Abstract
There is substantial evidence that disordered sleep negatively impacts both physical and psychological health. The evidence for the negative effects of the two most prevalent sleep disorders, insomnia and obstructive sleep apnea (OSA), on overall health is particularly strong (Centers for Disease Control and Prevention, 2014; Edinger, Grubber, Ulmer, Zervakis, & Olsen, 2016). Insomnia is defined as dissatisfaction with sleep quantity or quality with severity of sleep onset latency (time awake before sleep onset), time awake after sleep onset, or early morning awakenings of at least 30 min, at a frequency of 3 or more nights per week, for a duration of 3 months or more that is not directly attributable to substance use or another psychiatric disorder (American Psychiatric Association, 2013). OSA is a condition during which five or more obstructive apneas or hypopneas, an occurrence during which the airway is either partially or fully closed, occur per hour of sleep, with the presence of nocturnal breathing disturbances (i.e., snoring, snorting/gasping, or breathing pauses) or daytime sleepiness, fatigue, or unrefreshing sleep (American Psychiatric Association, 2013).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
There is substantial evidence that disordered sleep negatively impacts both physical and psychological health. The evidence for the negative effects of the two most prevalent sleep disorders, insomnia and obstructive sleep apnea (OSA), on overall health is particularly strong (Centers for Disease Control and Prevention, 2014; Edinger, Grubber, Ulmer, Zervakis, & Olsen, 2016). Insomnia is defined as dissatisfaction with sleep quantity or quality with severity of sleep onset latency (time awake before sleep onset), time awake after sleep onset, or early morning awakenings of at least 30 min, at a frequency of three or more nights per week, for a duration of three months or more that is not directly attributable to substance use or another psychiatric disorder (American Psychiatric Association, 2013). OSA is a condition during which five or more obstructive apneas or hypopneas, an occurrence during which the airway is either partially or fully closed, occur per hour of sleep, with the presence of nocturnal breathing disturbances (i.e., snoring, snorting/gasping, or breathing pauses) or daytime sleepiness, fatigue, or unrefreshing sleep (American Psychiatric Association, 2013).
Recent estimates suggest that up to 30% of adults suffer from insomnia (Budhiraja, Roth, Hudgel, Budhiraja, & Drake, 2011; Roth et al., 2011) and 17–22% of adults suffer from OSA (Franklin & Lindberg, 2015). Not only are these disorders prevalent but they also place individuals at risk for a wide variety of serious chronic health conditions, such as heart attack, stroke, and type 2 diabetes, as well as increased risk of death compared to those without these disorders (Cappuccio, D'Elia, Strazzullo, & Miller, 2010b; Marshall, Wong, Cullen, Knuiman, & Grunstein, 2014; Taylor et al., 2007). Given the widespread impact untreated insomnia and OSA can have on overall health, it is not surprising that these disorders are associated with significant direct and indirect costs at both the individual and the societal level (AlGhanim, Comondore, Fleetham, Marra, & Ayas, 2008; Ayas et al., 2006; McCrae, Bramoweth, Williams, Roth, & Mosti, 2014; Ozminkowski, Wang, & Walsh, 2007).
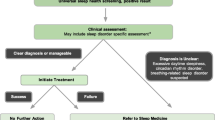
The primary care setting likely provides the best opportunity to screen for common sleep disorders like insomnia and OSA, as this is often where sleep problems are first reported (Israel & Lieberman, 2004). In fact, OSA is most frequently diagnosed by primary care providers (PCPs) (Namen et al., 2002), and patients with insomnia typically first seek help in primary care (Aikens & Rouse, 2005). Estimates suggest that up to 50% of patients in primary care settings may be affected by insomnia (Arroll et al., 2012), and up to 10% of primary care patients are likely to meet the full criteria for an insomnia disorder (Arroll et al., 2012; Goodie & Hunter, 2014). Despite the frequency at which primary care patients experience disordered sleep, screening, assessment, and treatment/management of sleep disorders, like chronic insomnia and OSA, in the primary care setting, are often limited (Edinger et al., 2016; Lugtenberg, Zegers-van Schaick, Westert, & Burgers, 2009; Sake, Wong, Bartlett, & Saini, 2017). Infrequent assessment of sleep disorders in primary care results in as few as 1.2% of primary care patients being diagnosed with insomnia (Goodie & Hunter, 2014). These findings suggest that a substantial number of patients are not only at risk of their sleep disorders going undetected and untreated but are also at increased risk for developing chronic health problems related to their untreated sleep problems.
Behavioral medicine providers are well equipped to provide medical professionals and the general public with information about sleep disorders and associated health outcomes, assess for a variety of sleep complaints, facilitate inter-professional consultation and collaboration, and provide effective treatment for insomnia (Edinger et al., 2016; McDaniel et al., 2014). There is compelling evidence that providing behavioral medicine interventions within primary care instead of a specialty mental health clinic increases engagement in insomnia treatment (Bartels et al., 2004; Goodie & Hunter, 2014). Given insomnia and OSA are most often reported in primary care and given that patients are more likely to engage in behavioral medicine interventions offered in a primary care setting, offering a sleep-focused behavioral medicine evaluation for common sleep disorders in primary care clinics via an integrated behavioral medicine provider offers the best opportunity to engage patients in treatment.
The Negative Effects of Insomnia and Obstructive Sleep Apnea on Health
The definition of abnormal sleep duration varies widely; however, the National Sleep Foundation and the American Academy of Sleep Medicine/Sleep Research Society define normative sleep to be between 7 and 9 h per night (Hirshkowitz et al., 2015; Watson et al., 2015). Definitions of short sleep in the research literature range from less than 7 h per night to less than 4 h per night, and long sleep definitions range from greater than 8 h per night to greater than 12 h per night (Cappuccio, Cooper, D'Elia, Strazzullo, & Miller, 2011; Cappuccio et al., 2010b). Abnormal sleep , which could be a consequence of insomnia, OSA, or other sleep, psychiatric, or medical disorders, has a variety of deleterious health effects. Both long and short sleep increase the risk for self-rated poor health, chronic disease, and mortality (Cappuccio et al., 2011, 2010b). While recent evidence suggests that long sleep (typically 9+ hours/night) may be more detrimental to health than short sleep (Cai et al., 2015), there is also a notable amount of data that suggests short sleep increases engagement in a variety of negative health behaviors (e.g., increased intake of unhealthy foods, physical inactivity, substance use) and increases risk for many negative health outcomes, such as obesity, high cholesterol, type 2 diabetes, hypertension, heart attacks, and death (Cappuccio et al., 2011; Gangwisch et al., 2010; Grandner, Patel, Gehrman, Perlis, & Pack, 2010; Liu et al., 2013; McKnight-Eily et al., 2011; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Shankar, Charumathi, & Kalidindi, 2011; Vgontzas, Liao, Bixler, Chrousos, & Vela-Bueno, 2009). While an insomnia diagnosis does not require short sleep to be present, short sleep is often a consequence of an insomnia disorder (Fernandez-Mendoza, 2017; Vgontzas et al., 2009, 2010).
Chronic insomnia has strong associations with poor outcomes across a variety of medical symptoms and disorders. Specifically, individuals with insomnia symptoms have significantly lower cardiorespiratory fitness (Strand et al., 2012) and are more likely to experience breathing problems, urinary problems, gastrointestinal problems, chronic pain (Taylor et al., 2007), metabolic syndrome (Troxel et al., 2010), type 2 diabetes (Cappuccio, D'Elia, Strazzullo, & Miller, 2010a), cardiovascular symptoms, myocardial infarctions, and cardiovascular disease (Conley & Redeker, 2015; D. J. Taylor et al., 2007) than individuals without insomnia symptoms. Individuals with insomnia are significantly more likely to experience decreased cognitive and occupational functioning (Fernandez-Mendoza, 2017), depression (Baglioni et al., 2011; Fernandez-Mendoza, 2017), worse perceived health (Fernandez-Mendoza, 2017), and lower quality of life (Baglioni et al., 2011) than individuals without insomnia. There is strong evidence suggesting that patients with both short sleep and insomnia are at further increased risk for these negative health outcomes, particularly if they are men. Studies indicate that men with insomnia are up to four times more likely to experience the abovementioned health outcomes than men without insomnia symptoms, whereas the risk of negative health outcomes for women is less clear (Fernandez-Mendoza, 2017; Vgontzas et al., 2010).
Given the myriad of physiological, cognitive, and emotional symptoms associated with insomnia and OSA, it is not surprising that individuals with insomnia report a significantly lower quality of life , defined by the World Health Organization as a subjective evaluation of one’s life within the context of larger culture in which they live and in relation to one’s individual goals, values, expectations, and standards (The WHOQOL Group, 1995). Reviews of empirical studies evaluating quality of life using multidimensional self-report measures indicate that individuals with insomnia report significantly lower occupational, physical, emotional, and social functioning (Ishak et al., 2012; Leger et al., 2012). These studies indicate that all domains that compose an individual’s quality of life are impacted by insomnia. In fact, the negative impact of insomnia on quality of life is so universal that similar findings are present in studies conducted throughout the world (Leger et al., 2012).
