Abstract
Acute kidney injury (AKI) is a life-threatening complication in patients with liver cirrhosis. AKI is frequently triggered by a precipitating event such as a bacterial infection, the use of diuretics, and/or gastrointestinal bleeding. Each of these precipitating conditions leads to a reduction in the effective circulating volume due to splanchnic arterial vasodilation, which impairs renal perfusion. Hepatorenal syndrome (HRS-AKI) is a particular type of AKI characterized by severe arterial vasoconstriction in response to severe splanchnic arterial vasodilation that cannot be solved by plasma volume expansion. HRS-AKI is the most life-threatening type of AKI and should be diagnosed and treated promptly. The differential diagnosis between HRS-AKI and acute tubular necrosis may be difficult. Pharmacological treatment of HRS-AKI with vasoconstrictors and albumin has been shown to be effective, but liver transplantation represents the best treatment of this condition. This chapter reviews the current knowledge on the pathophysiology and management of AKI and HRS-AKI in patients with cirrhosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Patients with liver cirrhosis have a higher risk of developing acute kidney injury (AKI) [1]. AKI is characterized by a wide spectrum of renal dysfunction, which may involve both a reduction in the glomerular filtration rate (GFR) and some degree of parenchymal kidney damage. AKI in patients with cirrhosis is associated with high morbidity and mortality, and prompt diagnosis and treatment of AKI is crucial in these patients. Type 1 hepatorenal syndrome (HRS-AKI) is a particular form of AKI characterized by severe renal vasoconstriction and associated with a poor prognosis.
Definition and Types of AKI in Patients with Cirrhosis
The definition of AKI requires four components : (a) a biomarker of renal function, (b) a baseline value for this biomarker, (c) a range of changes in this biomarker, and (d) a timeframe of when these changes occur. Serum creatinine (sCR) is still the most frequently used biomarker of renal function in patients with cirrhosis [2], and the definition of AKI is based on changes in sCR [3]. Previously, an increase in sCR of at least 50% from the baseline to a final value above 1.5 mg/dl has been used to define AKI in this patient population and has been shown to be a strong predictor of mortality [4,5,6]. However, more recently, new criteria have been proposed and validated in the general population, namely, the Kidney Disease Improving Global Outcomes (KDIGO) criteria [7]. These criteria define AKI as an absolute increase of sCR ≥ 0.3 mg/dl in 48 h or a percentage increase ≥ 50% that occurred (or is presumed to have occurred) in the previous 7 days. The KDIGO criteria also introduced urinary output criteria; however, their applicability in patients with cirrhosis has been questioned due to the avid fluid and sodium retention and oliguria observed in patients with advanced cirrhosis and ascites. Nonetheless, despite these changes, renal function may remain adequate [3]. The KDIGO criteria also provided a staging of AKI according to the increase in sCR: (i) stage 1, an increase in sCR of between 1.5- and 2-fold from baseline; (ii) stage 2, an increase in sCR from 2- to 3-fold from baseline; and (iii) stage 3, an increase in sCR above 3-fold or an increase above 4 mg/dl. The mortality rate increased in a stepwise manner according to the AKI stages. Finally, the progression of AKI to a higher stage was associated with an even worse survival rate [8, 9]. The potential benefit of the new criteria is early diagnosis of AKI to enable prompt treatment and reduce the risk of AKI progression, which is associated with a worse prognosis [8, 9]. Several studies have validated the KDIGO criteria based on sCR in patients with cirrhosis [8,9,10,11,12,13,14].
The new International Club of Ascites (ICA) AKI criteria proposed the use of modified KDIGO criteria to diagnose AKI in patients with cirrhosis (Table 9.1) [3]. The ICA also based the definition of AKI on the sCR value: the last available preadmission value of sCR obtained in the 3 months before admission. In cases for which no preadmission value of sCR is available, the admission value should be used as a baseline, as other strategies may lead to relevant bias [15]. The staging of the ICA-AKI criteria was similar to that provided by the KDIGO criteria (Table 9.1).
Traditionally, three types of AKI have been considered: (a) prerenal AKI, (b) intrinsic AKI, and (c) post renal AKI [1]. Hypovolemia and hepatorenal syndrome (HRS-AKI) are the two main types of prerenal AKI , whereas acute tubular necrosis (ATN-AKI) is the most common type of intrinsic AKI .
