Abstract
In patients with newly discovered macroprolactinomas, dopamine agonists are the treatment of choice to reduce prolactin levels and lactotroph hyperplasia. Cabergoline is an ergot derivative which demonstrates good efficacy for treatment of prolactinomas and is generally well tolerated by patients. Surgical intervention with a transsphenoidal resection performed by even the most experienced neurosurgeon is less likely to result in long-term biochemical remission and control of tumor growth when compared to medical therapy for prolactin-secreting tumors. An important consideration with use of dopamine agonists for prolactinomas is the development of psychotic symptoms. It is prudent to ascertain the patient’s past psychiatric history to determine whether dopamine agonists can be safely prescribed. If dopamine agonist therapy is initiated in the absence of any known prior psychosis, new signs and symptoms of psychosis should also be carefully monitored for the duration of therapy. In the case that discontinuation of dopamine agonist therapy is clinically warranted due to psychotic decompensation, secondary approaches for management include surgical intervention or radiation therapy for the prolactinoma and treatment of any underlying metabolic dysfunction due to hyperprolactinemia.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Macroprolactinoma
- Prolactinoma
- Lactotroph hyperplasia
- Hyperprolactinemia
- Pituitary adenoma
- Sellar mass
- Dopamine agonists
- Intolerance to dopamine agonists
- Cabergoline
- Cabergoline-induced psychosis
Case Presentation
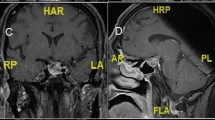
This is a 22-year-old male with no significant past medical history who presented with progressive vision loss for 3 months. Formal visual field testing was obtained through the patient’s ophthalmologist, which demonstrated a bitemporal hemianopsia . A pituitary MRI was ordered, which revealed a sellar and suprasellar mass measuring 42 × 30 × 38 mm in size with compression of the optic chiasm and invasion of the right cavernous sinus. The patient denied any complaints of headache. Biochemical testing was significant for elevated prolactin of 4064 ng/mL and a low free T4(FT4) of 0.8 ng/dL. The patient was exonerated of adrenal insufficiency through cosyntropin stimulation testing . The remainder of his pituitary function was unremarkable, and there was no evidence of cosecretion. The patient was started on cabergoline 0.5 mg daily for a presumed macroprolactinoma. A relatively higher dose was initiated given the urgency to preserve visual function. A prolactin and pituitary MRI were reevaluated after 2 weeks. The prolactin level was significantly reduced to 657 ng/mL. Repeat MRI showed a decrease in size of the macroprolactinoma now measuring 30 × 27 × 33 mm in size with decompression of the optic chiasm. The patient’s cabergoline dose was reduced to 0.5 mg twice weekly, as the proximity of the tumor to the optic chiasm became less concerning. He continued to demonstrate a good biochemical and tumor response. After 1 month of medical therapy, the prolactin level was 25 ng/mL. Normalization of the prolactin was later achieved by 8 months, but imaging of the pituitary was notable for stable residual tumor. Two years following a stable dose of cabergoline, the patient reported new-onset auditory hallucinations, delusions, and excessive anxiety, which profoundly impaired his social and professional interactions.
My Management Options
-
(a)
Start antipsychotic medications , and continue to treat the macroprolactinoma with cabergoline.
-
(b)
Discontinue the cabergoline and refer to neurosurgery to remove the residual tumor.
-
(c)
Discontinue the cabergoline and refer to radiation oncology for radiation therapy.
Assessment and Diagnosis
Prolactin is regulated primarily through dopamine via negative feedback. Dopamine is secreted from neurons of the arcuate nucleus in the tuberoinfundibular pathway and has an inhibitory effect on prolactin synthesis and secretion by binding to dopamine D2 receptors found on lactotrophs in the anterior pituitary gland [1]. In contrast, thyrotropin-releasing hormone has a smaller contribution to regulating prolactin through stimulation of prolactin release [2].
