Abstract
In this chapter, we explore the functional diversity concept and its importance in several ecological issues, especially maintenance of ecosystem services and conservation. We consider that Mexico’s species megadiversity should be reflected into a high functional diversity. However, our knowledge on this issue is still limited. Interest in the functional diversity approach has just increased in Mexico. Despite that, since the 1970s, ecophysiological research in Mexican ecosystems has had important pioneer contributions to our knowledge on functional traits in plants and its ecological importance. In this chapter, we review some case studies describing our knowledge of plant physiological diversity in different ecosystems, as examples of the high functional diversity in Mexico. Unfortunately anthropogenic disturbance is increasingly affecting species biodiversity, in particular the more vulnerable species and ecosystems. Increasing research on the functional traits of Mexican ecosystems will provide important information about species function at the ecosystem level and species vulnerability in the context of human disturbance and/or climatic change. Studies focused in functional diversity as an important component of biodiversity will provide us a solid base for planning on conservation decisions, restoration programs, and maintenance of ecosystem services.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Functional Diversity: Concept and Importance
Biodiversity is a multidimensional concept defining the variability of living beings, their functions and ecological complexes. It includes four components: species diversity, genetic diversity, ecological diversity, and functional or physiological diversity. Until recently functional diversity (FD) has been considered an important component of biodiversity. Diaz et al. (2007) define functional diversity as the type, range, and relative abundance of functional traits in a community. Functional traits are morphological, physiological, and phenological traits of individuals that influence growth, reproduction, and survivorship and therefore have an effect on fitness (Violle et al. 2007; Orozco-Segovia and Sanchez-Coronado 2009). Knowledge on functional traits is important to understand the mechanistic basis of species distribution, their response to environmental changes, their biotic interactions, and their role in ecosystem processes. For example, the relative abundance and diversity of species attributes in response to environmental gradients, biogeographic regions, or human disturbance, such as cattle grazing, change the functional diversity of ecosystems (Diaz et al. 2003, 2007). At a higher scale (communities), FD is the key to define the mechanisms that link biodiversity and ecosystems function (Diaz 2001 for a review; Cadotte et al. 2011).
As a growing and complex research area, there is not a unique methodology to measure FD. Most knowledge on FD has been generated at the individual and population levels since the 1970s, when technological advances allowed the possibility for ecophysiological studies . From these studies, common and meaningful traits have been collected and analyzed. To date, there are standardized protocols to measure some functional traits that are frequently measured in ecophysiological studies (Cornelisen et al. 2003). However, the selection of the system and meaningful traits to be measured depend on the study question; it is also important to include several traits to decrease the probability of species redundancy and to measure quantitative traits to capture interspecific variability (Orlandi Laureto et al. 2015). Estimations of functional diversity at more complex levels (communities) is not an easy task; therefore, indexes and models have been proposed as an approach to calculate FD (Carmona et al. 2018; Pakeman 2011).
Mexico is one of the megadiverse countries of the world; it harbors 12% of the word biodiversity, despite being a relatively small country, with 1.4% of the planet land surface. Its biological diversity is associated with the following factors : high variety of climates, complex geological history, topographic heterogeneity, and a geographical location in a wide range of latitude (14° 32′27″–32° 43′ 06″ N), resulting in a mosaic of environmental conditions. Environmental heterogeneity has led to a great variety of habitats where a high number of organisms have evolved and coexist. Furthermore, Mexico is the site of confluence of the Nearctic and Neotropical biogeographic regions, where boreal and tropical organisms converge, increasing biodiversity of species and ecosystems. Under this scenario, it is not hard to think that Mexican biodiversity is associated with a high functional diversity that might evolve in response to this highly heterogeneous environment.
Although we require more research to be focused on functional diversity to build a good scientific basis to preserve Mexico’s biodiversity, conservation measurements should be a priority. In this chapter, we include some case studies describing our knowledge on plant physiological diversity for different ecosystems, as a sample of the high functional diversity in Mexico.
2 Functional Diversity in Seasonally Dry Forest: Plants with Crassulacean Acid Metabolism
2.1 Plants with Crassulacean Acid Metabolism in Seasonally Dry Forests
Crassulacean acid metabolism (CAM) is an adaptation for some plants to concentrate atmospheric carbon dioxide (CO2) within their cells (Winter and Smith 1996; Lüttge 2004; Andrade et al. 2007). CAM is common in plants living in environments with long periods without water but also exists in aquatic plants that inhabit places where CO2 becomes scarce during the day (Keeley and Rundel 2003). Nonaquatic CAM species assimilate CO2 when air temperature is lower and relative humidity is higher, which is generally at night; this allows less water loss by transpiration and permits high water use efficiency. CAM plants have greater water use efficiency than C3 and C4 plants under similar conditions; therefore, they are distinctive of arid and semiarid regions and of forest canopies (Drennan and Nobel 2000; Winter et al. 2005; Andrade et al. 2009). In Mexico, CAM plants are emblematic , revealed in many cultural aspects, and they have even gone beyond borders: Opuntia spp. are the most extensively cultivated CAM crop worldwide, exceeding that of the better known pineapple (Nobel 2002; de la Barrera and Andrade 2005).
Mexican CAM plants inhabit in virtually all terrestrial ecosystems below 2000 m asl (Körner et al. 1991; Vargas-Soto et al. 2009), but the great aridity of the Mexican territory significantly favors them. In fact, Mexico is the center of origin and diversification of cacti and other CAM species, and, because most of these species are endemic, they are particularly vulnerable to changes in land use. In tropical areas, CAM species are terrestrial in short-statured communities such as dunes and dry deciduous forests and predominantly epiphytes or hemiepiphytes in taller forests (Ricalde et al. 2010; Valdez-Hernández et al. 2015). In fact, epiphytic CAM species probably also outnumber terrestrial CAM species in Mexico (Andrade et al. 2007; Silvera and Lasso 2016; Zotz 2016). In seasonally dry forests, where dry season can last several weeks, CAM species comprise important functional groups, which can be source of water and food for animals during drought; this is because they have leaves or stems structurally transformed to store or trap water (Martínez-Ramos 2008).
At a global scale, tropical seasonally dry forests are the less understood and represent one of the most threatened ecosystems (Miles et al. 2006). In Mexico, they are also the less protected even with their floristic richness and endemism (Trejo and Dirzo 2002; Dick and Wright 2005). This type of vegetation is characterized, depending of the precipitation, by having 25–100% of the composing tree species losing their leaves during the dry season (Miranda and Hernández 1963), when light intensity and air temperatures can change dramatically (Graham and Andrade 2004). Dry deciduous forests of Mexico are rich in CAM species, which are from diverse families and at high densities, and in some forests, some terrestrial species can even be ecologically the most important species (Ricalde et al. 2010). Moreover, abundance of nitrogen fixer legumes in some dry forests can induce growth in accompanying terrestrial CAM plants since epiphytic CAM species receive most of their nitrogen from atmospheric sources (Santiago et al. 2017). Additionally, relative capacitance (the capacity of the tissues to maintain their water potentials when the water content decreases) is higher for terrestrial CAM species than for CAM epiphytes (Andrade et al. 2009).
2.2 Terrestrial CAM Species
Terrestrial CAM plants have succulent stems or leaves that store water. Also, rosette forms of species from several CAM families have a role to capture rainfall and even fog (Martorell and Ezcurra 2002). So, these specialized storage tissues can transport water to maintain turgor in the photosynthetic tissues during drought (Goldstein et al. 1991; Lüttge 2004; Andrade et al. 2009). In the rainy season, CAM photosynthesis can enhance; many studies reveal that the greatest tissue acid accumulation (a proxy for CO2 uptake) of terrestrial CAM plants in seasonally dry forests takes place during the rainy season (Andrade et al. 2006; Cervera et al. 2007; Vargas-Soto et al. 2009; González-Salvatierra et al. 2010; Ricalde et al. 2010), with lower air temperatures and vapor pressure deficits than during the rest of the year.
Seedlings of terrestrial CAM plants cannot have enough water in their tissues to endure long dry seasons but have higher survival probabilities under the canopy of nurse plants (Esparza-Olguín et al. 2002; Flores et al. 2004; Cervera et al. 2006). Although this phenomenon has been widely documented in different sites (Valiente-Banuet and Ezcurra 1991; Godínez-Álvarez et al. 1999, 2003), few studies have measured microenvironmental and physiological differences for seedlings and adults, which provide information for conservation purposes . For instance, seedlings of the rare cactus Mammillaria gaumeri do not survive under more than 20% of ambient light; however, adults increase growth at exposed locations (40–80% of ambient light), indicating that some disturbances can be beneficial for adults of this species (Cervera et al. 2006, 2007).
The enzyme that fixes CO2 at night in CAM plants, phosphoenolpyruvate carboxylase (PEPc) , does not discriminate against 13CO2, whereas ribulose bisphosphate carboxylase (RuBisCO) , which fixes carbon during the day, favors 12C fixation. Then, studies on carbon isotopic composition (δ13C) provide information on the proportion of CO2 fixed during the night or day (Griffiths 1992). CAM plants with CO2 uptake exclusively at night, by PEPc, would have δ13C values around −11 °/oo (close to atmospheric values), whereas if all CO2 is fixed by RuBisCO the values of δ13C would be about −27 °/oo (O’Leary 1988). In northern Yucatan, tissue δ13C values for terrestrial CAM plants of the coastal dunes are 2 °/oo higher than CAM plants from the tropical dry deciduous forests (Ricalde et al. 2010), which indicates greater CO2 fixation through the CAM pathway in sites with less water.
During the dry season, the lack of water and high temperatures can affect growth and reproduction of CAM species; high temperatures increase respiratory rates, and plant tissues must invest photosynthetic products to repair the photosynthetic apparatus from photoinhibitory damage, which leads to lower investment against pathogens and predators (Cervantes et al. 2005; Andrade et al. 2009). Optimal diurnal/nocturnal temperature for CO2 uptake of some species in seasonal dry forests is 30/20 °C (Nobel 1985; Nobel and de la Barrera 2002). Temperatures above or below this optimal, especially the nocturnal ones, reduce growth in CAM plants (Andrade et al. 2007).
A specific group of terrestrial CAM plants belongs to the genus Clusia, with species with C3, C3/CAM, and CAM pathways (Winter and Smith 1996; Winter et al. 2005; Lüttge 2006). In Mexico, there is a preponderance of C3/CAM Clusia species , but in seasonally dry forests, all species are CAM (Vargas-Soto et al. 2009).
2.3 Epiphytic CAM
Vascular epiphytes inhabit a very dynamic microenvironment, where light, nutrients, and water can change drastically in time and space (Benzing 1990; Lüttge 2008; Zotz 2016). In seasonal dry forests, epiphytes are prevalently CAM, but they have also evolved several morphophysiological adaptations such as succulence, reduced stomatal size and density, and specialized root, leaf, and stem structures (Winter et al. 2005; Andrade et al. 2009; Petter et al. 2015; Zotz 2016). Epiphytes from seasonally dry forests of Mexico also represent a main group of interest for physiological studies even though they are not as diverse, abundant, or important in biomass as in wet forests.
Vertical distribution of epiphytes in the dry forests is more related to light than to host tree species; most species are concentrated in the middle part of the host trees, mainly on the main stem or in branches near the main stem (Cervantes et al. 2005; Reyes-García et al. 2008; Cach-Pérez et al. 2013; Chilpa-Galván et al. 2013; de la Rosa-Manzano et al. 2014a). Some studies suggest that species distributed within the lower canopy avoid excess radiation (Griffiths and Maxwell 1999; Reyes-García and Griffiths 2009; Reyes-García et al. 2012). Indeed, species within the upper canopy can be more tolerant to high light but paradoxically may also be able to obtain more water (rainfall, fog, and dew) than species in the lower canopy (Andrade 2003; Graham and Andrade 2004; Reyes-García et al. 2012). However, although microclimatic conditions can contribute to the vertical distribution of CAM epiphytes in tropical dry forests, factors such as seed dispersers, bark traits, competition, facilitation, and other variables need further investigation to determine their role on such distribution (Benzing 1990; Chilpa-Galván et al. 2017).
