Abstract
The importance of gut microbiome in influencing human health has been widely assessed. The gut microbiome may vary according to several extrinsic factors, among which diet can be considered one of the most important. Substrates provided through diet are metabolized by the gut microbiome, with the possible production of beneficial or harmful metabolites. In the past decades, dietary habits in the Western world have strongly changed, with an increase in the consumption of foods of animal origin and a decrease in the intake of fiber and complex polysaccharides. These changes in the diet impacted our microbial symbionts, possibly playing a role in the development of several diseases. The understanding of these relationships will allow, in a next future, a targeted modulation of the gut microbiome through ad hoc dietary interventions for therapeutic or preventive purposes. In this chapter, recent findings about the existing interconnections between gut microbiome, diet, and human health are discussed, highlighting possible future perspectives.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
6.1 The Human Gut Microbiome
Gut microbiota of healthy adults is commonly dominated by two bacterial phyla, Firmicutes and Bacteroidetes, with interindividual variability in their proportions. Proteobacteria, Actinobacteria, and Verrucomicrobia are present at lower levels (Lozupone et al. 2012). In spite of the great inter- and intraindividual variability, in the past years, a classification of subjects based on the most abundant genera in their gut microbiome was proposed. Some years ago, it was observed that all the subjects may be classified in three discrete clusters, named “enterotypes,” based on the prevalence of Prevotella, Bacteroides, or Ruminococcus in their gut microbiome (Arumungam et al. 2011). However, the “enterotype” concept was lately criticized, since a rigorous categorization may lead to an oversimplified vision of the gut microbiome (Knights et al. 2014; Jeffery et al. 2012). On the contrary, although this classification may be attractive for understanding microbial variation in health and disease, the existence of a smooth gradient of the dominant taxa is more plausible, where the abundance of dominant genera varies continuously in the human population going from an enterotype to another. The important role of gut microbiota in influencing human well-being is widely recognized. It plays a primary function in host health by shaping the development of the immune system, metabolizing dietary nutrients and drugs, and synthesizing vitamins, bioactive molecules, and other beneficial or detrimental metabolites.
In the past decades, gut dysbiosis has been linked to the development of several kinds of diseases, including obesity (Turnbaugh et al. 2006; Le Chatelier et al. 2013), diabetes (Qin et al. 2012), inflammatory bowel disease (Marchesi et al. 2016), and cardiovascular diseases (Koeth et al. 2013).
Moreover, the gut microbiome may influence human behavior by the bidirectional communication path between the gastrointestinal (GI) and the central nervous system, namely, the gut-brain axis. This happens by a microbiome-mediated production of molecules that have neuroactive effects, such as serotonin and γ-aminobutyric acid (GABA). Nutrients and microbial metabolites interact with the enteroendocrine cells (EECs) located along the GI tract and containing most of the nutrient receptors. Interactions with EEC receptors are crucial in mechanisms such as the regulation of appetite and insulin secretion (Furness et al. 2013). Indeed, recent studies suggest that bacterial proteins may influence the appetite-controlling pathways, acting locally in the gut with a short-term effect on satiation (Breton et al. 2016). Moreover, plasmatic levels of specific bacterial proteins may activate host anorexigenic circuitries with a long-term regulation of the feeding pattern (Breton et al. 2016).
6.2 Diet-Induced Signatures in Gut Microbiota Composition and Functions in Rural Populations Around the World
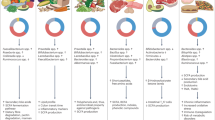
Diet can be considered as the primary factor influencing gut microbiota composition and functionality. The first studies highlighting the key role played by diet found an association of Bacteroides with a high-fat/low-fiber diet, while a high abundance of Prevotella was linked to a diet rich in fiber and low in foods of animal origin (David et al. 2014; Wu et al. 2011). Indeed, a short-term dietary switch from high-fat/low-fiber to high-fiber/low-fat diet causes reproducible changes in the gut microbiome that are, however, not long-lasting. On the contrary, long-term, habitual diet is the primary factor shaping our gut microbiome (David et al. 2014; Wu et al. 2011). Over recent decades, modern dietary patterns in Western countries have undergone major compositional changes, with an increase in the consumption of red meat, high-fat foods, and refined sugars. This “Westernization” of the diet, together with changes in the lifestyle, has surely partially contributed to the higher incidence of inflammatory disorders, such as obesity, diabetes, cardiovascular diseases, and allergies. The study of agrarian populations living in South America or Africa helped to understand the complex relationships between habitual diet and the gut microbiome (Table 6.1). Comparison of the gut microbiomes of agrarian and Western populations may help in understanding how dietary changes have affected the gut symbionts. All these studies consistently found that the composition of the gut microbiota dramatically differs between urbanized, Western people eating a high-fat and protein diet and rural populations still consuming a subsistent, agrarian diet, with low intake of products of animal origin and high consumption of fruit, vegetables, fibrous tubers, and roots. Most of these studies highlighted the loss of microbial diversity in Westernized populations (Clemente et al. 2015; Martínez et al. 2015; Obregon-Tito et al. 2015; Schnorr et al. 2014; De Filippo et al. 2010) and overlapping signatures in gut microbiota composition of the traditional population studied (Table 6.1). Prevotella, Xylanibacter, Treponema, Succinivibrio, Lachnospira, and other fiber-degrading bacteria were reported as enriched in traditional populations consuming an agrarian diet (Fig. 6.1). Treponema is a genus belonging to the Spirochaetes. It was only reported to be present in the gut microbiota of nonhuman primates (Gomez et al. 2016a; Ley et al. 2008; Ochman et al. 2010) and of human traditional populations (Schnorr et al. 2014; De Filippo et al. 2010; Obregon-Tito et al. 2015; Gomez et al. 2016b; Ou et al. 2013) suggesting that these symbionts were lost in urban, Westernized people. The genomes of two distinct strains of Treponema from gut metagenomes of South Americans were reconstructed (Obregon-Tito et al. 2015). These strains turned out to be clearly different from known pathogenic Treponema strains with their genomic potential suggesting a selective adaptation to the gut environment including the capability to use complex polysaccharides, abundant in the diet of non-Westernized subjects.
