Abstract
We tested the hypothesis that ERβ is involved in respiratory control in female mice. We used young adult (5–6 months-old) and aged (17–18 months-old) ERβKO or wild-type controls (WT) female mice to assess arterial blood pressure (via a tail-cuff sensor) and indices of respiratory pattern (sighs and apneas – recorded by whole body plethysmography at rest). We also measured respiratory parameters at rest and in response to brief (<10 min) exposure to hypoxia (12% O2) or hypercapnia (5% CO2). Because ERβ is localized in mitochondria, and because estradiol and ERβ agonist increase mitochondrial O2 consumption, we assessed the mitochondrial respiration (with a high-resolution oxygraph system) and the in vitro activity of the complex I of the electron transfer chain in samples of brain cortex in aged wild-type and ERβKO female mice. Compared to young WT mice, young ERβKO mice had elevated arterial blood pressure, but similar ventilatory responses to hypoxia and hypercapnia. In old ERβKO female mice compared to old WT mice, the arterial blood pressure was lower, the frequency of sighs was higher and the frequency of apneas was lower, and the hypoxic and hypercapnic ventilatory responses were reduced. In old ERβKO mice mitochondrial respiration and complex I activities in the brain cortex were lower than in WT mice. We conclude that ERβ has age-specific effects on vascular and respiratory functions in female mice.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Through their effects in the central nervous system, steroid hormones such as estradiol or progesterone play an essential role in the regulation of sexual behavior (Brinton et al. 2008; Micevych et al. 2015). These hormones are also involved in processes governing mood or cognition (Marrocco and McEwen 2016), and regulate homeostatic functions such as thermoregulation (Charkoudian et al. 2017) or respiratory control (Boukari et al. 2016, 2017; Behan and Wenninger 2008). Because the occurrence of sleep apnea is higher in men than in women and increases after menopause in women (Block et al. 1979; Bixler et al. 2001), it is of clinical relevance to better understand the effects of ovarian hormones on respiratory regulation. Earlier studies performed in cats suggested that the main effect of estradiol was limited to increase the expression of the progesterone receptor, thereby increasing the respiratory effect of progesterone (Bayliss et al. 1990). Thus, the effects of estradiol, and the role of the classical estradiol receptors (ERα and ERβ) in respiratory control remain poorly investigated. Nonetheless, we have reported that in newborn rats, long-term treatment with estradiol slightly reduced respiratory frequency and increased the frequency and length of apnea under resting conditions (Lefter et al. 2008). During hypoxia, estradiol treatment suppressed the normal decrease of body temperature, without altering the ventilatory response (Lefter et al. 2008). Furthermore, we showed recently that estradiol protects against the increased chemoreflex activity and respiratory instabilities induced by exposure to intermittent hypoxia (Laouafa et al. 2017b), conceivably due to protective effects of estradiol against the oxidative stress induced by intermittent hypoxia (Laouafa et al. 2017b), and likely involve effect of estradiol on mitochondrial respiration and production of reactive oxygen species in female rats exposed to intermittent hypoxia (Laouafa et al. 2017a). These results indicate that estradiol could have important roles on respiratory regulation that remains poorly understood. The effects of estradiol could be more important under specific pathophysiological conditions, and/or may be specific to sex- or age.
Classical ERs belong to the superfamily of steroid receptors, acting as transcription factors able to modulate the expression of target genes that have an “Estrogen Response Element” in their promoter region (Harris 2007). Two ERs have been described, ERα and ERβ, that are coded by different genes (Kuiper et al. 1996). We previously reported by immunohistochemistry that ERβ is expressed in the peripheral chemoreceptors of adult rats (Joseph et al. 2006), and ERβ mRNA and protein are densely expressed in central areas involved in respiratory control including the nucleus tractus solitarius, locus coeruleus, and the parabrachial nucleus (Shughrue et al. 1997; Shughrue and Merchenthaler 2001).