Based on this physiology, dopamine agonists are the treatment of choice to reduce prolactin levels and lactotroph hyperplasia . Cabergoline is an ergot derivative which demonstrates good efficacy for treatment of prolactinomas due to its high binding affinity for dopamine D2 receptors. Cabergoline is often preferred to bromocriptine for managing prolactinomas due to superior efficacy [3] probably due to greater selectivity for the dopamine D2 receptor , longer half-life, and better tolerated side effects, such as nausea and orthostasis.
An additional concern with the use of dopamine agonists for prolactinomas is the development of psychotic symptoms [4]. Based on the elimination half-life of cabergoline, systemic levels of the medication may be present for a few weeks. A single dose of cabergoline can have lasting effects on the prolactin for 21 days [5]. Cases of psychosis have been reported with use of cabergoline and bromocriptine for treatment of prolactinomas [6, 7]. Some reports have suggested that psychotic symptoms decrease after withdrawal of the dopamine agonist. Currently, there are no data to suggest differential effects on the severity of psychotic symptoms between cabergoline and bromocriptine based on varying pharmacokinetic profiles, and both medications should be used with caution, in consultation with psychiatry, or avoided, if psychosis is suspected.
A clear dose-dependent relationship between cabergoline use and psychotic symptoms remains unknown. Higher doses of cabergoline are used to treat Parkinson’s disease , and cases of psychosis are similarly reported in these patients [8]. Regardless of the dose, symptoms of psychosis need to be monitored cautiously . Lowering the dose of cabergoline may be a plausible strategy. However, cases of psychosis have been reported on low doses of dopamine agonists [9], and there are no safety data related to psychosis to support this management and is not generally advised. In addition, there are reports of persistent psychiatric decompensation despite the addition of an antipsychotic drug to a dopamine agonist, and therefore, concomitant use of the two agents is not typically recommended for management of a prolactinoma as well [10, 11]. The risks of psychiatric decompensation may outweigh the potential risks of a prolactinoma, as there are alternative approaches for safely managing prolactinomas.
While there is no clear mechanism for psychosis, dopamine hypersensitivity is hypothesized to play a key role. In this regard, dopamine antagonists are commonly used to treat antipsychotic disorders and demonstrate efficacy in psychosis by blocking the dopamine receptors in the mesolimbic pathway. Common symptoms of psychotic disorders include delusions and hallucinations as well as loss of emotion, speech, or motivation. Through their mechanism of action to block dopamine D2 receptors , dopamine antagonist use can result in unwanted side effects, such as medication-induced hyperprolactinemia by blocking the tonic inhibition of prolactin, thereby leading to increased prolactin levels. In addition, other classes of psychiatric medications, such as SSRIs, may also have unintended consequences of hyperprolactinemia [12].
While in this scenario, cabergoline was initiated first to treat a macroprolactinoma, prior reports exploring the commencement of cabergoline following antipsychotic therapy for medication-induced hyperprolactinemia are important to understand the interaction between these two classes of medications. Some reports had suggested that dopamine agonists, such as cabergoline, could be safely administered to treat medication-induced hyperprolactinemia secondary to antipsychotic use [13, 14]. However, in light of new evidence to suggest that dopamine agonists have the potential to exacerbate psychosis, this management is not recommended. While limited data are available evaluating the causal relationship between cabergoline and psychiatric disease, case reports suggest a significant and worrisome association is present [15,16,17]. Moreover, additional links of dopamine agonists to heightened impulsivity [18, 19] and mania [17, 20] in patients treated for prolactinomas have also been described in the literature.
Management
Surgical intervention with a transsphenoidal resection even performed by the most experienced neurosurgeon is less likely to result in long-term biochemical remission and control of tumor growth [21,22,23,24,25] when compared to medical therapy for prolactin-secreting tumors. Therefore, first-line treatment for a macroprolactinoma is dopamine agonist therapy. Alternatives to medical therapy may be warranted under special clinical circumstances, such as intolerance to dopamine agonist therapy , absolute or relative contraindication to dopamine agonist therapy, or poor response (either by biochemical or tumor measures) to dopamine agonist therapy. In this case, this patient was considered to have a contraindication to dopamine agonists based on the exacerbation of his psychosis . While surgical management could be offered as the next step, there were other considerations unique to this case, such as the patient’s age and aggressive nature of the tumor. The early onset of his disease presentation and characteristics of tumor aggressiveness (cavernous sinus invasion) increase the probability for potential recurrence that may require additional long-term treatment.
