Abstract
Radiologic imaging is a crucial part of the evaluation of women presenting with infertility. Imaging techniques that provide insight into causes and factors contributing to infertility include ultrasound, hysterosalpingography, hysterosalpingo-contrast sonography, and magnetic resonance imaging. In this chapter, we discuss imaging technologies for disorders of the uterus, fallopian tubes, and ovaries which may impact fertility and pregnancy outcomes, with consideration of test sensitivity and specificity, cost, discomfort, utilization of contrast media, and ionizing radiation. Hysterosalpingo-contrast sonography is an efficient modality for multiple aspects of female reproductive anatomy and is advocated as a first-line test for most infertile women. The specific questions and concerns for each patient should be considered to select the most appropriate test or sequence of tests.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Infertility
- Imaging
- Hysterosalpingo-contrast sonography
- Hysterosalpingography
- Ultrasound
- Recurrent pregnancy loss
- Uterine anomalies
- Asherman syndrome
- Leiomyoma
- Hydrosalpinx
- Ovary
1 Uterine Imaging
Uterine factor infertility comprises a small portion of infertility diagnoses. Uterine abnormalities that may impact fertility and pregnancy outcomes include congenital uterine anomalies, uterine leiomyomata, adenomyosis, endometrial polyps, and uterine synechiae. No single imaging modality is optimal for evaluating all of these, and when there is a finding of concern, multiple imaging tests may be required to ascertain the correct diagnosis and determine optimal management.
1.1 Uterine Anomalies
The uterus and fallopian tubes are formed in utero by fusion of the bilateral Mullerian ducts, followed by canalization and resorption of the septum between these two tubes, under the influence of the HOX family of genes [1]. Approximately 5.5% of women have an anomaly of the formation of the uterus, and thus these are among the most common congenital anomalies [2]. There is wide variability among anomalous uteri, as well as varying impact on reproduction and options for treatment. Multiple classification systems for uterine anomalies have been developed over the past 40 years [3,4,5,6,7], with increasing emphasis on objective imaging findings for classification. It is vital to correctly classify each uterine anomaly in order to counsel a woman about her risks and select appropriate candidates for surgery. In Table 6.1, the most common types of uterine anomalies are described according to the ESHRE/with their impact on reproductive outcomes and amenability to surgical repair. Reproductive outcomes appear to be improved after hysteroscopic surgery for uterine septum [8], and this procedure may be considered in women with a history of infertility, pregnancy loss, or poor pregnancy outcome [9]. Historically, reunification procedures were commonly performed for bicornuate and didelphys uteri, but these invasive procedures have not been demonstrated to increase the chances of a live birth and have largely been abandoned [10].
Imaging techniques for uterine anomalies are summarized in Table 6.2, including their sensitivities and specificities as well as positive predictive value (PPV) and negative predictive value (NPV) , relative to a gold standard of laparoscopy and hysteroscopy. In the evaluation of a woman with infertility, two-dimensional (2D), transvaginal ultrasound is often the first test performed, due to its availability, low cost, and lack of radiation, as well as its ability to provide information on a number of anatomic findings that impact fertility, as discussed later in the chapter. 2D ultrasound offers high specificity, but relatively poor sensitivity for uterine anomalies, and is dependent upon the skill of the operator. Three-dimensional ultrasound improves sensitivity in the diagnosis of uterine anomalies but is less widely available than 2D ultrasound. Image acquisition is typically simple, but processing and interpretation of images requires training and experience [11]. If available, a 3D ultrasound image may be acquired at the time of standard 2D ultrasound and reviewed later if concerns arise. Alternatively, 3D ultrasound may be used as an adjunct to 2D sonographic imaging in the case of indeterminate findings or clinical suspicion.
Saline infusion sonohysterography , also known as hysterosalpingo-contrast sonography (HyCoSy) , similarly provides improved sensitivity and accuracy in diagnosing uterine anomalies, as compared with 2D ultrasound. It is relatively low cost and requires specialized training of the operator. Compared with hysterosalpingography, it is less likely to cause pain or infection [11]. This technique is also advantageous in definitively diagnosing other intracavitary lesions. Some authors advocate the use of HyCoSy as a standard, first-line tool in the investigation of infertility [12], while others suggest this modality should be reserved for those with concerning findings on 2D ultrasound or hysterosalpingography [9].
Hysterosalpingography (HSG) is frequently performed in the evaluation of the infertile woman, as a primary assessment of tubal patency. This test is performed in the radiology suite. The cervix is canalized with a catheter, and radio-opaque contrast material is injected through the cervix to fill the uterus and ultimately spill from the fallopian tubes into the uterine cavity. An abnormal internal uterine contour may be noted. However, because HSG does not define the external contour of the uterus, this technique cannot distinguish a bicornuate from a subseptate uterus (Fig. 6.1) nor a unicornuate uterus from a uterine didelphys or complete uterine septum [11].
MRI (magnetic resonance imaging) offers a slight increase in accuracy of diagnosis of Mullerian anomalies relative to 3D ultrasound [13]. The internal and external contours of both uterus and cervix can be completely imaged with MRI . While intravenous contrast is not necessary, T2-weighted images and use of vaginal gel allow for optimal contrast imaging. Both axial and oblique coronal images should be obtained and reviewed, with 4–5 mm slice thickness and a 24–26 cm field of view. Because of the association between uterine and renal anomalies, consideration should be given to imaging the kidneys as well at the time of MRI [14]. Because of increased cost, MRI should be reserved for cases in which less expensive techniques have not adequately defined uterine and cervical anatomy, and a key management decision relies on the distinction, e.g., a complete uterine septum (Fig. 6.2) versus didelphys uterus (Fig. 6.3) in a patient with recurrent pregnancy loss . In this case, MRI imaging confirmation would enable hysteroscopic septum incision.