In the present study, we tested the hypothesis that ERβ is involved in respiratory control in female mice. We used young adult (5–6 months-old) and aged (17–18 months-old) ERβKO female mice to assess respiratory parameters at rest and in response to brief (<10 min) exposure to hypoxia (12% O2) or hypercapnia (5% CO2). Because previous studies showed elevated arterial pressure in young ERβKO female mice (Zhu et al. 2002), we also recorded arterial pressure in young and aged mice. Finally, because ERβ is localized to mitochondria, and because ERβ agonist and estradiol increase mitochondrial O2 consumption (Irwin et al. 2012), we assessed the mitochondrial respiration and the in vitro activity of the complex I of the electron transfer chain in samples of brain cortex in aged wild-type and ERβKO female mice.
15.2 Material and Methods
We used female ERβKO mice (Krege et al. 1998) (B6.129P2-Esr2tm1Unc/J; n = 9) and their recommended wild-type control (WT: C57BL/6 J; n = 7) from The Jackson laboratory. The mice were kept in our animal care facility under standard conditions with food and water available ad-libitum. All experiments were approved by our local ethic committee (Ref: 2014–156) and strictly adhered to the rules of the Canadian Council on Animal Care. We used the same mice at the age of 5–6 months (young) and 17–18 months (old) to perform measurement of arterial pressure and respiratory recordings. Old mice were sacrificed a few days after the last measurements and brain samples were used to assess mitochondrial respiration and in vitro activities of mitochondrial complex I.
15.2.1 Measurements of Arterial Pressure
Arterial blood pressure was measured as described previously in female rats (Laouafa et al. 2017b) by the tail cuff method and volume-pressure recording (CODA system – Kent Scientific, Torrington, CT, USA). Conscious mice were placed in a restrainer tube over a warmed blanket. After 30 min of habituation, several recordings were performed, separated at least by 5 min. We report the mean of the 3 lowest values for systolic, diastolic, and mean arterial pressures.
15.2.2 Respiratory and Metabolic Recordings
All recordings were performed as previously described for adult mice (Boukari et al. 2016; Marcouiller et al. 2014). Briefly, animals were placed in a whole-body plethysmograph chamber (Emka Technologies – Paris, France), flushed with fresh room air. For each animal, the flow rate was adjusted to avoid excessive built-up of CO2 within the recording chamber. Flow rate fluctuated between 160 and 250 ml/min, CO2 was measured in the outflowing air and was between 0.4 and 0.6%. Mice were left undisturbed for 4 h under room air to ensure a period of normoxic recordings representative of a resting state, during which mice spend much of their time asleep (Marcouiller et al. 2014; Bastianini et al. 2017). A subsampling pump was connected to the chamber for analysis of O2%, CO2%, and water pressure using dedicated gas analyzers (FC-10, CA-10 and RH-300 respectively, Sable Systems International, Las vegas, NV, USA). During recordings, values of O2%, CO2% and water pressure in the inflowing gas line were periodically measured and later used for calculation of O2 consumption and CO2 production using standard equations (Boukari et al. 2016). Rectal temperature was measured at the end of the normoxic recording period. Following the 4 h periods of recordings, the animals were exposed to brief periods of hypoxia (12% O2–10 mins) and hypercapnia (5% CO2–10 min) in randomized order, with 10–15 minutes between each test.
15.2.3 Analysis of Respiratory and Metabolic Rate Variables
All respiratory and metabolic variables were analyzed as performed previously in adult rats or mice (Laouafa et al. 2017b; Boukari et al. 2016; Marcouiller et al. 2014), to report respiratory frequency, tidal volume, minute ventilation, O2 consumption rate, and CO2 production under normoxic conditions and in response to hypoxia or hypercapnia. Because exposure to hypoxia was short (<10 min) and measurements are typically taken around the 5th minute of exposure, we estimated that rectal temperature and metabolic rate were similar in hypoxia and normoxia. We used standard criteria to report sigh, post-sigh apnea, and spontaneous apnea frequency under resting conditions (Laouafa et al. 2017b; Boukari et al. 2016; Marcouiller et al. 2014).