More definitive treatment with radiation should be considered when disease chronicity or aggressive features are a concern. Long-term consequences of radiation therapy should be discussed with the patient, the most common of which is hypopituitarism. The development of a secondary malignancy or neurologic symptoms , such as optic neuropathy and stroke are less common. Hypopituitarism can be managed with hormone replacement, and the usual onset does not occur on average until many years from treatment [26].
As approximately 90% of prolactinomas respond to dopamine agonist therapy , few cases require use of surgery or radiation. In this regard, data on the efficacy of transsphenoidal resection and radiation therapy is limited. One retrospective study following surgical intervention for prolactinoma reported an initial remission rate of 53%, which later declined to 43%, and the overall recurrence rate was 19% [27]. Other prospective studies demonstrate similar recurrence rates [21]. Data also show that prolactin-secreting macroadenomas and invasive adenomas tend to have reduced rates of remission and complete eradication of tumor [22, 28] when compared to microprolactinomas. Certainly, surgical intervention may be an option in situations where debulking is necessary or to facilitate adjunctive radiation [29]. In a series of 128 patients followed after receiving gamma knife radiation, 67 cases achieved clinical cure. Most patients demonstrated tumor control, but about 1/3 of patients followed for over 2 years still had evidence of hyperprolactinemia [30]. Another center’s experience with radiation similarly showed good tumor control and less frequent biochemical control among prolactinomas treated with radiation and followed up to a median of 6 years [31]. More recent data on proton therapy demonstrated that about 98% of all adenomas respond to treatment; however, two of three patients that had radiographic progression were prolactin-secreting adenomas . Biochemical remission was achieved in 38% of the prolactinoma cases by 5 years, a rate much lower than the other subtypes of adenomas [32]. Overall, response to radiation for prolactinomas tends to be less frequent and delayed when compared to other adenomas.
In addition, treatment of the underlying psychiatric disease should be pursued in consultation with the psychiatrist. Elevated prolactin levels are present in over 70% of patients on risperidone [33, 34] and may be elevated to a lesser extent in other atypical antipsychotics, such as olanzapine and clozapine. As clozapine has a weak binding affinity to the dopamine D2 receptor, hyperprolactinemia is rare [35], but its use may be limited by considerable side effects. Using an antipsychotic agent which tends to have fewer effects on the prolactin may be preferred, but ultimately the patient should be treated as necessary to stabilize the psychosis.
Aripiprazole is an ideal choice to dually manage psychosis and hyperprolactinemia if tolerated by the patient [34, 36] and administered in consultation with the managing psychiatrist. Aripiprazole’s mechanism of action as a partial dopamine agonist gives it the advantage to treat psychotic symptoms while lowering prolactin levels [37, 38]. Further investigation into aripiprazole’s effect on tumor size is needed , but isolated case reports suggest a beneficial reduction in prolactinoma size [39].
Outcome
Indeed, this case demonstrates the initial efficacy of cabergoline , as there was a rapid response in the prolactin level and tumor size. The patient’s clinical course was interrupted by serious psychiatric complaints, which prompted the immediate discontinuation of cabergoline. He was referred to psychiatry and was newly diagnosed with psychosis. The exacerbation of his psychotic symptoms had a significant impact on his life, leading to social isolation and a temporary leave of absence from his career. Upon further questioning, the patient expressed that mild anxiety and paranoia existed in the years prior to diagnosis of his macroprolactinoma. He was able to manage his symptoms conservatively through coping strategies and, therefore, never brought these symptoms to the attention of his care providers, as may be a common scenario in high-functioning individuals.