Additionally, epiphytes with CAM have a morphology and physiology modified to tolerate water scarcity and high light incidence (Benzing 1990; Zotz and Hietz 2001). For instance, epiphytic bromeliads may store water in succulent tissues or in water-storing tanks and may absorb water and nutrients through modified leaf trichomes (Benzing 1990; Reyes-García et al. 2012). Epiphytic orchids have water storage capacity in their stems or pseudobulbs and on specialized roots with a highly absorptive velamen (de la Rosa et al. 2014b). Also, epiphytic bromeliad densities are inversely related to vapor pressure deficit (VPD , Cach-Pérez et al. 2013). Low values of VPD diminish the rate of water loss and allow a high probability for deposition of dew (Chilpa-Galván et al. 2013; de la Rosa et al. 2014b). In the north of Yucatan, water table can be very shallow, and, in locations where trees can reach it, low-VPD canopy islands emerge with high epiphyte densities (Chilpa-Galván et al. 2013).
In seasonally dry forests, the open canopy of trees and pinnate leaves offer poor protection to epiphytes against high incident light. Furthermore, trees also lose their leaves during the dry season with a consequent increase in photooxidative stress in epiphytic leaves, which carries a decrease in the quantum efficiency of photosystem II and a decline in CO2 assimilation (Valdez-Hernández et al. 2015). However, it has been reported that epiphytes with CAM avoid the production of the harmful reactive oxygen species, reducing photoinhibition and oxidative stress (Niewiadomska and Borland 2008). CAM is advantageous to cope with drought because it creates high CO2 concentrations in the cytosol and chloroplasts, favoring Rubisco’s carboxylation activity over its oxygenase activity, maintaining electron transport, and preventing damage to photosystems (Niewiadomska and Borland 2008). Moreover, epiphytic bromeliads increased the production of antioxidant compounds and flavonoids as a response to high light and other stressful conditions (Saito and Harborne 1983; González-Salvatierra et al. 2010). Also, leaf trichomes on bromeliads are highly reflective and could have an important role in photoprotection of leaves exposed to direct sunlight (Pierce 2007). For instance, individuals of tank species on deciduous hosts in short-statured dry forests have larger trichomes compared to species on host in taller less drier forests (Cach-Pérez et al. 2016).
Epiphytic orchids have also diverse mechanisms to remove or avoid excess light and heat, including a reduction of leaf area to increase heat flux by conduction and convection, carotenoid production, and photosystem II heat dissipation of absorbed energy (Vaz et al. 2004; Adams et al. 2008; de la Rosa-Manzano et al. 2014b, 2015). Not surprisingly, epiphytic orchids produce more carotenoids (such as lutein and neoxanthin) during the dry season than during the wet and early dry seasons in tropical dry forests ; also, the concentrations of these pigment are higher in leaves of orchids from the deciduous forest than those from the semi-deciduous forest (de la Rosa-Manzano et al. 2015). These differences in carotenoid compositions between epiphytic plants growing at high vs. low light are similar to the general plant responses reported by Demmig-Adams and Adams (1992).
Additionally, orchid leaves from a dry deciduous forest have higher zeaxanthin retention and lower values of maximum quantum efficiency of photosystem II (Fv/Fm) during the dry season than orchids from a semi-deciduous forest (de la Rosa-Manzano et al. 2015). These changes are related to high incident light and the prolonged dry period that occur in the tropical dry deciduous forests, when practically all trees shed their leaves. Moreover, in contrast to terrestrial CAM species , for epiphytes CAM photosynthesis enhances during the early dry season, particularly in the dry deciduous forest, when lower air temperatures, light, and minimal VPD occur than during the rest of the year (de la Rosa-Manzano et al. 2014b, 2015); indeed, dew and fog have higher deposition rates during this season (Andrade 2003; Reyes- García et al. 2012), which are conditions that favored a higher stomatal conductance to increase CO2 uptake.
Epiphytes have a distinct isotopic composition of nitrogen (15N) values in a tropical dry deciduous forest because they must obtain most of their N from the atmospheric sources than for decaying organic matter (Santiago et al. 2017). Low 15N values in epiphytes have been reported for other forests and for organic N in precipitation of non-polluted areas (Stewart et al. 1995; Cornell et al. 1995; Hietz et al. 1999).
It would be important to study multiple stress factors for CAM epiphytes in combination to measure plasticity and predict plant responses to future climate change (Prasch and Sonnewald 2015; de la Rosa-Manzano et al. 2017), vital information for conservation, and restoration programs. Since the future of epiphytes is uncertain in this changing world (Zotz 2016), research that highlights the potential of these species as ornamentals or biological indicators is extremely important.
3 Functional Diversity in Drylands: Environmental Tolerance and Species Adaptation in Mexican Drylands
Diversity and abundance of response traits change in plant communities in response to historical, biogeographical, and environmental local effects and more recently to man-made disturbances and global change. Functional diversity in plant communities of dryland ecosystems reflects the different adaptive responses to water limitations . Low and infrequent precipitation amounts and rainfall events or pulses with extreme high temperatures are the main selective factors for the different functional groups in dryland ecosystems (Noy-Meir 1973; Schwinning and Ehleringer 2001; Reynolds et al. 2004). In Mexican drylands, plant functional groups are numerous considering the different adaptive responses of leaves, stems, and roots to selective environmental and biological factors (Shreve 1942; Miranda and Hernández-Xolocotzi 1963). Response traits have an effect and influence ecosystem function as species ecophysiological traits are affected by the environment. For example, functional diversity in Mexican dryland species correlate with morpho-structural proxies of drought-enduring mechanisms such as leaf and leaflet size and life span, stem height and numbers, and root and water storage characteristics (Miranda and Hernández-Xolocotzi 1963) that are a consequence of the diverse adaptive ecophysiological responses, such as temperature and drought tolerances, photosynthetic capability, and water use efficiency (Flexas et al. 2002; Castellanos et al. 2010; Ryel et al. 2010; Medrano et al. 2015).
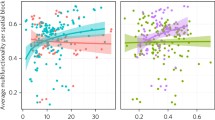
Mexican drylands are widespread and cover about 55–60% of the country, most of which correspond to the Northern Chihuahuan and Sonoran deserts. In Northwestern Mexico in the Sonoran Desert, the Neotropical and Temperate biogeographic regions meet in the Central Region of Sonora (Rzedowski 1978; Castellanos et al. 2010) and sustain some of the largest diversity of plant functional types . An example of the functional diversity in that region is the presence of one of the most abundant vine floras in the world (Castellanos 1991; Rundel and Franklin 1991; Molina-Freaner et al. 2004), even though vine species diversity are known to decrease from the tropics to temperate regions (Gentry 1983; Ewers et al. 1991). Vine species are present in the region and their diversity increase toward the high rainfall end of a precipitation gradient . Measured for C3 species only, mean vines species increase their water use efficiency in the driest sites, but their mean water use efficiency was lower in sites with higher annual rainfall, as measured by isotopic 13C discrimination (Table 23.1). Differential leaf isotopic carbon discrimination by the photosynthetic enzyme RUBISCO (ribulose bisphosphate carboxylase-oxygenase ) and stomatal control is correlated to water use efficiency and reflect the different long-term physiological mechanisms that control water loss, such as smaller diameter in xylem cells and hydraulic conductivity and stomatal regulation to minimize leaf transpiration under the region harsh environments (Castellanos 1991; Molina-Freaner and Tinoco-Ojanguren 1997; Castellanos et al. 1999; Molina-Freaner et al. 2004). As in other studies, we found larger variability in the functional diversity within site than between sites.
Drylands species have a number of other different functional strategies and attributes that allow them to adapt to the limiting water conditions of dryland habitats, such as the increasing niche differentiation within their biogeographic distribution range . Jatropha species are able to differentiate their species distribution ranges, within the arid and subtropical ecosystems in Northwestern Mexico, along both precipitation and temperature gradients (Fig. 23.1). J. cuneata, a species characteristic of some of the most arid regions of Mexico, has a lower and much narrower range of precipitation in their habitats. On the contrary, J. cordata, a characteristic species to subtropical shrublands and deciduous forests in Northwestern Mexico (INEGI 2000), has a wider range of precipitation in the habitats it is found. Both Jatropha species are drought evaders, and their fleshy roots and stems may store important amounts of water, such that when water is available, they sustain some of the highest leaf water potentials during the day, compared to other species in the same site (Table 23.2). Poor stomatal control of Jatropha leaves, however, may be responsible for their rapid leaf senescence, soon at the end of the rainfall season (Castellanos pers. obs.). Jatropha also characterize the contribution of species from the subtropical biogeographic regions to the large diversity and contrasting physiological responses and adaptations of plant functional types in the drylands of Mexico.
3.1 Threatens to Functional Diversity in Mexican Drylands: Global Change and Land Use Cover Change
The large contribution of Neotropical floristic elements to the functional diversity of Mexican drylands may also be the major drawback to their richness and functional diversity in the future. Given the current scenarios and the major drivers of global change, increasing atmospheric CO2 concentration, GH gases, and land use changes and deforestation, their effects are more notorious in Mexican drylands. Global change will increase annual temperatures, decrease rainfall amount, and change their seasonality (Seager et al. 2007b, 2009). Global change will have unknown but likely differentiated effects on species niche distribution (Villaseñor and Téllez-Valdés 2004; Moghaddam-Gheshlagh et al. 2017); their abundance, composition, and diversity (Archer et al. 1995; Archer and Predick 2008); biogeochemical cycles (Delgado-Baquerizo et al. 2013; Yuan and Chen 2015); and ecosystem function (Smith et al. 1997; Pan 2000; Chapin 2003). In the Central Region of Sonora, for example, Jatropha species, J. cuneata and J. cordata, have easily differentiated distribution niches along a rainfall, but not a temperature gradient (Fig. 23.1). Given the proposed global change scenarios of increasing temperature and decreasing rainfall in the region (Seager et al. 2007a, b), it is expected that J. cuneata will expand their range of distribution, and J. cordata will be the most affected, decreasing their distribution and abundance in the future, as current populations are commonly found in habitats with 350 mm annual precipitation or more. It is clear that many more ecophysiological studies are needed to determine the extent and severity of local effects of global change in the different plant species in Mexican drylands.
Change in arid land use is the main driver of global change in Mexican drylands. For more than 400 years, cattle grazing has been a major activity in arid plant communities and have induced changes in species abundance and composition, reducing the most palatable and increasing the more unpalatable (McIntyre et al. 2003; Callaway et al. 2005; Díaz et al. 2007; Dorrough et al. 2007; Hanke et al. 2014). In some drylands plant communities, overgrazing was detected, probably accentuated by a period of prolonged droughts, during the 1850s (Archer et al. 1995; Buffington and Herbel 1965; Seager et al. 2007a). During the mid-1950s, introduction of exotic grasses and rotation of grazing lands increased cattle stocking level in ranches (Bravo-Peña et al. 2010; Castellanos et al. 2010; Brenner 2011). Some of the ecological and environmental consequences of those management practices are still unknown and unmeasured.
Functional diversity is reduced after exotic buffelgrass savannas are established. Bulldozing of plant communities to establish buffelgrass grasslands or savannas removes most of herbs, shrubs, and most species of trees, reducing plant diversity (Saucedo-Monarque et al. 1997; Castellanos et al. 2002). Once buffelgrass is established, there may be some reestablishment of trees and shrubs (Castellanos et al. 2010; Tinoco-Ojanguren et al. 2013), although some others may be inhibited (Morales-Romero and Molina-Freaner 2008; Morales-Romero et al. 2012). In sites were buffelgrass savannas were established, many other functional processes may be changed. We have documented changes in ecosystem productivity (Hinojo-Hinojo et al. 2016), nitrogen mineralization (Celaya-Michel and Castellanos-Villegas 2011; Celaya-Michel et al. 2015), soil carbon accumulation (Castellanos et al. 2010; Morales-Romero et al. 2015), and soil moisture distribution, recharge, and availability in the soil profile (Castellanos et al. 2016). Water availability after prolonged rainfall periods increases in the soil profile under buffelgrass canopies, because of their limited rooting depth, and can be used by the remaining deep-rooted dominant tree and shrub species . Increasing the storage and availability of water in the deeper soil profile of the savanna (Fig. 23.2) decouple rainfall season and the time of water use by the remaining species and extend the amount of time during the year in which the species remain productive (Hinojo-Hinojo et al. submitted). It is noteworthy that changes in land cover and disturbance will reduce shallow water consumption and increase infiltration and volumetric soil moisture deeper in the soil profile. Increase in deeper soil moisture in subtropical drylands decouple plant activity from rainfall periods and extend the period of photosynthetic carbon gain and ecosystem productivity of deep-rooted perennial shrubs and trees (Scott et al. 2006, 2014), when other environmental factors like below zero temperatures or cloudiness are not limiting. Some species of dominant tree species in the drylands of Mexico are known to help redistribute water from deep to shallow roots and facilitate the growth and activity of annual and herbaceous perennial plants (Hultine et al. 2003, 2004).
