Abstract
Echinoderms possess an incredible regenerative capacity. Within this phylum, holothurians, better known as sea cucumbers, can regenerate most of their internal and external organs. While regeneration has been studied in several species, the most recent and extensive studies have been done in the species Holothuria glaberrima, the focus of most of our discussion. This chapter presents the model system and integrates the work that has been done to determine the major steps that take place, during regeneration of the intestinal and nervous system, from wound healing to the reestablishment of original function. We describe the cellular and molecular events associated with the regeneration processes and also describe the techniques that have been used, discuss the results, and explain the gaps in our knowledge that remain. We expect that the information provided here paves the road for new and young investigators to continue the study of the amazing potential of regeneration in members of the Echinodermata and how these studies will shed some light into the mechanisms that are common to many regenerative processes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Echinodermata
The phylum Echinodermata comprises animals with some fascinating characteristics. For one they are invertebrate members of the Deuterostomata clade, sharing similar embryological development with members of the Chordata. Even though their adult pentaradial symmetry might be perceived as a primitive trait, their embryological development identifies them as members of the vertebrate evolutionary branch. Moreover, modern genetic analyses have confirmed their close phylogenetic position to vertebrates (Cannon et al. 2014; Holland 2015; Perseke et al. 2013; Satoh et al. 2014). Only one other phylum, the Hemichordata, shares with them the characteristics of being non-chordate deuterostomes. In fact, their phylogenetic position can be considered as ideal for the comparative analyses necessary to understand the evolution of vertebrates. There are five classes within the phylum Echinodermata: Crinoidea (sea lilies), Asteroidea (sea stars), Ophiuroidea (brittle stars), Echinoidea (sea urchins), and Holothuroidea (sea cucumbers). Echinoderms are marine animals and can be found in all marine habitats from the coastal areas to the benthic depths and from the tropical to the polar regions.
Echinoderms are unique in many other ways. They possess a calcium endoskeleton that is very developed in some groups such as in sea urchins while less so in holothurians. They have a water vascular system, whose major function is thought to be nutrient transport and gas exchange. They display a mutable collagenous tissue (MCT) that has “muscle-like” properties, allowing for the reversible changes of tensile strength under the control of the nervous system; for review see Wilkie (2002). Finally, the focus of this chapter is that echinoderms feature an incredible regenerative capacity.
1.2 Regeneration in Echinoderms
All echinoderms have the capacity to regenerate lost body parts. In some groups, the regenerative capacity is far more developed than in others. For example, sea urchins, where regeneration capacity is less developed, can still regenerate their damaged spines and tube feet and can repair holes in their test. Sea stars and brittle stars are well known for being able to regenerate their arms and in some species for being able to regenerate a complete organism from a severed arm. However, it is in holothurians where the regenerating capacity is most developed. The species Leptosynapta crassipatina (Holothuroidea, Apodida) serves as a fitting example to highlight the regenerative capacity of holothurians. Organisms of this species can regenerate a whole organism from a disk from the oral complex (Smith 1971b). Many holothurian species can regenerate two organisms from one that has been cut in half. This process can occur in nature (fission) and has been studied in the laboratory by scientists interested in the regenerative response. In fact, many holothurians use fission as their means of asexual reproduction. This process has been thoroughly documented in the holothurian Cladolabes schmeltzii (Holothuroidea, Dendrochirotida) using electron microscopy (Kamenev and Dolmatov 2015, 2017; Kamenev et al. 2013). When animals are ready to reproduce, they divide into two regions of about the same size, and each region regenerates the missing part. Therefore, the anterior part can regenerate the posterior part and vice versa.
The holothurian Thyone briareus (Holothuroidea, Dendrochirotida) can autotomize into two segments, both of which can regenerate a complete animal (Pearse 1909). However, in other species only one end of the animal has been shown to survive and regenerate a complete animal. This is the case for Leptosynapta crassipatina (Smith 1971a, b). A systematic analysis of the minimal region required to regenerate the organism was done, shedding some light into the likely progenitor cells required to undergo this process. In the case of Leptosynapta, if cut in half, only the anterior part will regenerate and form a whole organism (the missing posterior region), while the posterior part is not able to regenerate.
A more complete review of the autotomy process and the regeneration capacities of different holothurian orders can be found in a previous review (Garcia-Arraras and Greenberg 2001). In summary, these experiments highlight that the regenerating region must contain the necessary cells and gene activation network information necessary to rebuild the cells and tissues of the entire lost component. However, the results of many of these experiments, performed years ago, must be analyzed with the prism of our accumulated knowledge on stem cells and dedifferentiation to fully understand the regenerative mechanisms of echinoderms.
2 Digestive Tract Regeneration
Most studies on digestive tract regeneration, particularly those in vertebrate, refer strictly to the regeneration of the mucosa or luminal layer. There are important reasons for this. First, the luminal epithelial layer is known to be constantly formed and regenerated. Second, problems in the renewal of this layer cause a series of diseases, such as ulcers or cancers. However, whole organ regeneration, such as regeneration of the intestine or the stomach, has not been widely studied, probably for an obvious reason: not many organisms can achieve this task. Thus, it is only a selected group of animals where this event has been documented: planaria, tunicates, the echinoderms, and in some amphibians. Here we focus on the regeneration of the complete digestive tract in holothurians. For this we provide the available information on the cellular and molecular processes that bring about this process.
The regeneration of the digestive tract, following evisceration, has been studied in some species. The original studies were performed early last century by Fausta Bertolini at the Stazione Zoologica in Naples, Italy. In her work, using various holothurian species, she described the formation of the new intestine following evisceration (Bertolini 1930, 1932). Even with the technical limitations of the time, she was able to illustrate some of the cellular events that underlie the formation of the new intestine at the free end of the remaining mesentery. Later histological studies, using different species, confirmed the mesentery as the main tissue involved in intestinal regeneration and extended the description of the regenerative processes (Bai 1971; Dawbin 1949; Kille 1935; Leibson 1992; Mosher 1956; Smith 1971a; Tracey 1972). In the 1990s our laboratory revisited the use of holothurians, focusing on the species Holothuria glaberrima (Holothuroidea, Aspidochirotida), to study intestinal regeneration, and began applying modern technologies to answer some of the remaining questions. Since then we have developed and extended this model system to probe into the cellular and molecular basis of regenerative processes. Our laboratory has published various reviews that provide additional details on sea cucumber evisceration and on various aspects of the regenerative processes that might be of interest to investigators (Garcia-Arraras and Greenberg 2001; Mashanov and Garcia-Arraras 2011; Mashanov et al. 2014a).
2.1 Holothurian Digestive Tract
Prior to describing the intestinal regeneration model, it is necessary to describe the anatomy/histology of H. glaberrima digestive tract. At the macroscopic level, the digestive tract consists of a short esophagus adjacent to the circumoral ring that opens to the mouth at the anterior region. At the posterior end of the esophagus begins a long curving intestine. This can be subdivided into three segments: a descending intestine, an ascending intestine, and a second descending intestine. The first two sections show a smaller diameter and have been labeled as descending and ascending small intestines, while the last segment is larger in diameter and has been described as the descending large intestine. The main difference between the descending and the ascending small intestine lies in the latter association with two other visceral organs: the hemal system and the right respiratory tree. The large intestine continues into the cloaca at the posterior end of the animal. The digestive tract is attached to the body wall by mesenteries, in a similar manner to the vertebrate digestive tract. The anterior part of the tract, the esophagus and descending intestine are attached by a dorsal mesentery, the ascending small intestine by a lateral mesentery, while the descending large intestine is attached by a ventral mesentery. In reality, the three mesenteries are all a continuous structure that changes the localization of its attachment to the body wall as one moves along the digestive tract.
At the histological level, the digestive tract is made up of three main tissue layers: the mesothelium, a connective tissue layer, and the luminal epithelium (Fig. 13.1). The mesothelium itself can be divided into two tissue types; the most external layer is made up of monociliated coelomic cells named peritoneocytes that are attached to each other via specialized cell junctions and extend processes that anchor them in the underlying basal lamina. The most internal layer of the mesothelium is a muscle layer that can have both longitudinal and circular components and also lie above the basal lamina. This basal lamina separates the mesothelium from the underlying connective tissue layer. The connective tissue layer corresponds to the submucosa of vertebrates and as the name implies is characterized by having a large amount of connective tissue where few, scattered cells can be found. The luminal epithelium corresponds to the mucosa of vertebrates. This is a pseudostratified epithelium that is mainly formed by columnar cells or enterocytes whose apical ends face the luminal cavity. However, other cell types can be found among the enterocytes, including goblet-like cells and peptide-containing cells similar to vertebrate enteroendocrine cells (Garcia-Arraras et al. 1998; Garcia-Arraras et al. 2001).
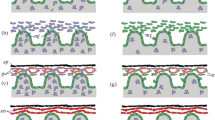
Holothurian digestive system and stages of the regenerating digestive system. (a) Diagram of a cross section through the echinoderm posterior intestine demonstrating its basic organization. Cross section through the stages of regeneration of the posterior intestine at (b) 3 dpe, (c) 7 dpe, (d) 14 dpe, (e) 21 dpe, and (f) 28 dpe. DPE days post-evisceration, CE coelomic epithelium, CT connective tissue, LE luminal epithelium
The mesothelial layer is continuous with the mesothelial layer of the mesentery as is the connective tissue layer. Thus, the mesentery is formed by two mesothelial layers separated by a small amount of connective tissue. The mesentery mesothelium is itself continuous with the mesothelium that forms the coelomic epithelia of the peritoneum lining the coelomic cavity.
