Abstract
Adenosine receptors A1, A2A, A2B, and A3 are effector proteins triggered by the endogenous nucleoside adenosine to exert its numerous vital physiological effects, behaving like a guardian angel. This chapter offers an overview of the updated knowledge concerning the structure, distribution, and signal transduction of adenosine receptors. They are a family of G protein-coupled receptors widely distributed through the body, from central nervous system to peripheral organs, important and ubiquitous regulators of numerous cellular signaling. Their presence on every cell renders them an attractive opportunity for the pharmacological research and development of new drugs but also a challenge in the difficulty to produce tissue-selective ligands avoided of side effects. To aid this process, several efforts have been invested to reveal the molecular structure and the consequent mechanism of ligand binding of these receptors, and until now more than 30 structures have been published for the human A2A subtype. Finally, the principal adenosine receptor signaling pathways including adenylyl cyclase, phospholipase C, inositol triphosphate, diacylglycerol, phosphatidylinositol 3-kinase, and mitogen-activated protein kinases determining their effects on several transcription factors, such as hypoxia-inducible factor 1, cyclic AMP (cAMP)-responsive elements, nuclear factor-kB, and exchange protein directly activated by cAMP as the most relevant, are presented.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Adenosine is a purine nucleoside released by almost all cells mediating its effects through activation of four G protein-coupled adenosine receptors , classified as A1, A2A, A2B, and A3 (Borea et al. 2016). The first demonstration of their existence has been offered more than 40 years ago by the observation that methylxanthines such as caffeine and theophylline were able to antagonize the cardiac and cerebral effects of adenosine. These receptors are characterized by different affinity for adenosine, G protein coupling, as well as intracellular signal transduction inside cells. In general adenosine interacts with A1, A2A, or A3 subtypes with an EC50 in the range 10 nM–1 μM, while activation of the A2B subtype needs concentrations higher than 10 μM, rarely obtained in physiological conditions but present in hypoxic/injured tissues (Eltzschig 2009). Anyway, the affinity of adenosine to its receptors may also depend on the effect investigated, e.g., cAMP level determination versus MAPK activation or the number of receptors expressed (Chen et al. 2013). Specifically, on the one hand, A1 and A3 adenosine receptors show high and low affinity for adenosine, respectively, and are able to reduce adenylyl cyclase activity. On the other hand, A2A and A2B subtypes display high and low affinity for the nucleoside, respectively, and activate adenylyl cyclase, thus stimulating cyclic AMP (cAMP) levels (Fredholm et al. 2011; Borea et al. 2017). Adenosine receptors are present in every organ, tissue, and cell of the body rendering them attractive targets for the research and development of new drugs in many pathological conditions related with raised adenosine levels (Gessi et al. 2011). Anyway this wide distribution implies the lack of specificity of a given receptor subtype that may be present in both tissues involved in disease but also in healthy organs with consequent side effects, rendering difficult the development of drugs for specific medical needs. In this chapter updated informations concerning the molecular structure, distribution, and signal transduction of adenosine receptors are provided.
3.2 Molecular Structures of Adenosine Receptors
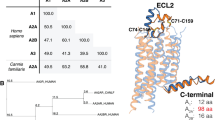
Adenosine receptors have been cloned in the beginning of the 1990s and deeply pharmacologically characterized and consist of a similar structure represented by a core domain crossing the plasma membrane seven times, with an extracellular N-terminus, an intracellular C-terminus, and three intracellular and three extracellular loops (IL and EL, respectively) of different lengths and functions among the four adenosine receptor subtypes (Fredholm et al. 2000). These domains give specific characteristics important for receptor-ligand interactions. Specifically, the EL1, EL2, and EL3 of GPCRs contribute significantly to receptor function as evidenced by crystal structures, and cysteine amino acids forming disulfide bonds in the EL domains of GPCRs are important not only in ligand binding but also in receptor stability and function (Avlani et al. 2007; Schiedel et al. 2011). The N-terminus presents one or more glycosylation sites, while the C-terminus possesses phosphorylation and palmitoylation loci, which are important for receptor desensitization and internalization. Specifically, mutation studies revealed that glycosylation is relevant for the recruitment of receptors to the plasma membrane, while palmitoylation sites, located at the end of helix 8 and absent in A2A adenosine receptors, influence receptor degradation. Depalmitoylation of A3 adenosine receptors, in contrast to what happens for A1 subtype, induces a fast receptor desensitization through GPCR kinase phosphorylation induction (Piirainen et al. 2011). Adenosine receptors are characterized by a high homology sequence among them, ranging from 41% to 58% of sequence identity for the human species, with the most conserved region being in the extracellular region of the receptor reaching 71%.
3.2.1 A1 Adenosine Receptors
A1 adenosine receptors are 326 amino acid long distributed among 7 transmembrane domains (TM) of which TM3 and TM7 result strictly conserved sequences for ligand interaction with the receptor, as reported from mutagenesis studies (Jespers et al. 2018). The A1AR orthosteric site is found inside the TM packet, but also EL2 has been implicated in the ligand affinity and signal transduction (Peeters et al. 2012; Nguyen et al. 2016a, b). In addition, in the A1 adenosine receptor EL2, the presence of an allosteric site has been reported through molecular modeling characterization (Narlawar et al. 2010). Recently, the crystal structure of A1 adenosine receptors bound to a selective covalent antagonist has been revealed (Glukhova et al. 2017). Interestingly, significant differences with respect to already presented A2A adenosine receptor structure indicate a different conformation of EL2 and a bigger extracellular cavity presenting an alternative binding pocket accepting both orthosteric and allosteric molecules. It has been suggested that this configuration confers ligand selectivity instead of the simple amino acid sequence. From this knowledge more selective drugs could be projected with both agonist and allosteric properties, useful for the therapy of neuropathic pain, ischemia-reperfusion damage, and renal pathologies (Glukhova et al. 2017; Cheng et al. 2017).
As for allosteric sites located on the EL region, a crystal structure with an allosteric modulator has not been provided but through mutagenesis studies the amino acid sequence responsible for these ligands involved in the binding site of A1AR allosteric modulators (Jespers et al. 2018) has been reported.
3.2.2 A2A Adenosine Receptors
A2A adenosine receptors in human species are 412 amino acid long, but this number may slightly change from 409 to 412 in other species (de Lera Ruiz et al. 2014). At variance with other adenosine subtypes, it presents a long carboxy-terminal domain, responsible for a major molecular weight (45 kDa) with respect to the other adenosine subtypes (Preti et al. 2015). A2A adenosine receptors are formed by 7 TM of 20–27 amino acids with TM3 and EL2 containing cysteine residues giving a disulfide bond . In addition an extra short TM8 domain is present toward the membrane cytoplasmic surface (Jaakola and IJzerman 2010; de Lera Ruiz et al. 2014). Interestingly, two new cholesterol-binding sites have been described on it, one of which interacts with cholesterol only when bound to an inverse agonist, as demonstrated through numerous high-resolution crystal structure studies (Rouviere et al. 2017). Indeed, the last 10 years have seen a huge development of novel crystallization strategies that have introduced enormous changes in the knowledge of structural biology of GPCRs. Specifically, the A2A adenosine receptor has been one of the best studied and characterized by a structural point of view, having more than 30 structures been described (Carpenter and Lebon 2017). In particular, crystal structures of A2A adenosine receptors have been solved in complex with both agonists and antagonists, which provide informations concerning the binding sites and the conformational changes occurring following ligand-receptor interactions (Jaakola et al. 2008; Xu et al. 2011; Lebon et al. 2011, 2015; Doré et al. 2011; Hino et al. 2012; Congreve et al. 2012; Liu et al. 2012; Carpenter et al. 2016; Jazayeri et al. 2017; Carpenter and Lebon 2017). Specifically, the most observed phenomenon taking place after binding of the agonist is a contraction of the binding site due to TM3, 5, 6, and 7 rearrangements (Jespers et al. 2018). In addition an outward rotation of TM6 on the cytoplasmic side, consequent to receptor activation, allows G protein activation and signal transduction propagation. In addition it has been revealed that the ribose moiety is a key component of A2A receptor agonists that helps to stabilize the intermediate-active state before the occurrence of the fully active receptor conformation, following G protein coupling (Carpenter and Lebon 2017). Numerous mutagenesis studies investigating the ligand binding of A2A adenosine receptors have been performed. Interestingly, from them, the relevance of a glutamic acid and a histidine in TM1 and TM7, respectively, has been found taking part into the agonist binding process. In addition a relevant role for H bonds in ligand binding affinity has been revealed following the observation that loss of interactions between ligand and water is reflected in worsen affinity of both agonists and antagonists (Jespers et al. 2018). Overall from the data emerging by complementary techniques such as crystal structures as well as X-rays and mutagenesis studies, it is possible today to address a structure-based rationale design of new ligands interacting with A2A adenosine receptors (Jespers et al. 2017).
