Abstract
Transient global brain ischemia, induced by cardiac arrest and resuscitation, results in reperfusion injury leading to delayed selective neuronal cell loss and post-resuscitation mortality. This study determined the effects of post-resuscitation hypotension and hypothermia on long-term survival following cardiac arrest and resuscitation. The capillary density was also determined. Based on the mean arterial blood pressure (MABP) at 1 h of recovery, the normotension group (MABP 80–120 mmHg) and hypotension group (MABP <80 mmHg) were defined. The overall survival was determined at 4 days of recovery. Brain microvascular density was assessed using immunohistochemistry of the glucose transporter, GLUT-1. The pre-arrest MABP was similar in each group; at 1 h after resuscitation, the MABP in the normotension groups was about 80% of their pre-arrest values; the hypotension group had a significantly lower MABP compared to the normotension group. The overall survival rate was lower in the hypotension group compared to the normotension group (36%, 4/11 vs. 67%, 14/21) under the normothermic condition. Brain blood flow in the hypotension group was lower (33% decrease) compared to the normotension group at 1-h post-resuscitation. Compared to the pre-arrest baseline, the capillary density was significantly increased at 14 days of recovery (355 ± 42 vs. 469 ± 50, number/mm2) in the cortex. The capillary density in hippocampus was also increased at 4–30 days following cardiac arrest and resuscitation. Our results suggest that rats able to maintain their post-resuscitation blood pressure at normotension, had higher brain blood flow during the early recovery phase, and improved survival outcome following cardiac arrest and resuscitation. In addition, cardiac arrest and resuscitation induced angiogenesis in brain in the first month of recovery.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
The brain is extremely sensitive to hypoxia and ischemia. Transient global brain ischemia induced by cardiac arrest and resuscitation results in reperfusion injury in the central nervous system leading to post-resuscitation mortality and morbidity . The incidence of cardiac arrest is still high and the outcome following cardiac arrest and resuscitation is poor. The American Heart Association reported that about 326,200 out-of-hospital cardiac arrests occurred in the USA during 2011, with only about 10% of the patients surviving to discharge from hospital, many of them suffering from neurological deficits.
In rat studies, following cardiac arrest and resuscitation , the non-surviving animals died from cardiorespiratory collapse, suggesting involvement of brainstem function, in particular the maintenance and regulation of cardiovascular and respiratory functions. We earlier reported damage of brainstem function with respect to respiratory regulation in rats [1, 2]. The maintenance of arterial blood pressure during the early recovery phase may also reflect the integrity of brainstem function following cardiac arrest and resuscitation. In addition, the higher blood pressure may be related to the higher blood flow in brain.
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that regulates the adaptive response to hypoxia, such as angiogenesis , and the resultant increased capillary density that typically occurs over a 3-week time course [3]. We have reported that, following cardiac arrest and resuscitation, HIF-1α accumulates as early as 1 h of recovery and the elevated HIF-1 levels are sustained for at least 1 week, irrespective of tissue hypoxia (indicated by hypoxic marker EF-5), which lasted for 2 days [4]. However, it is unknown whether there is an angiogenic response following an ischemia/reperfusion insult accompanied by a short period of hypoxia.
In this study we investigated the association between arterial blood pressure during the early recovery phase and long-term survival following cardiac arrest and resuscitation . Post-resuscitation brain capillary density was also determined.
2 Methods
2.1 Animals and Induction of Transient Global Ischemia
The experimental protocol was approved by the Animal Care and Use Committee at Case Western Reserve University. Male Fisher rats (3 months old) were used in these experiments. Transient global brain ischemia was achieved using a rat model of cardiac arrest and resuscitation, as described previously [1]. In brief, rats were anesthetized with isoflurane and cannulae were placed in the femoral artery and external jugular vein. Cardiac arrest was induced in the conscious rat by the rapid sequential intra-atrial injection of D-tubocurare (0.3 mg) and ice-cold KCl solution (0.5 M; 0.12 ml/100 g of body weight). Resuscitation was initiated at 10 min after arrest. The animal was orotracheally intubated and ventilation was begun simultaneously with chest compressions and the intravenous administration of normal saline. Once a spontaneous heart beat returned, epinephrine (4–10 μg) was administered intravenously, the animal was considered to be resuscitated when mean blood pressure rose above 80% of pre-arrest value. The duration of ischemia was about 12 min. Non-arrested rats went through the same surgical procedures except cardiac arrest. Based on the mean arterial blood pressure (MABP) at 1 h recovery, the normotension group (MABP 80–120 mmHg) and hypotension group (MABP <80 mmHg) were defined. In a separate group of rats, brain blood flows were measured in non-arrested rats and in rats at 1-h post-resuscitation. The overall survival was determined at 4 days of recovery .
