Abstract
Pomalidomide (originally CC-4047 or 3-amino-thalidomide) is a derivative of thalidomide that is antiangiogenic and also acts as immunomodulatory. Pomalidomide, the recent immunomodulatory agent (IMiD), has shown substantial in vitro antiproliferative and proapoptotic effects. In vivo studies have suggested limited cross-resistance between lenalidomide and pomalidomide. Moreover, pomalidomide achieved very convincing responses in relapsed and refractory multiple myeloma (RRMM) patients, including those, who are refractory to both lenalidomide and bortezomib. Since pomalidomide plus low-dose dexamethasone has shown better responses, progression-free survival (PFS) and overall survival (OS) than high-dose dexamethasone or pomalidomide alone, subsequent trials have pursued or are still investigating pomalidomide triplet combinations, using cyclophosphamide or other novel agents, such as proteasome inhibitors (PI: bortezomib, carfilzomib) or antibodies, like elotuzumab or daratumumab. Pomalidomide has also been assessed in AL amyloidosis, MPNs (myelofibrosis [MF]), Waldenstrom’s macroglobulinemia, solid tumors (sarcoma, lung cancer), or HIV, and—for AL amyloidosis and MF—has already been proven to be remarkably active. Due to its potency, pomalidomide was approved for RRMM by the US Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) in 2013 and for drug combination with low-dose dexamethasone in 2015. In June 2017, the FDA further expanded approval for pomalidomide in combination with daratumumab and low-dose dexamethasone for patients with RRMM.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The accelerated approval in 2013 for the treatment of patients with RRMM, who had received at least two prior therapies, including lenalidomide and bortezomib, and had demonstrated disease progression on their last antimyeloma treatment, was based on the results of the CC-4047-MM-002 trial, a multicenter, randomized, open-label study in 221 patients with RRMM, who had previously received lenalidomide and bortezomib, but were refractory to their last myeloma treatment (Richardson et al. 2009). The treatment arms were pomalidomide alone or pomalidomide plus low-dose dexamethasone. The efficacy results demonstrated an overall response rate (ORR) of 7% in patients treated with pomalidomide alone as compared to 29% in those treated with pomalidomide plus low-dose dexamethasone. The median response duration was not evaluable (rather short) in the pomalidomide monotherapy arm versus 7.4 months in the pomalidomide plus low-dose dexamethasone arm. As MM is a so far incurable disease with an unfavorable clinical outcome under conventional chemotherapy (e.g., with melphalan or bendamustin alone), the introduction of novel agents, like PIs or IMiDs, demonstrated to substantially prolong survival in MM patients. Among these novel agents, especially pomalidomide constitutes a valuable option, including high-risk and/or refractory patients, since pomalidomide combinations have proven their potential and efficacy in PI- and IMiD-refractory patients.
2 Structure and Mechanism of Action
The structurally related parent compound of pomalidomide, namely thalidomide, was discovered to inhibit angiogenesis in 1994. Pomalidomide, the latest IMiD, suggests at least incomplete cross-resistance among thalidomide or lenalidomide, and—albeit all three IMiDs have similar structures—they differ markedly in their potency and side effects (Fig. 1). Further, structure–activity studies led to the first report in 2001 (D’Amato et al. 2001), demonstrating that pomalidomide was able to directly inhibit the tumor cell and vascular compartment of MM. Compared with thalidomide and lenalidomide, pomalidomide has stronger direct antiproliferative effects on myeloma tumor cells. Moreover, IMiDs have shown to have a pleiotropic mechanism of action: antiangiogenetic, anti-inflammatory and immunomodulatory activity on T cells, natural killer (NK) cells, monocytes (Mitsiades and Chen-Kiang 2013; Görgün et al. 2010; Gandhi et al. 2014), and effects induced on the bone marrow (BM) microenvironment (BMM) and cell proliferation (Fig. 2). In addition and like other drugs in this group, pomalidomide can decrease vascular endothelial growth factor (VEGF) and basic fibroblast growth factor-2 (bFGF) levels resulting in an inhibition of angiogenesis. MM is characterized by increased BM angiogenesis. However, it is not clear, whether this inhibition of angiogenesis contributes to the overall tumor effect of IMiDs in MM (Kortüm et al. 2015).
