Abstract
Over the last years, targeted anti-cancer therapy with small-molecule inhibitors and antibodies moved to the forefront as a strategy to treat hematological cancers. These novel agents showed outstanding effects in treatment of patients, often irrespective of their underlying genetic features. However, evolution and selection of subclones with continuous treatment leads to disease relapse and resistance toward these novel drugs. Venetoclax (ABT-199) is a novel, orally bioavailable small-molecule inhibitor for selective targeting of B-cell lymphoma 2 (BCL2). Venetoclax is in clinical development and shows high efficacy and safety in particular in the treatment of chronic lymphocytic leukemia (CLL), but preliminarily also in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). The most important and impressive outcomes of venetoclax treatment include a rapid induction of apoptosis and drastic reduction of the tumor bulk within a few hours after administration. Venetoclax was approved by the FDA and EMA in 2016 for patients with previously treated CLL with del(17p13) and patients failing B cell receptor signaling inhibitors (EMA only), on the basis of a single-arm phase II trial demonstrating a tremendous response rate of 79% with complete remission in 20% of cases and an estimated 1-year progression-free survival of 72%. This review focuses on the mode of action, the preclinical models, and outcomes from various clinical trials with venetoclax in different hematologic cancers as well as future development.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Background: The Balance between Anti-apoptotic and Pro-apoptotic Proteins
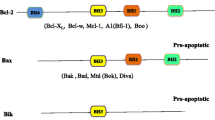
BCL2 family proteins play a major role in the regulation of cell death, and BCL2 has been the first anti-apoptotic gene discovered in 1985 (Tsujimoto et al. 1985). The BCL2 family is highly conserved and contains more than a dozen proteins that are key regulators of the mechanism of intrinsic programmed cell death. The BCL2 family is clustered into three main functional groups, the pro-survival and anti-apoptotic proteins BCL2, MCL1, BCL-XL and BCL-W (O’Connor et al. 1998; Gibson et al. 1996; Boise et al. 1993; Opferman et al. 2005; Opferman et al. 2003), the multi-BH domain pro-apoptotic proteins BAX and BAK and the pro-apoptotic BH3-only proteins BIM, tBID; BAD, PUMA; NOXA and HRK (Inohara et al. 1997; Datta et al. 2002; Oda et al. 2000; O’Connor et al. 1998; Wei et al. 2000; Korsmeyer et al. 2000) that trigger and execute the ‘suicidal’ cell death. In healthy cells, the balance between cell survival and cell death requires the dynamic binding interactions between the pro-apoptotic and anti-apoptotic proteins (Fig. 1). However, in various malignancies, the anti-apoptotic proteins are frequently overexpressed, leading to defective apoptosis (Robertson et al. 1996).
Overview of pro- and anti-apoptotic molecules. a Cell death signals trigger BID and BIM to activate BAX and BAK, which in turn initiate MOMP and lead to apoptosis. b Anti-apoptotic molecules, including BCL2, antagonize both activator and effector molecules and block the apoptotic cascade. c Cell death signals also activate sensitizer molecules, which antagonize anti-apoptotic molecules and release the block on apoptosis. This physiologic role is pharmacologically recapitulated by BH3-mimetic drugs such as venetoclax
In cancers, anti-apoptotic BCL2 is upregulated by various mechanisms. The t(14;18) chromosomal translocation which is a genetic hallmark of follicular lymphoma (a subtype of indolent non-Hodgkin lymphoma) includes juxtapositioning of BCL2 in the IGHV locus, activating BCL2 at the transcriptional level (Tsujimoto et al. 1985). Amplification of chromosome 18q21 resulting in high BCL2 levels is observed in small-cell lung cancers (SCLC) (Monni et al. 1997) and mantle cell lymphoma (MCL) (Bentz et al. 2000). In chronic lymphocytic leukemia (CLL), the most common cytogenetic abnormality is the del(13q14), the minimally deleted region of which includes the BCL2 repressors, microRNAs 15 and 16 (Cimmino et al. 2005). Moreover, hypomethylation of BCL2 in CLL also contributes to BCL2 upregulation due to epigenetic dysregulation (Hanada et al. 1993; Cahill and Rosenquist 2013). On the other hand, defects in expression of pro-apoptotic members result in a loss of the tumor suppressive function and lead to an imbalance between pro-and anti-apoptotic BCL2 family proteins. Homozygous deletions or inactivating mutations of BAX and BID (Meijerink et al. 1998; Lee et al. 2004) or defective expression of BID and PUMA due to loss of p53 function also tip the balance toward anti-apoptotic proteins (Sturm et al. 2000; Miyashita and Reed 1995).
In summary, prevention of apoptosis is one of the hallmarks of cancer cells, which in addition to sustaining survival of the malignant clone, impacts treatment outcome and progression of the disease (Hanahan and Weinberg 2011).
2 Pharmacology and Evolution of BH3 Mimetics
2.1 BCL2 Inhibitors
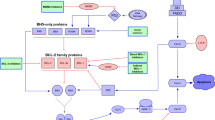
In cancer, apoptosis is prevented by the formation of a heterodimer through binding of the pro-apoptotic protein’s BH3 domain into the hydrophobic cleft of anti-apoptotic proteins. The era of BCL2 inhibitors started with the design of anti-sense oligonucleotides to knockdown BCL2 (Reed et al. 1990), followed by BH3 mimetics which bind to the hydrophobic groove of the anti-apoptotic proteins, stabilizing the pro-apoptotic proteins to carry out their function (Fig. 2a).
a Summary of molecules which interplay in mitochondrial apoptosis. Venetoclax acts as a BH3 mimetic and inhibits BCL2. The activation of BAX and BAK and their delocalization to the outer mitochondrial membrane induces cytochrome c release by depolarization and caspase activation. In healthy cells, BCL2 represses the activation of BAX and BAK. Figure is adopted from Roberts et al. (2017). b Chemical structure of venetoclax (C45H50ClN7O7S(4-(4-{[2-(2-(4-chlorophenyl)-4,4-dimethylcyclohex-2H-pyrrolo[2,3-b]pyridine-5-yloxy)benzamide))
Oblimersen, the antisense oligonucleotide that was designed to specifically target BCL2 showed only limited efficacy either in monotherapy (O’Brien et al. 2005) or in combination with chemotherapy (O’Brien et al. 2009). Inhibitors of BCL2 derived from natural substances such as AT-101 (Balakrishnan et al. 2009) and synthetic inhibitors such as obatoclax showed modest responses in CLL patients (Brown et al. 2015).