Following discontinuation of the cabergoline, the prolactin level rose to 838 ng/dL. In addition, the prolactin rose further to 992 ng/dL after olanzapine, an atypical antipsychotic and dopamine antagonist, was initiated to treat his auditory hallucinations and delusions. Aripiprazole was considered to manage the psychotic symptoms, but he did not tolerate this well. Olanzapine was continued to provide optimal psychiatric benefit. He was simultaneously referred to radiation oncology for further management of the macroprolactinoma.
The patient was advised to undergo fractionated radiation, which was tolerated well. Pituitary MRI at 6-, 12-, and 24-months postradiation demonstrated stability of the tumor. Two years following radiation, the prolactin level had decreased by half to 428 ng/dL, while the patient actively remained on a dopamine antagonist for his psychiatric illness. Overall, his social and professional impairment had been slowly improving with titration of his dopamine antagonist treatment.
The patient subsequently developed fatigue and decreased libido. A morning testosterone level was 19 ng/dL, which was consistent with male hypogonadism. The hypogonadism probably developed after prolactin levels rose due to withdrawal of cabergoline therapy. In males, hypogonadism may be the initial presenting symptoms in cases of hyperprolactinemia [40]. Hyperprolactinemia contributes to hypogonadism through suppressive effects on gonadotrophin-releasing hormone. Testosterone replacement was started. A normal testosterone level was achieved (336 ng/dL) despite ongoing biochemical evidence of hyperprolactinemia, and symptoms related to hypogonadism eventually resolved.
Clinical Pearls and Pitfalls
-
Prior to starting a dopamine agonist, physicians should screen patients for psychiatric disease. When a psychiatric disorder is suspected, referral to a psychiatrist is recommended to determine if starting a dopamine agonist is clinically feasible. Use of a dopamine agonist is not recommended in psychotic patients and may have detrimental effects on social and professional functioning.
-
Dopamine agonists can unmask an underlying psychotic disorder. If an unknown psychiatric condition is exacerbated after starting a dopamine agonist, the medication should be discontinued.
-
Antipsychotic agents functioning as dopamine antagonists may contribute to medication-induced hyperprolactinemia. Any underlying psychiatric disorder should be treated as deemed appropriate by the psychiatrist. The preferred antipsychotic to treat symptoms of psychosis in the setting of hyperprolactinemia is aripiprazole [41], which functions as a partial dopamine agonist and may reduce prolactin levels. Data are lacking as to aripiprazole’s effects on tumor growth.
-
Surgery and/or radiation therapy can be effective second-line treatment strategies for macroprolactinomas not amenable to medical therapy. When there is residual tissue or aggressive features, transsphenoidal resection may not be entirely effective but can be considered for debulking, and radiation, either single dose or fractionated, may be the preferred option.
-
In the setting of continued hyperprolactinemia following radiation, conservative monitoring is sufficient if the patient does not demonstrate any prolactin-related symptoms, as it is expected that tumor growth will stabilize and prolactin levels will gradually decline with radiation therapy.
-
If patients cannot tolerate dopamine agonists, secondary symptoms should be treated via alternative methods. For example, male hypogonadism secondary to hyperprolactinemia can be alternatively treated with testosterone replacement, rather than with cabergoline. Female hypogonadism may require oral contraceptive therapy. Long-standing hypogonadism may have deleterious effects on quality of life and bone health and should be addressed [42, 43].
References
Leblanc H, Lachelin GC, Abu-Fadil S, Yen SS. Effects of dopamine infusion on pituitary hormone secretion in humans. J Clin Endocrinol Metab. 1976;43:668–74.
Jacobs LS, Snyder PJ, Wilber JF, Utiger RD, Daughaday WH. Increased serum prolactin after administration of synthetic thyrotropin releasing hormone (TRH) in man. J Clin Endocrinol Metab. 1971;33:996–8.
Biller BM, Molitch ME, Vance ML, Cannistraro KB, Davis KR, Simons JA, Schoenfelder JR, Klibanski A. Treatment of prolactin-secreting macroadenomas with the once-weekly dopamine agonist cabergoline. J Clin Endocrinol Metab. 1996;81:2338–43.