Thalidomide, lenalidomide, and pomalidomide structures. Albeit these three IMiDs are structurally similar, they are functionally different, resulting in different potencies. Pomalidomide is the most potent IMiD with approximately 100 times the strength of thalidomide and 10 times the potency of lenalidomide (Raza et al. 2017)
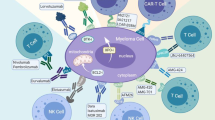
Mechanism of action of pomalidomide. Pomalidomide has a pleiotropic mechanism of action. Binding to cereblon (CRBN) is an important component required for the antimyeloma activity of immunomodulatory drugs (IMiDs). CRBN forms an E3 ubiquitin ligase complex that ubiquitinates substrates targeting them for proteolysis. IMiDs potentiate the ubiquitination and proteolysis of two specific proteins, Ikaros (IKZF1) and Aiolos (IKZF3). They are important transcription factors for B cell differentiation. Knockdown of Ikaros and Aiolos in myeloma cells induces myeloma cell cytotoxicity and downregulation of interferon regulatory factor 4 (IRF4), which also is critical for myeloma cell survival. The immunomodulatory activity of IMiDs is characterized by an enhancement of CD4+ and CD8+ T cell co-stimulation. Moreover, enhancing interleukin 2 (IL-2) and interferon (IFN ) production, the activity of natural killer cells (NK cells) is increased. Another important component of the mechanism of action of pomalidomide is the downregulation of the interaction between MM cells and the bone marrow (BM) microenvironment including BM stroma cells (BMSCs). This interaction could result, for example, in cell adhesion-mediated drug resistance. Furthermore, MM is characterized by an increased BM angiogenesis. IMiDs decrease vascular endothelial growth factor (VEGF) and basic fibroblast growth factor-2 (bFGF) levels resulting in an inhibition of angiogenesis. However, it is not clear whether this restraint of angiogenesis contributes to the overall tumor effect of IMiDs in MM. Additionally, pomalidomide inhibits proinflammatory cytokines, for example tumor necrosis factor α (TNF α), interleukin 6 (IL-6), and interleukin 12 (IL-12), increasing the levels of other interleukins with anti-inflammatory nature, such as interleukin 10 (IL-10) (Kortüm et al. 2015; Ríos-Tamayo et al. 2017; Zhu et al. 2013)
Additionally, pomalidomide inhibits proinflammatory cytokines (e.g., TNF α, IL-6, IL-12), increasing the levels of other interleukins with anti-inflammatory properties (such as IL-10) (Ríos-Tamayo et al. 2017).
The immunomodulatory activity of IMiDs is characterized by an enhancement of CD4+ and CD8+ T cell co-stimulation. Both lenalidomide and pomalidomide are more potent than thalidomide in inducing T cell proliferation and enhancing IL-2 and interferon γ (IFN γ) production (Zhu et al. 2013).
Indirect antimyeloma activity of IMiDs is postulated to be mediated by alteration of the interaction between MM cells and non-myeloma cells in the BMM, including BM stromal cells (BMSCs), osteoclasts, and immune cells. This interaction can result in cell adhesion-mediated drug resistance (CAM-DR). The crosstalk between MM cells and BMSCs can be altered by IMiDs, which may downregulate various cell surface adhesion molecules and decreases cell migration (Kortüm et al. 2015).
Another antiproliferative mode of action for thalidomide and its analogs is binding to cereblon (CRBN) (Lopez-Girona et al. 2012). CRBN forms an E3 ubiquitin ligase complex, that ubiquitinates substrates targeting them for proteolysis. IMiDs potentiate the ubiquitination and proteolysis of two specific proteins, Ikaros (IKZF1) and Aiolos (IKZF3), which are important transcription factors for B cell differentiation. Knockdown of Ikaros and Aiolos in myeloma cells induces myeloma cell cytotoxicity and leads to the downregulation of transcription factors like the interferon regulatory factor 4 (IRF4), which is also critical for myeloma cell survival (Kortüm et al. 2015). Albeit these findings, the precise molecular mechanism of action and all targets, through which IMiDs exert their antitumor effects, remain to be fully elucidated.
3 Preclinical Data
In vitro, IMiDs antagonize angiogenesis and expression of TNF-α and IL-6, while they facilitate production of IL-2 and IFN-γ and enhance T and NK cell proliferation and activity. Albeit all precise mechanisms of their action are not entirely revealed, IMiDs seem to induce downregulation of cytokine signaling (Görgün et al. 2010). Moreover, Görkün et al. demonstrated that the tumor suppressor molecule SOCS1 plays an important role in the tumor cell-immune cell-BMM interaction in MM. Importantly, lenalidomide and pomalidomide induced epigenetic modifications of SOCS1 gene in MM cells, as well as SOCS1-mediated modulation of the cytokine signaling in effector cells. Therefore, characterization of molecular mechanisms of IMiDs on immune cells in the BMM needs to be further defined to suggest that novel immune-based targeted therapies, such as the combination of IMiDs with epigenetic modulating drugs (e.g., histone deacetylase inhibitors [HDACi] and/or demethylating agents), may improve MM therapy. Given the promising clinical activity of pomalidomide even in lenalidomide-refractory MM, current efforts therefore attempt to delineate direct and epigenetic mechanisms to account for important differences (Görgün et al. 2010). Several preclinical and clinical studies have also demonstrated that threshold levels of CRBN expression are important to induce response to IMiDs (Schuster et al. 2012; Sehgal et al. 2015): CRBN depletion is initially cytotoxic to human myeloma cells, but surviving cells with stable CRBN depletion become highly resistant to both lenalidomide and pomalidomide, but not to the unrelated drugs bortezomib, dexamethasone, and/or melphalan. Acquired depletion of CRBN was described to be the primary genetic event of myeloma cell lines cultured to be sensitive or resistant to lenalidomide or pomalidomide. Gene expression changes induced by lenalidomide were substantially suppressed in the presence of CRBN depletion, demonstrating that CRBN is required for lenalidomide activity. Patients exposed and resistant to lenalidomide had lower CRBN levels in paired samples before and after therapy, suggesting that CRBN is an essential requirement for IMiD activity and possibly a useful biomarker for the clinical assessment of IMiDs’ antimyeloma efficacy. Other recent studies have confirmed that threshold levels of CRBN expression are required for response to IMiD therapy (Schuster et al. 2012, Krönke). However, Seghal et al. suggested that baseline levels of Ikaros and Aiolos protein in tumor cells did not correlate with response or survival. They showed that pomalidomide led to rapid decline of Ikaros in T and NK cells in vivo, and, further, that therapy-induced activation of CD8+ T cells correlated with clinical response. These data suggest that pomalidomide leads to strong and rapid immunomodulatory effects involving both innate and adaptive immunities, even in heavily pretreated MM, which correlates with clinical antitumor effects. Another point of interest, which needs further investigation, is the possibility of resensitization of MM cells to pomalidomide and other antimyeloma agents, e.g., with use of the CXCR4 inhibitor plerixafor or others. CXCR4 is a metabotropic chemokine receptor with potent chemotactic activity. It may act as an inductor of the BM crosstalk, which leads to disease progression and CAM-DR. Prior data suggested that CXCR4, CXCR7, and their ligand CXCL12 may act as valid targets to antagonize CAM-DR in MM, and that antimyeloma combinations with the CXCL12 antagonist NOX-A12 or the CXCR4 inhibitor plerixafor may improve therapeutic responses due to adhesion interference of MM cells to BMSCs (Waldschmidt et al. 2017).