1.2 Leiomyoma
Uterine leiomyomata are an extremely common finding among women of reproductive age, with a cumulative incidence of nearly 60% in black women and approximately 30% in white women by age 40 [15]. Leiomyoma are classified as submucosal, intramural, or subserosal based on their location relative to the myometrium, with subclassifications for the proportion of each fibroid in each location [16]. The impact of intramural and subserosal uterine fibroids on fertility is somewhat controversial, and it is not certain whether myomectomy for these types of fibroids improves the chances of successful pregnancy. In contrast, hysteroscopic myomectomy for submucosal leiomyomata has been demonstrated to increase the chances of clinical pregnancy in infertile women [17,18,19]. Therefore, a precise delineation of the location of uterine is imperative for selecting candidates who are likely to benefit from surgical management.
Uterine leiomyomata are often initially diagnosed on 2D ultrasound , which allows measurement of the size of fibroids but is often inadequate for classification of the location. Hysterosalpingography may identify intracavitary filling defects that might represent leiomyoma but cannot differentiate submucosal fibroids from endometrial polyps, as noted in Fig. 6.4 [20]. Saline infusion sonohysterography has nearly 100% sensitivity, and approximately 90% specificity for diagnosing submucosal fibroids [21, 22], and is the optimal second imaging study for characterization of uterine fibroids.
While MRI provides high resolution imaging of uterine fibroids with clear classification of location (Fig. 6.5), its high cost mandates selective utilization. T2-weighted images provide the greatest contrast between leiomyoma and surrounding myometrium; T1-weighted images should also be obtained to differentiate uterine fibroids from other types of pelvic masses [23]. Unlike ultrasound, MRI has high accuracy in differentiating benign leiomyoma from uterine malignancies [24] and adenomyosis [25, 26], particularly with the use of diffusion-weighted imaging. Utilization of MRI for confirmation of an ultrasound diagnosis of fibroids prior to abdominal or laparoscopic myomectomy may avoid unnecessary surgeries for adenomyosis and inadvertent spread of malignant cells.
1.3 Adenomyosis
Adenomyosis is the growth of endometrial glands and stroma within the uterine myometrium, which can lead to menorrhagia and dysmenorrhea. It may be focal, creating an adenomyoma, or diffuse throughout the uterus. While it is more commonly found in parous women, adenomyosis has been hypothesized to contribute to infertility [27] and is associated with decreased chances of clinical pregnancy among women undergoing in vitro fertilization [28, 29]. Hysterectomy is the definitive treatment of adenomyosis, but in infertile women, conservative uterine surgery [30, 31] and the use of GnRH agonists [32, 33] have been found to be beneficial in small studies.
Hysterosalpingography may provide evidence of adenomyosis with small outpouchings from the uterine cavity representing the invasion of endometrial glands into the myometrium (Fig. 6.6). Transvaginal ultrasound findings suggestive of adenomyosis include myometrial heterogeneity and myometrial anechoic cysts [34], as well as enlargement of the uterine corpus and asymmetric anterior or posterior myometrial thickening [35]. A meta-analysis found transvaginal ultrasound to be 82.5% sensitive and 84.6% specific for the diagnosis of adenomyosis.
MRI offers decreased sensitivity (46%) but increased specificity (99%) in the diagnosis of adenomyosis, leading to a positive predictive value of 92% among women with an enlarged uterus planning hysterectomy [36]. MRI findings include a junctional zone between the endometrium that is thicker than 12 mm, poorly defined margins of the lesion, and the absence of deformity of the endometrium, as demonstrated in Fig. 6.7. In contrast, Fig. 6.8 depicts MRI findings consistent with focal adenomyosis. When managing adenomyosis in a patient with infertility, the high specificity of MRI allows certainty in diagnosis to facilitate appropriate medical or surgical treatment.
1.4 Endometrial Polyps
Endometrial polyps are outgrowths of the endometrium that project into the uterine cavity. They are found in approximately 10% of women with infertility [37] and may present with abnormal uterine bleeding. Among premenopausal women, over 98% are benign, while less than 2% represent cancerous or premalignant lesions [38]. While polyps may spontaneously regress, and clearly pregnancy can be achieved in the presence of polyps, a randomized controlled trial has demonstrated higher pregnancy rates with intrauterine insemination among women who underwent hysteroscopic resection of endometrial polyps than in those whose polyps were left in situ [39]. Because hysteroscopic resection of endometrial polyps is a low risk procedure that can be performed in an office setting, many authors advocate that endometrial polyps be removed prior to fertility treatment [40, 41].
Saline infusion sonohysterography offers the optimal visualization of endometrial polyps, with sensitivity and specificity at 90% or greater, as shown in Fig. 6.9. This technique has greater sensitivity than transvaginal ultrasound, and there is no statistically significant increase with the use of 3D versus 2D sonohysterography [22, 42]. HSG cannot differentiate endometrial polyps from submucosal fibroids. MRI is not indicated in the evaluation of suspected endometrial polyps.