ABT-737 was a highly specific small-molecule inhibitor of BCL-xL, BCL2, and BCL-W with EC50 of 78.7, 30.3, and 197.8 nM in cell-free assays. The drug was designed by a strategy of combining screening using nuclear magnetic resonance, structure-based design, and combinatory chemical synthesis (Oltersdorf et al. 2005). Preclinical data showed ABT-737 to trigger BAX- and BAK-mediated apoptosis in various cancer cell lines and xenograft models (van Delft et al. 2006). Refractoriness to ABT-737 treatment was associated with the upregulation of MCL1 (van Delft et al. 2006). However, the therapeutic use of ABT-737 was limited due to its lack of oral bioavailability and the induction of thrombocytopenia, and higher incidences of transaminitis were observed in the treatment owing to a lower binding affinity of the drugs to BCL2 (Wilson et al. 2010).
A precursor of ABT-199 is navitoclax (ABT-263), a first-in-class dual inhibitor of BCL2 and BCL-xL. Navitoclax is structurally related to ABT-737 and inhibits BCL2 and BCL-xL with EC50 values of 60 and 20 nM, respectively (Tse et al. 2008). Navitoclax has been evaluated in clinical phase I and II trials in B-cell lymphomas as well as in solid tumors (Gandhi et al. 2011; Tolcher et al. 2015); however, the strong inhibition of BCL-xL induced a rapid decrease in circulating platelets due to a direct toxic effect. This concentration-dependent grade 3 and 4 thrombocytopenia affected treatment with high drug doses (Gandhi et al. 2011; Kaefer et al. 2014). Navitoclax was explored in a phase I study in relapsed/refractory CLL. In this trial, nine of 29 patients achieved a response with a median PFS of 25 months (Roberts et al. 2012). A phase 2 study of rituximab with or without navitoclax in untreated CLL reported ORR of 55% (Kipps et al. 2015). However, due to the thrombocytopenia, navitoclax never entered clinical phase III trials in spite of being proven efficient in BCL2 dependent malignancies (Roberts et al. 2012).
Venetoclax (ABT-199, GDC-0199) is a selective, potent, orally bioavailable BCL2 inhibitor. The structural formula is C45H50ClN7O7S (4-(4-{[2-(2-(4-chlorophenyl)-4,4-dimethylcyclohex-2H-pyrrolo[2,3-b]pyridine-5-yloxy)benzamide)), with a molecular mass of Mr = 868.4 g/mol (Fig. 2b). In comparison to navitoclax, venetoclax lacks a thiophenyl unit located at the P4 hotspot which re-engineers the BH3-binding domain. The pharmacokinetics of venetoclax is described by a Ki < 0.01 nM in cell-free assays, and it has 4800 times higher potency for BCL2 than for BCL-xL(Ki = 48 nM) and BCL-W (Ki = 245 nM) (Fig. 2). There is no activity described targeting MCL-1 (Ki > 444 nM) (Souers et al. 2013). The half-life time of venetoclax is 16–19 h (Roberts et al. 2016). ABT-199 was studied in various cellular models and primary patient samples ex vivo, where it induced apoptosis and its sensitivity strongly correlated with the expression of BCL2 (Anderson et al. 2016; Fischer et al. 2015; Varadarajan et al. 2013). The dosing in clinical trials is performed daily and ranges between 300 and 900 mg/day. The peak plasma concentration of venetoclax is achieved 5–8 h after drug uptake. If venetoclax is taken with a fat meal, the mean maximum observed plasma concentration (Cmax) and area under the plasma concentration—time curve (AUC) are fourfold increased as its intestinal uptake and absorbance are accelerated. Venetoclax is metabolized by CYP3A4/5 and is a substrate of the P-glycoprotein pump (Agarwal et al. 2016). The use of CYP3A inhibitors leads to an accumulation and dose increase and therefore should be avoided (Agarwal et al. 2016, 2017). Due to the high degree of specificity and comparatively lower toxicity, only venetoclax among all the BCL2 inhibitors successfully reached the market.
2.2 MCL1 Inhibitors
MCL1 is an anti-apoptotic member of the BCL2 family of proteins. MCL1 is frequently upregulated in various cancers and hence considered as a promising therapeutic target. Moreover, transcriptional upregulation or amplification of MCL1 is described to be an important mechanism, driving resistance to BCL2 inhibitors (Beroukhim et al. 2010). As MCL1 has a binding pocket for BAK, several compound screens have been performed to develop competitive inhibitors to disrupt this interaction (Varadarajan et al. 2013). However, MCL1 inhibition is known to be embryonically lethal (Rinkenberger et al. 2000), and MCL1 is also important for survival of hematopoietic stem cells (Opferman et al. 2005), which might limit the therapeutic window due to toxicity. After TW-37 which showed potential efficacy in in vitro models by disruption of MCL1 binding to BAK (Varadarajan et al. 2013), development of an amenable inhibitor had been challenging. Recent findings presented S63845 with potent anti-tumor activity in vitro and in vivo (Kotschy et al. 2016). The inhibitor binds to the BH3 binding groove of MCL1 and was efficacious in the pre-clinical setting in different hematopoietic cancers such as multiple myeloma, acute myeloid leukemia, and lymphomas as well as in solid cancers. Interestingly, S63845 showed no adverse effect on hematopoietic progenitor cells which points to MCL1 as a treatment option for MCL1 dependent tumors and in drug combination with BCL2 inhibitors (Kotschy et al. 2016) (Table 1).
3 Preclinical Studies Using Venetoclax
A number of preclinical studies reported the tremendous effects of venetoclax in vitro. The major mode of action of venetoclax is the activation of BAK and BAX and subsequent mitochondrial cytochrome C release leading to apoptosis. Treatment with venetoclax was reported in various cellular models to functionally validate its mode of action. Jurkat T-cells lacking BAX showed no response to treatment with different concentrations of venetoclax (Vogler et al. 2013). Apoptosis induction by venetoclax in peripheral blood samples from CLL cases was confirmed by externalization of phosphatidylserine. Comparison of venetoclax with ABT-737 and ABT-263 to analyze their susceptibility to affect platelets showed markedly reduced toxicity of venetoclax in comparison to ABT-737 or ABT-263 (Vogler et al. 2013).
Furthermore, during preclinical development, high single-agent cell-killing was demonstrated in non-Hodgkin’s lymphoma (NHL) cell lines including those from diffused large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and follicular lymphoma (FL). Notably, NHL cell lines carrying the t(14;18) BCL2 gain showed higher sensitivity toward ABT-199 treatment than cell lines without the alteration (Souers et al. 2013). Multiple myeloma cell lines showed sensitivity to venetoclax correlating with the expression profile of BCL2, BCL-xL, and MCL1 which were predictive for treatment response. Interestingly, the co-expression of BCL2 and BCL-xL resulted in resistance to venetoclax monotherapy but still showed response to BCL-xL inhibitors.
Remarkable effects were also demonstrated for ABT-199 treatment of AML and pediatric ALL cell lines (Fischer et al. 2015). Also, studies using xenografts of AML (Pan et al. 2014), ALL (Frismantas et al. 2017), and B-cell lymphomas with venetoclax as monotherapy or in combination with rituximab and bendamustine (Souers et al. 2013) highlighted the efficacy of venetoclax as well as its safety in combination treatments. In line with these findings, studies using MLL-rearranged ALL primary samples in vitro (Alford et al. 2015) and in vivo (Khaw et al. 2016) demonstrated the potent single-agent activity of venetoclax in these tumor entities.