Neuronal cells have been described in all intestinal layers (Diaz-Balzac et al. 2007, 2010b, 2012, 2014, 2016; Garcia-Arraras et al. 2001). Cell bodies in the mesothelium appear to be primarily associated with the innervation of the mesothelial muscle. These cells are not associated into the ganglion-like structures found in the myenteric plexus of vertebrates but are isolated or grouped in small numbers and dispersed along the intestinal length. Neuron-like cells have also been identified within the connective tissue of the intestine and have been associated with the modulation of intestinal mutability. Neuron-like cells have also been described within the luminal layer, and some of these appear to extend long processes into the connective tissue layer, suggesting that they might represent sensory cells in the luminal epithelia that convey information to the animal. Neuron-like cells have also been detected within the mesothelium of the mesentery. These are mainly found among large number of parallel fiber tracts that extend from the body wall to the intestine (Tossas et al. 2014). Although glial or support cells might be found in the enteric nervous system, their presence has not been determined due to a lack of appropriate markers that specifically identify this putative cell population.
The digestive tract is very similar in composition throughout all of its regions. However, the size of the layers varies within each region. For example, the esophagus has a very developed muscle layer that is much reduced in the rest of the digestive tract. In contrast, the connective tissue layer in the large intestine is the widest when compared to the rest of the digestive tract.
2.2 Evisceration
Many holothurian species activate an evisceration process when stressed or in danger (Hyman 1955; Emson and Wilkie 1980). This process involves the forceful ejection of their viscera, either anteriorly by the loss of the anterior part of the animal including tentacles, nerve ring, and Aristotle’s lantern or the posterior evisceration of the intestinal system through the cloaca. The organs that can be eviscerated include the esophagus, intestines, hemal system, respiratory trees, nerve ring, gonads, and others associated with the aquapharyngeal complex. However, the eviscerated organs depend on the species and its particular evisceration mode. Most of the organs eviscerated are those associated with the digestive tract. In holothurians, the digestive tract is principally made up of an extended intestinal system; thus this system comprises the main component of the eviscerated contents. The digestive system is also the first organ to be regenerated. Visceral evisceration is often mistaken with the ejection of Cuvierian tubules. These tubular structures are found in the posterior end, attached to the base of the respiratory trees in the cloaca and are filled with an adhesive substance. They are ejected through the cloaca when the organism feels threatened and form a sticky network that can deter the aggressor or predator. Cuvierian tubules are then regenerated, and this regenerative process has been documented by various investigators (VandenSpiegel et al. 2000).
While the ejection of the Cuvierian tubules is strictly a defense mechanism, the purpose of the evisceration reaction is less clear. Some investigators suggest it is a defense mechanism against predators; however, evisceration often can occur when animals are exposed to stressful situations such as high temperatures or low oxygen concentrations. Whatever evisceration is intended to achieve, the process, together with the ensuing regeneration, has been exploited by researchers to study organ regeneration.
Our laboratory has developed the use of the sea cucumber Holothuria glaberrima, a local species found in the rocky intertidal zone throughout the Caribbean, as a model system to study intestinal regeneration. H. glaberrima can be easily induced to eviscerate by intracoelomic injections of 0.35 ml KCl. They eviscerate posteriorly, via the cloaca, and eject their intestinal system (small and large), the hemal system, the right respiratory tree, and, when in season, most of the gonads. The rupture sites occur between the descending small intestine and the esophagus and between the large intestine and the cloaca. Autotomy of the intestine-mesentery connections occurs close to the intestine; thus, the ejected intestine contains a very small portion of the mesentery, while most of the mesentery remains attached to the body wall at one end and free within the coelomic cavity at the other end.
Intestine regeneration in this species provides an excellent biological system for studying regeneration. Advantages of this model include the following:
-
(i)
Evisceration is easily induced by KCl injections and follows a fixed pattern that diminishes the individual variability due to surgical amputations required by other regeneration models.
-
(ii)
The pattern of regeneration can be divided into several stages and involves the organization of specific cells and tissues into a functional organ.
-
(iii)
Regeneration occurs along the torn edge of the mesentery, physically isolated from other tissues of the organism.
-
(iv)
A complex organ in an adult organism is formed in less than 3 weeks.
-
(v)
The size of the organism is small enough for maintenance in indoor aquaria but large enough to obtain the quantities of tissue needed for the cellular and molecular experiments.
-
(vi)
The organism has a soft body wall that facilitates dissection.
-
(vii)
It is extremely abundant in local waters.
2.3 Regeneration of the Digestive Tract
2.3.1 Wound Healing
Following evisceration there are three regions of the digestive tract that would need some type of immediate repair. Two of these are the area of the esophagus where the small intestine was attached and the area of the cloaca where the large intestine was attached. These regions are points of contact with the external environment and need to be sealed off rapidly to prevent the influx of bacteria or other pathogens into the coelomic cavity. The third region is the rupture margin of the mesentery where the intestine, both small and large, was attached. The wound healing response following evisceration has not been studied in detail in H. glaberrima. However, information from other echinoderm species provides a glimpse of what could be occurring.
2.3.2 Cellular Mechanisms
We have shown that, following autotomy of the digestive tube, regeneration begins with the formation of a thickening at the free end of the remaining mesentery. This thickening grows in size until it forms a solid rod of tissue resembling a blastema that extends from the esophagus on the oral side of the animal to the cloaca on the posterior end. Intestinal regeneration involves cellular dedifferentiation (Candelaria et al. 2006), cell division (Garcia-Arraras et al. 1998, 2011), apoptosis (Mashanov et al. 2010b), cell migration (Cabrera-Serrano and Garcia-Arraras 2004; Garcia-Arraras et al. 1998), and ECM remodeling (Quinones et al. 2002) among other events associated with regenerative processes.
Dedifferentiation
The first morphological indication of the regeneration process is the dedifferentiation of the muscle cells in the mesentery adjacent to the injury/autotomy site (Candelaria et al. 2006; Garcia-Arraras et al. 2011). In this process, muscle cells become fragmented, and the contractile apparatus is degraded and ejected from the cell in membrane-bound structures that have been named spindle-like structures (SLSs) because of their morphology (Garcia-Arraras and Dolmatov 2010). This dedifferentiation occurs in a temporal and spatial sequence. From its initiation at the tip of the mesentery at about days 1–2, it peaks at days 5–7 following evisceration. More interestingly, the dedifferentiation process occurs in a gradient, beginning at the tip of the mesentery and moving toward the body wall. Thus, at about 3 days post-evisceration (dpe), the mesenterial area near the injury site has many SLSs, while the rest of the mesentery has the usual muscle fiber component. A few days later, the mesenterial area adjacent to the now larger rudiment is devoid of muscle cells and SLSs, the middle of the mesentery has few muscle fibers but an abundant amount of SLSs, while the area near the body wall has the normal-looking muscle fibers and few if any SLSs. We have hypothesized that these dedifferentiated muscle cells provide the precursors for the formation of the new intestine.
Cell death and cell proliferation
Two other processes are associated with the initial formation of the rudiment: cell death (apoptosis) and cell proliferation. An increase in apoptosis has been documented using the TUNEL method, from 3 dpe (the earlier stage measured). Cell division has been documented using BrdU. Although some cell division is observed 24 h after evisceration, it is not until a few days later (5–7 dpe) when the peak in cell division is observed, most of it taking place in the mesothelium of the growing rudiment.
Dedifferentiation, apoptosis, and proliferation occur within some spatial differences. While dedifferentiation takes place within the mesenterial tissue and, as explained above, spreads in a gradient farther and farther from the regenerating structure, both apoptosis and proliferation mainly occur within the regenerating structure or in the mesenterial region close to it. A more discrete spatial difference has also been documented between apoptosis and cell proliferation at 7 dpe, where areas of the regenerating rudiment with high levels of apoptosis tend to be the same areas where less cell proliferation occurs and vice versa (Mashanov et al. 2010b).
Our working hypothesis of these initial events associated with the early formation of the intestinal rudiment is the following: evisceration induces the dedifferentiation of the muscle cells of the mesentery close to the injury site. This triggers a feed forward process where dedifferentiating cells induce other cells to dedifferentiate, thus producing the gradient in dedifferentiation that begins at the mesentery tip and extends toward the opposite end of the mesentery. Dedifferentiated cells then migrate toward the growing intestinal rudiment where they enter a proliferative state (some proliferation takes place during the migration, though it is within the mesothelium of the rudiment where it peaks). Apoptosis, activated by the injury, and possibly by the remodeling process, serves to provide the rudiment with a particular morphology and, as has been documented in other species/regenerative processes, might be serving as an inducer of cell proliferation.
Epithelial to mesenchymal transition (EMT)
As the regenerating rudiment grows in size, a process of epithelial to mesenchymal transition is observed (Garcia-Arraras et al. 2011). The basal lamina of the intestinal rudiment appears discontinuous and is not visible at certain sites. At the same time, cells of the rudiment mesothelium dissociate and ingress into the rudiment connective tissue, forgoing their epithelial phenotype for a new roundish mesenchymal-type cell. The initial EMT takes place at the tip of the mesentery, that is, at the opposite end to the rudiment attachment to the mesentery; however, as time passes and the rudiment continues to grow, some cells appear to ingress in many areas of the rudiment and are not circumscribed to the tip. The final destination or the role of these incoming cells is unclear, although some have been observed to migrate and apparently settle at the connective tissue side of the luminal epithelium basal lamina.
