Abstract
The attenuation of light with increasing depth, along with reduced exposure to wave stress, plays an important role in vertically structuring coral reef communities. Benthic photosynthetic organisms exhibit different depth distributions and abundance patterns which cause changes in community composition of associated reef fauna. This vertical zonation in coral reef community structure suggests special adaptations in response to the changing environmental regime with depth including changes in light intensity, light spectrum, and angular distribution. At the lower depth limits of mesophotic coral ecosystems (MCEs), both light and temperature can become limiting factors with the latter playing an important role at higher latitudes. The available evidence indicates that different species can exhibit distinct and sometimes opposing photophysiological adaptations with increasing depth. Some zooxanthellate corals appear to maximize ambient light utilization at the expense of efficiency, while others appear to maximize efficiency. Coral holobiont adaptations to mesophotic depths include changes in colony morphology, algal symbionts, pigment physiology, skeletal properties, and metabolic strategy. Given the scarcity of physiological studies at depths >60 m, the current understanding of how obligate zooxanthellate corals and other light-dependent organisms can inhabit such a broad depth distribution is far from complete. This chapter summarizes the ecologically relevant aspects of light and temperature regimes of MCEs, as well as the depth-related photophysiological and adaptive strategies of coral holobionts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Mesophotic coral ecosystems (MCEs) are warm water, light-dependent coral reef communities starting at 30–40 m and extending to the bottom of the photic zone, which varies by location (Hinderstein et al. 2010). The lower depth limit of MCEs can reach >150 m in some locations but can be considerably shallower in lower-light habitats or higher-latitude locations (Kahng et al. 2010). MCEs represent a direct extension of shallow-water coral reef ecosystems. However, the upper depth limit attributed to MCEs does not represent a static physiological boundary for marine organisms but corresponds to the depth limit of conventional SCUBA diving (Kahng et al. 2014) and therefore the traditional limits of most knowledge available for coral reef science (Loya et al. 2016; Turner et al. 2017).
Beginning in shallow water and increasing with depth, the abundance patterns of sessile benthic taxa on coral reef ecosystems including shifts in dominant species of zooxanthellate coral and benthic algae are well documented (reviewed in Kahng et al. 2010, 2014). This change in photosynthetic species composition includes a conspicuous reduction in the abundance of dominant shallow-water coral taxa (e.g., Acropora, Porites, and Pocillopora) at deeper depths (e.g., >60 m) (Kahng et al. 2010). While a few depth-generalist species appear to span most or all of the depth range of zooxanthellate corals in the Caribbean, at other MCE locations, depth specialists, which are cryptic or absent in shallow water, dominate the zooxanthellate coral community in the lower photic zone (Kahng et al. 2010; Englebert et al. 2014). Where studied crustose coralline algae are commonly reported as the dominant phototrophic taxa at the deepest depths (e.g., >200 m) (reviewed in Kahng et al. 2017).
Of the physical factors (e.g., hydrodynamic regime, sedimentation, and topography) influencing this vertical zonation (reviewed in Kahng et al. 2010), gradients of both light and temperature play major roles in structuring communities. In general, there is a correlation between the water clarity by location and the observed lower depth limit of zooxanthellate corals and some macroalgae (Kahng et al. 2010). With increasing latitude, lower daily solar insolation during winter is thought to limit the depth distribution of corals (Muir et al. 2015a). Where there is reduced light due to lower water clarity, an upward shift in the coral community can be observed with deeper coral fauna occurring at shallower depths (e.g., Acevedo et al. 1989). In warm, turbid waters, light attenuation is a primary factor limiting photosynthetic organisms (including zooxanthellate corals) to relatively shallow depths (Kirk 2011), although, in some locations with relatively clear water, other factors (e.g., lack of suitable substrate and low temperature) may restrict lower depth distributions.
Both the change in photosynthetic species composition with depth and low light availability at extreme depths suggest that phototrophic depth specialists have physiological adaptations and capabilities lacking in their shallow-water counterparts. While special adaptations affect post-settlement fitness and therefore vertical zonation of community structure, larval behavior in the form of preferential settlement based on depth-related cues may also reinforce these patterns (Grigg 1965; Baird et al. 2003; Putnam et al. 2008). This chapter summarizes the ecologically relevant changes in light (intensity, spectrum, and angular distribution) and temperature regimes with depth and the adaptation strategies enabling zooxanthellate corals to thrive in low-light habitats, as well as the lower depth limits for light-dependent corals.
2 Light Regime
2.1 Intensity of Photosynthetically Available Radiation
Many biotic and abiotic factors vary predictably with depth and can influence the upper and lower depth distribution of mesophotic organisms. For photosynthetic organisms, light regime (along with thermal and hydrodynamic regimes) is a primary factor influencing the vertical zonation of community structure (reviewed in Kahng et al. 2010). For zooxanthellate corals and algae, the underwater light field controls rates of primary productivity, growth, and calcification (reviewed in Barnes and Chalker 1990; Falkowski et al. 1990). As light travels through the water column, it is absorbed and scattered by particles that reduce its intensity and change its spectral composition. When light (E 0) entering the ocean reaches a particular depth (z), its intensity (E z) can be modeled by Beer’s law:
where K d is the diffuse attenuation coefficient of downwelling light. K d primarily results from the combined effects of absorption and scattering, which are inherent optical properties of water (West et al. 2016). K d also depends on the directionality of the instantaneous light field (technically classifying it as an apparent optical property), but this dependence is weak, especially at mesophotic depths, away from boundary effects (Mobley 1994). For purposes of this review, K d is assumed to be a good representation of the optical water quality.
The exponential decay of downwelling light (Fig. 42.1) eventually limits the distribution of obligate photosynthetic organisms (Kirk 2011). In Eq. 42.1, E and K d are both functions of wavelength λ, but for practical purposes attenuation can be represented by scalar irradiance K d(PAR), which is calculated from the integrated value of E across the wavelength range of photosynthetically available radiation or PAR (400–700 nm). Assuming that an obligate photosynthetic taxon has a minimum absolute threshold of light intensity (E min) required for basal metabolism (i.e., survival), the compensation depth limit for this taxon can be derived from Eq. 42.1 and represented as follows (Kahng et al. 2010):
Variability in photosynthetically available light. The intensity of PAR decreases exponentially with depth. For all sites, PAR intensity was calculated for the 400–700 nm range of K d curves obtained from the following sources: Bermuda oceanic (thin gray lines) is the Bermuda Atlantic Time-series Study (BATS) from the SeaBASS database (Werdell and Bailey 2002) for all months between 2000 and 2012 (n = 791), collected 0–120 m; Hawaiʻi oceanic is from Kalohi Channel collected 0–55 m, measured between 395 and 710 nm at 19 wavelengths (Kahng et al. 2012b); Hawaiʻi coastal is from south Oahu collected 0–30 m, measured between 395 and 710 nm at 19 wavelengths (Kahng et al. 2012b); Bahamas oceanic is from the SeaBASS database (collection depth N/A) measured between 412 and 683 nm at 7 wavelengths; Bahamas coastal K d value calculated from irradiance measurements at 0–20 m (measured wavelengths unknown) from Fig. 2 in Lesser et al. (2009); and pure water is from Pope and Fry (1997) measured between 380 and 727.5 nm at 140 wavelengths. Measurement of surface light was used from the dataset presented in Fig. 5b of Akkaynak et al. (2017), measured between 305 and 710 nm at 15 wavelengths
The greatest viable depth z min for a given species will vary more strongly with K d(PAR) than with surface PAR (E 0). However, additional environmental factors (e.g., thermal regime and space competition) may restrict depth distribution independent of K d(PAR) and E 0.
Angle and orientation of local topography can also affect the amount of ambient light available to the benthos. On flat or gently sloping areas, sessile organisms can be exposed to light throughout the day, but on a steep slope, light is limited because the slope obstructs the light (i.e., shades the benthos) for a portion of the day (Brakel 1979). Reflectance and scattering properties of the substrata can further influence the amount of light available to the photosynthetic community (Kahng et al. 2012b).
In the ocean, annual solar insolation reaching the surface of the ocean is determined by latitude and atmospheric conditions (e.g., cloud cover) (reviewed in Kirk 2011). On shorter timescales, seasonal variations in both solar zenith angle and day length cause the daily solar insolation reaching the top of the atmosphere to vary in a predicable manner. Independent of latitude, daily variations in atmospheric and sea surface conditions moderate the daily irradiance entering the ocean.
2.1.1 Air-Sea Interface
When sunlight hits the ocean surface, some of it reflects back into the air reducing the amount transmitted into the seawater. Because the refractive index of seawater (n sw = 1.33) is larger than the refractive index of air (n air = 1), the angle of transmission θ t becomes smaller than the original angle of incidence θ i (Snell’s law, Fig. 42.2a):
and the light transmitted into the ocean “bends” toward the vertical direction (0°). While Snell’s law governs the angles of incident, reflected, and transmitted light travelling from one medium to another, the quantity of incident light that will be reflected/scattered at the air-sea boundary is governed by the Fresnel reflectance equation. Under calm surface conditions, almost all incident light perpendicular to the sea surface (e.g., θi = 0°) is transmitted into the ocean. As the incidence angle becomes larger, the ratio of light reflected from the surface increases, decreasing the portion that is transmitted into the water (Fig. 42.2b).
(a) Snell’s law. Light refracts, or bends, when travelling from one medium to another with different indices of refraction. When sunlight at incidence angle θ i reaches the surface of the ocean, the part that is transmitted through bends toward the vertical direction (θ t) because the refractive index of seawater (n sw ≈ 1.33) is larger than that of air (n a = 1). The angle of reflected light θ r is always equal to θ i. (b) Fresnel reflectance. The fraction of light that is reflected from the air-sea interface is governed by Fresnel’s reflectance function. For unpolarized water-incident light reflectance increases rapidly around incidence angles of θ i = 30°. For air-incident light reflectance increases substantially when θ i exceeds 65°
The amount of light reflected at the surface is heavily influenced by wind (reviewed in Kirk 2011). During periods of light winds, surface waves can act like an optical lens focusing light and substantially amplifying its intensity over short (sub-second) timescales to depths of at least 35 m (Stramska and Dickey 1998; Veal et al. 2010). At higher velocities, winds increase surface roughness and inject small air bubbles into the water (e.g., white caps and sea foam). Both factors can dramatically increase the surface area of air-water interfaces and the potential for multiple angle reflections. Thus, for small angles of incidence (e.g., θ i ≈ 0°), reflectance increases, and the amount of light transmitted into the ocean is reduced. For example, at solar noon, the brightness of the white caps on the sea surface results from incident light being reflected and not transmitted into the ocean. For high angles of incidence (e.g., θ i > 75°), the amount of light reflected is reduced, and the amount of light transmitted into the ocean is increased (Kirk 2011).
2.1.2 Optical Water Quality
Just as surface irradiance can vary based on weather conditions, the absorption and scattering of water (and therefore K d) can also vary across space and time depending on the amount of dissolved and particulate matter suspended in the water column (reviewed in Kirk 2011). In general, optical water quality is correlated with primary productivity, terrigenous influences (e.g., riverine discharge), and hydrodynamic conditions, which can suspend particles from the seafloor into the water column. The clearest surface waters are found in oligotrophic, subtropical gyres far away from landmasses and upwelling. Coastal waters generally have higher primary productivity and suspended particulate matter, and hence lower optical water quality (i.e., faster light attenuation with depth) than adjacent open ocean locations (Fig. 42.1). However, even oligotrophic, oceanic locations far from terrigenous influences [e.g., the Bermuda Atlantic Time-Series Study (BATS) Hydrostation S] can experience considerable variability in optical water quality over time (Fig. 42.1). For many locations, offshore oceanic water serves as the source water for the coastal zone and the end member for maximum water clarity for many MCEs.
2.2 Spectrum
The attenuation of light through seawater is wavelength dependent, and some wavelengths (perceived by humans as different colors) penetrate the water better than others (reviewed in Kirk 2011). In pure water, longer wavelengths >570 nm (red-yellow light) are quickly attenuated with depth, while short wavelengths <500 nm (blue and UVA light) penetrate deeper (Fig. 42.3; Smith and Baker 1981; Pope and Fry 1997; Mason et al. 2016). In oligotrophic waters, the low concentration of phytoplankton, their light-absorbing pigments (e.g., chlorophyll), and the associated production of dissolved organic matter (DOM) result in optical properties similar to pure water, with the light spectra being predominately blue at depth (Fig. 42.3; Kirk 2011). Unlike in coastal waters, ultraviolet radiation (<400 nm) penetrates relatively well in oligotrophic waters and remains a significant fraction of irradiance at depth (reviewed in Tedetti and Sempéré 2006) (Fig. 42.4).
Diffuse downwelling attenuation coefficients. The attenuation of light through seawater is wavelength dependent. In pure water, longer wavelengths attenuate faster with depth, while shorter wavelengths penetrate further. See Fig. 42.1 for data sources, plus the following: Eilat is from Station A (collection depth 0–120 m) measured between 305 and 710 nm at 15 wavelengths (R. Tamir, unpubl. data)
Ultraviolet radiation (UVA) at depth. UVA (320–400 nm) is a significant component of the total light regime in oligotrophic waters, but not in some coastal waters. Data sources same as in Fig. 42.1 plus the following: Hawaiʻi oceanic is from Station Aloha north of Oahu from the SeaBASS database (Werdell and Bailey 2002), measured between 305 and 683 nm at 11 wavelengths collected between 0 and 148 m
In more productive coastal and temperate waters, ultraviolet radiation (UVR) and blue light (short wavelengths) are more quickly attenuated, leaving green as the dominant light at depth in these waters (reviewed in Kirk 2011). The predominant wavelength of light reaching the lower photic zone is strongly influenced by suspended particulate matter (i.e., tripton) and the concentration of CDOM (colored or chromophoric dissolved organic matter) – also known as gilvin (dissolved yellow substance) (Booth and Morrow 1997; Stomp et al. 2007). Because CDOM strongly absorbs ultraviolet and blue wavelengths, low concentrations of CDOM can alter light spectra at depth and shift the predominant wavelength at depth toward longer wavelengths (Bricaud et al. 1981; Stomp et al. 2007). In the lower photic zone, these spectral differences can become amplified. For example, the spectral composition reported from a coastal MCE location in the Bahamas (Lesser et al. 2009) appears significantly different from adjacent offshore oceanic waters and other MCE locations (Figs. 42.4 and 42.5). Typically, CDOM concentrations are correlated with primary production and proximity to riverine input, but small, persistent differences also exist between ocean basins on a macroscopic scale because of differences in riverine discharge of terrigenous DOM among ocean basins (Carder et al. 1989; Opsahl and Benner 1997; Kirk 2011). For example, the Atlantic Ocean receives 3.6 times more riverine water discharge and has 2.6 times greater terrigenous DOM concentrations than the Pacific Ocean (Opsahl and Benner 1997).
Light spectrum of the lower photic zone. The relative intensity of light for each wavelength in the lower photic zone (where PAR is reduced to 1% of surface irradiance at each respective location). In more productive coastal areas, the predominant wavelength at depth shifts toward longer wavelengths due to the presence of phytoplankton and CDOM. (Data sources same as in Fig. 42.3)
Seasonal variability in thermal stratification, primary productivity, terrigenous influence, and photooxidation of CDOM can cause seasonal changes in optical water quality, especially in coastal waters (Kirk 2011). Episodic events such as diazotroph blooms during extended periods of calm, mesoscale eddies and storms causing deepwater mixing can also change the optical water quality. Even in the oligotrophic, open ocean, there can be considerable variability in optical water quality across time (e.g., BATS Hydrostation S in Fig. 42.3).
