Abstract
Defensins are small, cysteine-rich peptides that are ubiquitously present in all plants. They are important components of the plant immune system and serve as first line of defense against invading pathogens. Plant defensins share conserved tetradisulfide connectivity but vary in their sequence, net charge, and hydrophobicity. A number of plant defensins with potent broad-spectrum antifungal activity have been identified and characterized. Studies conducted during the past decade have highlighted the diverse modes of action (MOA) of a few antifungal defensins. Constitutive expression of these defensins has been demonstrated to confer in planta resistance to several economically important fungal and oomycete pathogens in transgenic crops. Here, we provide a brief review of recent findings that have contributed to our current understanding of the MOA of these peptides and their deployment for disease resistance in crops.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Plants are continuously exposed to a plethora of potentially harmful pathogens. Fungal and oomycete pathogens of considerable economic importance impose major constraints globally on agricultural production and pose a clear threat to food security (Collinge et al. 2010). Plants lack a somatic adaptive immune system to protect themselves from pathogen attack and therefore must rely on their sophisticated innate immune system for defense against these pathogens (Jones and Dangl 2006). The innate immunity of plants comprises fortification of cell wall, hypersensitive response, and production of antimicrobial compounds and antimicrobial peptides (AMPs). AMPs serve as one of the first lines of defense against pathogen invasion and make up the crucial effector arm of the plant’s immune system (van der Weerden et al. 2013).
Defensins represent a large family of AMPs in all higher plants and are at the forefront of their defense against pathogens. Plant defensins are cysteine-rich cationic peptides of 45–54 amino acids. First isolated from wheat and barley seeds in 1990 (Colilla et al. 1990; Mendez et al. 1990), plant defensins have since been discovered in several phyla of plant kingdom. They have been identified in a variety of plant tissues and are either ubiquitously or conditionally expressed in response to various biological or environmental cues. Based on their subcellular localization, plant defensins have been designated either as class I or class II. Class I defensins are secreted to the apoplast and synthesized by plant cells as precursor proteins comprising of the secretory signal peptide followed by the mature peptide. Class II defensins, localized to the vacuole and expressed in floral tissue of plants from Solanaceae and Poaceae families, are synthesized containing an additional carboxy-terminal propeptide (CTPP) (Lay et al. 2003).This C-terminal propeptide may serve dual function, i.e., it protects against autocytoxicity by neutralizing the deleterious cationicity of the peptides during export to the vacuole and acts as a chaperone to assist in folding (Lay et al. 2014). Plant defensins share a conserved 3D structure consisting of one α-helix and three antiparallel β-strands that are connected by four disulfide bonds forming a cysteine-stabilized αβ (CSαβ) motif (Broekaert et al. 1997; Thomma et al. 2002). The structure of each plant defensin is also characterized by the occurrence of a functionally important signature γ-core motif GXCX3-9C (where G is glycine, C is cysteine, and X is any amino acid) that is conserved among all antimicrobial peptides containing disulfide bonds. Despite their structural similarity, plant defensins exhibit very low sequence homology outside the eight conserved cysteines. This divergence in primary sequences may account for the multi-functionality of plant defensins including antifungal and antibacterial activity, proteinase inhibitor activity, pollen tube guidance and discharge of male gametes, zinc tolerance, and plant development (Carvalho Ade and Gomes 2009). During the past decade, significant inroads into understanding the structure-activity relationships and MOA of a few antifungal plant defensins have been made. This chapter highlights current knowledge of their MOA and their deployment for improving plant resistance to fungal and oomycete pathogens.
6.2 MOA of Antifungal Plant Defensins
To fully comprehend the roles of defensins in plant defense and to harness their potential for engineering disease-resistant crops, it is important to unravel the MOA of antifungal plant defensins. First studies aimed at unraveling the MOA of defensins revealed interactions with fungus-specific membrane components (Thevissen et al. 1997, 2000, 2004). Defensins permeabilize fungal plasma membrane, induce Ca2+ influx, and disrupt a tip-focused Ca2+ gradient essential for polar growth of hyphal tips (Thevissen et al. 1996, 1997, 1999). In 2004, we presented evidence that defensin MsDef1 from Medicago sativa blocks the L-type calcium channel in mammalian cells (Spelbrink et al. 2004). It remains to be determined, however, if a fungal calcium channel plays a functional role in the antifungal action of MsDef1. Using Neurospora crassa expressing the Ca2+ reporter aequorin, MsDef1 and the cognate peptides containing its γ-core motif were each shown to perturb Ca2+ homeostasis in a highly specific and distinct manner (Munoz et al. 2014). Recently, Arabidopsis thaliana defensin AtPDF2.3 has been shown to block voltage-gated potassium channels expressed in frog oocytes indicating the role for potassium transport and/or homeostasis in the antifungal action of this defensin (Vriens et al. 2016).