Untreated OSA is also associated with a number of negative health outcomes . Specifically, longitudinal studies suggest that untreated OSA is positively associated with body mass index (BMI), diabetes mellitus (Gottlieb et al., 2010), high systolic blood pressure, hypertension (Gottlieb et al., 2010; Redline et al., 2010), and low high-density lipoprotein cholesterol (HDL; i.e., “good” cholesterol; (Centers for Disease Control and Prevention, 2015; Gottlieb et al., 2010). Findings suggest that, for men, risk of heart failure and stroke increases as OSA severity increases (Gottlieb et al., 2010; Marshall et al., 2014; Redline et al., 2010). Individuals with moderate to severe OSA are also 2.5 times more likely to be diagnosed with cancer and 3.4 times more likely to die from cancer than individuals without OSA (Marshall et al., 2014). All-cause mortality risk is up to 4.2 times higher for men and women with moderate to severe OSA than those without OSA. As with risk for heart failure and stroke, mortality risk (e.g., risk of death) increases with severity of OSA. These findings suggest that identification and treatment of OSA can significantly reduce a patient’s morbidity and mortality risk, especially for men (Gottlieb et al., 2010; Marshall et al., 2014; Redline et al., 2010). Studies evaluating the impact of untreated OSA on quality of life suggest that individuals with untreated OSA also report lower quality of life than both those without OSA (Yang et al., 2000) and those with OSA treated with continuous positive airway pressures (CPAP; (Batool-Anwar et al., 2016; Chai-Coetzer et al., 2013).
Untreated insomnia and OSA are both linked to a wide variety of negative health outcomes and decreased quality of life, suggesting that screening for sleep disorders and providing insomnia treatment in the primary care setting are important to maximize patient well-being and minimize risk for chronic disease. Behavioral medicine providers are particularly well equipped to perform both of these tasks. Greater integration of behavioral medicine providers and primary care significantly increases access to assessment of sleep disorders and non-pharmacological, evidence-based treatment of chronic insomnia that would otherwise only be accessible to patients receiving care in specialty mental health and sleep disorder clinics.
Insomnia Treatment: Recommendations and Practice
The American College of Physicians (Qaseem et al., 2016) suggests more efficient and effective delivery of adapted insomnia treatments provided by behavioral medicine providers (e.g., clinical psychologists, licensed clinical social workers) within the primary care setting is key to providing care to the largest number of patients suffering from insomnia with evidence-based treatment. Fortunately, behavioral medicine providers within primary care have a particularly effective tool with which to treat primary care patients with insomnia: cognitive behavioral therapy for insomnia (CBTI) . CBTI is an evidence-based psychotherapy, typically delivered in individual sessions (20–50 min) as well as in group format (60–90 min). CBTI utilizes multiple components, including psychoeducation about sleep and insomnia, stimulus control, sleep restriction, cognitive therapy and cognitive restructuring, relaxation techniques, and sleep hygiene. Stimulus control in the context of CBTI involves strengthening the association of the bed and bedroom environment to sleep via going to bed and waking at specific times, getting out of bed when not sleeping and not returning until sleepy, and eliminating behaviors other than sleep and sexual activity in the bed. Sleep restriction involves temporarily restricting time in bed to approximate the duration of time the individual is currently sleeping and gradually expanding the total allowable time in bed until the individual reaches a satisfactory duration of quality of sleep. Cognitive therapy and cognitive restructuring involve enacting cognitive behavioral techniques to reduce worry about sleep and correcting inaccurate and maladaptive thoughts and beliefs about sleep, while relaxation techniques, such as progressive muscle relaxation and mindful breathing, are used to prepare the body for sleep. The sleep hygiene component of CBTI involves changing lifestyle factors that may be contributing to insomnia symptoms (e.g., use of caffeine or tobacco products close to bedtime, eating large meals close to bedtime, daytime napping). Individuals also typically maintain a daily sleep log that documents nightly bedtime, sleep onset latency, number of awakenings, and duration of time awake after sleep onset. This allows a clinician or, with education, the patient to calculate their time in bed, total sleep time, and sleep efficiency and to customize and titrate their prescribed sleep schedule.
CBTI has a strong evidence base (Jacobs, Pace-Schott, Stickgold, & Otto, 2004; Koffel, Koffel, & Gehrman, 2015; Mitchell, Gehrman, Perlis, & Umscheid, 2012; Morin, Culbert, & Schwartz, 1994; Morin et al., 2009; Smith et al., 2002; Taylor et al., 2014; Trauer, Qian, Doyle, Rajaratnam, & Cunnington, 2015; Wu, Appleman, Salazar, & Ong, 2015) and is effective in a variety of settings and formats (Manber et al., 2012; Trockel, Karlin, Taylor, & Manber, 2014). There is evidence that the benefits of CBTI extend beyond improving sleep quality to improvements in medical and psychological health (Baglioni et al., 2011; Blom et al., 2015; Conley & Redeker, 2015; Roane & Taylor, 2008; Troxel et al., 2010; Watanabe et al., 2011). Due to the strong evidence base, multiple health organizations and institutions recommend CBTI as the first-line treatment for insomnia, including the National Institutes of Health (National Institutes of Health, 2005), the Department of Veterans Affairs (2007), the British Association for Psychopharmacology (Wilson et al., 2010), and most recently the American College of Physicians (Qaseem et al., 2016). Treatment recommendations and guidelines disseminated by these institutions emphasize that CBTI be provided prior to pharmacologic interventions for most individuals with insomnia disorder.
A recent review of pharmacologic treatments for insomnia disorder conducted by the American College of Physicians outlines the existing evidence base for medications approved by the Federal Drug Administration (FDA ) for use in the treatment of insomnia disorder (Wilt et al., 2016). The authors identified several classes of medications that are prescribed to treat insomnia disorders, including nonbenzodiazepine “Z” drugs (i.e., eszopiclone [Lunesta], zaleplon [Sonata], zolpidem [Ambien], zolpidem extended-release [Ambien CR], zolpidem sublingual [Edluar], and zolpidem sublingual [Intermezzo]), an orexin receptor antagonist (suvorexant [Belsomra]), melatonin, a melatonin receptor agonist (ramelteon [Rozerem], and a tricyclic antidepressant (doxepin [Silenor]), and benzodiazepines including estazolam (Prosom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Based on the reviewed evidence, the authors conclude that eszopiclone, zolpidem, and suvorexant may improve short-term global outcomes and sleep variables. However, they clarify that the absolute effect of these medications on insomnia symptoms were not sufficient to indicate remission of symptoms, and there was evidence for harms.
A subsequent comprehensive review and clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults published by the American Academy of Sleep Medicine provides only weak recommendations for the use of suvorexant, eszopiclone, zaleplon, zolpidem, triazolam, temazepam, ramelteon, and doxepin for treatment of insomnia disorder (Sateia, Buysse, Krystal, Neubauer, & Heald, 2017). They clarify that while these medications may be more helpful than no treatment, CBTI has comparable efficacy and more durable treatment effects after treatment is discontinued than these medications . The authors recommend against the use of trazodone, tiagabine, diphenhydramine, melatonin, tryptophan, and valerian for treatment of insomnia disorder.
Despite the plethora of empirical evidence for CBTI as the recommended insomnia treatment over sleep medications (Manber et al., 2012; Sateia et al., 2017; Trockel et al., 2014; Wilson et al., 2010), CBTI is not consistently offered as the first-line treatment for insomnia. A recent review of VA electronic medical record data discovered that, of over 5000 veterans who received a recommendation for insomnia treatment, only 2% were referred to CBTI (Bramoweth et al., 2017). Improved integration of behavioral medicine and primary care has the potential to improve treatment of insomnia with CBTI and reduce referrals to specialty clinics. However, there are numerous barriers, at the provider, patient, and system level, which prevent adequate integration and implementation of both sleep disorder screening and CBTI within primary care.
Barriers to Integration
Provider-Level Barriers
A key provider barrier that can hinder implementation of evidence-based sleep treatments, especially insomnia , within the primary care setting is a lack of familiarity with current treatment recommendations (Edinger et al., 2016; Grol & Wensing, 2013; Lugtenberg et al., 2009; Sake et al., 2017). Research findings suggest that PCPs often do not evaluate for sleep-related problems during a typical exam (Edinger et al., 2016; Lugtenberg et al., 2009; Sake et al., 2017), in part due to lack of knowledge and training about sleep disorders and treatment (Edinger et al., 2016; Grol & Wensing, 2013; Lugtenberg et al., 2009; Sake et al., 2017) and in part due to insomnia symptoms not being a provider priority for patient care (Sake et al., 2017). Provider-reported barriers to implementing clinical guidelines for evaluation and treatment of sleep disorders include lack of awareness/familiarity with guidelines for treatment of sleep disorders, like those disseminated by the American Academy of Sleep Medicine (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008) and more recently the American College of Physicians (Qaseem et al., 2016), lack of agreement with guidelines/recommendations, and lack of outcome expectancy (Lugtenberg et al., 2009). A study which specifically assessed the management of insomnia within primary care suggested that, while 67% of primary care providers (PCPs) surveyed referred their patients with an insomnia complaint to a psychologist for “specific advice and treatment,” 82% were hesitant to recommend non-pharmacological treatment, like CBTI, because they expected patients to decline more time-intensive treatments (Sake et al., 2017). Even when PCPs evaluate for sleep symptoms and determine that treatment for insomnia is needed, they often focus on identifying the underlying cause of insomnia (e.g., depression, PTSD, chronic low back pain, fibromyalgia) rather than considering insomnia as a diagnosis in need of its own treatment plan (Cheung, Bartlett, Armour, Glozier, & Saini, 2014; Davy, Middlemass, & Siriwardena, 2015). This is particularly problematic given that current diagnostic coding systems like the ICD-10 and the DSM-5 recognize insomnia as a disorder necessitating treatment independent of treatment for comorbid disorders (American Psychiatric Association, 2013; World Health Organization, 1992). These barriers often result in increased rates of prescription of sedative-hypnotic medications rather than referral to non-pharmacological treatments such as CBTI. Additionally, deprioritizing insomnia symptoms often leads to patients feeling frustrated, despite understanding that their insomnia may have multiple causes (Araujo, Jarrin, Leanza, Vallieres, & Morin, 2017; Davy et al., 2015).