4 Biomarkers
Acquired depletion of CRBN has been demonstrated to be the primary genetic event of myeloma cell lines cultured to be sensitive or resistant to IMiDs. Gene expression changes induced by lenalidomide were substantially suppressed in the presence of CRBN depletion, demonstrating that CRBN is vital for IMiD activity. Zhu et al. also showed that patients exposed and resistant to lenalidomide had lower CRBN levels in paired samples before and after therapy, suggesting that CRBN is a useful biomarker for the clinical assessment of IMiDs’ antimyeloma efficacy (Zhu et al. 2011). Other recent studies have confirmed that threshold levels of CRBN expression are required for response to IMiD therapy (Schuster et al. 2012).
Across six cohorts—of the phase II trials at Mayo in 345 MM patients receiving pomalidomide at doses of 2 or 4 mg/day (d)—confirmed responses of PR or better in 34%. Responses and duration of response (DOR) in those with high-risk molecular markers included (del)17p in 19 of 56 (34%): DOR 8.2 months; t(4;14): 6 of 24 (25%): DOR 4.8 months; t(14;16): 7 of 11 (64%): DOR 9.5 months and deletion 13 by cytogenetics: 13 of 37 (35%): DOR 8.2 months. In a multivariate analysis, LDH > ULN, number of prior regimens, and prior bortezomib therapy were predictive of a shorter time to progression and factors associated with a poor OS following initiation of pomalidomide therapy included ß2-microglobuline levels > 5.5 mg/l, LDH > ULN, number of prior regimens, and prior bortezomib therapy. In general and as true for almost all antimyeloma agents, number and types of prior regimens were the strongest predictors of pomalidomide response and survival, with best responses in patients who were the least heavily pretreated (Lacy 2013).
5 Clinical Data
The results of the CC-4047-MM-002 trial, a multicenter, randomized, open-label study with RRMM 221 patients, who had previously received lenalidomide and bortezomib and were refractory to their last line of treatment, led to pomalidomide’s accelerated FDA approval in 2013 (Richardson et al. 2009). The treatment arms were pomalidomide alone or pomalidomide plus low-dose dexamethasone. The efficacy results showed superior ORR with pomalidomide/low-dose dexamethasone of 29% versus 7% with pomalidomide alone, with a substantial median response duration of 7.4 months.
A phase I dose-escalation study determined the maximum tolerated dose (MTD) of pomalidomide on days 1–21 of a 28-day cycle in 38 patients with RRMM (Richardson et al. 2013). Pretreatment had been substantial with a median of 6 prior therapies, including 63% who were refractory to both lenalidomide and bortezomib. There were four dose-limiting toxicities (grade 4 neutropenia) at 5 mg/d; therefore, the MTD was specified at 4 mg/d. Among the 38 patients enrolled (including 22 with added dexamethasone), 42% achieved minimal response (MR) or better, 21% PR or better, and 3% CR. Median duration of response, PFS, and OS were 4.6, 4.6, and 18.3 months, respectively.
The subsequent multicenter, phase II randomized study assessed two different pomalidomide dose schedules [4 mg for 21 vs. 28 days (21/28 vs. 28/28)] combined with dexamethasone in 84 advanced MM patients. The median number of prior therapy lines was again substantial with 5 and the ORR was 35% (arm 21/28) and 34% (arm 28/28), thus very similar, irrespective of the number of prior lines and level of refractoriness. Median duration of response, time to disease progression, and PFS were 7.3, 5.4, and 4.6 months, respectively. At 23 months of follow-up, median OS was 14.9 months (Leleu et al. 2013). This phase II trial suggested that 4 mg pomalidomide, given on days 1–21 of a 28-day cycle and combined with dexamethasone, was efficacious, well tolerated, allowed a “1-week-IMiD-rest” period and the blood count and patient to recuperate, which therefore determined the dose and schedule of choice.
5.1 High-Risk Patients
The International Myeloma Working Group (IMWG) published a consensus guideline on the treatment of MM patients with high-risk cytogenetics: Therein, cytogenetic abnormalities such as del(17p), t(4;14), t(14;16), t(14;20), gain(1q), and nonhyperdiploidy were specified as high risk, and patients with multiple abnormalities demonstrate more dismal therapy responses, earlier disease recurrence, and decreased PFS and OS (Sonneveld et al. 2016). Of note, pomalidomide in RRMM patients with high-risk cytogenetics was assessed in the phase III MM-003 study, an associated subanalysis and several phase II and phase I/II studies.