Extracellular matrix remodeling
Concomitant with the increase in growth of the intestinal rudiment, there are also drastic changes in the composition of the extracellular matrix. This remodeling primarily involves the activation of matrix metalloproteases and the loss of the collagen fibrils, in a process that closely follows the dedifferentiation gradient (Quinones et al. 2002). Thus, collagen fibers are first lost within the connective tissue of the rudiment, and as time goes on, fibers are lost from the connective tissue of the mesentery that is adjacent to the intestinal rudiment. Similar to the wave of dedifferentiation, the remodeling of the ECM continues within the mesentery with loss of collagen in the central region, and in some cases the loss can extend to areas close to the body wall. Other components, such as fibronectin and glycosaminoglycan while losing some of their organization, remain within the mesentery and growing rudiment throughout the regeneration process (Quinones et al. 2002; Vazquez-Velez et al. 2016). Functional studies where ECM remodeling was slowed down by metalloproteinase inhibitors or where cell-ECM interactions were disrupted by ERGD peptides caused a negative effect in the process of inhibition (Cabrera-Serrano and Garcia-Arraras 2004; Quinones et al. 2002). These experiments highlight the important role played by the ECM in the regeneration of the intestine.
Cell migration
Two cell migration events have been proposed to occur during intestinal regeneration. First, dedifferentiated mesothelial cells forming the coelomic epithelium are thought to migrate as an epithelial sheet toward the forming intestinal rudiment. These cells eventually form part of the regeneration epithelium of the rudiment, undergo cell division, and eventually differentiate into the mesothelium of the new intestine. The evidence for this migration is admittedly rather weak, and the phenomenon needs to be better studied with live action imaging and migration markers. The second migration event is the migration of cells along the mesentery connective tissue. Cells undergoing this migration are either coming from the body wall connective tissue or might be coelomocytes from the coelomic cavity. The event has been shown to take place using immunocytochemical markers that identify a spherulocyte population (Garcia-Arraras et al. 2006) and has been shown to be disrupted by ERGD peptides that interfere with the binding of cells to ECM molecules (Cabrera-Serrano and Garcia-Arraras 2004). This event, however, occurs relatively late in regeneration and is probably associated with the formation of the lumen either with the restoration of the ECM or with the activation of the immune system to protect the tissue from microorganism found in the newly forming luminal cavity.
Lumen formation
By the end of the first week of regeneration, the free margin of the mesentery has become a solid rod of tissue that extends along the anterior-posterior axis of the organism. At the anterior end, the rudiment connects with the (uneviscerated) remnants of the esophagus, and at the posterior end, it connects with the cloaca. This sets the stage for the formation of the luminal layer, which takes place during the second week of regeneration.
The formation of the luminal epithelial layer brings about the formation of the lumen. This process takes place as luminal cells from the cloaca and esophagus proliferate and invade the connective tissue of the regenerating intestine rudiment (Fig. 13.1). It is possible that, as has been shown in other species, the dividing luminal cells are partly dedifferentiated and lose their connection to the basal lamina, although keeping their connection with their neighbors (Odintsova et al. 2005; Shukalyuk and Dolmatov 2001). In experiments using BrdU, we have quantified the level of cell proliferation at the invading tip and shown it to be almost twice the proliferation of the luminal epithelial cells where the lumen has already formed. The cell migration in the connective tissue has two immediate consequences: first they open up the cavity that will become the intestinal lumen and second, they form the luminal cell layer. The incoming cells from the esophagus move posteriorly, while those from the cloaca move anteriorly so that in no more than a week, the two tips of incoming cells meet medially and form the complete lumen of the regenerated intestine. Thus, by 14 dpe the lumen has formed, and the mucosal epithelium has regenerated, establishing the basic layout of the intestine.
Cellular differentiation
The early regenerating rudiment is composed of two tissue layers that are contiguous with the mesentery layers where they originated: a connective tissue layer that has increased in size as regeneration proceeds and an epithelial layer that, although contiguous with the mesentery mesothelium, differs dramatically from it. During the early stages of regeneration, the cells in the epithelial layer of the regenerating rudiment are rounded or spherical cells that show a high proliferative index. These cells are at least partially dedifferentiated, having lost muscle and peritoneocyte markers. But they retain other markers, such as immunoreactivity to the antibody MESO1, an undetermined epitope that identifies most, if not all, cells of the mesothelium (Garcia-Arraras et al. 2011). Nonetheless, soon after the formation of the rudiment, some of these epithelial cells begin the process of redifferentiation. The process of myogenesis has been studied in some detail (Murray and Garcia-Arraras 2004). In this process, the coelomic epithelial cells in the regenerating rudiment move toward the basal lamina and begin expressing muscle markers. This is a step-by-step process that is accompanied by morphological changes, where cells elongate, express the proteins of the contractile apparatus, and eventually form the muscle fibers. Thus, cell movement toward the basal lamina begins at about 4 dpe, when these cells begin to express some muscle cell markers, but organized contractile apparatus filaments, as determined by phalloidin binding, do not appear until a few days later, once cells have acquired the elongated, muscle-like morphology (Murray and Garcia-Arraras 2004). BrdU labeling has shown that newly formed muscle cells originate from dividing precursors and that most of the muscle cells are born during the second week of regeneration.
A similar process is assumed to occur in the formation of neurons of the enteric plexus (Tossas et al. 2014). However, this differentiation has not been studied in detail, in part due to the smaller number of neurons (when compared with muscle cells) in the organ and to the difficulty in identifying them. The appearance of neurons in the mesothelium enteric nervous system occurs later than the appearance of muscle cells; neuronal phenotypes begin appearing at about 10 dpe and continue for the next 2 weeks.
A fast transition between cell proliferation and beginning of differentiation has also been documented in the luminal epithelium. Here cells with a high proliferating index are found at the tip of the migrating cells that originated from the esophagus. These cells do not show any of the available differentiated cell markers. Nonetheless, cells in the luminal epithelium that have formed a few days before from the rapidly proliferating/migrating cells already show markers that identify them as neuroendocrine cells (unpublished observation).
Tissue layer proportioning
Once the lumen has formed, the basic layout of the intestine is completed. The three tissue layers, mesothelium, connective tissue, and luminal epithelium, are formed, and a hollow tube extending from the esophagus to the cloaca is ready for the passage of the food/nutrients. None of the studies have address the recovery of intestinal function; nonetheless, once the continuous lumen is formed, mud, algae, and other particles have been detected within the lumen of regenerating animals, implying that the animas have ingested these particles and are in the process of digestion.
This three-layer structure continues to be modified even after the lumen has formed. We have previously named this stage the stage of tissue layer proportioning (Garcia-Arraras et al. 1998). For example, although all tissue layers are present, their contribution to the complete organ does not clearly represent what is found in the normal intestine. Additionally, the initial intestine that forms resembles a small intestine throughout its length, and it takes some additional time for the width of the connective tissue in the posterior part of the organ to acquire the proportions of a large intestine. Similarly, changes are observed within the muscle component of the mesothelium (Murray and Garcia-Arraras 2004). As cells differentiate forming the muscle precursors and eventually the muscle fibers, a single layer of the mainly circular-oriented fibers is formed. This muscle layer continues growing in width, but a clear organization of its fibers is not discernible. It is not until about 4 weeks into regeneration that the two muscle layers that constitute the normal intestine (circular and longitudinal) can be identified.
Intestinal enlargement
Roughly 1 month following evisceration, the intestinal system of the holothurian has formed what can be considered a smaller copy of the original organ. Arguably, not all tissue components are identical to those of the normal pre-eviscerated intestine, but all identifiable cell types and structures are present in a closely similar organization and proportional size. Once this stage is acquired, the next step is a general growth in the size of the structure in order for it to acquire the expected size. This process has not been studied in the laboratory. We have hypothesized that in the normal environment, the later processes of regeneration might occur at a faster pace than in the aquaria due to access to nutrients and other environmental inputs that might be lacking in the lab setting; however, this has not been documented.
2.3.3 Species Differences
Intestinal regeneration following evisceration is a common process exhibited by holothurians. Some species, such as Apostichopus japonicus (Holothuroidea, Synallactida), follow a process of evisceration and regeneration very similar to that observed in H. glaberrima (Leibson 1992; Shukalyuk and Dolmatov 2001). However, the evisceration route as well as the regeneration process can show some differences depending on the species. For example, both Sclerodactyla briareus (previously known as Thyone briareus) and Eupentacta fraudatrix eviscerate anteriorly ejecting all their viscera together with their anterior nerve ring, introvert, and tentacles. While regeneration of the posterior intestinal segment in E. fraudatrix follows a pathway similar to that described for H. glaberrima, the regeneration of the anterior segment is somewhat different. The lumen epithelia in this segment are formed by transdifferentiation of the coelomic epithelial cells (Mashanov et al. 2005). This itself is an interesting process where the luminal epithelium has two different origins: an endodermal origin in the posterior intestine and a mesodermal origin in the anterior intestinal segment.
2.3.4 Molecular Basis of Intestinal Regeneration
As the cellular processes that bring about intestinal regeneration are elucidated, research focus has been directed at characterizing the underlying molecular events. The primary focus of this research has been to identify the genes that are differentially expressed between regenerating and normal (non-regenerating) intestines. The techniques that have been used in this approach have dramatically changed over the last two decades, as better and more sensitive methods are made available. Early studies were initially done using a selected candidate approach looking at particular genes that, because of their involvement in embryological development or in other wound healing or regenerative processes, seemed good candidates to be involved in intestinal regeneration. Example of this approach was the characterization of holothurian Hox genes (Mendez et al. 2000).