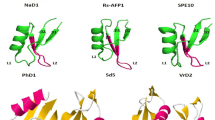
Some defensins bind with high affinity to specific sphingolipids present in the fungal cell wall and/or plasma membrane of their target fungi (Thevissen et al. 2003, 2005, 2007; Aerts et al. 2008). Sphingolipids are important structural components of the fungal cell wall and plasma membrane and serve as second messengers regulating delicate balance between cell death and survival (Thevissen et al. 2006). Plant defensins RsAFP2 from Raphanus sativus, MsDef1 from M. sativa, and Psd1 from Pisum sativum bind specifically to fungal cell wall localized glucosylceramide (GlcCer) (Fig. 6.1). Importantly, RsAFP2 does not interact with soybean or human GlcCer, suggesting that its preferential binding to yeast GlcCer may be due to structural differences. RsAFP2/GlcCer interaction has been shown to result in the induction of cell wall stress, accumulation of ceramides and reactive oxygen species (ROS), and ultimately cell death (De Coninck et al. 2013; van der Weerden et al. 2013). The ability of MsDef1 to disrupt a Ca2+ gradient in N. crassa is also dependent on its ability to interact with GlcCer (Munoz et al. 2014). Dahlia merckii defensin DmAMP1 targets a different sphingolipid mannose-(inositol phosphate)2-ceramide in fungal cells (Thevissen et al. 2000). At present, very little is known regarding the biochemical steps downstream of defensin/sphingolipid interaction that lead to fungal growth arrest or cell death.
Amino acid sequence comparison of sphingolipid (MsDef1, Psd1, RsAFP2, and DmAMP1)- and phospholipid (MtDef4, NaD2, TPP3, NsD7, NaD1, HsAFP1, and MtDef5)-binding defensins. The presence of eight cysteines and the γ-core motif (GXCX3-9C, where X is any amino acid) in each defensin is shown in red and blue, respectively. Each defensin comprises one α-helix and three β-strands as shown below the amino acid sequences
During the past decade, evidence has emerged that some antifungal plant defensins bind to a variety of bioactive plasma membrane resident phospholipids, induce membrane disruption, and gain entry into fungal cells (Lobo et al. 2007; van der Weerden et al. 2008, 2010). The phospholipid-binding plant defensins shown in Fig. 6.1 have received much attention lately, for studies aimed at unraveling their MOA. These include NaD1 from Nicotiana alata, TPP3 from tomato (Baxter et al. 2015), NsD7 from N. suaveolens (Kvansakul et al. 2016), and MtDef4 and MtDef5 from M. truncatula (Sagaram et al. 2013; Islam et al. 2017). These defensins bind to different membrane phospholipids (Fig. 6.2). MtDef4 and NsD7 target phosphatidic acid (PA), a precursor for the biosynthesis of other phospholipids and a regulator of membrane-cytoskeleton interactions and membrane curvature. They also bind to a lesser extent to other bioactive phospholipids, in particular, phosphatidylinositol mono- and bisphosphates. MtDef4 mutants that fail to bind PA also fail to gain entry into fungal cells and show much reduced or complete loss of antifungal activity. Recently, HsAFP1 from Heuchera sanguinea has also been shown to bind PA (Cools et al. 2017). HsAFP1 mutant that exhibits much reduced PA binding also exhibits loss of antifungal activity greater than twofold. Structural analysis of the NsD7-PA complex has revealed a double helix forming right-handed coiled oligomeric defensin fibril, and PA is required for oligomerization. It remains to be determined if MtDef4 forms oligomeric complexes in the presence of PA. Interaction with PA is important for the antifungal activity of these defensins.