The MM-002-study was a multicenter, randomized, open-label dose-escalation study conducted to determine the MTD, safety, and efficacy of pomalidomide–dexamethasone in patients with RRMM, who had received both bortezomib and lenalidomide. The subanalysis reported on the use of pomalidomide versus pomalidomide–dexamethasone in patients with high-risk cytogenetics (Table 1), showing favorable responses, PFS, and OS also in high-risk patients (Richardson et al. 2012). Common grade 3/4 AEs (in >10% of patients) were neutropenia, thrombocytopenia, back pain, fatigue, renal failure, urinary tract infection, and leukopenia. Grade 3/4 adverse events (AEs) were similar in high- and standard-risk patients.
The MM-003 study was a phase III, multicenter, randomized, open-label study that compared the efficacy and safety of pomalidomide with low- versus high-dose dexamethasone in patients with MM, who were refractory after more than two previous treatments, including bortezomib and lenalidomide (San Miguel et al. 2013). Dimopoulos et al. updated these results with a median follow-up of 15.4 months: Pomalidomide–dexamethasone significantly improved PFS as compared to high-dose dexamethasone alone, including high-risk patients with del(17p) or t(4;14). The median PFS in the pomalidomide–dexamethasone arm for patients with del(17p) was 4.6 months versus 1.1 months with high-dose dexamethasone and 2.8 months versus 1.9 months in patients with t(4;14). Among standard-risk patients, the median PFS with pomalidomide–dexamethasone was 4.2 months versus 2.3 months with high-dose dexamethasone. The median OS for patients with del(17p) was 12.6 months (pom–dex) versus 7.7 months (high dex) and 7.5 months versus 4.9 months in patients with t(4;14). For standard-risk patients, OS in the pom–dex arm was 14.0 months versus 9.0 months for patients with high-dose dexamethasone. However, it should be noted that 46% of high-risk patients and 64% of standard-risk patients enrolled in the high-dose dexamethasone arm subsequently received pomalidomide (Table 2); thus without this “crossover,” the observed differences would have been even more striking (Dimopoulos et al. 2015).
5.2 Patients with Renal Failure
For patients with impaired renal function or renal failure, it is always a challenge to induce a suitable therapy, which is both efficient and well tolerated. Ramasamy et al. performed a phase II study (MM-013) of pomalidomide–dexamethasone in 81 patients with RRMM with moderate or severe renal impairment (RI), including patients on dialysis, who had received ≥1 prior treatment including lenalidomide. Patients were stratified in arm A with moderate RI (estimated glomerular filtration rate, eGFR ≥ 30 to < 45 ml/min), arm B with severe RI without dialysis (eGFR < 30), and arm C with severe RI requiring dialysis (eGFR < 30). The median number of cycles was 6 (range: 1–21), ORR was 32.1% (moderate RI: 39.4%, severe RI without dialysis: 32.4%, severe RI requiring dialysis: 14.3%), and median PFS was 6.5, 4.2, and 2.4 months, respectively. The median OS was 16.4 months in patients in arm A, 11.8 months in arm B, and 5.2 months in arm C. The authors conclude that pomalidomide dosed at 4 mg on a 21/28-day schedule was a valuable therapy option and can be safely administered with low-dose dexamethasone in patients with moderate or severe RI, including those on hemodialysis (Ramasamy et al. 2015). Post hoc analysis and prospective evaluations of other clinical trials fortified this study (Siegel et al. 2012; Matous et al. 2014). Thus, pomalidomide is a suitable treatment option for patients with severe RI, even requiring dialysis. As pomalidomide can be eliminated from the blood circulation by hemodialysis, on dialysis days, patients should take their pomalidomide medication following hemodialysis (IMNOVID®: summary of product characteristics; Celgene, http://www.fachinfo.de; last revised: September 2016).
5.3 AL Amyloidosis and Other Disease Entities
Although previous studies could not show a survival advantage for patients with AL amyloidosis responding to salvage treatment with pomalidomide, Palladini et al. assessed the safety and efficacy in a phase II trial of pomalidomide–dexamethasone in 28 AL amyloidosis patients who were previously exposed to bortezomib, alkylators, and other immunomodulatory drugs. In a dose-escalation phase, three patients received 2 mg pomalidomide/d, with no dose-limiting toxicity and the remaining patients received 4 mg/d. Pomalidomide was administered continuously, and dexamethasone was given once per week at doses of 20 or 40 mg. Fifteen patients experienced grade 3/4 AEs; the most common were fluid retention and infections. Hematologic response was observed in 68% of patients (VGPR or CR in 29%), as well as a gratifying OS. Median time to response was short with 1 month. This trial confirmed that pomalidomide–dexamethasone was a rapidly active regime and may prolong survival in responding, heavily pretreated patients with AL amyloidosis (Palladini et al. 2017).
Pomalidomide is not only a relevant treatment option for MM or AL amyloidosis. There are also several clinical trials in other entities, like soft tissue sarcoma, medulloblastoma, sickle cell anemia, Waldenstrom’s macroglobulinemia, myelofibrosis, Kaposi sarcoma. In the future, these trials will hopefully elucidate, whether and to what extent pomalidomide is a profitable treatment option in these challenging to treat diseases.