4 Ecophysiological Tolerance of Ferns in the Cloud Forest
4.1 The Cloud Forest
Low and often-twisted deciduous and evergreen trees and high abundance and biomass of epiphytes characterize the cloud forest (Hietz and Hietz-Seifert 1995; Crausbay and Martin 2016). Cloud forest is distributed in fragmented form between 1000 and 3500 m of altitude, in rainy and cold mountains with high frequency of clouds and mist that limit the incidence of sunlight inside the forest (Lüttge 2008). Cloud forest collects large amounts of rain; however, there are periods without rain that can last several weeks and cause seedling mortality (Engelbrecht et al. 2005).
Climate changes and human disturbances have affected the distribution and decreased the extension of cloud forest (Foster 2001; Crausbay and Martin 2016). It has been predicted that the cloud forest will change to new climatic zones , but it will not move as a unit because it is composed of many species and possibly each one will respond in a particular way to the human-induced global change. However, the data commonly used to model the potential distribution of the species is made with average values of the climate, without taking into account the physiological tolerance of the plants to the physical conditions of the microhabitat, which can differ substantially from the regional climate (Foster 2001; Zotz and Bader 2009). Therefore, an important task is to know the physiological responses of plants to the environmental conditions of the ecosystems where they live.
4.2 Ecological Groups of the Ferns in the Cloud Forest
Light intensity is one of the most important environmental factors that influence the abundance, growth, and survival of plants in Tropical Mountain Forests (Poorter and Arets 2003; Lüttge 2008). Different light environments caused by the fall of trees and edges of the forest favors the coexistence of species with different light requirements (Chazdon et al. 1996). Shade-tolerant plants have low phenotypic plasticity in response to changes in the light environment, compared to sun-tolerant plants. Shade-tolerant plants show low photosynthetic and respiration rates and are slow growing, while light-demanding plants show higher photosynthetic and respiration rates and are fast growing (Chazdon and Smith 1996; Poorter and Arets 2003). In addition, the species are able to acclimatize physiologically to the seasonal climatic changes of the Tropical Mountain Forests (Chazdon et al. 1996). In ferns, interspecific differentiation in the response of the spore to the physical environment and tolerance to desiccation in the gametophyte has been associated with the preference of the habitat of the sporophyte (Pérez-García et al. 1982; Watkins et al. al. 2007; Testo and Watkins 2013; Riaño et al. 2015). Similarly, differences in distribution and physiological response between sporophytes of fern species that inhabit the same community support the hypothesis that the differentiation of niches promotes the coexistence of species (Hietz and Briones 1998; Arens and Sánchez-Baracaldo 1998; Durand and Goldstein 2001; Saldaña et al. 2010; Volkova et al. 2009, 2010; Bystriakova et al. 2011; Riaño and Briones 2013, 2015).
Several authors have made contributions to recognize functional groups in ferns. In the middle of the last century, the ferns of Singapore and Malaysia were divided according to the habitat where they grow in terrestrial sun , terrestrial shadows, rocks, banks of gullies, aquatic, and mountain species (Holtum 1954). The physiological behavior of terrestrial cloud forest ferns characterizes them as shade plants because they tend to show low photosynthetic and respiration rates and are slow growing (Chazdon and Smith 1996; Poorter and Arets 2003). It has been hypothesized that the tolerance of ferns to low light environments is due to the action of phytochrome PHY3 and neochrome, because both photoreceptors are sensitive to blue and red light (Kawai et al. 2003; Li et al. 2014). However, there are important differences between species of tropical terrestrial ferns in the distribution and degree of shade tolerance (Durand and Goldstein 2001; Jones et al. 2007; Bystriakova et al. 2011; Riaño and Briones 2013, but see Volkova et al. 2010). In a cloud forest where several species of ferns coexist, the arborescent fern Alsophila firma was abundant in gullies and humid sites, the arborescent Cyathea divergens and the herbaceous Blechnum schiedeanum occupied moderately open sites, while the arborescent Lophosoria quadripinnata and the herbaceous B. appendiculatum preferred more open sites (Riaño and Briones 2013; López-Romero et al. 2016).
With respect to humidity, epiphytic species have been grouped into poikilohydric and homohydric hygrophytes, mesophytic, and xerophytic that tolerate drought or evade it (Benzing 1990). In relation to light, the epiphytes have been categorized as exposed with total or almost total exposure to the sun, sun with growth in half shadow and shade-tolerant to deep shadow (Pittendrigh 1948; Benzing 1990). The epiphytic species in the cloud forest prefer different zones in the sporophyte. These zones are distinguished by their height, thickness, and inclination of the branches and composition of the substrate accumulated in the branches (Johansson 1974; Kelly 1985; Ter Steege and Cornelissen 1989; Hietz and Hietz-Seifert 1995; Hietz and Briones 1998; Rudolph et al. 1998). The distribution of the biomass of the epiphytic fern species in a cloud forest was unequal in the sporophyte and allowed to classify them based on their apparent luminance requirement (Hietz and Briones 1998). The deep shade-tolerant type composed of Trichomanes bucinatum and Asplenium cuspidatum showed 85–100% of the biomass confined to the base and bark of the trunks. The sun type or tolerant to medium shade formed by Elaphoglossum glaucum, E. petiolatum, Phlebodium areolatum, and Polypodium puberulum distributed 80–100% of its biomass in the upper trunk and branches thick >20 cm and medium thick >5 cm diameter. The exposure type was composed of Pleopeltis mexicana and Polypodium plebeium with 70–95% of its biomass on relatively thick branches of 5–20 cm and thin <5 cm in diameter (Hietz and Briones 1998).
4.3 Ecophysiological Tolerance of Ferns of the Cloud Forest
The spore, gametophyte, and sporophyte are the most important phases of the life cycle of the ferns (Sharpe and Mehltreter 2010). The spore is a haploid cell dispersed by the wind, but due to its small size (25–50 μm), it can remain in the pores of the soil and keep dormant forming a bank of spores (Pérez-García et al. 1982; Sharpe and Mehltreter 2010). The gametophyte is a laminar and tiny plant with rootlets, without vascular tissue or stomata, has little capacity to store water, and can be poikilohydric but requires water for fertilization, while the sporophyte is a conspicuous perennial plant, with vascular tissue, rhizome, roots, and leaves with stomata and sori (Farrar et al. 2008; Watkins et al. 2010). Due to these differences, the sporophyte and gametophyte can have independent environmental tolerances and optimally succeed in different habitats (Warne and Lloyd 1980). The physiological limitations of the ferns determine that many species grow under the shade of the canopy or in humid places of the cloud forest (Hietz and Briones 1998; Riaño and Briones 2013; López-Romero et al. 2016). However, ferns are physiologically competitive and have a large number of adaptive characters resistant to abiotic stress, so that they can colonize shady sites, as well as open areas and can survive in flooded and degraded lands (Hietz and Briones 2004; Hietz 2010; Mehltreter et al. 2010; Watkins et al. 2010).
4.3.1 Ecophysiological Tolerance of the Spore Germination
Light and water are the resources that mostly limit the germination of the fern spores, and their success in germinating has an important influence on the distribution of the species. The spores of species whose sporophytes grow in shade environments germinate better in conditions similar to the interior of the forest, such as lower temperature, low light, and high availability of water, while those that are distributed in open or exposed environments germinate in higher values of temperature, light, and water availability (Warne and Lloyd 1980; Pérez-García and Riba 1982; Riaño et al. 2015; López-Romero et al. 2016). The light promotes the germination of the spores of the ferns through a system of phytochromes . Phytochrome-dependent germination can be photoreversible with a far-red irradiation and can be photoreversible by brief irradiation with blue or UV light (Raghavan 1989). With the phytochrome system, the spore detects light in the gaps forest and the proximity to the soil surface, which allows the spores to germinate under favorable conditions for the growth of the gametophyte and sporophyte (Pérez-García et al. 2007). No spores germinated under far-red light or darkness; but germination was high under white light in four species of tropical ferns (Pérez-García et al. 2007). The temperature for the germination of fern spores ranges between 15 and 30 °C, and the optimum germination temperature of five arborescent species was 25 °C (Pérez-García and Riba 1982). Lophosoria quadripinnata and Trichipteris bicrenata had the highest tolerance to extreme temperatures in a thermal gradient of 11–35 °C, while Trichipteris scabriuscula had the lowest tolerance; Nephelea mexicana and Cyathea fulva had an intermediate tolerance (Pérez-García and Riba 1982). The spores of Alsophila firma, Lophosoria quadripinnata, and Cyathea divergens seeded at 13 °C, 21 °C, and 25 °C increased germination with the increase in temperature, but L. quadripinnata had higher thermal tolerance compared to A. firma and C. divergens (Salvador 2014). In six ornamental fern species, it was found that the best substrate for germination was the mixture of peat pH 6 and perlite, reaching 100% germination of Cyathea cooperi, Pteris cretica, and P. incompleta, but with mixture of peat pH 4 and pearlite germination decreased significantly in Cyathea cooperi, C. contaminans, and C. tsangii, and the lowest germination percentages of all species , including Asplenium nidus, were obtained in sand substrate (Barros et al. 2008). In Dryopteris munchii, twice as many gametophytes were obtained in spores germinated in soils derived from volcanic toba, in comparison with those obtained in chromic luvisol soil (Jaramillo and Mendoza 2004).
The differences observed in the frequency of individuals in the understory and the correspondence of the spore’s physiological behavior with respect to water availability support the hypothesis that the coexistence of five sympatric species in a cloud forest occurs by differentiation in the ecological niche from early stages of the individual’s development (Riaño et al. 2015; López-Romero et al. 2016). The germination of the spore of the herbaceous species Blechnum appendiculatum and B. schiedeanum and the arborescent tree ferns Alsophila firma, Lophosoria quadripinnata, and Cyathea divergens was controlled by exposure to light and water availability. The spores of all the species did not germinate under darkness, and the germination was smaller with far-red light, compared with white light. All species germinated at very low photon flux density (0.04 μmol m−2 s−1) and increased germination with the amount of light received, but the saturation of germination was different between species. The decrease in available water from 0 to −0.7 MPa reduced germination in all species, and the water requirements for germination were associated with the habitat preference where sporophytes grow. The increase in canopy opening from 0.6% to 19.1% decreased the germination capacity of the three species of tree ferns, but L. quadripinnata was the least affected (Riaño et al. 2015).
4.3.2 Ecophysiological Tolerance of the Gametophyte
The gametophyte of a fern may have a broader environmental tolerance than its sporophyte and is physiologically similar to the gametophyte of a bryophyte (Ong et al. 1998; Farrar et al. 2008). The distribution of fern gametophytes depends on multiple environmental conditions and resources, and disturbance is also important (Farrar et al. 2008). In sites with low light and no disturbance in a humid forest, no gametophytes were established, while in high light sites, the density of gametophytes was increased by the removal of the litter and when the topsoil layer was removed and exposed (Watkins et al. 2007). The gametophytes of the arborescent ferns Alsophila firma, Cyathea divergens, and Lophosoria quadripinnata when they grew with 42% and 85% solar radiation in shade houses and with 7% and 23% of solar radiation in the understory of a cloud forest decreased the quantum yield (proportion of the light used in photosynthesis) and increased the electron transport rate of photosynthesis in treatments with higher solar radiation; however, solar radiation decreased the survival of the three species, and L. quadripinnata had higher survival in comparison with A. firma and C. divergens in the shade house with higher solar radiation (Salvador 2014). Gametophytes of the same three species subjected to>96% RH did not reduce the quantum yield, but it decreased strongly to 69% and 35% of relative humidity and gametophytes tolerated direct solar radiation up to 30 min (Riaño and Briones 2015). The high dependence of gametophytes from tree ferns to humid environments could explain why these species, and possibly other species with similar life histories, are restricted to humid forests (Bystriakova et al. 2011; Ramírez-Barahona et al. 2011; Riaño and Briones 2015).