3.2.3 A2B Adenosine Receptors
A2B adenosine receptors in human species are 328 amino acid long, organized following the typical GPCR architecture consisting of 7 TM domains presenting the highest homology between A2B and the other adenosine receptors. This core is formed by hydrophobic amino acids linked by three EL and three IL and terminates with an extracellular N-terminus and an intracellular C-terminus. Combination of homology modeling of rhodopsin GPCR structure and mutational studies of the A2B adenosine receptors leads to the knowledge of its binding site, where TM regions 3, 5, 6, and 7 are involved in agonist and antagonist recognition (Beukers et al. 2000, 2004; Aherne et al. 2011). Interestingly, the EL2 of A2B receptor, the longest of all the other adenosine receptor subtypes, presents four cysteine amino acids (C154, C166, C167, C171) responsible for disulfide bonds connecting EL and TM domains . Interestingly, only disulfide bond occurring between C171 in EL2 and C78 present in TM3 is essential for A2B adenosine receptor-ligand binding and function, and it may also play a role in the transport of the receptors toward the membrane. As for the other cysteine residues in the ECL2 of the A2B receptor, they may have different functions in comparison to the role that they play in the A2A receptor (Schiedel et al. 2011). In addition subsequent site-directed mutagenesis studies have reported that introducing ECL2 of A2A adenosine receptors in the structure of A2B adenosine receptors provides a mutant A2B receptor that displays higher affinity for both agonist and antagonists, thus suggesting that ECL2 is crucial for ligand binding. Therefore the major length of ECL2 in the A2B adenosine subtype is responsible for the lower affinity of ligands to it in comparison to A2A receptors, because it may hamper the ligand interaction to the binding site (Schiedel et al. 2011; Seibt et al. 2013; da Rocha Lapa et al. 2014). Other mutational studies have discovered the amino acids involved in ligand binding of three different classes of molecules including xanthine, adenosine, and aminopyridine derivatives. In particular, the amino acids Asn282 and His280 by forming H bond stabilize the binding site as occurs in the A2A adenosine receptor. Trp247, Val250, and especially Ser279 are crucial for adenosine binding. Leu81, Asn186, and Val250 are important for binding of the xanthine antagonists (Thimm et al. 2013).
3.2.4 A3 Adenosine Receptors
A3 adenosine receptors in human species are 318 amino acid long. As with the other adenosine receptors, the A3 is constituted by seven TM domains with an intracellular C-terminal sequence containing six Ser and Thr amino acids undergoing phosphorylation by GPCR kinases during rapid receptor desensitization occurring in the order of minutes. Specifically, this process triggered following agonist binding to the A3 adenosine receptors causes subsequent internalization through clathrin-coated pits in rat A3 adenosine receptors (Palmer and Stiles 2000; Trincavelli et al. 2002a, b; Madi et al. 2003; Pugliese et al. 2007; Jacobson et al. 2018). However, the fast desensitization has not been observed in A1, A2A, and A2B receptor subtypes where this process takes place after hours. The reason for this discrepance has been attributed to the lack of Ser and Thr residues in the C-terminus, for example, of the A1 subtype. Another reason explaining the rapid desensitization of A3 receptors resides in the presence of Cys amino acids in its C-terminus tail, crucial for GRK activation. As the sequence identity between rat and human A3 receptors is only 72%, this point has been recently addressed. Specifically, it has been shown that the C-terminus of the human subtype is not involved in βarr2 recruitment, receptor desensitization, and internalization , suggesting that other different regions of the human A3 adenosine receptors, either cytosolic or exposed upon receptor activation, are involved in this process. It has been observed that C-terminal truncation, in combination with mutation of the “DRY” motif located at the boundary between TM3 and IL-2, significantly decreased βarr2 recruitment (Storme et al. 2018). Interestingly, mutational studies demonstrated that the active shape of the human A3 receptor needs the highly conserved Trp (W6.48) in TM6, important to activate signal transduction pathways, to interact with β-arrestin2, and to undergo receptor internalization (Gao et al. 2002; Stoddart et al. 2014). Furthermore, use of a novel fluorescent A3 agonist has allowed for the observation of co-localization with internalized receptor βarr3 complexes (Stoddart et al. 2015).
3.2.5 Adenosine Receptor Heteromers
Homomer, oligomer, and heteromer formation has been recently recognized as a common phenomenon affecting numerous GPCRs including adenosine receptors (Ferré et al. 2010a, b; Navarro et al. 2010a, b, 2016b; Brugarolas et al. 2014). The possibility of homo- or hetero-oligomer formation lies on, at least in part, high receptor levels (Fredholm et al. 2011). Specifically, GPCR heteromers are new entities for signal transduction with different functions if compared to homomers. In the field of adenosine receptors, A1-A2A oligomers are present in neural tissue, comprising two different receptors coupled to two different G proteins (Brugarolas et al. 2014; Navarro et al. 2016b). In particular the A1 component, through Gi and the A2A part via Gs, confers to the heteromer the possibility to signal in an opposite way on cyclic adenosine monophosphate (cAMP) intracellular pathway. Therefore, this complex constitutes a cell surface sensor of adenosine concentration, distinguishing between low and high nucleoside concentration (Navarro et al. 2016b). Indeed the A1 unit of this complex interacts with Gi/o protein, thus decreasing cAMP levels, PKA, and GABA uptake, when adenosine levels are low. The A2A monomer of the heteromer takes place in cAMP signaling when adenosine levels increase, due to its inhibition of A1 component and activation of Gs proteins, thus obtaining GABA uptake increase (Cristóvão-Ferreira et al. 2013). In addition various physiological process, such as glutamate release, may be regulated on the basis of adenosine concentration (Ciruela et al. 2006). Heteromerization has been described as a general process involving other receptors inside adenosine receptor family including A3ARs, forming homodimers and A1-A3 heterodimers (Kim and Jacobson 2006; Hill et al. 2014). In addition, heteromerization involves also the interaction of adenosine receptors with other GPCRs. For example, A1 may form oligomers with P2Y1 (Yoshioka et al. 2001), D1 dopamine (Ginés et al. 2000), and mGlu1αR receptors (Ciruela et al. 2001). As for A2A receptors, the most studied combination in this field is represented by the A2A-D2 dopamine complex, detected in the striatum, and a viable therapeutic target in PD (Fuxe et al. 2005, 2007; Ferré et al. 2010b; Navarro et al. 2016a). In addition they may oligomerize with mGlu5 (Ferré et al. 2002), P2Y1 (Arellano et al. 2009), and cannabinoid CB1 receptors (Carriba et al. 2007).
3.3 Distribution of Adenosine Receptors
Adenosine receptors are widely distributed throughout the body spanning from the central nervous system, cardiovascular apparatus, respiratory tract, gastrointestinal tissue, and immune system to different organs or tissues including the kidney, bone, joints, eyes, and skin, suggesting a wide influence of adenosine in almost all physiological processes (Peleli et al. 2017). This distribution reflects a significative function of adenosine in the neurons, heart, and kidney.
3.3.1 A1 Adenosine Receptors
In the brain A1 adenosine receptors are highly distributed in different regions, including the cortex, hippocampus, cerebellum and spinal cord, autonomic nerve terminals, and glial cells (Chen et al. 2013; Ballesteros-Yáñez et al. 2018). In the heart, A1 adenosine receptor expression has been detected with higher levels in atria and less in the ventricular myocardium (Varani et al. 2017). At vascular level A1 adenosine receptors are present on coronary smooth muscle arteries and endothelial cells (Headrick et al. 2013). Furthermore, A1 adenosine receptors are found in the lung endothelial cells, in smooth muscle cells of airway, in alveolar epithelial cells, and in macrophages (Sun et al. 2005). In the kidney, A1 adenosine receptors are located in the collecting ducts of the papilla, inner medulla, and cells of the juxtaglomerular apparatus (Varani et al. 2017; Soni et al. 2017). A1 adenosine receptors are expressed in pancreas tissues and adipocytes (Meriño et al. 2017). As for immune system, A1 adenosine receptors are present on different immune cells, such as neutrophils, eosinophils, macrophages, and monocytes (Sachdeva and Gupta 2013; Boros et al. 2016). A1 adenosine receptors have also been localized in the retina , intestine, skeletal muscle, and vascular cells of skeletal muscle (Varani et al. 2017).