15.2.4 Mitochondrial Respiration Recordings on Permeabilized Brain Samples
In old mice, a few days after the respiratory measurements, the animals were sacrificed with an overdose of anesthetic (ketamine/xylasine), the brain cortex was rapidly dissected and immediately used to perform measurements of mitochondrial respiratory functions using an O2K Oroboros respirometer (Oroboros Instruments, Innsbruck, Austria).
After calibration of the Oroboros chambers, cortex samples were weighted (2–3 mg), and recordings of O2 consumption performed at 37 °C in a respiration buffer (0.5 mM EGTA, 3 mM MgCl2, 60 mM potassium lactobionate, 10 mM KH2PO4, 20 mM Hepes, 110 mM sucrose, 1 g/l BSA). Based on a previous study establishing the optimal conditions for measurements of mitochondrial respiration in permeabilized brain samples (Herbst and Holloway 2015), the samples are incubated in the recording chamber for 20 min with saponin (50 μg/ml). For each sample, we have differentiated mitochondrial respiration linked to NADH oxidation through the mitochondrial respiratory complex I (using pyruvate 5 mM, malate 0.2 mM, and glutamate 10 mM as substrates), and mitochondrial respiration linked to FADH2 oxidation through the mitochondrial respiratory complex II (succinate 5 mM and rotenone 4 μM to block complex I activity). After equilibration with the substrates, ADP (500 μM) is added to the chambers to measure O2 consumption under normal phosphorylating state (ATP synthesis – mitochondrial respiratory state 3). We then add cytochrome-c to assess the integrity of mitochondrial membranes, then oligomycin (2 μg/mg tissue) was added to block ATP synthesis and measure O2 consumption due to leakage of protons in non-phosphorylating state (no ATP synthesis – state 4). Uncoupling is further exaggerated by adding graded bolus of carbonyl cyanide m-chlorophenylhydrazone (CCCP – 0.5 μM in 1 μl/bolus) until reaching stable O2 consumption. We then blocked the activity of the complex 3 with antimycin A (2.5 μM) to measure non-mitochondrial O2 consumption due to cytosolic oxidases. Finally, the maximum activity of the complex 4 (cytochrome c oxidase) is measured by using ascorbate (2 mM) and tetramethyl-p-phenylenediamine dihydrochloride (TMPD; 0.5 mM).
15.2.5 In vitro Activity of the Mitochondrial Complex I
Frozen cortex (20 mg/ml) were homogenized in 100 mM phosphate buffer KPI (100 mM K2HP04, 100 mM KH2PO4, PH7,4) + EDTA 2 mM. Then, the tissues were centrifuged for 10 min at 1500 g, and the supernatant was collected and kept frozen at −80 °C. The concentration of proteins was determined by a standard colorimetric BCA assay kit (Thermo Scientific, catalogue # 23225) and complex I activity was normalized to protein concentration.
Complex I activity was assessed by measuring the disappearance rate of NADH by spectrophotometry. Briefly, the day of measurement, a solution containing 50 mM KPI buffer, 1 mM EDTA, 100 μM decylubiquinone, and 3.75 mg/ml BSA (10%) was prepared. A similar solution was prepared with rotenone (10 μM: inhibitor of complex I activity). 4 μl of each sample was added in two different wells. In the first well, we added 250 μl of solution containing rotenone, and in the second well we added 250 μl of cocktail without rotenone. We then added 3 mM of NADH into each well, and read the absorbance at 340 nm every 15 s for 3 min to monitor the rate of NADH disappearance. Since NADH can be consumed by complex I of the mitochondria and by cytochrome b5 reductase, the activity of the complex I was obtained by subtracting the slope of the well with rotenone (activity without complex I) from the slope of the well without rotenone (total activity).
15.2.6 Statistics
For arterial blood pressure, we used a student’s t-test to assess if significant differences appeared between WT and ERβKO mice at each age. For respiratory variables at rest, we used a one-way ANOVA to assess if significant differences appear between groups (Young WT, young ERβKO, old WT, old ERβKO). When the ANOVA reported a significant effect (P < 0.05) we used a Fisher’s LSD test to assess differences between WT and ERβKO mice with each age, and differences between old and young mice for each genotype. For the hypoxic or hypercapnic ventilatory response, we used 2 way-ANOVA for repeated measures, with genotype and gas exposure as grouping variables, followed by a post-hoc Fisher LSD test if significant effect of genotype or significant interaction between genotype and gas exposure appeared. All analyses were performed using GraphPad Prism 7. All values are reported as mean ± sem.