In CLL, treatment with ibrutinib, a novel small-molecule inhibitor of BTK showed impressive clinical activity with durable responses (Byrd et al. 2014; Byrd et al. 2015); however, subsets of patients did not achieve deep remission or cure. To address a possible synergism between venetoclax and ibrutinib, ex vivo serial samples of CLL patients under ibrutinib treatment were treated with venetoclax. The combination resulted in high cytotoxicity in vitro and confirmed a possible synergy between venetoclax and ibrutinib. Of functional relevance, the decrease of MCL1 and BCL-xL mediated by ibrutinib augmented the response to inhibition of BCL2 through venetoclax, strongly endorsing for a clinical trial with this combination therapy (Cervantes-Gomez et al. 2015).
Importantly, on the other hand, various studies were performed to assess the safety profile of venetoclax and their impact on non-tumor hematopoietic lineages using genetically modified murine models. Pre-B-cells and immature B-cells were found to highly depend on BCL-xL (Motoyama et al. 1995) with a low expression of BCL2 in these subsets; however, a gradual switch in BCL2 expression in pro-B-cell precursors directed their maturation (Merino et al. 1994). In order to validate the mode of action of ABT-199 on normal lymphocyte subsets, knockout mice models carrying knockouts of apoptotic players (Bim, Bax, Bak, Puma, Noxa) were analyzed (Khaw et al. 2014).
Analysis of the in vitro sensitivity of lymphocyte subsets identified human peripheral B-cells of healthy donors to be more sensitive to venetoclax treatment than CD4+ or CD8+ T-cells and granulocytes. These data were also confirmed by analyzing murine lymphocyte subsets (Khaw et al. 2014). In contrast to cells of the B-cell lineage, the T-cell lineage and granulocytes were resistant to venetoclax treatment. In-depth analysis of T-cell subsets revealed a higher susceptibility of double-negative (CD4- CD8-) thymocytes and mature peripheral T-cells than CD4 and CD8 subsets to venetoclax treatment, correlating with their reliance on BCL2 (Gratiot-Deans et al. 1994; Veis et al. 1993).
4 Clinical Efficacy of Venetoclax in Hematological Malignancies
4.1 Venetoclax for Treatment of Poor-Risk CLL
CLL is a B-cell malignancy, where clonal CD5+ CD19+ CD23+ B-cells are present in the peripheral blood and infiltrate lymphoid organs such as lymph nodes, spleen, and bone marrow. The mechanisms underlying CLL pathogenesis are not fully resolved, and the clinical course of CLL is highly diverse. Recently, there was a paradigm change in treatment of CLL from chemoimmunotherapy-based treatments to the use of small-molecule inhibitors targeting key survival mechanisms especially in cases with high-risk genetic aberrations. The three main FDA/EMA approved small-molecule inhibitors with proven efficacy are ibrutinib, targeting BTK; idelalisib, targeting PI3K-δ; and venetoclax targeting BCL2.
4.2 Phase I and Phase II Trials with Venetoclax as a Single-Agent in CLL
In spite of variations in BCL2 expression levels between patient samples, almost all CLL tumors express high levels of BCL2 primarily due to the prevalence of del(13q14), harboring the BCL2 repressors, miR-15 and miR-16 (Cimmino et al. 2005).
A phase I first-in-human dose escalation clinical trial was initiated to determine the dosings of venetoclax in patients with relapsed/refractory CLL, SLL, or B-NHL (Roberts et al. 2016). To address the safety and pharmacokinetic profile, the first group was treated with an escalating dose, in which 56 patients received treatment with eight different doses ranging from initial doses between 20 and 50 mg venetoclax and received a weekly increase up to 1200 mg per day. In the second group, 60 patients were treated in a stepwise weekly ramp-up of up to 400 mg per day. Treatment with venetoclax resulted in an overall response rate (ORR) of 79% in the relapsed/refractory CLL with poor prognostic clinical factors (ORR of 77% in the dose escalation cohort, ORR of 82% in the expansion cohort) (Roberts et al. 2016). Strikingly, with venetoclax treatment, a rapid reduction of absolute lymphocyte counts occurred within 6–24 h after a single 20 mg dosing, where apoptotic CLL cells were detected in peripheral blood. Reduction in tumor burden was detected in blood, lymph nodes, and bone marrow; however, tumor lysis syndrome (TLS) occurred only in two patients with lymphadenopathy. Twenty percentage of patients achieved complete remission, and among them, 5% were negative for MRD by flow cytometry, which has never been observed with BTK or PI3 K inhibitors treatments (Woyach and Johnson 2015). Notably, tumor lysis syndrome emerged as dose-limiting toxicity in these early data and dedicated risk mitigation strategies have been implemented including a slow ramp-up dosing and prophylactic measures to allow for save treatment initiation (for details please see below).
In the dose escalation protocol, 60 patients were treated with a weekly dose ramp-up of 20–400 mg daily and no tumor lysis syndrome was observed. Progression-free survival of 69% was reported after 15 months. The grade 3/4 adverse effects included neutropenia in 40% of the patients and grade 1/2 adverse effects were associated with the gastrointestinal system. Nevertheless, infections due to neutropenia remained lower compared to treatment with chemoimmunotherapy. The efficacy of venetoclax was irrespective of the cytogenetics and TP53 mutation status. After a median observation time of 17 months, 41 patients (35%) showed progressive disease and among these 18 patients (16%) developed Richter’s transformation.
Due to the promising results in poor-risk CLL, a pivotal phase II clinical trial was initiated, accruing relapsed/refractory CLL patients with del(17p). In this multicenter open-label study, 107 patients were enrolled between 2013 and 2014 and treated with venetoclax with a weekly dose escalation from 20 to 400 mg over 4 weeks (20, 50, 100, 200, 400 mg) and continued until disease progression. Overall response after an observation time of 12 months was 79.4% (85 of 107 patients), and in 8% of patients, complete remission was achieved. Estimated PFS and OS after 12 months were 72 and 86.7%, respectively (Fig. 3) (Stilgenbauer et al. 2016).
a Cumulative incidence of overall response and CR by independent review-committee assessment. b Cumulative incidence of minimal residual disease-negative status in peripheral blood for all patients and for patients achieving CR or CRi by independent review-committee assessment. Kaplan–Meier curves for c overall survival, d progression-free survival (n = 107), e duration of overall response for all responders by independent review-committee assessment (n = 85), and f duration of overall response for all responders separated by response subgroups (independently assessed). CR: complete remission; Cri: CR with incomplete recovery of blood counts; nPR: nodular partial response (Stilgenbauer et al 2016; Huber et al. 2017)
Responses were durable, and majority of the patients showed reduction in absolute lymphocyte count, target lymph node lesion diameter, and bone marrow infiltrate at a median of 0.3 months of treatment (Fig. 4). Management of tumor lysis syndrome was by prophylaxis. Laboratory TLS was observed in five patients during dose escalation and in one patient in the third week; however, no clinical TLS appeared in these cases. The most common adverse effect of higher grade was neutropenia in 40% of patients, which was handled with dose reductions, G-CSF administration, or prophylactic antibiotic regimens. The results of this pivotal trial led to FDA approval of venetoclax in April 2016 for the treatment of previously treated CLL patients with the 17p deletion (Deeks 2016).