2.2 Measurement of Brain Blood Flow
Regional brain (cortex, hippocampus, brainstem and cerebellum) blood flow was measured using [14C] iodoantipyrene (IAP) autoradiograph, as previously described [5]. In brief, the femoral artery catheter was attached to a withdrawal syringe pump set to a withdraw rate at 1.6 ml/min. A bolus of 25 μCi of [14C] IAP was injected intra-arterially 3 s after the pump was started. The rat was decapitated and the pump stopped simultaneously 10 s later. The brain was quickly removed, frozen and stored at −80 °C. The reference arterial blood was collected and its radioactive content was determined. For autoradiography, each frozen brain was sectioned (20 μm) in a cryostat at the levels of atlas plate 13, 30 and 69 [6]. Brain sections and [14C]-Micro-scale standards were placed on glass slides, covered with an autographic film and exposed for 21 days. The images were analyzed using a BIOQUANT image analysis system (R&M Biometries). Optical densities were converted to nCi per gram using standard curves generated from [14C]-Micro-scale standards. The blood flow was calculated by the equation: Blood flow (ml/g/min) = Tissue (nCi/g) × pump rate (ml/min)/Reference blood (nCi).
2.3 Determination of Cerebral Capillary Density
Brain microvascular density was assessed using immunohistochemistry of the glucose transporter, GLUT-1. At 4, 14 and 30 days after resuscitation rats were perfused and fixed. Brain capillaries (<15 μm) were identified by positive stain of GLUT-1 and capillary density (number/mm2) was determined in the frontal cortex and CA1 region of the hippocampus, as described previously (Benderro and LaManna 2011; [7, 8]). Perfusion, paraffin-embedded sectioning and capillary density determination of the mouse cerebral cortex, as described by Tsipis et al., was strictly adhered to for the purposes of the present study [8]. Coronal serial sections (5 μm) of frontal cortex (levels of Bregma 1.20–0.20 mm, (Paxinos and Franklin 2003)) and hippocampal sections (levels of Bregma −2.80 to −3.60 mm, [6]) were made on a microtome and stained for GLUT-1. For each brain, at least four different GLUT-1 stained sections were averaged for quantification. Each quantified section was at least 50 μm apart from the subsequent quantified section .
2.4 Statistical Analysis
Data are expressed as mean ± SD. Statistical analyses were performed using SPSS V20 for Windows. The comparison between any two groups was analyzed with a t-test for paired sample, two-tailed. The survival analysis was performed using a Wilcoxon (Gehan) survival analysis. Significance was considered at the level of p < 0.05.
3 Results
3.1 Arterial Blood Pressure and Brain Blood Flow
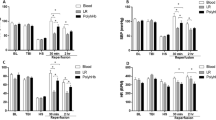
As shown in Fig. 1a, the pre-arrest MABP was similar (~110 mmHg) in the normotension group and the hypotension group. At 1 h following cardiac arrest and resuscitation, the MABP was 90 ± 12 mmHg (mean ± SD, n = 21) and 61 ± 17 mmHg (n = 14) in the normotension group and hypotension group, respectively. The cerebral blood flow decreased significantly in both the normotension and hypotension groups at 1-h post-resuscitation compared to the non-arrested controls (ml/mg/min, 0.62 ± 0.10, n = 4 and 0.42 ± 0.02, n = 4, respectively vs. 1.48 ± 0.08, n = 7). However, the normotension group had significantly higher (about 47%) cerebral blood flows compared to the hypotension group (Fig. 1b). The non-arrested blood flows (ml/mg/min) in the hippocampus, brain stem and cerebellum were 0.89 ± 0.22, 1.11 ± 0.26 and 0.85 ± 0.23, respectively. A similar profile of post-resuscitation blood flow change was observed between the normotension group and the hypotension group in these regions of brain. For example, at 1-h post-resuscitation, the blood flows in the brainstem was significantly higher in the normotension group compared to the hypotension group (ml/mg/min, 0.92 ± 0.07 vs. 0.56 ± 0.08, t-test, p < 0.05).
(a) Mean blood pressure in the normotension (n = 21) and hypotension (n = 14) groups before cardiac arrest and at 1 h following resuscitation. (b) Blood flow in cortical brain in the non-arrested controls (n = 7) and at 1-h post-resuscitation in the normotension and the hypotension groups (n = 4 each group). *significance vs. compared to the pre-arrest baseline. # indicates significant difference compared to the normotension group (t-test, p < 0.05)
3.2 Overall Survival
Overall survival rates were examined for 4 days following cardiac arrest and re-suscitation in the normotension and hypotension groups (Fig. 2). The 4-day survival rate was significantly higher (Wilcoxon (Gehan) survival analysis, p < 0.05) in the normotension group (67%, 14/21) compared to the hypotension group (36%, 4/11). In these animal experiments, most deaths occurred within the first 2 days following cardiac and resuscitation .
3.3 Post-resuscitation Cerebral Capillary Density
As shown in Fig. 3, microvascular density (N/mm2) was determined by GLUT-1 positive capillary profiles identified in the cerebral cortex and CA1 region of hippocampus. Compared to the pre-arrest baseline, the capillary density was significantly increased at 14 days of recovery (355 ± 42 vs. 469 ± 50, mean ± SD, n = 7 each) in the cortex. The baseline of capillary density in the CA1 region of hippocampus was higher (472 ± 10) compared to that of the cortex. The hippocampal capillary density was also significantly increased (about 15%, t-test, p < 0.05) at 4 days (539 ± 41) and 30 days (551 ± 49) post-resuscitation, respectively, compared to the pre-attest baseline .