2.3 Angular Distribution
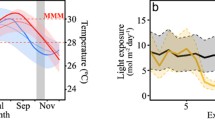
For the light transmitted into the seawater, the angle of transmission (θ t) is reduced relative to the original incident angle (θ i), thereby “bending” the light toward the vertical direction (Eq. 42.3 and Fig. 42.2a). In concert with the high transmission of low-angle incident light (high solar elevation) and low transmission of high-angle incident light (Fig. 42.2b), downwelling light concentrates around the vertical axis. In shallow water (e.g., at 5 m), the instantaneous light field is highly directional and dependent on the incident angle of the sun’s rays, which varies by time of day (Fig. 42.6a). On a relative scale, the angular distribution of instantaneous light field becomes more diffuse with increasing depth due to multiple scattering. However, light transmitted at higher angles is reduced (e.g., Frade et al. 2008) because it must travel through more water per unit depth and is subject to greater attenuation than light transmitted at small angles (θ~0°). With increasing depth, the instantaneous light field becomes increasingly symmetrical about the vertical axis (θ = 0°) regardless of the time of day. Although the daily light field (integrated over 24 h) becomes more diffuse with increasing depth (Fig. 42.6b), above a minimum threshold, it also becomes more directional at depth with the highest intensity of light near θ = 0°. For light irradiance above a minimum threshold (e.g., 0.5% of surface), the range of angles of incoming light above the threshold (angular width) narrows with depth (Fig. 42.6c). In shallow water, this angular width is 360° (irradiance exceeds minimum threshold from all directions). Below a certain depth (not calculated in the example in Fig 42.6c), this angular width starts to decrease very quickly (approximately exponentially). The decrease in angular width decelerates in deep water before eventually reaching zero.
Angular distribution of light. (a) Relative radiance distributions for PAR at selected depths and times of day. Arrow lengths indicate the relative radiance intensity for each direction at a given time and depth (not comparable between times and depths). (b) Angular distribution of total diel-integrated PAR radiance (mol photons m−2 sr−1 day−1) for the same depths. Note the diffuse nature of the light field with increasing depth. (c) Despite the fact that the light becomes more diffuse with depth, the angular width of the light field (i.e., the angles at which radiance exceeds 0.5% of surface light level) decreases approximately exponentially with depth. Data were generated for the Au’au channel, Hawaii (20.9407 N, 156.7573 W) on June 21 using Hydrolight 5.3 for the default parameters of the “new” Case 1 model, assuming clear marine air, 75% relative humidity, 50 km visibility, 7 m s−1 wind speed, bottom boundary specified with an average coral Lambertian spectral reflectance. The total daily PAR radiance plots were obtained by integrating radiance at each zenith and azimuth angles across wavelengths, with respect to time. The radiance distributions are not symmetrical around the vertical because the hourly intervals of Hydrolight runs are not equal before and after solar noon
The sharply truncated angular distribution of light available to the benthos at depth can be illustrated by a phenomenon called Snell’s window. In accordance with Eq. 42.3, light transmitted into the ocean bends toward the vertical direction compressing all air incident light into an angle of view of ~97° underwater (within the critical angle θ c = 48.6° from the vertical). For an observer looking at the sea surface from depth, this phenomenon results in a circular window of light surrounded beyond the critical angle by darkness (i.e., the attenuated reflection originating from weakly upwelled light) (Fig. 42.7).
Snell’s cone. (a) An observer looking up to the sea surface from depth sees a dark reflection of the sea bottom that surrounds a small circular window of light called Snell’s cone (or an optical manhole). The light entering the water at large angles bends toward the vertical, creating the circular window of light. Rays exceeding the critical angle of θ c = 48.6° are from upwelled light (very weak) that is reflected downward at the sea surface. (b) A branching Acropora pharaonis colony on an MCE at 44 m with Snell’s cone (Photo credit: T. Shlesinger)
3 Temperature Regime
In the tropics and subtropics, open ocean temperature exhibits a predictable pattern with depth rapidly decreasing below the mixed layer (usually <100 m) followed by a more gradual decrease to abyssal depths (Emery and Dewar 1982; Ohno et al. 2009; Holte et al. 2017). During seasonal periods of sea surface temperature (SST) warming, the depth of the mixed layer is generally shallower as increasing thermal stratification opposes wind-driven mixing (Fig. 42.8a). During seasonal periods of SST cooling, thermal stratification weakens. In many regions, seasonal SST cooling can be correlated with stronger wind events that cause deeper mixing of the water column (Fig. 42.8b). At higher latitudes large seasonal variations in SST occur with deeper mixing especially during the winter (Ohno et al. 2009; Holte et al. 2017).
Monthly temperature profiles measured at Station Aloha, Hawaiʻi. (a) Progression of temperature during the warming season (March–October 2002) and (b) cooling season (September 2001–March 2002). (c) Monthly temperature profiles (January 2001–November 2016; n = 164) illustrating the contrast between the consistent seasonal pattern SST and the lack of seasonal consistency below the surface mix layer (Data from Hawaii Ocean Time-Series program (http://hahana.soest.hawaii.edu/hot/hot-dogs/) Karl and Lukas 1996). Arrows in (a) and (b) indicate the temporal progression of the temperature profiles
Below the seasonal thermocline in the open ocean, the predictable seasonal pattern of SST warming and cooling does not exist. At these depths the water is warmed via vertical mixing with the warmer water above it. Warming events are caused by episodic deep mixing events, which are more probable during seasonal SST cooling and weaker thermal stratification (Ohno et al. 2009; Holte et al. 2017). In general, warm surface water can mix to deeper depths when SST is colder (Fig. 42.8c). For example, at 150 m depth at the Hawaii Ocean Time-series’ Station Aloha (located 100 km north of Oahu), the warmest temperatures typically occur in late winter and early spring when SST is the coldest. In general, these dynamics govern the seasonal dynamics of the open ocean subtropical gyres, which feed the insular shelves and continental shelves where many MCEs are located.
In enclosed, low-latitude marginal seas (e.g., the Red Sea and Persian Gulf), shallower bathymetry, less heat capacity, and lack of connectivity with polar, deepwater formation result in warm bottom waters year-round (Tomczak and Godfrey 2003). Seasonal SST warming and cooling cause greater variability in shallow water compared to more stable, warm deep waters (Biton and Gildor 2011). In the Gulf of Eilat/Aqaba, winter vertical mixing is driven by a loss of surface water buoyancy from a combination of summer evaporation (increase in salinity) and subsequent winter cooling.
Mesoscale eddies can also influence the thermal regime and sea surface height on nearby coastlines, especially around oceanic islands and their associated MCEs. The vast majority of these eddies are nonlinear and entrain/trap water within their core (Chelton et al. 2011). Anticyclonic eddies cause surface waters to converge toward the eddy’s center raising sea surface height and lowering the thermocline in the core. Cyclonic eddies cause waters to diverge away from the center raising the thermocline in the core and stimulating primary productivity (McGillicuddy et al. 1998; Seki et al. 2001). Mesoscale eddies are a common feature in the open ocean except at the equator (Chelton et al. 2011). They generally propagate westward (5–12 cm s−1 in the subtropics) with an average lifespan of ~32 weeks and propagation distance of ~550 km. However, many can last over a year and cross entire ocean basins. Eddy size and propagation speed vary inversely with latitude. The effects of a nearby mesoscale eddy can alter the normal thermocline structure and tide levels for several weeks (e.g., Firing and Merrifield 2004).
In many locations at mesophotic depths, the benthic communities are subject to rapid influxes of cooler, deeper water. These cold-water intrusions are often associated with internal waves coinciding with the lunar semidiurnal (M2) tidal frequency (e.g., Wolanski and Delesalle 1995; Wolanski and Deleersnijder 1998; Wolanski et al. 2004; Leichter et al. 2006; Kahng et al. 2012a; Leichter et al. 2012). Internal waves are often generated as tidal flow interacts with seafloor topography causing vertical oscillations in the water column’s isopycnals (i.e., thermal structure). These internal waves travel along density boundaries/interfaces including the thermocline beneath the surface mixed layer. Upwelling of sub-thermocline water can also be caused by a variety of other interactions with surface currents and topographic features including island wakes, tidal jets, and land-sea boundary shear (Wolanski and Hamner 1988).
These short duration cold-water pulses vary in magnitude by location and can be very pronounced below the mixed layer depth. At some MCE locations, rapid temperature decreases of 2–6 °C lasting for ~1 to 2 h can be common (Wolanski and Pickard 1983; Leichter et al. 2008, 2012; Kahng et al. 2012a; Bongaerts et al. 2015b). In areas of high internal wave energy dissipation, extreme thermocline fluctuations (e.g., >100 m) can occur (e.g., Wolanski et al. 2004). For many locations exposed to internal waves, the thermal variability at mesophotic depths is far greater than in shallow water (Fig. 42.9). For example, the benthos at 100 m in the Auʻau Channel in Hawaiʻi often experiences a greater temperature range in a single day than the benthos in shallow water experiences all year (Fig. 42.10). In general, these temperature variations do not reach shallow water (<10 m) except in areas with large breaking internal waves. During periods of seasonal cooling, when the mixed layer deepens, the internal waves propagate along the deeper and more gradual density boundary thereby weakening (or potentially removing) their impact on shallower waters on a seasonal basis (e.g., Leichter et al. 2012; Smith et al. 2016).
Short-term thermal variability. Temperature in the Auʻau Channel, Hawaiʻi (90–110 m depth) from November 5–8, 2007. (Data from Kahng et al. 2012a, temperature recorded every 90 s)
In addition to being colder, the sub-thermocline water is often associated with higher concentrations of inorganic nutrients, which can enhance primary productivity in the euphotic zone (e.g., Wolanski et al. 1988; Leichter et al. 2003; Letelier et al. 2004). Aggregations of zooplankton and phytoplankton can also be associated with density boundaries and fronts and be transported with them due to their buoyant properties (Leichter et al. 1998; Wolanski and Hamner 1988; Pineda 1999; McManus et al. 2005). These cold-water intrusions are generally viewed as ecologically beneficial and are linked to inorganic nutrient enrichment, enhanced bottom currents, heterotrophic feeding, and lower rates of coral bleaching during periods of anomalously high SST (Leichter et al. 1998, 2006, 2012; Roder et al. 2010; Jantzen et al. 2013; Wall et al. 2015). However, extreme cold-water intrusions may cause lethal stress and limit the lower depth distribution of sessile, warm-water fauna where they occur (Wolanski et al. 2004).
4 Photophysiology and Adaptions to Light Regimes at Depth
4.1 Adaptation to Light Intensity
In shallow water, adaptation (both ontogenetic and phylogenetic) to high light irradiance dominates the photophysiology of zooxanthellate corals. Symbiodinium acclimated to high light increase their photoprotective pigments, which convert light energy to heat (i.e., nonphotochemical quenching or NPQ), increase antioxidant capacity, and adopt self-shading morphologies at a cellular level (MacIntyre et al. 2002; Lesser 2006; reviewed in Falkowski and Raven 2007). Some coral hosts also employ self-shading mechanisms via gross colony morphology (Kaniewska et al. 2008; Kaniewska et al. 2014), tissue thickness (Kaniewska et al. 2011), and contraction and/or retraction of polyps and tissue (Brown et al. 2002; Levy et al. 2003). Biochemically, some coral hosts also accumulate UV-absorbing mycosporine-like amino acids (MAAs) in exposed tissues (reviewed in Shick and Dunlap 2002), produce heat shock proteins (Brown et al. 2002), and express fluorescent proteins (FPs) and chromoproteins (CPs) of the green fluorescent protein family, which can play a photoprotective role (Salih et al. 2000; Dove et al. 2001; Smith et al. 2013). These host pigments are responsible for the pronounced color polymorphism of reef corals (Gittins et al. 2015, Quick et al. 2018). In shallow-water corals, the tissue concentration of the sunscreen proteins are regulated in response to increased light intensity, particularly in the blue spectral range (D’Angelo et al. 2008, 2012).
Although Palmer et al. (2009) hypothesized antioxidant functions for coral FPs and CPs, the experimental support is very tenuous. The H2O2 scavenging experiments by Palmer et al. (2009) lacked positive (e.g., catalase) and negative controls. Therefore, any FP-/CP-specific activity cannot be distinguished from unspecific scavenging of the protein scaffold. Furthermore, the apparently positive correlation between the level of tissue fluorescence and H2O2 scavenging was disproportionately influenced by an individual outlier in each of the datasets (Fig. 3 in Palmer et al. 2009), and the R 2 values were accordingly low (R 2 = 0.08 − 0.34).
In low-light conditions associated with deeper depths and shaded habitats, corals and their endosymbiotic algae acclimate in reciprocal ways (reviewed in Falkowski et al. 1990; Kirk 2011). Shade-acclimated plants decrease their photoprotective pigments and increase light-harvesting pigments at a cellular level by increasing the number and size (absorption cross section) of the photosynthetic units (PSU) (Kirk 2011). For most phytoplankton, the increase in light-harvesting pigments is achieved by increasing the size of the PSU antennae, but not the number of PSUs (Dubinsky and Stambler 2009). Despite being a classic mechanism for optimizing light absorption and enhancing quantum efficiency in the lower photic zone (Chang et al. 1983; Prézelin 1987; Iglesias-Prieto and Trench 1997; Hutchings et al. 2008), an increase the number of PSUs per cell has not been observed to date on MCEs (Mass et al. 2007; Einbinder et al. 2016). Within the same coral species (i.e., Stylophora pistillata), both stable and increasing zooxanthellae densities have been associated with photoacclimatization to deeper depths (Falkowski et al. 1990; Titlyanov et al. 2001; Mass et al. 2007) indicating that depth-related acclimatization mechanisms can vary due to other environmental factors (Winters et al. 2009; but see Keshavmurthy et al. 2013). In low light, the xanthophyll cycle, an important photoprotection mechanism (NPQ), can be converted to light-harvesting state in some algae (reviewed in Goss and Jakob 2010). Diatoxanthin (Dtx) is epoxidized into diadinoxanthin (Ddx) removing the quenching pigment and transforming the antennae system from a heat dissipation state to a light-harvesting state (Goss et al. 2006).
Per unit surface area, some shade-acclimated corals can exhibit greater photosynthetic capabilities than their high light-acclimated counterparts (reviewed in Falkowski et al. 1990). However, shade-acclimated corals reach their maximum rates of gross photosynthesis (P max) at lower irradiance than their high light-acclimated counterparts (e.g., Einbinder et al. 2016). In some shade-acclimated algae, a reduction in the carboxylation capacity limits P max compared to high light-acclimated conspecifics (Falkowski and Raven 2007). For shade-acclimated corals, photosynthetic efficiency or P max normalized per unit chlorophyll a (chl a) is lower due to self-shading of light-harvesting centers. Also, an increase of antennae size leads to a decrease in quantum efficiency due to a slower rate of energy transfer to the reaction center (RC) (Croce and van Amerongen 2014).
While an increase in light-harvesting pigments with depth (traditionally called shade adaptation or shade acclimation) has been demonstrated in both shallow-water corals and depth-generalist mesophotic corals, stable or decreasing pigment concentrations with depth has also been demonstrated in mesophotic coral species (reviewed in Kahng et al. 2010). Some corals in the lower photic zone consistently exhibit lower areal concentrations of photosynthetic pigments than their shallow-water counterparts (Kahng et al. 2012b). At intermediate depths increasing photosynthetic pigment concentrations per unit area to maximize utilization of ambient light is potentially advantageous, but this form of shade acclimation eventually becomes self-limiting due to the package effect (Kirk 1975; Bissett et al. 1997) as the incremental gain in light harvesting per unit pigment diminishes (Falkowski et al. 1990; Stambler and Dubinsky 2007). Theoretically, there is a limit to enhancing light absorption through an increase in effective antenna cross section as intracellular self-shading (from increased pigment density) negates gains in quantum efficiency (Falkowski and Dubinsky 1981). In an energy- or resource-limited environment, the incremental cost of producing and maintaining additional pigments may outweigh the energetic gain in incremental light harvesting.
An alternative way to enhance quantum efficiency is to modify the pathway of excitation energy trapping and its transfer. The challenge with a very low-light environment is that the balance between the rates of trapping photons to the rate of the back reactions (decay rates) for the S states may not be positive, thereby preventing oxygen evolution via catalytic water oxidation (McEvoy and Brudvig 2006; Nelson and Yocum 2006; Cox et al. 2013; Shen 2015). Assuming a single electron is moved by photosystem (PS) II per a quanta of energy, four positive charges have to be concurrently created to produce an oxygen molecule via the five S-stages of the oxygen-evolving complex (OEC) where the S1 state is the resting state of the enzyme under no light (Joliot et al. 1969; Kok et al. 1970). Under very low light, the enzyme may never cycle to the S4 state (due to lack of protons) and thus never release an oxygen molecule. In order for the symbiont to have a positive contribution to the overall energy balance of this symbiosis, the organism must outcompete the back reaction.