Another early approach was the use of differential display to identify transcripts that were preferentially expressed in regenerating tissues (Roig-López et al. 2000). Using this technique several holothurian genes were identified that could be playing various roles in the intestinal regeneration process. These genes include actin isoforms (Roig-López et al. 2000) and serum amyloid A (Santiago et al. 2000).
As methodologies improved, the next advance was the preparation of cDNA libraries of intestines at various stages of regeneration, the cloning and random sequencing of hundreds of genes (Rojas-Cartagena et al. 2007), and the preparation of “in-house” microarrays (Ortiz-Pineda et al. 2009). The random sequencing of clones produced by itself new candidate genes that could be probed for their role in the regenerative process. Thus, ependymin (Suarez-Castillo et al. 2004; Suarez-Castillo and Garcia-Arraras 2007), ubiquitin-proteasome system genes (Pasten et al. 2012a, b), Centaurin (Rojas-Cartagena et al. 2007), and melanotransferrin (Hernandez-Pasos et al. 2017; Rojas-Cartagena et al. 2007) were among the genes characterized and whose expression was further studied using PCR to show their differential levels of expression at particular regeneration stages. However, it was the gene expression profiling using custom-made microchips that produced a surprising result, showing that hundreds of genes (which accounted to over 70% of the studied genes) were differentially expressed during regenerative stages (Ortiz-Pineda et al. 2009). Many of the differentially expressed genes showed no similarities to genes in the databanks (at the time of publication), while others were related to known genes involved in regeneration-related processes, wound healing, cell proliferation, differentiation, morphological changes, cell survival, stress response, immune activation, and neoplastic transformations. The differentially expressed genes included genes associated with developmental processes such as growth factors (TGF-β, BMP, WNT, myotrophin) and transcription factors (homeobox, Krueppel-like, forkhead), with the cytoskeleton (actin, tubulin myosin, gelsolin) and with extracellular matrix (matrix metalloproteases, laminin, echinonectin, collagen, tenascin). Among the differentially expressed genes that were validated were Wnt9, homeobox genes, metalloproteinases, actins, tenascin, as well as several unknowns.
More recently new technologies of transcriptome analyses and RNAseq have been applied to our system and to other species of regenerating holothurians increasing exponentially the number of genes that are associated with regenerative responses. In A. japonicus, next-generation sequencing of regenerating body wall and intestine identified almost 7000 genes, and hundreds of these were found to be differentially expressed (Sun et al. 2011). Further studies by the same group using RNAseq reported over 2400 genes to be upregulated and over 1000 genes to be downregulated when compared to the normal intestine (Sun et al. 2013).
Nonetheless, in future studies, the large number of differentially expressed genes could be tailored down to those that might really play a significant role in regeneration. At present, most of the comparisons made to identify differentially expressed genes were made by comparing the regenerating intestine to the normal intestine. However, these two organs are comprised of different tissues and/or of disproportionate contributions of tissue layers to the whole organ. For example, the normal intestine has a large amount of luminal epithelium, while the early rudiment of the regenerating intestine lacks completely this tissue layer. Thus, a gene preferentially expressed in luminal epithelial cells will then appear as underexpressed in a RNAseq comparison. To minimize this problem, we are now comparing early regenerating intestine to the normal mesentery, since most of the tissue component of the early regenerating intestine is the mesentery that remains from the eviscerated intestine. By doing so, we have (1) identified genes that appeared to be differentially expressed between the normal and regenerating intestine but are not differentially expressed between the normal mesentery and the regenerating intestine, suggesting that these are genes that are preferentially expressed in the mesentery, (2) reduced the number of differentially expressed genes, and (3) obtained a listing of genes that appear to be highly expressed from very early in the mesentery that will give rise to the intestine (Garcia-Arraras et al. unpublished results).
Two recent developments will also serve to advance future studies in intestinal regeneration using holothurians: first, the establishment of in vitro culture systems for isolated cells and organ explants of holothurians (Bello et al. 2015), and second, the increasing availability of echinoderm genomes and gene sequences (Long et al. 2016). In fact, the first holothurian genome (of A. japonicus) has recently been published (Zhang et al. 2017). In this study, investigators identified a specific tandem-duplicated prostatic secretory protein of 94 amino acids (PSP94)-like gene family and proposed that the genes in this family are somewhat involved in the remarkable regenerative capacities of the holothurians.
2.3.5 Gene Expression Patterns
Several gene products have been localized in the regenerating intestine: proteins using immunohistochemistry and mRNA by in situ hybridization. The expression of these gene products provides important information on the cellular events and the cellular changes that are underpinning the regenerative process. Among the gene products that have been identified are survivin, mortalin, and translationally controlled tumor protein (TCTP) (Mashanov et al. 2010b, 2012). These genes, associated with processes of cell proliferation and cell death, are expressed abundantly in the mesothelium of the growing intestinal rudiment at stages of regeneration that correlate with the peak of cell proliferation and apoptosis. In situ hybridization signals of the genes which encode soluble signaling proteins (WNT9 and BMP1/TLL) are also observed in the mesothelium of the intestinal rudiment and in the mesothelium of the mesentery adjacent to the growing rudiment during the early stages of regeneration. Although each gene has a particular temporal and spatial expression pattern, they all are highly expressed in the rudiment mesothelium at 7 dpe (not necessarily in the same cell types).
We have also identified the expression of the holothurian orthologues of the four so-called Yamanaka factors, Myc, SoxB1, Klf1/2/4, and Oct1/2/11. These are transcription factors that can induce pluripotency when expressed in mammalian somatic cells. In the early regenerating mesentery (2 dpe), only Myc is extensively expressed. Klf1/2/4 and Oct1/2/11 are only expressed in scattered cells, while no SoxB1 expression is observed. At later times (7 dpe) the expressions of Klf1/2/4 and Oct1/2/11 increase in the mesothelium, but SoxB1 expression remains low or undetectable.
These studies provide evidence that the gene expression profile of the mesothelial cells within the growing intestinal rudiment differs significantly from the mesothelium of the normal mesentery. This correlates with what is observed in terms of tissue organization, where the mesothelium in the normal mesentery is composed of externally localized coelomic epithelium with an underlying muscle layer and with scattered neuronal cells, all organized above a basal lamina, while during regeneration this mesothelium becomes a simple epithelium of specialized cells without any muscle layer. This fits with our working hypothesis that as the mesentery cells dedifferentiate and migrate into the growing rudiment, they also undergo partial reprogramming, expressing genes that are associated with embryological and oncological processes. Thus, the mesothelial cells in the rudiment can be considered a regenerative epithelium with very distinct characteristics.
2.3.6 Functional Studies
We have used pharmacological tools to determine the functional role of gene products. In this respect, the focus has been to expose animals to available modulators (both inhibitors and/or activators) known to modify the activity of a protein or signaling pathway. These drugs are usually injected intracoelomically, during the regeneration period, and the outcome, in terms of rudiment growth or other parameters, is determined. Thus, the effect of modulators on cell division, cell death, cell dedifferentiation, and ECM remodeling can be determined. Inhibitors of proteinase activity, both MMPs (Quinones et al. 2002) and the proteasome degradation pathway (Pasten et al. 2012b), inhibit the regenerative process, causing a delay in the formation of the intestinal rudiment, determined by a smaller rudiment being formed. More recent experiments have shown a role of WNT in the early stages of regeneration, where injections of EGCG, a WNT pathway inhibitor, cause a reduction in the size of the regenerating rudiment, while LiCl, a WNT pathway activator, causes an increase in the rudiment size (Bello et al. unpublisehd results).
3 RNC Regeneration
3.1 Holothurian Nervous System
Echinoderm nervous systems follow the pentamerous body organization of the animal’s body plan. In holothurians, the nervous system is composed of an anterior circumoral nerve ring from which five radial nerve cords (RNCs) extend posteriorly. The ring and radial nerve cords comprise the central nervous system (CNS) (Fig. 13.2). Each RNC is physically divided by a thin connective tissue layer into two components, the ectoneural radial nerve cord (ERNC) and the hyponeural radial nerve cord (HRNC). This connective tissue boundary contains several short neural bridges that allow the passage of neuroepithelia and fibers from one component to the other (Mashanov et al. 2006). This physical subdivision also correlates with a functional difference for each component. The ectoneural component of the RNC innervates the body wall, podia, and viscera. On the other hand, the hyponeural component of the RNC innervates the longitudinal (LM) and circular muscles (CM). This gives the HRNC a majorly motor function, while the ERNC has both sensory and motor functions. Peripheral nerves that arise from both the hyponeural and the ectoneural components connect the CNS to other organs and structures. These peripheral nerves, plus the nervous component associated with different organs, comprise the peripheral nervous system (PNS) (for review see Mashanov et al. 2016). Among the PNS components that have been best studied are the podial nerves (Diaz-Balzac et al. 2010a), the enteric nervous system (Diaz-Balzac et al. 2007, 2014, 2016; Diaz-Miranda et al. 1995; 1996; Garcia-Arraras et al. 1991a, b, 2001; Garcia-Arraras and Viruet 1993), and the mutable connective tissue plexus (Diaz-Balzac et al. 2007, 2012, 2016).