Absolute change from baseline in peripheral absolute lymphocyte count in patients with a baseline absolute lymphocyte count > 5 × 109 cells/L (n = 87) (a) and unidimensional nodal diameter (n = 96) (b). Thresholds of 4 × 109 cells/L (a) and 15 mm (b) corresponded to requirements for complete remission. Line length indicates absolute best change from baseline; each line represents one patient, with patients arranged in descending order of baseline measurement. Nodal measurements were computed tomography scan-derived, consisting unidimensional diameters of largest target lesions for patients who had at least one follow-up computed tomography scan on study. Response categories were assessed by an independent review committee (Stilgenbauer et al 2016; Huber et al. 2017)
To date, limited clinical data is available regarding the sequential use of the novel drugs or synergism of inhibitors in combinations. Various drug combinations are currently being tested to improve response rates and with an aim for deep remission or even cure.
4.3 Venetoclax in Combination Therapy for the Treatment of CLL
Since the combination of venetoclax with rituximab enhanced the efficacy of venetoclax in preclinical models, a phase Ib study was initiated where relapsed/refractory CLL patients were continuously treated with venetoclax and received a single dose of rituximab every six weeks. Primary aim of the combination study was to address the maximum tolerable dose and safety of the combination, and further objectives were to assess the pharmacokinetic profile, efficacy, overall response, and PFS (Seymour et al. 2017). Twenty-five (51%) of 49 patients had a complete remission and 28 (57%) patients achieved undetectable bone marrow MRD. The most common adverse effects included grade 1/2 gastrointestinal events and grade 3/4 neutropenia in 26 (53%) patients, similar to that of venetoclax monotherapy. Remarkably, 42 (86%) patients responded (ORR) to the combination, and a 2-year progression-free survival was achieved in 82% (Seymour et al. 2017).
Most recently, the first randomized phase III data on venetoclax became available from the MURANO trial. This showed profound improvement in PFS (primary endpoint), clinical response rate, MRD response, and OS in relapsed/refractory CLL patients treated with venetoclax plus rituximab (VR) compared to bendamustine and rituximab (BR). Of the 389 patients enrolled, 27% had a del(17p13), 60% received one prior therapy, and 15% were refractory to fludarabine. At interim analysis (median follow-up of 23.8 months), PFS was significantly prolonged for VR compared to BR arm (median PFS not reached vs. 17 months, Fig. 5). The ORR for VR was 93.3% compared to 68% for BR, and CR/CRi was achieved in 26.8% versus 8.2%, respectively. MRD negativity was attained in 83.5% of cases treated with VR compared to 23.1% treated with BR. Comparable number of fatal AEs (5%) and Richter’s transformation (3%) was observed in both treatment arms. The dramatic efficacy of VR treatment combined with favorable tolerability will lead to approval of this combination therapy for relapsed/refractory CLL (Seymour et al. 2017) (Fig. 5).
Progression-free survival of the interim analysis of the pivotal phase III MURANO trial. The combination of venetoclax with rituximab showed a dramatic prolongation of PFS in comparison to bendamustine and rituximab (Seymour et al. 2017)
Furthermore, a prospective, open-label multicenter randomized phase 3 trial was initiated to compare the efficacy of obinutuzumab combined with venetoclax versus obinutuzumab with chlorambucil (Clb) in the front line treatment of CLL patients with comorbidity (CLL14 trial of the GCLLSG). The safety run-in phase of the trial included 12 patients who received the experimental arm (obinutuzumab with venetoclax). The safety and efficacy of 6 weekly cycles of obinutuzumab and daily treatment with venetoclax appeared well tolerated and showed excellent efficacy; therefore, the randomized phase III trial is currently fully accrued, and follow-up is ongoing (Fischer et al. 2015).
Several lines of evidence support the combination of venetoclax with other small-molecule inhibitors in CLL. Ongoing clinical phase II trials are recruiting participants for combination treatment of ibrutinib, venetoclax, and obinutuzumab for first-line treatment of CLL (CLL13, GAIA trial; NCT02950051). The study protocol compares several combinations such as standard chemotherapy (FCR/BR) versus rituximab with venetoclax (RVe) versus obinutuzumab with venetoclax versus obinutuzumab with ibrutinib and venetoclax (GIVe) in previously untreated fit patients without del(17p13) or TP53 mutation (NCT02758665). Furthermore, the combination of venetoclax with ibrutinib (NCT02756897) is being studied for the treatment of relapsed/refractory CLL. In the CLL-BAG trial, the sequential regimen of bendamustine for debulking of tumor cells is followed by ABT-199 and GA101-induction and -maintenance therapy. Interim results after the induction phase report tremendous success with an overall response rate in 97% of treated patients and 89% of patients were MRD-negative (NCT02401503) (Cramer et al. 2017). Even more impressive results are expected from these trials on treatment-naïve patients as well as relapsed/refractory diseases (Table 2).
4.4 Venetoclax for the Treatment of Acute Lymphoblastic Leukemia (ALL)
Treatment of pediatric B-cell precursor ALL (BCP-ALL) has evolved to be more and more successful over the past decades as survival rates of patients have improved to more than 80% (Pui et al. 2015). However, decreased tolerance to therapy (toxicity) and minimal residual disease positivity with subsequent relapse remain issues associated with poor outcome. As BCP-ALL is a very heterogeneous disease, ideal biomarkers for early stratification of patients into groups which would potentially benefit from treatment with venetoclax are required. In particular, the prognosis of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph(+) ALL) remains unfavorable. Ph(+) ALL occurs in 25% of adults and in only 3% of pediatric ALL patients (Liu-Dumlao et al. 2012). The current treatment regimen includes multi-chemotherapy which is complemented with the kinase inhibitors imatinib or dasatinib. A pre-clinical trial in xenograft mice demonstrated that the combination of dasatinib and venetoclax is synergistic and tolerable in vivo and that the anti-leukemic effects were vastly improved (Leonard et al. 2016).
Two clinical trials are planned to study the efficacy of venetoclax monotherapy in ALL. A phase I study to address the safety and pharmacokinetics of venetoclax monotherapy in pediatric and young adult patients will recruit patients with relapsed/refractory ALL (NCT03236857). Another open-label phase I dose-escalating study will recruit participants and analyze safety and pharmacokinetics of venetoclax, navitoclax, and chemotherapy in relapsed acute lymphoblastic leukemia (NCT03181126).