5.4 Pomalidomide in Combination Schedules
The introduction of novel agents and their combination have generated major advances in MM. Nevertheless, their immediate use in first-line and subsequent therapies makes the treatment of subsequent relapses a challenge, since MM may remain incurable and patients will ultimately acquire resistance to prior agents. Once patients are no longer responsive to IMiDs and bortezomib, the prognosis is grave and new agents, respectively the approval and use of well tolerable triplet or quadruple therapies, are needed. Furthermore, there is a lack of new therapies for patients with high-risk cytogenetics and RI, for which pomalidomide is a promising option. Currently, there are 139 trials that include pomalidomide and which are registered at ClinicalTrials.gov: 103 (103/139 = 74%) of these involve MM patients (out of currently 2228 clinical trials for the treatment of MM: 103/2220 = 4.6%).
5.4.1 Pomalidomide–Proteasome Inhibitor–Dexamethasone (P-VD) Combination
The combination of pomalidomide, bortezomib, and low-dose dexamethasone (P-VD) has been evaluated in several phase I/II clinical trials for the treatment of RRMM patients. Lacy et al. reported the results from a phase I/II study evaluating the safety and efficacy of P-VD in 50 patients with RRMM. In the phase I trial involving n = 9 patients, dose level I doses of pomalidomide 4 mg on days 1–21, bortezomib 1.0 mg/m2 (1.3 mg/m2 in dose level 2) i.v. on days 1, 8, 15, and 22, and dexamethasone 40 mg on days 1, 8, 15, and 22 in 28-day cycles were given. In the phase II part, 41 patients were treated. The median age was 66 years and 51% were female. The median number of prior treatment lines was 3, 100% had received prior lenalidomide, 68% had received prior SCT, 17% had received thalidomide, 56% alkylators, 57% bortezomib, and 25% were high risk by Mayo Stratification for Myeloma And Risk-Adapted Therapy (mSMART). Confirmed response occurred in 34/42 (81%) evaluable patients, including 3 stringent complete responses (sCR), 5 CRs, 8 VGPRs, and 18 PR. Among 11 evaluable high-risk patients, 9 (82%) achieved confirmed response. Median PFS was 17.7 months. At median follow-up of 9 months, 72% of patients were progression-free, 96% of patients were alive, and 66% had remained on study (Lacy et al. 2014). Richardson et al. presented another multicenter, open-label, randomized phase III study (MM-007; OPTIMISMM) comparing P-VD to bortezomib/low-dose dexamethasone (VD) alone in RRMM patients (EHA, June 2016), and this study has completed recruitment and is expected to confirm highly promising results with more extended treatment periods, PFS, and possibly also OS with P-VD versus VD alone (Richardson et al. 2015).
Furthermore, the combination of pomalidomide, carfilzomib, and low-dose dexamethasone (PCfzD) is evaluated in several phase I/II clinical trials for the treatment of RRMM (Bringhen et al., Jakubowiak et al., Rosenbaum et al., Shah et al.). Dosing varied for the combination in these trials, ORR for this combination ranged from 64 to 84%, and median PFS ranged from 9.2 to 16.8 months (Bringhen et al. 2016; Jakubowiak et al. 2017; Shah et al. 2015).
The results of these trials verify the benefit of new treatment combinations involving pomalidomide in triplets; therefore, the approval of P-VD and PCfzD in RRMM is being anticipated.
5.4.2 Pomalidomide–Cyclophosphamide–Dexamethasone (PCycloD) Combination
The combination of pomalidomide with cyclophosphamide and steroid (dexamethasone or prednisone) is a promising option to improve efficacy and treatment response in RRMM patients. The aim of a study performed by Baz et al. was to assess the safety and efficacy of adding oral weekly cyclophosphamide to the standard treatment pom–dex. A dose-escalation phase I study was performed to determine the recommended phase II dose of cyclophosphamide in combination with pom–dex (arm A). This was followed by a randomized, multicenter phase II study enrolling patients with lenalidomide-refractory MM. Patients were randomized (1:1) to receive pomalidomide 4 mg on days 1–21 of a 28-day cycle in combination with weekly dexamethasone 40 mg (20 mg, if patients were > 75 years or unable to tolerate 40 mg weekly) (arm B) or pomalidomide, cyclophosphamide, and dexamethasone (PCycloD), using cyclophosphamide with 400 mg orally on days 1, 8, and 15 (arm C). The primary endpoint was ORR. Eighty patients were enrolled (10 in the phase I part and 70 randomized in the phase II part: 36 in arm B and 34 in arm C). The ORR in arm B and C was 38.9% (95% CI: 23–54.8%) versus 64.7% (95% CI: 48.6–80.8%), and the median PFS was 4.4 (95% CI, 2.3–5.7) and 9.5 months (95% CI, 4.6–14), respectively. Toxicity was predominantly hematologic, but not statistically higher in arm C. The combination of PCycloD results in substantially improved ORR and PFS as compared to pom–dex alone in patients with lenalidomide-refractory MM and thus should be considered to enhance responses and prolong progression (Baz et al. 2016).