Hypovolemia is the most common type of AKI in patients with cirrhosis followed by ATN-AKI and HRS-AKI. Post renal AKI is rare in patients with cirrhosis. AKI associated with bacterial infections can show characteristics of hypovolemia, HRS-AKI, or ATN-AKI according to the clinical scenario [16].
HRS-AKI is the most life-threatening type of AKI and requires prompt diagnosis and treatment [17]. It is characterized by a severe vasoconstriction of renal arterioles not responding to fluid administration [18, 19]. The incidence of HRS in the natural history of cirrhosis is estimated to be 18% after 1 year and 39% after 5 years [20]. Classically, two clinical types of HRS can be identified [18, 19]:
-
1.
Type 1 HRS , characterized by a rapidly progressive reduction of renal function, is classically defined by a doubling of the initial serum creatinine (sCR) concentration to more than 226 mmol/l (2.5 mg/dl) in less than 2 weeks
-
2.
Type 2 HRS , moderate renal failure (sCR from 133 to 226 mmol/l or from 1.5 to 2.5 mg/dl), with a steady or slowly progressive course, is usually associated with refractory ascites
With the adoption of the ICA-AKI criteria, the cutoff of 2.5 mg/dl required for the diagnosis of type 1 HRS was removed, and it is now defined as HRS-AKI [3]. Conversely, type 2 HRS has been considered to be a form of chronic kidney disease, although this definition is still a matter of debate [21].
Pathophysiology of AKI and HRS in Patients with Cirrhosis
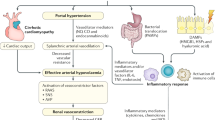
The following factors render patients with cirrhosis susceptible to the development of AKI and HRS-AKI (Fig. 9.1):
-
(a)
Severe splanchnic arterial vasodilation
-
(b)
Reduction in cardiac output
-
(c)
Systemic inflammation
The “peripheral arterial vasodilation hypothesis” has been considered for several years as the main pathophysiological mechanism of renal dysfunction in patients with cirrhosis [22]. Portal hypertension causes the release of vasodilators in the splanchnic circulation such as nitric oxide (NO), carbon monoxide (CO), adenomedullin, glucagon, and prostacyclin. The splanchnic arterial vasodilation causes a reduction in the effective circulating volume with subsequent stimulation of baroreceptors and, thus, activation of vasoconstrictor systems including production of catecholamine, activation of the renin-angiotensin-aldosterone system, and nonosmotic release of arginine vasopressin. This results in increased heart rate and cardiac output, with the development of a hyperdynamic circulation, as well as in the retention of sodium and water in the kidney (which causes the development of ascites and peripheral edema). Vasoconstrictor systems ensure the restoration of effective circulating volume. However, in the advanced stages of liver disease, the further increase of splanchnic vasodilation cannot be compensated by an increase in vasoconstrictor system activity. At this time, further water and sodium retention causes the formation of ascites and/or the development of dilutional hyponatremia. In the most advanced stages, the maximal activity of the vasoconstrictor systems leads to severe renal vasoconstriction, which is the cause of HRS. A precipitating event that can further worsen splanchnic vasodilation, such as a bacterial infection, or the administration of some medications, including nonsteroidal anti-inflammatory drugs (NSAIDs) or angiotensin-converting enzyme inhibitors, can trigger the development of AKI [12, 23,24,25,26].
In the early years of the twenty-first century, a new hypothesis was added to the splanchnic arterial vasodilation hypothesis. In fact, three hemodynamic studies demonstrated reduced cardiac output in patients who develop HRS-AKI. The first study compared the baseline characteristics of patients who developed HRS after an episode of SBP with those of patients who did not do so [27]. Patients with HRS had significantly higher levels of plasma renin activity and lower cardiac output as compared with patients who did not develop HRS. It is interesting that the concentrations of tumor necrosis factor alpha (TNF-α) were significantly higher in patients who developed HRS, highlighting the role of inflammation. The second study was performed in patients with cirrhosis, ascites, and normal renal function. Significantly lower cardiac output and mean arterial pressure and significantly higher plasma renin activity levels were found in patients who had developed HRS vs. those who had not developed HRS [28]. Furthermore, when HRS occurred, a further reduction in cardiac output was observed, suggesting that HRS is the result of a decrease in cardiac output in the setting of severe arterial vasodilation. In the third study, again, a reduction in the cardiac index was found to be a strong predictor of HRS development [29]. The mechanism of cardiac alterations in patients with cirrhosis is still unclear, but specific cardiac abnormalities including systolic and diastolic dysfunction, changes in electrophysiological repolarization, and enlargement of cardiac chambers were found in affected patients. Overall, these abnormalities are commonly referred to as “cirrhotic cardiomyopathy ” [30].