Holothurian nervous system and stages of the regenerating RNC. (a) Diagram of a longitudinal section through the echinoderm radial nerve cord and body wall demonstrating its basic organization. Cross section through the stages of regeneration of the radial nerve cord and body wall at (b) 3 dpe, (c) 7 dpe, (d) 14 dpe, (e) 21 dpe, and (f) 28 dpe. DPI days post-injury, ERNC ectoneural radial nerve cord, HRNC hyponeural radial nerve cord, RNC radial nerve cord
The RNCs are ganglionated nerves, meaning that they have both neuronal bodies and fibers within them. They present a similar organization throughout the CNS, where the neuronal somata are found in the outer borders of the RNC, while the interior of the structure is a neuropil made by the fibers originating from the neurons. Radial glia, with elongations that extend from basal to apical ends, provide mechanical support. The glial bodies are interspersed among the neuronal soma. The nerve ring is itself a sort of ganglionated nerve, although only representing the ectoneural component. The neurons appear to be homogeneously distributed along the CNS components; there are neither ganglia nor large accumulations of neuronal bodies, although the nerve ring appears to have a slightly larger proportion of neurons than the RNCs (Mashanov et al. 2006, 2009, 2010a). The neurons express a wide range of neurotransmitters, from classical neurotransmitters, such as acetylcholine, catecholamines, and GABA (Diaz-Balzac et al. 2014, 2016), to neuropeptides such as GFSKLFYaide, a neuropeptide within the SALMFamide neuropeptide family (Diaz-Miranda et al. 1992; Elphick et al. 1991; Diaz-Miranda and Garcia-Arraras 1995). Within the neuropil, evidence for the presence of neural tracts, analogous to those in the vertebrate NS, has recently been provided (Diaz-Balzac et al. 2016).
3.2 Radial Nerve Cord Transection
The methodology used to transect the RNC of two sea cucumber species differs as a reflection of their regenerative potential. Specimens of E. fraudatrix can be transected by cutting the RNC with scissors from the external part of the body (Mashanov et al. 2008). The position of the RNC can be determined by the rows of podial feet that lie external to the RNC. Once lesioned, the animals contract the body wall, closing the injury site and proceed to heal the injured area.
Specimens of H. glaberrima, in contrast, are not able to heal a wound that traverses the animal body wall and exposes the coelomic space to the outside environment. Therefore, in this species, a more complex method needs to be used to transect the RNC. Animals are eviscerated and then anesthetized. Once the animals become flaccid, the body wall is pushed from the outside through the cloaca, using a glass rod. This partial eversion of the body wall exposes the coelomic side of the body wall with its clearly defined pairs of longitudinal muscles. The RNCs lie within the body wall between the longitudinal muscle pairs and can be sectioned using a razor blade taking the precaution not to cut open the body wall to the outside of the animal. Once the transection is done, the body wall is brought back through the cloaca, and the animals are left to regenerate. It is important, particularly for experiments that might last weeks, to label the body wall next to the transection with India ink or some other permanent stain, since it might be difficult to localize the transected area once the regenerative process has advanced.
3.3 Regeneration of the Nervous System
Holothurians are known to be able to regenerate most of their body parts and thus can regenerate the neural components within those body parts. Such regenerative prowess is exemplified by the sea cucumber Sclerodactyla briareus, previously known as Thyone briareus. These organisms can eviscerate their anterior portion or introvert which includes, among many other organs, their nerve ring (Garcia-Arraras and Greenberg 2001; Kille 1935). A new nerve ring is regenerated and reconnected to the RNCs in a few weeks. The original experiments describing regeneration of the anterior end of this species were done in the early part of last century. Thus, they lack the details of cellular and molecular processes that can be gained with the use of modern techniques.
More recent studies have focused on the regeneration of transected RNCs. It has been documented that the reconnection of the transected RNCs occurs without scarring a fact that contrast with the extensive scarring and lack of reconnection that takes place in mammals. This capacity has been documented in at least two species, H. glaberrima (San Miguel-Ruiz and Garcia-Arraras 2007; San Miguel-Ruiz et al. 2009) and in Eupentacta fraudatrix (Mashanov et al. 2008). This process is characterized by its timing and precision, since it only takes about a month to complete, and the final process is a RNC that is practically indistinguishable from its uninjured counterpart. The initial studies done in E. fraudatrix (Mashanov et al. 2008) and H. glaberrima (San Miguel-Ruiz et al. 2009) are complimentary to each other. The former provides a detailed anatomical description of the process, and the cellular components involved, at the electron microscope. The latter provides information on cellular events that take place during the regeneration process and in particular on neuronal cells. The H. glaberrima model has been further explored in terms of the presence and role of the glial component and most importantly of the molecular changes that underlie the regenerative response.
3.3.1 Neurodegeneration
In both studied species, following transection, the cut edges of the RNC swell out and protrude into the coelomic cavity. However, even in the face of this discontinuity in the RNC, both ectoneural and hyponeural components retain their organization. Nonetheless, signs of neurodegeneration are observed: less densely packed nerve processes, swollen or distorted nerve fibers, secondary lysosomes, and apoptotic cells. The latter are particularly abundant in the stump edges (Mashanov et al. 2013). In addition, spherule-containing cells, which are rarely observed within the RNC of normal animals, infiltrate the wounded area (San Miguel-Ruiz et al. 2009).
3.3.2 Initial Growth
The initial steps in regeneration are characterized by two interconnected events, the accumulation of ECM in the wound edges and the formation of an elongation from the ectoneural portion of the RNC. The exposed RNC is slowly covered by the new ECM and by the coelomic epithelium, although it is still separated by the injury gap. It is not clear which cell types are responsible for the deposition of the new ECM or what is the component of the new connective tissue. Nonetheless, transcriptomic analyses have shown that genes responsible for ECM molecules comprise some of the most upregulated genes during the early process of RNC regeneration (Mashanov et al. 2014b).
The origin and formation of the elongations that extend from the ectoneural region of the RNC of both stumps have been better studied. These are the result of an active process of cell dedifferentiation that originates at the RNC stump and eventually extends more distally from the injury site. Most of the dedifferentiating cells are the radial glia, although additional dedifferentiation of other cell types (i.e., neurons) has not been excluded. The dedifferentiation process has been described in detailed using electron microscopy and immunohistochemical markers. In brief, the cells remain within the adluminal region of both ectoneural and hyponeural component and remain attached to each other by intercellular junctions. The glial cytoskeleton undergoes radical changes, where the intermediate filaments are fragmented and either broken down internally or phagocytized by the adjacent cells. These dedifferentiated glial cells make up most of the tubular outgrowth from the ectoneural component that extends into the newly formed ECM to bridge the injury gap and reconnect with ectoneural extension originating from the opposite stump. The migrating dedifferentiated glial cells at the tip of the extension are closely followed by the growing neuronal fiber possibly originating from neurons whose fibers have been severed by the transection.
Cell proliferation which plays a minor role in the initial healing process of the nerve stump increases during this stage. Dividing cells are observed in the area that is extending from the ectoneural tissue as well as in adjacent areas. The dedifferentiated glial cells are thought to be the main cells undergoing proliferation.
It is important to state that there are no clear delimitations between stages. Some cell events are continuous but can change either temporally and/or spatially at different stages. For example, signs of neurodegeneration continue to be found even as regenerative processes begin. These neurodegenerative events spread from the areas close to the cut stumps to more distal areas. In this scenario, cellular apoptotic cells are observed from early regeneration near the exposed stumps, possibly in injured cells that were too damaged to survive, and then increase in number in more distal areas at later stages of regeneration. It is thought that the initial apoptosis is a direct effect of the lesion-caused damage, while the later apoptotic event might be due to remodeling of the cellular architecture of the RNC.
3.3.3 Reconnection
Eventually, as the tubular growths from both nerve stumps elongate and meet, they form the initial bridge joining the two RCM through the injury gap. This is probably the most complex step given the difficulty of reconnecting two nerve stumps to recreate a RNC without creating a scar or abnormal connection. This process required not only a complex signaling of cellular factors but also of ECM and interactions between the neurons, glia, and their surrounding tissues.
At this stage three different but contiguous areas can be found: first, the space within the injury gap which is mainly formed by dedifferentiated glial cells and the newly regrowth of neuronal fibers; adjacent to this is the area undergoing dedifferentiation, which can extend to about 500μm from the injured stumps; finally, the area of the RNC that does not undergo any visible change in its structure or cell composition following the injury. This spatial order reflects the temporal process that is taking place for the formation of the new RNC.
It is during this stage that the peak of cell proliferation takes place, being the injury site area the place where the highest numbers of dividing cells are observed. Cellular proliferation is also documented in the adjacent area that is undergoing dedifferentiation but rarely in areas of the RNC distal to the injury site. Cellular proliferation takes place in both ectoneural and hyponeural RNC subdivisions.
3.3.4 Differentiation and Growth
The next step in the regenerative process is the redifferentiation of cells into the components of the normal RNC within the regenerated area that now expands the injury gap and eventually the growth of the structure to reach the size of the adjacent noninjured area. At this stage, the connective tissue band that separates the ectoneural from the hyponeural component becomes visible within the regenerated area. During this stage, all cellular events that were documented return to basal (or close to basal) levels. Thus, both cell proliferation and apoptosis are reduced, as well as the infiltration of spherule-containing cells.
Finally, with a completely reconnected RNC, the final step of regeneration occurs as the subpopulations of neurons appear in the RNC as evidenced by their specific neurochemistry. Therefore, at the end, the new RNC is identical or, at least, very similar to the original RNC in terms of the neuronal subpopulations and radial glial numbers and organization, with no scar left at the transection site.