5.4.3 Pomalidomide–Antibody–Dexamethasone Combination
In June 2017, the FDA approved the anti-CD38 antibody (Ab) daratumumab in combination with pomalidomide and dexamethasone for the treatment of MM patients, who had received at least two prior therapies, including lenalidomide and a PI. Relevant for the approval was the trial of Chari et al. in which daratumumab–pom–dex (Dara-PD) was evaluated in RRMM patients with two or more prior lines of therapy, who were refractory to their last treatment. Patients received daratumumab 16 mg/kg at the recommended dosing schedule, pomalidomide 4 mg daily for 21 days of each 28-day cycles, and dexamethasone 40 mg weekly. Safety was the primary endpoint. ORR and minimal residual disease (MRD) by next-generation sequencing were secondary endpoints. Patients (n = 103) received a median of four (range: 1–13) prior therapies; 76% received three or more prior therapies. The safety profile of Dara-PD was similar to that of pom–dex alone, with the exception of daratumumab-specific infusion-related reactions (IRR: 50%) and a higher incidence of neutropenia, although without an increase in infections. The ORR was 60% and was generally consistent across subgroups (58% in double-refractory patients). Among patients with a CR or better, 29% were MRD negative at a threshold of 10−5. At a median follow-up of 13.1 months, the median PFS was 8.8 (95% CI: 4.6–15.4) months and median OS was 17.5 (95% CI: 13.3-not reached) months. The estimated 12-month survival rate was 66% (95% CI: 55.6–74.8).
Aside from increased neutropenia, the safety profile of Dara-PD was consistent with that of the individual therapies. Deep, durable responses were observed in heavily pretreated patients (Chari et al. 2017).
Likewise, there are several trials ongoing proving the value of adding antibodies like elotuzumab and nivolumab to pom–dex in triplets or in quadruple combinations (pom–dex plus PI and Ab or pom–dex plus two abs). These combinations might further enhance responses, PFS, and OS, enrich the options in the treatment of RRMM patients, and enhance the possibilities of patient-individualized therapy approaches.
6 Toxicity
The most common side effects of pomalidomide reported in clinical trials have been fatigue and asthenia, neutropenia, anemia, constipation, nausea, diarrhea, dyspnea, upper respiratory tract infections, back pain, and pyrexia. In the comparative analysis of six sequential phase II trials at Mayo in 345 patients receiving pomalidomide at doses of 2 or 4 mg/d, most common toxicities (grade ≥ 3) were neutropenia (31%), anemia (16%), thrombocytopenia (12%), pneumonia (8%), and fatigue (8%). Venous thromboembolism (VTE) was seen in ten patients (3%; Lacy et al. 2012). Moreover, a brief review on two patients who developed pulmonary toxicity related to pomalidomide was consistent with previously published reports on pulmonary toxicity related to thalidomide and lenalidomide. It was suggested that this very rare toxicity should readily be recognized by clinicians in patients with pulmonary complaints and no identifiable infectious source and that timely withdrawal of the medication leads to rapid resolution of symptoms without long-term sequelae (Geyer et al. 2011). In general, pomalidomide induces less aesthesia and neuropathy than thalidomide and is more likely to induce neutropenia than thalidomide, but this side effect is usually well manageable with dose reduction. Subsets of MM patients, who are sensitive to the myelosuppressive effect of lenalidomide and have trouble tolerating even low doses, may do well with pomalidomide, suggesting that its myelosuppressive effect is less pronounced. Skin rash which might be observed with lenalidomide (Wäsch et al. 2012) is rarely seen with pomalidomide (Lacy 2013).
Pomalidomide is approved by the FDA and EMA with a boxed warning alerting patients and health care professionals that the drug can cause embryo-fetal toxicity and VTE. Because of this embryo-fetal risk, pomalidomide is available only through a restricted distribution program called the POMALYST Risk Evaluation and Mitigation Strategy (REMS) program. Prescribers must be certified with the POMALYSTREMS program by enrolling and complying with the REMS requirements. Patients must sign a patient–physician agreement form and comply with the REMS requirements. Female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements. Males must comply with contraception requirements. Pharmacies must be certified with the POMALYSTREMS program, must only dispense to patients, who are authorized to receive pomalidomide, and comply with REMS (requirements on http://www.fda.gov).
7 Drug Interactions
CYP1A2 and CYP3A4 were identified as the most important enzymes metabolizing IMiDs. Further, pomalidomide is a substrate of p-glycoprotein (p-gp). It is not to be expected that pomalidomide causes drug interactions by inhibiting or inducing P450-isoenzymes, if it is administered simultaneously with other substrates of CYP1A2 or CYP3A4. Furthermore, the concomitant application of ketoconazole (strong CYP3A4 and p-gp inhibitor) and carbamazepine (strong CYP3A4/5 inductor) showed no significant impact on the exposition of pomalidomide. Indeed, co-administration of strong inhibitors of CYP1A2 (e.g., fluvoxamine, ciprofloxacin, or enoxacin) increases the plasma levels of pomalidomide. If concomitant treatment is unavoidable, the dose of pomalidomide should be decreased by 50%. Cigarette smoking may reduce pomalidomide exposure via CYP1A2 induction. Therefore, patients should be advised that smoking may reduce the efficacy of pomalidomide (IMNOVID®: summary of product characteristics; Celgene, http://www.fachinfo.de; last revised: September 2016).
8 Summary and Perspectives
Although new agents have significantly improved the prognosis in MM, novel therapies are constantly needed. Pomalidomide is effective and well tolerated in patients with advanced, refractory MM and potentially provides an unmet clinical need in patients with previously treated MM. The use of pomalidomide and low-dose dexamethasone, and their combination with other active agents, warrants further clinical testing. Moreover, the response in cytogenetically high-risk patients (Richardson et al. 2012) and with organ impairment, such as RI (Ramasamy et al. 2015), is currently confounded by low patient numbers and needs to be further investigated.