An efficient low-light energy transfer model was postulated by Einbinder et al. (2016) based on novel photosynthetic characteristics measured from deep (65 m) for Stylophora pistillata colonies with a Symbiodinium clade distinct from shallow (3 m) conspecific colonies. A model of a closely arranged PSII array combined with PCP (peridinin-chlorophyll a protein) soluble antenna that funnels the light to PSI can create an efficient low-light energy transfer system that is no longer based on a membrane-embedded light-harvesting system, but a membrane-attached light-harvesting system. Under low-light intensities, this energy transfer increases photosynthetic quantum efficiency (Joliot and Joliot 1964, 2003). Combining this distinct light-harvesting antenna organization with a lower PSI content will enable a cooperative effect (Einbinder et al. 2016).
To minimize self-shading at an intercellular level, some coral species exhibit a monolayer zooxanthellae arrangement in deep water (in contrast to the multilayer zooxanthellae arrangement in shallow-water corals) (Dustan 1979; Schlichter et al. 1986). Also, the distribution of photosynthetic pigments over a greater number of symbionts reduces the package effect and increases light absorption efficiency (a∗) (Scheufen et al. 2017). At a colony level, corals growing in low light construct less self-shading colony morphologies including wider spacing between branches/plates and more flattened growth forms at depth compared to conspecifics in shallower water (Fig. 42.11; Kühlmann 1983; Anthony et al. 2005; Hoogenboom et al. 2008).
Changes in coral colony morphology with depth. (a) Spherical colony of Platygyra lamellina in a shallow reef (3 m) compared to (b) a flat conspecific colony in an MCE (44 m). (c) The branching coral Stylophora pistillata in shallow reef (1.5 m) compared to (d) an MCE (46 m). Branching corals may display thinner branches with greater space between them in MCEs compared to shallow reefs. (e) Turbinaria reniformis in a shallow reef (5 m) with typical foliose coral morphology with upright unifacial laminae in contrast to (f) flat colony of Turbinaria reniformis in an MCE (41 m). (g) Goniastrea retiformis colony with a massive to sub-massive growth form in shallow reef (2 m) compared to (h) an encrusting foliose morphology in an MCE (43 m). All photos taken at the Gulf of Eilat/Aqaba, Red Sea (Photo credits: T. Shlesinger)
4.2 Chromatic Adaption and Fluorescent Pigments
In addition to the decrease in light intensity, the change in light spectra with increasing depth has led to distinct niches and pigment physiology of algal taxa in the lower photic zones across various aquatic habitats (Falkowski and Laroche 1991; Stomp et al. 2007). For the lower depth distributions of benthic macroalgal taxa in oligotrophic waters, the general pattern from brown (phaeophytes) to green (chlorophytes) to red (rhodophytes) at the deepest depths suggests phylogenetic chromatic adaptation to a particular spectral character (Dring 1981; Kirk 2011; but see Ramus 1983). Compared to algae, relatively few studies have investigated chromatic adaptation in zooxanthellate corals. Because reef-building corals only host dinoflagellates as major photosynthetic endosymbionts (Baker 2003; LaJuenesse et al. 2005), the potential for chromatic adaptation is focused on the genus Symbiodinium. All dinoflagellates including Symbiodinium are phylogenetically constrained in terms of their available photosynthetic machinery including their light-harvesting pigments (reviewed in Schnepf and Elbrächter 1999).
Despite the pronounced changes in spectra with increasing depth, physiological evidence for chromatic adaptation in mesophotic corals remains somewhat limited. In several shallow-water corals, depth-dependent distribution of MAAs has been demonstrated along with acclimation to UV exposure (reviewed in Shick and Dunlap 2002). MAAs absorb UV light, which can induce photochemical damage, and are assumed to originate from the algal partner in microalgal-invertebrate symbiosis.
The negative effects that red light can have on Symbiodinium indicate that at least some Symbiodinium are adapted to the absence (or near absence) of red light which characterizes the underwater light field in general including in shallow water (Kinzie et al. 1984; Wijgerde et al. 2014). In a controlled experiment using Stylophora pistillata, Mass et al. (2010) demonstrated that corals, acclimated to a restricted spectrum of blue light (at 40 m) versus full spectrum PAR (at 3 m), exhibited higher photosynthetic performance in their respective spectral habitats independent of light intensity.
Based on absorption characteristics, an increase in chl c 2 (with a blue-shifted peak compared to peridinin) would be expected for dinoflagellate taxa chromatically adapted to blue light in the lower photic zone (Iglesias-Prieto and Trench 1994). The available evidence from mesophotic corals conflicts with this expectation of chromatic adaptation. Chl c 2/chl a ratios decline with increasing depths for depth-generalist coral species (spanning from shallow to mesophotic depths), and depth-specialist coral species dominating the lower photic zone (i.e., agariciids) exhibit lower ratios than their shallow-water counterparts (Lesser et al. 2010; Nir et al. 2011; Kahng et al. 2012b; Einbinder et al. 2016).
Based on the overlap between the emission spectra of host FPs and the absorption spectra of photosynthetic pigments, FPs had been hypothesized to enhance photosynthesis (Schlichter et al. 1986; Schlichter and Fricke 1991; Salih et al. 2000). However, coral FPs (donor) are not energetically coupled to the light-harvesting pigments (acceptor) of Symbiodinium (reviewed in Dubinsky and Falkowski 2011) because Förster resonance energy transfer (FRET) decreases exponentially (\( E=1/\left\{1+{\left[\frac{r}{R_0}\right]}^6\right\} \)) with donor-to-acceptor separation distance (r), where R 0 is the Förster distance of this pair of donor and acceptor, i.e., the distance at which the energy transfer efficiency is 50% (Förster 1960). Without intracellular coupling, the efficiency of energy transfer from host FPs to symbiont light-harvesting centers becomes mathematically minute.
Despite the limitations imposed by spatial physics, Smith et al. (2017) proposed that photoconvertible red fluorescent proteins (pcRFPs) provide adaptive value to corals at intermediate depths by showing that the proportion of corals with pcRFPs increases with depth (to at least 45 m) and by demonstrating increased long-term survivorship of color morphs with strong pcRFP expression compared to conspecific color morphs without them. The yellow-orange wavelengths emitted by pcRFPs lie in-between the blue and red absorption peaks of the chlorophyll-peridinin complex in Symbiodinium (Devred et al. 2013; Croce and van Amerongen 2014) and can stimulate photosynthesis deeper in heavily pigmented coral tissues (Bollati et al. 2017; Smith et al. 2017). This mechanism would enable the symbiont community deeper in the coral tissue (which is shaded by near-surface symbionts and deprived of ambient blue light) to increase their levels of photosynthesis. Downwelling yellow-orange light (>560 μm) quickly attenuates from the light spectrum with increasing depth and is essentially absent below 30–40 m in clear oligotrophic waters (Kahng et al. 2012b; Smith et al. 2017). While the conversion of blue-to-orange wavelengths by pcRFP is efficient (Bollati et al. 2017), the subsequent utilization of this light by photosynthetic pigments is not due to nondirectional emission (i.e., Lambertian diffusion) and distance between donor (pcRFP) and acceptor (Symbiodinium chl). Given the loss of energy involved, this mechanism would add value only if the near-surface symbiont community is somehow unable to utilize the incident blue light or is rate limited due to other factors such as diffusion limitation in dense aggregations of near-surface symbionts.
For substrates and metabolites exchanged with the external environment, some evidence from the micro environments in the tissue of shallow-water corals shows that surface symbionts would be less diffusion limited than symbionts deeper in the tissue layer (300–500 μm deep) (Kühl et al. 1995; de Beer et al. 2000; Brodersen et al. 2014). The coral skeleton acts as a barrier to diffusion causing metabolites (e.g., oxygen) to accumulate in lower tissue layers (Kühl et al. 1995; Brodersen et al. 2014). In deeper water with less light, less diffusion limitation would be expected during the daytime, but the same general concentration gradients would be expected.
Other evidence suggests that conditions deeper in the tissue may be more favorable for photosynthesis. Kühl et al. (1995) reported a sub-tissue surface gross photosynthesis maximum in the tissue below 500 μm, and Brodersen et al. (2014) detected a sub-tissue surface maximum (around 300 μm depth) in quantum efficiency under low light. While exchange of substrates and metabolites with the external environment may be more limiting in deeper tissue, diffusion-limited exchange (between host and symbiont) of vital compounds such as coral host N- and P-rich metabolites and photosynthate may have the opposite gradient with more diffusion limitation associated with a near-surface symbiont community.
This light redistribution concept is somewhat analogous to the redistribution of high solar flux on the surface of the mantle of tridacnid clams to Symbiodinium in deeper layers of tissue (Holt et al. 2014). Bragg-reflective iridocytes efficiently scatter light in a wavelength-specific and angle-dependent manner. Depending on their size and microstructure, iridocytes reflect a small percentage the same color that they forward scatter preferentially (Ghoshal et al. 2016). Other wavelengths are scattered at higher angles. High incoming solar flux can be redistributed over a greater area (deeper in the tissue) for efficient utilization and obviate the need for nonproductive photoprotection (e.g., NPQ) (Holt et al. 2014).
The concept of chromatic adaptation to utilize “leftover” light, which either lies outside the absorption peak of overshading plants or is a byproduct of photosynthesis itself (i.e., red chl fluorescence), also has precedence. In the low-light environment of endolithic algae (Ostreobidineae) below the layer of coral tissue (Marcelino and Verbruggen 2016), near-infrared and infrared wavelengths (700–900 nm) dominate the light field and are enhanced over incident irradiance in some corals (Magnusson et al. 2007). These wavelengths are not utilized by Symbiodinium but lay within the theoretical limits (<900 nm) for natural photosynthesis (van Grondelle and Boeker 2017). They readily penetrate coral tissue well into the coral skeleton where they may be at least partially utilized by chromatically adapted Ostreobium. Due to the limited penetration of these longer wavelengths through the water column in stark contrast to the deep depth distribution of Ostreobium, the adaptive value of these near-infrared absorbing pigments is debatable (Ralph et al. 2007). For the tropical plant Begonia which inhabits the very low-light environment of the forest floor, the light regime is predominantly green (Jacobs et al. 2016). The thylakoid tissue of Begonia is structured to create multilayered photonic structures which directly enhance the capture of green light and increases quantum yield 10–15% under low-light conditions. While photonic structures are well represented in a diversity of phyla and have been demonstrated to enhance photosynthesis in other algae (Vukusic and Sambles 2003; Toster et al. 2013; De Tommasi 2016), whether corals or Symbiodinium employ analogous photonic structures is unknown.
4.3 Gross Morphology
In shallow waters where available light is not limiting, corals can exhibit a diversity of colony morphologies to optimize different strategies (e.g., resistance to wave stress, maximize surface area for mass transfer and photosynthesis, and reduce internal light field via self-shading) (reviewed in Todd 2008). Daily light flux from all incident angles is sufficient to support photosynthetic organisms (E θ > E min for all θ) due to change in incident angle throughout the day and diffusive scattering from both particles in the water column and the seafloor. For example, coral and algae can grow on the undersides of horizontal substrates, which are completely shaded from direct sunlight.
With increasing depth, a reduction in self-shading morphologies is common although some species can be phenotypically stable (Willis 1985). For shallow-water branching and foliaceous corals, acclimation to small increases in depth (<10 m) can be associated with greater spacing between branches or plates, less vertical growth, fewer branches or convolutions, and less surface area (Jaubert 1977; Willis 1985; Muko et al. 2000; Kruszynski et al. 2007; Kaniewska et al. 2008). Across larger in situ-depth gradients (~20 to 40 m), intraspecific micromorphology at deeper locations includes lower calical relief, fewer septa, and lower corallite density (Wijsman-Best 1974; Willis 1985; Beltrán-Torres and Carricart-Ganivet 1993; Muko et al. 2000; Klaus et al. 2007). Reciprocal transplants of Goniastrea pectinata in shallow water (between 3 and 7 m) have confirmed acclimation of corallite morphology leading to less self-shading at slightly deeper depths (Ow and Todd 2010).
Across much larger depth gradients, the incident angles of light sufficient to drive photosynthesis (i.e., above a minimum threshold for a given taxon) become limited to a range of angles symmetric about the vertical axis (angular width) (Fig. 42.6). The angular width for driving daily net positive photosynthesis becomes increasingly narrow with increasing depth. This narrowing angular width correlates with the flattening of coral skeletons with increasing depth (i.e., more perpendicular to the vertical axis) (Fig. 42.11; Fricke and Schuhmacher 1983; Kühlmann 1983; Titlyanov and Titlyanova 2002; Muir et al. 2015b). If light is limiting at depth, there is adaptive value in eliminating colony self-shading and maximizing solar flux per unit area of coral surface.
In deeper water where the angular width of light above the threshold for net positive photosynthesis is very narrow (Fig. 42.6), coral skeleton orientations which deviate away from being perpendicular (θ = 90°) to the vertical axis will reduce their daily solar flux per unit surface area by a factor of sin θ (E θ = E z,θ = 90°|sin θ|). At depths, where E z is close to the compensation depth (E z~E min), the angle of the coral skeleton must be very close to |θ| = 90° (i.e., horizontally oriented plates) to maintain net positive photosynthesis, and the potential angular deviation from the perpendicular plane becomes highly constrained. This relationship holds for both foliose/plating (concave: |θ| < 90°) and mounding (convex: |θ| > 90°) coral morphologies (Fig. 42.11). At their maximum depth distribution, obligate zooxanthellate corals (commonly agariciids) exclusively exhibit thin horizontal plates (Kahng and Maragos 2006; Kahng et al. 2012b; Englebert et al. 2014; Englebert et al. 2017). These corals grow to expand surface area rather than volume resulting in very thin skeletal plates requiring minimal calcification (Anthony and Hoegh-Guldberg 2003; Kahng 2013; Luck et al. 2013).
4.4 Role of Calcium Carbonate Skeletons and Light Scattering
The reflective properties of calcium carbonate play an important role in increasing the light-harvesting efficiency of photosynthetic marine organisms including corals, calcareous green algae, and coralline red algae, as well as phototrophs growing on calcium carbonate substrate (Enríquez et al. 2005; Kahng et al. 2012b, 2017). In corals, the aragonite skeleton redirects diffuse light back into the overlying tissue enhancing scalar irradiance in the tissue, increasing the path length of photons within the tissue, and raising the absorption efficiency of light-harvesting pigments in endosymbiotic algae (Wangpraseurt et al. 2014). The scalar irradiance at the surface of the coral tissue can be three to five times enhanced over the incident downwelling irradiance outside the tissue (Kühl et al. 1995). The reflective properties of skeletons allow corals to absorb two to five times more light than isolated symbionts (with the same density of pigments) and harvest the same amount of incident radiation as the leaf of a terrestrial plant with six times less pigment density (Enríquez et al. 2005). This enhancement effect is greater at lower pigment densities and decreases with increasing pigmentation.
Under conditions of high light in shallow water and reduced pigmentation due to bleaching, this absorption efficiency can magnify susceptibility to photo-damage (Enríquez et al. 2005; Terán et al. 2010; Marcelino et al. 2013). This light enhancement effect does not apply to harmful UVR (Reef et al. 2009). While coral skeletons are highly reflective for PAR and infrared, they readily absorb UVR and weakly fluoresce yellow light. This property allows corals to enhance PAR for photosynthesis without the harmful effects of enhancing UVR, which readily penetrates oligotrophic waters.
Scattering properties differ among coral taxa due to variations in colony morphology and skeletal construction (Marcelino et al. 2013; Enríquez et al. 2017). Due to their micromorphology (e.g., concave parallel septal cavities), the skeletons of some agariciid species enhance light absorption more than the skeletons of their shallow-water counterparts (i.e., Porites spp.) independent of photosynthetic pigment concentrations (Kahng et al. 2012b; Enríquez et al. 2017). This adaptation may help these deepwater specialists dominate the coral taxa in the lower photic zone. For calcareous and coralline algae that dominate the flora in the lower photic zone (reviewed in Kahng et al. 2010; Kirk 2011; Kahng et al. 2017), the calcium carbonate underlying their tissues enhance their light-harvesting efficiency in an analogous fashion to corals. The superior reflectance of calcite versus aragonite (Gaffey 1986) may play a role in the deeper depth distribution of coralline red algae (calcitic) relative to calcareous green algae (aragonitic). Although non-calcareous, endolithic green algae (Ostreobidineae) have also been reported as abundant below 200 m (Aponte and Ballantine 2001), they inhabit calcium carbonate substrate and may benefit from its reflective properties.