In comparison with the sporophyte , there have been very few studies on the photochemistry and carbon metabolism in the gametophyte. The CO2 assimilation rate of the gametophytes of four fern species (of the genera Cibotium, Todea, Pyrrosia, and Thelypteris) ranged between 0.03 and 0.18 μmol CO2 g−1 s−1 (Farrar et al. 2008). Photosynthetic rate of the gametophyte could be compared with that of a young sporophyte in an epiphytic fern, obtaining values of 0.18 and 0.14 μmol CO2 g−1 s−1, respectively (Sakamaki and Ino 1999). The gametophyte of 15 fern species recovered their physiological functioning after dehydration, and the capacity for recovery was variable among the species (Farrar et al. 2008). The quantum yield of the gametophyte of an epiphytic fern species that grew in exposed environments recovered almost completely after 24 h of desiccation, while it decreased strongly in the same period of desiccation in another species of terrestrial fern that inhabited shaded sites (Farrar et al. 2008). The gametophytes of the tropical epiphytic ferns Phlebodium pseudoaureum and Microgramma reptans recovered the initial values of quantum yield after exposure to treatments of low relative humidity ; however, the gametophytes of Diplazium striatastrum, a terrestrial species that grows in humid environments within the forest, were unable to recover their quantum yield values (Watkins et al. 2007). The gametophyte of Asplenium scolopendrium var. americanum, a rare species with low abundance in a Temperate Forest, could not tolerate a relative humidity <90%, in comparison with other species of the same genus and common in the same forest that presented physiological tolerance to desiccation (Testo and Watkins 2013). These authors attributed interspecific differences in relative humidity tolerance to habitat preference among species. The gametophytes of Asplenium trichomanes, A. scolopendrium, and Ceterach officinarum showed similar capacity to acclimate their photosynthetic apparatus to the increase of the quantity of light modifying the content of photoprotective pigments (Fernández-Marín et al. 2012). The pigments of the gametophytes of these three species were not qualitatively different from those of the sporophytes of seven species of epiphytic ferns (Tauz et al. 2001).
4.3.3 Ecophysiological Tolerance of the Sporophyte
The sporophyte leaf of ferns behaves like the leaf of other vascular plants (Hietz and Briones 1998; Watkins et al. 2010). The cuticle and stomata of the leaves of the sporophytes help them to avoid the loss of water, but the low capacity to drive and store water causes restrictions to the transpiration and carbon gain, in comparison with the angiosperms (Woodhouse and Nobel 1982; Watkins et al. 2010).
Chlorophyll fluorescence data show that the sporophyte leaf possesses quantum yield values and electron transport rates similar to the other vascular plants (Maxwell and Johnson 2000). The maximum quantum yield or predawn yield of the arborescent cloud forest ferns is at the optimum value of most non-stressed plants and is similar to the arborescent ferns of humid forests, non-arborescent terrestrial ferns of tropical and humid forests, and epiphytic ferns of tropical forests (Durand and Goldstein 2001; Zhang et al. 2009; Huang et al. 2011), but it is slightly larger than that of arborescent ferns of sclerophyllous moist forests (Volkova et al. 2010). On the other hand, the maximum quantum yield of the unstressed fronds of the epiphytic ferns of a cloud forest was scarcely less than that of tree ferns coexisting in the same forest and of epiphytic ferns in a tropical rainforest (Zhang et al. 2009). The apparent maximum rate of electron transport (ETRmax) of the tree ferns Alsophila firma, Cyathea divergens, and Lophosoria quadripinnata of a cloud forest was low compared to species of environments with high light availability, such as semi-deciduous rainforests and sandy coasts (Geβer et al. 2005, 2008; Riaño and Briones 2013) and sun ferns (Wong et al. 2012); however, they showed similar values to the highest values of ETR or ETRmax of arborescent and herbaceous ferns of the temperate rainforest and temperate evergreen forest (Bystriskova et al. 2011; Saldaña et al. 2010; Wong et al. 2012). The ETR light saturation values of the tree ferns Alsophila firma, Cyathea divergens, and Lophosoria quadripinnata of a cloud forest were lower than those of sunny environments (Geβer et al. 2005, 2008; Riaño y Briones 2013) but similar to those of three species of the genus Blechnum growing in an open canopy in a temperate evergreen forest (Saldaña et al. 2010).
The CO2 assimilation rate of sporophytes ranges between 0.6 and 15 μmol m−2 s−1, which is similar to that of vascular shade plants (Hietz and Briones 2004; Hietz 2010; Mehltreter et al. 2010). The maximum photosynthesis (Amax) of three tree fern species of a cloud forest was higher than Amax of the moss, similar to that of other terrestrial fern species occupying closed sites and shaded leaves of deciduous trees, and less than that of tree ferns of the humid forest, epiphytes of exposed sites, and tropical and herbaceous trees C3 and C4 (Durand and Goldstein 2001; Larcher 2003; Hietz and Briones 2004; Lüttge 2008; Volkova et al. 2010; Riaño and Briones 2013). The Amax of the epiphytic ferns of the deep shadow group of a cloud forest was similar to that of mosses, shadow epiphytes, and terrestrial ferns of closed sites, while the Amax of the epiphytic ferns of the exposure group was similar to other epiphytes of the same group and the Amax of the epiphytic ferns of the exposure and tolerant groups was similar to the Amax of terrestrial ferns of closed sites and shade leaves of deciduous and tropical trees (Hietz and Briones 2001). Zhang et al. (2009) also found no differences in Amax between two species of epiphytic ferns and two species of terrestrial ferns in a tropical rainforest. Riaño and Briones (2013) showed that the density of photosynthetic flux to saturation of photosynthesis (PPFDsatA) of tree ferns Alsophila firma, Cyathea divergens, and Lophosoria quadripinnata of a cloud forest growing in a closed site was similar to that of shade plants, other terrestrial and arborescent ferns of closed sites, and shadow epiphytes , indicating the efficiency of tree ferns to absorb the scarce available light and assimilate CO2 in shaded habitats by the canopy (Durand and Goldstein 2001). The PPFDsatA of the epiphytic ferns of a cloud forest was higher than that of shade plants and terrestrial ferns of closed sites, but the light compensation point (LCP) of the epiphytic ferns was similar to that of both groups (Hietz and Briones 2001). Despite their low photosynthetic rate, possibly the result of a deficiency of nutrients, the high PPFDsatA values of the epiphytic ferns of the cloud forest allow them to take advantage of high amounts of light in the hours or areas with high light in the canopy. On the other hand, the low LCP values of the epiphytic ferns make it easier for them to assimilate carbon during the frequent cloudy days or when they grow in shaded places in the canopy forest (Briones and Hietz 2001). Three herbaceous ferns of the genus Blechnum of a temperate forest that differ in the range of the light environment that they occupy in the undergrowth adjusted the leaf area in correspondence with the habitat that they occupied, being more plastic the species with greater ecological amplitude, but they did not show differences in the rates of CO2 assimilation and respiration in the dark (Saldaña et al. 2010). In a New Zealand tropical forest, two tree ferns with a fast height growth rate of the genus Cyathea had high electron transport rates , in comparison with two species of the same genus and Dicksonia squarrosa (Bystriakova et al. 2011).
Like other plant species, the arborescent ferns of the cloud forest respond to the decrease in water availability by decreasing their water potential, possibly due to the increase in transpiration and/or decrease in water conduction by the stems, and their leaves thickening in the dry season (Riaño and Briones 2013). The decrease of the hydric state of the arborescent ferns of the cloud forest can be caused by the relatively high temperature and evaporative demand of the air during the dry season, in addition to the decrease of the hydric potential of the soil. However, even in the dry season, the soil water potential of a cloud forest can remain high, between −0.01 and −0.26 MPa. The decrease in water potential of the arborescent ferns Dicksonia antarctica and Cyathea australis of a sclerophyllous wet forest in response to the sudden exposure of high levels of light was not associated with changes in water availability, but probably with an increase in transpiration due to the increase in leaf temperature and possible decrease in water conduction (Volkova et al. 2009). The epiphytic ferns of a cloud forest that tolerate deep shade and grow at the base of the trunks showed no obvious adaptations to cope with drought (Hietz and Briones 1998). The leaves of Trichomanes bucinatum were completely dried after a few hours in moderately dry air, and the low ability to contain water caused the quantum yield to decrease faster compared to the epiphytes that grow in more exposed sites (Hietz and Briones 2001). Despite losing more than 90% of the relative water content, the quantum yield of T. buccinatum was able to return to half its capacity in less than 5 min and up to 90% after 1 h. Most of the plants that inhabit the canopy show adaptations to the xeric environment (Benzing 1990). The shade-tolerant and exposure-tolerant epiphytic ferns of a cloud forest showed leathery leaves , succulent rhizomes, low rates of uncontrolled water loss, leaf scales, and high cellular elasticity (Hietz and Briones 1998). The flow of water from the atmosphere to the leaves could be an important source of water for cloud forest ferns, but the cuticle of the leaves being very efficient for the control of water loss could be limiting for the absorption of liquid water or water vapor. Both the epiphytic ferns of the tolerant type and the exposure type of the cloud forest showed values of total water potential similar to those of the epiphytic bromeliads (Hietz and Briones 1998). The epiphytic ferns of the exposure type of a cloud forest diminished their values of total water potential in response to the drought, in comparison with the ferns of the tolerant shade type (Hietz and Briones 1998). Most vascular plants close their stomata before loss of cell turgor with increasing drought ; however, the epiphytic ferns of the exposure group of a cloud forest had the capacity to tolerate the loss of water beyond the point of turgor loss before the stomatal closure (Hietz and Briones 1998).
In conclusion, the spores of the ferns have mechanisms to detect the environment and initiate germination when conditions are favorable in the cloud forest. The specialization to humid habitats of the gametophyte of tree ferns possibly restricts the distribution of that group to humid places. The physiological tolerance of the gametophytes of the epiphytic ferns of the cloud forest is practically unknown. The differences in the plasticity and relatively high physiological tolerance of the sporophyte facilitate the coexistence of the fern species in the cloud forest. The physiological performance as shade plants of terrestrial and epiphytes ferns makes them vulnerable to fragmentation and changes in the use of the land in the cloud forest.
5 Functional Diversity in the Tropical Rainforest: Piper, a Case Study
The tropical rainforest (TRF) is one of the most complex ecosystems and supports the highest biodiversity and productivity on earth. This forest is found along the equatorial region, where climate is more stable, hot, and wet. In Mexico, TRF has an extension of 91, 566 km2 (4.7%), representing 45% of its original surface. Since 1960 its importance as a natural resource and the high rate of fragmentation and decrease of its original surface raised the necessity to study the biodiversity and the dynamics of change and regeneration of the TRF as the basis for restoration and conservation alternatives.
Natural disturbance , produced by gaps formation, and succession of TRF are dynamic processes in which spatial and temporal variability of the light environment plays a fundamental role, becoming a selective factor for plant attributes of these communities. To understand these processes, in 1970, Gómez-Pompa et al. initiated a series of ecological and ecophysiological pioneer studies of TRF plants at “Los Tuxtlas ,” a biological reserve in Veracruz, Mexico, and proposed a model to study its dynamics and to establish the scientific basis for future conservation (Gomez-Pompa et al. 1972). The theoretical framework of the research was published in the paper “The tropical rainforest: a non-renewable resource ” published in 1972. The approach to fulfill their goal included ecological and physiological studies of species with differential distribution in diverse successional stages. Considering the high biodiversity of the TRF, they selected a group of species of the genus Piper as a model system to understand functional diversity and its ecological importance (Gómez-Pompa et al. 1976; Gómez Pompa and del Amo 1985; Field and Vázquez-Yanes 1993).
Piper species have a worldwide distribution, containing a high number of species. At “Los Tuxtlas” reserve, Piper has 12 species differentially distributed in habitats with variable light availability and successional stages (Gómez-Pompa 1971; Fig. 23.3), becoming a good system to study the mechanisms underlying the distribution of TRF species, as well as the ecological and evolutionary meaning of physiological and morphological responses of plants to light (Gómez-Pompa et al. 1971; Field and Vázquez-Yanes 1993). The studies initiated addressing the ecophysiology of germination and further were diversified to several topics including seedlings survival, establishment, photosynthesis, mineral nutrition, and growth (Vázquez-Yanes 1976, 1985; Field and Vázquez-Yanes 1993). These studies generated a great interest for ecophysiological studies of the TRF plants in Mexico and other countries (Denslow et al. 1990; Field and Vázquez-Yanes 1993; Nicotra et al. 1997). Furthermore the studies provide the basis for understanding the mechanisms of TRF species for adaptation to different rainforest habitats and their relation with the species distribution and role in ecological succession.
Temporal and spatial distribution of nine Piper species of the tropical rainforest at Los Tuxtlas, Veracruz, Mexico. The bar indicates the amplitude of distribution of the species under different light environments along time and gap size axis. Species are indicated by numbers: (1) and (2) Piper umbellatum and P. peltata, (3) P. auritum, (4) P. aduncum, (5) and (6) P. hispidum and related forms of the species, (7) P. amalago, (8) P. lapathifolium, and (9) P. aequale. (Figure modified from Gómez-Pompa and Vázquez-Yanes 1985)
5.1 Physiology of Germination
Tropical rainforest pioneer species produce small seeds that may persist in the rainforest floor, until a canopy disturbance originates a gap and favorable conditions for their germination and establishment. The studies of germination of Piper, and other pioneer species from Los Tuxtlas Veracruz, in response to light and temperature clearly establish the role of light quality and temperature on the control of germination of photoblastic species (Vázquez-Yanes and Smith 1982). The studies have also provided a better understanding of the mechanisms of light use and acclimation in TRF plants, as well as its importance for species distribution in different successional stages (Vázquez-Yanes and Orozco- Segovia 1987, 1990).