References
Baz RC, Martin TG 3rd, Lin HY, Zhao X, Shain KH, Cho HJ, Wolf JL, Mahindra A, Chari A, Sullivan DM, Nardelli LA, Lau K, Alsina M, Jagannath S (2016) Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood 127(21):2561–2568
Bringhen S, Magarotto V, Liberati M et al (2016) A multicenter, open label phase I/II study of carfilzomib, pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma (MM) patients [Oral]. In: Oral presented at: 58th annual meeting and exposition of the American Society of Hematology (ASH); December 3–6, 2016; San Diego, CA, USA
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, Weiss BM, Krishnan A, Lentzsch S, Comenzo R, Wang J, Nottage K, Chiu C, Khokhar NZ, Ahmadi T, Lonial S (2017) Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 130(8):974–981
D’Amato R, Lentzsch S, Anderson KC, Rogers MS (2001) Mechanism of action of thalidomide and 3-aminothalidomide in multiple myeloma. Semin Oncol 28:597–601
Dimopoulos MA, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H, Moreau P, Banos A, Oriol A, Garderet L, Cavo M, Ivanova V, Alegre A, Martinez-Lopez J, Chen C, Spencer A, Knop S, Bahlis NJ, Renner C, Yu X, Hong K, Sternas L, Jacques C, Zaki MH, San Miguel JF (2015) Cytogenetics and long-term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low-dose dexamethasone. Haematologica 100(10):1327–1333
Geyer HL, Viggiano RW, Lacy MQ, Witzig TE, Leslie KO, Mikhael JR, Stewart K (2011) Acute lung toxicity related to pomalidomide. Chest 140:529–533
Gandhi AK, Kang J, Havens CG et al (2014) Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 164(6):811–821
Görgün G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, Cirstea D, Santo L, Hu Y, Tai YT, Nahar S, Mimura N, Fabre C, Raje N, Munshi N, Richardson P, Anderson KC (2010) Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 116:3227–3237
IMNOVID®: summary of product characteristics; Celgene, http://www.fachinfo.de; last revised September 2016
Jakubowiak AJ, Rosenbaum CA, Stephens L et al (2017) Final results of phase (PH) 1/2 study of carfilzomib; pomalidomide; and dexamethasone (KPD) in patients (PTS) with relapsed/refractory multiple myeloma (RRMM): a multi-center MMRC study [Meeting Abstract]. In: Presented at: 22nd Congress of the European Hematology Association (EHA), June 22–25, 2017, Madrid, Spain. Poster#P680
Kortüm KM, Zhu YX, Shi CX, Jedlowski P, Stewart AK (2015) Cereblon binding molecules in multiple myeloma. Blood Rev 29:329–334
Lacy MQ, Kumar SK, LaPlant BR, Laumann K, Gertz MA, Hayman SR, Buadi FK, Dispenzieri A, Lust JA, Russell S, Dingli D, Zeldenrust SR, Fonseca R, Bergsagel PL, Stewart K, Roy V, Sher T, Chanan-Khan A, Reeder C, Rajkumar SV, Mikhael JR (2012) Pomalidomide plus low-dose dexamethasone (Pom/Dex) in relapsed myeloma: long term follow up and factors predicting outcome in 345 patients. Blood 120:201
Lacy MQ (2013) “IM iD” eally treating multiple myeloma. Blood 121:1926–1928
Lacy MQ, LaPlant BR, Laumann KM et al (2014) Pomalidomide, bortezomib, and dexamethasone for patients with relapsed lenalidomide refractory multiple myeloma. ASH Annual Meeting. Abstract 304. Presented December 8, 2014
Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, Mathiot C, Petillon MO, Macro M, Roussel M, Pegourie B, Kolb B, Stoppa AM, Hennache B, Bréchignac S, Meuleman N, Thielemans B, Garderet L, Royer B, Hulin C, Benboubker L, Decaux O, Escoffre-Barbe M, Michallet M, Caillot D, Fermand JP, Avet-Loiseau H, Facon T (2013) Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myélome 2009–02. Blood 121:1968–1975
Lopez-Girona A, Mendy D, Ito T et al (2012) Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26(11):2326–2335
Matous J, Siegel DS, Lonial S et al (2014) MM-008: a phase 1 trial evaluating pharmacokinetics and tolerability of pomalidomide + low-dose dexamethasone (LoDEX) in patients (Pts) with relapsed or refractory multiple myeloma and renal impairment [poster]. In: Poster presented at: 56th annual meeting and exposition of the American Society of hematology (ASH); December 6–9, 2014; San Francisco, CA, USA
Mitsiades CS, Chen-Kiang S (2013) Immunomodulation as a therapeutic strategy in the treatment of multiple myeloma. Crit Rev Oncol Hematol 88(Suppl 1):S5–S13
Palladini G, Milani P, Foli A, Basset M, Russo F, Perlini S, Merlini G (2017) A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood 129(15):2120–2123
Ramasamy K, Dimopoulos MA, Van de Donk NWCJ, et al. (2015) Safety of treatment (Tx) with pomalidomide (POM) and low-dose dexamethasone (LoDEX) in patients (Pts) with relapsed or refractory multiple myeloma (RRMM) and renal impairment (RI), including those on dialysis [meeting abstract]. In: Presented at: 57th annual meeting and exposition of the American Society of Hematology (ASH); December 5–8, 2015, Orlando FL, USA. Abstract# 374