New data suggest that systemic inflammation is likely to play a central role in both promoting splanchnic arterial vasodilation and reducing cardiac output. In patients with cirrhosis, the main driver of chronic inflammation is the translocation of bacteria from intestinal lumen to systemic circulation [31]. This pathological process is the result of increased gut permeability, intestinal bacterial overgrowth, and changes in microbiome. The translocation of bacteria or bacterial products (pathogen-associated molecular patterns [PAMPs]) stimulates pattern recognition receptors (PRRs) such as toll-like receptors (TLRs) on immune cells, thereby stimulating the production of inflammatory cytokines including TNF-alpha, interleukin-6 (IL-6), and interleukin-1beta (IL-1beta). Studies performed on an experimental model of cirrhosis suggest that these proinflammatory mediators cause oxidative stress and stimulate the synthesis of NO, further enhancing splanchnic arterial vasodilation [32]. Intestinal decontamination with norfloxacin administration leads to a reduction in the inflammatory mediator plasma concentration as well as a decrease in NO synthesis. This suggests that bacterial translocation is the cause of the inflammatory response. Proinflammatory cytokines are also involved in the pathogenesis of cardiac dysfunction in cirrhosis. In fact, TNF-alpha stimulates the production of NO in the cardiac tissue of cirrhotic rats by exerting a negative inotropic effect [33]. Interestingly, TNF-alpha knockout mice and those treated with anti-TNF-alpha antibodies demonstrated restored cardiac contractility. Finally, it has recently been shown that PAMPs may directly cause renal damage due to the activation of TLR-4 and local inflammation. The latter was demonstrated in both experimental and clinical studies [34, 35]. All these data have led experts in this field to introduce a new hypothesis for the development of AKI and other organ failures in patients with cirrhosis: the “systemic inflammation hypothesis” [36]. According to this hypothesis, systemic inflammation is the main driver of AKI. A superimposed precipitating event, such as a bacterial infection, can cause systemic inflammation with a subsequent further increase in splanchnic arterial vasodilation, a depression of cardiac contractility, and a reduction of the effective circulating volume, resulting in renal hypoperfusion. Systemic inflammation can also damage the kidney directly due to the action of inflammatory mediators [16].
Epidemiology and Clinical Features of AKI in Patients with Cirrhosis
The prevalence of AKI in hospitalized patients with cirrhosis is variable according to the criteria used, and ranges from 20 to 50% [1, 13]. About two-thirds of episodes are community-acquired, whereas the remaining are nosocomial [13]. Most of the cases are diagnosed while in stage 1, and progression of AKI occurs in 20–50% of patients [8,9,10]. AKI occurs more frequently in patients with ascites and bacterial infections (the most common precipitating event of AKI) [23, 24]. Of the infections, spontaneous bacterial peritonitis (SBP) is the one most frequently associated with the development of AKI and HRS-AKI. Other predisposing factors are less common and include gastrointestinal bleeding or acute alcohol consumption. However, AKI has a significant negative prognostic impact on these subgroups of patients [37, 38]. The spectrum of clinical manifestations of AKI may be very different, and sometimes the precipitating event (bacterial infection, GI bleeding, etc.) is the main clinical manifestation. However, sometimes, oliguria and a worsening of ascites may be the trigger. Finally, it should be remembered that hepatic encephalopathy may be the first manifestation of AKI.
Management and Differential Diagnosis of AKI in Cirrhosis
AKI should be managed according to the algorithm provided by the ICA [3] (Fig. 9.2) . This algorithm differentiates between the management of patients according to two groups: those with AKI stage 1 and those with AKI stage 2 or 3.