Phospholipid-binding profile of defensins using protein-lipid overlay assay. (A) NaD1 binds to a broad range of bioactive phospholipids with a strong preference for phosphatidylinositol 4,5-bisphosphate PI(4,5)P2. (B) TPP3 binds specifically to PI(4,5)P2. (A, B, reproduced from Baxter et al. 2015). (C) MtDef4 bound preferentially to phosphatidic acid and PI(3,5)P2. (Reproduced from Sagaram et al. 2013). (D) MtDef5 binds to a range of bioactive phospholipids with a strong preference to PI3P, PI4P, and PI5P. (Reproduced from Islam et al. 2017)
NaD1 and TPP3 bind to plasma membrane-localized phosphoinositides, in particular, phosphatidylinositol 4,5-bisphosphate (PIP2), a key mediator of cytoskeletal-membrane interactions (Fig. 6.2a, b). NaD1 also binds to other phospholipids and thus appears to be more promiscuous than TPP3 (Poon et al. 2014; Baxter et al. 2015). In absence of phospholipids, NaD1 has been shown to form dimers in solution, and dimerization enhances its antifungal activity (Lay et al. 2012). However, in presence of PIP2, structural analysis has shown that 7 dimers cooperatively bind to the anionic head groups of 14 molecules of PIP2 and form arch-shaped “cationic grip” configuration, and this PIP2-mediated oligomerization is important for fungal plasma membrane permeabilization. NaD1 thus employs a unique PIP2-dependent mechanism to disrupt fungal plasma membrane. NaD1 also forms oligomers in solution in presence of PIP2 (Fig. 6.3A), and these oligomers lead to the formation of fibrils as observed by transmission electron microscopy. TPP3, which shares 63% sequence identity with NaD1, binds specifically to PIP2, and structural analysis has shown that it too forms a dimeric cationic grip only in presence of this phospholipid (Fig. 6.3B). This interaction with PIP2 also leads to higher order oligomerization of this defensin and formation of string-like fibrils (Poon et al. 2014; Baxter et al. 2015). Whether TPP3 and NaD1 form fibrils in contact with the fungal plasma membrane in vivo is not known.
Oligomerization profile of defensins using protein cross-linking analysis. (A, B) NaD1 and TPP3 form oligomers in the presence of PIP2. (Reproduced from Baxter et al. 2015). (C) MtDef4 forms oligomers in the presence of phosphatidic acid. (Reproduced from Sagaram et al. 2013). (D) MtDef5 forms oligomers in the presence of PI3P, PI4P, and PI5P. (Reproduced from Islam et al. 2017)
Another phospholipid-binding antifungal defensin MtDef5 has been recently studied in our lab (Islam et al. 2017). It is a novel bi-domain defensin which consists of 2 defensin monomers, 50 amino acids each, linked by a 7-amino acid peptide sequence APKKVEP (Fig. 6.1). It carries a net charge of +16 and exhibits broad-spectrum antifungal activity against filamentous fungi at submicromolar concentrations. MOA studies have shown that MtDef5 is a highly promiscuous defensin which binds to a number of phospholipids but with a strong preference for phosphatidylinositol 3-phosphates (PI3P), PI4P, and PI5P, substrates for synthesis of PIP2 and contributors to polar tip growth in fungi (Fig. 6.2D).The phospholipid-binding profile of MtDef5 is different from those of MtDef4 and NsD7 which bind to PA and also different from those of NaD1and TPP3 which bind preferentially to PIP2. MtDef5 forms oligomers in presence of PIP, but surprisingly, it also oligomerizes in presence of PI and PA to which it has relatively weak binding (Fig. 6.3D). Similarly, both NaD1 and NsD7 have also been reported to oligomerize in presence of PA and PIP2 (Poon et al. 2014; Baxter et al. 2015; Kvansakul et al. 2016) (Fig. 6.3A, C). In preliminary studies, MtDef5/PIP complexes form nanonet-like structures when observed under the transmission electron microscope. Mutagenesis studies have revealed that cationic amino acids present in the γ-core motif are involved in PIP binding and oligomerization of this defensin and facilitating membrane disruption and fungal killing by this protein (Islam et al. 2017).