Raza S, Safyan RA, Lentzsch S (2017) Immunomodulatory drugs (IMiDs) in multiple myeloma. Curr Cancer Drug Targets 17(9):846–857
Ríos-Tamayo R, Martín-García A, Alarcón-Payer C, Sánchez-Rodríguez D, del Valle Díaz de la Guardia AM, García Collado CG, Jiménez Morales A, Jurado Chacón M, Cabeza Barrera J (2017) Pomalidomide in the treatment of multiple myeloma: design, development and place in therapy. Drug Des Devel Ther 11:2399–2408
Richardson P, Baz R et al (2009) A phase 1/2 multi-center, randomized, open label dose escalation study to determine the maximum tolerated dose, safety and efficacy of pomalidomide alone or in combination with low-dose dexamethasone in patients with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide and bortezomib. Blood 114:301
Richardson P, Jakubowiak A, Bahlis NJ, Siegel DS, Chen CI, Chen M, Zaki M, Larkins G, Anderson K (2012) Treatment outcomes with pomalidomide (POM) in combination with low-dose dexamethasone (LoDex) in patients with relapsed and refractory multiple myeloma (RRMM) and del(17p13) and/or t(4;14) (p16;q32) cytogenetic abnormalities who have received prior therapy with lenalidomide (LEN) and bortezomib (BORT). Blood 120:4053
Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, Sullivan D, Alsina M, Schlossman R, Ghobrial IM, Doss D, Loughney N, McBride L, Bilotti E, Anand P, Nardelli L, Wear S, Larkins G, Chen M, Zaki MH, Jacques C, Anderson KC (2013) Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood 121:1961–1967
Richardson PG, Bensmaine A, Doerr T, Wang J, Zaki M (2015) MM-007: a phase 3 trial comparing the efficacy and safety of pomalidomide (POM) bortezomib (BORT) and low-dose-dexamethasone (LoDEX[PVD]) versus BORT and LoDEX (VD) in subjects with relapsed or refractory multiple myeloma (RRMM) [Poster]. In: Poster presented at: 2015 annual meeting of the American Society of Clinical Oncology (ASCO); May 29–June 2, 2015; Chicago, IL USA
San Miguel J, Weisel K, Moreau P et al (2013) Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 14(11):1055–1066
Schuster SR, Kortuem KM, Zhu YX, Braggio E, Shi C-X, Bruins L, Schmidt J, Ahmann G, Kumar SK, Rajkumar SV, Mikhael JR, Roy V, LaPlant BR, Laumann K, Barlogie B, Shaughnessy JD Jr, Fonseca R, Bergsagel L, Lacy MQ, Stewart K (2012) Cereblon expression predicts response, progression free and overall survival after pomalidomide and dexamethasone therapy in multiple myeloma. Blood 120:194
Sehgal K, Das R, Zhang L, Verma R, Deng Y, Kocoglu M, Vasquez J, Koduru S, Ren Y, Wang M, Couto S, Breider M, Hansel D, Seropian S, Cooper D, Thakurta A, Yao X, Dhodapkar KM, Dhodapkar MV (2015) Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: impact of immune activation and cereblon targets. Blood 125(26):4042–4051
Shah JJ, Stadtmauer EA, Abonour R, Cohen AD, Bensinger WI, Gasparetto C, Kaufman JL, Lentzsch S, Vogl DT, Gomes CL, Pascucci N, Smith DD, Orlowski RZ, Durie BG (2015) Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 126(20):2284–2290
Siegel DS, Richardson PG, Baz R, Chen M, Zaki M, Anderson KC (2012) Pomalidomide with low-dose dexamethasone in patients with relapsed and refractory multiple myeloma: impact of renal function on patient outcomes [Poster]. In: Poster presented at: 54th annual meeting of the American Society of Hematology (ASH); December 8–11, 2012, Atlanta, GA, USA
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle RA, Caers J, Hillengass J, San Miguel J, van de Donk NW, Einsele H, Bladé J, Durie BG, Goldschmidt H, Mateos MV, Palumbo A, Orlowski R (2016) Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 127(24):2955–2962
Waldschmidt JM, Simon A, Wider D, Müller SJ, Follo M, Ihorst G, Decker S, Lorenz J, Chatterjee M, Azab AK, Duyster J, Wäsch R, Engelhardt M (2017) CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br J Haematol 179(1):36–49
Wäsch R, Jakob T, Technau K, Finke J, Engelhardt M (2012) Stevens-Johnson/toxic epidermal necrolysis overlap syndrome following lenalidomide treatment for multiple myeloma relapse after allogeneic transplantation. Ann Hematol 91:287–289
Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK (2011) Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 118:4771–4779
Zhu YX, Kortuem KM, Stewart AK (2013) Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma 54(4):683–687
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Engelhardt, M., Ajayi, S., Reinhardt, H., Müller, S.J., Dold, S.M., Wäsch, R. (2018). Pomalidomide. In: Martens, U. (eds) Small Molecules in Hematology. Recent Results in Cancer Research, vol 212. Springer, Cham. https://doi.org/10.1007/978-3-319-91439-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-91439-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91438-1
Online ISBN: 978-3-319-91439-8
eBook Packages: MedicineMedicine (R0)