Piper species produce a large number of small seeds with specific light requirements for germination. The seeds are nondormant after dispersal, and under appropriate light, temperature and humidity conditions may germinate immediately. However, if seeds are dispersed in the rainforest floor or buried in the soil, germination is inhibited by enforced dormancy and remains viable for more than 1 year, until light and/or temperature conditions trigger germination (Orozco-Segovia and Vázquez-Yanes 1990; for a review see Vázquez-Yanes and Orozco-Segovia 1993). Photoblastic seeds sense the light environment through phytochrome pigments, which function as a signal-transducing photoreceptor of light quality. Phytochrome has two forms: an active form (Pfr) and one inactive (Pr) which are interconverted by the amount of red and far-red light in the environment (R/FR ratio); this ratio increase when canopy opens, triggering seed germination, and modulating development and growth of plants (Vázquez-Yanes and Smith 1982; Orozco-Segovia and Vázquez-Yanes 1989).
In mature forest, when the canopy opens by natural or anthropogenic disturbance, changes in the daily temperature alternation in the soil and changes in the amount and quality of the light reaching the ground are detected by seeds. The fine perception sensing these changes in their surroundings allows the seeds to recognize the arrival of specific favorable conditions for germination and establishment (Vázquez-Yanes 1976; Vázquez-Yanes and Orozco-Segovia 1993).
Although for germination and establishment all Piper species are triggered by light, seeds of pioneer trees common in secondary forest such as Piper auritum, P. sanctum, P. aduncum, P. marginatum, P. umbellatum, and P. aff. yzabalanum require large gaps for dormancy break and germination. On the other hand, P. lapathifolium, P. nitidum, and P. amalago, species common in mature forest, have partial photoblastism, larger seeds, and short viability. Piper hispidum is the more plastic species; its seeds germinate in a wide range of light conditions, from shade of the understory to secondary forest, and it can be found in all these successional stages (Vázquez-Yanes and Orozco-Segovia 1982; Orozco-Segovia and Vázquez-Yanes 1989).
Piper seedlings growth rates also reflect their differential ability to develop under contrasting light conditions. A study on growth responses of P. aequale, P. auritum, and P. hispidum seedlings to contrasting natural and experimental light environments reveals that P. auritum had the best growth response under high light; P. aequale grew better at intermediate light levels, whereas P. hispidum showed more plasticity and grew well along the whole range of light conditions (Sanchez-Coronado et al. 1990). This study reveals how the species have a different degree of adaptation in physiological traits to germinate and grow in gaps of different size.
5.2 Photosynthesis and Light Environment
The light environment of the TRF is characterized by high spatial and temporal variability. The light varies from full sun in large gaps, open areas, and top of the canopy to less than 5% of full sun in the understory; spatially, light levels also varied according to gaps size. Although understory is characterized by low light conditions, short periods of direct light (sunflecks) that pass through the canopy openings reach the plants, making their light environment highly dynamic with a widely temporal variation, from few seconds to minutes; sunflecks also present a high variation in peak photon flux density (Chazdon 1988).
Photosynthetic capacity among Piper species varied according to their ability to grow under different light conditions (Walters and Field 1987; Chazdon et al. 1989). Large gap species show leaves with the highest photosynthetic capacity, taking advantage of the high light found in these environments; this capacity is combined with high leaf area, high nitrogen content, and short leaf life span (<1 year). On the other hand, understory species have leaves with the lowest photosynthetic capacity, small area, lower nitrogen content, and longevity of 3–5 years (Chazdon et al. 1989, Table 23.3). Differences among species in the way they gain and invest their resources allow Piper species to fall in a continuum between photosynthetic maximization on one side and cost maximization on the other (Field and Vázquez-Yanes 1993). P. hispidum, one of the species with wider distribution in different light conditions, produced leaves with photosynthetic and cost features nearly along the entire range; leaf longevity also showed plasticity lasting from 6 months to 2 years (Field and Vázquez-Yanes 1993).
Sunflecks represent an important resource for understory plants, as they provide 10–80% of the total photon flux density available for photosynthesis (Chazdon 1988). For Adenocaulon bicolor, an understory herb, 30–70% of the total daily carbon gain occurs during sunflecks (Pfitsch and Pearcy 1989). Therefore the capacity of understory plants to utilize this resource may have a strong influence on their growth and reproduction (Pfitsch and Pearcy 1989).
The ability of plants to utilize sunflecks depends, at a physiological time scale, on the photosynthetic induction state, a process determined by the response dynamics of photosynthetic enzymes and stomatal opening. The completion of the induction process may last 20 min under continuous high light, but it may be able to be attained under dynamic light conditions (Pearcy 1990). When a sunfleck arrives at the leaf surface of an understory plant, an increase in CO2 assimilation occurs, and it continues for at least 1 min after the sunfleck has passed as post illumination CO2 fixation; the state of induction before the sunfleck determines the maximum assimilation rate during the sunfleck (Pearcy 1990).
The role of dynamic stomatal and biochemical responses to variable light on carbon gain was studied in two Piper species with contrasting distribution: the pioneer tree, P. auritum, and a shade-tolerant shrub, P. aequale. The species where acclimated to high and low light to determine the influence of photosynthetic acclimation on dynamic photosynthetic responses.
Results showed that acclimation to contrasting light environments had a significant effect on stomatal response dynamics; for both species, the integrated stomatal conductance (gs) response to lightflecks was smaller when plants were acclimated to a light regime different to its natural environments (Tinoco-Ojanguren and Pearcy 1992, Fig. 23.4).
The steady-state and the dynamic responses of CO2 assimilation (A) and gs to light (light curves and lightflecks, respectively) showed contrasting results; for shade-acclimated plants, the steady-state responses were identical; in contrast, the response to lightflecks was significantly different (Tinoco-Ojanguren and Pearcy 1992). The shade-tolerant species P. aequale showed larger and faster gs response than the gap species P. auritum. The response of P. aequale allowed this species to keep higher gs and intercellular CO2 concentration during a series of consecutive lightflecks and therefore improves assimilation by 30–200%, depending on the sunfleck duration. In comparison, the gap species presented a small stomatal response to lightflecks and no improvement in C gain. In contrast for high light-acclimated plants, gs response to lightflecks was greater in P. auritum than P. aequale.
Vapor pressure deficit (VPD) had a significant effect on the stomatal response to lightflecks. Increases in VPD had decreased the magnitude and dynamics of the response, exhibiting a more conservative behavior in terms of water loss, at the expenses of carbon gain. The effect was different among species and between sun- and shade-acclimated plants. Sun plants of P. auritum were more sensitive to VPD (Tinoco-Ojanguren and Pearcy 1993). These results suggest a possible adaptive significance of the stomatal response for the species studied. Shade-acclimated plants of P. aequale showed a stomatal response that would be expected for a species that maximize C gain in an environment where light is the main limiting factor. Sun acclimated plants of P. auritum had a stomatal behavior that would be expected for a species that maximizes C gain and water loss in an environment where light is not limiting but water stress may be an important limitation.
6 Implications of Functional Diversity in Conservation
The case studies presented in this chapter are just an example of plant functional diversity found at different phases of their life cycle and at different complexity levels. There is still the necessity for increasing our knowledge to predict how climatic change, fragmentation, land use change, introduction of exotic plants, and other anthropogenic disturbances are affecting most species directly or indirectly, as many biotic interactions are affected by these disturbances. The importance of knowledge on functional traits for conservation issues has led to propose a research discipline: conservation physiology, which has been defined in a broad sense as a scientific discipline that encompasses functions and mechanisms at all scales (from molecules to biosphere) and their responses to environmental changes; it also includes the development of strategies to understand, manage, and restore populations and ecosystems (Cooke et al. 2013). This definition assumes that for real advancements in conservation and resources management, this discipline must also be integrative with other disciplines. In a rapidly changing world, efforts should be focused to the responses of plant species (including invasive species) to environmental changes to develop models for well-characterized functional diversity for management and restoration proposals , especially for keystone species, vulnerable species such as epiphytes, rare and endemic species, and some seedlings that require host trees and nurses.
Mexico has provided to the world several globally important cultivated plants such as maize, beans, cacao, prickle cactus, and tomato, which are the result of selection from its high plant diversity, together with the empirical botanical knowledge of its inhabitants. Presently, there is a pressing need to understand physiological mechanisms that led this diversity of cultivated plants and their ancestors to persist and an urgency to implement management practices for its conservation.
Plant physiological research in Mexico is helping to explain questions of interest at various scales, from molecules (Vargas-Soto et al. 2009; Ricalde et al. 2010; de la Rosa-Manzano et al. 2015; Santiago et al. 2017) to whole plant processes (Cervera et al. 2006, 2007; Riaño and Briones 2013; Riaño-Ospina et al. 2015), to communities and ecosystems (Reyes-García 2012; Cach-Pérez et al. 2013; Chilpa-Galván et al. 2013; Méndez-Barroso et al. 2014; Sanaphre-Villanueva et al. 2017), for functional diversity of seeds and its application to restoration (Orozco–Segovia and Sanchez– Coronado 2009).