Management of acute kidney injury in cirrhosis. (Modified from Ref. [3]). AKI acute kidney injury, NSAIDs nonsteroidal anti-inflammatory drugs, HRS hepatorenal syndrome
In both groups, the first steps are to identify and treat potential precipitating factors. Thus, diuretics should be tapered or withdrawn, and a precise diagnostic workup for infection should be performed (paracentesis to rule out SBP, chest X-ray, urinalysis, and blood, urine and ascitic fluid cultures). All potential nephrotoxic drugs (NSAIDs, vasodilators, angiotensin-converting enzyme inhibitors, etc.) should be withdrawn. In patients with AKI stage 1, volume expansion should be administered with crystalloids in cases of dehydration (diarrhea or overdiuresis), packed red blood cells in cases of GI bleeding, and albumin in patients with SBP (1.5 g/kg on day 1 and 1 g/kg on day 3) [4]. In cases of progression to a higher stage, patients should receive the treatment provided for patients with AKI stage > 1. Diuretics should be withdrawn, and albumin should be administered at a dose of 1 g/kg per day for 2 days. In cases in which there is no response, the main differential diagnosis is between HRS-AKI and ATN-AKI (other types of AKI are quite rare). Patients with ascites, and without several pathological factors including signs of shock, the use of a nephrotoxic drug, and macroscopic signs of kidney parenchymal damage (normal renal ultrasound, no sign of proteinuria, no sign of hematuria) meet the criteria for HRS-AKI (Table 9.2) . It should be highlighted that new biomarkers of renal tubular damage have recently become available. Among these biomarkers, urinary neutrophil gelatinase-associated lipocalin (NGAL) is the most investigated in patients with cirrhosis. Urinary NGAL was found to be significantly higher in patients with ATN-AKI than in those with HRS-AKI, with the lowest levels demonstrated in patients with hypovolemic AKI [39]. More recently, it has been suggested that a combination of several urinary biomarkers such as kidney injury molecule 1, interleukin-18, liver fatty acid-binding protein, and albumin may improve the differential diagnosis among ATN-AKI and other types of AKI. Further studies are needed before urinary biomarkers are included in a diagnostic algorithm of AKI; however, this diagnostic approach is very promising.
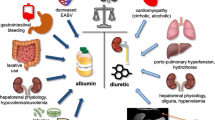
Management of HRS-AKI
General management of HRS-AKI should include monitoring the patient’s parameters, such as fluid balance, arterial pressure, and vital signs. Treatment of bacterial infections should be started as soon as possible. There are no data supporting the use of empirical antibiotic treatment for unproven infections. When terlipressin is administered, beta-blockers should be discontinued [40]. Paracentesis with albumin administration can be performed in a patient with HRS and tense ascites, but removing more than 51 per paracentesis is not recommended. The use of diuretics should be avoided, but furosemide may be useful in treating central volume overload. Figure 9.3 summarizes available treatments for patients with HRS according to the pathogenesis of the condition.
Vasoconstrictors Plus Albumin
The combination of arterial vasoconstrictors with albumin is the most effective and investigated treatment for HRS-AKI [41]. The rationale behind the use of vasoconstrictors is to counteract splanchnic arterial vasodilation, while albumin expands the effective blood volume. However, both clinical and experimental studies suggest that the positive effects of albumin are not only mediated by plasma volume expansion. It has been demonstrated that in patients with cirrhosis and SBP, albumin in comparison to hydroxyethyl starch is capable of increasing cardiac stroke volume and systemic vascular resistance [42]. Conversely, no difference was found before and after the administration of hydroxyethyl starch, suggesting that albumin may improve cardiac output and vascular resistance with mechanisms other than plasma expansion. Experimental animal studies demonstrated that albumin is able to restore cardiac contractility in cirrhotic rats and, by a reduction of the TNF-α-induced activation of the NF-κB-iNOS pathway, diminish oxidative stress in the cardiac tissue [43]. In fact, albumin has several non-oncotic properties such as the capacity to bind and inactivate PAMPs, NO, and reactive oxygen species [44]. The importance of the combination of using albumin and vasoconstrictors is supported by a lower rate of positive response when each of these drugs is administered alone [45].
Three types of vasoconstrictors are currently available for the treatment of HRS: terlipressin, noradrenaline, and the combination of midodrine + octreotide.