3.3.2 A2A Adenosine Receptors
A2A adenosine receptors are mostly expressed in selected areas of the central nervous system as well as in peripheral immune cells. Specifically, concerning brain regions A2A adenosine receptors are expressed at high level in striatal neurons, while lower presence has been detected in extra-striatal and in glial cells (Fredholm et al. 2011; Boison et al. 2012; Borea et al. 2017). In particular, they are numerous in the caudate and putamen, in the nucleus accumbens, as well as in the olfactory tubercle. The presence of A2A adenosine receptors has been demonstrated in the heart, in both atria and ventricle and in coronary vessels, but also in the lung and liver. Finally, high expression of A2A adenosine receptors has been reported in platelets, lymphocytes, neutrophils, monocytes, macrophages, dendritic cells, vascular smooth muscle , and endothelial cells (Gessi et al. 2000).
3.3.3 A2B Adenosine Receptors
At the central level, the A2B adenosine receptors are expressed in astrocytes, neurons, and microglia (Koupenova et al. 2012; Merighi et al. 2015; Pedata et al. 2016). As for the periphery, they are found in the bowel, bladder, lung, vas deferens, and different cell types including fibroblasts; smooth muscle, endothelial, alveolar epithelial, chromaffin, and taste cells; platelets; myocardial cells; and retinal, intestinal and pulmonary epithelial, and endothelial cells. A2B adenosine receptors are expressed in several immune cells including mast cells, macrophages , lymphocytes, neutrophils, and dendritic cells (Aherne et al. 2011).
3.3.4 A3 Adenosine Receptors
A3 adenosine receptors are present in several cells and tissues with a different degree of expression at central and peripheral level. In the brain tissue, they are present in low amount in the thalamus, hypothalamus, and hippocampus. At cellular level they are expressed in motor nerve terminals, microglia, astrocytes, cortex, and retinal ganglion cells while at cerebral vascular level in the pial and intercerebral arteries (Janes et al. 2014; Borea et al. 2016). A3 adenosine receptors are present in the coronary and carotid artery and in the heart but only at low level. At the periphery A3 adenosine receptors have been demonstrated in lung parenchyma and bronchi, enteric neurons and colonic mucosa, and epithelial cells. Finally, A3 adenosine receptors have a wide distribution in immune and inflammatory cells including lymphocytes, neutrophils, eosinophils, monocytes, macrophages, dendritic cells, foam cells, mast cells, splenocytes, bone marrow cells, lymph nodes, synoviocytes, chondrocytes, and osteoblasts. Interestingly, A3 adenosine receptors are overexpressed in different cancer tissues such as the colon, liver, lung, melanoma, and glioblastoma (Borea et al. 2015).
3.4 Signal Transduction of Adenosine Receptors
All adenosine receptors are coupled to G proteins and trigger several transduction pathways that may differ depending on the specific cell activated (Fredholm et al. 2001).
3.4.1 A1 Adenosine Receptors
The Gi-coupled A1 adenosine receptor inhibits adenylyl cyclase (AC) activity thus decreasing cAMP levels. This leads to the inhibition of cAMP-dependent protein kinase A (PKA) activation and cAMP-responsive element-binding protein 1 (CREB-1) phosphorylation, resulting in the reduction of CREB transcriptional activation. In addition it also induces phospholipase C (PLC)-β stimulation, by link to Gq proteins, thus rising diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) that, through interaction with its cytoplasmic receptor, rises intracellular Ca2+ concentrations, which activate calcium-dependent protein kinases (PKC) and/or other calcium-binding proteins. PKC may be phosphorylated also by DAG. In addition, βγ subunits of Gi/o protein are involved by A1 adenosine receptor to induce PLC activation (Biber et al. 1997). In addition A1 adenosine receptor enrolls pertussis-toxin-sensitive potassium (K) and KATP channels, expressed in neurons and myocardium , while reduces Ca2+ channels of Q, P, and N type. Recently, it has been reported that it increases PC12 cell damage following intermittent hypoxia through PKC and KATP mediators (Mei et al. 2018). Furthermore, the first report describing the link between A1 adenosine receptor and the family of mitogen-activated protein kinase (MAPK) indicated the stimulation by it of extracellular signal-regulated kinase (ERK) (Schulte and Fredholm 2000) (Fig. 3.1). Since then many studies have found different effects on MAPK modulation depending on the cell investigated. For example, it has been reported that A1 adenosine receptor in brain neurons increases p38 to reduce apoptosis in a rat model of brain injury (Zhai et al. 2016). Accordingly, it activates p38 and also c-Jun N-terminal kinase (JNK) in hippocampal neurons, thus inducing clathrin-mediated internalization of GluA2 and GluA1 subunits responsible for synaptic depression that caused hippocampal neurodegeneration after hypoxia/cerebral ischemia (Brust et al. 2006; Liang et al. 2008; Chen et al. 2014). Previous data in the hippocampus demonstrated that the increase in p38 phosphorylation induced by A1 receptor was involved in brain-derived neurotrophic factor (BDNF) generation (Katoh-Semba et al. 2009). In astrocytes, A1 adenosine receptor reduces ERK and AKT, thus provoking the inhibition of LPS-induced hypoxia-inducible factor (HIF)-1α activation with reduction of genes involved in inflammation and hypoxic injury (Gessi et al. 2013). In ear cochlea, it inhibits p38, ERK, and JNK activation and decreases cisplatin-induced signal transducer and activator of transcription (STAT-1) phosphorylation, thus reducing apoptosis and inflammation. This mechanism may be relevant to provide otoprotection against ototoxicity induced by this chemotherapeutic drug (Kaur et al. 2016). In cardiomyocytes, A1 adenosine receptor phosphorylates p38, present downstream the mitochondrial K(ATP) channel, protecting cells from hypoxia injury (Leshem-Lev et al. 2010). Accordingly, in these cells it activates p38, ERK, and JNK phosphorylation, producing an increase of tissue transglutaminase (TG2) and cytoprotection (Vyas et al. 2016). An increase of p38 was also discovered in the reduction by A1 adenosine receptor of beta-adrenergic-induced contractile function as a mechanism of adenoprotection (Fenton et al. 2010). In mouse coronary artery smooth muscle cells, it activates the PKC-alpha transduction pathway, causing ERK phosphorylation (Ansari et al. 2009). In foam cells, A1 adenosine receptor contributed to atherosclerosis by inducing HIF-1 accumulation through an increase of p38 and AKT phosphorylation (Gessi et al. 2010a). In contrast in neutrophils, it reduces p38 thus decreasing chemotaxis (Xu et al. 2017). Together, these data indicate that modulation of MAPK signaling, especially the one related to p38 phosphorylation, by A1 adenosine receptor occurs in different organs and tissues thus affecting numerous pathological processes.