4.5 Treatment of Non-Hodgkin’s Lymphomas (NHL) and Multiple Myeloma (MM) with Venetoclax
Non-Hodgkin’s lymphoma describes a group of B- and T-cell lymphomas, which range from indolent to aggressive types. The low-grade or indolent subtypes include follicular lymphoma (FL) and CLL, while the high-grade or aggressive NHLs include diseases such as diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL). While the standard chemo-immuno therapy has improved the outcome of patients diagnosed with aggressive non-Hodgkin’s lymphomas, treatment after disease relapse remains challenging.
Venetoclax monotherapy is being assessed in a clinical trial for pediatric and young patients with NHL (NCT03236857), and even further clinical trials are planned to study venetoclax in combination (NCT03181126). A clinical phase III trial is currently investigating in the combination of venetoclax and ibrutinib in MCL patients (NCT03112174). An ongoing phase I single-arm study investigates the combination of venetoclax with bendamustine and rituximab. After completion of the treatment cycles with bendamustine, rituximab, and venetoclax, venetoclax is continued as monotherapy for two more years. In a phase I first in human trial of 106 recruited patients with relapsed/refractory FL (29), DLBCL (34), MCL (28), Waldenstroem macroglobulinea (4), marginal zone lymphoma (3) and DLBCL derived from Richter´s syndrome (3), an ORR of 44% and median PFS of 6 months was achieved. 14 patients had a complete response, 33 patients showed a partial response, and 32 patients had a stable disease. In this dose escalation study, the MCL cases achieved durable response with 800mg while 1200mg was the effective single agent dose of venetoclax for FL and DLBCL patients (Davids et al. 2017).
A follow-up phase II single-arm study is currently recruiting participants for the combination of venetoclax with obinutuzumab in relapsed/refractory DLBCL. Here, the combination treatment is repeated for three cycles, and if complete or partial responses are obtained, the patients will receive stem cell transplantation (NCT02987400). Several trials aiming for the combination treatment of venetoclax with rituximab or obinutuzumab, cyclophosphamide, etoposide, prednisone, and others are under investigation.
Venetoclax demonstrated cell killing in multiple myeloma cell lines and primary tumor cells (Kumar et al. 2015), and several clinical studies with venetoclax are currently ongoing for the treatment of multiple myeloma. Relapsed/refractory patients receive combinations of venetoclax and current standard therapy with dexamethasone and bortezomib (NCT02755597; NCT01794507). Other trials combine venetoclax with daratumumab, a CD38 antibody (NCT03314181). In the monotherapy approach, 21% of patients responded to venetoclax treatment. Notably, 40% of these patients carried the t(11;14) translocation, were refractory to bortezomib and lenalidomide, and were treated with at least four prior regimens (Kumar et al. 2015). Due to good safety and efficacy, a phase Ib trial was initiated to study the combination treatment of venetoclax with bortezomib and dexamethasone. Sixty-six heavily pretreated patients were enrolled. The overall response rate was 66%, and the median time to progression was 9.7 months. The results were irrespective of the t(11;14) status, with manageable adverse effects and an acceptable safety profile (Moreau et al. 2017).
4.6 Venetoclax for Treatment of Myeloid Malignancies
Acute myeloid leukemia is an aggressive malignancy of the myeloid progenitor cells. AML cases have variable outcome after chemotherapy due to the enormous clinical and molecular heterogeneity. Over decades, the induction therapy of combined anthracycline plus cytarabine cytotoxic agents has remained the standard of treatment with little improvement in survival with the additions of novel agents. Though the manipulation of intensity and duration of treatment modestly improved survival incertain patient subsets, a consolidation chemotherapy often including stem cell transplant remains necessary (Fernandez et al. 2009). Venetoclax demonstrated high efficiency in preclinical AML models as well as synergy with anthracyclines (Teh et al. 2017).
Venetoclax achieved remarkable results in early clinical trials in AML. The M13-387 phase Ib trial evaluated the safety and the maximum tolerable dose of venetoclax in treatment naïve elderly AML patients not eligible for standard induction chemotherapy. Patients were treated in two arms, a ramp-up of venetoclax with decitabine or 5-azacitidine. Nineteen of 22 patients completed the first 28-day cycle with venetoclax. The response to treatment was 75% in the decitabine and 70% in the 5-azacitidine arms (DiNardo et al. 2015). Also, venetoclax as a single agent in heavily pretreated AML patients resulted in an overall response rate of 19% (Konopleva et al. 2016). Based on these results, further clinical trials were initiated for the treatment of AML. A phase III study was initiated to assess treatment with Venetoclax in combination with azacitidine, where patients not eligible for intensive therapy were randomized to venetoclax with azacitidine or placebo with azacitidine arms. Interim results present an impressive overall response rate of 69% with a complete response (CR) of 60% in high-risk AML patients (NCT03236857; DiNardo et al. 2015).
5 Venetoclax in Solid Tumors
As various cancers show dependency on anti-apoptotic BCL2 family members for their survival, the use of venetoclax extends beyond that of hematologic malignancies.
BCL2 is overexpressed in 85% of estrogen receptor-positive (ER) breast cancers. ER was found to bind two estrogen response elements (EREs) in the coding regions of BCL2, thereby enhancing BCL2 expression in these tumors (Perillo et al. 2000). Preclinical data generated in ER-positive breast cancer xenografts showed venetoclax to be highly efficacious. Also, venetoclax was found to synergize with PI3K and mTOR inhibitors enhancing its apoptotic effect (Vaillant et al. 2013). To explore more effective therapeutic strategies, safety and efficiency of venetoclax were tested in a phase Ib study in ER+ BCL2+ breast cancer patients. The results were heterogeneous, as four patients had a partial response and five has a stable disease with a clinical benefit rate of 69%. Venetoclax treatment of ER+ breast cancers is further being validated in phase II clinical trials which are ongoing (NCT02391480).
Small-cell lung cancer (SCLC) is a high proliferating cancer with a low doubling time, rapid metastasis, and quick relapse after treatment. First-line chemotherapy with platinum-based agents and etoposide remains without long-term success, and multiple-targeted approaches using receptor tyrosine kinase (RTK) inhibitors are being investigated. Though the RTK inhibitors were of therapeutic relevance, the curative potential was minimal (Niederst et al. 2013). Assessment of the efficacy of venetoclax in SCLC treatment is still in the pre-clinical phase. The expression of BCL2 was comprehensively assessed in different lung cancer cell lines, and BCL2 inhibitors were found to synergize with anthracyclines providing a promising strategy for treatment (Inoue-Yamauchi et al. 2017). Venetoclax was also addressed in combination with BET inhibitor ABBV-075, since BET is known to regulate key oncogenes as MYC, CCND2, and BCL2L1 and enhance complex formation of pro-apoptotic BIM and BCL2 (Lam et al. 2017). The combination treatment proved to be highly synergistic and therefore might be a possible rationale for treatment of SCLC patients with high BCL2 expression.