15.3 Results
15.3.1 Arterial Pressure in Young and Old WT and ERβKO Female Mice
As previously reported in ERβKO mice (Zhu et al. 2002), the systolic, mean, and diastolic arterial pressures were elevated in young ERβKO compared to wild-type (Fig.15.1 – upper panels). However, in older mice there was an opposite effect, with ERβKO mice showing reduced diastolic and mean arterial pressures than wild-type (Fig. 15.1 – lower panels).
15.3.2 Body Weight, Respiratory and Metabolic Variables at Rest in Young and Old WT and ERβKO Female Mice
Body weight was higher in ERβKO vs wild-type mice at both ages (Table 15.1), the mean of the difference between the WT and KO mice was 1.8 ± 0.8 g in young mice, and 14.2 ± 2.7 g in older mice, thus indicating a gradual increase in body weight during aging in ERβKO mice. Previous studies reported an increased body weight in ERβKO mice, which was associated with increased fat mass when mice reached 10–12 months of age (Seidlova-Wuttke et al. 2012) but not in younger (4 months-old) animals (Ohlsson et al. 2000). This suggests an important role of ERβ to avoid excessive adipose tissue accumulation during aging in female mice, but this remains poorly documented (Davis et al. 2013).
All respiratory and metabolic parameters measured at rest were similar between WT and ERβKO mice. Compared to young ERβKO, in old KO mice respiratory frequency was higher, and CO2 production rate was lower (Table 15.1). Interestingly, in old ERβKO mice the frequency of sigh was more elevated than in WT mice and in young KO mice (Fig. 15.2). The frequency of post-sigh apnea was reduced by aging, and in aged ERβKO mice the frequency of post-sigh and spontaneous apnea were drastically reduced compared to aged WT mice.
Typical respiratory recordings from an old WT and an old ERβKO mice showing the occurrence of a sigh followed by apneas in the WT but not in the ERβKO mice. Lower panels: frequency of sigh, post-sigh apnea, and spontaneous apnea recorded at rest in young and old WT and ERβKO mice. *p < 0.05 ERβKO vs WT (same age). °, °°p < 0.05 and p < 0.01 old vs young (same genotype)
15.3.3 Hypoxic and Hypercapnic Ventilatory Responses in Young and Old WT and ERβKO Female Mice
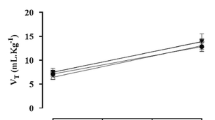
During the exposures to hypoxia and hypercapnia in young mice, there were no differences between wild-type and ERβKO (Fig. 15.3). In aged mice, compared to wild-type controls ERβKO aged mice had a reduced ventilatory response to hypoxia due to a lower tidal volume (Fig. 15.4 – upper rows). In response to CO2 exposure, minute ventilation was 39% lower in ERβKO compared to wild-type mice (Fig. 15.4 lower rows), again due to a lower elevation of tidal volume in response to the CO2 exposure.
15.3.4 Mitochondrial Respiration and In vitro Activity of Electron Transport Chain Complexes in Young and Old WT and ERβKO Female Mice
Mitochondrial respiration was measured only in old WT and ERβKO mice using permeabilized cortical brain samples. When the respiratory activity was activated by substrates of complex I, the mitochondrial O2 consumption was lower in ERβKO mice compared to WT control (Fig. 15.5). This was not observed with substrates of complex II, and there was no significant difference for the maximum activity of complex IV (cytochrome oxidase) between wild-type and ERβKO mice.