Ali S, Miller KK, Freudenreich O. Management of psychosis associated with a prolactinoma: case report and review of the literature. Psychosomatics. 2010;51:370–6.
DA agonists—ergot derivatives: cabergoline: management of Parkinson’s disease. Mov Disord. 2002;17 Suppl 4:S68–71.
Cabeza GA, Flores LF, Iniguez IE, Calarco ZE, Valencia PF. Acute psychosis secondary to bromocriptine treatment in a patient with a prolactinoma. Rev Invest Clin. 1984;36:147–9.
Peter SA, Autz A, Jean-Simon ML. Bromocriptine-induced schizophrenia. J Natl Med Assoc. 1993;85:700–1.
Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–73.
Turner TH, Cookson JC, Wass JA, Drury PL, Price PA, Besser GM. Psychotic reactions during treatment of pituitary tumours with dopamine agonists. Br Med J (Clin Res Ed). 1984;289:1101–3.
Nieman DH, Sutterland AL, Otten J, Becker HE, Drent ML, van der Gaag M, Birchwood M, de Haan L. Treating prolactinoma and psychosis: medication and cognitive behavioural therapy. BMJ Case Rep. 2011;1–5.
Robbins RJ, Kern PA, Thompson TL 2nd. Interactions between thioridazine and bromocriptine in a patient with a prolactin-secreting pituitary adenoma. Am J Med. 1984;76:921–3.
Papakostas GI, Miller KK, Petersen T, Sklarsky KG, Hilliker SE, Klibanski A, Fava M. Serum prolactin levels among outpatients with major depressive disorder during the acute phase of treatment with fluoxetine. J Clin Psychiatry. 2006;67:952–7.
Tollin SR. Use of the dopamine agonists bromocriptine and cabergoline in the management of risperidone-induced hyperprolactinemia in patients with psychotic disorders. J Endocrinol Invest. 2000;23:765–70.
Cavallaro R, Cocchi F, Angelone SM, Lattuada E, Smeraldi E. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry. 2004;65:187–90.
Bilal L, Ching C. Cabergoline-induced psychosis in a patient with undiagnosed depression. J Neuropsychiatry Clin Neurosci. 2012;24:E54.
Chang SC, Chen CH, Lu ML. Cabergoline-induced psychotic exacerbation in schizophrenic patients. Gen Hosp Psychiatry. 2008;30:378–80.
Harris YT, Harris AZ, Deasis JM, Ferrando SJ, Reddy N, Young RC. Cabergoline associated with first episode mania. Psychosomatics. 2012;53:595–600.
Barake M, Evins AE, Stoeckel L, Pachas GN, Nachtigall LB, Miller KK, Biller BM, Tritos NA, Klibanski A. Investigation of impulsivity in patients on dopamine agonist therapy for hyperprolactinemia: a pilot study. Pituitary. 2014;17:150–6.
Martinkova J, Trejbalova L, Sasikova M, Benetin J, Valkovic P. Impulse control disorders associated with dopaminergic medication in patients with pituitary adenomas. Clin Neuropharmacol. 2011;34:179–81.
Yuksel RN, Elyas Kaya Z, Dilbaz N, Cingi Yirun M. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6:229–31.
Feigenbaum SL, Downey DE, Wilson CB, Jaffe RB. Transsphenoidal pituitary resection for preoperative diagnosis of prolactin-secreting pituitary adenoma in women: long term follow-up. J Clin Endocrinol Metab. 1996;81:1711–9.
Randall RV, Laws ER Jr, Abboud CF, Ebersold MJ, Kao PC, Scheithauer BW. Transsphenoidal microsurgical treatment of prolactin-producing pituitary adenomas. Results in 100 patients. Mayo Clin Proc. 1983;58:108–21.
Schlechte JA, Sherman BM, Chapler FK, VanGilder J. Long term follow-up of women with surgically treated prolactin-secreting pituitary tumors. J Clin Endocrinol Metab. 1986;62:1296–301.
Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983;309:280–3.