6 Toxicity and Adverse Effects Associated with BCL2 Inhibitor Treatments
6.1 Thrombocytopenia
BH3 mimetics such as ABT-767 and ABT-263 inhibit BCL2, as well as BCL-xL and BCL-W. Since thrombocytes depend on BCL-xL for survival, treatment with these inhibitors leads to reduction in platelets and dose-dependent thrombocytopenia due to direct toxic anti-platelet effects. Assessment of safety and tolerability of navitoclax documented the prevalence of thrombocytopenia in different tumor entities. In a clinical trial on 39 SCLC patients, 41% developed grade III–VI thrombocytopenia upon treatment with navitoclax (Rudin et al. 2012). In a phase II study of CLL patients, 36% of patients suffered from grad III–IV thrombocytopenia (Kipps et al. 2015). This mode of action of these drugs on platelets was clarified to be mediated by apoptosis cell death, as well as decreased calcium flux, reducing the activation of platelets (Vogler et al. 2013). To circumvent severe thrombocytopenia, the initial dose of navitoclax was kept low within the first seven days of treatment. However, these side effects limited the clinical development of navitoclax. Venetoclax being a highly specific for BCL2 with lower affinity toward BCL-xL did not induce dose-dependent thrombocytopenia.
6.2 Tumor Lysis Syndrome (TLS)
The tumor lysis syndrome is one of the major risk issues in venetoclax treatment, owing to the very high potency of the drug. Due to the rapid response, with abrupt onset at 6–8 h following dosing, venetoclax is prone to induce tumor lysis syndrome, depending on tumor mass, comorbidities (in particular renal function), and treatment dose (Cheson et al. 2017). TLS results from rapid cell death, wherein tumor cells release their metabolites, nucleic acids, and intracellular ions into the blood stream, thereby potentially inducing metabolic dysfunction. The efflux of cellular metabolites leads to a disbalance of the blood homeostasis with an increase in uric acid, potassium, and phosphoric acid and decrease in calcium levels. If the renal excretion is affected or delayed, an accumulation of these metabolites occurs in blood. The incidence of TLS is increased in tumors with a high tumor burden or high cell turnover (Cheson et al. 2017; Crombie and Davids 2017). The most common criteria for subdivision into clinical and laboratory TLS were defined in 2004 by Cairo et al. (2004). Laboratory TLS includes at least two or more biochemical variables being increased or reduced by a factor of more than 25%; furthermore, TLS appears within three days prior or seven days after initiation of therapy. TLS causes hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia and can lead to acute renal failure and cardiac events of life-threatening potential. If laboratory TLS is accompanied by clinically relevant events such as creatinine increase, seizures, or cardiac dysfunction, a clinical TLS is diagnosed. Prophylaxis and treatment of TLS includes hydration, diuresis, monitoring of electrolytes, and prevention of hypouricemia with allopurinol or rasburicase (Howard et al. 2011).
For treatment with venetoclax, a treatment risk stratification is implemented where patients are grouped according to their TLS risk. Low risk for TLS is defined by small nodal disease, a low ALS < 25 × 109/L. Medium risk includes an ALS > 25 × 109/L or a lymph node with more than 5 cm diameter. The high-risk group for TLS is a radiological tumor > 10 cm in diameter and a ALS > 25 × 109/L (Roberts et al. 2016). Therapy with venetoclax is initiated with a low starting dose, and patients pass through a weekly dose ramp-up from 20 to 50, 100, 200, and 400 mg to ensure safety and tolerability. Laboratory monitoring of blood counts and clinical chemistry is mandatory during treatment initiation, and immediate action is required in case of relevant abnormalities. Detailed information and guidance on TLS management are beyond the scope of this article and are available in the prescription information of venetoclax. Meticulous adherence to the guidelines is required to deliver venetoclax therapy safely.
7 Venetoclax Drug Interactions
7.1 Interaction with CYP3A4 Inhibitors
The routes of elimination of venetoclax were tested by administration of a 200 mg single dose of 14C (100 µCi) venetoclax to four healthy volunteers. The recovery of total radioactive dose (100% ± 5%) was through feces as the major route of drug elimination. The major metabolite M27 was formed by oxidation cytochrome P450 isoform 3A4 (CYP3A4) (Choo et al. 2014; Li et al. 2016). Since various drugs such as protease inhibitors, anti-fungal agents, macrolide antibiotics, and anti-depressants are described to be inhibitors of CYP3A4, possibility of drug interactions was tested using ketoconazole as a representative agent. Twelve NHL patients were enrolled for a phase I, open-label study, where patients received venetoclax and ketoconazole, the strong CYP3A4 inhibitor. Inhibition of CYP3A4 led to a significant increase in the mean maximum observed plasma concentration Cmax and area under the plasma concentration-time curve (AUC∞) by 2.3-fold and 6.4-fold, respectively.
Similarly, also, simultaneous treatment with venetoclax and CYP3A4 inducers such as Rifampin led to an increase in the AUC and Cmax of venetoclax (Agarwal et al. 2016). These studies suggest the need to avoid concomitant use of strong and moderate inhibitors or inducers of CYP3A4 during the venetoclax ramp-up phase in patients (Agarwal et al. 2017). Also, venetoclax dosage should be reduced by 25–50% when co-administering the CYP3A4 modulators after dose escalation.
7.2 Interaction with P-Glycoprotein Inhibitors
Venetoclax has been described to be a substrate of P-glycoproteins (P-gp) based on in vitro studies. Also, venetoclax was shown to inhibit P-glycoprotein at the transcriptional and protein levels (Weiss et al. 2016). In a clinical trial in healthy volunteers, the effect of venetoclax on the pharmacokinetics of digoxin, a P-gp inhibitor was evaluated. Co-administration of digoxin and venetoclax increased digoxin maximum observed plasma concentration (Cmax) by 35% and area under the plasma concentration-time curve (AUC0–∞) by 9%. The study indicated that venetoclax can increase the concentrations of P-gp substrates. The study suggested administration of narrow therapeutic index P-gp substrates, six hours prior to venetoclax to minimize the potential interaction.
8 Biomarkers of Resistance to Venetoclax Treatment
Biomarkers are important to predict the response and efficacy to venetoclax therapy and for early assignment of combination or alternative treatments for effective therapy.
Due to the high specificity of venetoclax, upregulation of alternative anti-apoptotic is described to confer resistance to therapy. Several preclinical and ex vivo studies analyzed the ratio between BCL2 and MCL1 which was of clinical importance to predict venetoclax treatment response. Studies of multiple myeloma xenografts showed that the overexpression of BCL2 and MCL1 led to resistance toward BCL2 inhibitors treatment (Punnoose et al. 2016). Upregulation of BCL-xL or MCL1 or both is also contributing to venetoclax resistance in lymphoma cell lines (Tahir et al. 2017).