3.3.5 Cellular Mechanisms
Neurogenesis
A key process that has been studied in the holothurian RNC regeneration model has been the process of neurogenesis. Particular effort has been focused on determining what cell type or types constitute the neuronal precursors and whether these cells are dividing. Dividing cells were documented by electron microscopy and by incorporation of BrdU (Mashanov et al. 2008; San Miguel-Ruiz et al. 2009). Moreover, co-localization of BrdU incorporation and neuronal markers expression was observed, providing strong evidence that neurons (at least a subpopulation) originate from dividing precursors. Further experiments suggest that the neuronal precursors are the dedifferentiated glial cells. Thus, the available data suggests that the radial glial cells dedifferentiate early in the regenerative process and migrate to the injury area as part of a tubular outgrowth. These dedifferentiated cells can divide either in transit or as they reach the regenerating structure. At some point, and under unknown signaling pathways, the dedifferentiated glia can differentiate into either radial glia or neurons. The mechanisms by which neurons differentiate into the many subpopulations are not known. However, an interesting finding by our group (San Miguel-Ruiz et al. 2009) is that at least a subpopulation of neuropeptide expressing neurons appears to originate from nondividing precursors.
Role of glia
As has been described above, it becomes evident that glial cells play a pivotal role in RNC regeneration. Such role has been best described by Mashanov et al. (2013), where the use of two antibodies that label glial populations (RS—Reissner substance and ERG1—a monoclonal that labels echinoderm radial glia) provides a detailed description of the changes that the glial population undergoes following RNC transection. This includes the loss of basal processes, the degradation of intermediate filaments, and the acquisition of a flattened morphology that incorporate into a tubular outgrowth that bridges the injury gap between the two stumps. These cells maintain their ERG1 immunoreactivity but decrease the expression of RS, and in those cells at the tip of the tubular outgrowth, there is a complete loss of RS expression. Glial cells eventually restore the RNC epithelial organization and reexpress both ERG1 and RS immunoreactivity. The regenerative role of the glial cells depends not only on the dedifferentiation response but also in their proliferative capacity as it has been shown that they account for most of the cells that are undergoing cell division in the regenerating RNC (Mashanov et al. 2013). BrdU birth-dating experiments have shown that glial cells give rise both to the new glia and to the neurons in the regenerated area of the RNC. Among the roles that glial cells might be taking in the regenerative response is the creation of a permissive environment for axon growth, as the tubular outgrowth composed of dedifferentiated glia precedes and is closely associated with numerous neuronal fibers in the process of reconnecting the two RNC stumps.
3.3.6 Molecular Basis
Recent genomic studies have identified the gene expression profile important for the process of RNC regeneration (Mashanov et al. 2014b). This RNAseq project produced a reference library of ~70,000 contigs out of which ~24,000 had significant BLAST similarities; most of them with the sea urchin, Strongylocentrotus purpuratus (Echinoidea, Camarodonta), predicted protein sequences. As with the gene profiling studies of intestinal regeneration, thousands of genes were found to be differentially expressed between normal and regenerating animals. Not surprisingly, the most dominant categories of genes throughout the whole process of regeneration were those involved in developmental morphogenesis. It is important to note that within the GO gene categories that were most prominent for the different regenerative stages, the most drastic changes were observed in genes involved in the synthesis and organization of the extracellular matrix (ECM) components, ECM remodeling, and cell-cell and cell-ECM interaction. When analyzed at individual regeneration phases during the growth phase, those categories related to DNA synthesis and protein translation were overrepresented, in the reconnection phase and in the differentiation phase.
Rather than focusing on single genes, this study aimed to determine functional gene groups that might be associated with neural regeneration and neurogenesis. Some of the major findings were: (1) Many of the differentially expressed genes in early regenerative stages were genes associated with oncogenesis (WNT, WNT receptors, survivin). (2) At all regeneration stages, some of the most highly overexpressed genes were those associated with ECM components and ECM-cell interactions (collagen, MMPs, TIMPs, integrins, selectins). (3) Surprisingly, most genes associated with pluripotency were not differentially expressed (Klf2, Sox2, Oct1, Lgr5, Bmi-1, Ephb2, Msi2, Sall4, Zic3, Lin28) (Mashanov et al. 2015a, 2017a). Only Myc was found to be overexpressed at all regenerative stages. (4) Most neurogenesis-related genes were already expressed in the uninjured RNC and did not show an overexpression during regeneration (Thbs4, Musashi, DCLK1, Meis2, Nurr1, NeuroD1, Rein, FoxJ1, Prox1, WNT3, NeuN). Only Sox11 and BMP-7 were upregulated, while Prom1, Isl1, and Lhx3 were downregulated. (5) Genes associated with the production of glutamate or with possible steps in glutamate excitotoxicity (GCPII, Abat, Glur1, Glur4) were downregulated during regeneration, suggesting that such downregulation might be important in the survival of neurons following injury.
3.3.7 Gene Expression Patterns
The temporal and spatial expression of gene products has also been studied during regeneration of the RNC. Immunohistochemical studies have shown that various populations of cell fibers regenerate across the injury gap. These include neuropeptide-containing neuronal fibers labeled by anti-galanin and anti-GFSKLYFa (neuropeptides) or with the antibody RN1 (now known to recognize a STARD lipid-binding protein) (Rosado-Olivieri et al. 2017; San Miguel-Ruiz et al. 2009). Similarly, neuronal cell bodies labeled with an anti-Nurr1 antibody have been shown to appear in the regenerated RNC, possibly originating from dividing precursors (San Miguel-Ruiz et al. 2009).
To determine the role of the Yamanaka factors in inducing the dedifferentiation (and possible reprogramming) of the radial glia that was observed in the RNC, their expression was probed during regeneration using in situ regeneration. These genes were found to be expressed in the apical region of both ectoneural and hyponeural components of normal and regenerating animals as well as in the glial tubular structure that is formed during regeneration. However, when compared to their expression patterns did not vary greatly during regeneration nor when compared with normal non-transected RNCs.
3.3.8 Functional Studies
Two important functional studies have been performed using the transection model of RNC regeneration in H. glaberrima. The first was aimed at determining the role of cell division versus cell dedifferentiation/migration in the regeneration of the severed RNC (Mashanov et al. 2015b). The treatment of regenerating animals with a cell proliferation inhibitor significantly reduced cell division without any observable effect on RNC regeneration. The results suggest that the dedifferentiated cells that migrate into the injury site make a much higher contribution of the cellular elements that populate the regenerated nerve and that this mechanism can restore the injury site even when cell proliferation is reduced (Mashanov et al. 2015b, 2017b).
The second functional study addresses the role of Myc in RNC regeneration (Mashanov et al. 2015c). In this study Myc expression is downregulated by electroporation of Myc dsRNA. The results of the experiment are a decrease in radial glia dedifferentiation and in cell death. Therefore, the Myc expression that has been previously documented to take place in the regenerating RNC is involved in inducing the two main processes observed early in the regeneration process, cell apoptosis and cell dedifferentiation.
While the latter experiment provides some insight into the function of one pivotal transcription factor to the regeneration process, its main value lies elsewhere. The study provides the first time that RNA interference-mediated gene knockdown is used in adult echinoderms. Therefore, the development of this technique opens the door for other gene functional studies that hitherto could not be performed until this date.