References
Adams WW III, Zarter CR, Mueh KE, Amiard VSE, Demmig-Adams B (2008) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, WWIII A, Matto A (eds) Photoprotection, photoinhibition, gene regulation and environment. Springer, Berlin, pp 49–64
Andrade JL (2003) Dew deposition on epiphytic bromeliad leaves: an important event in a Mexican tropical dry deciduous forest. J Trop Ecol 19:479–488
Andrade JL, Rengifo E, Ricalde MF, Simá JL, Cervera JC, Vargas-Soto G (2006) Microambientes de luz, crecimiento y fotosíntesis de la pitahaya (Hylocereus undatus) en un agrosistema de Yucatán, México. Agrociencia 40:687–697
Andrade JL, de la Barrera E, Reyes-García C, Ricalde MF, Vargas-Soto G, Cervera JC (2007) El metabolismo ácido de las crasuláceas: diversidad, fisiología ambiental y productividad. Bol Soc Bot Mex 81:37–51
Andrade JL, Cervera JC, Graham EA (2009) Microenvironments, water relations and productivity of CAM plants. In: de la Barrera E, Smith WK (eds) Perspectives in biophysical plant ecophysiology: a tribute to Park S Nobel. Universidad Nacional Autónoma de México, Mexico, pp 95–120
Archer SR, Predick KI (2008) Climate change and ecosystems of the Southwestern United States. Rangelands 30:23–28
Archer S, Schimel DS, Holland EA (1995) Mechanisms of shrubland expansion: land use, climate or CO2? Clim Chang 29:91–99
Arens NC, Sanchez-Baracaldo PS (1998) Distribution of tree ferns (Cyatheaceae) across the succesional mosaic in an Andean cloud forest, Nariño, Colombia. Am Fern J 88:60–71
Barros A, Salinero C, Vela P, Sainz MJ (2008) Método rápido para la propagación de helechos ornamentales. Actas Hortic 52:1–5
Benzing DH (1990) Vascular epiphytes general biology and related biota. Cambridge University Press, Cambridge
Bravo-Peña LC, Doode-Matsumoto S, Castellanos-Villegas AE, Espejel-Carbajal I (2010) Políticas rurales y pérdida de cobertura vegetal Elementos para reformular instrumentos de fomento agropecuario relacionados con la apertura de praderas ganaderas. Región Soc 22:3–35 doi:1870–3925
Brenner JC (2011) Pasture conversion, private ranchers, and the invasive exotic Buffelgrass (Pennisetum ciliare) in Mexico’s Sonoran Desert. Ann Assoc Am Geogr 101:84–106. https://doi.org/10.1080/000456082010518040
Buffington LC, Herbel CH (1965) Vegetational changes on a semidesert grassland range from 1958 to 1963. Ecol Monogr 35:139–164
Bystriakova N, Bader A, Coomes D (2011) Long-term fern dynamics linked to disturbance and shade tolerance. J of Veg Sci 22: 72–44
Cach-Pérez MJ, Andrade JL, Chilpa-Galván N, Tamayo-Chim M, Orellana R, Reyes-García C (2013) Climatic and structural factors influencing epiphytic bromeliad community assemblage along a gradient of water-limited environments in the Yucatan Peninsula, Mexico. Trop Conserv Sci 6:283–302
Cach-Pérez MJ, Andrade JL, Cetzal-Ix W, Reyes-García C (2016) Environmental influence on inter- and intraspecific variation in density and morphology of stomata and trichomes of epiphytic bromeliads of the Yucatan Peninsula. Bot J Linn Soc 181:441–458
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087
Callaway RM, Kikodze D, Chiboshvili M, Khetsuriani L (2005) Unpalatable plants protect neighbors from grazing and increase plant community diversity. Ecology 86:1856–1862
Carmona CP, de Bello F, Mason NWH, Lepš L (2018) Traits without borders: integrating functional diversity across scales. Trends Ecol Evol 31:382–394. https://doi.org/10.1016/j.tree.2016.02.003
Castellanos AE (1991) Photosynthesis and gas exchange of vines. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 171–214
Castellanos AE, Tinoco-Ojanguren C, Molina-Freaner FE (1999) Microenvironmental heterogeneity and space utilization by desert vines within their host trees. Ann Bot 84:145–153
Castellanos AE, Yanes G, Valdéz-Zamudio D (2002) Drought – tolerant exotic buffel – grass and desertification. In: Tellman B (ed) Weeds across borders. Arizona-Sonora Desert Museum, Tucson
Castellanos AE, Bravo LC, Koch GW, Llano JM, Lopez D, Mendez R, Rodriguez JC, Romo JR, Sisk T, Yanes G (2010) Impactos Ecologicos por el Uso del Terreno en el Funcionamiento de Ecosistemas Aridos Semi-Aridos de Sonora. In: Molina-Freaner F, Van Devender TR (eds) Diversidad Biologica del Estado de Sonora. CONABIO – UNAM, México, pp 157–186
Castellanos AE, Celaya-Michel H, Rodríguez JC, Wilcox BP (2016) Ecohydrological changes in semiarid ecosystems transformed from Shrubland to Buffelgrass savanna. Ecohydrology 9:1663–1674. https://doi.org/10.1002/eco1756
Celaya-Michel H, Castellanos-Villegas AE (2011) Mineralización de nitrógeno en el suelo de zonas áridas y semiárida. Terra Latinoam 29:343–356
Celaya-Michel H, García-Oliva F, Rodríguez JC, Castellanos-Villegas AE (2015) Cambios en el almacenamiento de nitrógeno y agua en el suelo de un matorral desértico transformado a sabana de buffel (Pennisetum ciliare (L) Link). Terra Latinoam 33:79–93
Cervantes S, Graham E, Andrade JL (2005) Light microhabitats, growth and photosynthesis of an epiphytic bromeliad in a tropical dry forest. Plant Ecol 179:107–118
Cervera JC, Andrade JL, Simá JL, Graham EA (2006) Microhabitats, germination, and establishment for Mammillaria gaumeri (Cactaceae), a rare species from Yucatan. Int J Plant Sci 167:311–319
Cervera JC, Andrade JL, Graham EA, Durán R, Jackson PC, Simá JL (2007) Photosynthesis and optimal light microhabitats for a rare cactus, Mammillaria gaumeri, in two tropical ecosystem. Biotropica 39:620–627
Chapin FS (2003) Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann Bot 91:455–463
Chazdon R (1988) Sunflecks and their importance to forest understory plants. Adv Ecol Res 18:1–63
Chazdon RL, Field CB (1987) Determinants of photosynthetic capacity in six rainforest Piper species. Oecologia 73:222–230
Chazdon RI, William K, Field CB (1989) Interactions between crown structure and light environments in five rain forest Piper species. Am J Bot 75:1459–1475
Chazdon RL, Smith AP (1996) Tropical Forest Plant Ecophysiology. Chapman & Hall. New York.
Chazdon RL, Pearcy RW, Lee DW, Fetcher N (1996) Photosynthetic response of tropical forests plants to contrasting light environments. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman & Hall, New York, pp 5–55
Chilpa-Galván N, Tamayo-Chim M, Andrade JL, Reyes-García C (2013) Water table depth may influence the asymmetric arrangement of epiphytic bromeliads in a tropical dry forest. Plant Ecol 214:1037–1048
Chilpa-Galván N, Zotz G, Sánchez-Fuente GJ, Espadas-Manrique C, Andrade JL, Reyes-García C (2017) Drought, post-dispersal seed predation, and the establishment of epiphytic bromeliads (Tillandsia spp). Biotropica 49:770–773
Cornell S, Rendell A, Jickells T (1995) Atmospheric inputs of dissolved organic nitrogen to the oceans. Nature 376:243–246
Cornellisen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan D, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–338
Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Con Physiol 1:10.1093/conphys/cot001
Crausbay SD, Martin PH (2016) Natural disturbance, vegetation patterns and ecological dynamics in tropical montane forests. J Trop Ecol 32:1–20
de la Barrera E, Andrade JL (2005) Challenges to plant megadiversity: how environmental physiology can help? New Phytol 167:5–8
de la Rosa-Manzano E, Andrade JL, Zotz G, Reyes-Garcia C (2014a) Epiphytic orchids in tropical dry forests of Yucatan, Mexico – species occurrence, abundance and correlations with host tree characteristics and environmental conditions. Flora 209:100–109
de la Rosa-Manzano E, Andrade JL, Zotz G, Reyes-García C (2014b) Respuestas fisiológicas a la sequía de cinco especies de orquídeas epífitas en dos selvas secas de la Península de Yucatán. Bot Sci 92:607–616
de la Rosa-Manzano E, Andrade JL, García-Mendoza E, Zotz G, Reyes-García C (2015) Photoprotection related to xanthophyll cycle pigments in epiphytic orchids acclimated at different light microenvironments in two tropical dry forests of the Yucatan Peninsula, Mexico. Planta 242:1425–1438
de la Rosa-Manzano E, Andrade JL, Zotz G, Reyes-García C (2017) Physiological plasticity of epiphytic orchids from two contrasting tropical dry forests. Acta Oecol 85:25–32
Delgado-Baquerizo M, Maestre FT, Gallardo A, Bowker MA, Wallenstein MD, Quero JL, Ochoa V, Gonzalo B, García-Gómez M, Soliveres S, García-Palacios P, Berdugo M, Valencia E, Escolar C, Arredondo T, Barraza-Zepeda C, Bran D, Carreira JA, Chaieb M, Conceição AA, Derak M, Eldridge DJ, Escudero A, Espinosa CI, Gaitán J, Gatica MG, Gómez-González S, Guzman E, Gutiérrez JR, Florentino A, Hepper E, Hernández RM, Huber-Sannwald E, Jankju M, Liu J, Mau RL, Miriti M, Monerris J, Naseri K, Noumi Z, Polo V, Prina A, Pucheta E, Ramírez E, Ramírez-Collantes DA, Romão R, Tighe M, Torres D, Torres-Díaz C, Ungar ED, Val J, Wamiti W, Wang D, Zaady E (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502:672–676
Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Denslow SJ, Schultz JC, Vitousek PM (1990) Growth responses of tropical shrubs to treefall gap environments. Ecology 71:165–179
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Díaz S, Symsstad AJ, Chapin FS, Wardle DA, Huenneke LF (2003) Functional diversity revealed by removal experiments. Trends Ecol Evol 18:140–146
Díaz S, Lavorel S, McIntyre S, Falczuk V, Casanoves F, Milchunas DG, Skarpe C, Rusch G, Sternberg M, Noy-Meir I, Landsberg J, Shang W, Clark H, Campbell BD (2007) Plant trait responses to grazing – a global synthesis. Glob Chang Biol 13:313–341
Dick CW, Wright SJ (2005) Tropical mountain cradles of dry forest diversity. PNAS 102:10757–10758
Dorrough JW, Ash JE, Bruce S, McIntyre S (2007) From plant neighbourhood to landscape scales: how grazing modifies native and exotic plant species richness in grassland. Plant Ecol 191:185–198
Drennan PM, Nobel PS (2000) Responses of CAM species to increasing atmospheric CO2 concentrations. Plant Cell Environ 23:767–781
Durand LG, Goldstein G (2001) Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 126:345–354
Engelbrecht BMJ, Kurssar TA, Tyree MT (2005) Drought effects on seedling survival in a tropical moist forest. Trees 19:312–321
Esparza-Olguín L, Valverde T, Vilchis-Anaya E (2002) Demographic analysis of a rare columnar cactus (Neobuxbaumia macrocephala) in the Tehuacán Valley. Mex Conserv Biol 103:349–359
Ewers FW, Fisher JB, Fichtner K (1991) Water flux and xylem structure in vines. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, p 127
Farrar DR, Dassler C, Watkins JE, Chanda S (2008) Gametophyte ecology. In: Ranker TA, Haufler CH (eds) Biology and evolution of ferns and lycophytes. Cambridge University Press, New York, pp 222–256
Fernández-Marín B, Arroyo A SJ, Becerril M, García-Plazaola I (2012) Do fern gametophytes have the capacity for irradiance acclimation? Biol Plant 56:351–356
Field CB, Vázquez-Yanes C (1993) Species of the genus Piper provide a model to study how plants can grow in different kinds of rainforest habitat. Interciencia 18(5):230–236
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H (2002) Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct Plant Biol 29:461–471
Flores J, Briones O, Flores A, Sánchez-Colón S (2004) Effect of predation and solar exposure on the emergence and survival of desert seedlings of contrasting life-forms. J Arid Environ 58:1–18
Foster P (2001) The potential negative impacts of global climate change on tropical montane cloud forest. Earth–Sci Rev 55:73–106
Geβler A, Duarte HM, Franco AC, Lüttge U, Demattos EA, Nahm M, Rodrigues PJFP, Scarano FR, Rennenberg H (2005) Ecophysiology of selected tree species in different plant communities at the periphery of the Atlantic Forest of SE-Brazil III three legume trees in a semi-deciduous dry forest. Trees 19:523–530
Geβler A, Nietschke R, Demattos EA, Zaluar HLT, Scarano FR, Rennenberg H, Lüttge U (2008) Comparison of the performance of three different ecophysiological life forms in a sandy coastal restinga ecosystem of SE-Brazil: a nodulated N2- fixing C3-shrub (Andira legalis (Vell) Toledo), a CAM-shrub (Clusia hilariana Schltdl) and a tap root C3-hemicryptophyte (Allagoptera arenaria (Gomes) O Ktze). Trees 22:105–119
Gentry AH (1983) Lianas and the “paradox” of contrasting latitudinal gradients in wood and litter production. Trop Ecol 24:63–67
Godínez-Álvarez H, Valiente-Banuet A, Valiente-Banuet L (1999) Biotic interactions and the population dynamics of the long-lived columnar cactus Neobuxbaumia tetetzo in the Tehuacán Valley, Mexico. Can J Bot 77:203–208