Microscale light regulation in corals also occurs in the coral tissue. Differences in the refractive index between tissue layers can cause incident photons to be scattered and even trapped (due to internal reflections) within the tissue (Wangpraseurt et al. 2014). Photons can also be internally reflected (Fresnel reflection) due to differences in refractive indices between tissue layers and between the tissue and overlying water. The former increases the photon path length per vertical distance travelled, increasing the average residence time of photons within the tissue layer, and thereby raises the probability of photon absorption by photosynthetic pigments. In concert with the coral skeleton, the latter can lead to photon trapping or wave guiding for obliquely scattered photons and promote lateral light transfer within the coral tissue. This anisotropic propagation of light within the coral tissue may facilitate light-harvesting efficiency in low-light habitats (Brodersen et al. 2014; Wangpraseurt et al. 2014).
4.5 Algal Symbionts
As in shallow water, obligate zooxanthellate corals at depth are energetically dependent on their algal endosymbionts (i.e., Symbiodinium) for their autotrophic capabilities. Both depth-generalist subclades with broad depth distributions and depth-specialist subclades have been found in MCEs (reviewed in Kahng et al. 2017). Independent of environmental conditions, most coral hosts appear to exhibit specificity in their symbioses with one Symbiodinium type, which does not change over time (Baker 2003; Goulet 2006, 2007; Bongaerts et al. 2011a). Consistent with this pattern, multiple depth-generalist coral species, which also inhabit mesophotic depths, appear to maintain the same Symbiodinium type across a large depth range (Bongaerts et al. 2011b, 2015b; Cooper et al. 2011; Nir et al. 2011; Ziegler et al. 2015). However, some coral species are consistently associated with multiple symbiont types (i.e., polymorphic) and exhibit depth- and irradiance-related partitioning in the relative frequencies of clades and subclades (Rowan and Knowlton 1995; Rowan et al. 1997; Bongaerts et al. 2015a). Symbiodinium in MCEs is comprehensively reviewed in Goulet et al. (2019), Chap. 30 of this book.
In addition to Symbiodinium, endolithic green algae (Ostreobidineae) can contribute photosynthate to coral hosts and may play a significant metabolic role for corals at depth (Odum and Odum 1955; Schlichter et al. 1997; Fine and Loya 2002). Endolithic algae colonize the coral skeleton below the layer of host coral tissue and live in an environment of very low light that can be enriched in red and infrared wavelengths (Magnusson et al. 2007). Their very low metabolic rates likely facilitate their ability to inhabit these extremely low-light habitats (Shashar and Stambler 1992). Recent multi-marker metabarcoding of coral skeleton endoliths has revealed that the endolithic algae community is far more taxonomically diverse at the family level than previously recognized (Marcelino and Verbruggen 2016). Ostreobium (which was previously thought to consist of only three species) has several separate evolutionary lineages that predate the first scleractinian corals.
Green sulfur bacteria (GSB; Chlorobiaceae) with their bacterial chlorophylls have also been detected in the endolithic community of shallow-water corals (Magnusson et al. 2007; Ralph et al. 2007; Cai et al. 2017). GSB are obligate photoautotrophs (anoxygenic photosynthesis) that are strictly anaerobic and occupy the lowermost part of light-stratified environments with their very sensitive light receptors (chlorosomes) (reviewed in Imhoff 2014). Bacterial chlorophylls strongly absorb both UVA and infrared wavelengths (Croce and van Amerongen 2014). While the latter is generally limited to shallow water due to rapid attenuation in seawater, the former can remain a significant fraction of the available light in the lower photic zone (Fig. 42.4). Metagenomic analyses confirm the widespread presence of GSB in shallow-water corals and their nitrogen-fixing capabilities (Cai et al. 2017). Given their ability to inhabit the low-light regions deep within coral skeletons below the layer of endolithic algae (Ralph et al. 2007), their presence in mesophotic corals as potential symbionts is quite probable.
Interestingly, photosynthesis may not be the only function of algal endosymbionts in mesophotic corals. The depth distributions of active algal endosymbionts in antipatharians, benthic foraminifera, and cyanosponges extend far below the photic zone and suggest that algal endosymbionts may perform other adaptive functions for their hosts or may even be parasitic (Grzymski et al. 2002; Usher 2008; Wagner et al. 2010). Below the photic zone, Kahng et al. (2014) hypothesized that algal endosymbionts may be assimilating nitrate (instead of inorganic carbon) in environments where their heterotrophic hosts are limited by the availability of reduced forms of bioavailable nitrogen.
5 Metabolic Adaptations
5.1 Energetics and Elemental Resources
Like all living organisms, corals require both energy (phototrophy and/or chemotrophy) and elemental resources through assimilation of organic matter (organoheterotrophy) and/or inorganic (lithoautotrophy) compounds. Survival, growth, and reproduction can be limited by either factor independently. Energetics depends on both energy input (e.g., organic carbon from photosynthesis or heterotrophy) and metabolic demand (i.e., energy output) with each affecting growth and growth efficiency. Required elemental resources consist of all elements needed to build organic molecules. In general, hydrogen, oxygen, carbon, and sulfur are not limiting in the marine environment because bioavailable forms of each are readily available from seawater (Libes 2011). However, in oligotrophic waters, organisms are often subject to (bioavailable) nitrogen limitation and sometime phosphorus or iron limitation (Atkinson and Falter 2003; Karl and Church 2017). In well-lit shallow-water habitats, the uptake of inorganic nutrients (e.g., nitrogen and phosphorous) and dissolved organic nutrients (e.g., urea and amino acids) has been shown to limit coral growth due to suboptimal mass transfer, which is a function of not only nutrient concentration but also water velocity and drag (reviewed in Atkinson 2011; Suzuki and Casareto 2011). In other words, current speed can compensate for low inorganic nutrient concentrations and facilitate faster growth.
In low-light conditions, some corals lower metabolic demand via slower rates of growth, less calcification, and reduced respiration (Huston 1985; Anthony and Hoegh-Guldberg 2003; Grigg 2006; Cooper et al. 2011). At depth, some mesophotic corals exhibit morphological adaptations, which reduce basal metabolism thereby increasing growth efficiency. These adaptations include thinner tissue (less biomass per unit area) and lower surface area-to-volume ratios, which slows metabolite exchange rates and reduces respiration per unit surface area (reviewed in Falkowski et al. 1990; Anthony and Hoegh-Guldberg 2003). Lower surface area-to-volume ratios can be achieved by having larger polyps and/or lower polyp density per unit area. However, such morphological features may also influence hydrodynamic boundary layer effects, drag, and therefore mass transfer rates. For several coral species, polyp density decreases with depth or in shaded habitat (e.g., Dustan 1979; Lasker 1981; Dinesen 1983; Villinski 2003; Einbinder et al. 2009). Some of the deepest obligate zooxanthellate corals (i.e., Leptoseris spp.) exhibit relatively large polyps without tentacles and extremely low polyp densities (Fig. 42.12; Luck et al. 2013; Englebert et al. 2017). However, all corals at depth and in low light do not uniformly exhibit these morphologies. For example, many shade-dwelling zooxanthellate corals in shallow water exhibit small polyps (Dinesen 1983).
Low polyp density and relatively large polyps in Leptoseris spp. (a–c) skeletal specimens collected at 80–130 m depth with 0.82, 1.24, and 1.22 polyps per cm2 (a, b, and c, respectively). (d) Leptoseris sp. in situ at depth of 50 m. Scale bars represent 1 cm (Photo credits: (a-c) Takaaki Watanabe, (d) T. Shlesinger)
5.2 Light and Growth
Since light is presumed to be the primary environmental factor controlling growth rates of zooxanthellate corals via light-enhanced calcification (reviewed in Allemand et al. 2011), growth rates in the lower photic zone can be expected to be very slow. Intraspecific growth rates decrease with increasing latitude and decreasing PAR (Harriott 1999; Lough et al. 2016). The exponential attenuation of light with increasing depth also decreases intraspecific growth rates (Huston 1985; Grigg 2006) and fecundity (Shlesinger et al. 2018). In the Au‘au Channel of Hawaiʻi, the growth rate of Porites lobata declines exponentially with depth from 13.5 mm year−1 at 6 m to 3.0 mm year−1 at 60 m (Grigg 2006). Similar declines in growth rates have been reported for other species across analogous depth gradients (e.g., Dustan 1979; Huston 1985; Weinstein et al. 2016). However, there is currently a dearth of data on the growth rates of zooxanthellate corals below normoxic SCUBA diving depths (>60 m). For many years, the lone species measured at greater depth was Leptoseris fragilis, which grows 0.2–0.8 mm year−1 at 90–120 m in the Red Sea (Fricke et al. 1987). This evidence suggested that zooxanthellate coral growth rates are uniformly very slow at lower mesophotic depths. However, an 11 mm year−1 radial growth rate reported for a single colony of Leptoseris hawaiiensis at 90 m in Hawaiʻi demonstrates the potential for moderate growth for an obligate zooxanthellate coral in the lower photic zone (Kahng 2013). In Curacao, recent transplant experiments of Agaricia grahamae confirmed moderate growth rates are possible at mesophotic depths (up to 30.8 mm year−1 at 60 m and up to 4.1 mm year−1 at 80–100 m) (Bongaerts et al. 2015b). At their deepest depths, agariciids (including Leptoseris and Agaricia) form thin horizontal plates that grow radially, and some species do not increase in thickness over time. This efficient growth strategy maximizes surface area while minimizing calcification. While these delicate colonies would be highly susceptible to breakage in shallow water, they are protected from wave stress given their depth distribution (Luck et al. 2013).
In shallow, oligotrophic waters where light energy is plentiful, the primary benefit of heterotrophy for zooxanthellate corals is likely the acquisition of limiting elemental resources (e.g., bioavailable nitrogen and phosphorus). For a few depth-generalist species, growth and reproduction evidence is consistent with the premise that elemental resources are more limiting than energetics in shallow water in contrast to deeper water. For Madracis mirabilis in Jamaica, growth rates are bimodal with depth, with the fastest growth rates at 10 and 30 m and slower growth at 20 and 45 m (Leichter et al. 2006). In the US Virgin Islands, Orbicella faveolata exhibits higher fecundity at 40 m compared to shallower depths, and Porites astreoides exhibits a constant fecundity with depth (5–37 m) (Holstein et al. 2015, 2016). While these data indicate that light energy may not always be the sole limiting factor for growth and reproduction with depth, the potential causes of faster growth or higher fecundity at depth (i.e., increased availability of inorganic nutrients, increased availability of heterotrophic resources, or reduced exposure to shallow-water stressors) have not been isolated.
Anecdotally, long-term cultures of several deepwater Leptoseris spp. colonies (collected 70–130 m) growing under a simulated deepwater light regime (programmed using an AquaIllumination AI Hydra 52 LED light system) at the Waikiki Aquarium in Honolulu, Hawaiʻi, have shown that zooxanthellate corals can survive and grow for several years without allochthonous POM (S. Kahng, unpubl. data). The aquarium facility is fed with filtered groundwater (from a 14 m well) which has very low levels of dissolved organic matter, but high concentrations of inorganic nutrients (Atkinson et al. 1995). Therefore, corals growing in such cultures essentially have no heterotrophic resources to supplement energetics.
In theory, when dissolved inorganic nutrients are readily available, zooxanthellate corals should be able to acquire all necessary elemental resources via their algal symbionts, which can readily assimilate them from seawater. Below the surface mixed layer, the increased availability of inorganic nutrients below may reduce or even obviate the need for heterotrophy as a source for elemental resources. The extent to which energetic subsidies from heterotrophy are required by zooxanthellate corals in the lower photic zone to compensate for low levels of light is likely species specific and remains largely unresolved.
6 Evidence for Increased Heterotrophy
Given their mixotrophic capabilities, zooxanthellate corals are thought to increase their metabolic reliance on heterotrophy in low-light habitats to compensate for reduced rates of photosynthesis (reviewed in Houlbrèque and Ferrier-Pagès 2009). Early investigations from in situ respirometry (Fricke et al. 1987), carbon budgets and models (Falkowski et al. 1984; Muscatine et al. 1984; Dubinsky and Jokiel 1994), and bulk stable isotopes (Muscatine et al. 1989) have provided consistent support for an increased reliance on heterotrophy under low-light conditions at mesophotic depths. Heterotrophic carbon is estimated to supply up to 15–35% of daily metabolic demand in healthy corals and up to 100% in some species of bleached corals (reviewed in Houlbrèque and Ferrier-Pagès 2009). Heterotrophic plasticity is thought to be species specific with differential feeding capabilities recorded among zooxanthellate corals. Where measured, feeding rates are not related to surface area-to-volume ratio or polyp size (reviewed in Houlbrèque and Ferrier-Pagès 2009). While zooxanthellate corals clearly benefit from heterotrophy in many ways, direct evidence for the relative importance of heterotrophy for depth-specialist zooxanthellate corals in the lower photic zone remains somewhat sparse (but see Crandall et al. 2016).
Recent investigations regarding the role of heterotrophy at mesophotic depths have relied heavily on the interpretation of stable isotopic values and have generated a collection of conflicting and potentially confusing conclusions. Therefore, a comprehensive review of the available evidence to date is warranted. Both bulk and compound-specific carbon stable isotopes for coral host tissue (δ13Ch), zooxanthellae (δ13Cz), and coral skeleton (δ13Cs) have been used as proxies for measuring relative rates of photosynthesis and relative contributions of autotrophy versus heterotrophy. The use of δ15N in zooxanthellate corals has also been used to identify nitrogen sources and trophic status. However, interpretation of bulk isotopic values can be problematic due to heterogeneous distribution of isotopic values within total organic matter (Benner et al. 1987).
Applications of dietary biomarkers such as lipids have also been used in marine trophic studies to determine cycling and transfer of material (Parrish 2013). Because lipid composition can be taxa specific, lipid classes (e.g., wax esters, triglycerides, phosolipids, and sterols) and their constituents (various types of fatty acids and alcohols) can be used as dietary tracers for heterotrophy (Houlbrèque and Ferrier-Pagès 2009; Tolosa et al. 2011; reviewed in Parrish 2013). Their different functional roles (as energy reserves or membrane structural components) enable feeding studies to trace the fate and determine the effects of heterotrophic input (Treignier et al. 2008). Integrating compound-specific stable isotopic analysis with dietary biomarkers can eliminate much of the inherent uncertainty associated with bulk stable isotopic values and can clarify nutrient acquisition strategies for corals (Parrish 2013; Crandall et al. 2016). For example, Treignier et al. (2009) demonstrated effects of light and food source on the δ13C signature of various lipid components providing insight in the relative importance of photosynthesis, direct absorption from diet, and de novo synthesis for each.
6.1 Host and Symbiont Carbon Stable Isotopes
The bulk tissue δ13C values for consumers are similar to those of its food source (i.e., endmembers) (reviewed in Peterson and Fry 1987; Peterson 1999). For corals relying primarily on (photolitho-)autotrophy for their supply of organic carbon, the bulk carbon stable isotopic composition of their tissue (δ13Ch) should be similar to that of their zooxanthellae (δ13Cz). Conversely, for corals relying heavily on (chemoorgano-)heterotrophy for their supply of organic carbon, their δ13Ch values should be similar to that of the organic matter being consumed (e.g., plankton), which has more negative δ13C values.
For eight coral species over a depth range of 1–50 m, Muscatine et al. (1989) applied the diffusion depletion hypothesis (D’elia et al. 1983), to provide modest support for an increase in heterotrophy with depth. Due to translocation of photosynthate, the coral δ13Ch should be similar to their zooxanthellae δ13Cz in shallow water, but decrease with depth as corals rely more on consuming particulate organic matter (i.e., heterotrophy). Muscatine et al. (1989) reported an increasing difference between bulk δ13Ch and δ13Cz with depth indicating a greater reliance on heterotrophy.