Previous experiences and assumptions also influence PCPs willingness to discuss sleep problems with patients. In a recent survey, the vast majority of PCPs cited barriers to discussing sleep problems with patients, such as past experiences with prescription-seeking patients/beliefs that patients expect medications to be the only useful treatment for sleep problems (96%), patients wanting a “quick fix” (79%), patients being disinterested in changing their lifestyle, and patients being hesitant to engage in time-intensive non-pharmacological treatments (83%), as well as difficulty de-prescribing sedative-hypnotics (75%; (Sake et al., 2017). These findings are consistent with previous findings that 25–50% of PCPs perceive both patients’ preferences for treatment and patients’ ability (or inability) to engage in non-pharmacological treatment, like CBTI, are key barriers to implementing national standards of care for sleep disorders (Lugtenberg et al., 2009). Qualitative research indicates that providers often resort to prescribing sedative-hypnotic medications despite ambivalence about their use—perhaps to avoid confrontation with a patient or even to show sympathy (Cheung et al., 2014). Quantitative studies seem to support these perceptions as well. Secondary analyses of medical records data indicate that patients with sleep complaints are much more likely to be prescribed with sedative-hypnotic medications than to be referred to cognitive behavioral insomnia treatment (Bramoweth, 2017; Bramoweth et al., 2017). However, as providers become more aware of treatments like CBTI and these treatments become more easily accessible, the ratio of medications to CBTI will reduce.
Patient-Level Barriers
Research findings have identified the primary patient-level barrier to be poor communication and reporting of sleep-related complaints with providers. In fact, up to 52% patients with sleep disturbance are hesitant to discuss sleep problems with their PCP (Aikens & Rouse, 2005; Andrews, Coviello, Hurley, Rose, & Redeker, 2013), and as few as 5% of patients report symptoms or seek treatment for their sleep complaints from their healthcare providers (Erman, 2004). Counter to many PCPs’ perceptions that patients are looking for “a quick fix” (Lugtenberg et al., 2009), patient hesitancy to discuss sleep problems was often related to fear that PCPs would prescribe “another pill,” which is undesirable due to the adverse effects associated with sedative-hypnotic medications (Andrews et al., 2013). Also inconsistent with PCPs’ perception that patients would be resistant to more time-intensive or non-pharmacological treatments (Lugtenberg et al., 2009; Sake et al., 2017), most patients with insomnia were interested in learning more about sleep and behavioral treatment strategies (Aikens & Rouse, 2005). This inconsistency between patient- and provider-reported barriers in discussing sleep problems and treatments is notable, as it indicates that a substantial number of patients may be interested in and benefit from non-pharmacological treatment, like CBTI , but are not provided with this opportunity simply due to inaccurate assumptions by both patient and provider and poor communication during health exams.
Systems-Level Barriers
A variety of systemic barriers can occur when implementing change, such as the integration of sleep disorder evaluations and delivery of CBTI or related treatments within primary care. These barriers can occur at numerous points in the integration process. Focus groups with PCPs indicate that systemic barriers to the implementation of clinical guidelines (e.g., American College of Physicians guidelines on management of chronic insomnia; Qaseem et al., 2016) and key recommendations for sleep disorders include lack of time, lack of resources, organizational constraints, and lack of reimbursement (Lugtenberg et al., 2009). Additional key limiting factors relate to physical infrastructure (e.g., lack of physical office space within primary care for a behavioral medicine clinician to conduct same-day evaluations for patients reporting insomnia/behavioral sleep problems during primary care appointments), poor clinic design (e.g., lack of clear local screening and/or treatment guidelines, CBTI provider offices located in a different building or within the general mental health clinic), and inadequate information technology (e.g., lack of or outdated electronic health records). Some provider-reported variables may also represent a lack of systemic support for the integration of behavioral medicine and primary care. Insufficient and inadequate opportunities for education and training on the value of assessing and treating sleep disorder may contribute to knowledge deficits, perceived ineffectiveness of treatments, and low self-efficacy to deliver sleep-related care. Similarly, provider-reported implementation barriers, such as failure to implement recommended sleep guidelines due to guidelines that are unclear, incomplete, outdated, or too complex, may represent systematic failures to provide clear, realistic, behavioral guidelines for sleep disorder screening and treatment. Limited support at the system level, whether related to financial constraints, limited physical resources, insufficient staffing, or inadequate clinic space all reduce the likelihood of successful integration of evidence-based sleep-related care with primary care.
Approaches to Integration
In recent years, a myriad of efforts to improve integration of evidence-based behavioral interventions in primary care have occurred. General approaches to improve integration include professional-oriented methods, organizational methods, and regulatory methods (Grol, Bosch, & Wensing, 2013). Provider-focused methods most frequently target improvements in education and information, as well as integration and implementation of evidence-based care. These methods have evidenced varying levels of success; no single strategy or program is effective or appropriate for all settings. Simply educating PCPs and the public about the symptoms, consequences, and treatment of sleep disorders and offering multidiscipline training opportunities can be helpful in increasing the effective and efficient integration of sleep medicine and primary care (Schmitz, 2016). Overcoming the education and information barriers about sleep can help to increase the frequency of sleep-related screening and assessment, improve accurate detection of symptoms, and facilitate treatment planning and initiation. Regular screening for sleep disorders is vital, given up to 95% of patients do not report or seek treatment for sleep complaints to their healthcare providers (Erman, 2004). Valid, reliable screening measures for sleep disorders include the Insomnia Severity Index (ISI; Bastien, Vallieres, & Morin, 2001; Morin, Belleville, Belanger, & Ivers, 2011), the Epworth Sleepiness Scale (ESS; Johns, 1991, 1992), and the STOP-BANG Questionnaire (Chung et al., 2012). The ISI is a 7-item questionnaire that assesses symptoms like difficulty falling asleep, staying asleep, and waking up too early as well as satisfaction with sleep and the impact of poor sleep on daytime function. A score ≥ 10 (range 0–28) is usually indicative of further assessment, and a score ≥ 15 indicates moderate–severe clinical symptoms. The ESS, an 8-item measure, is the most common assessment of daytime sleepiness, a key symptom of OSA and other sleep disorders. It assesses the likelihood of falling asleep in various situations, such as sitting and reading, watching TV, or while in the car stopped at a red light. A score > 10 (range 0–24) is indicative of excessive daytime sleepiness, and further evaluation is likely needed. The STOP-BANG is an 8-item questionnaire to assess risk for OSA and focuses on both self-reported or observed symptoms (i.e., snoring, daytime sleepiness, gasping or breathing pauses during sleep) and objective factors (i.e., high blood pressure, body mass index >35, age > 50, neck size >16 [female]/17 [male], gender [male]). Yes to three to four questions is considered intermediate risk of OSA, and five to eight is high risk.
Some common provider-level integration strategies include the distribution of educational materials, such as clinical practice guidelines, audiovisual materials, and printed handouts with information about current evidence and recommendations. Clinical practice guidelines, like those disseminated by the American Academy of Sleep Medicine (Schutte-Rodin et al., 2008) and the American College of Physicians (Qaseem et al., 2016), provide in-depth guidance about the treatment of insomnia utilizing cognitive behavioral interventions . However, helpful information is also available at sleep association websites like the Society of Behavioral Sleep Medicine (www.behavioralsleep.org), the American Academy of Sleep Medicine (www.aasmnet.org), and the National Sleep Foundation (sleepfoundation.org and sleep.org). Development of consensus strategies are challenging as each provider will have different learning style and learning preferences as well as access to different learning opportunities.
Conferences, lectures, and workshops that offer continuing education credits are also common approaches for making improvements and/or changes in clinical practice via provider education and training. The annual meeting of the Associated Professional Sleep Societies offers numerous opportunities to learn about advances in assessment and treatment of sleep disorders and to network with colleagues (sleepmeeting.org). Additionally, many academic societies like the Association for Behavioral and Cognitive Therapies (abct.org) and Society of Behavioral Medicine (www.sbm.org/about/special-interest-groups/sleep) now have special interest groups focused on sleep that provide opportunities to learn about evidence-based practices for insomnia and other sleep disorders. Evidence suggests that small-scale educational meetings (e.g., healthcare teams and clinics) and traditional methods, like grand rounds, are more helpful for implementing change in specific settings than large conferences or online learning (Wensing, Fluit, & Grol, 2013); localized application of educational strategies also allows for the development of implementation plans that are tailored to the individual needs of providers. For individualized and in-depth training, both basic and advanced, organizations like the Society of Behavioral Sleep Medicine (www.behavioralsleep.org) and the University of Pennsylvania (www.med.upenn.edu/bsm) offer training for community providers, and the Center for Deployment Psychology (deploymentpsych.org/training) and the Department of Veterans Affairs offer training for clinicians working with military service members and veterans.