1.5 Uterine Synechiae
Uterine synechiae are adherent fibrous bands crossing the uterine cavity, initially described by Asherman in 1948 in association with amenorrhea [43]. They are found in approximately 7% of women with infertility. Surgical procedures involving the uterine cavity are the primary risk factor, with the highest rates of adhesion formation after dilation and evacuation procedures for intrauterine fetal demise, dilation, and curettage for retained products of conception and postpartum dilation and curettage [44]. The risk of uterine synechiae among women who have undergone dilation and curettage to manage a miscarriage may be as high as 19% [45]. Uterine synechiae may present clinically with changes in menstrual bleeding patterns including amenorrhea or hypomenorrhea, infertility, cyclic pelvic, or recurrent pregnancy loss [46]. Treatment is surgical, with hysteroscopic lysis of adhesions.
Saline sonohysterography is the optimal imaging method for uterine synechiae, with sensitivity of 82–100% and specificity of 99–100% [47]. Uterine synechiae appear as hyperechoic bands crossing the uterine cavity (Fig. 6.10). Hysterosalpingography has demonstrated approximately 80% sensitivity and specificity for uterine synechiae, leading to a positive predictive value of 63% and negative predictive value of 84% in infertile women [48]. While 2D ultrasound lacks adequate sensitivity, relatively new data suggests that 3D ultrasound findings including irregularity at the endometrial margin, partial thinning and endometrial defects, and hyperechoic lesions are highly predictive of a hysteroscopic diagnosis of intrauterine adhesions [49]. While MRI can be considered when the severity of intrauterine adhesions prevents catheter passage for saline-infused sonohysterography , this test has not been demonstrated to provide additional value in ascertaining individuals who may benefit from hysteroscopic evaluation and treatment.
2 Fallopian Tube Imaging
Evaluation of fallopian tube patency remains a cornerstone of the female fertility assessment. Hysterosalpingogram has long been the mainstay of this evaluation. Distal tubal obstruction with and the degree of hydrosalpinx can clearly be delineated by HSG (Fig. 6.11). Women with bilateral hydrosalpinges are presumed to have extremely low chances of conception without in vitro fertilization, and women with unilateral hydrosalpinx are 75% less likely to conceive with intrauterine insemination than those with bilateral tubal patency [50]. In women with hydrosalpinges , salpingectomy improves the odds of ongoing pregnancy with IVF [51]. Salpingectomy for proximal tubal obstruction has been hypothesized to improve the chance of spontaneous pregnancy in women with unilateral hydrosalpinx [52].
However, the specificity of HSG for proximal tubal obstruction is limited, as 60% of women with unilateral proximal tubal obstruction on initial HSG will have bilateral tubal patency on repeat HSG [53]. Tubal spasm is the hypothesized mechanism for this [54].
Various radiologic techniques have been proposed for the evaluation and management of proximal tubal obstruction. These include selective salpingography, in which a catheter is fluoroscopically guided to the cornu where proximal obstruction is detected and contrast material directly injected, tubal catheterization with a soft, Teflon catheter, and canalization with a guide-wire. Each of these techniques has relatively high success in achieving tubal patency, and approximately 40% of treated patients achieve spontaneous pregnancies within 1 year [55, 56]. However, an absence of randomized controlled trials or high quality observational studies comparing treated to untreated patients makes it impossible to assess whether these treatments actually improve the odds of successful pregnancy. Indeed, Ferraiolo et al. [57] found that 21% of women with bilateral proximal tubal obstruction that could not be relieved by selective salpingography spontaneously conceived an intrauterine pregnancy within 1 year after the procedure. Women with untreated unilateral proximal tubal obstruction on HSG have similar rates of clinical pregnancy with intrauterine insemination to women with bilateral tubal patency [50].
Assessment of tubal patency by HyCoSy requires specialized training, and the technique involves instillation of air bubbles, which appear hyperechoic on ultrasound, through the fallopian tubes after completion of the uterine cavity assessment. Two meta-analyses have demonstrated 95–98% sensitivity and 90–93% specificity of HyCoSy for tubal obstruction relative to the gold standard of laparoscopic evaluation, which was equivalent to HSG [58, 59]. In the first meta-analysis, the addition of contrast media to HyCoSy did not improve diagnostic accuracy. Use of this technology to diagnose hydrosalpinx to select candidates for surgical treatment prior to in vitro fertilization may also prevent unnecessary surgeries, as only hydrosalpinges visible on ultrasound appear to impact the outcome of IVF [60, 61]. Some authors have noted higher pain scores in women undergoing HyCoSy relative to HSG [62], while others found the opposite with hysteron-foam-sonography [63]. Some authors advocate for the use of HyCoSy rather than HSG for assessment of tubal patency due to its ability to evaluate all pelvic anatomy and avoidance of radiation [64].
3 Ovarian Imaging
3.1 Infertility Evaluation and Monitoring
Transvaginal ultrasound is the standard of evaluation of the ovaries in the infertile female. Initial ultrasound is best performed in the early follicular phase in order to assess antral follicle count, a key measure of ovarian reserve, as well as ovarian volumes, in the absence of a dominant follicle or corpus luteum cyst that could impact these measurements (Fig. 6.12). Antral follicle count predicts response to gonadotropins in in vitro fertilization cycles [65] and has been correlated with chances of live birth [66].