Kristof RA, Schramm J, Redel L, Neuloh G, Wichers M, Klingmuller D. Endocrinological outcome following first time transsphenoidal surgery for GH-, ACTH-, and PRL-secreting pituitary adenomas. Acta Neurochir (Wien). 2002;144:555–561; discussion 561.
Snyder PJ, Fowble BF, Schatz NJ, Savino PJ, Gennarelli TA. Hypopituitarism following radiation therapy of pituitary adenomas. Am J Med. 1986;81:457–62.
Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, Buchfelder M. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008;158:11–8.
Primeau V, Raftopoulos C, Maiter D. Outcomes of transsphenoidal surgery in prolactinomas: improvement of hormonal control in dopamine agonist-resistant patients. Eur J Endocrinol. 2012;166:779–86.
Shimon I, Sosa E, Mendoza V, Greenman Y, Tirosh A, Espinosa E, Popovic V, Glezer A, Bronstein MD, Mercado M. Giant prolactinomas larger than 60 mm in size: a cohort of massive and aggressive prolactin-secreting pituitary adenomas. Pituitary. 2016;19:429–36.
Pan L, Zhang N, Wang EM, Wang BJ, Dai JZ, Cai PW. Gamma knife radiosurgery as a primary treatment for prolactinomas. J Neurosurg. 2000;93(Suppl 3):10–3.
Wilson PJ, Williams JR, Smee RI. Single-centre experience of stereotactic radiosurgery and fractionated stereotactic radiotherapy for prolactinomas with the linear accelerator. J Med Imaging Radiat Oncol. 2015;59:371–8.
Wattson DA, Tanguturi SK, Spiegel DY, Niemierko A, Biller BM, Nachtigall LB, Bussiere MR, Swearingen B, Chapman PH, Loeffler JS, Shih HA. Outcomes of proton therapy for patients with functional pituitary adenomas. Int J Radiat Oncol Biol Phys. 2014;90:532–9.
Molitch ME. Medication-induced hyperprolactinemia. Mayo Clin Proc. 2005;80:1050–7.
Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60:681–90.
Volavka J, Czobor P, Cooper TB, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychiatry. 2004;65:57–61.
Hoffer ZS, Roth RL, Mathews M. Evidence for the partial dopamine-receptor agonist aripiprazole as a first-line treatment of psychosis in patients with iatrogenic or tumorogenic hyperprolactinemia. Psychosomatics. 2009;50:317–24.
Byerly M, Suppes T, Tran QV, Baker RA. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: recent developments and current perspectives. J Clin Psychopharmacol. 2007;27:639–61.
Sheldrick AJ, Grunder G. Aripiprazole reduces serum prolactin in a woman with prolactinoma and acute psychosis. Pharmacopsychiatry. 2008;41:160.
Bakker IC, Schubart CD, Zelissen PM. Successful treatment of a prolactinoma with the antipsychotic drug aripiprazole. Endocrinol Diabetes Metab Case Rep. 2016;2016:160028.
Tsigkaropoulou E, Peppa M, Zompola C, Rizos E, Xelioti I, Chatziioannou S, Filippopoulou A, Lykouras L. Hypogonadism due to hyperprolactinemia and subsequent first episode of psychosis. Gend Med. 2012;9:56–60.
Wix-Ramos RJ, Paez R, Capote E, Ezequiel U. Pituitary microadenoma treated with antipsychotic drug aripiprazole. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:58–60.
Klibanski A, Neer RM, Beitins IZ, Ridgway EC, Zervas NT, McArthur JW. Decreased bone density in hyperprolactinemic women. N Engl J Med. 1980;303:1511–4.
Greenspan SL, Neer RM, Ridgway EC, Klibanski A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1986;104:777–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Srinivasa, S. (2018). Macroprolactinoma: Alternatives to Dopamine Agonist Therapy for Treatment of Macroprolactinomas in Patients Who Present with Psychotic Symptoms. In: Nachtigall, L. (eds) Pituitary Tumors. Springer, Cham. https://doi.org/10.1007/978-3-319-90909-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-90909-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90907-3
Online ISBN: 978-3-319-90909-7
eBook Packages: MedicineMedicine (R0)