References
Bai MM (1971) Regeneration in the holothurian, Holothuria scabra Jager. Indian J Exp Biol 9(4):467–471
Bello SA, Abreu-Irizarry RJ, Garcia-Arraras JE (2015) Primary cell cultures of regenerating holothurian tissues. Methods Mol Biol 1189:283–297. https://doi.org/10.1007/978-1-4939-1164-6_19
Bertolini F (1930) Rigenerazione dell’apparato digerente nello Stichopus regalis. Pubb Staz Zool Napoli 10:439–448
Bertolini F (1932) Rigenerazione dell’apparato digerente nelle Holothuria. Pubb Staz Zool Napoli 11:432–444
Cabrera-Serrano A, Garcia-Arraras JE (2004) RGD-containing peptides inhibit intestinal regeneration in the sea cucumber Holothuria glaberrima. Dev Dyn 231(1):171–178. https://doi.org/10.1002/dvdy.20112
Candelaria AG, Murray G, File SK, Garcia-Arraras JE (2006) Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima. Cell Tissue Res 325(1):55–65. https://doi.org/10.1007/s00441-006-0170-z
Cannon JT, Kocot KM, Waits DS, Weese DA, Swalla BJ, Santos SR, Halanych KM (2014) Phylogenomic resolution of the hemichordate and echinoderm clade. Curr Biol 24(23):2827–2832. https://doi.org/10.1016/j.cub.2014.10.016
Dawbin WH (1949) Auto-evisceration and the regeneration of viscera in the holothurian Stichopus mollis (Hutton). Trans R Soc N Z 77(4):497–523
Diaz-Balzac CA, Santacana-Laffitte G, San Miguel-Ruiz JE, Tossas K, Valentin-Tirado G, Rives-Sanchez M, Mesleh A, Torres II, Garcia-Arraras JE (2007) Identification of nerve plexi in connective tissues of the sea cucumber Holothuria glaberrima by using a novel nerve-specific antibody. Biol Bull 213(1):28–42. https://doi.org/10.2307/25066616
Diaz-Balzac CA, Abreu-Arbelo JE, Garcia-Arraras JE (2010a) Neuroanatomy of the tube feet and tentacles in Holothuria glaberrima (Holothuroidea, Echinodermata). Zoomorphology 129(1):33–43. https://doi.org/10.1007/s00435-009-0098-4
Diaz-Balzac CA, Mejias W, Jimenez LB, Garcia-Arraras JE (2010b) The catecholaminergic nerve plexus of Holothuroidea. Zoomorphology 129(2):99–109. https://doi.org/10.1007/s00435-010-0103-y
Diaz-Balzac CA, Lazaro-Pena MI, Garcia-Rivera EM, Gonzalez CI, Garcia-Arraras JE (2012) Calbindin-D32k is localized to a subpopulation of neurons in the nervous system of the sea cucumber Holothuria glaberrima (Echinodermata). PLoS One 7(3):e32689. https://doi.org/10.1371/journal.pone.0032689
Diaz-Balzac CA, Vazquez-Figueroa LD, Garcia-Arraras JE (2014) Novel markers identify nervous system components of the holothurian nervous system. Invert Neurosci 14(2):113–125. https://doi.org/10.1007/s10158-014-0169-1
Diaz-Balzac CA, Lazaro-Pena MI, Vazquez-Figueroa LD, Diaz-Balzac RJ, Garcia-Arraras JE (2016) Holothurian Nervous System Diversity Revealed by Neuroanatomical Analysis. PLoS One 11(3):e0151129. https://doi.org/10.1371/journal.pone.0151129
Diaz-Miranda L, Garcia-Arraras JE (1995) Pharmacological action of the heptapeptide GFSKLYFamide in the muscle of the sea cucumber Holothuria glaberrima (Echinodermata). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 110(2):171–176
Diaz-Miranda L, Price DA, Greenberg MJ, Lee TD, Doble KE, Garcia-Arraras JE (1992) Characterization of two novel neuropeptides from the sea cucumber Holothuria glaberrima. Biol Bull 182(2):241–247. https://doi.org/10.2307/1542117
Diaz-Miranda L, Blanco RE, Garcia-Arraras JE (1995) Localization of the heptapeptide GFSKLYFamide in the sea cucumber Holothuria glaberrima (Echinodermata): a light and electron microscopic study. J Comp Neurol 352(4):626–640. https://doi.org/10.1002/cne.903520410
Diaz-Miranda L, Pardo-Reoyo CF, Martinez R, Garcia-Arraras JE (1996) Galanin-like immunoreactivity in the sea cucumber Holothuria glaberrima. Cell Tissue Res 286(3):385–391
Elphick MR, Reeve JR Jr, Burke RD, Thorndyke MC (1991) Isolation of the neuropeptide SALMFamide-1 from starfish using a new antiserum. Peptides 12(3):455–459
Emson RH, Wilkie IC (1980) Fission and autotomy in echinoderms. Oceanogr Mar Biol Annu Rev 18:155–250
Garcia-Arraras JE, Dolmatov IY (2010) Echinoderms: potential model systems for studies on muscle regeneration. Curr Pharm Des 16(8):942–955
Garcia-Arraras JE, Greenberg MJ (2001) Visceral regeneration in holothurians. Microsc Res Tech 55(6):438–451. https://doi.org/10.1002/jemt.1189
Garcia-Arraras JE, Viruet E (1993) Enteric nerve fibers of holothurians are recognized by an antibody to acetylated alpha-tubulin. Neurosci Lett 157(2):153–156
Garcia-Arraras JE, Enamorado-Ayala I, Torres-Avillan I, Rivera V (1991a) FMRFamide-like immunoreactivity in cells and fibers of the holothurian nervous system. Neurosci Lett 132(2):199–202
Garcia-Arraras JE, Torres-Avillan I, Ortiz-Miranda S (1991b) Cells in the intestinal system of holothurians (Echinodermata) express cholecystokinin-like immunoreactivity. Gen Comp Endocrinol 83(2):233–242
Garcia-Arraras JE, Estrada-Rodgers L, Santiago R, Torres II, Diaz-Miranda L, Torres-Avillan I (1998) Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea:Echinodermata). J Exp Zool 281(4):288–304
Garcia-Arraras JE, Rojas-Soto M, Jimenez LB, Diaz-Miranda L (2001) The enteric nervous system of echinoderms: unexpected complexity revealed by neurochemical analysis. J Exp Biol 204(Pt 5):865–873
Garcia-Arraras JE, Schenk C, Rodrigues-Ramirez R, Torres II, Valentin G, Candelaria AG (2006) Spherulocytes in the echinoderm Holothuria glaberrima and their involvement in intestinal regeneration. Dev Dyn 235(12):3259–3267. https://doi.org/10.1002/dvdy.20983
Garcia-Arraras JE, Valentin-Tirado G, Flores JE, Rosa RJ, Rivera-Cruz A, San Miguel-Ruiz JE, Tossas K (2011) Cell dedifferentiation and epithelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Dev Biol 11:61. https://doi.org/10.1186/1471-213X-11-61
Hernandez-Pasos J, Valentin-Tirado G, Garcia-Arraras JE (2017) Melanotransferrin: new homolog genes and their differential expression during intestinal regeneration in the sea cucumber Holothuria glaberrima. J Exp Zool B Mol Dev Evol 328(3):259–274. https://doi.org/10.1002/jez.b.22731
Holland LZ (2015) Evolution of basal deuterostome nervous systems. J Exp Biol 218(Pt 4):637–645. https://doi.org/10.1242/jeb.109108
Hyman LH (1955) The invertebrates: echinodermata. McGraw-Hill, New York
Kamenev YO, Dolmatov IY (2015) Posterior regeneration following fission in the holothurian Cladolabes schmeltzii (Dendrochirotida: Holothuroidea). Microsc Res Tech 78(7):540–552. https://doi.org/10.1002/jemt.22507
Kamenev YO, Dolmatov IY (2017) Anterior regeneration after fission in the holothurian Cladolabes schmeltzii (Dendrochirotida: Holothuroidea). Microsc Res Tech 80(2):183–194. https://doi.org/10.1002/jemt.22786
Kamenev YO, Dolmatov IY, Frolova LT, Khang NA (2013) The morphology of the digestive tract and respiratory organs of the holothurian Cladolabes schmeltzii (Holothuroidea, Dendrochirotida). Tissue Cell 45(2):126–139. https://doi.org/10.1016/j.tice.2012.10.002
Kille FR (1935) Regeneration in Thyone briareus lesueur following induced autotomy. Biol Bull 69(1):82–108. https://doi.org/10.2307/1537360
Leibson NL (1992) Regeneration of digestive tube in holothurians Stichopus japonicus and Eupentacta fraudatrix. Monogr Dev Biol 23:51–61
Long KA, Nossa CW, Sewell MA, Putnam NH, Ryan JF (2016) Low coverage sequencing of three echinoderm genomes: the brittle star Ophionereis fasciata, the sea star Patiriella regularis, and the sea cucumber Australostichopus mollis. Gigascience 5:20. https://doi.org/10.1186/s13742-016-0125-6
Mashanov VS, Garcia-Arraras JE (2011) Gut regeneration in holothurians: a snapshot of recent developments. Biol Bull 221(1):93–109. https://doi.org/10.1086/BBLv221n1p93
Mashanov VS, Dolmatov IY, Heinzeller T (2005) Transdifferentiation in holothurian gut regeneration. Biol Bull 209(3):184–193. https://doi.org/10.2307/3593108
Mashanov VS, Zueva OR, Heinzeller T, Dolmatov IY (2006) Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology 125:27–38
Mashanov VS, Zueva OR, Heinzeller T (2008) Regeneration of the radial nerve cord in a holothurian: a promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue Cell 40(5):351–372. https://doi.org/10.1016/j.tice.2008.03.004
Mashanov VS, Zueva OR, Heinzeller T, Aschauer B, Naumann WW, Grondona JM, Cifuentes M, Garcia-Arraras JE (2009) The central nervous system of sea cucumbers (Echinodermata: Holothuroidea) shows positive immunostaining for a chordate glial secretion. Front Zool 6:11. https://doi.org/10.1186/1742-9994-6-11
Mashanov VS, Zueva OR, Garcia-Arraras JE (2010a) Organization of glial cells in the adult sea cucumber central nervous system. Glia 58(13):1581–1593. https://doi.org/10.1002/glia.21031
Mashanov VS, Zueva OR, Rojas-Catagena C, Garcia-Arraras JE (2010b) Visceral regeneration in a sea cucumber involves extensive expression of survivin and mortalin homologs in the mesothelium. BMC Dev Biol 10:117. https://doi.org/10.1186/1471-213X-10-117
Mashanov VS, Zueva OR, Garcia-Arraras JE (2012) Expression of Wnt9, TCTP, and Bmp1/Tll in sea cucumber visceral regeneration. Gene Expr Patterns 12(1–2):24–35. https://doi.org/10.1016/j.gep.2011.10.003