Antral follicle count and ovarian volume are elements of the ovarian morphology criterion for polycystic ovary syndrome, although societies debate on the antral follicle count that defines polycystic ovaries. The Endocrine Society requires an antral follicle count of 12 per ovary [67], while the Androgen Excess and Polycystic Ovary Syndrome Society utilizes 25 per ovary [68]. Both concur on an ovarian volume of at least 10 mL.
Ultrasound monitoring of the number and size of follicles in in vitro fertilization is the standard of care, for determining cycle cancelation, gonadotropin dose changes, risk of ovarian hyperstimulation syndrome , and timing of ovulation trigger [69]. In Fig. 6.13 is an ovary with multiple maturing follicles after treatment with gonadotropins, while Fig. 6.14 demonstrates the appearance of the ovary in ovarian hyperstimulation syndrome, with multiple corpus luteum cysts. Ultrasound monitoring may be used with clomiphene citrate in order to determine the timing of intrauterine insemination, but this has not been demonstrated to improve the chances of pregnancy compared to urinary LH monitoring [70, 71].
3.2 Ovarian Cysts
Ovarian cysts and other adnexal masses are common findings in women with infertility. While they may present with pain, often they are found incidentally, most commonly with transvaginal ultrasound performed in the evaluation of infertility [72]. The primary goal of imaging of ovarian cysts is to determine which cysts present with a significant risk of malignancy so that they may be appropriately managed surgically. A secondary goal is to determine whether an ovarian cyst contributes to the patient’s infertility, and if so, determine an optimal management strategy.
The LR2 prediction model , developed from the International Ovarian Tumor Analysis study , uses patient age and ultrasound findings to predict the likelihood of malignancy in adnexal masses. Ascites, blood flow in a papillary projection, maximal diameter of the solid component, irregular internal cyst walls, and acoustic shadows each increase the risk of malignancy [73]. The LR2 model has demonstrated 94% sensitivity and 82% specificity for ovarian cancer, significantly better than a model that incorporates the serum markers CA-125 and HE4 [74] and can be performed by the clinician to determine which patients should be referred to gynecologic oncology. Figure 6.15 depicts a complex cyst with cystic and solid components and a papillary projection, highly concerning for malignancy.
Follicular cysts, corpora lutea, endometriomas, and dermoid cysts (mature cystic teratomas) are among the most common benign ovarian cysts found on ultrasound. Thirty to fifty percent of those who have both dysmenorrhea and infertility have endometriosis, and 20–40% of women with endometriosis will have an endometrioma [75]. Endometriomas may be unilocular or multilocular, and most commonly have a homogeneous, ground-glass appearance on ultrasound (Fig. 6.16), and may have hyperechoic wall nodules [76]. Transvaginal ultrasound is highly sensitive (87–99%) and specific (92–99%) for endometrioma, with a predictive value equivalent to MRI [77].
Endometriomas may have a negative impact on the surrounding ovarian stroma and follicles, as decreased follicular number and density has been reported in ovaries with endometriomas [78]. While measures of ovarian reserve are lower in women with endometriomas [79], the presence of an endometriomas does not decrease the chances of live birth with in vitro fertilization [80]. Moreover, it is well established that surgical excision of endometriomas decreases ovarian reserve [72], and a meta-analysis found that neither medical nor surgical treatment of endometrioma prior to in vitro fertilization improved the odds of clinical pregnancy [81].
Dermoid cysts , also known as mature cystic teratomas , are comprised of a variety of cell types and have a complex appearance on ultrasound, with bright calcifications and echogenic sebaceous material, as shown in Fig. 6.17 [82]. While studies of the impact of dermoid cysts on fertility are very limited, surgical resection of dermoid cysts has been reported to decrease ovarian reserve to a greater degree than cystectomy for endometrioma [83], and the presence of a dermoid does not appear to decrease ovarian response to gonadotropin stimulation for IVF [84]. Therefore, dermoid cysts do not require surgical treatment in the infertile patient.
4 Endometriosis
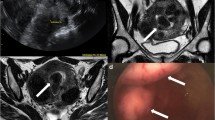
As previously noted, the majority of cases of endometriosis are not associated with an ovarian endometrioma. Transvaginal ultrasound may detect large nodules of endometriosis , and the sliding sign technique (applying pressure of the vaginal ultrasound transducer to the posterior fornix to determine if the uterus moves independently from the rectum) demonstrates high sensitivity and specificity for deeply infiltrating endometriosis in the rectovaginal septum [85]. However, MRI ultimately demonstrates greater sensitivity for deeply infiltrating endometriosis than ultrasound, 94% versus 79% [77], as well as for posterior implants involving the uterosacral ligaments and cul-de-sac [76]. MRI can detect small endometrial implants on T1-weighted images, and adhesions resulting from endometriosis are visible on both T1- and T2-weighted images. In Fig. 6.18, a posterior endometrioma can be visualized on T2-weighted imaging, as well as deeply infiltrating endometriosis in the rectovaginal septum, as indicated by the arrow.
MRI may also improve identification of hematosalpinx due to endometriosis, as seen in Fig. 6.19. The detailed, 3-dimensional imaging provided by MRI is highly predictive of surgical findings [76]. Prospective studies are needed to demonstrate the utility of MRI assessment for endometriosis among infertile women, in order to select the optimal treatment for each woman.