Schematic picture of A1 adenosine receptor signaling cascade . Adenosine activates A1R to reduce AC activity and cAMP levels thus blocking PKA and CREB while stimulates PLC-β and Ca2+. In addition adenosine triggers K+ channels and inhibits Q, P, N, and Ca2+ channels. p38, ERK1/2, and JNK1/2 phosphorylation is determined by A1R stimulation
3.4.2 A2A Adenosine Receptors
The Gs-coupled A2A adenosine receptor stimulates AC activity, thereby increasing cAMP levels, with consequent PKA phosphorylation that causes activation of numerous proteins, including receptors, phosphodiesterases, cAMP-responsive element-binding protein (CREB), and dopamine- and cAMP-regulated phosphoprotein (DARPP-32) (Preti et al. 2015). Interestingly, in hepatocyte membranes two different cAMP-responsive macrocomplexes activated by adenosine have been demonstrated that contain their own sequestered cAMP pools to generate their selective effects. One of these complexes responds to A2A adenosine receptor that activates AC6, linked to A-kinase-anchoring proteins (AKAP)79/150, to produce cAMP available for AKAP79/150-tethered proteins, named protein kinase A (PKA) and phosphodiesterase 3A (PDE3A). The other complex responds to A2B adenosine receptor, and the novel generated cAMP does not diffuse between these “signalosomes,” thus suggesting that a spatiotemporal regulation of cAMP exists in the cell to obtain receptor-specific responses (Guinzberg et al. 2017). In addition, in the brain, A2A adenosine receptor regulates a specific neuron type of Gs protein named Golf, which is also related to AC (Kull et al. 2000). In the rat tail artery, it promotes noradrenaline release through both PKC and PKA recruitment (Fresco et al. 2004). A2A adenosine receptor may also bind, through its long C-terminus , to various accessory proteins including D2 dopamine receptors , α-actinin, ADP ribosylation factor nucleotide site opener (ARNO), ubiquitin-specific protease (USP4), and translin-associated protein X (TRAX) (Baraldi et al. 2008). Importantly, A2A adenosine receptor plays a role in the regulation of MAPK affecting the transduction pathway of several cells from different organs and tissues (Baraldi et al. 2008; Chen et al. 2013) (Fig. 3.2). In neutrophils, A2A adenosine receptor by increasing cAMP decreases phosphorylation of p38, ERK, PI3K/AKT, Hck, and Syk, thus inducing inhibition of their functions (Giambelluca and Pouliot 2017). Accordingly, in the same cells, the agonist ATL313 was able to suppress selectin-mediated activation of Src kinases (SFKs) and p38, thus reducing cell adhesion (Yago et al. 2015). In contrast, an increase in ERK, nuclear factor (NF)-κB, and pSTAT was involved in the reduction of inflammatory cytokines produced by methotrexate through A2A receptor activation in T cells (Ma et al. 2018). In dermal fibroblasts the A2A receptor increases collagen (col) 1 and 3 production via cAMP, PKA, ERK, p38, and AKT pathways, confirming data obtained in hepatic stellate cells where collagen 1 production was influenced also by A2A receptor-mediated ERK activity (Chan et al. 2006; Che et al. 2007; Shaikh and Cronstein 2016). It is known that also Wnt signaling is important in fibrosis where cAMP and Wnt pathways may converge. In this context it has been found that A2A receptor increases synthesis of collagen 3 through the activation of β-catenin, suggesting a role for this subtype in dermal fibrosis and scarring (Shaikh and Cronstein 2016). In normal skin col1 is more expressed than col3 that increases in immature scars and is then replaced by col1 in mature scars, a process regulated by A2A receptor and Epac2. At nanomolar levels of adenosine, the receptor via PKA induces col1 and reduces col3 production, respectively. At higher levels, the raised cAMP levels promote Epac2 signaling producing col3 (Perez-Aso et al. 2012, 2014). In mice adipose tissue, A2A receptor stimulation induces an increase in p38 phosphorylation, thus resulting in improvements in glucose homeostasis and adipose tissue inflammation (DeOliveira et al. 2017). In the brain, following ischemia-reperfusion (IR) damage , a huge increase of adenosine stimulates A2A receptor to potentiate neuronal injury by increasing ERK and consequently stimulating microglial activation, glial tumor necrosis factor-alpha (TNF-α) and BDNF, glutamate, inducible nitric oxide synthase (iNOS), as well as apoptosis (Mohamed et al. 2016). In an vitro model of osteoclast, differentiation occurs through activation of A2A receptor activation of PKA and ERK1/2, thus inhibiting NF-κB nuclear translocation (Mediero et al. 2013). In cancer cells, A2A receptor activation stimulates proliferation phospholipase C (PLC), protein kinase C-delta (PKC-δ), ERK, JNK, and AKT (Gessi et al. 2017). Accordingly, the same effect was reached by combination of TLR2 and adenosine receptor agonists , through ERK stimulation, in oral squamous carcinoma cells (Palani et al. 2018).
3.4.3 A2B Adenosine Receptors
The Gs-coupled A2B adenosine receptor activates AC, causing phosphorylation of PKA and recruitment of various effectors like guanine nucleotide exchange factor 2 (Epac), directly stimulated by cAMP. However it has been recently reported that a complex constituted by A2B receptor stimulates AC5 bound to D-AKAP2 to generate cAMP, activating two other tethered proteins named Epac2 and PDE3B (Guinzberg et al. 2017). Epac activation by A2B receptor stimulation has been previously reported to affect cell proliferation in human umbilical vascular endothelial cells and to induce early gene expression decreasing cell proliferation in human coronary artery smooth muscle cells (Fang and Olah 2007; Mayer et al. 2011). In addition, by enrolling Gq proteins, it triggers PLC activation , thus determining Ca2+ increase, and through βγ subunits modulates ion channels. Furthermore, A2B adenosine receptor presents numerous binding actors that influence its responses and effects such as netrin-1, E3KARP-ezrin-PKA, SNARE, NF-κB 1/P105, and α-actinin-1. Specifically, netrin-1 is a neuron protein hypoxia-dependent, which by binding to A2B adenosine receptor reduces neutrophil migration and consequent inflammation (Rosenberger et al. 2009). SNARE protein is responsible for the translocation of the receptor from the cytoplasm to the cell membrane in the presence of an agonist through a mechanism involving (Wang et al. 2004) a structure composed by E3KARP (NHERF2) and ezrin which fixes A2B adenosine receptor at cell surface (Sitaraman et al. 2002). In particular, A2A and A2B receptor dimerization is induced by α-actinin-1 promoting expression of the A2B subtype on the cell surface (Moriyama and Sitkovsky 2010). In addition, it interacts with P105 then blocking NF-kB inflammatory effects (Sun et al. 2012). MAPK and AKT are target also for A2B receptor in different cells thus regulating numerous pathophysiological functions (Sun and Huang 2016) (Fig. 3.3). In cardiac fibroblasts its stimulation reduced fibroblast proliferation and α-SMA expression generated by endothelin or angiotensin II, through a pathway dependent on cAMP, Epac, PI3K, and AKT signaling, thus contrasting cardiac fibrosis (Phosri et al. 2017, 2018). In bone A2B subtype stimulation decreases ERK1/2, p38, and NF-κB induced by RANKL, thus contributing to the reduction of osteoclastogenesis (Kim et al. 2017). In human coronary artery smooth muscle cells, the A2B adenosine receptor, cAMP, and PKA signaling decrease cell growth by inhibiting ERK1/2, AKT, and Skp2 stimulators of the cell cycle regulator cyclin D (Dubey et al. 2015). In the placenta A2B receptor activation depresses trophoblast migration through MAPK signaling inhibition and lower proMMP-2 levels, suggesting a role for it in placenta formation and preeclampsia (Darashchonak et al. 2014). In glioblastoma cells prostatic acid phosphatase increases proliferation in a HIF-2α-dependent manner, requiring activation of A2B receptors AKT and ERK pathways, suggesting this receptor subtype as a target for antiglioblastoma therapies (Liu et al. 2014). In microglia A2B receptor increases IL-10 through p38 phosphorylation as well as IL-6 secretion and cell proliferation, through PLC, PKC-ε, PKC-δ, and p38 pathways, thus indicating their role in microglial activation and neuroinflammation (Koscso et al. 2012; Merighi et al. 2017). In enterochromaffin cells this subtype increases serotonin hypoxic synthesis and release through MAPK, CREB, and tryptophan hydroxylase-1 stimulation, a signaling having relevance in inflammatory bowel disease (Chin et al. 2012; Dammen et al. 2013). In HEK293 cells and in cardiomyocytes, A2B receptor inhibited superoxide generation from mitochondrial complex I via Gi/o protein, ERK, PI3K, and NOS signaling having a role in ischemic preconditioning (Yang et al. 2011). In foam cells it accumulates HIF-1α through involvement of ERK, p38, and AKT and induces VEGF and IL-8 secretion , playing a role in atherosclerosis development (Gessi et al. 2010a).