Terlipressin , a vasopressin analog, is the most investigated vasoconstrictor in this field. Three randomized controlled trials found that the combination of terlipressin plus albumin is more effective than albumin alone in the treatment of HRS [46,47,48]. The use of terlipressin and albumin in combination for the management of HRS is reported to be successful in 34–54% of cases. Terlipressin can be administered both as intravenous boluses (starting from 0.5–1 mg every 4–6 h to a maximum dose of 2 mg every 4 h) and as a continuous intravenous infusion (starting from 2 mg/day to a maximum dose of 12 mg/day). Continuous intravenous infusion is associated with a significantly lower incidence of side effects than bolus administration [49]. It has also been demonstrated that continuous infusion is effective at a lower dose compared to intravenous boluses. These findings are consistent with the short half-life of terlipressin, lasting 3–4 h [50]. Doses of terlipressin should be increased in a stepwise manner if serum creatinine does not decrease at least 25% after 3 days of treatment [51]. Albumin should be administered at the dose of 20–40 g/day. Usually, full response to treatment occurs within 14 days. After discontinuation of terlipressin and albumin, a recurrence of HRS can be observed in about 20% of patients with type 1 HRS, and retreatment is usually effective. Conversely, recurrence of HRS is quite common in patients with type 2 HRS, and treatment should be reserved for the most severe patients (sCR > 2 mg/dl). Some patients with AKI-HRS may require long-term treatment with terlipressin and albumin [52], and a specific LT allocation policy has been suggested for these patients [53]. Several predicting factors of a positive response to treatment were found, including baseline sCR, bilirubin, and the delta increase in mean arterial pressure on day 3 [54, 55]. Additionally, patients who responded to treatment with terlipressin plus albumin demonstrated a better survival rate than non-responders [49]. In a recent meta-analysis of randomized trials, the use of terlipressin was associated with a trend toward an improvement in survival vs. those treated with placebo [56].
The usual adverse effects of treatment with terlipressin include diarrhea, abdominal cramps, nausea, and headache. Also, some severe side effects, such as angina, cardiac arrhythmia, and intestinal ischemia, have been described. Patients with severe hypertension, ischemic heart disease, and peripheral vascular disease should not be treated with terlipressin.
Midodrine (an α1-agonist drug) combined with octreotide (a somatostatin analog) in combination with albumin infusion has been demonstrated as effective in treating HRS-AKI [57]. Midodrine is administered orally at a dose of 2.5 mg t.i.d., which can be increased to 12.5 mg t.i.d. if there is not a reduction in sCR of at least 25%, compared to baseline at day 3 of treatment. The starting dose of octreotide is 100 mcg t.i.d., and it can be increased to a maximum of 200 mcg t.i.d. The albumin dose is the same as that provided for terlipressin. In a randomized controlled trial, the combination of terlipressin plus albumin was significantly more effective than the combination of midodrine plus octreotide and albumin in treating HRS-AKI (an improvement in renal function of 70 vs. 29%, respectively; p = 0.01) [58]. Thus, this treatment should be considered only in patients with contraindications to terlipressin.
The administration of norepinephrine (administered at a dose of 0.5–3 mg/h) plus albumin has been investigated for treatment in HRS-AKI. The efficacy of noradrenaline was similar to that of terlipressin in treating HRS-AKI [59]. Norepinephrine is cheaper than terlipressin. However, it should be administered in a central venous line and under continuous monitoring, such that its use is limited to patients admitted to the intensive care unit. The treatment with vasoconstrictors plus albumin should be continued until sCR reaches a value below 1.5 mg/dl.
Liver Transplantation
Liver transplantation (LT) represents the best treatment for HRS-AKI [60]. Unfortunately, the timing of the transplantation procedure is unpredictable, and liver transplant candidates (LTCs) with HRS-AKI should be treated with vasoconstrictor plus albumin while on the waiting list. In fact, LTCs with HRS-AKI responding to terlipressin and albumin while on the waiting list demonstrated a better posttransplantation course, a shorter period of hospitalization, and less requirement for renal replacement therapy (RRT) after LT [61]. Conversely, in a recent case control study, the use of terlipressin and albumin in patients with type 2 HRS is questioned, as no differences were found in terms of post-LT outcomes between patients treated with terlipressin and those not treated with it [62].