Mitochondrial function in samples of permeabilized brain cortex in old (17–18 months) WT and ERβKO female mice. Upper panels: O2 consumption rate (pmol O2/second/mg tissue) with substrates of complex I (left – NADH-linked respiration), and substrates of complex II (right – FADH2- linked respiration). PMG: Pyruvate, malate, glutamate (substrates of complex I), SR succinate, rotenone (substrate of complex II and blockade of complex I), ADP adenosine di-phosphate, OligoM Oligomycin A, CCCP carbonyl cyanide m-chlorophenylhydrazone. See text for further details. Lower panels: activity of complex I and IV of the electron transport chain (see text). *, **p < 0.05, and p < 0.01 ERβKO vs WT
The activity of complex I measured in vitro by the disappearance rate of NADH was lower in ERβKO mice compared to WT (Fig. 15.5).
15.4 Discussion
The present studies show that deletion of ERβ in female mice leads to age-specific alterations of the cardio-vascular and respiratory systems. Interestingly, while elevated blood pressure was present in young ERβKO female mice, our results indicate that this effect does not persist in older ERβKO mice. In fact, in old ERβKO mice arterial blood pressure was lower than in old WT. In contrast, alterations in the respiratory pattern and chemoreflex functions appeared in old, but not in young ERβKO female mice. In older mice, our results also show a reduced mitochondrial respiration in brain cortex through alteration of complex I activity.
15.4.1 Role of ERβ on the Cardiovascular System in Old Vs Young Female Mice
A recent systematic review based on the analysis of 88 studies (mostly on animal models or tissues) provides a comprehensive overview on the role of ERβ in the female cardiovascular system (Muka et al. 2016). ERβ is expressed in endothelial cells, and has an important role by a direct, vasodilatory action on blood vessels. ERβ regulates nitric oxide (NO) bio-availability and inflammatory markers in arterial walls, thus contributing to the regulation of arterial pressure (Muka et al. 2016). Our results showing elevated blood pressure in young female ERβKO mice are in line with previous studies, showing similar results (Zhu et al. 2002). We’ve been surprised to observe that arterial pressure is reduced in old ERβKO mice compared to their wild-type controls, and we are not aware of previous published report showing this effect. While it has been concluded that ERβ plays a vasodilatory role (Muka et al. 2016), some studies have reported that vasodilation mediated by an ERβ agonist does not require NO signaling, and that ERβ might downregulate ERα- and NO-mediated vasodilation (Cruz et al. 2006). Furthermore, in a mouse model of accelerated senescence, age profoundly impacts the effect of estrogen-mediated regulation of NO and oxidative stress systems. In young females, estrogen increases the expression of endothelial NO synthase (eNOS) and decreases oxidative stress, but in aged mice estradiol treatment does not modulate eNOS expression. In isolated aorta of aged mice estradiol increases the expression of the NADPH oxidase subunit NOX1, and increases generation of superoxide anion (O2 −, one of the major reactive oxygen species) by NADPH oxidase (Novensa et al. 2011). Interestingly, such reversal of estrogen action on the vascular system in aged mice was associated to increased expression level of ERβ and reduced expression level of ERα, leading to a high ratio of ERβ/ERα expression, (Novensa et al. 2011). It has been reported that ERβ inhibits ERα-dependent gene expression, and might thus opposes the vascular effects of ERα (Matthews and Gustafsson 2003). Since ERα is a potent modulator of vasodilation and NO synthesis (Traupe et al. 2007), we might therefore hypothesize that in aged ERβKO mice ERα signaling in blood vessels is increased, leading to vasodilation and reduced blood pressure. This scenario remains speculative, but we believe that it helps understanding our results.