The effectiveness of most educational methods is positive, but their effect is, at best, modest in magnitude (Wensing et al., 2013). Cochrane reviews suggest that educational interventions only result in an approximate 5% improvement in professional performance (Farmer et al., 2008; Forsetlund et al., 2009; O'Brien et al., 2007; Wensing et al., 2013). To be effective and influence change, information and education efforts about implementing innovative and evidence-based care typically need to be repeated at regular intervals and delivered in various modalities (Grol & Wensing, 2013). Ensuring the content provided is relevant to clinical practice, establishing specific learning goals (Burke & Hutchins, 2007), and increasing active participation in trainings (Beaudry, 1989) enhance motivation and learning (Burke & Hutchins, 2007). Education provided by key opinion leaders, individuals well respected by their peers and colleagues, as well as individuals with authority and prestige, can also enhance education and awareness efforts (Grol & Wensing, 2013). Overall, these findings indicate that, while improving provider knowledge and skills can be useful, education alone is rarely sufficient for successful integration.
Audit and feedback and clinical reminders are two additional methods that can improve the implementation of evidence-based care. Audit and feedback involves tracking clinical practice behaviors over a specific time period and providing clinicians with feedback about their performance (van der Weijden, Wensing, Eccles, & Grol, 2013). The existing research suggest that inclusion of data about the performance of a provider’s colleagues as part of the feedback process, as well as provision of achievable benchmarks, improves the impact of feedback on behavior change (Balas et al., 1996; van der Weijden et al., 2013). Similar to some educational efforts, audit and feedback in the context of behavior change can also be enhanced if it is personalized, provided by a respected colleague, and related to patient-specific characteristics and outcomes rather than general health outcomes (Winkens et al., 1995, 1996; Winkens, Pop, Grol, Kester, & Knottnerus, 1992). Unfortunately, the optimal quantity of data for feedback has yet to be determined.
Clinical reminders are reminders to follow specific recommendations for clinical practices that are typically delivered verbally or electronically (van der Weijden et al., 2013). When using clinical reminders to improve integration, it is important to consider the costs and benefits of different reminder intervals, as the optimal timing of reminders differs based on the target behavior. For example, simultaneous reminders , provided at the moment of patient contact, can increase a desired behavior (e.g., screening for OSA and insomnia) or decrease an undesired behavior (e.g., writing a prescription for a sedative hypnotic prior to recommending CBTI). Reminders that occur between patient contacts can help to correct or improve patient care or assist with follow-up on important test results (e.g., discuss findings from an overnight sleep study). With advancements in electronic health records, clinical reminders are often easy to program and customize; however, it is important to consider the overall number and frequency of reminders, as they can become burdensome for providers. An overwhelming number or frequency of reminders may result in decreased completion rates, thereby decreasing their effectiveness as an integration tool. Considering the current knowledge and evidence, clinical reminders appear to be more feasible than audit and feedback for increasing adherence to clinical practice guidelines for insomnia (van der Weijden et al., 2013).
At the organizational and systemic level, improvement of evidence-based care can be facilitated by revision of professional roles—shifts and changes of job descriptions among health professionals and expansion of roles to include new tasks (e.g., CBTI delivered in primary care by nurses; (Grol et al., 2013). Additionally, primary care providers have indicated that brief, reliable screening instruments for sleep disorders (e.g., Insomnia Severity Index, Epworth Sleepiness Scale, STOP-BANG Questionnaire) and building a screening for sleep disorders into the check-in process would likely result in increased compliance with sleep-related guidelines (Sake et al., 2017). Formal integration of services, such as the integration of behavioral medicine and primary care, can also lead to better adoption of clinical practice guidelines (Falloon, Arroll, Elley, & Fernando 3rd, 2011). The optimal systemic approach or combination of approaches will likely vary by system.
Improving Integration Through Treatment Delivery Innovations
Numerous factors limit patient access to sleep disorder screening and CBTI, such as an insufficient availability of trained providers, distance to care and travel limitations, wait times for appointments, and method of treatment delivery (Manber, Simpson, & Bootzin, 2015). Stepped care models , in which patients are triaged and offered the least intensive treatment appropriate for their reported symptoms (O'Donohue & Draper, 2011), offer a variety of possible solutions to these barriers (Espie, 2009). Alternative treatment approaches to traditional CBTI, which is in-person, individual treatment delivered by a psychologist , can improve access to non-pharmacological insomnia treatments. Feasible alternatives include group-based treatment, briefer courses of treatment, and treatment via self-directed methods like workbooks, telephone, and the Internet. Offering a variety of treatments options can be especially valuable for improving access to insomnia treatment in an integrated care setting.
The qualifications and training necessary for a provider to deliver CBTI are frequently discussed topics in the field of behavioral sleep medicine , which is largely due to the widely recognized deficit of behavioral sleep medicine providers. Since at least the early 2000s, there have been several efforts to better understand the provider insufficiency problem and to develop solutions (Fields, Schutte-Rodin, Perlis, & Myers, 2013). Master’s level providers (MLP) , like nurse practitioners (NP) and physician assistants (PA), are promising CBTI providers due their ability to conduct medical assessments, make differential diagnoses and treatment plans, deliver care in both general and specialized settings, and conceptualize treatment in a biopsychosocial manner, all of which are important components of evaluating and treating insomnia. Also, insomnia treatment delivered by MLPs , specifically NPs and PAs, may appear to be more medical than mental health in nature to patients than treatment provided by psychologists or other MLPs like social workers or mental health counselors; this approach could reduce treatment-related stigma. While findings indicate that medical providers can effectively provide CBTI, provision of CBTI by non-doctoral level medical providers is not without its own challenges. For MLPs to be qualified to provide treatments, proper training in CBTI, as well as appropriate supervision, credentialing, and continuing education may be required (Manber et al., 2012); required qualifications for MLPs will likely differ across state and federal practice laws.
Group CBTI provided in a primary care setting can overcome several common barriers to the treatment of insomnia: provider availability, treatment accessibility, and mental health stigma. There is evidence that group CBTI lead by primary care nurses supervised by experienced psychologists can significantly reduce patients’ insomnia symptoms (Espie et al., 2007). A recent meta-analysis of group-based CBTI suggests moderate to large effect sizes for reductions in sleep onset latency, times awake after sleep onset, and improvements in sleep efficiency, with improvements lasting 3–12 months (E. A. Koffel et al., 2015). Groups typically included less than ten individuals who attended four to eight 60–120-min sessions. Longer session and treatment duration was associated with larger treatment effects.
While group treatment can overcome many common barriers to care, key challenges associated with this modality, such as longer wait time and stigma, remain. There are often longer wait times for groups compared to individual treatment, as individuals may have to wait several weeks until the next series of sessions begins (Manber et al., 2015). This is particularly problematic because longer wait times could result in dropout before treatment even begins. Patient and provider perceptions about treatment may also interfere with the implementation of group treatment within the primary care setting. Despite evidence to the contrary, group treatment is often seen as less effective and credible than individual treatment by the patients and providers alike. Referring to group treatment as a workshop or class may reduce stigma and improve treatment acceptability and participation (Manber et al., 2015).
Brief treatments are a key part of a stepped care approach to treating insomnia (Espie, 2009). Several studies have focused on delivering CBTI in a briefer format . Evidence suggests that patients who engaged in a two-session CBTI group report feeling more rested and evidence lower insomnia severity and reduced time awake after sleep onset, as well as higher sleep efficiency and better sleep quality at 3-month follow-up compared to those attending two sleep hygiene groups (Edinger & Sampson, 2003). Brief behavioral treatment for insomnia (BBTI) was developed due to the known limitations of CBTI, namely, the lack of trained providers, and the duration, intensity, and cost of six to eight individual treatment sessions (Buysse et al., 2011; Troxel, Germain, & Buysse, 2012). The goal of BBTI was to develop a robust insomnia treatment that was relevant in a broad, general medical setting. BBTI is based on the behavioral components of CBTI—stimulus control and sleep restriction—and is intended to involve two in-person sessions and two phone follow-ups delivered by MLPs . The behavioral components of CBTI were reduced to four basic rules: (1) reduce time in bed; (2) get up at the same time every day, regardless of sleep duration,; (3) do not go to bed unless sleepy; and (4) do not stay in bed unless asleep (e.g., get out of bed if awake >30 min (Buysse et al., 2011)). These four rules are introduced in an initial in-person session lasting 45–60 min, followed by a brief ~30 min in-person follow-up 2 weeks later. Two brief, ~20 min, phone calls take place after each session and serve as opportunities to review treatment rules and adherence, as well as to adjust the prescribed sleep schedule. Evidence suggests that BBTI delivered by mental health MLPs and social workers effectively reduces insomnia symptoms (Buysse et al., 2011; Germain et al., 2014).
Alternative evidence-based methods for delivery of insomnia treatment, such as telephone-based care and self-directed care (e.g., paper and online/mobile-based care), can further help to increase access to care and fit well within a stepped care model . These interventions are often more readily accessible to patients and may be preferable for those who experience barriers related to distance to a provider or competing time demands due to work and/or child-care responsibilities. Others may simply want to try a lower intensity treatment with less contact with a provider that still offers a strong evidence base.