Subsequent investigations of bulk δ13Ch and δ13Cz have reported conflicting trends (Alamaru et al. 2009; Maier et al. 2010; Crandall et al. 2016) with increasing depth and identified the potential for additional δ13C tissue dynamics including light-dependent rates of fractionation during photosynthesis (Swart et al. 2005), recycling of carbon between coral host and symbionts (Alamaru et al. 2009; Einbinder et al. 2009), and host carbon retention (Tremblay et al. 2014). Increased nitrate and phosphate availability which can differ with depth may also affect δ13Ch and δ13Cz across time, especially if the N:P ratios are not balanced (Tanaka et al. 2017). These factors confound use of bulk δ13Ch and δ13Cz values to directly interpret heterotrophy with depth (e.g., as in Lesser et al. 2010). Irradiance conditions alone do not strongly influence the percentage of photosynthate translocation as complex interactions between autotrophy and heterotrophy alter how nutrients are acquired and shared (Tremblay et al. 2014).
Instead of increasing with depth, heterotrophic feeding may simply be a function of species-specific capability and food availability (Maier et al. 2010; Seemann 2013). Using δ13C compound-specific stable isotopic analysis of sterols, Crandall et al. (2016) demonstrated that while Montastraea cavernosa does not increase heterotrophic feeding with depth (to 60 m), Agaricia spp. appears to rely on heterotrophy at both shallow and mesophotic depths. Heterotrophic capabilities and plasticity under low light appear to be species specific (reviewed in Houlbrèque and Ferrier-Pagès 2009; Seemann 2013). While reduced light may increase rates of heterotrophic feeding in some species (Muscatine et al. 1984; Palardy et al. 2005), light intensity does not influence feeding rates of other species (Langlois and Hoogenboom 2014).
6.2 Skeleton Carbon Stable Isotopes
While the origins of δ13Cs variation in zooxanthellate scleractinian corals remain a matter of considerable debate (McConnaughey 2003; Sun et al. 2008), there is general agreement that increasing solar insolation results in more positive δ13Cs due to fractionation during photosynthesis (Swart et al. 2005). Normalizing δ13Cs to δ18Os to correct for kinetic effects (Heikoop et al. 2000), Lesser et al. (2010) used the transformed δ13Cs values to calculate theoretical photosynthesis-to-respiration (P:R) ratios (sensu McConnaughey et al. 1997) for Montastraea cavernosa in the Bahamas and reported a declining trend with depth. Although photosynthesis often affects δ13Cs more than respiration (McConnaughey 2003), no correlation between δ13Cs and the P:R ratio has been found to date in experiments that have directly measured P:R (Swart et al. 1996, 2005). Therefore, the relationship between δ13Cs and P:R ratio remains hypothetical.
6.3 Tissue Nitrogen Stable Isotopes
The use of bulk δ15N in zooxanthellate corals to determine trophic status is more complex than in pure animal systems (i.e., non-plant/algae). For strict heterotrophs, δ15N is affected by food source (equilibrium effect) and trophic enrichment where δ15N values become more positive with trophic level (Peterson and Fry 1987). In zooxanthellate corals, light levels affect rates of fractionation with greater fractionation in low-light levels (Heikoop et al. 1998). Numerous factors can influence bulk δ15N including variability in nitrogen sources (both organic and inorganic) with depth, eutrophication (Risk et al. 2009), zooxanthellae density (Heikoop et al. 2000), coral bleaching (Rodrigues and Grottoli 2006), and endosymbiotic nitrogen-fixing cyanobacteria (Lema et al. 2012; Benavides et al. 2016). Within a single colony, holobiont δ15N values can also vary by location due to uneven heterotrophic feeding (e.g., greater feeding on the tips of coral branches) (Maier et al. 2010).
Another factor influencing δ15N in zooxanthellate corals is phosphate availability which influences the amount of inorganic nitrogen assimilated from seawater (Tanaka et al. 2015). Phosphate availability also influences the assimilation of diazotroph-derived bioavailable nitrogen (δ15N = 0‰) (Bednarz et al. 2017). Since phosphate concentrations generally increase with depth below the surface mix layer (e.g., Letelier et al. 2004), these nitrogen source dynamics differentially affect corals at mesophotic depths and can complicate the analysis and interpretation of depth trends in δ15N that span multiple nutrient regimes.
For some corals, there is a general trend of holobiont δ15N values decreasing with increasing depth, which has been interpreted as the autotrophic signature overwhelming any heterotrophic signature (Muscatine and Kaplan 1994; Heikoop et al. 1998; Maier et al. 2010; Baker et al. 2011). Theoretically, at extreme depths with very low-light levels, the heterotrophic signature should eventually prevail and reflect more positive holobiont δ15N (Heikoop et al. 1998). For the largest depth gradients studied, the corals showed no consistent depth trend in δ15Nh or δ15Nz indicating additional δ15N dynamics must be considered (Alamaru et al. 2009; Lesser et al. 2010). In the only controlled experiment to date, Reynaud et al. (2009) also reported no trend in δ15Nh or δ15Nz across a range of low to moderate light. Unexpectedly, fed coral colonies actually exhibited more negative δ15Nh and δ15Nz compared to starved colonies indicating that nitrogen recycling between host and symbiont can overwhelm any heterotrophic signature (Reynaud et al. 2009). This level of nitrogen recycling varies by coral species (Tanaka et al. 2015).
Given the multiple of factors influencing bulk carbon and nitrogen stable isotopic values for zooxanthellate corals and the multiple trends of these factors with depth, in situ studies attempting to isolate heterotrophic signatures with depth are fraught with many potentially confounding factors. Such studies must be interpreted in concert with all relevant studies including the most recent and ongoing advancements in the field of stable isotopic analyses.
6.4 Comparative Morphology and Depth Distributions
Facultatively zooxanthellate corals, obligate heterotrophic (azooxanthellate) corals, and other anthozoans, which rely on currents for passive suspension feeding, generally share common morphological features including functional tentacles for capturing particulate organic matter and tall and/or ramose morphologies designed to penetrate the benthic boundary layer (Etnoyer and Morgan 2005; Lumsden et al. 2007). Zooxanthellate corals abundant in the lower photic zone tend to exhibit contrasting features, which appear inconsistent with optimal passive suspension feeding, including horizontal plate-like morphologies, low or decreasing polyp density with depth, and polyps which lack tentacles (Dinesen 1980; Fricke and Schuhmacher 1983; Fricke et al. 1987; Goldberg 2002; Einbinder et al. 2009; Holstein et al. 2015). These features characterize many of the species from the family Agariciidae, which dominates the zooxanthellate coral community found in the lower photic zone throughout the Indo-Pacific Ocean and in parts of the Caribbean (reviewed in Kahng et al. 2010, 2014; Englebert et al. 2017). For example, polyp densities for several deepwater (>70 m) Leptoseris spp. have been commonly measured at 0.1–2.2 polyps per cm2 (S. Kahng, unpubl. data) (Fig. 42.12).
If heterotrophic capabilities play a significant role in extending the depth distribution of zooxanthellate corals, species with morphologies facilitating passive suspension feeding could be expected to thrive deeper (in less light) than other species. Similar to antipatharians which are strictly heterotrophic, gorgonians commonly have tall and ramose morphologies and high polyp densities which can facilitate passive suspension feeding (Fabricius and Alderslade 2001; Won et al. 2001; Brugler et al. 2013). However, zooxanthellate gorgonians have much shallower depth distributions than zooxanthellate scleractinians with flattened morphologies (reviewed in Kahng et al. 2010), and the gorgonians common to the lower photic zone (>60–70 m) are exclusively azooxanthellate (e.g., Bridge et al. 2012). While facultative zooxanthellate scleractinians (e.g., Oculina varicosa, Astrangia danae, and Madracis pharensis) also have ramose colony morphologies and can inhabit depths below the photic zone, the zooxanthellate morphs of these species exhibit much shallower lower depth distributions than their obligate zooxanthellate counterparts (Kahng et al. 2010).
7 Role of Light and Temperature in the Lower Depth Limits of MCEs
The depths at which benthic photosynthetic taxa thrive are deepest in oligotrophic tropical and subtropical waters due to their superior optical water clarity (Kirk 2011). Obligate photosynthetic taxa have been observed living in situ at extreme depths, for example, 165 m for Leptoseris spp. at Johnston Atoll (Maragos and Jokiel 1986) and 312 m for crustose coralline algae at Pitcairn Island (Friedlander et al. 2014). While the decrease in light intensity with increasing depth is very rapid in shallow waters, it is very gradual in the lower photic zone. Zooxanthellate corals at their lower depth limit at Johnston Atoll, Gulf of Eilat/Aqaba, and Hawaiʻi Island are very small in size and found on barren substrate (reviewed in Kahng et al. 2010). A gradual decrease in conspecific colony size with increasing depth and the presence of only small colonies at their lower depth limit would be consistent with slow/stunted growth and light limitation for an obligate photosynthetic organism. In other locations (i.e., Enewetak, Palau, and the Au‘au Channel), zooxanthellate corals at their lower depth limit can be relatively large (Colin et al. 1986; Wolanski et al. 2004; Kahng et al. 2012b). These observations are not consistent with a gradual decrease in light eventually limiting the lower depth distribution of a photosynthetic organism, but instead suggest a sharp threshold-related, depth limit.
In some subtropical locations with clear, oligotrophic waters, low temperatures can limit the depths to which zooxanthellate corals and other warm-water, sessile benthic fauna extend (reviewed in Kahng et al. 2010, 2012a, 2014). For example, in Hawaiʻi, the community structure of sessile benthic organisms exhibits a vertical zonation with an abrupt cessation of warm-water fauna at a location-specific depth (Kahng and Kelley 2007). In the Au‘au Channel, both dominant zooxanthellate and azooxanthellate mesophotic corals (e.g., Antipathes griggi, A. grandis, and Carijoa riisei) abruptly cease at ~115 m (Kahng and Kelley 2007). The shared lower depth limit across several taxa suggests a common mechanism. The replacement of warm-water azooxanthellate corals by closely related taxa of similar morphology and trophic ecology at deeper depths (e.g., Acanthopathes undulata, Myriopathes spp., Stichopathes spp., and Corallium spp.) suggests that neither resource limitation, competition, top-down control, nor mechanical disturbance are determining factors.
For ectothermic tropical organisms, exposure to low temperatures has been shown to limit their geographic distribution at high latitudes (Pörtner 2002, 2010). An analogous decrease in temperature with depth eventually limits the distribution of tropical organisms in deeper waters. All organisms live within a limited range of body temperatures due to optimized structural and kinetic coordination of molecular, cellular, and systemic processes with functional constraints at temperature extremes (Pörtner and Farrell 2008). The physiological basis by which temperature limits the geographic and depth distribution of sessile, ectothermic fauna is aerobic capacity, which decreases and eventually goes to zero outside an optimal temperature range (Kassahn et al. 2009; Pörtner 2010). While environmental controls on oxygen- and capacity-limited thermal tolerance (i.e., OCLTT) apply to all living organisms (Bozinovic and Pörtner 2015), these constraints can have a pronounced effect on the depth limits of sessile, ectothermic tropical fauna, which cannot migrate to warmer waters during low-temperature events.
While distribution limits for zooxanthellate corals and their thermal regime have been relatively well studied at high latitude (reviewed in Kleypas et al. 1999; Kahng et al. 2010), there is considerably less data on the thermal regime at their lower depth distribution. Cold tolerance is both species-specific (e.g., Higuchi et al. 2015; Kemp et al. 2016) and population-specific within a species indicating the importance of adaption to the local environment (Howells et al. 2013). From both in situ observations and laboratory experiments, a wide range of low-temperature tolerances has been reported with high-latitude species exhibiting the most resilience to cold temperature exposure (Davis 1982; Porter et al. 1982; Roberts et al. 1982; Coles and Fadlallah 1991; Muscatine et al. 1991; Harriott and Banks 2002; Macintyre 2003; Hoegh-Guldberg et al. 2005; Chen et al. 2009; Thomson and Frisch 2010; Kemp et al. 2011). For example, the coral reef community at Iki Island, Japan (33.5°N), regularly experiences low temperatures of 13 °C every winter (Yamano et al. 2001). In contrast, prolonged exposure to temperatures of only 18 °C can cause mortality in cold-sensitive species at lower latitudes (e.g., Jokiel and Coles 1977; Laboy-Nieves et al. 2001). Where measured, the annual thermal regime at the lower depth limit of zooxanthellate corals remains considerably warmer (Kahng et al. 2012a; Bongaerts et al. 2015b) than previously reported lethal exposures to cold temperatures. This disconnect indicates that lethal, cold-water temperatures do not always limit the depth distribution of warm-water fauna (but see Wolanski et al. 2004).
Within an optimal temperature range, growth and development scale approximately linearly with increasing temperature (Jarošík et al. 2004; Neuheimer and Taggart 2007; Neuheimer et al. 2011). A narrower thermal tolerance window is often associated with reproduction (van der Have 2002; Pörtner and Farrell 2008; Dixon et al. 2009) and decouples the lower survival limit from the lower limit of reproduction and development and therefore natural distribution. Such restrictive thermal thresholds limiting reproduction, but not adult survival, are widely reported for other ectothermic organisms. For viable development, the temperature ranges are much narrower than the ranges for the performance of adult physiological traits (van der Have 2002; Dixon et al. 2009). Low temperature-induced, reversible inactivation of regulatory proteins involved in first-order reactions (e.g., cell cycle or DNA replication) could be responsible for threshold character of thermal limits on reproduction and development (van der Have 2002). Adult physiological traits depend broadly on metabolic efficiency and metabolic pathways, which are not directly linked to cell cycle or DNA replication. Therefore, the narrow thermal niche for reproduction makes thermal habitat (and depth) selection critical and high selectivity by larvae probable (van der Have 2002). Genotypes associated with larval behavior settling below depths of viable reproduction would quickly exit the gene pool. Thus, the lower depth limits of warm water sessile invertebrates, including zooxanthellate corals, may be heavily influenced by larval settlement behavior.
8 Summary
Zooxanthellate corals exhibit a variety of strategies for growing in deeper water where the light available to drive photosynthesis decreases to a very small fraction of surface irradiance. For shallow-water species, which also inhabit intermediate depths, shade adaptation (e.g., increase in pigment concentrations) appears to be a common strategy for increasing utilization of ambient light (energy input) at the expense of photosynthetic efficiency. This strategy suggests that the coral growth may be light limited, but the holobiont is not necessarily efficiency limited. For many depth-specialist species, efficient use of energy and elemental resources appears to drive adaptation strategies in the lower photic zone. Minimal investments in tissue biomass, pigments, and calcification may optimize return on investment (i.e., increased fitness). Superior efficiency allows them to grow faster and compete more effectively in very low-light habitats as obligate photosynthetic organisms and inhabit depths where shallow-water counterparts may have difficulties reproducing or surviving. The collective evidence indicates that different species can exhibit distinct and sometimes opposing photophysiological adaptations with increasing depth. Zooxanthellate corals do not conform to a uniform set of adaptations or consistent trophic or metabolic strategy at depth. The gradient of light and other environmental factors with increasing depth create heterogeneity in habitat niches and opportunity for adaptations and specialization. As more mesophotic habitats are studied and molecular genetic tools clarify identification of species, the recognized biodiversity on coral reefs is expected to grow and expand our understanding of the ecological secrets allowing obligate zooxanthellate corals to thrive at extreme depths.