Godínez-Álvarez H, Valverde T, Ortega-Baez P (2003) Demographic trends in the Cactaceae. Bot Rev 93:173–203
Goldstein G, Andrade JL, Nobel PS (1991) Differences in water relations parameters for the chlorenchyma and the parenchyma of Opuntia ficus-indica under wet versus dry condition. Aust J Plant Physiol 18:95–107
Gómez Pompa A, del Amo S (1985) Investigaciones sobre la regeneración de selvas altas en Veracruz, México II Editorial Alhambra Mexicana. SA de CV, México 421 pp
Gómez Pompa A, Vázquez-Yanez C, del Amo S, Butanda A (eds) (1976) Investigaciones sobre la regeneración de selvas altas en Veracruz, México CECSA, CNEB. INIREB, México 640 pp
Gómez- Pompa A, Vázquez-Yanez C, Guevara S (1972) The tropical rain forest: a nonrenewable resource. Science 177:762–765
Gómez-Pompa A (1971) Posible papel de la vegetación secundaria en la evolución de la flora tropical. Biotropica 3:125–135
González-Salvatierra C, Andrade JL, Orellana R, Peña-Rodríguez LM, Reyes-García C (2010) Microambientes de luz y morfología y fisiología foliar de Bromelia karatas (Bromeliaceae) en una selva baja caducifolia de Yucatán, México. Bot Sci 91:75–84
Graham E, Andrade JL (2004) Drought tolerance associated with vertical stratification of two co-occurring epiphytic bromeliads in a tropical dry forest. Am J Bot 91:699–706
Griffiths H (1992) Carbon isotope discrimination and the integration of carbon assimilation pathways in terrestrial CAM plants. Plant Cell Environ 15:1051–1062
Griffiths H, Maxwell K (1999) In memory of C S Pittendrigh: does exposure in forest canopies relate to photoprotective strategies in epiphytic bromeliads? Funct Ecol 13:15–23
Hanke W, Böhner J, Dreber N, Jürgens N, Schmiedel U, Wesuls D, Dengler J (2014) The impact of livestock grazing on plant diversity: an analysis across dryland ecosystems and scales in southern Africa. Ecol Appl 24:1188–1203. https://doi.org/10.1890/13-03771
Hietz P (2010) Fern adaptations to xeric environments. In: Mehltreter K, Walker LR, Sharpe JM (eds) Fern ecology. Cambridge University Press, Cambridge
Hietz P, Briones O (1998) Correlation between water relations and within-canopy distribution of epiphytic ferns in a Mexican cloud forest. Oecologia 114:305–316
Hietz P, Briones O (2001) Photosynthesis, chlorophyll fluorescence and within-canopy distribution of epiphytic ferns in a Mexican cloud forest. Plant Biol 3:279–287
Hietz P, Briones O (2004) Adaptaciones y bases fisiológicas de la distribución de los helechos epifitos en un bosque de niebla. In: Cabrera HM (ed) Fisiología Ecológica de Plantas Mecanismos y Respuestas al Estrés en Ecosistemas. Ediciones Universitarias de Valparaíso, Valparaíso
Hietz P, Hietz-Seifer U (1995) Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. J Veg Sci 6:487–498
Hietz P, Wanek W, Popp M (1999) Stable isotopic composition of carbon and nitrogen and nitrogen content in vascular epiphytes along an altitudinal transect. Plant Cell Environ 22:1435–1443
Hinojo-Hinojo C, Castellanos AE, Rodriguez JC, Delgado-Balbuena J, Romo-León JR, Celaya-Michel H, Huxman TE (2016) Carbon and water fluxes in an exotic Buffelgrass savanna rangeland. Ecol Manag 69:334–341. https://doi.org/10.1016/jrama201604002
Holttum RE (1954) A revised flora of Malaya: an illustrated systematic account of the Malayan flora, including commonly cultivated plants, vol II. Government Printing Office, Singapore
Huang D, Wu L, Chen JR, Dong L (2011) Morphological plasticity, photosynthesis and chlorophyll fluorescence of Athyrium pachyphlebium at different shade levels. Photosynthetica 49:611–618
Hultine KR, Cable WL, Burgess SSO, Williams DG (2003) Hydraulic redistribution by deep roots of a Chihuahuan Desert phreatophyte. Tree Physiol 23:353–360
Hultine KR, Scott RL, Cable WL, Goodrich DC, Williams DG (2004) Hydraulic redistribution by a dominant, warm-desert phreatophyte: seasonal patterns and response to precipitation pulses. Funct Ecol 18:530–538
INEGI (2000) Inventario Forestal Nacional Serie III INEGI http://mapasinegobmx/temashtml?seleccion=Vegetación. Accessed June 12, 2008
Jaramillo IR, Mendoza A (2004) Apogamia en Dryopteris munchii (Dryopteridaceae). Polibotánica 18:99–110
Johansson D (1974) Ecology of vascular epiphytes in West African rain forest. Acta Phytogeographica Suecica 59:1–129
Jones MM, Olivas Rojas P, Tuomisto H, Clark DB (2007) Environmental and neighborhood effects on tree fern distributions in a neotropical lowland rain forest. J Veg Sci 18:13–24
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato T, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290
Keeley JE, Rundel PW (2003) Evolution of CAM and C4 carbon-concentrating mechanisms. Int J Plant Sci 164(S3):S55–S77
Kelly DL (1985) Epiphytes and climbers of a Jamaican rain forest: vertical distribution, life forms and life history. J Biogeogr 12:223–241
Körner C, Farquhar GD, Wong SC (1991) Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 88:30–40
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer, Berlin
Li FW, Villarreal JC, Kelly S, Rothfels CJ, Melkonian M, Frangedaki E, Ruhsman M, Sigel EM, Der JP, Pitterman J, Burge DO, Pokorny L, Larsson A, Chen T, Weststran S, Thomas P, Carpenter E, Zhang Y, Tian Z, Chen L, Yan Z, Zhu Y, Sun X, Wang J, Stevenson DW, Crandall-Stotler BJ, Shaw AJ, Wong GKS, Mathews S, Pryer KM (2014) Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proc Natl Acad Sci 111:6672–6677
López-Romero JM, Riaño K, Briones O (2016) Germinación y frecuencia de esporofitos de dos especies simpátricas de Blechnum (Blechnaceae). Acta Bot Mex 117:47–58
Lüttge U (2004) Ecophysiology of crassulacean acid metabolism (CAM). Ann Bot 93:629–652
Lüttge U (2006) Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree in the neotropics. New Phytol 171:7–25
Lüttge U (2008) Physiological ecology of tropical plants. Springer, Berlin
Martínez-Ramos M (2008) Grupos funcionales In: Capital natural de México, vol I: Conocimiento actual de la biodiversidad Conabio, México, pp 365–412
Martorell C, Ezcurra E (2002) Rosette scrub occurrence and fog availability in arid mountains of Mexico. J Veg Sci 13:651–662
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 53:2131–2142
McIntyre S, Heard KM, Martin TG (2003) The relative importance of cattle grazing in subtropical grasslands: does it reduce or enhance plant biodiversity? J Appl Ecol 40:445–457
Medrano H, Tomás M, Martorell S, Flexas J, Hernández E, Rosselló J, Pou A, Escalona J-M, Bota J (2015) From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J 3:220–228
Mehltreter K, Walker LR, Sharpe JM (eds) (2010) Fern ecology. Cambridge University Press, Cambridge
Méndez-Barroso LA, Vivoni ER, Robles-Morua A, Mascaro G, Yépez EA, Rodríguez JC, Watts CJ, Garatuza-Payán J, Saíz-Hernández JA (2014) A modeling approach reveals differences in evapotranspiration and its partitioning in two semiarid ecosystems in Northwest Mexico. Water Resour Res 50:3229–3252
Miles L, Newton AC, Defries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Miranda F, Hernández XE (1963) Los tipos de vegetación de México y su clasificación. Bol Soc Bot Mex 28:29–179
Moghaddam-Gheshlagh A, Hernández-Verdugo S, Rueda-Puente O, Soria-Ruíz J, Parra-Terrazas S, Pacheco-Olvera A, Mafakheri N (2017) Climate change impact on Olneya tesota a gray (ironwood) distribution in Sonoran desert using MaxEnt modeling approach. J Wildl Biodivers 1:110–117
Molina-Freaner F, Tinoco-Ojanguren C (1997) Vines of a desert plant community in central Sonora, Mexico. Biotropica 29:46–56
Molina-Freaner F, Castillo-Gamez R, Tinoco-Ojanguren C, Castellanos AE (2004) Vine species diversity across environmental gradients in Northwestern Mexico. Biodivers Conserv 13:1853–1874
Morales-Romero D, Molina-Freaner F (2008) Influence of buffelgrass pasture conversion on the regeneration and reproduction of the columnar cactus, Pachycereus pecten-aboriginum, in northwestern Mexico. J Arid Environ 72:228–237
Morales-Romero D, Godinez-Alvarez H, Campo-Alves J, Molina-Freaner F (2012) Effects of land conversion on the regeneration of Pachycereus pecten-aboriginum and its consequences on the population dynamics in northwestern Mexico. J Arid Environ 77:123–129. https://doi.org/10.1016/jjaridenv201109005
Morales-Romero D, Campo J, Godinez-Alvarez H, Molina-Freaner F (2015) Soil carbon, nitrogen and phosphorus changes from conversion of thornscrub to buffelgrass pasture in northwestern Mexico. Agric Ecosyst Environ 199:231–237
Nicotra AB, Chazdon RL, Schlichting (1997) Patterns of genotypic variation and phenotypic plasticity of light response in two tropical Piper (Piperaceae) species. Am J Bot 84:1542–1552
Niewiadomska E, Borland AM (2008) Crassulacean acid metabolism: a cause or consequence of oxidative stress in planta? In: Lüttge U, Beyschlag W, Murata J (eds) Progress in botany, vol 69. Springer, Berlin, pp 247–266
Nobel PS (1985) PAR, water and temperature limitations on the productivity of cultivated Agave fourcroydes (henequen). J Appl Ecol 22:157–173
Nobel PS (2002) Cacti: biology and uses. University of California Press, Berkeley
Nobel PS, de la Barrera E (2002) High temperatures and net CO2 uptake, growth, and stem damage for the hemiepiphytic cactus Hylocereus undatus. Biotropica 34:225–231
Noy-Meir I (1973) Desert ecosystems: environment and producers. Ann Rev Ecol Syst 4:25–51
O’Leary MH (1988) Carbon isotopes in photosynthesis fractionation techniques may reveal new aspects carbon of dynamics in plants. Bioscience 38:328–336
Ong BL, Koh CKK, Wee YC (1998) Effects of CO2 on growth and photosynthesis of Pyrrosai piloselloides (L) price gametophytes. Photosynthetica 35:21–27
Orlandi Laureto LM, Cianciaruso MV, Soares D, Menezes S (2015) Functional diversity: an overview of its history and applicability. Nat Conservacao 13:112–116
Orozco–Segovia A, Sanchez– Coronado ME (2009) Functional diversity in seeds and its implications for ecosystem functionality and restoration ecology. In: Gamboa-de Buen A, Orozco-Segovia A, Cruz-Garcia F (eds) Functional diversity of plant reproduction. Research Signpost, Kerala, pp 175–216
Orozco-Segovia A, Vázquez-Yanes C (1989) Light effect on seed germination in Piper L. Acta Ecol 10(2):123–146
Orozco-Segovia A, Vazquez-Yanes C (1990) Effect of moisture on seed longevity in seed of some tropical rain forest species. Biotropica 22:215–216
Pakeman RJ (2011) Functional diversity indices reveal the impacts of land use intensification on plant community assembly. J Ecol 99:1152–1161
Pan Y (2000) Plant functional types: their relevance to ecosystem properties and global change. Ecol Eng 16:305–307
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41:421–453
Pérez-García B, Riba R (1982) Germinación de esporas de Cyatheaceae bajo diversas temperaturas. Biotropica 14:281–287
Pérez-García B, Orozco-Segovia A, Riba R (1982) El banco de esporas de helechos en el suelo de Los Tuxtlas, Ver. Bol Soc Bot Méx 43:89–92
Pérez-García B, Mendoza-Ruiz A, Sánchez-Coronado ME, Orozco-Segovia A (2007) Effect of light and temperature on germination of spores of four tropical fern species. Acta Oecol 32:172–179
Petter G, Wagner K, Wanek W, Sánchez-Delgado EJ, Zotz G, Sarmento-Cabral J, Kreft J (2015) Functional leaf traits of vascular epiphytes: vertical trends within the forest, intra- and interspecific trait variability, and taxonomic signals. Funct Ecol 30:188–198
Pfitsch WA, Pearcy RW (1989) Daily carbon gain by Adenocaulon bicolor (Asteraceae), a redwood forest herb, in relation to its light environment. Oecologia 80:465–470
Pierce S (2007) The jeweled armor of Tillandsia – multifaceted or elongated trichomes provide photoprotection. Aliso 23:44–52
Pittendrigh CS (1948) The bromeliad-anopheles-malaria complex in Trinidad I-the bromeliad flora. Evolution 2:58–89
Poorter L, Arets EJMM (2003) Light environment and tree strategies in a Bolivian tropical moist forest: an evaluation of the light partitioning hypothesis. Plant Ecol 166:295–306
Prasch CM, Sonnewald U (2015) Signaling events in plants: stress factors in combination change the picture. Environ Exp Bot 114:4–14
Raghavan V (1989) Physiology of spore germination. In: Barlow PW, Bray D, Green PB, Slack JMW (eds) Development and cell biology series. University Press, Cambridge
Ramírez-Barahona S, Luna-Vega I, Tejero-Díez D (2011) Species richness, endemism, and conservation of American tree fern (Cyatheales). Biodivers Conserv 20:59–72