While treatment protocols, such as BBTI , involve phone care as a secondary or supportive aspect of care to in-person treatment delivery (Buysse et al., 2011), there is good preliminary support for CBTI delivered solely via telephone . One such study compared telephone-based CBTI, plus four information modules that were mailed to participants, to information that would typically be provided in a primary care setting (Arnedt et al., 2013). The four modules provided to patients included information on (1) behavioral strategies based on stimulus control and sleep restriction; (2) sleep hygiene focusing on education on how behaviors, substances, and environmental factors can impact sleep; (3) cognitive therapy focusing on dysfunctional beliefs about sleep that can contribute to insomnia; and (4) relapse prevention. Treatment involved 4–8 weekly 15–60-min phone calls with experienced clinical psychologists. The control group participants were mailed with informational material about CBTI, developed by the American Academy of Sleep Medicine (www.aasmnet.org/resources/pdf/products/understandinginsomnia_web.pdf) and received one 15–20-min phone call during which a clinician reviewed the provided information. Consistent with previous CBTI studies, patients in the treatment group achieved significant improvements, with large effect sizes, for sleep onset latency, time awake after sleep onset, and sleep efficiency, as well as for total sleep time and sleep quality, with continued treatment gains at 12-week follow-up. Although the information control condition resulted in significantly lower response and remission rates compared to the treatment condition, participants in the information control condition also evidenced a significant reduction in insomnia symptoms. This finding may indicate that an even less clinically intensive intervention, such as basic sleep hygiene guidelines or the four rules of BBTI , plus a brief phone call with a provider may be a useful entry-level treatment for many patients (Arnedt et al., 2013).
At the lowest level of the stepped-care pyramid exist interventions that provide the widest access to care and are easiest to disseminate—self-directed interventions . These approaches include written materials such as books and workbooks like Quiet Your Mind and Get to Sleep (Carney & Manber, 2009), The Insomnia Answer (Glovinsky & Spielman, 2006), The Insomnia Workbook: A Comprehensive Guide to Getting the Sleep You Need (Silberman, 2009), No More Sleepless Nights (Hauri & Linde, 1996), Say Good Night to Insomnia (Jacobs, 2009), and Improve Your Sleep: A Self-Guided Approach for Veterans with Insomnia (Ulmer, Farrell-Carnahan, Hughes, Manber, & Tatum, 2016). Also, audiovisual interventions are available through interactive online and mobile platforms; two of the most well-studied are Sleep Healthy Using the Internet (SHUTi™; www.myshuti.com) and Sleepio ™ (www.sleepio.com). A meta-analysis of self-directed insomnia interventions found posttreatment improvements similar to other CBTI trials for key variables like sleep onset latency and sleep efficiency (Ho et al., 2015). Notably, some key methodological flaws in these studies, such as inconsistent use of intent-to-treat analyses and lower than usual dropout rates (e.g., some studies reported 0% drop out), may limit the generalizability of reported results (Manber et al., 2015). Evidence suggests that self-directed interventions that offered some clinician contact result in increased effectiveness. This may be due to the therapeutic elements of clinician contact, such as increased accountability, support and encouragement, added structure, ability to ask questions and voice concerns, and customization of treatment recommendations that are missing from purely self-directed approaches (Manber et al., 2015).
Perhaps the self-directed interventions with the most promise for wide dissemination are Internet- and mobile-based treatments. These interventions can serve as stand-alone treatments or as enhancements for in-person, and other, treatment modalities. Preliminary findings from the Department of Veterans Affairs shows that the use of the VA-developed mobile application, CBTI Coach, in conjunction with in-person treatment was acceptable to patients and did not compromise the effectiveness of in-person CBTI (Koffel et al., 2016). Two Internet-based interventions, SHUTi™ (Ritterband et al., 2009, 2017) and Sleepio™ (Espie et al., 2012), also offer robust treatment options, and both are supported by large randomized clinical trials, conducted in generalizable samples across several countries. Recently, a study using SHUTi showed continued treatment response at 1-year follow-up (Ritterband et al., 2017). With the ever-increasing availability of Internet access, these interventions may soon serve as the first-line treatment for many individuals. There is even preliminary evidence to suggest that Internet-based insomnia treatments can be effective when accessed through Internet connections provided in a community setting (e.g., library, community medical, or mental health center; Feuerstein et al., 2017). This could offer a cost-effective treatment option to individuals without regular Internet access.
Self-directed treatment approaches are not without their own challenges and limitations. One of the key limitations to self-directed approaches is the lack of follow-up procedures for those who experience a suboptimal treatment response. It is important that self-directed approaches to the treatment of insomnia offer information and advice about appropriate follow-up with a provider for those who do not experience a satisfactory response to treatment. If self-directed approaches are recommended by providers as a part of a stepped care approach, it is important that providers plan a timely follow-up to assess treatment response and evaluate the need for additional or alternative treatment.
Summary
As sleep disorders are most frequently reported and diagnosed in the primary care setting , the integration of behavioral medicine and primary care allows for the identification and treatment of sleep disorders to occur in a more efficient and effective manner. Untreated OSA and insomnia increase risk for chronic medical conditions and all-cause mortality and reduce quality of life; therefore, the negative impact of failing to provide treatment to individuals suffering from these disorders cannot be underestimated. Lack of provider knowledge about treatment guidelines and the efficacy of recommended treatments, de-prioritizing sleep concerns, and (potentially inaccurate) expectations about patient openness to non-pharmacological sleep treatments, particularly insomnia, serve as significant barriers to the provision of behavioral sleep treatments in the primary care setting. Patient hesitancy to discuss sleep-related concerns with their PCPs, barriers due to time, travel, finances, and competing demands, as well as limited willingness to engage in care due to stigma, serve as additional obstacles to care. Systematic barriers to integration of behavioral sleep medicine and evidence-based treatment guidelines for insomnia into the primary care setting often include infrastructure, resource, technology, and financial constraints.
Research findings suggest that a variety of different strategies may aid the integration of behavioral sleep medicine and primary care. Educating PCPs and the public about symptoms, consequences, and treatment of sleep disorder and offering appropriate and timely provider trainings related to the identification and treatment of sleep disorders are important but typically not sufficient successful integration. Some promising interventions involve educational efforts that provide recommendations that are applicable to a provider’s clinical practice and can be delivered repeatedly over time. Providing feedback on specific performance metrics, the use of electronic clinical reminders, and the provision of financial incentives may also improve the implementation and integration of evidence-based care. Insomnia treatments have also been adapted to overcome numerous barriers and more easily integrate into the primary care environment, increase accessibility, and decrease provider burden; evidence supports CBTI delivered via group, telephone, and through Internet and mobile applications (either self-directed or therapist-assisted). CBTI can also be effectively delivered by MLPs in a briefer format, consisting of as few as two clinic visits.
Regardless of best efforts and improved practices at various levels, key components to successful integration of behavioral sleep medicine within primary care settings are the awareness of the innovation (i.e., assessment, diagnosis, and treatment of sleep disorder in primary care), consistent and continued systemic support for newly integrated clinical practices, interest in and involvement of providers (behavioral medicine and medical) and patients (Manber et al., 2015), and implementation of regular provider reminders and feedback. The methods described above will help to promote awareness of sleep disorders within primary care, emphasize the importance of screening for sleep disorders, and provide cognitive behavioral insomnia treatment to patients in primary care settings.
References
Aikens, J. E., & Rouse, M. E. (2005). Help-seeking for insomnia among adult patients in primary care. The Journal of the American Board of Family Practice, 18(4), 257–261 doi:18/4/257 [pii].
AlGhanim, N., Comondore, V. R., Fleetham, J., Marra, C. A., & Ayas, N. T. (2008). The economic impact of obstructive sleep apnea. Lung, 186(1), 7–12. https://doi.org/10.1007/s00408-007-9055-5
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Andrews, L. K., Coviello, J., Hurley, E., Rose, L., & Redeker, N. S. (2013). “I'd eat a bucket of nails if you told me it would help me sleep:” Perceptions of insomnia and its treatment in patients with stable heart failure. Heart & Lung: The Journal of Critical Care, 42(5), 339–345. https://doi.org/10.1016/j.hrtlng.2013.05.003
Araujo, T., Jarrin, D. C., Leanza, Y., Vallieres, A., & Morin, C. M. (2017). Qualitative studies of insomnia: Current state of knowledge in the field. Sleep Medicine Reviews, 31, 58–69.
Arnedt, J. T., Cuddihy, L., Swanson, L. M., Pickett, S., Aikens, J., & Chervin, R. D. (2013). Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. https://doi.org/10.5665/sleep.2448
Arroll, B., Fernando, A., 3rd, Falloon, K., Goodyear-Smith, F., Samaranayake, C., & Warman, G. (2012). Prevalence of causes of insomnia in primary care: A cross-sectional study. The British Journal of General Practice: The Journal of the Royal College of General Practitioners, 62(595), e99–e103. https://doi.org/10.3399/bjgp12X625157
Ayas, N. T., FitzGerald, J. M., Fleetham, J. A., White, D. P., Schulzer, M., Ryan, C. F., … Marra, C. A. (2006). Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Archives of Internal Medicine, 166(9), 977–984 doi:166/9/977 [pii].