3.4.4 A3 Adenosine Receptors
The Gi-coupled A3 adenosine receptor inhibits AC, thus reducing cAMP accumulation, while through Gq coupling stimulates PLC, thereby increasing Ca2+ release from intracellular stores in different cellular models (Gessi et al. 2008; Borea et al. 2015). Other signal transductors coupled to this receptor subtype include the monomeric G protein RhoA and phospholipase D as well as sarcolemmal KATP channels, to produce cardioprotection (Borea et al. 2015). In addition a role for PKC has been reported in both early and delayed preconditioning (Borea et al. 2016). Specifically, in cardiac mast cells , A2B/A3 receptor stimulation leads to activation of aldehyde dehydrogenase type 2, via PKC-ɛ, thus reducing renin release and the activation of renin-angiotensin system (Koda et al. 2010). Concerning delayed preconditioning, A3 receptor activation exerts a protective role through PKC-δ (Zhao and Kukreja 2003). A pro-survival intracellular cascade involving ERK, PI3K, and AKT is enrolled by it to decrease caspase-3 activity and apoptosis (Hussain et al. 2014). Another relevant effect induced by A3 receptor activation is neuroprotection through PLC, PKC, or intracellular Ca2+ sequestration giving synaptic depression following oxygen-glucose deprivation as well as a reduction in AMPA receptors on hippocampal neurons (Dennis et al. 2011). It is well known that A3 receptor intracellular transduction occurs through modulation of MAPKs in numerous cellular models (Merighi et al. 2010; Jacobson et al. 2018) (Fig. 3.4). This receptor induced ERK1/2 and cell proliferation in human fetal astrocytes, CHO cells expressing the human A3AR (CHO-hA3), microglia, colon carcinoma, glioblastoma, melanoma, and foam cells (Neary et al. 1998; Schulte and Fredholm 2000, 2002; Hammarberg et al. 2003; Merighi et al. 2006, 2007a, b, Gessi et al. 2010a, b; Soares et al. 2014). In contrast, a decrease of ERK activation has been reported in melanoma, prostate cancer, and glioma cells, reducing proliferation as well as decreasing TNF-α release in RAW 264.7 cells (Madi et al. 2003; Martin et al. 2006; Jajoo et al. 2009; Kim et al. 2012). A3 receptor activation modulates also p38 in several cell types such as CHO-hA3, human synoviocytes, melanoma, glioblastoma, and colon carcinoma (Merighi et al. 2005b, 2006, 2007b; Varani et al. 2010). In addition it regulates JNK, in microglia and glioblastoma cells, to increase cell migration and matrix metalloproteinase-9 (MMP-9) secretion, respectively (Gessi et al. 2010b; Ohsawa et al. 2012). Interestingly, A3 receptor increases chemoresistance induced by multiple resistance-associated protein-1 (MRP1) transporter through a pathway involving PI3K/AKT and MEK/ERK1/2 (Torres et al. 2016; Uribe et al. 2017). Another effect induced by this subtype through AKT phosphorylation was protection from apoptosis in RBL-2H3 and stimulation of MMP-9 in glioblastoma cells (Gao et al. 2001; Merighi et al. 2005a, 2007a; Gessi et al. 2010b). In melanoma the same pathway modulated by A3 receptor decreased proliferation and increased pigmentation (Madi et al. 2013). Anti-inflammatory effects are produced by its modulation of PI3K/AKT and NF-κB transduction systems in BV2 microglial cells, monocytes, arthritis, and mesothelioma (Haskó et al. 1998; la Sala et al. 2005; Fishman et al. 2006; Lee et al. 2006, 2011; Madi et al. 2007; Varani et al. 2011). Instead reduction of AKT has been reported in murine astrocytes to decrease HIF-1α accumulation (Gessi et al. 2013). Accordingly, A3 receptor inhibits angiogenesis in endothelial cells through PI3K/AKT/mammalian target of rapamycin (mTOR) signaling decrease (Kim et al. 2013). Finally, the inhibition of PKA mediated by A3 receptor stimulation raised glycogen synthase kinase-3β (GSK-3β), inducing beta-catenin reduction; decrease of its transcriptional gene products, such as cyclin D1 and c-Myc; as well as reduction of NF-κB DNA-binding capacity in melanoma, hepatocellular carcinoma, and synoviocytes from RA patients and in adjuvant-induced arthritis rats (Fishman et al. 2002, 2004; Bar-Yehuda et al. 2008; Ochaion et al. 2008). Accordingly, following a reduction of A3 receptor expression in colon cells after ulcerative colitis due to miR-206 activity, increased NF-κB, and related cytokines in the mouse colon, has been observed resulting in a proinflammatory event (Wu et al. 2016).
Schematic picture of A3 adenosine receptor signaling cascade. Interaction of adenosine with A3R reduces AC activity and cAMP levels and stimulates GSK-3β with consequent reduction of β-catenin, cyclin D1, and c-Myc. Stimulation of A3R activates PLC-β and Ca2+, RhoA, and PLD. p38, ERK1/2, and JNK1/2 are phosphorylated through A3R activation
3.5 Conclusion
Adenosine receptors are important targets for drug development in several pathologies spanning from ischemic brain and heart injury, pain, neurodegenerative diseases, cancer, and inflammation, and for this reason there is a big interest in the development of novel selective and potent molecules targeting this system. This issue today may be better afforded, thanks to the improvement in the knowledge about the structure of receptor subtypes, which are the targets of new drugs. During the last 10 years, the crystallization approach has dramatically revealed the biological structure of GPCRs, and the A2A receptor has been the pioneer in this process, followed by the A1 subtype. In the next future, continued energy to reveal the structures of all four adenosine receptor subtypes in the three distinct activation states is fundamental to better improve the rational drug design process to develop novel molecules. From the extensive literature mentioned in this chapter, it is evident that adenosine modulates different intracellular signaling pathways involving MAPK and AKT to produce its pathophysiological effects. The regulation of these cascades is not univocal meaning that stimulation or inhibition of specific kinases may occur differentially depending on the receptor subtype involved and the cell system investigated. It is auspicable that future drugs coming from the adenosinergic field could exploit separated signaling pathway linked to a specific adenosine subtype, thus avoiding or limiting side effects.
References
Aherne CM, Kewley EM, Eltzschig HK (2011) The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta Biomembr 1808:1329–1339
Ansari HR, Teng B, Nadeem A et al (2009) A1 adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 297:H1032–H1039
Arellano RO, Garay E, Vázquez-Cuevas F (2009) Functional interaction between native G protein-coupled purinergic receptors in Xenopus follicles. Proc Natl Acad Sci U S A 106:16680–16685
Avlani VA, Gregory KJ, Morton CJ et al (2007) Critical role for the second extracellular loop in the binding of both Orthosteric and allosteric G protein-coupled receptor ligands. J Biol Chem 282:25677–25686
Ballesteros-Yáñez I, Castillo CA, Merighi S, Gessi S (2018) The role of adenosine receptors in psychostimulant addiction. Front Pharmacol 8:985
Baraldi PG, Tabrizi MA, Gessi S, Borea PA (2008) Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem Rev 108:238–263
Bar-Yehuda S, Stemmer SM, Madi L et al (2008) The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol 33:287–295
Beukers MW, den Dulk H, van Tilburg EW et al (2000) Why are A(2B) receptors low-affinity adenosine receptors? Mutation of Asn273 to Tyr increases affinity of human A(2B) receptor for 2-(1-Hexynyl)adenosine. Mol Pharmacol 58:1349–1356
Beukers MW, van Oppenraaij J, van der Hoorn PPW et al (2004) Random mutagenesis of the human adenosine A2B receptor followed by growth selection in yeast. Identification of constitutively active and gain of function mutations. Mol Pharmacol 65:702–710
Biber K, Klotz KN, Berger M, Gebicke-Härter PJ, van Calker D (1997) Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci 17:4956–4964
Boison D, Singer P, Shen H-Y et al (2012) Adenosine hypothesis of schizophrenia – opportunities for pharmacotherapy. Neuropharmacology 62:1527–1543
Borea PA, Varani K, Vincenzi F et al (2015) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102
Borea PA, Gessi S, Merighi S, Varani K (2016) Adenosine as a multi-Signalling Guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci 37:419–434
Borea PA, Gessi S, Merighi S et al (2017) Pathological overproduction: the bad side of adenosine. Br J Pharmacol 174:1945–1960
Boros D, Thompson J, Larson D (2016) Adenosine regulation of the immune response initiated by ischemia reperfusion injury. Perfusion 31:103–110
Brugarolas M, Navarro G, Martínez-Pinilla E et al (2014) G-protein-coupled receptor Heteromers as key players in the molecular architecture of the central nervous system. CNS Neurosci Ther 20:703–709