From the studies described above, it is becoming increasingly clear that plant defensins which gain entry into fungal cells utilize a broad “phospholipid code” to identify and attack fungal membranes as part of the first line of plant defense (Baxter et al. 2017). However, one outstanding issue which needs to be addressed is whether phospholipid binding is required for their antifungal activity. Bleackley and colleagues have addressed this issue by analyzing the phospholipid binding and antifungal activity of a series of NaD1 chimeras with NaD2 that exhibits poor antifungal activity (Bleackley et al. 2016). These chimeras were produced by replacing the sequence between the two neighboring cysteine residues of NaD1 with the corresponding sequence of NaD2. Surprisingly, some of the chimeras that lost PIP2 binding retained their ability to inhibit fungal growth suggesting mechanisms other than phospholipid binding exist for antifungal activity.
Defensin NaD1 has been shown to permeabilize the plasma membrane of Fusarium oxysporum f. sp. vasinfectum ultimately leading to granulation of the cytoplasm and cell death (van der Weerden et al. 2008) (Fig. 6.4A). Similarly, MtDef4 rapidly permeabilizes plasma membrane of F. graminearum where it accumulates in the cytoplasm that eventually leads to death (Sagaram et al. 2013) (Fig. 6.4B). The important question related to the antifungal action of a specific plant defensin is whether its MOA is conserved in different fungi. Surprisingly, we have found that mechanisms used by MtDef4 to inhibit the growth of F. graminearum and N. crassa are not the same even though these two fungi belong to the same phylum Ascomycota, subphylum Pezizomycotina, and order Sordariomycetes (El-Mounadi et al. 2016).When used at minimal inhibitory concentration, MtDef4 permeabilizes the plasma membrane of F. graminearum but not N. crassa (Fig. 6.5). After its internalization, MtDef4 is localized to vesicular bodies in the conidia and germlings of N. crassa but shows diffuse cytoplasmic localization in those of F. graminearum. Further, cellular uptake of MtDef4 into N. crassa is energy dependent and involves endocytosis, whereas it is only partially energy dependent in F. graminearum. Brefeldin A (an ER to Golgi transport inhibitor) and filipin (a lipid raft-mediated endocytosis inhibitor) significantly inhibit internalization of MtDef4 in N. crassa but not in F. graminearum. In fungi, PA is generated mainly through the action of phospholipase D (PLD). N. crassa and F. graminearum each express three PLDs, namely, PLD1, PLD, and PLDA. Surprisingly, the plasma membrane-localized PLD1 is required for entry of this defensin in N. crassa, but not in F. graminearum (El-Mounadi et al. 2016). These findings indicate that the cell wall and plasma membrane compositions are different even in closely related fungi and markedly influence the antifungal activity of plant defensins. They also raise the possibility that pathogenic and saprophytic fungi respond differently to challenge by a specific antifungal plant defensin.
Immunogold labeling of defensins (A) Micrograph of NaD1-treated hyphae of F. oxysporum f. sp. vasinfectum. NaD1 has been internalized at a high concentration inside a treated hypha. (Reproduced from van der Weerden et al. 2008). (B) Micrograph of MtDef4-treated F. graminearum hypha. MtDef4 internalized at a high concentration inside the fungal cell. (Reproduced from Sagaram et al. 2013)
Permeabilization of fungal plasma membrane by plant defensins. (A, B) MtDef4 permeabilizes the plasma membrane of F. graminearum but not of N. crassa as revealed by SYTOX green uptake assay. (Reproduced from El-Mounadi et al. 2016). (C–D) MtDef5, in contrast, permeabilizes the plasma membrane of both fungi. (Reproduced from Islam et al. 2017)
Deeper knowledge of different MOA employed by defensins for fungal killing highlighted here will undoubtedly enable rational design and exploitation of these peptides for engineering disease-resistant crops, a topic discussed below.