References
Acevedo R, Morelock J, Olivieri RA (1989) Modification of coral reef zonation by terrigenous sediment stress. Palaios 4:92–100
Akkaynak D, Treibitz T, Shlesinger T, Tamir R, Loya Y, Iluz D (2017) What is the space of attenuation coefficients in underwater computer vision? In: Proceedings of the IEEE Computer Vision and Pattern Recognition Conference (CVPR), Honolulu, pp 568–577
Alamaru A, Yam R, Shemesh A, Loya Y (2009) Trophic biology of Stylophora pistillata larvae: evidence from stable isotope analysis. Mar Ecol Prog Ser 383:85–94
Allemand D, Tambutté É, Zoccola D, Tambutté S (2011) Coral calcification, cells to reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 119–150
Anthony KRN, Hoegh-Guldberg O (2003) Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys. Funct Ecol 17:246–259
Anthony KRN, Hoogenboom MO, Connolly SR (2005) Adaptive variation in coral geometry and the optimization of internal colony light climates. Funct Ecol 19:17–26
Aponte NE, Ballantine DL (2001) Depth distribution of algal species on the deep insular fore reef at Lee Stocking Island, Bahamas. Deep-Sea Res I Oceanogr Res Pap 48:2185–2194
Atkinson MJ (2011) Biogeochemistry of nutrients. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 199–206
Atkinson MJ, Falter JL (2003) Coral reefs. In: Black K, Shimmield G (eds) Biogeochemistry of marine systems. CRC Press, Boca Raton, pp 40–64
Atkinson MJ, Carlson B, Crow GL (1995) Coral growth in high-nutrient, low-pH seawater: a case study of corals cultured at the Waikiki aquarium, Honolulu, Hawaii. Coral Reefs 14:215–223
Baird AH, Babcock RC, Mundy CP (2003) Habitat selection by larvae influences the depth distribution of six common coral species. Mar Ecol Prog Ser 252:289–293
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689
Baker DM, Kim K, Andras JP, Sparks JP (2011) Light-mediated 15N fractionation in Caribbean gorgonian octocorals: implications for pollution monitoring. Coral Reefs 30:709–717
Barnes DJ, Chalker BE (1990) Calcification and photosynthesis in reef-building corals and algae. In: Dubinsky Z (ed) Ecosystems of the world: coral reefs. Elsevier, Amsterdam, pp 109–131
Bednarz VN, Grover R, Maguer J-F, Fine M, Ferrier-Pagès C (2017) The assimilation of diazotroph-derived nitrogen by scleractinian corals depends on their metabolic status. mBio 8:e02058–e02016
Beltrán-Torres AU, Carricart-Ganivet JP (1993) Skeletal morphologic variation in Montastraea cavernosa (Cnidaria: Scleractinia) at Isla Verde coral reef, Veracruz, Mexico. Rev Biol Trop 41:559–562
Benavides M, Houlbrèque F, Camps M, Lorrain A, Grosso O, Bonnet S (2016) Diazotrophs: a non-negligible source of nitrogen for the tropical coral Stylophora pistillata. J Exp Biol 219:2608–2612
Benner R, Fogel ML, Sprague EK, Hodson RE (1987) Depletion of 13C in lignin and its implications for stable carbon isotope studies. Nature 329:708–710
Bissett WP, Patch JS, Carder KL, Lee ZP (1997) Pigment packaging and Chl a-specific absorption in high-light oceanic waters. Limnol Oceanogr 42:961–968
Biton E, Gildor H (2011) The general circulation of the Gulf of Aqaba (Gulf of Eilat) revisited: the interplay between the exchange flow through the straits of Tiran and surface fluxes. J Geophys Res 116:C08020
Bollati E, Plimmer D, D’Angelo C, Wiedenmann J (2017) FRET-mediated long-range wavelength transformation by photoconvertible fluorescent proteins as an efficient mechanism to generate orange-red light in symbiotic deep water corals. IJMS 18:1174
Bongaerts P, Riginos C, Hay KB, van Oppen MJH, Hoegh-Guldberg O, Dove SG (2011a) Adaptive divergence in a scleractinian coral: physiological adaptation of Seriatopora hystrix to shallow and deep reef habitats. BMC Evol Biol 11:303
Bongaerts P, Sampayo EM, Bridge TCL, Ridgway T, Vermeulen F, Englebert N, Webster JM, Hoegh-Guldberg O (2011b) Symbiodinium diversity in mesophotic coral communities on the Great Barrier Reef: a first assessment. Mar Ecol Prog Ser 439:117–126
Bongaerts P, Carmichael M, Hay KB, Tonk L, Frade PR, Hoegh-Guldberg O (2015a) Prevalent endosymbiont zonation shapes the depth distributions of scleractinian coral species. R Soc Open Sci 2:140297
Bongaerts P, Frade PR, Hay KB, Englebert N, Latijnhouwers KR, Bak RP, Vermeij MJ, Hoegh-Guldberg O (2015b) Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci Rep 5:7652
Booth CR, Morrow JH (1997) The penetration of UV into natural waters. Photochem Photobiol 65:254–257
Bozinovic F, Pörtner HO (2015) Physiological ecology meets climate change. Ecol Evol 5:1025–1030
Brakel WH (1979) Small-scale spatial variation in light available to coral reef benthos: quantum irradiance measurements from a Jamaican reef. Bull Mar Sci 29:406–413
Bricaud A, Morel A, Prieur L (1981) Absorption by dissolved organic matter of the sea (yellow substance) in the UV and visible domains. Limnol Oceanogr 26:43–53
Bridge TCL, Fabricius KE, Bongaerts P, Wallace CC, Muir PR, Done TJ, Webster JM (2012) Diversity of Scleractinia and Octocorallia in the mesophotic zone of the Great Barrier Reef, Australia. Coral Reefs 31:179–189
Brodersen KE, Lichtenberg M, Ralph PJ, Kühl M, Wangpraseurt D (2014) Radiative energy budget reveals high photosynthetic efficiency in symbiont-bearing corals. J R Soc Interface 11:20130997
Brown BE, Downs CA, Dunne RP, Gibb SW (2002) Preliminary evidence for tissue retraction as a factor in photoprotection of corals incapable of xanthophyll cycling. J Exp Mar Biol Ecol 277:129–144
Brugler MR, Opresko DM, France SC (2013) The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zool J Linnean Soc 169:312–361
Cai L, Zhou G, Tian R-M, Tong H, Zhang W, Sun J, Ding W, Wong YH, Xie JY, Qiu J-W, Sheng L, Huang H, Qian P-Y (2017) Metagenomic analysis reveals a green sulfur bacterium as a potential coral symbiont. Sci Rep 7:9320
Carder KL, Steward RG, Harvey GR, Ortner PB (1989) Marine humic and fulvic acids: their effects on remote sensing of ocean chlorophyll. Limnol Oceanogr 34:68–81
Chang SS, Prézelin BB, Trench RK (1983) Mechanisms of photoadaptation in three strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar Biol 76:219–229
Chelton DB, Schlax MG, Samelson RM (2011) Global observations of nonlinear mesoscale eddies. Prog Oceanogr 91:167–216
Chen TR, Yu KF, Shi Q, Li S, Price GJ, Wang R, Zhao MX, Chen TG, Zhao JX (2009) Twenty-five years of change in scleractinian coral communities of Daya Bay (northern South China Sea) and its response to the 2008 AD extreme cold climate event. Chin Sci Bull 54:2107–2117
Coles SL, Fadlallah YH (1991) Reef coral survival and mortality at low temperatures in the Arabian Gulf: new species-specific lower temperature limits. Coral Reefs 9:231–237
Colin PL, Devaney DM, Hillis-Colinvaux L, Suchanek TH, Harrison JT (1986) Geology and biological zonation of the reef slope, 50–360 m depth at Enewetak Atoll, Marshall Islands. Bull Mar Sci 38:111–128
Cooper TF, Ulstrup KE, Dandan SS, Heyward AJ, Kühl M, Muirhead A, O’Leary RA, Ziersen BEF, van Oppen MJH (2011) Niche specialization of reef-building corals in the mesophotic zone: metabolic trade-offs between divergent Symbiodinium types. Proc R Soc B 278:1840–1850
Cox N, Pantazis DA, Neese F, Lubitz W (2013) Biological water oxidation. Acc Chem Res 46:1588–1596
Crandall JB, Teece MA, Estes BA, Manfrino C, Ciesla JH (2016) Nutrient acquisition strategies in mesophotic hard corals using compound specific stable isotope analysis of sterols. J Exp Mar Biol Ecol 474:133–141
Croce R, van Amerongen H (2014) Natural strategies for photosynthetic light harvesting. Nat Chem Biol 10:492–501
D’Angelo C, Denzel A, Vogt A, Matz MV, Oswald F, Salih A, Nienhaus GU, Wiedenmann J (2008) Blue light regulation of host pigment in reef-building corals. Mar Ecol Prog Ser 364:97–106
D’Angelo C, Smith EG, Oswald F, Burt J, Tchernov D, Wiedenmann J (2012) Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 31:1045–1056
D’elia CF, Domotor SL, Webb KL (1983) Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar Biol 75:157–167
Davis GE (1982) A century of natural change in coral distribution at the Dry Tortugas: a comparison of reef maps from 1881 and 1976. Bull Mar Sci 32:608–623
de Beer D, Kühl M, Stambler N, Vaki L (2000) A microsensor study of light enhanced Ca2+ uptake and photosynthesis in the reef-building hermatypic coral Favia sp. Mar Ecol Prog Ser 194:75–85
De Tommasi E (2016) Light manipulation by single cells: the case of diatoms. J Spectrosc 2016:2490128
Devred E, Turpie KR, Moses W, Klemas VV, Moisan T, Babin M, Toro-Farmer G, Forget M-H, Jo Y-H (2013) Future retrievals of water column bio-optical properties using the Hyperspectral Infrared Imager (HyspIRI). Remote Sens 5:6812–6837
Dinesen ZD (1980) A revision of the coral genus Leptoseris (Scleractinia: Fungiina: Agariciidae). Mem Queensland Mus 20:181–235
Dinesen ZD (1983) Shade-dwelling corals of the Great Barrier Reef. Mar Ecol Prog Ser 10:173–185
Dixon AFG, Hon KA, Keil P, Kotela MAA, Šizling AL, Jarošík V (2009) Relationship between the minimum and maximum temperature thresholds for development in insects. Funct Ecol 23:257–264
Dove SG, Hoegh-Guldberg O, Ranganathan S (2001) Major colour patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 19:197–204
Dring MJ (1981) Chromatic adaption of photosynthesis in benthic marine algae: an examination of its ecological significance using a theoretical model. Limnol Oceanogr 26:271–284
Dubinsky Z, Falkowski PG (2011) Light as a source of information and energy in zooxanthellate corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 107–118
Dubinsky Z, Jokiel PL (1994) Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac Sci 48:313–324
Dubinsky Z, Stambler N (2009) Photoacclimation processes in phytoplankton: mechanisms, consequences, and applications. Aquat Microb Ecol 56:163–176
Dustan P (1979) Distribution of zooxanthellae and photosynthetic chloroplast pigments of the reef-building coral Montastraea annularis Ellis and Solander in relation to depth on a West Indian coral reef. Bull Mar Sci 29:79–95
Einbinder S, Mass T, Brokovich E, Dubinsky Z, Erez J, Tchernov D (2009) Changes in morphology and diet of the coral Stylophora pistillata along a depth gradient. Mar Ecol Prog Ser 381:167–174
Einbinder S, Gruber DF, Salomon E, Liran O, Keren N, Tchernov D (2016) Novel adaptive photosynthetic characteristics of mesophotic symbiotic microalgae within the reef-building coral, Stylophora pistillata. Front Mar Sci 3:195
Emery WJ, Dewar JS (1982) Mean temperature-salinity, salinity-depth and temperature-depth curves for the North Atlantic and the North Pacific. Prog Oceanogr 11:219–305
Englebert N, Bongaerts P, Muir PR, Hay KB, Hoegh-Guldberg O (2014) Deepest zooxanthellate corals of the Great Barrier Reef and Coral Sea. Mar Biodivers 45:1–2
Englebert N, Bongaerts P, Muir PR, Hay KB, Pichon M, Hoegh-Guldberg O (2017) Lower mesophotic coral communities (60–125 m depth) of the Northern Great Barrier Reef and Coral Sea. PLoS ONE 12:e0170336
Enríquez S, Méndez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol Oceanogr 50:1025–1032
Enríquez S, Méndez ER, Hoegh-Guldberg O, Iglesias-Prieto R (2017) Key functional role of the optical properties of coral skeletons in coral ecology and evolution. Proc R Soc B 284:20161667
Etnoyer P, Morgan LE (2005) Habitat-forming deep-sea corals in the Northeast Pacific Ocean. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 331–343
Fabricius KK, Alderslade PP (2001) Soft corals and sea fans: a comprehensive guide to the tropical shallow water genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science (AIMS), Townsville
Falkowski PG, Dubinsky Z (1981) Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature 289:172–174
Falkowski PG, Laroche J (1991) Acclimation to spectral irradiance in algae. J Phycol 27:8–14
Falkowski PG, Raven JA (2007) Aquatic photosynthesis. Princeton University Press, Princeton
Falkowski PG, Dubinsky Z, Muscatine L, Porter JW (1984) Light and the bioenergetics of a symbiotic coral. Bioscience 34:705–709
Falkowski PG, Jokiel PL, Kinsey RR (1990) Irradiance and corals. In: Dubinsky Z (ed) Ecosystems of the world: coral reefs. Elsevier, Amsterdam, pp 89–107
Fine M, Loya Y (2002) Endolithic algae: an alternative source of photoassimilates during coral bleaching. Proc R Soc B 269:1205–1210
Firing YL, Merrifield MA (2004) Extreme sea level events at Hawaii: influence of mesoscale eddies. Geophys Res Lett 31:L24306
Förster TH (1960) Transfer mechanisms of electronic excitation energy. Radiat Res Suppl:326–339
Frade PR, Bongaerts P, Winkelhagen AJS, Tonk L, Bak RPM (2008) In situ photobiology of corals over large depth ranges: a multivariate analysis on the roles of environment, host, and algal symbiont. Limnol Oceanogr 53:2711–2723
Fricke HW, Schuhmacher H (1983) The depth limits of Red Sea stony corals: an ecophysiological problem (a deep diving survey by submersible). Mar Ecol 4:163–194
Fricke HW, Vareschi E, Schlichter D (1987) Photoecology of the coral Leptoseris fragilis in the Red Sea twilight zone (an experimental study by submersible). Oecologia 73:371–381
Friedlander AM, Caselle JE, Ballesteros E, Brown EK, Turchik A, Sala E (2014) The real bounty: marine biodiversity in the Pitcairn Islands. PLoS ONE 9:e100142
Gaffey SJ (1986) Spectral reflectance of carbonate minerals in the visible and near-infrared (0.35–2.55 microns): calcite, aragonite, and dolomite. Am Miner 71:151–162
Ghoshal A, Eck E, Gordon M, Morse DE (2016) Wavelength-specific forward scattering of light by Bragg-reflective iridocytes in giant clams. J R Soc Interface 13:20160285
Gittins JR, D’Angelo C, Oswald F, Edwards RJ, Wiedenmann J (2015) Fluorescent protein-mediated colour polymorphism in reef corals: multicopy genes extend the adaptation/acclimatization potential to variable light environments. Mol Ecol 24:453–465
Goldberg WM (2002) Feeding behavior, epidermal structure and mucus cytochemistry of the scleractinian Mycetophyllia reesi, a coral without tentacles. Tissue Cell 34:232–245
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Goss R, Pinto EA, Wilhelm C, Richter M (2006) The importance of a highly active and ΔpH-regulated diatoxanthin epoxidase for the regulation of the PS II antenna function in diadinoxanthin cycle containing algae. J Plant Physiol 163:1008–1021
Goulet TL (2006) Most corals may not change their symbionts. Mar Ecol Prog Ser 321:1–7
Goulet TL (2007) Most scleractinian corals and octocorals host a single symbiotic zooxanthella clade. Mar Ecol Prog Ser 335:243–248
Goulet TL, Lucas MQ, Schizas NV (2019) Symbiodiniaceae genetic diversity and symbioses with hosts from shallow to mesophotic coral ecosystems. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic coral ecosystems. Springer, New York, pp 537–551
Grigg RW (1965) Ecological studies of black coral in Hawaii. Pac Sci 19:244–260
Grigg RW (2006) Depth limit for reef building corals in the Auʻau channel, S.E. Hawaii. Coral Reefs 25:77–84