Functional analysis of the cell’s response to venetoclax using BH3 profiling of primary CLL cells showed a significant association between apoptotic priming by venetoclax ex vivo to clinical response to venetoclax. Precisely, the extent of mitochondrial depolarization by a BIM BH3 peptide in vitro correlated with percentage reduction of CLL in the blood following venetoclax treatment suggesting its use as a potential biomarker for early risk stratification (Anderson et al. 2016).
Furthermore, acquired missense mutations in the BH3 binding groove of resistant cell lines were detected to interfere with drug binding capacity. Also, acquired mutations in the pro-apoptotic BAX gene limiting its anchoring to the mitochondria were also described to interfere with apoptosis induction by venetoclax (Fresquet et al. 2014).
9 Summary and Future Perspectives
The EMA and FDA approval of venetoclax, a novel, and highly specific BCL2 inhibitor already indicates its high therapeutic potential in CLL and potentially also in various other malignancies. Venetoclax demonstrated tremendous success in treatment of poor risk, relapsed/refractory CLL, and in the future may be revolutionizing treatment of various other hematological malignancies. With precision medicine evolving to a real-world paradigm, there is an absolute need for novel and specific targeted therapies. The use of targeted combination therapies will further improve the landscape of treatment options for patients who are refractory to conventional therapies and may eventually lead to novel approaches en route toward the cure of cancer.
References
Abulwerdi F et al (2014) A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther 13:565–75
Agarwal SK, Hu B, Chien D, Wong S, Salem A (2016) Evaluation of Rifampin’s transporter inhibitory and CYP3A inductive effects on the pharmacokinetics of venetoclax, a BCL-2 inhibitor: results of a single- and multiple-dose study. J Clin Pharmacol 56:1335–1343
Agarwal SK et al (2017) Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br J Clin Pharmacol 83:846–854
Albershardt TC et al (2011) Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem 286:24882–24895
Alford SE et al (2015) BH3 inhibitor sensitivity and Bcl-2 dependence in primary acute lymphoblastic leukemia cells. Cancer Res 75:1366–1375
Anderson MA et al (2016) The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 127:3215–3224
Bai L et al (2014) BM-1197: a novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PLoS ONE 9:e99404
Balakrishnan K, Burger JA, Wierda WG, Gandhi V (2009) AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood 113:149–153
Bentz M et al (2000) t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes, Chromosom Cancer 27:285–294
Beroukhim R et al (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463:899–905
Boise LH et al (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608
Brown JR et al (2015) Obatoclax in combination with fludarabine and rituximab is well-tolerated and shows promising clinical activity in relapsed chronic lymphocytic leukemia. Leuk Lymphoma 56:3336–3342
Byrd JC et al (2015) Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 125
Byrd JC et al (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371:213–223
Cahill N, Rosenquist R (2013) Uncovering the DNA methylome in chronic lymphocytic leukemia. Epigenetics 8:138–148
Cairo MS, Bishop M (2004) Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 127:3–11
Cervantes-Gomez F et al (2015) Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 21:3705–3715
Cheson BD et al (2017) Tumor lysis syndrome in chronic lymphocytic leukemia with novel targeted agents. Oncologist 22:1283–1291
Choo EF et al (2014) The role of lymphatic transport on the systemic bioavailability of the BCL-2 protein family inhibitors navitoclax (ABT-263) and ABT-199. Drug Metab Dispos 42:207–212
Cimmino A et al (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci 102:13944–13949
Cohen NA et al (2012) A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem Biol 19:1175–1186
Cramer P et al (2017) Bendamustine (B), followed by obinutuzumab (G) and venetoclax (A) in patients with chronic lymphocytic leukemia (Cll): Cll2-bag trial of the german CLL study group (GCLLSG). Hematol Oncol 35:25–27
Crombie J, Davids MS (2017) Venetoclax for the treatment of patients with chronic lymphocytic leukemia. Futur Oncol 13:1223–1232
Datta SR et al (2002) Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell 3:631–643
Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S, Kipps TJ, Anderson MA, Salem AH, Dunbar M, Zhu M, Peale F, Ross JA, Gressick L, Desai M, Kim SY, Verdugo M, Humerickhouse RA, Gordon GB, Gerecitano JF (2017) Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol 35(8):826–833
Deeks ED (2016) Venetoclax: first global approval. Drugs 76:979–987
DiNardo C et al (2015) A phase 1b study of venetoclax (ABT-199/GDC-0199) in combination with decitabine or azacitidine in treatment-naive patients with acute myelogenous leukemia who are ≥ to 65 years and not eligible for standard induction therapy. Blood 126
Fernandez HF et al (2009) Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 361:1249–1259
Fischer K et al (2015) Results of the safety run-in phase of CLL14 (BO25323): a prospective, open-label, multicenter randomized phase III trial to compare the efficacy and safety of obinutuzumab and venetoclax (GDC-0199/ABT-199) with obinutuzumab and chlorambucil in patients w…. Blood 126
Fischer U et al (2015) Genomics and drug profiling of fatal TCF3-HLF − positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet 47:1020–1029
Fresquet V, Rieger M, Carolis C, Garcia-Barchino MJ, Martinez-Climent JA (2014) Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood 123:4111–4119
Frismantas V et al (2017) Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood 129:e26–e37
Gandhi L et al (2011) Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 29:909–916
Gibson CJ, Davids MS (2015) BCL-2 antagonism to target the intrinsic mitochondrial pathway of apoptosis. Clin Cancer Res 21:5021–5029
Gibson L et al (1996) bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene 13:665–675
Gratiot-Deans J, Merinot R, Nurezt G, Turkau LA (1994) Bcl-2 expression during T-cell development: early loss and late return occur at specific stages of commitment to differentiation and survival. 91, 10685–10689
Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC (1993) bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood 82:1820–1828
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Howard SC, Jones DP, Pui C-H (2011) The tumor lysis syndrome. N Engl J Med 364:1844–1854
Huber H, Edenhofer S, Estenfelder S, Stilgenbauer S (2017) Profile of venetoclax and its potential in the context of treatment of relapsed or refractory chronic lymphocytic leukemia. Onco Targets Ther 10:645–656
Inohara N, Ding L, Chen S, Núñez G (1997) Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J 16:1686–1694
Inoue-Yamauchi A et al (2017) Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat Commun 8:16078
Kaefer A et al (2014) Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother Pharmacol 74:593–602
Khaw SL et al (2014) Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia 28:1207–1215
Khaw SL et al (2016) Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood 128:1382–1395
Kipps TJ et al (2015) A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma 56:2826–2833
Konopleva M et al (2016) Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 6:1106–1117
Korsmeyer SJ et al (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7:1166–1173
Kotschy A et al (2016) The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538:477–482
Kumar SK et al (2015) Safety and efficacy of venetoclax (ABT-199/GDC-0199) monotherapy for relapsed/refractory multiple myeloma: phase 1 preliminary results. Blood 126