6.3 Deployment of Plant Defensins to Engineer Disease-Resistant Plants
Combatting plant fungal and oomycete diseases by varietal genetic resistance, management practices, and fungicide application is the current norm. Novel technologies that could augment the above disease control strategies, however, will be required to stay ahead of fast-evolving pathogens and changing climate. Plant defensins with their potent broad-spectrum antifungal and anti-oomycete activity combined with nontoxicity to humans hold potential for use as antifungal agents in transgenic crops. Several labs including ours have reported enhanced resistance to various plant fungal and oomycete pathogens in transgenic plants expressing plant defensins. The reader is referred to excellent reviews on this topic (Kaur et al. 2011; DeConinck et al. 2013). While majority of these studies demonstrated in planta efficacy of defensins in controlled environment of a growth chamber or a greenhouse, few have shown resistance to fungal and/or oomycete pathogens in the field. One of the pioneer studies, first reported in 2000, demonstrated that constitutive expression of alfAFP (MsDef1) controlled Verticillium wilt caused by fungus V. dahliae in field-grown potato (Gao et al. 2000). Almost a decade later, constitutive expression of N. megalosiphon defensin NmDef02 was shown to confer resistance to an oomycete pathogen Phytophthora infestans, causal agent of potato late blight, in the field (Portieles et al. 2010). In both studies, defensin peptides were secreted to the apoplast in transgenic lines and field efficacy of these lines correlated with the peptide expression levels. In another exciting study, expression of vacuole-targeted NaD1 was shown to provide substantial field level resistance to F. oxysporum f. sp. vasinfectum and V. dahliae in transgenic cotton (Fig. 6.6A). When compared with non-transgenic control lines, transgenic cotton lines had increased survival rate and produced two- to fourfold increase in lint yield under disease pressure. In non-diseased soil, transgenic lines showed no negative impact on agronomic characteristics relative to non-transgenic lines (Gasper et al. 2014). Collectively, these studies demonstrate that antifungal defensins can be used to engineer resistance to economically important fungal and oomycete pathogens.
Disease resistance in transgenic lines overexpressing defensins. (A) Transgenic cotton line D1 expressing NaD1 showed resistance to F. oxysporum f. sp. vasinfectum compared to non-transgenic Coker parent line. (Reproduced from Gasper et al. 2014). (B) MtDef4 overexpressing transgenic wheat lines conferred resistance to Puccinia triticina. Transgenic lines BW-A-11, BW-B-4, BW-F-10, and XC9–104-1 in comparison to their respective non-transgenic controls BW and XC9. (Reproduced from Kaur et al. 2017)
Targeting a specific defensin to the appropriate subcellular compartment to match the lifestyle of a fungal or oomycete pathogen is the key for design of effective disease control strategies. We have shown that targeting MtDef4 to the apoplast, but not to the intracellular compartments, is necessary to control an obligate biotrophic oomycete Hyaloperonospora arabidopsidis causing downy mildew in transgenic Arabidopsis thaliana (Kaur et al. 2012). The efficacy of apoplast-targeted MtDef4 to confer resistance to an obligate biotroph Puccinia triticina, causal fungal pathogen of leaf rust, was also demonstrated in transgenic wheat (Kaur et al. 2017) (Fig. 6.6B). It is proposed that antifungal defensin optimally expressed in the extracellular milieu makes direct contact with the biotrophic pathogen and impedes its growth. In a recently published study, transgenic peanuts overexpressing apoplast-targeted MsDef1 or MtDef4 exhibit near immunity to Aspergillus flavus and accumulate drastically reduced levels of aflatoxins (Sharma et al. 2017). Aflatoxin contamination caused by A. flavus infection of peanuts poses a major food safety problem for people in sub-Saharan Africa and South Asia. This finding clearly indicates that the defensin technology when employed strategically could have important implications to mitigate the toxin levels.
Expression of defensins using strong constitutive promoters in transgenic plants provides yield advantage under epidemic conditions but can also result in deleterious side effects in the absence of a disease. In such cases, expression of defensins using tissue-specific or pathogen-inducible promoters will be crucial for commercially viable deployment of defensin-mediated resistance. A number of such tissue-specific and pathogen-inducible promoters are available to choose from to match the target pathogen’s lifestyle. For root-colonizing pathogens, for example, expression of defensins using root-specific promoters might be sufficient to confer optimal resistance without the deleterious effects of their constitutive expression. Recently, kernel-specific zein promoter was used to express RNA interference gene cassette directed against the aflatoxin biosynthesis pathway for development of A. flavus-resistant maize with much reduced aflatoxin accumulation (Thakare et al. 2017). This promoter might prove useful for expression of A. flavus inhibitory defensins in transgenic maize kernels to reduce aflatoxin levels. The Lem2 promoter known to be expressed in lemma and palea of florets in wheat and barley might prove useful for expression of antifungal defensins and conferring resistance to Fusarium graminearum. Interestingly, Lem2 promoter is also induced by Fusarium infection (Abebe et al. 2005, 2006). Pathogen-inducible promoters such as OsPR10a (Hwang et al. 2008) and GER4c (Himmelbach et al. 2010) induced by pathogens and defense hormones can also be tested for resistance to economically important fungal and oomycete pathogens in transgenic cereals. In addition, synthetic designer promoters that are responsive to a number of phytohormones (Liu et al. 2011) could also be used for appropriate targeting of defensins. With much greater understanding of the MOA of sequence divergent antifungal plant defensins in recent years and the availability of tools for their pathogen-inducible or tissue-specific expression and subcellular localization, we are in an excellent position to engineer durable, agronomically useful level of fungal resistance in transgenic crops.