Mashanov VS, Zueva OR, Garcia-Arraras JE (2013) Radial glial cells play a key role in echinoderm neural regeneration. BMC Biol 11:49. https://doi.org/10.1186/1741-7007-11-49
Mashanov VS, Zueva O, Garcia-Arraras JE (2014a) Postembryonic organogenesis of the digestive tube: why does it occur in worms and sea cucumbers but fail in humans? Curr Top Dev Biol 108:185–216. https://doi.org/10.1016/B978-0-12-391498-9.00006-1
Mashanov VS, Zueva OR, Garcia-Arraras JE (2014b) Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC Genomics 15:357. https://doi.org/10.1186/1471-2164-15-357
Mashanov VS, Zueva OR, Garcia-Arraras JE (2015a) Expression of pluripotency factors in echinoderm regeneration. Cell Tissue Res 359(2):521–536. https://doi.org/10.1007/s00441-014-2040-4
Mashanov VS, Zueva OR, Garcia-Arraras JE (2015b) Heterogeneous generation of new cells in the adult echinoderm nervous system. Front Neuroanat 9:123. https://doi.org/10.3389/fnana.2015.00123
Mashanov VS, Zueva OR, Garcia-Arraras JE (2015c) Myc regulates programmed cell death and radial glia dedifferentiation after neural injury in an echinoderm. BMC Dev Biol 15:24. https://doi.org/10.1186/s12861-015-0071-z
Mashanov V, Zueva O, Rubilar T, Epherra L, García-Arrarás JE (2016) Echinodermata. In: Schmidt-Rhaesa A, Harzsch S, Purschke G (eds) Structure and evolution of invertebrate nervous systems. Oxford University Press, Oxford
Mashanov V, Zueva O, Mashanova D, Garcia-Arraras JE (2017a) Expression of stem cell factors in the adult sea cucumber digestive tube. Cell Tissue Res 370(3):427–440. https://doi.org/10.1007/s00441-017-2692-y
Mashanov VS, Zueva OR, Garcia-Arraras JE (2017b) Inhibition of cell proliferation does not slow down echinoderm neural regeneration. Front Zool 14:12. https://doi.org/10.1186/s12983-017-0196-y
Mendez AT, Roig-Lopez JL, Santiago P, Santiago C, Garcia-Arraras JE (2000) Identification of Hox gene sequences in the sea cucumber Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). Mar Biotechnol (NY) 2(3):231–240. https://doi.org/10.1007/s101269900027
Mosher C (1956) Observation on evisceration and visceral regeneration in the sea cucumber Actinopyga agassizi Selenka. Zoologica (NY) 41:17–26
Murray G, Garcia-Arraras JE (2004) Myogenesis during holothurian intestinal regeneration. Cell Tissue Res 318(3):515–524. https://doi.org/10.1007/s00441-004-0978-3
Odintsova NA, Dolmatov IY, Mashanov VS (2005) Regenerating holothurian tissues as a source of cells for long-term cell cultures. Mar Biol 146(5):915–921. https://doi.org/10.1007/s00227-004-1495-3
Ortiz-Pineda PA, Ramirez-Gomez F, Perez-Ortiz J, Gonzalez-Diaz S, Santiago-De Jesus F, Hernandez-Pasos J, Del Valle-Avila C, Rojas-Cartagena C, Suarez-Castillo EC, Tossas K, Mendez-Merced AT, Roig-Lopez JL, Ortiz-Zuazaga H, Garcia-Arraras JE (2009) Gene expression profiling of intestinal regeneration in the sea cucumber. BMC Genomics 10:262. https://doi.org/10.1186/1471-2164-10-262
Pasten C, Ortiz-Pineda PA, Garcia-Arraras JE (2012a) Ubiquitin-proteasome system components are upregulated during intestinal regeneration. Genesis 50(4):350–365. https://doi.org/10.1002/dvg.20803
Pasten C, Rosa R, Ortiz S, Gonzalez S, Garcia-Arraras JE (2012b) Characterization of proteolytic activities during intestinal regeneration of the sea cucumber, Holothuria glaberrima. Int J Dev Biol 56(9):681–691. https://doi.org/10.1387/ijdb.113473cp
Pearse AS (1909) Autotomy in holothurians. Biol Bull 18:42–49
Perseke M, Golombek A, Schlegel M, Struck TH (2013) The impact of mitochondrial genome analyses on the understanding of deuterostome phylogeny. Mol Phylogenet Evol 66(3):898–905. https://doi.org/10.1016/j.ympev.2012.11.019
Quinones JL, Rosa R, Ruiz DL, Garcia-Arraras JE (2002) Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev Biol 250(1):181–197
Roig-López JL, Santiago P, Jiménez B, Santiago C, García-Arrarás JE (2000) Strategies to identify differentially expressed genes during regeneration. In: Barker M (ed) Echinoderms 2000. Swelts & Zeitlinger, Lisse, pp 49–54
Rojas-Cartagena C, Ortiz-Pineda P, Ramirez-Gomez F, Suarez-Castillo EC, Matos-Cruz V, Rodriguez C, Ortiz-Zuazaga H, Garcia-Arraras JE (2007) Distinct profiles of expressed sequence tags during intestinal regeneration in the sea cucumber Holothuria glaberrima. Physiol Genomics 31(2):203–215. https://doi.org/10.1152/physiolgenomics.00228.2006
Rosado-Olivieri EA, Ramos-Ortiz GA, Hernandez-Pasos J, Diaz-Balzac CA, Vazquez-Rosa E, Valentin-Tirado G, Vega IE, Garcia-Arraras JE (2017) A START-domain-containing protein is a novel marker of nervous system components of the sea cucumber Holothuria glaberrima. Comp Biochem Physiol B Biochem Mol Biol 214:57–65. https://doi.org/10.1016/j.cbpb.2017.08.004
San Miguel-Ruiz JE, Garcia-Arraras JE (2007) Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev Biol 7:115. https://doi.org/10.1186/1471-213X-7-115
San Miguel-Ruiz JE, Maldonado-Soto AR, Garcia-Arraras JE (2009) Regeneration of the radial nerve cord in the sea cucumber Holothuria glaberrima. BMC Dev Biol 9:3. https://doi.org/10.1186/1471-213X-9-3
Santiago P, Roig-Lopez JL, Santiago C, Garcia-Arraras JE (2000) Serum amyloid A protein in an echinoderm: its primary structure and expression during intestinal regeneration in the sea cucumber Holothuria glaberrima. J Exp Zool 288(4):335–344. https://doi.org/10.1002/1097-010X(20001215)288:4<335::AID-JEZ6>3.0.CO;2-1
Satoh N, Rokhsar D, Rokhsar D, Nishikawa T (2014) Chordate evolution and the three-phylum system. Proc Biol Sci 281(1794):20141729. https://doi.org/10.1098/rspb.2014.1729
Shukalyuk AI, Dolmatov IY (2001) Regeneration of the digestive tube in the holothurian Apostichopus japonicus after evisceration. Russ J Mar Biol 27(3):168–173. https://doi.org/10.1023/A:1016717502616
Smith GN Jr (1971a) Regeneration in the sea cucumber Leptosynapta. I. The process of regeneration. J Exp Zool 177(3):319–329. https://doi.org/10.1002/jez.1401770306
Smith GN Jr (1971b) Regeneration in the sea cucumber Leptosynapta. II. The regenerative capacity. J Exp Zool 177(3):331–342. https://doi.org/10.1002/jez.1401770307
Suarez-Castillo EC, Garcia-Arraras JE (2007) Molecular evolution of the ependymin protein family: a necessary update. BMC Evol Biol 7:23. https://doi.org/10.1186/1471-2148-7-23
Suarez-Castillo EC, Medina-Ortiz WE, Roig-Lopez JL, Garcia-Arraras JE (2004) Ependymin, a gene involved in regeneration and neuroplasticity in vertebrates, is overexpressed during regeneration in the echinoderm Holothuria glaberrima. Gene 334:133–143. https://doi.org/10.1016/j.gene.2004.03.023
Sun L, Chen M, Yang H, Wang T, Liu B, Shu C, Gardiner DM (2011) Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comp Biochem Physiol Part D Genomics Proteomics 6(2):195–205. https://doi.org/10.1016/j.cbd.2011.03.002
Sun L, Yang H, Chen M, Ma D, Lin C (2013) RNA-Seq reveals dynamic changes of gene expression in key stages of intestine regeneration in the sea cucumber Apostichopus japonicus [corrected]. PLoS One 8(8):e69441. https://doi.org/10.1371/journal.pone.0069441
Tossas K, Qi-Huang S, Cuyar E, Garcia-Arraras JE (2014) Temporal and spatial analysis of enteric nervous system regeneration in the sea cucumber Holothuria glaberrima. Regeneration (Oxf) 1(3):10–26. https://doi.org/10.1002/reg2.15
Tracey DJ (1972) Evisceration and regeneration in Thyone okeni (Bell, 1884). Proc Linnean Soc N S W 97:72–81
VandenSpiegel D, Jangoux M, Flammang P (2000) Maintaining the line of defense: regeneration of Cuvierian tubules in the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea). Biol Bull 198(1):34–49. https://doi.org/10.2307/1542802
Vazquez-Velez GE, Rodriguez-Molina JF, Quinones-Frias MC, Pagan M, Garcia-Arraras JE (2016) A proteoglycan-like molecule offers insights into ground substance changes during holothurian intestinal regeneration. J Histochem Cytochem 64(6):381–393. https://doi.org/10.1369/0022155416645781
Wilkie IC (2002) Is muscle involved in the mechanical adaptability of echinoderm mutable collagenous tissue? J Exp Biol 205(Pt 2):159–165
Zhang X, Sun L, Yuan J, Sun Y, Gao Y, Zhang L, Li S, Dai H, Hamel JF, Liu C, Yu Y, Liu S, Lin W, Guo K, Jin S, Xu P, Storey KB, Huan P, Zhang T, Zhou Y, Zhang J, Lin C, Li X, Xing L, Huo D, Sun M, Wang L, Mercier A, Li F, Yang H, Xiang J (2017) The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol 15(10):e2003790. https://doi.org/10.1371/journal.pbio.2003790
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
García-Arrarás, J.E., Lázaro-Peña, M.I., Díaz-Balzac, C.A. (2018). Holothurians as a Model System to Study Regeneration. In: Kloc, M., Kubiak, J. (eds) Marine Organisms as Model Systems in Biology and Medicine. Results and Problems in Cell Differentiation, vol 65. Springer, Cham. https://doi.org/10.1007/978-3-319-92486-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-92486-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92485-4
Online ISBN: 978-3-319-92486-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)