Brust TB, Cayabyab FS, Zhou N, MacVicar BA (2006) p38 mitogen-activated protein kinase contributes to adenosine A1 receptor-mediated synaptic depression in area CA1 of the rat Hippocampus. J Neurosci 26:12427–12438
Carpenter B, Lebon G (2017) Human adenosine A2A receptor: molecular mechanism of ligand binding and activation. Front Pharmacol 8:898
Carpenter B, Nehmé R, Warne T et al (2016) Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 536:104–107
Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Müller C, Woods AS, Hope BT, Ciruela F, Casadó V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S (2007) Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32:2249–2259
Chan ESL, Fernandez P, Merchant AA et al (2006) Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum 54:2632–2642
Che J, Chan ESL, Cronstein BN (2007) Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling Cascade or p38 mitogen-activated protein kinase signaling path. Mol Pharmacol 72:1626–1636
Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 12:265–286
Chen Z, Xiong C, Pancyr C et al (2014) Prolonged adenosine A1 receptor activation in hypoxia and Pial vessel disruption focal cortical ischemia facilitates Clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential Regulat. J Neurosci 34:9621–9643
Cheng RKY, Segala E, Robertson N et al (2017) Structures of human A1 and A2A adenosine receptors with Xanthines reveal determinants of selectivity. Structure 25:1275–1285.e4
Chin A, Svejda B, Gustafsson BI et al (2012) The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol 302:G397–G405
Ciruela F, Casadó V, Rodrigues RJ et al (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor Heteromers. J Neurosci 26:2080–2087
Ciruela F, Escriche M, Burgueno J, Angulo E, Casado V, Soloviev MM, Canela EI, Mallol J, Chan WY, Lluis C, McIlhinney RA, Franco R (2001) Metabotropic glutamate 1alpha and adenosine A1 receptors assemble into functionally interacting complexes. J Biol Chem 276:18345–18351
Congreve M, Andrews SP, Doré AS et al (2012) Discovery of 1,2,4-Triazine derivatives as adenosine A2A antagonists using structure based drug design. J Med Chem 55:1898–1903
Cristóvão-Ferreira S, Navarro G, Brugarolas M et al (2013) A1R–A2AR heteromers coupled to Gs and Gi/0 proteins modulate GABA transport into astrocytes. Purinergic Signal 9:433–449
da Rocha Lapa F, Macedo-Júnior SJ, Luiz Cerutti M, Santos ARS (2014) Pharmacology of adenosine receptors and their signaling role in immunity and inflammation. In: Gowder SJT (ed) Pharmacology and therapeutics. InTech, Rijeka, pp 85–130
Dammen R, Haugen M, Svejda B et al (2013) The stimulatory adenosine receptor ADORA2B regulates serotonin (5-HT) synthesis and release in oxygen-depleted EC cells in inflammatory bowel disease. PLoS One 8:e62607
Darashchonak N, Sarisin A, Kleppa M-J et al (2014) Activation of adenosine A2B receptor impairs properties of trophoblast cells and involves mitogen-activated protein (MAP) kinase signaling. Placenta 35:763–771
de Lera Ruiz M, Lim Y-H, Zheng J (2014) Adenosine A2A receptor as a drug discovery target. J Med Chem 57:3623–3650
Dennis SH, Jaafari N, Cimarosti H et al (2011) Oxygen/glucose deprivation induces a reduction in synaptic AMPA receptors on hippocampal CA3 neurons mediated by mGluR1 and adenosine A3 receptors. J Neurosci 31:11941–11952
DeOliveira CC, Paiva Caria CR, Ferreira Gotardo EM et al (2017) Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur J Pharmacol 799:154–159
Doré AS, Robertson N, Errey JC et al (2011) Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19:1283–1293
Dubey RK, Fingerle J, Gillespie DG et al (2015) Adenosine attenuates human coronary artery smooth muscle cell proliferation by inhibiting multiple signaling pathways that converge on cyclin D. Hypertension 66:1207–1219
Eltzschig HK (2009) Adenosine: an old drug newly discovered. Anesthesiology 111:904–915
Fang Y, Olah ME (2007) Cyclic AMP-dependent, protein kinase A-independent activation of extracellular signal-regulated kinase 1/2 following adenosine receptor stimulation in human umbilical vein endothelial cells: role of exchange protein activated by cAMP 1 (Epac1). J Pharmacol Exp Ther 322:1189–1200
Fenton RA, Shea LG, Doddi C, Dobson JG (2010) Myocardial adenosine A1 -receptor-mediated adenoprotection involves phospholipase C, PKC-ε, and p38 MAPK, but not HSP27. Am J Physiol Circ Physiol 298:H1671–H1678
Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, Casadó V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F (2002) Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A 99:11940–11945
Ferré S, Lluís C, Justinova Z et al (2010a) Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol 160:443–453
Ferré S, Navarro G, Casadó V et al (2010b) G protein-coupled receptor heteromers as new targets for drug development. Prog Mol Biol Transl Sci 91:41–52
Fishman P, Bar-Yehuda S, Madi L, Cohn I (2002) A3 adenosine receptor as a target for cancer therapy. Anti-Cancer Drugs 13:437–443
Fishman P, Bar-Yehuda S, Ohana G et al (2004) An agonist to the A3 adenosine receptor inhibits colon carcinoma growth in mice via modulation of GSK-3 beta and NF-kappa B. Oncogene 23:2465–2471
Fishman P, Bar-Yehuda S, Madi L et al (2006) The PI3K-NF-kappaB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Res Ther 8:R33
Fredholm BB, Arslan G, Halldner L et al (2000) Structure and function of adenosine receptors and their genes. Naunyn Schmiedeberg’s Arch Pharmacol 362:364–374
Fredholm BB, IJzerman AP, Jacobson KA et al (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Fredholm BB, IJzerman AP, Jacobson KA et al (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev 63:1–34
Fresco P, Diniz C, Gonçalves J (2004) Facilitation of noradrenaline release by activation of adenosine A2A receptors triggers both phospholipase C and adenylate cyclase pathways in rat tail artery. Cardiovasc Res 63:739–746
Fuxe K, Ferré S, Canals M et al (2005) Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci 26:209–220
Fuxe K, Ferré S, Genedani S et al (2007) Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav 92:210–217
Gao Z, Li BS, Day YJ, Linden J (2001) A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol Pharmacol 59:76–82
Gao Z-G, Chen A, Barak D et al (2002) Identification by site-directed mutagenesis of residues involved in ligand recognition and activation of the human A3 adenosine receptor. J Biol Chem 277:19056–19063
Gessi S, Varani K, Merighi S et al (2000) A2A adenosine receptors in human peripheral blood cells. Br J Pharmacol 129:2–11
Gessi S, Merighi S, Varani K et al (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117:123–140
Gessi S, Fogli E, Sacchetto V et al (2010a) Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler Thromb Vasc Biol 30:90–97
Gessi S, Sacchetto V, Fogli E et al (2010b) Modulation of metalloproteinase-9 in U87MG glioblastoma cells by A3 adenosine receptors. Biochem Pharmacol 79:1483–1495
Gessi S, Merighi S, Fazzi D et al (2011) Adenosine receptor targeting in health and disease. Expert Opin Investig Drugs 20:1591–1609
Gessi S, Merighi S, Stefanelli A et al (2013) A1 and A3 adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol Res 76:157–170
Gessi S, Bencivenni S, Battistello E et al (2017) Inhibition of A2A adenosine receptor signaling in Cancer cells proliferation by the novel antagonist TP455. Front Pharmacol 8:888
Giambelluca MS, Pouliot M (2017) Early tyrosine phosphorylation events following adenosine A2A receptor in human neutrophils: identification of regulated pathways. J Leukoc Biol 102:829–836
Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati LF, Verniera P, Lluis C, Ferré S, Fuxe K, Franco R (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci U S A 97:8606–8611
Glukhova A, Thal DM, Nguyen AT et al (2017) Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell 168:867–877.e13
Guinzberg R, Díaz-Cruz A, Acosta-Trujillo C et al (2017) Newly synthesized cAMP is integrated at a membrane protein complex signalosome to ensure receptor response specificity. FEBS J 284:258–276
Hammarberg C, Schulte G, Fredholm BB (2003) Evidence for functional adenosine A3 receptors in microglia cells. J Neurochem 86:1051–1054
Haskó G, Németh ZH, Vizi ES et al (1998) An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol 358:261–268
Headrick JP, Ashton KJ, Rose’Meyer RB, Peart JN (2013) Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Ther 140:92–111
Hill SJ, May LT, Kellam B, Woolard J (2014) Allosteric interactions at adenosine A1 and A3 receptors: new insights into the role of small molecules and receptor dimerization. Br J Pharmacol 171:1102–1113
Hino T, Arakawa T, Iwanari H et al (2012) G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 482:237–240