References
Abebe T, Skadsen RW, Kaeppler HF (2005) A proximal upstream sequence controls tissue-specific expression of Lem2, a salicylate-inducible barley lectin-like gene. Planta 221:170–183
Abebe T, Skadsen R, Patel M, Kaeppler H (2006) The Lem2 gene promoter of barley directs cell- and development-specific expression of gfp in transgenic plants. Plant Biotechnol J 4:35–44
Aerts AM, François IEJA, Cammue BPA, Thevissen K (2008) The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci 65:2069–2079
Baxter AA, Richter V, Lay FT, Poon IKH, Adda CG, Veneer PK, Phan TK, Bleackley MR, Anderson MA, Kvansakul M, Hulett MD (2015) The tomato defensin TPP3 binds phosphatidylinositol (4,5)-bisphosphate via a conserved dimeric cationic grip conformation to mediate cell lysis. Mol Cell Biol 35:1964–1978
Baxter AA, Poon IKH, Hulett MD (2017) The lure of the lipids: how defensins exploit membrane phospholipids to induce cytolysis in target cells. Cell Death Dis 8:e2712
Bleackley MR, Payne JAE, Hayes BME, Durek T, Craik DJ, Shafee TMA, Poon IKH, Hulett MD, van der Weerden NL, Anderson MA (2016) Nicotiana alata defensin chimeras reveal differences in the mechanism of fungal and tumour cell killing and an enhanced antifungal variant. Antimicrob Agents Chemother 60:6302–6312
Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K, De Samblanx GW, Osborn RW, Nielson K (1997) Antimicrobial peptides from plants. Crit Rev Plant Sci 16:297–323
Carvalho Ade O, Gomes VM (2009) Plant defensins-prospects for the biological functions and biotechnological properties. Peptides 30:1007–1020
Colilla FJ, Rocher A, Mendez E (1990) Gamma-purothionins: amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm. FEBS Lett 270:191–194
Collinge DB, Jorgensen HJ, Lund OS, Lyngkjaer MF (2010) Engineering pathogen resistance in crop plants: current trends and future prospects. Annu Rev Phytopathol 48:269–291
Cools TL, Vriens K, Struyfs C, Verbandt S, Ramada MHS, Brand GD, Block C Jr, Koch B, Traven A, Drijfhout JW, Demuyser L, Kucharikova S, Van Dijck P, Spasic D, Lammertyn J, Cammune BPA, Thevissen K (2017) The antifungal plant defensin HsAFP1 is a phosphatidic acid-interacting peptide inducing membrane permeabilization. Front Microbiol 8:2295. https://doi.org/10.3389/fmicb.2017.02295
De Coninck B, Cammue BPA, Thevissen K (2013) Modes of antifungal action and in planta functions of plant defensins and defensin-like peptides. Fungal Biol Rev 26:109–120
El-Mounadi K, Islam KT, Hernández-Ortiz P, Read ND, Shah DM (2016) Antifungal mechanisms of a plant defensin MtDef4 are not conserved between the ascomycete fungi Neurospora crassa and Fusarium graminearum. Mol Microbiol 100:542–559
Gao AG, Hakimi SM, Mittanck CA, Wu Y, Woerner BM, Stark DM, Shah DM, Liang J, Rommens CM (2000) Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotechnol 18:1307–1310
Gaspar YM, McKenna JA, McGinness BS, Hinch J, Poon S, Connelly AA, Anderson MA, Heath RL (2014) Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J Exp Bot 65:1541–1550
Himmelbach A, Liu L, Zierold U, Altschmied L, Maucher H, Beier F, Müller D, Hensel G, Heise A, Schützendübel A, Kumlehn J, Schweizer P (2010) Promoters of the barley germin-like GER4 gene cluster enable strong transgene expression in response to pathogen attack. Plant Cell 22:937–952
Hwang S-H, Lee IA, Yie SW, Hwang D-J (2008) Identification of an OsPR10a promoter region responsive to salicylic acid. Planta 227:1141–1150