5 Conclusions: A Practical Approach to Imaging
In a woman presenting with infertility, HyCoSy offers an opportunity to evaluate the uterus, fallopian tubes, and ovaries with a single, relatively low cost, minimally invasive test that can be performed in the office and does not require radiation exposure. As such, many authors advocate for this as the first-line test for all new infertility patients [12]. Because of the high sensitivity and specificity of this test, clinical management of abnormalities including adnexal masses, hydrosalpinges, and uterine anomalies can often be determined from HyCoSy alone. However, when specific abnormalities are suspected due to clinical history or uncertain findings, additional imaging may be beneficial to make a definitive diagnosis, and prior to surgery or other invasive procedures. Radiation exposure, need for contrast, patient discomfort, and cost are all important considerations in weighing imaging techniques, and a personalized approach is paramount, with selection of only those tests whose results will change clinical management.
References
Taylor HS. The role of HOX genes in the development and function of the female reproductive tract. Semin Reprod Med. 2000;18(1):81–9.
Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound Obstet Gynecol. 2011;38(4):371–82. https://doi.org/10.1002/uog.10056.
Buttram VC Jr, Gibbons WE. Mullerian anomalies: a proposed classification. (An analysis of 144 cases). Fertil Steril. 1979;32(1):40–6.
The American Fertility Society. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–55.
Oppelt P, Renner SP, Brucker S, Strissel PL, Strick R, Oppelt PG, Doerr HG, Schott GE, Hucke J, Wallwiener D, Beckmann MW. The VCUAM (Vagina Cervix Uterus Adnex-associated Malformation) classification: a new classification for genital malformations. Fertil Steril. 2005;84(5):1493–7. https://doi.org/10.1016/j.fertnstert.2005.05.036.
Acien P, Acien MI. The history of female genital tract malformation classifications and proposal of an updated system. Hum Reprod Update. 2011;17(5):693–705. https://doi.org/10.1093/humupd/dmr021.
Grimbizis GF, Gordts S, Di Spiezio Sardo A, Brucker S, De Angelis C, Gergolet M, Li TC, Tanos V, Brolmann H, Gianaroli L, Campo R. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod. 2013;28(8):2032–44. https://doi.org/10.1093/humrep/det098.
Freud A, Harlev A, Weintraub AY, Ohana E, Sheiner E. Reproductive outcomes following uterine septum resection. J Matern Fetal Neonatal Med. 2015;28(18):2141–4. https://doi.org/10.3109/14767058.2014.981746.
Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44–50. https://doi.org/10.1016/j.fertnstert.2015.03.019.
Sugiura-Ogasawara M, Lin BL, Aoki K, Maruyama T, Nakatsuka M, Ozawa N, Sugi T, Takeshita T, Nishida M. Does surgery improve live birth rates in patients with recurrent miscarriage caused by uterine anomalies? J Obstet Gynaecol. 2015;35(2):155–8. https://doi.org/10.3109/01443615.2014.936839.
Grimbizis GF, Di Spiezio Sardo A, Saravelos SH, Gordts S, Exacoustos C, Van Schoubroeck D, Bermejo C, Amso NN, Nargund G, Timmermann D, Athanasiadis A, Brucker S, De Angelis C, Gergolet M, Li TC, Tanos V, Tarlatzis B, Farquharson R, Gianaroli L, Campo R. The Thessaloniki ESHRE/ESGE consensus on diagnosis of female genital anomalies. Gynecol Surg. 2016;13:1–16. https://doi.org/10.1007/s10397-015-0909-1.
Groszmann YS, Benacerraf BR. Complete evaluation of anatomy and morphology of the infertile patient in a single visit; the modern infertility pelvic ultrasound examination. Fertil Steril. 2016;105(6):1381–93. https://doi.org/10.1016/j.fertnstert.2016.03.026.
Bermejo C, Martinez-Ten P, Recio M, Ruiz-Lopez L, Diaz D, Illescas T. Three-dimensional ultrasound and magnetic resonance imaging assessment of cervix and vagina in women with uterine malformations. Ultrasound Obstet Gynecol. 2014;43(3):336–45. https://doi.org/10.1002/uog.12536.
Robbins JB, Broadwell C, Chow LC, Parry JP, Sadowski EA. Mullerian duct anomalies: embryological development, classification, and MRI assessment. J Magn Reson Imaging. 2015;41(1):1–12. https://doi.org/10.1002/jmri.24771.
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–7.
Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13. https://doi.org/10.1016/j.ijgo.2010.11.011.
Practice Committee of the American Society for Reproductive Medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertil Steril. 2017;108(3):416–25. https://doi.org/10.1016/j.fertnstert.2017.06.034.
Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–23. https://doi.org/10.1016/j.fertnstert.2008.01.051.
Styer AK, Jin S, Liu D, Wang B, Polotsky AJ, Christianson MS, Vitek W, Engmann L, Hansen K, Wild R, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Christy A, Diamond MP, Eisenberg E, Zhang H, Santoro N, National Institute of Child Health and Human Development Reproductive Medicine Network. Association of uterine fibroids and pregnancy outcomes after ovarian stimulation-intrauterine insemination for unexplained infertility. Fertil Steril. 2017;107(3):756–762.e3. https://doi.org/10.1016/j.fertnstert.2016.12.012.
Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril. 2000;73(2):406–11.