Baglioni, C., Battagliese, G., Feige, B., Spiegelhalder, K., Nissen, C., Voderholzer, U., … Riemann, D. (2011). Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135, 10–19. https://doi.org/10.1016/j.jad.2011.01.011
Balas, E. A., Boren, S. A., Brown, G. D., Ewigman, B. G., Mitchell, J. A., & Perkoff, G. T. (1996). Effect of physician profiling on utilization. Meta-analysis of randomized clinical trials. Journal of General Internal Medicine, 11(10), 584–590.
Bartels, S. J., Coakley, E. H., Zubritsky, C., Ware, J. H., Miles, K. M., Arean, P. A., … PRISM-E Investigators. (2004). Improving access to geriatric mental health services: A randomized trial comparing treatment engagement with integrated versus enhanced referral care for depression, anxiety, and at-risk alcohol use. The American Journal of Psychiatry, 161(8), 1455–1462. https://doi.org/10.1176/appi.ajp.161.8.1455
Bastien, C. H., Vallieres, A., & Morin, C. M. (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307.
Batool-Anwar, S., Goodwin, J. L., Kushida, C. A., Walsh, J. A., Simon, R. D., Nichols, D. A., & Quan, S. F. (2016). Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). Journal of Sleep Research, 25(6), 731–738. https://doi.org/10.1111/jsr.12430
Beaudry, J. S. (1989). The effectiveness of continuing medical education: A quantitative synthesis. The Journal of Continuing Education in the Health Professions, 9, 285–307.
Blom, K., Jernelöv, S., Kraepelien, M., Bergdahl, M. O., Jungmarker, K., Ankartjärn, L., … Kaldo, V. (2015). Internet treatment addressing either insomnia or depression, for patients with both diagnoses: A randomized trial. Sleep, 38(2), 267–277. https://doi.org/10.5665/sleep.4412
Bramoweth, A. D. (2017). A step in the right direction: Moving toward increased access to insomnia care. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 13(2), 161–162. https://doi.org/10.5664/jcsm.6432
Bramoweth, A. D., Luther, J. F., Hanusa, B. H., Walker, J. D., Atwood, C. W., & Germain, A. (2017). Clinical characterization of insomnia among veterans with PTSD: Identifying risk factors for diagnosis and treatment with sedative-hypnotics. Defence and Peace Economics, Online 12 July 2017. https://doi.org/10.1080/10242694.2017.1349633
Bramoweth, A. D., Renqvist, J. G., Hanusa, B. H., Walker, J. D., Germain, A., & Atwood, C. W., Jr. (2017). Identifying the demographic and mental health factors that influence insomnia treatment recommendations within a veteran population. Behavioral Sleep Medicine, 1–12. https://doi.org/10.1080/15402002.2017.1318752
Budhiraja, R., Roth, T., Hudgel, D. W., Budhiraja, P., & Drake, C. L. (2011). Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep, 34, 859–867. https://doi.org/10.5665/SLEEP.1114
Burke, L. A., & Hutchins, H. M. (2007). Training transfer: An integrative literature review. Human Resources Development Review, 6(3), 263–296. https://doi.org/10.1177/1534484307303035
Buysse, D. J., Germain, A., Moul, D. E., Franzen, P. L., Brar, L. K., Fletcher, M. E., … Monk, T. H. (2011). Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine, 171(10), 887–895.
Cai, H., Shu, X. O., Xiang, Y. B., Yang, G., Li, H., Ji, B. T., … Zheng, W. (2015). Sleep duration and mortality: A prospective study of 113 138 middle-aged and elderly Chinese men and women. Sleep, 38(4), 529–536. https://doi.org/10.5665/sleep.4564
Cappuccio, F. P., Cooper, D., D'Elia, L., Strazzullo, P., & Miller, M. A. (2011). Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal, 32(12), 1484–1492. https://doi.org/10.1093/eurheartj/ehr007
Cappuccio, F. P., D'Elia, L., Strazzullo, P., & Miller, M. A. (2010a). Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care, 33(2), 414–420. https://doi.org/10.2337/dc09-1124
Cappuccio, F. P., D'Elia, L., Strazzullo, P., & Miller, M. A. (2010b). Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep, 33(5), 585–592.
Carney, C. E., & Manber, R. (2009). Quiet your mind and get to sleep: Solutions to insomnia for those with depression, anxiety or chronic pain. Oakland, CA: New Harbinger Publications, Inc.
Centers for Disease Control and Prevention. (2014). Key sleep disorders. Retrieved from https://www.cdc.gov/sleep/about_sleep/key_disorders.html
Centers for Disease Control and Prevention. (2015). LDL and HDL: “Bad” and “Good” cholesterol. Retrieved from https://www.cdc.gov/cholesterol/ldl_hdl.htm
Chai-Coetzer, C. L., Antic, N. A., Rowland, L. S., Reed, R. L., Esterman, A., Catcheside, P. G., … McEvoy, R. D. (2013). Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: A randomized trial. JAMA, 309(10), 997–1004. https://doi.org/10.1001/jama.2013.1823
Cheung, J. M., Bartlett, D. J., Armour, C. L., Glozier, N., & Saini, B. (2014). Insomnia patients' help-seeking experiences. Behavioral Sleep Medicine, 12(2), 106–122. https://doi.org/10.1080/15402002.2013.764529
Chung, F., Subramanyam, R., Liao, P., Sasaki, E., Shapiro, C., & Sun, Y. (2012). High STOP-bang score indicates a high probability of obstructive sleep apnoea. British Journal of Anaesthesia, 108(5), 768–775.
Conley, S., & Redeker, N. S. (2015). Cognitive behavioral therapy for insomnia in the context of cardiovascular conditions. Current Sleep Medicine Reports, 1(3), 157–165. https://doi.org/10.1007/s40675-015-0019-7
Davy, Z., Middlemass, J., & Siriwardena, A. N. (2015). Patients' and clinicians' experiences and perceptions of the primary care management of insomnia: Qualitative study. Health Expectations: An International Journal of Public Participation in Health Care and Health Policy, 18(5), 1371–1383. https://doi.org/10.1111/hex.12119
Department of Veterans Affairs. (2007). Department of veterans affairs pharmacy benefits management service and medical advisory panel: Guidance for treatment of insomnia in veterans in the primary care setting. Washington, DC: Department of Veterans Affairs.
Edinger, J. D., Grubber, J., Ulmer, C., Zervakis, J., & Olsen, M. (2016). A collaborative paradigm for improving management of sleep disorders in primary care: A randomized clinical trial. Sleep, 39(1), 237–247. https://doi.org/10.5665/sleep.5356
Edinger, J. D., & Sampson, W. S. (2003). A primary care friendly cognitive behavioral insomnia therapy. Sleep, 26(2), 177–182.
Erman, M. (2004). Clinical update – Diagnosis of insomnia in the primary care practice. Retrieved from http://www.medscape.org/viewarticle/478849
Espie, C. A. (2009). "stepped care": A health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep, 32, 1549–1558.
Espie, C. A., Kyle, S. D., Williams, C., Ong, J. C., Douglas, N. J., Hames, P., & Brown, J. S. (2012). A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep, 35, 769–781. https://doi.org/10.5665/sleep.1872
Espie, C. A., MacMahon, K. M., Kelly, H. L., Broomfield, N. M., Douglas, N. J., Engleman, H. M., … Wilson, P. (2007). Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep, 30(5), 574–584.
Falloon, K., Arroll, B., Elley, C. R., & Fernando, A., 3rd. (2011). The assessment and management of insomnia in primary care. BMJ (Clinical Research Ed.), 342, d2899.
Farmer, A. P., Legare, F., Turcot, L., Grimshaw, J., Harvey, E., McGowan, J. L., & Wolf, F. (2008). Printed educational materials: Effects on professional practice and health care outcomes. The Cochrane Database of Systematic Reviews, (3), CD004398. https://doi.org/10.1002/14651858.CD004398.pub2
Fernandez-Mendoza, J. (2017). The insomnia with short sleep duration phenotype: An update on its importance for health and prevention. Current Opinion in Psychiatry, 30(1), 56–63. https://doi.org/10.1097/YCO.0000000000000292
Feuerstein, S., Hodges, S. E., Keenaghan, B., Bessette, A., Forselius, E., & Morgan, P. T. (2017). Computerized cognitive behavioral therapy for insomnia in a community health setting. Journal of Clinical Sleep Medicine, 13(2), 267–274. https://doi.org/10.5664/jcsm.6460
Fields, B. G., Schutte-Rodin, S., Perlis, M. L., & Myers, M. (2013). Master's-level practitioners as cognitive behavioral therapy for insomnia providers: An underutilized resource. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 9(10), 1093–1096. https://doi.org/10.5664/jcsm.3096
Forsetlund, L., Bjorndal, A., Rashidian, A., Jamtvedt, G., O'Brien, M. A., Wolf, F., … Oxman, A. D. (2009). Continuing education meetings and workshops: Effects on professional practice and health care outcomes. The Cochrane Database of Systematic Reviews, (2), CD003030. https://doi.org/10.1002/14651858.CD003030.pub2
Franklin, K. A., & Lindberg, E. (2015). Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. Journal of Thoracic Disease, 7(8), 1311–1322. https://doi.org/10.3978/j.issn.2072-1439.2015.06.11
Gangwisch, J. E., Malaspina, D., Babiss, L. A., Opler, M. G., Posner, K., Shen, S., … Ginsberg, H. N. (2010). Short sleep duration as a risk factor for hypercholesterolemia: Analyses of the national longitudinal study of adolescent health. Sleep, 33(7), 956–961.