Islam KT, Velivelli SLS, Berg RH, Oakley B, Shah DM (2017) A novel bi-domain plant defensin MtDef5 with potent broad-spectrum antifungal activity binds to multiple phospholipids and forms oligomers. Sci Rep 7:16157
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323
Kaur J, Sagaram US, Shah D (2011) Can plant defensins be used to engineer durable commercially useful fungal resistance in crop plants? Fungal Biol Rev 25:128–135
Kaur J, Thokala M, Robert-Seilaniantz A, Zhao P, Peyret H, Berg H, Pandey S, Jones J, Shah D (2012) Subcellular targeting of an evolutionarily conserved plant defensin MtDef4.2 determines the outcome of plant-pathogen interaction in transgenic Arabidopsis. Mol Plant Pathol 13:1032–1046
Kaur J, Fellers J, Adholeya A, Velivelli SL, El-Mounadi K, Nersesian N, Clemente T, Shah D (2017) Expression of apoplast-targeted plant defensin MtDef4.2 confers resistance to leaf rust pathogen Puccinia triticina but does not affect mycorrhizal symbiosis in transgenic wheat. Transgenic Res 26:37–49
Kvansakul M, Lay FT, Adda CG, Veneer PK, Baxter AA, Phan TK, Poon IKH, Hulett MD (2016) Binding of phosphatidic acid by NsD7 mediates the formation of helical defensin-lipid oligomeric assemblies and membrane permeabilization. Proc Natl Acad Sci 113:11202–11207
Lay FT, Brugliera F, Anderson MA (2003) Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol 131:1283–1293
Lay FT, Mills GD, Poon IK, Cowieson NP, Kirby N, Baxter AA, van der Weerden NL, Dogovski C, Perugini MA, Anderson MA, Kvansakul M, Hulett MD (2012) Dimerization of plant defensin NaD1 enhances its antifungal activity. J Biol Chem 287:19961–19972
Lay FT, Poon S, McKenna JA, Connelly AA, Barbeta BL, McGinness BS, Fox JL, Daly NL, Craik DJ, Heath RL, Anderson MA (2014) The C-terminal propeptide of a plant defensin confers cytoprotective and subcellular targeting functions. BMC Plant Biol 14:41
Liu W, Mazarei M, Rudis MR, Fethe MH, Stewart CN (2011) Rapid in vivo analysis of synthetic promoters for plant pathogen phytosensing. BMC Biotechnol 11:108–108
Lobo DS, Pereira IB, Fragel-Madeira L, Medeiros LN, Cabral LM, Faria J, Bellio M, Campos RC, Linden R, Kurtenbach E (2007) Antifungal Pisum sativumdefensin 1 interacts with Neurospora crassa Cyclin F related to the cell cycle. Biochemistry 46:987–996
Mendez E, Moreno A, Colilla F, Pelaez F, Limas GG, Mendez R, Soriano F, Salinas M, de Haro C (1990) Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, gamma-hordothionin, from barley endosperm. Eur J Biochem 194:533–539
Munoz A, Chu M, Marris PI, Sagaram US, Kaur J, Shah DM, Read ND (2014) Specific domains of plant defensins differentially disrupt colony initiation, cell fusion and calcium homeostasis in Neurospora crassa. Mol Microbiol 92:1357–1374
Poon IKH, Baxter AA, Lay FT, Mills GD, Adda CG, Payne JAE, Phan TK, Ryan GF, White JA, Veneer PK, van der Weerden NL, Anderson MA, Kvansakul M, Hulett MD (2014) Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. elife 3:e01808
Portieles R, Ayra C, Gonzalez E, Gallo A, Rodriguez R, Chacon O, Lopez Y, Rodriguez M, Castillo J, Pujol M, Enriquez G, Borroto C, Trujillo L, Thomma BP, Borras-Hidalgo O (2010) NmDef02, a novel antimicrobial gene isolated from Nicotiana megalosiphon confers high-level pathogen resistance under greenhouse and field conditions. Plant Biotechnol J 8:678–690
Sagaram US, El-Mounadi K, Buchko GW, Berg HR, Kaur J, Pandurangi RS, Smith TJ, Shah DM (2013) Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: identification of an RGFRRR motif governing fungal cell entry. PLoS One 8(12):e82485