Grzymski J, Schofield OM, Falkowski PG, Bernhard JM (2002) The function of plastids in the deep-sea benthic foraminifer, Nonionella stella. Limnol Oceanogr 47:1569–1580
Harriott VJ (1999) Coral growth in subtropical eastern Australia. Coral Reefs 18:281–291
Harriott VJ, Banks SA (2002) Latitudinal variation in coral communities in eastern Australia: a qualitative biophysical model of factors regulating coral reefs. Coral Reefs 21:83–94
Heikoop JM, Dunn JJ, Risk MJ, Sandeman IM, Schwartz HP, Waltho N (1998) Relationship between light and the δ15N of coral tissue: examples from Jamaica and Zanzibar. Limnol Oceanogr 43:909–920
Heikoop JM, Dunn JJ, Risk MJ, Schwarcz HP, McConnaughey TA, Sandeman IM (2000) Separation of kinetic and metabolic isotope effects in carbon-13 records preserved in reef coral skeletons. Geochim Cosmochim Acta 64:975–987
Higuchi T, Agostini S, Casareto BE, Suzuki Y, Yuyama I (2015) The northern limit of corals of the genus Acropora in temperate zones is determined by their resilience to cold bleaching. Sci Rep 5:18467
Hinderstein L, Marr J, Martinez F, Dowgiallo M, Puglise K, Pyle R, Zawada D, Appeldoorn R (2010) Theme section on “Mesophotic coral ecosystems: characterization, ecology, and management.” Coral Reefs 29:247–251
Hoegh-Guldberg O, Fine M, Skirving W, Johnstone R, Dove SG, Strong A (2005) Coral bleaching following wintry weather. Limnol Oceanogr 50:265–271
Holstein DM, Smith TB, Gyory J, Paris CB (2015) Fertile fathoms: deep reproductive refugia for threatened shallow corals. Sci Rep 5:12407
Holstein DM, Smith TB, Paris CB (2016) Depth-independent reproduction in the reef coral Porites astreoides from shallow to mesophotic zones. PLoS ONE 11:e0146068
Holt AL, Vahidinia S, Gagnon YL, Morse DE, Sweeney AM (2014) Photosymbiotic giant clams are transformers of solar flux. J R Soc Interface 11:1–13
Holte J, Talley LD, Gilson J, Roemmich D (2017) An Argo mixed layer climatology and database. Geophys Res Lett 44:5618–2826
Hoogenboom MO, Connolly SR, Anthony KRN (2008) Interactions between morphological and physiological plasticity optimize energy acquisition in corals. Ecology 89:1144–1154
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17
Howells EJ, Berkelmans R, van Oppen MJH, Willis BL, Bay LK (2013) Historical thermal regimes define limits to coral acclimatization. Ecology 94:1078–1088
Huston M (1985) Variation in coral growth rates with depth at Discovery Bay, Jamaica. Coral Reefs 4:19–25
Hutchings P, Mike K, Hoegh-Guldberg O (2008) The Great Barrier Reef: biology, environment and management. CSIRO Publishing, Springer, Collingwood
Iglesias-Prieto R, Trench RK (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Iglesias-Prieto R, Trench RK (1997) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll–protein complexes to different photon-flux densities. Mar Biol 130:23–33
Imhoff JF (2014) Biology of green sulfur bacteria eLS. In: Encyclopedia of life sciences. Wiley, Chichester
Jacobs M, Lopez-Garcia M, Phrathep O-P, Lawson T, Oulton R, Whitney HM (2016) Photonic multilayer structure of Begonia chloroplasts enhances photosynthetic efficiency. Nat Plants 2:16162
Jantzen C, Schmidt GM, Wild C, Roder C, Khokiattiwong S, Richter C (2013) Benthic reef primary production in response to large amplitude internal waves at the Similan Islands (Andaman Sea, Thailand). PLoS ONE 8:e81834
Jarošík V, Kratochvíl L, Honék A, Dixon AF (2004) A general rule for the dependence of developmental rate on temperature in ectothermic animals. Proc R Soc B 271:219–221
Jaubert J (1977) Light, metabolism and growth forms of the hermatypic scleractinian coral Synaraea convexa Verrill in the lagoon of Moorea (French Polynesia). Proc 3rd Int Coral Reef Symp Miami 1:483–488
Jokiel PL, Coles SL (1977) Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar Biol 43:201–208
Joliot A, Joliot P (1964) Etude cinetique de la reaction photochimique liberant l’oxygene au cours de la photosynthese. C R Acad Sci 258:4622–4625
Joliot P, Joliot A (2003) Excitation transfer between photosynthetic units: the 1964 experiment. Photosynth Res 76:241–245
Joliot P, Barbieri G, Chabaud R (1969) Un nouveau modele des centres photochimiques du systeme II. Photochem Photobiol 10:309–329
Kahng SE (2013) Growth rate for a zooxanthellate coral (Leptoseris hawaiiensis) at 90 m. Galaxea J Coral Reef Stud 15:39–40
Kahng SE, Kelley C (2007) Vertical zonation of habitat forming benthic species on a deep photosynthetic reef (50–140 m) in the Auʻau channel, Hawaii. Coral Reefs 26:679–687
Kahng SE, Maragos JE (2006) The deepest zooxanthellate, scleractinian corals in the world? Coral Reefs 25:254
Kahng SE, García-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275
Kahng SE, Wagner D, Lantz C, Vetter O, Gove J, Merrifield M (2012a) Temperature related depth limits of warm-water corals. In: Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9C
Kahng SE, Hochberg EJ, Apprill A, Wagner D, Luck DG, Perez D, Bidigare RR (2012b) Efficient light harvesting in deep-water zooxanthellate corals. Mar Ecol Prog Ser 455:65–77
Kahng SE, Copus JM, Wagner D (2014) Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr Opin Environ Sustain 7:72–81
Kahng SE, Copus JM, Wagner D (2017) Mesophotic coral ecosystems. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine animal forests: the ecology of benthic biodiversity hotspots. Springer international publishing, Switzerland, pp 1–21
Kaniewska P, Anthony KRN, Hoegh-Guldberg O (2008) Variation in colony geometry modulates internal light levels in branching corals, Acropora humilis and Stylophora pistillata. Mar Biol 155:649–660
Kaniewska P, Magnusson SH, Anthony KRN, Reef R, Kühl M, Hoegh-Guldberg O (2011) Importance of macro- versus microstructure in modulating light levels inside coral colonies. J Phycol 47:846–860
Kaniewska P, Anthony KRN, Sampayo EM, Campbell PR, Hoegh-Guldberg O (2014) Implications of geometric plasticity for maximizing photosynthesis in branching corals. Mar Biol 161:313–328
Karl DM, Church MJ (2017) Ecosystem structure and dynamics in the North Pacific subtropical gyre: new views of an old ocean. Ecosystems 20:433–457
Karl DM, Lukas R (1996) The Hawaii Ocean Time-series (HOT) program: background, rationale and field implementation. Deep-Sea Res 43:129–156
Kassahn KS, Crozier RH, Pörtner HO, Caley MJ (2009) Animal performance and stress: responses and tolerance limits at different levels of biological organisation. Biol Rev 84:277–292
Kemp DW, Oakley CA, Thornhill DJ, Newcomb LA, Schmidt GW, Fitt WK (2011) Catastrophic mortality on inshore coral reefs of the Florida Keys due to severe low-temperature stress. Glob Chang Biol 17:3468–3477
Kemp DW, Colella MA, Bartlett LA, Ruzicka RR, Porter JW, Fitt WK (2016) Life after cold death: reef coral and coral reef responses to the 2010 cold water anomaly in the Florida Keys. Ecosphere 7:e01373
Keshavmurthy S, Yang S-Y, Alamaru A, Chuang Y-Y, Pichon M, Obura D, Fontana S, De Palmas S, Stefani F, Benzoni F, MacDonald A, Noreen AME, Chen C, Wallace CC, Pillay RM, Denis V, Amri AY, Reimer JD, Mezaki T, Sheppard C, Loya Y, Abelson A, Mohammed MS, Baker AC, Mostafavi PG, Suharsono B, Chen CA (2013) DNA barcoding reveals the coral “laboratory-rat,” Stylophora pistillata encompasses multiple identities. Sci Rep 3:1520
Kinzie RA, Jokiel PL, York R (1984) Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar Biol 78:239–248
Kirk JTO (1975) A theoretical analysis of the contribution of algal cells to the attenuation of light within natural waters I. General treatment of suspensions of pigmented cells. New Phytol 75:11–20
Kirk JTO (2011) Light and photosynthesis in aquatic ecosystems. Cambridge University Press, New York
Klaus JS, Budd AF, Heikoop JM, Fouke BW (2007) Environmental controls on corallite morphology in the reef coral Montastraea annularis. Bull Mar Sci 80:233–260
Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution–I. A linear four step mechanism. Photochem Photobiol 11:457–475
Kruszyński KJ, Kaandorp JA, van Liere R (2007) A computational method for quantifying morphological variation in scleractinian corals. Coral Reefs 26:831–840
Kühl M, Cohen Y, Dalsgaard T, Jorgensen BB, Revsbech NP (1995) Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar Ecol Prog Ser 117:159–172
Kühlmann DHH (1983) Composition and ecology of deep-water coral associations. Helgol Mar Res 36:183–204
Laboy-Nieves EN, Klein E, Conde JE, Losada F, Cruz JJ, Bone D (2001) Mass mortality of tropical marine communities in Morrocoy, Venezuela. Bull Mar Sci 68:163–179
LaJuenesse TC, Lee S, Bush SL, Bruno JF (2005) Persistence of non-Caribbean algal symbionts in Indo-Pacific mushroom corals released to Jamaica 35 years ago. Coral Reefs 24:157–159
Langlois LA, Hoogenboom MO (2014) Capacity for short-term physiological acclimation to light does not control the lower depth distributions of branching corals. Mar Ecol Prog Ser 508:149–162
Lasker HR (1981) Phenotypic variation in the coral Montastraea cavernosa and its effects on colony energetics. Biol Bull 160:292
Leichter JJ, Shellenbarger G, Genovese SJ, Wing SR (1998) Breaking internal waves on a Florida (USA) coral reef: a plankton pump at work? Mar Ecol Prog Ser 166:83–97
Leichter JJ, Stewart HL, Miller SL (2003) Episodic nutrient transport to Florida coral reefs. Limnol Oceanogr 48:1394–1407
Leichter JJ, Helmuth B, Fischer AM (2006) Variation beneath the surface: quantifying complex thermal environments on coral reefs in the Caribbean, Bahamas and Florida. J Mar Res 64:563–588
Leichter JJ, Stokes MD, Genovese SJ (2008) Deep water macroalgal communities adjacent to the Florida Keys reef tract. Mar Ecol Prog Ser 356:123–138
Leichter JJ, Stokes MD, Hench JL, Witting J, Washburn L (2012) The island-scale internal wave climate of Moorea, French Polynesia. J Geophys Res 117:C06008
Lema KA, Willis BL, Bourne DG (2012) Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Appl Environ Microbiol 78:3136–3144
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375:1–8
Lesser MP, Slattery M, Stat M, Ojimi M, Gates RD, Grottoli A (2010) Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91:990–1003
Letelier RM, Karl DM, Abbott MR, Bidigare RR (2004) Light driven seasonal patterns of chlorophyll and nitrate in the lower euphotic zone of the North Pacific Subtropical Gyre. Limnol Oceanogr 49:508–519
Levy O, Dubinsky Z, Achituv Y (2003) Photobehavior of stony corals: responses to light spectra and intensity. J Exp Biol 206:4041–4049
Libes S (2011) Introduction to marine biogeochemistry. Elsevier Academic Press, New York
Lough JM, Cantin NE, Benthuysen JA, Cooper TF (2016) Environmental drivers of growth in massive Porites corals over 16 degrees of latitude along Australia’s northwest shelf. Limnol Oceanogr 61:684–700
Loya Y, Eyal G, Treibitz T, Lesser MP, Appeldoorn R (2016) Theme section on mesophotic coral ecosystems: advances in knowledge and future perspectives. Coral Reefs 35:1–9
Luck DG, Forsman ZH, Toonen RJ, Leicht SJ, Kahng SE (2013) Polyphyly and hidden species among Hawaiʻi’s dominant mesophotic coral genera, Leptoseris and Pavona (Scleractinia: Agariciidae). PeerJ 1:e132
Lumsden SE, Hourigan TF, Bruckner AW, Dorr G (2007) The state of deep coral ecosystems of the United States. NOAA Technical Memorandum CRCP–3, Silver Spring
Macintyre IG (2003) A classic marginal coral environment: tropical coral patches off North Carolina, USA. Coral Reefs 22:474
MacIntyre HL, Kana TM, Anning T, Geider RJ (2002) Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J Phycol 38:17–38
Magnusson SH, Fine M, Kühl M (2007) Light microclimate of endolithic phototrophs in the scleractinian corals Montipora monasteriata and Porites cylindrica. Mar Ecol Prog Ser 332:119–128
Maier C, Weinbauer MG, Pätzold J (2010) Stable isotopes reveal limitations in C and N assimilation in the Caribbean reef corals Madracis auretenra, M. carmabi and M. formosa. Mar Ecol Prog Ser 412:103–112
Maragos JE, Jokiel P (1986) Reef corals of Johnston Atoll: one of the world’s most isolated reefs. Coral Reefs 4:141–150
Marcelino VR, Verbruggen H (2016) Multi-marker metabarcoding of coral skeletons reveals a rich microbiome and diverse evolutionary origins of endolithic algae. Sci Rep 6:31508
Marcelino LA, Westneat MW, Stoyneva V, Henss J, Rogers JD, Radosevich A, Turzhitsky V, Siple M, Fang A, Swain TD, Fung J, Backman V (2013) Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. PLoS ONE 8:e61492
Mason JD, Cone MT, Fry ES (2016) Ultraviolet (250–550 nm) absorption spectrum of pure water. Appl Opt 55:7163–7172
Mass T, Einbinder S, Brokovich E, Shashar N, Vago R, Erez J, Dubinsky Z (2007) Photoacclimation of Stylophora pistillata to light extremes: metabolism and calcification. Mar Ecol Prog Ser 334:93–102
Mass T, Kline DI, Roopin M, Veal CJ, Cohen S, Iluz D, Levy O (2010) The spectral quality of light is a key driver of photosynthesis and photoadaptation in Stylophora pistillata colonies from different depths in the Red Sea. J Exp Biol 213:4084–4091
McConnaughey TA (2003) Sub-equilibrium oxygen-18 and carbon-13 levels in biological carbonates: carbonate and kinetic models. Coral Reefs 22:316–327
McConnaughey TA, Burdett J, Whelan JF, Paull CK (1997) Carbon isotopes in biological carbonates: respiration and photosynthesis. Geochim Cosmochim Acta 61:611–622
McEvoy JP, Brudvig GW (2006) Water-splitting chemistry of photosystem II. Chem Rev 106:4455–4483
McGillicuddy DJ Jr, Robinson AR, Siegel DA, Jannasch HW, Johnson R, Dickey TD, McNeil J, Michaels AF, Knap AH (1998) Influence of mesoscale eddies on new production in the Sargasso Sea. Nature 394:263–266
McManus MA, Cheriton OM, Drake PJ, Holliday D, Storlazzi CD, Donaghay PL, Greenlaw CF (2005) Effects of physical processes on structure and transport of thin zooplankton layers in the coastal ocean. Mar Ecol Prog Ser 301:199–215
Mobley CD (1994) Light and water: radiative transfer in natural waters. Academic Press, New York
Muir PR, Wallace CC, Done T, Aguirre JD (2015a) Limited scope for latitudinal extension of reef corals. Science 348:1135–1138
Muir PR, Wallace CC, Bridge TCL, Bongaerts P (2015b) Diverse staghorn coral fauna on the mesophotic reefs of North-east Australia. PLoS ONE 10:e0117933
Muko S, Kawasaki K, Sakai K, Takasu F, Shigesada N (2000) Morphological plasticity in the coral Porites sillimaniani and its adaptive significance. Bull Mar Sci 66:225–239
Muscatine L, Kaplan IR (1994) Resource partitioning by reef corals as determined from stable isotope composition II. δ15N of zooxanthellae and animal tissue versus depth. Pac Sci 48:304–312
Muscatine L, Falkowski PG, Porter JW, Dubinsky Z (1984) Fate of photosynthetic fixed carbon in light-and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc B 222:181–202
Muscatine L, Porter JW, Kaplan IR (1989) Resource partitioning by reef corals as determined from stable isotope composition. Mar Biol 100:185–193
Muscatine L, Grossman D, Doino J (1991) Release of symbiotic algae by tropical sea anemones and corals after cold shock. Mar Ecol Prog Ser 77:233–243
Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57:521–565
Neuheimer AB, Taggart CT (2007) The growing degree-day and fish size-at-age: the overlooked metric. Can J Fish Aquat Sci 64:375–385
Neuheimer AB, Thresher RE, Lyle JM, Semmens JM (2011) Tolerance limit for fish growth exceeded by warming waters. Nat Clim Chang 1:110–113
Nir O, Gruber DF, Einbinder S, Kark S, Tchernov D (2011) Changes in scleractinian coral Seriatopora hystrix morphology and its endocellular Symbiodinium characteristics along a bathymetric gradient from shallow to mesophotic reef. Coral Reefs 30:1089–1100
Odum HT, Odum EP (1955) Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol Monogr 25:291–320
Ohno Y, Iwasaka N, Kobashi F, Sato Y (2009) Mixed layer depth climatology of the North Pacific based on Argo observations. J Oceanogr 65:1–16
Opsahl S, Benner R (1997) Distribution and cycling of terrigenous dissolved organic matter in the ocean. Nature 386:480–482
Ow YX, Todd PA (2010) Light-induced morphological plasticity in the scleractinian coral Goniastrea pectinata and its functional significance. Coral Reefs 29:797–808
Palardy JE, Grottoli AG, Matthews KA (2005) Effect of upwelling, depth, morphology and polyp size on feeding in three species of Panamanian corals. Mar Ecol Prog Ser 300:79–89
Palmer CV, Modi CK, Mydlarz LD (2009) Coral fluorescent proteins as antioxidants. PLoS ONE 4:e7298
Parrish CC (2013) Lipids in marine ecosystems. ISRN Oceanogr 2013:604045
Peterson BJ (1999) Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecol 20:479–487
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Evol Syst 18:293–320
Pineda J (1999) Circulation and larval distribution in internal tidal bore warm fronts. Limnol Oceanogr 44:1400–1414
Pope RM, Fry ES (1997) Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl Opt 36:8710–8723
Porter JW, Battey JF, Smith GJ (1982) Perturbation and change in coral reef communities. Proc Natl Acad Sci U S A 79:1678
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Phys A 132:739–761
Pörtner HO (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Prézelin B (1987) Photosynthetic physiology of dinoflagellates. In: Taylor FJR (ed) The biology of dinoflagellates. Blackwell Scientific Publications, Oxford, pp 174–223
Putnam HM, Edmunds PJ, Fan TY (2008) Effect of temperature on the settlement choice and photophysiology of larvae from the reef coral Stylophora pistillata. Biol Bull 215:135–142
Quick C, D’Angelo C, Wiedenmann J (2018) Trade-offs associated with photoprotective green fluorescent protein expression as potential drivers of balancing selection for color polymorphism in reef corals. Front Mar Sci 5:11
Ralph PJ, Larkum AWD, Kühl M (2007) Photobiology of endolithic microorganisms in living coral skeletons: 1. Pigmentation, spectral reflectance and variable chlorophyll fluorescence analysis of endoliths in the massive corals Cyphastrea serailia, Porites lutea and Goniastrea australensis. Mar Biol 152:395–404
Ramus J (1983) A physiological test of the theory of complementary chromatic adaptation. II. Brown, green and red seaweeds. J Phycol 19:173–178
Reef R, Kaniewska P, Hoegh-Guldberg O (2009) Coral skeletons defend against ultraviolet radiation. PLoS ONE 4:e7995
Reynaud S, Martinez P, Houlbrèque F, Billy I, Allemand D, Ferrier-Pagès C (2009) Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: role of nitrogen recycling. Mar Ecol Prog Ser 392:103–110
Risk MJ, Lapointe BE, Sherwood OA, Bedford BJ (2009) The use of δ15N in assessing sewage stress on coral reefs. Mar Pollut Bull 58:793–802
Roberts HH, Rouse LJ Jr, Walker ND, Hudson JH (1982) Cold-water stress in Florida Bay and Northern Bahamas: a product of winter cold-air outbreaks. J Sediment Petrol 52:145–155
Roder C, Fillinger L, Jantzen C, Schmidt GM, Khokiattiwong S, Richter C (2010) Trophic response of corals to large amplitude internal waves. Mar Ecol Prog Ser 412:113–128
Rodrigues LJ, Grottoli AG (2006) Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim Cosmochim Acta 70:2781–2789
Rowan R, Knowlton N (1995) Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Natl Acad Sci U S A 92:2850–2853
Rowan R, Knowlton N, Baker AC, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408:850–853
Scheufen T, Iglesias-Prieto R, Enríquez S (2017) Changes in the number of symbionts and Symbiodinium cell pigmentation modulate differentially coral light absorption and photosynthetic performance. Front Mar Sci 4:309
Schlichter D, Fricke HW (1991) Mechanisms of amplification of photosynthetically active radiation in the symbiotic deep-water coral Leptoseris fragilis. Hydrobiologia 216:389–394
Schlichter D, Fricke HW, Weber W (1986) Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Mar Biol 91:403–407
Schlichter D, Kampmann H, Conrady S (1997) Trophic potential and photoecology of endolithic algae living within coral skeletons. Mar Ecol 18:299–317
Schnepf E, Elbrächter M (1999) Dinophyte chloroplasts and phylogeny—a review. Grana 38:81–97
Seemann J (2013) The use of 13C and 15N isotope labeling techniques to assess heterotrophy of corals. J Exp Mar Biol Ecol 442:88–95
Seki MP, Polovina JJ, Brainard RE, Bidigare RR, Leonard CL, Foley DG (2001) Biological enhancement at cyclonic eddies tracked with GOES thermal imagery in Hawaiian waters. Geophys Res Lett 28:1583–1586
Shashar N, Stambler N (1992) Endolithic algae within corals—life in an extreme environment. J Exp Mar Biol Ecol 163:277–286
Shen J-R (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Shick JM, Dunlap WC (2002) Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu Rev Physiol 64:223–262
Shlesinger T, Grinblat M, Rapuano H, Amit T, Loya Y (2018) Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology 99:421–437
Smith RC, Baker KS (1981) Optical properties of the clearest natural waters (200–800 nm). Appl Opt 20:177–184
Smith EG, D’Angelo C, Salih A, Wiedenmann J (2013) Screening by coral green fluorescent protein (GFP)-like chromoproteins supports a role in photoprotection of zooxanthellae. Coral Reefs 32:463–474
Smith KA, Rocheleau G, Merrifield MA, Jaramillo S, Pawlak G (2016) Temperature variability caused by internal tides in the coral reef ecosystem of Hanauma bay, Hawaiʻi. Cont Shelf Res 116:1–12
Smith EG, D’Angelo C, Sharon Y, Tchernov D, Wiedenmann J (2017) Acclimatization of symbiotic corals to mesophotic light environments through wavelength transformation by fluorescent protein pigments. Proc R Soc B 284:20170320
Stambler N, Dubinsky Z (2007) Marine phototrophs in the twilight zone. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments. Springer, Dordrecht, pp 81–97
Stomp M, Huisman J, Stal LJ, Matthijs HCP (2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME J 1:271–282
Stramska M, Dickey TD (1998) Short-term variability of the underwater light field in the oligotrophic ocean in response to surface waves and clouds. Deep-Sea Res I Oceanogr Res Pap 45:1393–1410
Sun D, Su R, McConnaughey TA, Bloemendal J (2008) Variability of skeletal growth and δ13C in massive corals from the South China Sea: effects of photosynthesis, respiration and human activities. Chem Geol 255:414–425
Suzuki Y, Casareto BE (2011) The role of dissolved organic nitrogen (DON) in coral biology and reef ecology. In: Dubinsy Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 207–214
Swart PK, Leder JJ, Szmant AM, Dodge RE (1996) The origin of variations in the isotopic record of scleractinian corals: II. Carbon. Geochim Cosmochim Acta 60:2871–2885
Swart PK, Szmant A, Porter JW, Dodge RE, Tougas JI, Southam JR (2005) The isotopic composition of respired carbon dioxide in scleractinian corals: implications for cycling of organic carbon in corals. Geochim Cosmochim Acta 69:1495–1509
Tanaka Y, Grottoli AG, Matsui Y, Suzuki A, Sakai K (2015) Partitioning of nitrogen sources to algal endosymbionts of corals with long-term 15N-labelling and a mixing model. Ecol Model 309:163–169
Tanaka Y, Grottoli AG, Matsui Y, Suzuki A, Sakai K (2017) Effects of nitrate and phosphate availability on the tissues and carbonate skeleton of scleractinian corals. Mar Ecol Prog Ser 570:101–112
Tedetti M, Sempéré R (2006) Penetration of ultraviolet radiation in the marine environment. A review. Photochem Photobiol 82:389–397
Terán E, Méndez ER, Enríquez S, Iglesias-Prieto R (2010) Multiple light scattering and absorption in reef-building corals. Appl Opt 49:5032–5042
Thomson DP, Frisch AJ (2010) Extraordinarily high coral cover on a nearshore, high-latitude reef in South-West Australia. Coral Reefs 29:923–927
Titlyanov EA, Titlyanova TV (2002) Reef-building corals—symbiotic autotrophic organisms: 2. Pathways and mechanisms of adaptation to light. Russ J Mar Biol 28:16–31
Titlyanov EA, Titlyanova TV, Yamazato K, van Woesik R (2001) Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J Exp Mar Biol Ecol 263:211–225
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337
Tolosa I, Treignier C, Grover R, Ferrier-Pages C (2011) Impact of feeding and short-term temperature stress on the content and isotopic signature of fatty acids, sterols, and alcohols in the scleractinian coral Turbinaria reniformis. Coral Reefs 30:763–774
Tomczak M, Godfrey JS (2003) Regional oceanography: an introduction. Daya Publishing House, Delhi
Toster J, Iyer KS, Xiang W, Rosei F, Spiccia L, Raston CL (2013) Diatom frustules as light traps enhance DSSC efficiency. Nanoscale 5:873–876
Treignier C, Grover R, Ferrier-Pages C, Tolosa I (2008) Effect of light and feeding on the fatty acid and sterol composition of zooxanthellae and host tissue isolated from the scleractinian coral Turbinaria reniformis. Limnol Oceanogr 53:2702–2710
Treignier C, Tolosa I, Grover R, Reynaud S (2009) Carbon isotope composition of fatty acids and sterols in the scleractinian coral Turbinaria reniformis: effect of light and feeding. Limnol Oceanogr 54:1933–1940
Tremblay P, Grover R, Maguer J-F, Hoogenboom MO, Ferrier-Pagès C (2014) Carbon translocation from symbiont to host depends on irradiance and food availability in the tropical coral Stylophora pistillata. Coral Reefs 33:1–13
Turner JA, Babcock RC, Hovey R, Kendrick GA (2017) Deep thinking: a systematic review of mesophotic coral ecosystems. ICES J Mar Sci 74:2309–2320
Usher KM (2008) The ecology and phylogeny of cyanobacterial symbionts in sponges. Mar Ecol 29:178–192
van der Have TM (2002) A proximate model for thermal tolerance in ectotherms. Oikos 98:141–155
van Grondelle R, Boeker E (2017) Limits on natural photosynthesis. J Phys Chem B 121:7229–7234
Veal CJ, Carmi M, Dishon G, Sharon Y, Michael K, Tchernov D, Hoegh-Guldberg O, Fine M (2010) Shallow-water wave lensing in coral reefs: a physical and biological case study. J Exp Biol 213:4304–4312
Villinski JT (2003) Depth-independent reproductive characteristics for the Caribbean reef-building coral Montastraea faveolata. Mar Biol 142:1043–1053
Vukusic P, Sambles JR (2003) Photonic structures in biology. Nature 424:852–852
Wagner D, Pochon X, Irwin L, Toonen RJ, Gates RD (2010) Azooxanthellate? Most Hawaiian black corals contain Symbiodinium. Proc R Soc B 278:1323–1328
Wall M, Putchim L, Schmidt GM, Jantzen C, Khokiattiwong S, Richter C (2015) Large-amplitude internal waves benefit corals during thermal stress. Proc R Soc B 282:20140650
Wangpraseurt D, Larkum AWD, Franklin J, Szabó M, Ralph PJ, Kühl M (2014) Lateral light transfer ensures efficient resource distribution in symbiont-bearing corals. J Exp Biol 217:489–498
Weinstein DK, Sharifi A, Klaus JS, Smith TB, Giri SJ, Helmle KP (2016) Coral growth, bioerosion, and secondary accretion of living orbicellid corals from mesophotic reefs in the US Virgin Islands. Mar Ecol Prog Ser 559:45–63
Werdell PJ, Bailey SW (2002) The SeaWiFS bio-optical archive and storage system (SeaBASS): current architecture and implementation. NASA Tech Memo 2002–211617, (Fargion GS, McClain CR (eds)) NASA Goddard Space Flight Center, Greenbelt, 45 p, http://seabass.gsfc.nasa.gov/
West AO, Nolan JM, Scott JT (2016) Optical water quality and human perceptions: a synthesis. WIREs Water 3:167–180
Wijgerde T, van Melis A, Silva CIF, Leal MC, Vogels L, Mutter C, Osinga R (2014) Red light represses the photophysiology of the scleractinian coral Stylophora pistillata. PLoS ONE 9:e92781
Wijsman-Best M (1974) Habitat-induced modification of reef corals (Faviidae) and its consequences for taxonomy. Proc 2nd Int Coral Reef Symp, Brisbane, Australia 2:217–228
Willis BL (1985) Phenotypic plasticity versus phenotypic stability in the reef corals Turbinaria mesenterina and Pavona cactus. Proc 5th Int Coral Reef Symp Tahiti 4:107–112
Winters G, Beer S, Ben Zvi B, Brickner I, Loya Y (2009) Spatial and temporal photoacclimation of Stylophora pistillata: zooxanthella size, pigmentation, location and clade. Mar Ecol Prog Ser 384:107–119
Wolanski E, Deleersnijder E (1998) Island-generated internal waves at Scott Reef, Western Australia. Cont Shelf Res 18:1649–1666
Wolanski E, Delesalle B (1995) Upwelling by internal waves, Tahiti, French Polynesia. Cont Shelf Res 15:357–368
Wolanski E, Hamner WM (1988) Topographically controlled fronts in the ocean and their biological influence. Science 241:177–181
Wolanski E, Pickard GL (1983) Upwelling by internal tides and Kelvin waves at the continental shelf break on the Great Barrier Reef. Aust Mar Freshwat Res 34:65–80
Wolanski E, Drew E, Abel KM, O’Brien J (1988) Tidal jets, nutrient upwelling and their influence on the productivity of the alga Halimeda in the Ribbon Reefs, Great Barrier Reef. Estuar Coast Shelf Sci 26:169–201
Wolanski E, Colin PL, Naithani J, Deleersnijder E, Golbuu Y (2004) Large amplitude, leaky, island-generated, internal waves around Palau, Micronesia. Estuar Coast Shelf Sci 60:705–716
Won J, Rho B, Song J (2001) A phylogenetic study of the Anthozoa (phylum Cnidaria) based on morphological and molecular characters. Coral Reefs 20:39–50
Yamano H, Hori K, Yamauchi M, Yamagawa O, Ohmura A (2001) Highest-latitude coral reef at Iki Island, Japan. Coral Reefs 20:9–12
Ziegler M, Roder CM, Büchel C, Voolstra CR (2015) Mesophotic coral depth acclimatization is a function of host-specific symbiont physiology. Front Mar Sci 2:4
Acknowledgments
The authors would like to thank the eight peer reviewers including F. Houlbrèque, P. Bongaerts, M. Hoogenboom, J. Leichter, and P. Swart whose thoughtful and detailed critique significantly improved the chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kahng, S.E. et al. (2019). Light, Temperature, Photosynthesis, Heterotrophy, and the Lower Depth Limits of Mesophotic Coral Ecosystems. In: Loya, Y., Puglise, K., Bridge, T. (eds) Mesophotic Coral Ecosystems. Coral Reefs of the World, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-319-92735-0_42
Download citation
DOI: https://doi.org/10.1007/978-3-319-92735-0_42
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92734-3
Online ISBN: 978-3-319-92735-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)