Hussain A, Gharanei AM, Nagra AS, Maddock HL (2014) Caspase inhibition via A3 adenosine receptors: a new Cardioprotective mechanism against myocardial infarction. Cardiovasc Drugs Ther 28:19–32
Jaakola V-P, IJzerman AP (2010) The crystallographic structure of the human adenosine A2A receptor in a high-affinity antagonist-bound state: implications for GPCR drug screening and design. Curr Opin Struct Biol 20:401–414
Jaakola V-P, Griffith MT, Hanson MA et al (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217
Jacobson KA, Merighi S, Varani K et al (2018) A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med Res Rev 38:1031–1072
Jajoo S, Mukherjea D, Watabe K, Ramkumar V (2009) Adenosine A(3) receptor suppresses prostate cancer metastasis by inhibiting NADPH oxidase activity. Neoplasia 11:1132–1145
Janes K, Esposito E, Doyle T et al (2014) A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain 155:2560–2567
Jazayeri A, Andrews SP, Marshall FH (2017) Structurally enabled discovery of adenosine A2A receptor antagonists. Chem Rev 117:21–37
Jespers W, Oliveira A, Prieto-Díaz R et al (2017) Structure-based design of potent and selective ligands at the four adenosine receptors. Molecules 22:1945
Jespers W, Schiedel AC, Heitman LH et al (2018) Structural mapping of adenosine receptor mutations: ligand binding and signaling mechanisms. Trends Pharmacol Sci 39:75–89
Katoh-Semba R, Kaneko R, Kitajima S et al (2009) Activation of p38 mitogen-activated protein kinase is required for in vivo brain-derived neurotrophic factor production in the rat hippocampus. Neuroscience 163:352–361
Kaur T, Borse V, Sheth S et al (2016) Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J Neurosci 36:3962–3977
Kim S-K, Jacobson KA (2006) Computational prediction of homodimerization of the A3 adenosine receptor. J Mol Graph Model 25:549–561
Kim TH, Kim YK, Woo JS (2012) The adenosine A3 receptor agonist cl-IB-MECA induces cell death through Ca2+/ROS-dependent down regulation of ERK and Akt in A172 human glioma cells. Neurochem Res 37:2667–2677
Kim GD, Oh J, Jeong LS, Lee SK (2013) Thio-Cl-IB-MECA, a novel A3 adenosine receptor agonist, suppresses angiogenesis by regulating PI3K/AKT/mTOR and ERK signaling in endothelial cells. Biochem Biophys Res Commun 437:79–86
Kim BH, Oh JH, Lee NK (2017) The inactivation of ERK1/2, p38 and NF-kB is involved in the down-regulation of Osteoclastogenesis and function by A2B adenosine receptor stimulation. Mol Cells 40:752–760
Koda K, Salazar-Rodriguez M, Corti F et al (2010) Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 122:771–781
Koscso B, Csoka B, Selmeczy Z et al (2012) Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. J Immunol 188:445–453
Koupenova M, Johnston-Cox H, Vezeridis A et al (2012) A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 125:354–363
Kull B, Svenningsson P, Fredholm BB (2000) Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol 58:771–777
la Sala A, Gadina M, Kelsall BL (2005) G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol 175:2994–2999
Lebon G, Warne T, Edwards PC et al (2011) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474:521–525
Lebon G, Edwards PC, Leslie AGW, Tate CG (2015) Molecular determinants of CGS21680 binding to the human adenosine A2A receptor. Mol Pharmacol 87:907–915
Lee JY, Jhun BS, Oh YT et al (2006) Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-α production through inhibition of PI 3-kinase/Akt and NF-κB activation in murine BV2 microglial cells. Neurosci Lett 396:1–6
Lee H-S, Chung H-J, Lee HW et al (2011) Suppression of inflammation response by a novel A3 adenosine receptor agonist thio-Cl-IB-MECA through inhibition of Akt and NF-κB signaling. Immunobiology 216:997–1003
Leshem-Lev D, Hochhauser E, Chanyshev B et al (2010) Adenosine A1 and A3 receptor agonists reduce hypoxic injury through the involvement of P38 MAPK. Mol Cell Biochem 345:153–160
Liang Y-C, Huang C-C, Hsu K-S (2008) A role of p38 mitogen-activated protein kinase in adenosine A1 receptor-mediated synaptic depotentiation in area CA1 of the rat hippocampus. Mol Brain 1:13
Liu W, Chun E, Thompson AA et al (2012) Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337:232–236
Liu T, Wang X, Bai Y et al (2014) The HIF-2alpha dependent induction of PAP and adenosine synthesis regulates glioblastoma stem cell function through the A2B adenosine receptor. Int J Biochem Cell Biol 49:8–16
Ma Y, Gao Z, Xu F et al (2018) A novel combination of astilbin and low-dose methotrexate respectively targeting A2AAR and its ligand adenosine for the treatment of collagen-induced arthritis. Biochem Pharmacol 153:269–281
Madi L, Bar-Yehuda S, Barer F et al (2003) A3 adenosine receptor activation in melanoma cells: association between receptor fate and tumor growth inhibition. J Biol Chem 278:42121–42130
Madi L, Cohen S, Ochayin A et al (2007) Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. J Rheumatol 34:20–26
Madi L, Rosenberg-Haggen B, Nyska A, Korenstein R (2013) Enhancing pigmentation via activation of A3 adenosine receptors in B16 melanoma cells and in human skin explants. Exp Dermatol 22:74–77
Martin L, Pingle SC, Hallam DM et al (2006) Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther 316:71–78
Mayer P, Hinze AV, Harst A, von Kügelgen I (2011) A2B receptors mediate the induction of early genes and inhibition of arterial smooth muscle cell proliferation via Epac. Cardiovasc Res 90:148–156
Mediero A, Perez-Aso M, Cronstein BN (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear translocation. Br J Pharmacol 169:1372–1388
Mei H-F, Poonit N, Zhang Y-C et al (2018) Activating adenosine A1 receptor accelerates PC12 cell injury via ADORA1/PKC/KATP pathway after intermittent hypoxia exposure. Mol Cell Biochem:1–10. https://doi.org/10.1007/s11010-018-3283-2
Merighi S, Benini A, Mirandola P et al (2005a) A3 adenosine receptor activation inhibits cell proliferation via phosphatidylinositol 3-kinase/Akt-dependent inhibition of the extracellular signal-regulated kinase 1/2 phosphorylation in A375 human melanoma cells. J Biol Chem 280:19516–19526
Merighi S, Benini A, Mirandola P et al (2005b) A3 adenosine receptors modulate hypoxia-inducible factor-1a expression in human A375 melanoma cells. Neoplasia 7:894–903
Merighi S, Benini A, Mirandola P et al (2006) Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem Pharmacol 72:19–31
Merighi S, Benini A, Mirandola P et al (2007a) Hypoxia inhibits paclitaxel-induced apoptosis through adenosine-mediated phosphorylation of bad in glioblastoma cells. Mol Pharmacol 72:162–172
Merighi S, Benini A, Mirandola P et al (2007b) Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol 72:395–406
Merighi S, Simioni C, Lane R, Ijzerman AP (2010) Regulation of second messenger systems and intracellular pathways. In: A3 adenosine receptors from cell biology to pharmacology and therapeutics. Springer, Dordrecht, pp 61–73
Merighi S, Borea PA, Stefanelli A et al (2015) A2A and A2B adenosine receptors affect HIF-1α signaling in activated primary microglial cells. Glia 63:1933–1952
Merighi S, Bencivenni S, Vincenzi F et al (2017) A2B adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol Res 117:9–19
Meriño M, Briones L, Palma V et al (2017) Rol de los receptores de adenosina en la interacción adipocito-macrófago durante la obesidad. Endocrinol Diabetes Nutr 64:317–327
Mohamed RA, Agha AM, Abdel-Rahman AA, Nassar NN (2016) Role of adenosine A2A receptor in cerebral ischemia reperfusion injury: signaling to phosphorylated extracellular signal-regulated protein kinase (pERK1/2). Neuroscience 314:145–159
Moriyama K, Sitkovsky MV (2010) Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem 285:39271–39288
Narlawar R, Lane JR, Doddareddy M et al (2010) Hybrid ortho/allosteric ligands for the adenosine A(1) receptor. J Med Chem 53:3028–3037
Navarro G, Ferré S, Cordomi A et al (2010a) Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J Biol Chem 285:27346–27359
Navarro G, Moreno E, Aymerich M et al (2010b) Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A 107:18676–18681
Navarro G, Borroto-Escuela DO, Fuxe K, Franco R (2016a) Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology 104:161–168
Navarro G, Cordomí A, Zelman-Femiak M et al (2016b) Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs. BMC Biol 14:26
Neary JT, McCarthy M, Kang Y, Zuniga S (1998) Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett 242:159–162
Nguyen ATN, Baltos J-A, Thomas T et al (2016a) Extracellular loop 2 of the adenosine A1 receptor has a key role in Orthosteric ligand affinity and agonist efficacy. Mol Pharmacol 90:703–714
Nguyen ATN, Vecchio EA, Thomas T et al (2016b) Role of the second extracellular loop of the adenosine A1 receptor on allosteric modulator binding, signaling, and cooperativity. Mol Pharmacol 90:715–725