Sharma KK, Pothana A, Prasad K, Shah D, Kaur J, Bhatnagar D, Chen ZY, Raruang Y, Cary JW, Rajasekaran K, Sudini HK, Bhatnagar-Mathur P (2017) Peanuts that keep aflatoxin at bay: a threshold that matters. Plant Biotechnol J 16:1024. https://doi.org/10.1111/pbi.12846
Spelbrink RG, Dilmac N, Allen A, Smith TJ, Shah DM, Hockerman GH (2004) Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol 135:2055–2067
Thakare D, Zhang J, Wing RA, Cotty PJ, Schmidt MA (2017) Aflatoxin-free transgenic maize using host-induced gene silencing. Sci Adv 3:e1602382
Thevissen K, Ghazi A, De Samblanx GW, Brownlee C, Osborn RW, Broekaert WF (1996) Fungal membrane responses induced by plant defensins and thionins. J Biol Chem 271:15018–15025
Thevissen K, Osborn RW, Acland DP, Broekaert WF (1997) Specific, high affinity binding sites for an antifungal plant defensin on Neurospora crassahyphae and microsomal membranes. J Biol Chem 272:32176–32181
Thevissen K, Terras FRG, Broekaert WF (1999) Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol 65:5451–5458
Thevissen K, Cammue BP, Lemaire K, Winderickx J, Dickson RC, Lester RL, Ferket KK, Van Even F, Parret AH, Broekaert WF (2000) A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii). Proc Natl Acad Sci U S A 97:9531–9536
Thevissen K, François IEJA, Takemoto JY, Ferket KKA, Meert EM, Cammue BPA (2003) DmAMP1, an antifungal palnt defensins from dahlia (Dahlia merckii), interats with sphingolipids from Saccharomyces cerevisiae. FEMS Microbiol Lett 226:169–173
Thevissen K, Warnecke DC, François IEJA, Leipelt M, Heinz E, Ott C, Zähringer U, Thomma BPHJ, Ferket KKA, Cammue BPA (2004) Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem 279:3900–3905
Thevissen K, Francois IE, Aerts AM, Cammue BP (2005) Fungal sphingolipids as targets for the development of selective antifungal therapeutics. Curr Drug Targets 6:923–928
Thevissen K, Francois IE, Winderickx J, Pannecouque C, Cammue BP (2006) Ceramide involvement in apoptosis and apoptotic diseases. Mini Rev Med Chem 6:699–709
Thevissen K, Kristensen H-H, Thomma BPHJ, Cammue BPA, François IEJA (2007) Therapeutic potential of antifungal plant and insect defensins. Drug Discov Today 12:966–971
Thomma BP, Cammue BP, Thevissen K (2002) Plant defensins. Planta 216(2):193–202
van der Weerden NL, Lay FT, Anderson MA (2008) The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem 283:14445–14452
van der Weerden NL, Hancock REW, Anderson MA (2010) Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J Biol Chem 285:37513–37520
van der Weerden NL, Bleackley MR, Anderson MA (2013) Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol Life Sci CMLS 70:3545–3570
Vriens K, Peigneur S, De Coninck B, Tytgat J, Cammue BPA, Thevissen K (2016) The antifungal plant defensin AtPDF2.3 from Arabidopsis thaliana blocks potassium channels. Sci Rep 6:32121
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kaur, J., Velivelli, S.L., Shah, D. (2018). Antifungal Plant Defensins: Insights into Modes of Action and Prospects for Engineering Disease-Resistant Plants. In: Gosal, S., Wani, S. (eds) Biotechnologies of Crop Improvement, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-319-90650-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-90650-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90649-2
Online ISBN: 978-3-319-90650-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)