Nass Duce M, Oz U, Ozer C, Yildiz A, Apaydin FD, Cil F. Diagnostic value of sonohysterography in the evaluation of submucosal fibroids and endometrial polyps. Aust N Z J Obstet Gynaecol. 2003;43(6):448–52.
Schwarzler P, Concin H, Bosch H, Berlinger A, Wohlgenannt K, Collins WP, Bourne TH. An evaluation of sonohysterography and diagnostic hysteroscopy for the assessment of intrauterine pathology. Ultrasound Obstet Gynecol. 1998;11(5):337–42. https://doi.org/10.1046/j.1469-0705.1998.11050337.x.
Testa AC, Di Legge A, Bonatti M, Manfredi R, Scambia G. Imaging techniques for evaluation of uterine myomas. Best Pract Res Clin Obstet Gynaecol. 2016;34:37–53. https://doi.org/10.1016/j.bpobgyn.2015.11.014.
Thomassin-Naggara I, Dechoux S, Bonneau C, Morel A, Rouzier R, Carette MF, Darai E, Bazot M. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol. 2013;23(8):2306–14. https://doi.org/10.1007/s00330-013-2819-9.
Jha RC, Zanello PA, Ascher SM, Rajan S. Diffusion-weighted imaging (DWI) of adenomyosis and fibroids of the uterus. Abdom Imaging. 2014;39(3):562–9. https://doi.org/10.1007/s00261-014-0095-z.
Yang Q, Zhang LH, Su J, Liu J. The utility of diffusion-weighted MR imaging in differentiation of uterine adenomyosis and leiomyoma. Eur J Radiol. 2011;79(2):e47–51. https://doi.org/10.1016/j.ejrad.2011.03.026.
Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18(4):374–92. https://doi.org/10.1093/humupd/dms006.
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014;29(5):964–77. https://doi.org/10.1093/humrep/deu041.
Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril. 2017;108(3):483–90. e483. https://doi.org/10.1016/j.fertnstert.2017.06.025.
Al Jama FE. Management of adenomyosis in subfertile women and pregnancy outcome. Oman Med J. 2011;26(3):178–81. https://doi.org/10.5001/omj.2011.43.
Wang PH, Fuh JL, Chao HT, Liu WM, Cheng MH, Chao KC. Is the surgical approach beneficial to subfertile women with symptomatic extensive adenomyosis? J Obstet Gynaecol Res. 2009;35(3):495–502. https://doi.org/10.1111/j.1447-0756.2008.00951.x.
Park CW, Choi MH, Yang KM, Song IO. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin Exp Reprod Med. 2016;43(3):169–73. https://doi.org/10.5653/cerm.2016.43.3.169.
Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol. 2013;29(12):1026–30. https://doi.org/10.3109/09513590.2013.824960.
Dueholm M, Lundorf E. Transvaginal ultrasound or MRI for diagnosis of adenomyosis. Curr Opin Obstet Gynecol. 2007;19(6):505–12. https://doi.org/10.1097/GCO.0b013e3282f1bf00.
Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. Am J Obstet Gynecol. 2009;201(1):107.e1–6. https://doi.org/10.1016/j.ajog.2009.03.021.
Stamatopoulos CP, Mikos T, Grimbizis GF, Dimitriadis AS, Efstratiou I, Stamatopoulos P, Tarlatzis BC. Value of magnetic resonance imaging in diagnosis of adenomyosis and myomas of the uterus. J Minim Invasive Gynecol. 2012;19(5):620–6. https://doi.org/10.1016/j.jmig.2012.06.003.
Sillo-Seidl G. The analysis of the endometrium of 1,000 sterile women. Hormones. 1971;2(2):70–5.
Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(5):1197–205. https://doi.org/10.1097/AOG.0b013e3181f74864.
Perez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, Engels V. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod. 2005;20(6):1632–5. https://doi.org/10.1093/humrep/deh822.
Afifi K, Anand S, Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):117–21. https://doi.org/10.1016/j.ejogrb.2010.04.005.
American Association of Gynecologic Laparoscopists. AAGL practice report: practice guidelines for the diagnosis and management of endometrial polyps. J Minim Invasive Gynecol. 2012;19(1):3–10. https://doi.org/10.1016/j.jmig.2011.09.003.
Nieuwenhuis LL, Hermans FJ, Bij de Vaate AJM, Leeflang MM, Brolmann HA, Hehenkamp WJ, Mol BWJ, Clark TJ, Huirne JA. Three-dimensional saline infusion sonography compared to two-dimensional saline infusion sonography for the diagnosis of focal intracavitary lesions. Cochrane Database Syst Rev. 2017;5:CD011126. https://doi.org/10.1002/14651858.CD011126.pub2.
Asherman JG. Amenorrhoea traumatica (atretica). J Obstet Gynaecol Br Emp. 1948;55(1):23–30.
March CM. Asherman’s syndrome. Semin Reprod Med. 2011;29:83–94. Epub 2011 Mar 24.
Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, Mol BW, Huirne JA. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20(2):262–78. https://doi.org/10.1093/humupd/dmt045.
Hanstede MM, van der Meij E, Goedemans L, Emanuel MH. Results of centralized Asherman surgery, 2003-2013. Fertil Steril. 2015;104(6):1561–8.e1. https://doi.org/10.1016/j.fertnstert.2015.08.039.