Ochaion A, Bar-Yehuda S, Cohen S et al (2008) The A3 adenosine receptor agonist CF502 inhibits the PI3K, PKB/Akt and NF-kappaB signaling pathway in synoviocytes from rheumatoid arthritis patients and in adjuvant-induced arthritis rats. Biochem Pharmacol 76:482–494
Ohsawa K, Sanagi T, Nakamura Y et al (2012) Adenosine A3 receptor is involved in ADP-induced microglial process extension and migration. J Neurochem 121:217–227
Palani CD, Ramanathapuram L, Lam-ubol A, Kurago ZB (2018) Toll-like receptor 2 induces adenosine receptor A2A and promotes human squamous carcinoma cell growth via extracellular signal regulated kinases 1/2. Oncotarget 9:6814–6829
Palmer TM, Stiles GL (2000) Identification of threonine residues controlling the agonist-dependent phosphorylation and desensitization of the rat A(3) adenosine receptor. Mol Pharmacol 57:539–545
Pedata F, Dettori I, Coppi E et al (2016) Purinergic signalling in brain ischemia. Neuropharmacology 104:105–130
Peeters MC, Wisse LE, Dinaj A et al (2012) The role of the second and third extracellular loops of the adenosine A1 receptor in activation and allosteric modulation. Biochem Pharmacol 84:76–87
Peleli M, Fredholm BB, Sobrevia L, Carlström M (2017) Pharmacological targeting of adenosine receptor signaling. Mol Asp Med 55:4–8
Perez-Aso M, Chiriboga L, Cronstein BN (2012) Pharmacological blockade of adenosine A2A receptors diminishes scarring. FASEB J 26:4254–4263
Perez-Aso M, Fernandez P, Mediero A et al (2014) Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J 28:802–812
Phosri S, Arieyawong A, Bunrukchai K et al (2017) Stimulation of adenosine A2B receptor inhibits Endothelin-1-induced cardiac fibroblast proliferation and α-smooth muscle actin synthesis through the cAMP/Epac/PI3K/Akt-signaling pathway. Front Pharmacol 8:428
Phosri S, Bunrukchai K, Parichatikanond W et al (2018) Epac is required for exogenous and endogenous stimulation of adenosine A2B receptor for inhibition of angiotensin II-induced collagen synthesis and myofibroblast differentiation. Purinergic Signal 14(2):141–156
Piirainen H, Ashok Y, Nanekar RT, Jaakola V-P (2011) Structural features of adenosine receptors: from crystal to function. Biochim Biophys Acta 1808:1233–1244
Preti D, Baraldi PG, Moorman AR et al (2015) History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med Res Rev 35:790–848
Pugliese AM, Coppi E, Volpini R et al (2007) Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem Pharmacol 74:768–779
Rosenberger P, Schwab JM, Mirakaj V et al (2009) Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 10:195–202
Rouviere E, Arnarez C, Yang L, Lyman E (2017) Identification of two new cholesterol interaction sites on the A2A adenosine receptor. Biophys J 113:2415–2424
Sachdeva S, Gupta M (2013) Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J 21:245–253
Schiedel AC, Hinz S, Thimm D et al (2011) The four cysteine residues in the second extracellular loop of the human adenosine A2B receptor: role in ligand binding and receptor function. Biochem Pharmacol 82:389–399
Schulte G, Fredholm BB (2000) Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol 58:477–482
Schulte G, Fredholm BB (2002) Signaling pathway from the human adenosine A(3) receptor expressed in Chinese hamster ovary cells to the extracellular signal-regulated kinase 1/2. Mol Pharmacol 62:1137–1146
Seibt BF, Schiedel AC, Thimm D et al (2013) The second extracellular loop of GPCRs determines subtype-selectivity and controls efficacy as evidenced by loop exchange study at A2 adenosine receptors. Biochem Pharmacol 85:1317–1329
Shaikh G, Cronstein B (2016) Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal 12:191–197
Sitaraman SV, Wang L, Wong M et al (2002) The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem 277:33188–33195
Soares AS, Costa VM, Diniz C, Fresco P (2014) The combination of Cl-IB-MECA with paclitaxel: a new anti-metastatic therapeutic strategy for melanoma. Cancer Chemother Pharmacol 74:847–860
Soni H, Peixoto-Neves D, Buddington RK, Adebiyi A (2017) Adenosine A1 receptor-operated calcium entry in renal afferent arterioles is dependent on postnatal maturation of TRPC3 channels. Am J Physiol Ren Physiol 313:F1216–F1222
Stoddart LA, Kellam B, Briddon SJ, Hill SJ (2014) Effect of a toggle switch mutation in TM6 of the human adenosine A3 receptor on Gi protein-dependent signalling and Gi-independent receptor internalization. Br J Pharmacol 171:3827–3844
Stoddart LA, Vernall AJ, Briddon SJ et al (2015) Direct visualisation of internalization of the adenosine A3 receptor and localization with arrestin3 using a fluorescent agonist. Neuropharmacology 98:68–77
Storme J, Cannaert A, Van Craenenbroeck K, Stove CP (2018) Molecular dissection of the human A 3 adenosine receptor coupling with β-arrestin2. Biochem Pharmacol 148:298–307
Sun Y, Huang P (2016) Adenosine A2B receptor: from cell biology to human diseases. Front Chem 4:37
Sun C-X, Young HW, Molina JG et al (2005) A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest 115:35–43
Sun Y, Duan Y, Eisenstein AS et al (2012) A novel mechanism of control of NFκB activation and inflammation involving A2B adenosine receptors. J Cell Sci 125:4507–4517
Thimm D, Schiedel AC, Sherbiny FF et al (2013) Ligand-specific binding and activation of the human adenosine a 2B receptor. Biochemistry 52:726–740
Torres A, Vargas Y, Uribe D et al (2016) Adenosine A3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget 7:67373–67386
Trincavelli ML, Tuscano D, Marroni M et al (2002a) Involvement of mitogen protein kinase cascade in agonist-mediated human A3 adenosine receptor regulation. Biochim Biophys Acta, Mol Cell Res 1591:55–62
Trincavelli ML, Tuscano D, Marroni M et al (2002b) A3 adenosine receptors in human astrocytoma cells: agonist-mediated desensitization, internalization, and down-regulation. Mol Pharmacol 62:1373–1384
Uribe D, Torres Á, Rocha JD et al (2017) Multidrug resistance in glioblastoma stem-like cells: role of the hypoxic microenvironment and adenosine signaling. Mol Asp Med 55:140–151
Varani K, Vincenzi F, Tosi A et al (2010) Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes. Br J Pharmacol 160:101–115
Varani K, Maniero S, Vincenzi F et al (2011) A3 receptors are overexpressed in pleura from patients with mesothelioma and reduce cell growth via Akt/nuclear factor-κB pathway. Am J Respir Crit Care Med 183:522–530
Varani K, Vincenzi F, Merighi S et al (2017) Biochemical and pharmacological role of A1 adenosine receptors and their modulation as novel therapeutic strategy. Adv Exp Med Biol 1051:193–232
Vyas FS, Hargreaves AJ, Bonner PLR et al (2016) A1 adenosine receptor-induced phosphorylation and modulation of transglutaminase 2 activity in H9c2 cells: a role in cell survival. Biochem Pharmacol 107:41–58
Wang L, Kolachala V, Walia B et al (2004) Agonist-induced polarized trafficking and surface expression of the adenosine 2b receptor in intestinal epithelial cells: role of SNARE proteins. Am J Physiol Gastrointest Liver Physiol 287:G1100–G1107
Wu W, He Y, Feng X et al (2016) MicroRNA-206 is involved in the pathogenesis of ulcerative colitis via regulation of adenosine A3 receptor. Oncotarget 8:705–721
Xu F, Wu H, Katritch V et al (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332:322–327
Xu X, Zheng S, Xiong Y et al (2017) Adenosine effectively restores endotoxin-induced inhibition of human neutrophil chemotaxis via A1 receptor-p38 pathway. Inflamm Res 66:353–364
Yago T, Tsukamoto H, Liu Z et al (2015) Multi-inhibitory effects of a 2A adenosine receptor signaling on neutrophil adhesion under flow. J Immunol 195:3880–3889
Yang X, Xin W, Yang X-M et al (2011) A 2B adenosine receptors inhibit superoxide production from mitochondrial complex I in rabbit cardiomyocytes via a mechanism sensitive to pertussis toxin. Br J Pharmacol 163:995–1006
Yoshioka K, Saitoh O, Nakata H (2001) Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci U S A 98:7617–7622
Zhai W, Chen D, Shen H et al (2016) A1 adenosine receptor attenuates intracerebral hemorrhage-induced secondary brain injury in rats by activating the P38-MAPKAP2-Hsp27 pathway. Mol Brain 9:66
Zhao TC, Kukreja RC (2003) Protein kinase C-delta mediates adenosine A3 receptor-induced delayed cardioprotection in mouse. Am J Physiol Heart Circ Physiol 285:H434–H441
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Merighi, S., Gessi, S., Borea, P.A. (2018). Adenosine Receptors: Structure, Distribution, and Signal Transduction. In: Borea, P., Varani, K., Gessi, S., Merighi, S., Vincenzi, F. (eds) The Adenosine Receptors. The Receptors, vol 34. Humana Press, Cham. https://doi.org/10.1007/978-3-319-90808-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-90808-3_3
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-90807-6
Online ISBN: 978-3-319-90808-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)