15.4.2 Role of ERβ on the Respiratory Control System in Old Vs Young Female Mice
Contrasting with data showing higher blood pressure in young ERβKO mice, there was no evidence of changes of the respiratory control system in these animals. However, compared to their wild-type controls, old ERβKO had several alterations, including higher frequency of sighs recorded during sleep, but a much lower occurrence of post-sigh and spontaneous apneas, and reduced hypoxic and hypercapnic ventilatory responses. As mentioned in the introduction, ERβ mRNA and protein are both expressed in brainstem areas involved in respiratory control (such as the nucleus tractus solitarius, locus coeruleus, parabrachial nucleus), in hypothalamic nuclei (Shughrue and Merchenthaler 2001; Shughrue et al. 1997), and in peripheral chemoreceptors (Joseph et al. 2006). The fact that ERβ is present in the peripheral chemoreceptors and in the nucleus tractus solitarius, the main projection site of the peripheral chemoreceptors in the brainstem (Finley and Katz 1992), lend support to the hypothesis that ERβ modulates responses elicited by activation of the peripheral chemoreceptor, which is in line with the drastically attenuated hypoxic response in ERβKO female mice. Interestingly, the hypercapnic ventilatory response was not completely abolished but reduced by about 50–55% in ERβKO mice compared to wild-type controls. Furthermore, responses to hypoxia and hypercapnia were both reduced owing to a lower tidal volume, and the magnitude of the difference observed between WT and ERβKO for both hypoxic and hypercapnic ventilatory responses were similar. These findings suggest a common mechanism might be responsible for the altered hypoxic and hypercapnic ventilatory responses, possibly involving a reduction of the peripheral chemoreceptor drive in response to hypoxia and hypercapnia. Alternatively, a central inhibition and/or altered integrative processes common to respiratory responses to hypoxia and hypercapnia could also explain these results.
Other interesting aspects of our data are the differences of sigh and apnea frequency in normoxia between WT and ERβKO mice. As reported for alterations of the chemoreflex responses, these effects are observed only in old mice. Sighs are very common, and play an important, but often overlooked, role to regulate the respiratory control system, and could possibly have functions extending well beyond this system (Ramirez 2014). Because they are readily observable in brainstem slices, sighs are likely an intrinsic property of the respiratory control system, and in most cases, in vitro and in vivo sighs are followed by a cessation of breathing, referred to as “post-sigh apneas” (Ramirez 2014). Interestingly, while sigh frequency was increased in ERβKO mice compared to WT controls, the frequency of post-sigh apnea was drastically reduced in ERβKO mice. While we could speculate on the significance of these findings in light of our most recent understanding of sigh functions and underlying neurobiology (Ramirez 2014), we prefer to remain cautious, and simply report this intriguing phenotype observed in aged ERβKO female mice.
15.4.3 Role of ERβ on Mitochondrial Respiration in Old Female Mice
Finally, our data shows that ERβ deletion reduces mitochondrial respiratory activity and activity of electron transfer chain complex I in brain cortex samples. These results are intriguing because at the level of the peripheral chemoreceptors recent data support the hypothesis that the mitochondrial activity contributes to oxygen sensing (Fernandez-Aguera et al. 2015; Chang 2017; McElroy and Chandel 2017). Therefore, if similar effects of ERβ deletion are present in the peripheral chemoreceptors of ERβKO mice, this may likely contribute to the reduced hypoxic ventilatory response in older mice. These data are in line with the effect of estradiol on mitochondrial function, which are mediated by ERα and ERβ (Yang et al. 2004; Irwin et al. 2012; Razmara et al. 2008). The modulations of mitochondrial function by E2 and ERs are thought to contribute to cardio-vascular and neurological protective effects in women before menopause, and to explain the longer life expectancy of women compared to men (Nilsen and Brinton 2004; Arnold et al. 2012; Irwin et al. 2012; Razmara et al. 2008).
15.5 Conclusion
We conclude that KO mice are a reliable model to assess the role of specific estradiol receptor on respiratory regulation. We started our studies with young ERβKO mice, and because there was no evidence for altered respiratory control system, we decided to keep these animals until they reached an older age. Our data reveal that in 17–18 months-old mice, the deletion of ERβ leads to several alterations of the respiratory control system, including reductions of chemoreflex functions. Because this is associated with a lower mitochondrial respiratory activity in the central nervous system, our results show that ERβ acts at different levels of the oxygen transport cascade (from pulmonary ventilation to mitochondrial O2 consumption). This may be relevant in pathological or physiological conditions in which O2 homeostasis is compromised (sleep apnea, altitude, lung diseases), and research efforts to further understand the role of estradiol and ERs in these conditions should be further developed.