Patients with HRS responding to treatment with vasoconstrictors plus albumin may be penalized by a current organ-distribution model based on MELD. In fact, patients with AKI-HRS have a higher mortality rate than other cirrhotic patients for any point of the MELD score [63]. Furthermore, patients showing continuous recurrence of HRS during any attempt to withdraw vasoconstrictors and albumin may be further disadvantaged [52]. In these two groups of patients, it has been suggested that the peak of sCR be used to estimate the MELD score (for responders to vasoconstrictors) and to compute the MELD score as provided for patients in dialysis (patients on long-term treatment with terlipressin) [53].
Transjugular Intrahepatic Portosystemic Shunt
Transjugular intrahepatic portosystemic shunt (TIPS ) is a technique used to create a shunt between the portal and hepatic veins in the liver. TIPS is usually well tolerated; however, some complications can occur, including thrombosis/occlusion of the shunt, fistulae, hemolysis, infections, and, more commonly, hepatic encephalopathy [51].
From a pathophysiological point of view, TIPS is beneficial, because it reduces portal hypertension and increases cardiac output. TIPS improves renal perfusion and water excretion and optimizes sodium and has been reported to reduce serum creatinine in selected patients with HRS [64, 65]. However, the applicability of TIPS in patients with HRS is very limited because many affected patients have contraindications to the use of TIPS. Furthermore, the available data regarding the use of TIPS in patients with HRS-AKI are mainly based on case series. Randomized controlled trials are necessary before TIPS can be implemented in clinical practice for patients with HRS.
Renal Replacement Therapy
The data pertaining to the use of RRT in patients with HRS-AKI are limited. However, if a patient does not respond to vasoconstrictors plus albumin, with volume overload, metabolic acidosis, severe hyperkalemia, and/or hyponatremia, RRT should be considered as an option, particularly for LTCs on the waiting list [56, 66]. No data are available regarding the optimal technique of RRT (intermittent hemodialysis vs. continuous RRT) in these patients. However, it has been suggested that continuous RRT may be the better option given the lower risk of hypotension as compared to the risk with intermittent hemodialysis. In patients who are not eligible for liver transplantation, the decision to perform RRT should be made on a case-by-case basis in order to avoid rendering futile treatment.
Key Points
-
1.
Patients with cirrhosis have a high risk of developing acute kidney injury (AKI), which is associated with a poor prognosis. The higher the stage of AKI, the higher the mortality rate.
-
2.
Bacterial infections (in particular, spontaneous bacterial peritonitis) are the most important trigger of AKI in patients with cirrhosis. Other triggers are an overdose of diuretics, the presence of gastrointestinal bleeding, and the use of nephrotoxic drugs (nonsteroidal anti-inflammatory drugs, ACE inhibitors, aminoglycosides, etc.).
-
3.
Hepatorenal syndrome AKI (HRS-AKI) is a specific type of AKI occurring in patients with cirrhosis and ascites. HRS-AKI is characterized by severe renal vasoconstriction with renal hypoperfusion, without macroscopic signs of intrinsic kidney injury.
-
4.
Liver transplantation is the optimal treatment for HRS-AKI. The use of vasoconstrictors plus albumin is the most effective medical treatment for HRS-AKI, being effective in about 50% of cases.
References
Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–77.
Piano S, Romano A, Di Pascoli M, Angeli P. Why and how to measure renal function in patients with liver disease. Liver Int. 2017;37:116–22.
Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–74.
Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–9.
Angeli P, Fasolato S, Mazza E, Okolicsanyi L, Maresio G, Velo E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98–104.
Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–10.
KDIGO. Clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138.
Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–9.
Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753–62.
Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–81.
Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131–7.
Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:1280–8.e1.
Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute on chronic liver failure. Clin Gastroenterol Hepatol. 2017;15:438–45.
Angeli P, Rodríguez E, Piano S, Ariza X, Morando F, Solà E, et al. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616–22.
Rosi S, Piano S, Frigo AC, Morando F, Fasolato S, Cavallin M, et al. New ICA criteria for the diagnosis of acute kidney injury in cirrhotic patients: can we use an imputed value of serum creatinine? Liver Int. 2015;35:2108–14.
Angeli P, Tonon M, Pilutti C, Morando F, Piano S. Sepsis-induced acute kidney injury in patients with cirrhosis. Hepatol Int. 2016;10:115–23.
Martin-Llahi M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488–96.
Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology. 1996;23:164–76.
Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8.
Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola AAV. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–36.
Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–9.
Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–7.
Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–9.
Terra C, Guevara M, Torre A, Gilabert R, Fernández J, Martín-Llahí M, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–53.
Ackerman Z, Cominelli F, Reynolds TB. Effect of misoprostol on ibuprofen-induced renal dysfunction in patients with decompensated cirrhosis: results of a double-blind placebo-controlled parallel group study. Am J Gastroenterol. 2002;97:2033–9.
Elia C, Graupera I, Barreto R, Solà E, Moreira R, Huelin P, et al. Severe acute kidney injury associated with non-steroidal anti-inflammatory drugs in cirrhosis: a case-control study. J Hepatol. 2015;63:593–600.
Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–8.
Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–47.
Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2009;59:105–10.
Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2014;11:177–86.
Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209.
Tazi KA, Moreau R, Hervé P, Dauvergne A, Cazals-Hatem D, Bert F, et al. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology. 2005;129:303–14.
Yang Y-Y, Liu H, Nam SW, Kunos G, Lee SS. Mechanisms of TNFα-induced cardiac dysfunction in cholestatic bile duct-ligated mice: interaction between TNFα and endocannabinoids. J Hepatol. 2010;53:298–306.
Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047–53.
Shah N, Mohamed FE, Jover-Cobos M, Macnaughtan J, Davies N, Moreau R, et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 2013;33:398–409.
Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–84.
Cárdenas A, Ginès P, Uriz J, Bessa X, Salmerón JM, Mas A, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34:671–6.
Altamirano J, Fagundes C, Dominguez M, García E, Michelena J, Cárdenas A, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71.e3.
Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267–73.
de Franchis R. Expanding consensus in portal hypertension. J Hepatol. 2015;63:743–52.
Cavallin M, Fasolato S, Marenco S, Piano S, Tonon M, Angeli P. The treatment of hepatorenal syndrome. Dig Dis. 2015;33:548–54.
Fernández J, Monteagudo J, Bargallo X, Jiménez W, Bosch J, Arroyo V, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–34.
Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S, Morando F, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology. 2013;57:266–76.
Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407.
Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–8.
Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–8.
Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–9.
Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O’Leary JG, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150:1579–89.e2.
Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983–92.
Escorsell À, Bandi JC, Moitinho E, Feu F, García-Pagán JC, Bosch J, et al. Time profile of the haemodynamic effects of terlipressin in portal hypertension. J Hepatol. 1997;26:621–7.
European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Piano S, Morando F, Fasolato S, Cavallin M, Boscato N, Boccagni P, et al. Continuous recurrence of type 1 hepatorenal syndrome and long-term treatment with terlipressin and albumin: a new exception to MELD score in the allocation system to liver transplantation? J Hepatol. 2011;55:491–6.
Angeli P, Gines P. Hepatorenal syndrome, MELD score and liver transplantation: an evolving issue with relevant implications for clinical practice. J Hepatol. 2012;57:1135–40.
Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–21.
Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–26.
Facciorusso A, Chandar AK, Murad MH, Prokop LJ, Muscatiello N, Kamath PS, et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:94–102.
Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–7.
Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567–74.
Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293–8.
Boyer TD, Sanyal AJ, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1*. Liver Transpl. 2011;17:1328–32.
Restuccia T, Ortega R, Guevara M, Ginès P, Alessandria C, Ozdogan O, et al. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140–6.
Rodriguez E, Henrique Pereira G, Solà E, Elia C, Barreto R, Pose E, et al. Treatment of type 2 hepatorenal syndrome in patients awaiting transplantation: effects on kidney function and transplantation outcomes. Liver Transpl. 2015;21:1347–54. https://doi.org/10.1002/lt.24210.
Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–9.
Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416–22.
Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55–64.
Francoz C, Nadim MK, Baron A, Prié D, Antoine C, Belghiti J, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology. 2014;59:1514–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Piano, S., Angeli, P. (2019). Acute Kidney Injury and Hepatorenal Syndrome. In: Bezinover, D., Saner, F. (eds) Critical Care for Potential Liver Transplant Candidates. Springer, Cham. https://doi.org/10.1007/978-3-319-92934-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-92934-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92933-0
Online ISBN: 978-3-319-92934-7
eBook Packages: MedicineMedicine (R0)