Reyes-García C, Griffiths H (2009) Strategies for survival of perennial bromeliads in seasonally dry forests. In: de la Barrera E, Smith WK (eds) Perspectives in biophysical plant ecophysiology: a tribute to Park S Nobel. Universidad Nacional Autónoma de México, Mexico, pp 121–151
Reyes-García C, Griffiths H, Rincón E, Huante P (2008) Niche differentiation in tank and atmospheric epiphytic bromeliads of a seasonally dry forest. Biotropica 40:168–175
Reyes-García C, Mejia-Chang M, Griffiths H (2012) High but not dry: diverse epiphytic bromeliad adaptations to exposure within a seasonally dry tropical forest community. New Phytol 193:745–754
Reynolds JF, Kemp PR, Ogle K, Fernandez RJ (2004) Modifying the ‘pulse-reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141:194–210
Riaño K, Briones O (2013) Leaf physiological response to light environment of three tree fern species in a Mexican cloud forest. J Trop Ecol 29:217–228
Riaño K, Briones O (2015) Sensitivity of three tree ferns during their first phase of life to the variation of solar radiation and water availability in the Mexican cloud forest. Am J Bot 102:1–10
Riaño K, Briones O, Pérez-García B (2015) Spore germination of three tree fern species in response to light, water potential and canopy openness. Am Fern J 105:59–72
Ricalde MF, Andrade JL, Durán R, Dupuy JM, Simá JL, Us-Santamaría R, Santiago L (2010) Environmental regulation of carbon isotope composition and crassulacean acid metabolism in three plant communities along a water availability gradient. Oecologia 164:871–880
Rudolph D, Rauer G, Nieder J, Barthlott W (1998) Distributional patterns of epiphytes in the canopy and phorophyte characteristics in a western Andean rain forest in Ecuador. Selbyana 19:27–33
Rundel PW, Franklin T (1991) Vines in arid and semi-arid ecosystems. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 337–356
Ryel RJ, Leffler AJ, Ivans C, Peek MS, Caldwell MM (2010) Functional differences in water-use patterns of contrasting life forms in Great Basin steppelands. Vadose Zone J 9:548–560
Rzedowski J (1978) Vegetación de México. LIMUSA, México
Saito N, Harborne JB (1983) A cyaniding glycoside giving scarlet coloration in plants of the Bromeliaceae. Phytochemistry 22:1735–1740
Sakamaki Y, Ino Y (1999) Contribution of fern gametophytes to the growth of produced sporophytes on the basis of carbon gain. Ecol Res 14:59–69
Saldaña AO, Hernandez C, Coopman RE, Bravo LA, Corcuera LJ (2010) Differences in light usage among three fern species of genus Blechnum of contrasting ecological breadth in forest light gradient. Ecol Res 25:273–281
Salvador MG (2014) Respuesta germinativa y efecto del sustrato sobre el crecimiento de gametofitos de helechos del Bosque Nublado de la Reserva El Riscal, Cofre de Perote, Veracruz. Tesis Licenciatura Instituto Tecnológico Superior de Zacapoaxtla
Sanaphre-Villanueva L, Dupuy JM, Andrade JL, Reyes-García C, Jackson PC, Paz H (2017) Patterns of plant functional variation and specialization along secondary succession and topography in a tropical dry forest. Environmental Research Letters 12:0550004. https://doi.org/10.1088/1748-9326/aa6baa
Sanchez-Coronado ME, Rincón E, Vázquez-Yanez C (1990) Growth responses of three contrasting Piper species growing under different light conditions. Can J Bot 68:1182–1186
Santiago LS, Silvera K, Andrade JL, Dawson T (2017) Functional strategies of tropical dry forest plants in relation to growth form and isotopic composition. Environ Res Lett 2:115006. https://doi.org/10.1088/1748-9326/aa8959
Saucedo-Monarque E, García-Moya E, Castellanos AE, Flores-Flores JL (1997) La riqueza, una variable de respuesta de la vegetación a la introducción del zacate buffel. Agrociencia 31:83–90
Schwinning S, Ehleringer JR (2001) Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J Ecol 89:464–480
Scott RL, Huxman TE, Williams DG, Goodrich DC (2006) Ecohydrological impacts of woody-plant encroachment: seasonal patterns of water and carbon dioxide exchange within a semiarid riparian environment. Glob Chang Biol 12:311–324
Scott RL, Huxman TE, Barron-Gafford GA, Jenerette GD, Young JM, Hamerlynck EP (2014) When vegetation change alters ecosystem water availability. Glob Chang Biol 20:2198–2210 doi:DOI:202222/GCB12511
Seager R, Ting M, Davis M, Cane M, Naik N, Miller J, Li C, Cook E, Stahle DW (2007a) Mexican drought: an observational, modeling and proxy reconstruction study of variability and climate change Lamont Doherty Earth Observatory. Columbia University, Palisades
Seager R, Ting M, Held IM, Kushnir Y, Lu J, Vecchi G, Huan H, Harnik N, Leetmaa A, Lau N, Li C, Velez J, Naik N (2007b) Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316:1181–1184
Seager R, Ting M, Davis M, Cane M, Naik N, Miller J, Li C, Cook E, Stahle DW (2009) Mexican drought: an observational, modeling and proxy reconstruction study of variability and climate change Atmosfera
Sharpe JM, Mehltreter K (2010) Ecological insights form fern population dynamics. In: Mehltreter K, Walker LR, Sharpe JM (eds) Fern ecology. Cambridge University Press, Cambridge
Shreve F (1942) The desert vegetation of North America. Bot Rev 8:195–246
Silvera K, Lasso E (2016) Ecophysiology and Crassulacean acid metabolism of tropical epiphytes. In: Goldstein G, Santiago LS (eds) Tropical tree physiology – adaptations and responses in a changing environment. Springer, Cham, pp 25–43
Smith TM, Shugart HH, Woodward FI (eds) (1997) Plant functional types their relevance to ecosystem properties and global change. Cambridge University Press, Cambridge
Stewart G, Schmidt S, Handley L, Turnbull M, Erskine P, Joly C (1995) 15N natural abundance of vascular rainforest epiphytes: implications for nitrogen source and acquisition. Plant Cell Environ 18:85–90
Tauz M, Hietz P, Briones O (2001) The significance of carotenoids and tocopherols in photoprotection of seven epiphytic fern species. Aust J Plant Physiol 28:775–783
ter Steege H, Cornelissen JHC (1989) Distribution and ecology of vascular epiphytes in lowland rain forest of Guayana. Biotropica 21:331–339
Testo WL, Watkins JE (2013) Understanding mechanisms of rarity in pteridophytes: competition and climate change threaten the rare fern Asplenium scolopendrium var americanum (Aspleniaceae). Am J Bot 100:2261–2270
Tinoco-Ojanguren C, Pearcy RW (1992) Dynamic stomatal behavior and its role in carbon gain during lightflecks of a gap phase and an understory species acclimated to high and low light. Oecologia 92:222–228
Tinoco-Ojanguren C, Pearcy RW (1993) Stomatal dynamics and its importance to carbon gain in two rainforest Piper species 1 VPD effects on the transient stomatal response to lightflecks. Oecologia 94:388–394
Tinoco-Ojanguren C, Díaz A, Martínez J, and Molina-Freaner F (2013) Species diversity and regeneration of native species in buffelgrass pastures from the thornscrub of Sonora, México. J Arid Environ 97:26–37
Trejo I, Dirzo R (2002) Floristic diversity of Mexican seasonally dry tropical forests. Biodivers Conserv 11:2063–2048
Valdez-Hernández M, González-Salvatierra C, Reyes-García C, Jackson PC, Andrade JL (2015) Physiological ecology of vascular plants. In: Islebe GA, Calmé S, León-Cortés JL, Schmook B (eds) Biodiversity and conservation of the Yucatan Peninsula. Springer, Cham, pp 97–129
Valiente-Banuet A, Ezcurra E (1991) Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacán Valley, Mexico. J Ecol 79:961–971
Vargas-Soto G, Andrade JL, Winter K (2009) Carbon isotope composition and mode of photosynthesis in Clusia species from Mexico. Photosynthetica 47:33–40
Vaz AP, Figueiredo-Ribeiro RC, Kerbauy G (2004) Photoperiod and temperature effects on in vitro growth and flowering of P pusilla, an epiphytic orchid plant. Physol Biochem 42:411–415
Vázquez Yanes C (1976) Estudios sobre ecofisiologia de la germinación en una zona calido-humeda de Mexico. In: Gómez-Pompa A, Del Amo S (eds) Investigaciones sobre la regeneración de selvas altas en Veracruz, México CECSA, CNEB. INIREB, México, pp 279–387
Vázquez Yanes C, Smith H (1982) Phytochrome control of seed germination in the tropical rain forest pioneer trees Cecropia obtusifolia and Piper auritum and its ecological significance. New Phytol 92:477–485
Vázquez-Yanes C, Orozco-Segovia A (1982) Germination of the seeds of a tropical rain forest shrub, Piper hispidum Sw (Piperaceae), under different qualities of light. Phyton 42:143–149
Vázquez-Yanes C, Orozco-Segovia A (1987) Light gap detection bt the photoblastic seeds of Cecropla obtusifolia and Piper auritum, two tropical rain forest trees. Biol Plant 29:234–236
Vázquez-Yanes C, Orozco-Segovia A (1990) Ecological significance of light controlled seed germination in two contrasting tropical habitats. Oecologia 83:171–175
Vázquez-Yanes C, Orozco-Segovia A (1993) Patterns of seed longevity and germination in the tropical rainforest. Annu Rev Ecol Syst 24:69–87
Villaseñor JL, Téllez-Valdés O (2004) Distribución potencial de las especies del género Jefea (Asteraceae) en México. An Inst Biol (serie Botánica) 75:205–224
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Volkova L, Bennett L, Tausz M (2009) Effects of sudden exposure to high light levels on two tree fern species Dicksonia antarctica (Dicksoniaceae) and Cyathea australis (Cyatheaceae) acclimated to different light intensities. Aust J Bot 57:562–571
Volkova L, Bennett LT, Tausz M (2010) Diurnal and seasonal variations in photosynthetic and morphological traits of the tree ferns Dicksonia antarctica (Discksoniaceae) and Cyathea australis (Cyatheaceae) in wet sclerophyll forests of Australia. Environ Exp Bot 70:11–19
Walters MB, Field CB (1987) Photosynthetic light acclimation in two rainforest Piper species with different ecological amplitudes. Oecologia 72:449–456
Warne TR, Lloyd RM (1980) The Role of Spore Germination and Gametophyte Development in Habitat Selection: Temperature Responses in Certain Temperature and Tropical Ferns. Bull of the Torrey Botanical Club 107:57–64
Watkins JE, Mack MK, Mulkey SS (2007) Gametophyte ecology and demography of epiphytic and terrestrial tropical ferns. Am J Bot 94:701–708
Watkins JE, Holbrook NM, Zwieniecki MA (2010) Hydraulic properties of fern sporophytes: consequences for ecological and evolutionary diversification. Am J Bot 97:2007–2019
Winter K, Smith JAC (1996) An introduction to crassulacean acid metabolism: biochemical principles and biological diversity. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism biochemistry, ecophysiology and evolution. Springer, Berlin, pp 1–13
Winter K, Aranda J, Holtum JAM (2005) Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Funct Plant Biol 32:381–388
Wong SL, Chen CW, Huang HW, Weng JH (2012) Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica 50:206–214
Woodhouse RW, Nobel P (1982) Stipe anatomy, water potential, and xylem conductances in seven species of ferns (Filicopsida). Am J Bot 69:135–140
Yuan ZY, Chen HY (2015) Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes nature. Climate Change 5:465–469
Zhang Q, Chen J-W, Li B-G, Ca K-F (2009) The effect of drought on photosynthesis in two epiphytic and two terrestrial tropical fern species. Photosynthetica 47:128–132
Zotz G (2016) Plants on plants – the biology of vascular epiphytes. Springer, Cham
Zotz G, Bader MY (2009) Epiphytic plants in a changing world-global: change effects on vascular and nonvascular epiphytes. In: Lütgge U, Beyschlag W, Büdel B, Francis D (eds) Progress in botany, vol 70. Springer-Verlag, Berlin, pp 147–170
Zotz G, Hietz P (2001) The physiological ecology of vascular epiphytes: current knowledge, open questions. J Exp Bot 52:2067–2078
Acknowledgments
A. C. V. acknowledges M. Martínez and O. Téllez-Valdés for the niche distribution, D. G. Williams for leaf 13C isotopic, and H. Celaya-Michel for the soil moisture data.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Tinoco-Ojanguren, C., Andrade, J.L., Briones, O., Castellanos, A.E. (2018). Functional Diversity in Plants: Implications for Conservation Issues of the Mexican Biodiversity. In: Ortega-Rubio, A. (eds) Mexican Natural Resources Management and Biodiversity Conservation. Springer, Cham. https://doi.org/10.1007/978-3-319-90584-6_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-90584-6_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90583-9
Online ISBN: 978-3-319-90584-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)