalence of physical and sexual aggression to caregivers in advanced Parkinson’s disease. Experience in the Palliative Care Program. Parkinsonism Relat Disord. 2016;24:141–2.
Wiglesworth A, Mosqueda L, Mulnard R, Liao S, Gibbs L, Fitzgerald W. Screening for abuse and neglect of people with dementia. J Am Geriatr Soc. 2010;58(3):493–500.
Fulmer T, Guadagno L, Bitondo Dyer C, Connolly MT. Progress in elder abuse screening and assessment instruments. J Am Geriatr Soc. 2004;52(2):297–304.
Holmes HM. Rational prescribing for patients with a reduced life expectancy. Clin Pharmacol Ther. 2009;85(1):103–7.
Abu Snineh M, Camicioli R, Miyasaki JM. Decisional capacity for advanced care directives in Parkinson’s disease with cognitive concerns. Parkinsonism Relat Disord. 2017;39:77–9.
Okonkwo OC, Griffith HR, Copeland JN, Belue K, Lanza S, Zamrini EY, et al. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71(19):1474–80.
Moye J, Karel MJ, Azar AR, Gurrera RJ. Capacity to consent to treatment: empirical comparison of three instruments in older adults with and without dementia. The Gerontologist. 2004;44(2):166–75.
Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–97.
Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004;363(9427):2105–15.
Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA. 1999;282(14):1365–70.
Volkert D, Chourdakis M, Faxen-Irving G, Frühwald T, Landi F, Suominen MH, et al. ESPEN guidelines on nutrition in dementia. Clin Nutr. 2015;34(6):1052–73.
Borsje P, Wetzels RB, Lucassen PL, Pot AM, Koopmans RT. The course of neuropsychiatric symptoms in community-dwelling patients with dementia: a systematic review. Int Psychogeriatr. 2015;27(3):385–405.
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–83.
Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762–9.
Malara A, De Biase GA, Bettarini F, Ceravolo F, Di Cello S, Garo M, et al. Pain assessment in elderly with behavioral and psychological symptoms of dementia. J Alzheimers Dis : JAD. 2016;50(4):1217–25.
Lichtner V, Dowding D, Esterhuizen P, Closs SJ, Long AF, Corbett A, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014;14:138.
Husebo BS, Ballard C, Aarsland D. Pain treatment of agitation in patients with dementia: a systematic review. Int J Geriatr Psychiatry. 2011;26(10):1012–8.
Husebo BS, Ballard C, Cohen-Mansfield J, Seifert R, Aarsland D. The response of agitated behavior to pain management in persons with dementia. Am J Geriatr Psychiatry. 2014;22(7):708–17.
Rodda J, Morgan S, Walker Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int Psychogeriatr. 2009;21(5):813–24.
Kishi T, Matsunaga S, Iwata N. The effects of memantine on behavioral disturbances in patients with Alzheimer’s disease: a meta-analysis. Neuropsychiatr Dis Treat. 2017;13:1909–28.
Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2):CD008191.
McClam TD, Marano CM, Rosenberg PB, Lyketsos CG. Interventions for neuropsychiatric symptoms in neurocognitive impairment due to Alzheimer’s disease: a review of the literature. Harv Rev Psychiatry. 2015;23(5):377–93.
Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The american psychiatric association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543–6.
Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry: Off J Am Assoc Geriatr Psychiatry. 2006;14(3):191–210.
Agar MR, Lawlor PG, Quinn S, Draper B, Caplan GA, Rowett D, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34–42.
Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–40.
Wolff JL, Spillman BC, Freedman VA, Kasper JD. A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med. 2016;176(3):372–9.
Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J Am Acad Nurse Pract. 2008;20(8):423–8.
Flynn Longmire CV, Knight BG. Confirmatory factor analysis of a brief version of the Zarit Burden Interview in Black and White dementia caregivers. Gerontologist. 2011;51(4):453–62.
Robinson BC. Validation of a caregiver strain index. J Gerontol. 1983;38(3):344–8.
Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4:9–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Caprio, T.V., Kosier, N. (2019). Dementia. In: Creutzfeldt, C., Kluger, B., Holloway, R. (eds) Neuropalliative Care. Springer, Cham. https://doi.org/10.1007/978-3-319-93215-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-93215-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93214-9
Online ISBN: 978-3-319-93215-6
eBook Packages: MedicineMedicine (R0)