Germain, A., Richardson, R., Stocker, R., Mammen, O., Hall, M., Bramoweth, A. D., … Buysse, D. J. (2014). Treatment for insomnia in combat-exposed OEF/OIF/OND military veterans: Preliminary randomized controlled trial. Behaviour Research and Therapy, 61, 78–88.
Glovinsky, P., & Spielman, A. (2006). The insomnia answer: A personalized program for identifying and overcoming the three types of insomnia. New York, NY: TarcherPerigee.
Goodie, J. L., & Hunter, C. L. (2014). Practical guidance for targeting insomnia in primary care settings. Cognitive and Behavioral Practice, 21(3), 261.
Gottlieb, D. J., Yenokyan, G., Newman, A. B., O'Connor, G. T., Punjabi, N. M., Quan, S. F., … Shahar, E. (2010). Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation, 122(4), 352–360. https://doi.org/10.1161/CIRCULATIONAHA.109.901801
Grandner, M. A., Patel, N. P., Gehrman, P. R., Perlis, M. L., & Pack, A. I. (2010). Problems associated with short sleep: Bridging the gap between laboratory and epidemiological studies. Sleep Medicine Reviews, 14(4), 239–247. https://doi.org/10.1016/j.smrv.2009.08.001
Grol, R., Bosch, M., & Wensing, M. (2013). Development and selection of strategies for improving patient care. In R. Grol, M. Wensing, M. Eccles, & D. Davis (Eds.), Improving patient care (2nd ed., pp. 165–184). Chichester, UK: Wiley. https://doi.org/10.1002/9781118525975.ch10
Grol, R., & Wensing, M. (2013). Dissemination of innovations. In R. Grol, M. Wensing, M. Eccles, & D. Davis (Eds.), Improving patient care: The implementation of change in health care (2nd ed., pp. 185–196). Chichester, UK: Wiley.
Hauri, P., & Linde, S. (1996). No more sleepless nights (Revised ed.). New York, NY: Wiley.
Hirshkowitz, M., Whiton, K., Albert, S. M., Alessi, C., Bruni, O., DonCarlos, L., … Adams Hillard, P. J. (2015). National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health: Journal of the National Sleep Foundation, 1(1), 40–43 Retrieved from https://doi.org/10.1016/j.sleh.2014.12.010
Ho, F. Y., Chung, K. F., Yeung, W. F., Ng, T. H., Kwan, K. S., Yung, K. P., & Cheng, S. K. (2015). Self-help cognitive-behavioral therapy for insomnia: A meta-analysis of randomized controlled trials. Sleep Medicine Reviews, 19, 17–28. https://doi.org/10.1016/j.smrv.2014.06.010
Ishak, W. W., Bagot, K., Thomas, S., Magakian, N., Bedwani, D., Larson, D., … Zaky, C. (2012). Quality of life in patients suffering from insomnia. Innovations in Clinical Neuroscience, 9(10), 13–26.
Israel, A. G., & Lieberman, J. A., 3rd. (2004). Tackling insomnia: Diagnostic and treatment issues in primary care. Postgraduate Medicine, 116(6 Suppl Insomnia), 7–13. https://doi.org/10.3810/pgm.12.2004.suppl38.257
Jacobs, G. D. (2009). Say good night to insomnia (Revised ed.). New York, NY: Henry Holt and Company, LLC.
Jacobs, G. D., Pace-Schott, E. F., Stickgold, R., & Otto, M. W. (2004). Cognitive behavior therapy and pharmacotherapy for insomnia: A randomized controlled trial and direct comparison. Archives of Internal Medicine, 164(17), 1888–1896. https://doi.org/10.1001/archinte.164.17.1888
Johns, M. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14, 540–545.
Johns, M. (1992). Reliability and factor analysis of the Epworth sleepiness scale. Sleep, 15, 376–381.
Koffel, E., Kuhn, E., Petsoulis, N., Erbes, C. R., Anders, S., Hoffman, J. E., … Polusny, M. A. (2016). A randomized controlled pilot study of CBT-I coach: Feasibility, acceptability, and potential impact of a mobile phone application for patients in cognitive behavioral therapy for insomnia. Health Informatics Journal. epub ahead of print. https://doi.org/10.1177/1460458216656472
Koffel, E. A., Koffel, J. B., & Gehrman, P. R. (2015). A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Medicine Reviews, 19, 6–16. https://doi.org/10.1016/j.smrv.2014.05.001
Leger, D., Morin, C. M., Uchiyama, M., Hakimi, Z., Cure, S., & Walsh, J. K. (2012). Chronic insomnia, quality-of-life, and utility scores: Comparison with good sleepers in a cross-sectional international survey. Sleep Medicine, 13(1), 43–51. https://doi.org/10.1016/j.sleep.2011.03.020
Liu, Y., Croft, J. B., Wheaton, A. G., Perry, G. S., Chapman, D. P., Strine, T. W., … Presley-Cantrell, L. (2013). Association between perceived insufficient sleep, frequent mental distress, obesity and chronic diseases among US adults, 2009 behavioral risk factor surveillance system. BMC Public Health, 13, 84. https://doi.org/10.1186/1471-2458-13-84
Lugtenberg, M., Zegers-van Schaick, J. M., Westert, G. P., & Burgers, J. S. (2009). Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implementation Science: IS, 4, 54. https://doi.org/10.1186/1748-5908-4-54
Manber, R., Carney, C., Edinger, J., Epstein, D., Friedman, L., Haynes, P. L., … Trockel, M. (2012). Dissemination of CBTI to the non-sleep specialist: Protocol development and training issues. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 8, 209–218. https://doi.org/10.5664/jcsm.1786
Manber, R., Simpson, N. S., & Bootzin, R. R. (2015). A step towards stepped care: Delivery of CBT-I with reduced clinician time. Sleep Medicine Reviews, 19, 3–5. https://doi.org/10.1016/j.smrv.2014.09.003
Marshall, N. S., Wong, K. K., Cullen, S. R., Knuiman, M. W., & Grunstein, R. R. (2014). Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton health study cohort. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 10(4), 355–362. https://doi.org/10.5664/jcsm.3600
McCrae, C. S., Bramoweth, A. D., Williams, J., Roth, A., & Mosti, C. (2014). Impact of cognitive behavioral treatment for insomnia on health care utilization and costs. Journal of Clinical Sleep Medicine, 10(2), 127–135.
McDaniel, S. H., Grus, C. L., Cubic, B. A., Hunter, C. L., Kearney, L. K., Schuman, C. C., … Johnson, S. B. (2014). Competencies for psychology practice in primary care. The American Psychologist, 69(4), 409–429. https://doi.org/10.1037/a0036072
McKnight-Eily, L. R., Eaton, D. K., Lowry, R., Croft, J. B., Presley-Cantrell, L., & Perry, G. S. (2011). Relationships between hours of sleep and health-risk behaviors in US adolescent students. Preventive Medicine, 53(4–5), 271–273. https://doi.org/10.1016/j.ypmed.2011.06.020
Mitchell, M. D., Gehrman, P., Perlis, M., & Umscheid, C. A. (2012). Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Family Practice, 13, 40. https://doi.org/10.1186/1471-2296-13-40
Morin, C. M., Belleville, G., Belanger, L., & Ivers, H. (2011). The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608.
Morin, C. M., Culbert, J. P., & Schwartz, S. M. (1994). Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. The American Journal of Psychiatry, 151(8), 1172–1180. https://doi.org/10.1176/ajp.151.8.1172
Morin, C. M., Vallieres, A., Guay, B., Ivers, H., Savard, J., Merette, C., … Baillargeon, L. (2009). Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA: The Journal of the American Medical Association, 301, 2005–2015. https://doi.org/10.1001/jama.2009.682
Namen, A. M., Dunagan, D. P., Fleischer, A., Tillett, J., Barnett, M., McCall, W. V., & Haponik, E. F. (2002). Increased physician-reported sleep apnea: The national ambulatory medical care survey. Chest, 121(6), 1741–1747.
National Institutes of Health. (2005). NIH state-of-the-science conference on manifestations and management of chronic insomnia. Bethesda, MD: National Institutes of Health. Retrieved from http://consensus.nih.gov/2005/insomniastatement.pdf
Nebes, R. D., Buysse, D. J., Halligan, E. M., Houck, P. R., & Monk, T. H. (2009). Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology.Series B, Psychological Sciences and Social Sciences, 64(2), 180–187. https://doi.org/10.1093/geronb/gbn037
O'Brien, M. A., Rogers, S., Jamtvedt, G., Oxman, A. D., Odgaard-Jensen, J., Kristoffersen, D. T., … Harvey, E. L. (2007). Educational outreach visits: Effects on professional practice and health care outcomes. The Cochrane Database of Systematic Reviews, (4). https://doi.org/10.1002/14651858.CD000409.pub2
O'Donohue, W., & Draper, C. (2011). The case for evidence-based stepped care as a part of a reformed delivery system. In W. O'Donohue & C. Draper (Eds.), Stepped care and e-health: Practical applications to behavioral disorders (1st ed., pp. 1–16). New York: Springer.
Ozminkowski, R. J., Wang, S., & Walsh, J. K. (2007). The direct and indirect costs of untreated insomnia in adults in the United States. Sleep, 30(3), 263–273.