Seshadri S, El-Toukhy T, Douiri A, Jayaprakasan K, Khalaf Y. Diagnostic accuracy of saline infusion sonography in the evaluation of uterine cavity abnormalities prior to assisted reproductive techniques: a systematic review and meta-analyses. Hum Reprod Update. 2015;21(2):262–74. https://doi.org/10.1093/humupd/dmu057.
Roma Dalfo A, Ubeda B, Ubeda A, Monzon M, Rotger R, Ramos R, Palacio A. Diagnostic value of hysterosalpingography in the detection of intrauterine abnormalities: a comparison with hysteroscopy. AJR Am J Roentgenol. 2004;183(5):1405–9. https://doi.org/10.2214/ajr.183.5.1831405.
Kim MJ, Lee Y, Lee C, Chun S, Kim A, Kim HY, Lee JY. Accuracy of three dimensional ultrasound and treatment outcomes of intrauterine adhesion in infertile women. Taiwan J Obstet Gynecol. 2015;54(6):737–41. https://doi.org/10.1016/j.tjog.2015.10.011.
Berker B, Sukur YE, Kahraman K, Atabekoglu CS, Sonmezer M, Ozmen B, Ates C. Impact of unilateral tubal blockage diagnosed by hysterosalpingography on the success rate of treatment with controlled ovarian stimulation and intrauterine insemination. J Obstet Gynaecol. 2014;34(2):127–30. https://doi.org/10.3109/01443615.2013.853030.
Johnson N, van Voorst S, Sowter MC, Strandell A, Mol BW. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev. 2010;(1):CD002125. https://doi.org/10.1002/14651858.CD002125.pub3.
Sagoskin AW, Lessey BA, Mottla GL, Richter KS, Chetkowski RJ, Chang AS, Levy MJ, Stillman RJ. Salpingectomy or proximal tubal occlusion of unilateral hydrosalpinx increases the potential for spontaneous pregnancy. Hum Reprod. 2003;18(12):2634–7.
Dessole S, Meloni GB, Capobianco G, Manzoni MA, Ambrosini G, Canalis GC. A second hysterosalpingography reduces the use of selective technique for treatment of a proximal tubal obstruction. Fertil Steril. 2000;73(5):1037–9.
Risquez F, Confino E. Transcervical tubal cannulation, past, present, and future. Fertil Steril. 1993;60(2):211–26.
Woolcott R, Petchpud A, O'Donnell P, Stanger J. Differential impact on pregnancy rate of selective salpingography, tubal catheterization and wire-guide recanalization in the treatment of proximal fallopian tube obstruction. Hum Reprod. 1995;10(6):1423–6.
Lazer T, Meltzer S, Saar-Ryss B, Liberty G, Rabinson Y, Friedler S. The place of selective hysterosalpingography and tubal canalization among sub-fertile patients diagnosed with proximal tubal occlusion. Arch Gynecol Obstet. 2016;293(5):1107–11. https://doi.org/10.1007/s00404-015-3998-1.
Ferraiolo A, Ferraro F, Remorgida V, Gorlero F, Capitanio GL, de Cecco L. Unexpected pregnancies after tubal recanalization failure with selective catheterization. Fertil Steril. 1995;63(2):299–302.
Maheux-Lacroix S, Boutin A, Moore L, Bergeron ME, Bujold E, Laberge P, Lemyre M, Dodin S. Hysterosalpingosonography for diagnosing tubal occlusion in subfertile women: a systematic review with meta-analysis. Hum Reprod. 2014;29(5):953–63. https://doi.org/10.1093/humrep/deu024.
Alcazar JL, Martinez-Astorquiza Corral T, Orozco R, Dominguez-Piriz J, Juez L, Errasti T. Three-dimensional hysterosalpingo-contrast-sonography for the assessment of tubal patency in women with infertility: a systematic review with meta-analysis. Gynecol Obstet Investig. 2016;81(4):289–95. https://doi.org/10.1159/000443955.
de Wit W, Gowrising CJ, Kuik DJ, Lens JW, Schats R. Only hydrosalpinges visible on ultrasound are associated with reduced implantation and pregnancy rates after in-vitro fertilization. Hum Reprod. 1998;13(6):1696–701.
Strandell A, Lindhard A, Waldenstrom U, Thorburn J. Hydrosalpinx and IVF outcome: cumulative results after salpingectomy in a randomized controlled trial. Hum Reprod. 2001;16(11):2403–10.
Socolov D, Boian I, Boiculese L, Tamba B, Anghelache-Lupascu I, Socolov R. Comparison of the pain experienced by infertile women undergoing hysterosalpingo contrast sonography or radiographic hysterosalpingography. Int J Gynaecol Obstet. 2010;111(3):256–9. https://doi.org/10.1016/j.ijgo.2010.07.018.
Dreyer K, Out R, Hompes PG, Mijatovic V. Hysterosalpingo-foam sonography, a less painful procedure for tubal patency testing during fertility workup compared with (serial) hysterosalpingography: a randomized controlled trial. Fertil Steril. 2014;102(3):821–5. https://doi.org/10.1016/j.fertnstert.2014.05.042.
Lo Monte G, Capobianco G, Piva I, Caserta D, Dessole S, Marci R. Hysterosalpingo contrast sonography (HyCoSy): let's make the point! Arch Gynecol Obstet. 2015;291(1):19–30. https://doi.org/10.1007/s00404-014-3465-4.