References
Arnold S, Victor MB, Beyer C (2012) Estrogen and the regulation of mitochondrial structure and function in the brain. J Steroid Biochem Mol Biol 131(1–2):2–9. https://doi.org/10.1016/j.jsbmb.2012.01.012
Bastianini S, Alvente S, Berteotti C, Lo Martire V, Silvani A, Swoap SJ, Valli A, Zoccoli G, Cohen G (2017) Accurate discrimination of the wake-sleep states of mice using non-invasive whole-body plethysmography. Sci Rep 7:41698. https://doi.org/10.1038/srep41698
Bayliss DA, Cidlowski JA, Millhorn DE (1990) The stimulation of respiration by progesterone in ovariectomized cat is mediated by an estrogen-dependent hypothalamic mechanism requiring gene expression. Endocrinology 126:519–527
Behan M, Wenninger JM (2008) Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol 164(1–2):213–221. https://doi.org/10.1016/j.resp.2008.06.006
Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A (2001) Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163(3 Pt 1):608–613
Block A, Boysen P, Wynne J, Hunt L (1979) Sleep apnea, hypopnea and oxygen desaturation in normal subjects. A strong male predominance. N Engl J Med 300:513–517
Boukari R, Rossignol O, Baldy C, Marcouiller F, Bairam A, Joseph V (2016) Membrane progesterone receptor-beta, but not -alpha, in dorsal brain stem establishes sex-specific chemoreflex responses and reduces apnea frequency in adult mice. J Appl Physiol 121(3):781–791. https://doi.org/10.1152/japplphysiol.00397.2016
Boukari R, Laouafa S, Ribon-Demars A, Bairam A, Joseph V (2017) Ovarian steroids act as respiratory stimulant and antioxidant against the causes and consequences of sleep-apnea in women. Respir Physiol Neurobiol 239:46–54. https://doi.org/10.1016/j.resp.2017.01.013
Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29(2):313–339. https://doi.org/10.1016/j.yfrne.2008.02.001
Chang AJ (2017) Acute oxygen sensing by the carotid body: from mitochondria to plasma membrane. J Appl Physiol 1985:jap 00398 02017. doi:https://doi.org/10.1152/japplphysiol.00398.2017
Charkoudian N, Hart ECJ, Barnes JN, Joyner MJ (2017) Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res 27(3):149–155. https://doi.org/10.1007/s10286-017-0420-z
Cruz MN, Douglas G, Gustafsson JA, Poston L, Kublickiene K (2006) Dilatory responses to estrogenic compounds in small femoral arteries of male and female estrogen receptor-beta knockout mice. Am J Physiol Heart Circ Physiol 290(2):H823–H829. https://doi.org/10.1152/ajpheart.00815.2005
Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y, Wang ZV, Khan SA, Clegg DJ (2013) The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2(3):227–242. https://doi.org/10.1016/j.molmet.2013.05.006
Fernandez-Aguera MC, Gao L, Gonzalez-Rodriguez P, Pintado CO, Arias-Mayenco I, Garcia-Flores P, Garcia-Perganeda A, Pascual A, Ortega-Saenz P, Lopez-Barneo J (2015) Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab 22(5):825–837. https://doi.org/10.1016/j.cmet.2015.09.004
Finley JC, Katz DM (1992) The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572(1–2):108–116
Harris HA (2007) Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol 21(1):1–13. https://doi.org/10.1210/me.2005-0459
Herbst EA, Holloway GP (2015) Permeabilization of brain tissue in situ enables multiregion analysis of mitochondrial function in a single mouse brain. J Physiol 593(4):787–801. https://doi.org/10.1113/jphysiol.2014.285379
Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD (2012) Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol 24(1):236–248. https://doi.org/10.1111/j.1365-2826.2011.02251.x
Joseph V, Doan VD, Morency CE, Lajeunesse Y, Bairam A (2006) Expression of sex-steroid receptors and steroidogenic enzymes in the carotid body of adult and newborn male rats. Brain Res 1073–1074:71–82. https://doi.org/10.1016/j.brainres.2005.12.075
Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O (1998) Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95(26):15677–15682
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93(12):5925–5930
Laouafa S, Bairam A, Soliz J, Roussel D, Joseph V (2017a) Estradiol receptor agonists α and β protect against brain mitochondrial dysfunction in a model of sleep apnea. FASEB J 31(1 Supplement):696.6–696.6