Qaseem, A., Kansagara, D., Forciea, M. A., Cooke, M., Denberg, T. D., & Clinical Guidelines Committee of the American College of Physicians. (2016). Management of chronic insomnia disorder in adults: A clinical practice guideline from the American college of physicians. Annals of Internal Medicine, 165(2), 125–133. https://doi.org/10.7326/M15-2175
Redline, S., Yenokyan, G., Gottlieb, D. J., Shahar, E., O'Connor, G. T., Resnick, H. E., … Punjabi, N. M. (2010). Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. American Journal of Respiratory and Critical Care Medicine, 182(2), 269–277. https://doi.org/10.1164/rccm.200911-1746OC
Ritterband, L. M., Thorndike, F. P., Gonder-Frederick, L. A., Magee, J. C., Bailey, E. T., Saylor, D. K., & Morin, C. M. (2009). Efficacy of an internet-based behavioral intervention for adults with insomnia. Archives of General Psychiatry, 66(7), 692–698.
Ritterband, L. M., Thorndike, F. P., Ingersoll, K. S., Lord, H. R., Gonder-Frederick, L., Frederick, C., … Morin, C. M. (2017). Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: A randomized clinical trial. JAMA Psychiatry, 74(1), 68–75. https://doi.org/10.1001/jamapsychiatry.2016.3249
Roane, B. M., & Taylor, D. J. (2008). Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep, 31, 1351–1356.
Roth, T., Coulouvrat, C., Hajak, G., Lakoma, M. D., Sampson, N. A., Shahly, V., … Kessler, R. C. (2011). Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: Results from the america insomnia survey. Biological Psychiatry, 69, 592–600. https://doi.org/10.1016/j.biopsych.2010.10.023
Sake, F. T., Wong, K., Bartlett, D. J., & Saini, B. (2017). Insomnia management in the australian primary care setting. Behavioral Sleep Medicine, 1–15. https://doi.org/10.1080/15402002.2016.1266491
Sateia, M. J., Buysse, D. J., Krystal, A. D., Neubauer, D. N., & Heald, J. L. (2017). Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American academy of sleep medicine clinical practice guideline. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 13(2), 307–349. https://doi.org/10.5664/jcsm.6470
Schmitz, M. F. (2016). The ACP guidelines for treatment of chronic insomnia: The challenge of implementation. Behavioral Sleep Medicine, 14(6), 699–700. https://doi.org/10.1080/15402002.2016.1220131
Schutte-Rodin, S., Broch, L., Buysse, D., Dorsey, C., & Sateia, M. (2008). Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 4, 487–504.
Shankar, A., Charumathi, S., & Kalidindi, S. (2011). Sleep duration and self-rated health: The national health interview survey 2008. Sleep, 34, 1173–1177. https://doi.org/10.5665/SLEEP.1232
Silberman, S. A. (2009). The insomnia workbook: A comprehensive guide to getting the sleep you need. Oakland, CA: New Harbinger Publications, Inc.
Smith, M. T., Perlis, M. L., Park, A., Smith, M. S., Pennington, J., Giles, D. E., & Buysse, D. J. (2002). Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. The American Journal of Psychiatry, 159, 5–11.
Strand, L. B., Laugsand, L. E., Wisløff, U., Nes, B. M., Vatten, L., & Janszky, I. (2012). Insomnia symptoms and cardiorespiratory fitness in healthy individuals: The Nord-tr\ondelag health study (HUNT). Sleep. https://doi.org/10.5665/sleep.2310
Taylor, D., Zimmerman, M., Gardner, C., Tatum, J., Bramoweth, A. D., Francetich, J., & Ruggero, C. (2014). A pilot randomized controlled trial of the effects of cognitive-behavioral therapy for insomnia on sleep and daytime functioning in college students. Behavior Therapy, 45(3), 376–389.
Taylor, D. J., Mallory, L. J., Lichstein, K. L., Durrence, H. H., Riedel, B. W., & Bush, A. J. (2007). Comorbidity of chronic insomnia with medical problems. Sleep, 30, 213–218.
The WHOQOL Group. (1995). The world health organization quality of life assessment (WHOQOL): Position paper from the world health organization. Social Science & Medicine, 41(10), 1403–1409.
Trauer, J. M., Qian, M. Y., Doyle, J. S., Rajaratnam, S. M., & Cunnington, D. (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163(3), 191–204. https://doi.org/10.7326/M14-2841
Trockel, M., Karlin, B. E., Taylor, C. B., & Manber, R. (2014). Cognitive behavioral therapy for insomnia with veterans: Evaluation of effectiveness and correlates of treatment outcomes. Behaviour Research and Therapy, 53, 41–46. https://doi.org/10.1016/j.brat.2013.11.006
Troxel, W. M., Buysse, D. J., Matthews, K. A., Kip, K. E., Strollo, P. J., Hall, M., … Reis, S. E. (2010). Sleep symptoms predict the development of the metabolic syndrome. Sleep, 33(12), 1633–1640.
Troxel, W. M., Germain, A., & Buysse, D. J. (2012). Clinical management of insomnia with brief behavioral treatment (BBTI). Behavioral Sleep Medicine, 10, 266–279. https://doi.org/10.1080/15402002.2011.607200
Ulmer, C. S., Farrell-Carnahan, L., Hughes, J. M., Manber, R., Tatum, J., & the Mid-Atlantic (VISN 6) Mental Illness Research, Education and Clinical Center (MIRECC). (2016). Improve your sleep: A self-guided approach for veterans with insomnia (Version 1 ed.). Department of Veterans Affairs
van der Weijden, T., Wensing, M., Eccles, M., & Grol, R. (2013). Feedback and reminders. In R. Grol, M. Wensing, M. Eccles, & D. Davis (Eds.), Improving patient care: The implementation of change in healthcare (2nd ed., pp. 210–223). Chichester, UK: Wiley.
Vgontzas, A. N., Liao, D., Bixler, E. O., Chrousos, G. P., & Vela-Bueno, A. (2009). Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep, 32(4), 491–497.
Vgontzas, A. N., Liao, D., Pejovic, S., Calhoun, S., Karataraki, M., Basta, M., … Bixler, E. O. (2010). Insomnia with short sleep duration and mortality: The penn state cohort. Sleep, 33(9), 1159–1164.
Watanabe, N., Furukawa, T. A., Shimodera, S., Morokuma, I., Katsuki, F., Fujita, H., … Perlis, M. L. (2011). Brief behavioral therapy for refractory insomnia in residual depression: An assessor-blind, randomized controlled trial. The Journal of Clinical Psychiatry, 72(12), 1651–1658.
Watson, N. F., Badr, M. S., Belenky, G., Bliwise, D. L., Buxton, O. M., Buysse, D., … Tasali, E. (2015). Recommended amount of sleep for a healthy adult: A joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep, 38(6), 843–844. https://doi.org/10.5665/sleep.4716
Wensing, M., Fluit, C., & Grol, R. (2013). Educational strategies. In R. Grol, M. Wensing, M. Eccles, & D. Davis (Eds.), Improving patient care: The implementation of change in health care (2nd ed., pp. 197–209). Chichester, UK: Wiley.
Wilson, S. J., Nutt, D. J., Alford, C., Argyropoulos, S. V., Baldwin, D. S., Bateson, A. N., … Wade, A. G. (2010). British association for psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology, 24(11), 1577–1601. https://doi.org/10.1177/0269881110379307
Wilt, T. J., MacDonald, R., Brasure, M., Olson, C. M., Carlyle, M., Fuchs, E., … Kane, R. L. (2016). Pharmacologic treatment of insomnia disorder: An evidence report for a clinical practice guideline by the American college of physicians. Annals of Internal Medicine, 1–10. https://doi.org/10.7326/M15-1781
Winkens, R. A., Pop, P., Bugter-Maessen, A. M., Grol, R. P., Kester, A. D., Beusmans, G. H., & Knottnerus, J. A. (1995). Randomised controlled trial of routine individual feedback to improve rationality and reduce numbers of test requests. Lancet (London, England), 345(8948), 498–502.
Winkens, R. A., Pop, P., Grol, R. P., Bugter-Maessen, A. M., Kester, A. D., Beusmans, G. H., & Knottnerus, J. A. (1996). Effects of routine individual feedback over nine years on general practitioners' requests for tests. BMJ (Clinical Research Ed.), 312(7029), 490.
Winkens, R. A., Pop, P., Grol, R. P., Kester, A. D., & Knottnerus, J. A. (1992). Effect of feedback on test ordering behaviour of general practitioners. BMJ (Clinical Research Ed.), 304(6834), 1093–1096.
World Health Organization. (1992). The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
Wu, J. Q., Appleman, E. R., Salazar, R. D., & Ong, J. C. (2015). Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions. JAMA Internal Med. https://doi.org/10.1001/jamainternmed.2015.3006
Yang, E. H., Hla, K. M., McHorney, C. A., Havighurst, T., Badr, M. S., & Weber, S. (2000). Sleep apnea and quality of life. Sleep, 23(4), 535–541.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Renqvist, J.G., Bramoweth, A.D. (2018). Improving Sleep Quality Through Integrated Care. In: Duckworth, M., O'Donohue, W. (eds) Behavioral Medicine and Integrated Care. Springer, Cham. https://doi.org/10.1007/978-3-319-93003-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-93003-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93002-2
Online ISBN: 978-3-319-93003-9
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)