Lam LT et al (2017) Vulnerability of small-cell lung cancer to apoptosis induced by the combination of BET bromodomain proteins and BCL2 inhibitors. Mol Cancer Ther 16:1511–1520
Lee JH et al (2004) Inactivating mutation of the pro-apoptotic geneBID in gastric cancer. J Pathol 202:439–445
Leonard JT et al (2016) Targeting BCL-2 and ABL/LYN in Philadelphia chromosome-positive acute lymphoblastic leukemia. Sci Transl Med 8, 354ra114-354ra114
Lessene G et al (2013) Structure-guided design of a selective BCL-XL inhibitor. Nat Chem Biol 9:390–397
Li CJ et al (2016) Novel Bruton’s tyrosine kinase inhibitor Bgb-3111 demonstrates potent activity in mantle cell lymphoma. Blood 128
Liu-Dumlao T, Kantarjian H, Thomas DA, O’Brien S, Ravandi F (2012) Philadelphia-positive acute lymphoblastic leukemia: current treatment options. Curr Oncol Rep 14:387–394
Meijerink JP et al (1998) Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood 91:2991–2997
Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nuñez G (1994) Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J 13:683–691
Miyashita T, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299
Monni O et al (1997) BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood 90
Moreau P et al (2017) Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood blood-2017-06-788323. https://doi.org/10.1182/blood-2017-06-788323
Motoyama N et al (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506–1510
NCT01794507. A study evaluating ABT-199 in multiple myeloma subjects who are receiving bortezomib and dexamethasone as standard therapy—full text view—ClinicalTrials.gov
NCT02391480. A study evaluating the safety and pharmacokinetics of ABBV-075 in subjects with cancer—full text view—ClinicalTrials.gov
NCT02755597. A study evaluating venetoclax (ABT-199) in multiple myeloma subjects who are receiving bortezomib and dexamethasone as standard therapy—full text view—ClinicalTrials.gov
NCT02758665. Trial of ibrutinib plus venetoclax plus obinutuzumab in patients with CLL (CLL2-GiVe)-full text view-ClinicalTrials.gov
NCT02987400. Combination of obinutuzumab and venetoclax in relapsed or refractory DLBCL—full text view—ClinicalTrials.gov
NCT03112174. Study of ibrutinib combined with venetoclax in subjects with mantle cell lymphoma (SYMPATICO)—full text view—ClinicalTrials.gov
NCT03181126 & AbbVie. A study of venetoclax in combination with navitoclax and chemotherapy in subjects with relapsed acute lymphoblastic leukemia—full text view—ClinicalTrials.gov
NCT03236857. A study of the safety and pharmacokinetics of venetoclax in pediatric and young adult patients with relapsed or refractory malignancies—full text view—ClinicalTrials.gov
NCT03314181. A study of combination therapy with venetoclax, daratumumab and dexamethasone (with and without bortezomib) in subjects with relapsed or refractory multiple myeloma—full text view—ClinicalTrials.gov
Nguyen M et al (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci 104:19512–19517
Niederst MJ, Engelman JA (2013) Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Sci Signal 6, re6
O’Brien SM et al (2005) Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol 23:7697–7702
O’Brien S et al (2009) 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol 27:5208–5212
O’Connor L et al (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J 17:384–395
Oda E et al (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058
Oltersdorf T et al (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677–681
Opferman JT et al (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671–676
Opferman JT et al (2005) Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307:1101–1104
Pan R et al (2014) Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 4:362–375
Park D et al (2013) Novel small-molecule inhibitors of Bcl-XL to treat lung cancer. Cancer Res 73:5485–5496
Perillo B, Sasso A, Abbondanza C, Palumbo G (2000) 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol 20:2890–2901
Pui C-H et al (2015) Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol 16:465–474
Punnoose EA et al (2016) Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther 15:1132–1144
Reed JC, Stein C, Subasinghe C, Haldar S, Croce CM, Yum S, Cohen J (1990) Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res 50:6565–6570
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ (2000) Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 14:23–27
Roberts AW et al (2012) Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 30:488–496
Roberts AW et al (2016) Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374:311–322
Roberts AW, Stilgenbauer S, Seymour JF, Huang DCS (2017) Venetoclax in patients with previously treated chronic lymphocytic leukemia. Clin Cancer Res 23:4527–4533
Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ (1996) Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia 10:456–459
Rudin CM et al (2012) Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 18:3163–3169
Seymour JF, Kipps TJ, Eichhorst BF, Hillmen P, D’Rozario JM, Assouline S, Owen CJ, Gerecitano J, Robak T, De la Serna J, Jaeger U, Cartron G, Montillo M, Humerickhouse R, Elizabet APK. Venetoclax plus rituximab is superior to bendamustine plus rituximab in patients with relapsed/refractory chronic lymphocytic leukemia—results from pre-planned interim analysis of the randomized phase 3 murano study. ASH Abstract 2017 (2017)
Seymour JF et al (2017) Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 18:230–240
Souers AJ et al (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19:202–208
Stilgenbauer S et al (2016) Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 17:768–778
Sturm I et al (2000) Impaired BAX protein expression in breast cancer: mutational analysis of the BAX and the p53 gene. Int J Cancer 87:517–521
Tahir SK et al (2017) Potential mechanisms of resistance to venetoclax and strategies to circumvent it. BMC Cancer 17:399
Teh T-C et al (2017) Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. https://doi.org/10.1038/leu.2017.243
Tolcher AW et al (2015) Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother Pharmacol 76:1025–1032
Tse C et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428
Tsujimoto Y, Cossman J, Jaffe E, Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228:1440–1443
Vaillant F et al (2013) Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 24:120–129
van Delft MF et al (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10:389–399
Varadarajan S et al (2013a) Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ 20:1475–1484
Varadarajan S et al (2013b) Sabutoclax (BI97C1) and BI112D1, putative inhibitors of MCL-1, induce mitochondrial fragmentation either upstream of or independent of apoptosis. Neoplasia 15:568–578
Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ (1993) Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J. Immunol. 151:2546–2554
Vogler M, Dinsdale D, Dyer MJS, Cohen GM (2013) ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. Br J Haematol 163:139–142
Wei MC et al (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 14:2060–2071
Weiss J, Gajek T, Köhler B, Haefeli W (2016) Venetoclax (ABT-199) might act as a perpetrator in pharmacokinetic drug-drug interactions. Pharmaceutics 8:5
Wilson WH et al (2010) Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 11:1149–1159
Woyach JA, Johnson AJ (2015) Targeted therapies in CLL: mechanisms of resistance and strategies for management. Blood 126
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Scheffold, A., Jebaraj, B.M.C., Stilgenbauer, S. (2018). Venetoclax: Targeting BCL2 in Hematological Cancers. In: Martens, U. (eds) Small Molecules in Hematology. Recent Results in Cancer Research, vol 212. Springer, Cham. https://doi.org/10.1007/978-3-319-91439-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-91439-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91438-1
Online ISBN: 978-3-319-91439-8
eBook Packages: MedicineMedicine (R0)