Mutlu MF, Erdem M, Erdem A, Yildiz S, Mutlu I, Arisoy O, Oktem M. Antral follicle count determines poor ovarian response better than anti-Mullerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet. 2013;30(5):657–65. https://doi.org/10.1007/s10815-013-9975-3.
Nelson SM, Fleming R, Gaudoin M, Choi B, Santo-Domingo K, Yao M. Antimullerian hormone levels and antral follicle count as prognostic indicators in a personalized prediction model of live birth. Fertil Steril. 2015;104(2):325–32. https://doi.org/10.1016/j.fertnstert.2015.04.032.
Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine S. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92. https://doi.org/10.1210/jc.2013-2350.
Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar-Morreale HF. Definition and significance of polycystic ovarian morphology: a task force report from the androgen excess and polycystic ovary syndrome society. Hum Reprod Update. 2014;20(3):334–52. https://doi.org/10.1093/humupd/dmt061.
Kwan I, Bhattacharya S, Kang A, Woolner A. Monitoring of stimulated cycles in assisted reproduction (IVF and ICSI). Cochrane Database Syst Rev. 2014;(8):CD005289. https://doi.org/10.1002/14651858.CD005289.pub3.
Zreik TG, Garcia-Velasco JA, Habboosh MS, Olive DL, Arici A. Prospective, randomized, crossover study to evaluate the benefit of human chorionic gonadotropin-timed versus urinary luteinizing hormone-timed intrauterine inseminations in clomiphene citrate-stimulated treatment cycles. Fertil Steril. 1999;71(6):1070–4.
Lewis V, Queenan J Jr, Hoeger K, Stevens J, Guzick DS. Clomiphene citrate monitoring for intrauterine insemination timing: a randomized trial. Fertil Steril. 2006;85(2):401–6. https://doi.org/10.1016/j.fertnstert.2005.07.1331.
Legendre G, Catala L, Moriniere C, Lacoeuille C, Boussion F, Sentilhes L, Descamps P. Relationship between ovarian cysts and infertility: what surgery and when? Fertil Steril. 2014;101(3):608–14. https://doi.org/10.1016/j.fertnstert.2014.01.021.
Timmerman D, Van Calster B, Testa AC, Guerriero S, Fischerova D, Lissoni AA, Van Holsbeke C, Fruscio R, Czekierdowski A, Jurkovic D, Savelli L, Vergote I, Bourne T, Van Huffel S, Valentin L. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: a temporal and external validation study by the IOTA group. Ultrasound Obstet Gynecol. 2010;36(2):226–34. https://doi.org/10.1002/uog.7636.
Kaijser J, Van Gorp T, Van Hoorde K, Van Holsbeke C, Sayasneh A, Vergote I, Bourne T, Timmerman D, Van Calster B. A comparison between an ultrasound based prediction model (LR2) and the risk of ovarian malignancy algorithm (ROMA) to assess the risk of malignancy in women with an adnexal mass. Gynecol Oncol. 2013;129(2):377–83. https://doi.org/10.1016/j.ygyno.2013.01.018.
Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):310–5.
Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: a review. Eur Radiol. 2006;16(2):285–98. https://doi.org/10.1007/s00330-005-2882-y.
Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591. https://doi.org/10.1002/14651858.CD009591.pub2.
Kitajima M, Defrere S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, Donnez J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011;96(3):685–91. https://doi.org/10.1016/j.fertnstert.2011.06.064.
Uncu G, Kasapoglu I, Ozerkan K, Seyhan A, Oral Yilmaztepe A, Ata B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod. 2013;28(8):2140–5. https://doi.org/10.1093/humrep/det123.
Benaglia L, Bermejo A, Somigliana E, Faulisi S, Ragni G, Fedele L, Garcia-Velasco JA. In vitro fertilization outcome in women with unoperated bilateral endometriomas. Fertil Steril. 2013;99(6):1714–9. https://doi.org/10.1016/j.fertnstert.2013.01.110.
Benschop L, Farquhar C, van der Poel N, Heineman MJ. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst Rev. 2010; (11):CD008571. https://doi.org/10.1002/14651858.CD008571.pub2.
Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475–90. https://doi.org/10.1148/radiographics.21.2.g01mr09475.
Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS, Kim SH. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Mullerian hormone levels. Fertil Steril. 2010;94(1):343–9. https://doi.org/10.1016/j.fertnstert.2009.02.022.
Caspi B, Weissman A, Zalel Y, Barash A, Tulandi T, Shoham Z. Ovarian stimulation and in vitro fertilization in women with mature cystic teratomas. Obstet Gynecol. 1998;92(6):979–81.
Hudelist G, Fritzer N, Staettner S, Tammaa A, Tinelli A, Sparic R, Keckstein J. Uterine sliding sign: a simple sonographic predictor for presence of deep infiltrating endometriosis of the rectum. Ultrasound Obstet Gynecol. 2013;41(6):692–5. https://doi.org/10.1002/uog.12431.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Johnstone, E.B., Olpin, J.D. (2018). Advanced Imaging Techniques Used in the Infertile Female. In: Carrell, D., Racowsky, C., Schlegel, P., DeCherney, A. (eds) Emerging Topics in Reproduction. Springer, Cham. https://doi.org/10.1007/978-3-319-90823-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-90823-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90822-9
Online ISBN: 978-3-319-90823-6
eBook Packages: MedicineMedicine (R0)