GLUT-1 immunohistochemistry (left panel, non-arrested and 14d post-resuscitation) and microvascular density (N/mm2) as identified by GLUT-1 positive endothelial cells (right panel) in the cerebral cortex following cardiac arrest and resuscitation. Values are mean ± SD, *significance vs. pre-arrest control group (t-test, p < 0.05), n = 7 for each group
4 Discussion
This study shows that rats able to maintain MABP at normotensive level during the early recovery phase, had improved survival outcome following cardiac arrest and resuscitation, and also had higher brain blood flow. We previously reported that, in rats, brain hypoperfusion lasted for days following cardiac arrest and resuscitation [5]. The ability to maintain higher blood pressure may reflect the less compromised brainstem function in these animals. Therefore, maintaining or even elevating arterial blood pressure may be beneficial to improve long-term survival following cardiac arrest and resuscitation. Hossmann and coworkers showed that cerebral perfusion pressure during reperfusion is important for recovery of neuronal electrical activity after global cerebral ischemia [9]. Brucken et al. demonstrated that brief inhalation of nitric oxide (iNO) during resuscitation increases resuscitation success and improves 7-day survival after cardiac arrest in rats. The induction of higher MAPs post-resuscitation was among the beneficial effects associated with iNO [10]. Safar and coworkers showed for the first time that long-term functional outcome can be improved after prolonged cardiac arrest with immediate and prolonged postarrest induced hypertension plus hemodilution and heparinization [11]. Recently the Neuroprotect post-CA trial has been designed to investigate whether a more aggressive hemodynamic strategy to obtain a MAP 85–100 mmHg reduces brain ischemia and improves outcome when compared with standard treatment (MAP 65 mmHg) in comatose post-resuscitation patients [12].
We also found that capillary density was increased in the cortex and hippocampus during the first month of recovery following cardiac arrest and resuscitation. The response and hence the mechanism of post-resuscitation angiogenesis may be related to the increased accumulation of HIF-1 and its target genes, which may be similar to hypoxia-induced angiogenesis.
In summary, rats able to maintain post-resuscitation normotension had higher brain blood flow and improved survival outcome; angiogenesis was induced in brain the first month following cardiac arrest and resuscitation.
References
Xu K, LaManna JC (2009) The loss of hypoxic ventilatory responses following resuscitation after cardiac arrest in rats is associated with failure of long-term survival. Brain Res 1258:59–64
Xu K, Puchowicz MA, Sun X et al (2010) Decreased brainstem function following cardiac arrest and resuscitation in aged rat. Brain Res 1328:181–189
Xu K, LaManna JC (2006) Chronic hypoxia and the cerebral circulation. J Appl Physiol 100:725–730
Chavez JC, LaManna JC (2002) Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci 22:8922–8931
Xu K, Puchowicz MA, Lust WD et al (2006) Adenosine treatment delays postischemic hippocampal CA1 loss after cardiac arrest and resuscitation in rats. Brain Res 1071:208–217
Palkovits M, Brownstein MJ (1988) Maps and guide to microdissection of rat brain. Elsevier, New York
Benderro GF, LaManna JC (2014) HIF-1α/COX-2 expression and mouse brain capillary remodeling during prolonged moderate hypoxia and subsequent re-oxygenation. Brain Res 1569:41–47
Tsipis CP, Sun X, Xu K et al (2014) Hypoxia-induced angiogenesis and capillary density determination. Methods Mol Biol 1135:69–80
Hossmann V, Hossmann KA (1973) Return of neuronal functions after prolonged cardiac arrest. Brain Res 60(2):423–438
Brucken A, Derwall M, Bleilevens C et al (2015) Brief inhalation of nitric oxide increases resuscitation success and improves 7-day-survival after cardiac arrest in rats: a randomized controlled animal study. Crit Care 19:408
Safar P, Stezoski W, Nemoto EM (1976) Amelioration of brain damage after 12 minutes’ cardiac arrest in dogs. Arch Neurol 33(2):91–95
Ameloot K, De Deyne C, Ferdinande B et al (2017) Mean arterial pressure of 65 mm hg versus 85–100 mm Hg in comatose survivors after cardiac arrest: rationale and study design of the Neuroprotect post-cardiac arrest trial. Am Heart J 191:91–98
Acknowledgments
This study was supported by NIH grant NINDS 1 R01 NS46074.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Xu, K., Puchowicz, M.A., LaManna, J.C. (2018). Post-resuscitation Arterial Blood Pressure on Survival and Change of Capillary Density Following Cardiac Arrest and Resuscitation in Rats. In: Thews, O., LaManna, J., Harrison, D. (eds) Oxygen Transport to Tissue XL. Advances in Experimental Medicine and Biology, vol 1072. Springer, Cham. https://doi.org/10.1007/978-3-319-91287-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-91287-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91285-1
Online ISBN: 978-3-319-91287-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)