Laouafa S, Ribon-Demars A, Marcouiller F, Roussel D, Bairam A, Pialoux V, Joseph V (2017b) Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep 40(8). https://doi.org/10.1093/sleep/zsx104
Lefter R, Doan VD, Joseph V (2008) Contrasting effects of estradiol and progesterone on respiratory pattern and hypoxic ventilatory response in newborn male rats. Respir Physiol Neurobiol 164(3):312–318. https://doi.org/10.1016/j.resp.2008.07.026
Marcouiller F, Boukari R, Laouafa S, Lavoie R, Joseph V (2014) The nuclear progesterone receptor reduces post-sigh apneas during sleep and increases the ventilatory response to hypercapnia in adult female mice. PLoS One 9(6):e100421. https://doi.org/10.1371/journal.pone.0100421
Marrocco J, McEwen BS (2016) Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci 18(4):373–383
Matthews J, Gustafsson JA (2003) Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv 3(5):281–292. https://doi.org/10.1124/mi.3.5.281
McElroy GS, Chandel NS (2017) Mitochondria control acute and chronic responses to hypoxia. Exp Cell Res 356(2):217–222. https://doi.org/10.1016/j.yexcr.2017.03.034
Micevych PE, Wong AM, Mittelman-Smith MA (2015) Estradiol membrane-initiated signaling and female reproduction. Compr Physiol 5(3):1211–1222. https://doi.org/10.1002/cphy.c140056
Muka T, Vargas KG, Jaspers L, Wen KX, Dhana K, Vitezova A, Nano J, Brahimaj A, Colpani V, Bano A, Kraja B, Zaciragic A, Bramer WM, van Dijk GM, Kavousi M, Franco OH (2016) Estrogen receptor beta actions in the female cardiovascular system: a systematic review of animal and human studies. Maturitas 86:28–43. https://doi.org/10.1016/j.maturitas.2016.01.009
Nilsen J, Brinton RD (2004) Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord 3(4):297–313
Novensa L, Novella S, Medina P, Segarra G, Castillo N, Heras M, Hermenegildo C, Dantas AP (2011) Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERalpha/ERbeta balance in female mice. PLoS One 6(9):e25335. https://doi.org/10.1371/journal.pone.0025335
Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA (2000) Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278(3):640–645. https://doi.org/10.1006/bbrc.2000.3827
Ramirez JM (2014) The integrative role of the sigh in psychology, physiology, pathology, and neurobiology. Prog Brain Res 209:91–129. https://doi.org/10.1016/B978-0-444-63274-6.00006-0
Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V (2008) Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharmacol Exp Ther 325(3):782–790. https://doi.org/10.1124/jpet.107.134072
Seidlova-Wuttke D, Nguyen BT, Wuttke W (2012) Long-term effects of ovariectomy on osteoporosis and obesity in estrogen-receptor-beta-deleted mice. Comp Med 62(1):8–13
Shughrue PJ, Merchenthaler I (2001) Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol 436(1):64–81
Shughrue PJ, Lane MV, Merchenthaler I (1997) Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388(4):507–525
Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M (2007) Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension 49(6):1364–1370. https://doi.org/10.1161/HYPERTENSIONAHA.106.081554
Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW (2004) Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA 101(12):4130–4135. https://doi.org/10.1073/pnas.0306948101
Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME (2002) Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295(5554):505–508. https://doi.org/10.1126/science.1065250
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Laouafa, S., Roussel, D., Marcouiller, F., Soliz, J., Bairam, A., Joseph, V. (2018). Role of Estradiol Receptor Beta (ERβ) on Arterial Pressure, Respiratory Chemoreflex and Mitochondrial Function in Young and Aged Female Mice. In: Gauda, E., Monteiro, M., Prabhakar, N., Wyatt, C., Schultz, H. (eds) Arterial Chemoreceptors. Advances in Experimental Medicine and Biology, vol 1071. Springer, Cham. https://doi.org/10.1007/978-3-319-91137-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-91137-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91136-6
Online ISBN: 978-3-319-91137-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)