Gomez et al. (2016b) characterized the gut microbiome of two traditional African populations (BaAka and Bantu) with different diet and lifestyle: BaAka are hunter-gatherers living in the rainforest and consuming a diet rich in fibrous tubers, such as manioc roots and wild yams, while Bantu have switched to a subsistence agriculture in the past century. Interestingly, when comparing their gut microbiomes with those of American control subjects, they found that Bantu showed a gut microbiota composition intermediate between BaAka and Americans. Indeed, a decrease in the abundance of fiber-degrading bacteria was observed from BaAka to Americans, with intermediate levels in Bantu, reflecting the different subsistence patterns and highlighting the effect of a recent transition to a more modern lifestyle in Bantu (Gomez et al. 2016b).
On the contrary, the gut microbiota of Western subjects is often enriched in bile-tolerant microorganisms, such as Bacteroides, Alistipes, and Bilophila, associated with the consumption of a diet rich in fat and protein and poor in indigestible carbohydrates (Fig. 6.1).
Such a difference in microbiota composition between rural and Western populations results in a different functionality. Indeed, metagenomes of Hadza hunter-gatherer and urbanized Italians showed a different potential activity (Rampelli et al. 2015). The Hadza microbiome contains a more diverse pool of genes that encodes for enzymes able to break down a broader set of polysaccharides compared to Italians. On the contrary, metabolic pathways related to the degradation of several xenobiotics of human origin (e.g., naphthalene, xylene, or benzoate), ubiquitous in industrialized environments, were enriched in the Italian gut microbiome (Rampelli et al. 2015). Moreover, Western diet, richer in fat and proteins, led to the selection of a gut microbiome with higher capacity to degrade amino acids and convert primary bile acids in secondary bile acids, as well as simple sugars (Yatsunenko et al. 2012).
6.3 Diet-Mediated Production of Beneficial or Detrimental Metabolites by the Gut Microbiome
Different dietary habits and gut microbiomes promote the production of a distinctive fecal metabolome in traditional and Western populations. Undigested dietary components reach the large intestine, where they are fermented by the microbial community to produce a wide pattern of metabolites, reflecting both the chemical diversity of the available substrates and the different metabolic potential of the gut microbiota. Most studies focused on fecal levels of short-chain fatty acids (SCFAs). The three most abundant SCFAs, acetic, propionic, and butyric acids, are produced by bacterial fermentation of indigestible polysaccharides and are normally present in a molar ratio of about 3:1:1 (Louis et al. 2014). Nondigestible carbohydrates include the structural polysaccharides of plant cell walls (non-starch polysaccharides, such as celluloses, xylans, pectins, and glucans), resistant starch, and soluble oligosaccharides (e.g., fructo-oligosaccharides) (Flint et al. 2012). SCFAs are an important energy source for human colonocytes and have been often associated with several health-promoting effects, such as anti-inflammatory and anticarcinogenic (Louis et al. 2014; O’Keefe 2016). Indeed, the abundance of fecal SCFAs was significantly higher in the agrarian and rural populations studied around the world (De Filippo et al. 2010; Ou et al. 2013; Schnorr et al. 2014), and this is undoubtedly correlated with the substantial enrichment of fiber in their diet, which selects for a fiber-degrading and SCFA-producing gut microbiota. Besides SCFAs, gut microbiota can produce other metabolites, with a potentially beneficial effect on the host health (Fig. 6.1).
Plant cell walls englobe a variety of complex micronutrients, collectively named phytochemicals, which are unabsorbed in the upper gastrointestinal tract. In the colon, microbial fermentation releases these compounds, and some bacteria can metabolize and convert them to a wide range on bioactive molecules (Cardona et al. 2013). As an example, the soy isoflavone, daidzein, can be converted to equol by some gut bacteria, such as species belonging to Faecalibacterium, Eubacterium, Bifidobacterium, and Clostridium. According to some scientific evidence, equol may possess anticarcinogenic properties (Cardona et al. 2013). However, due to the wide variety of chemical molecules commonly included in the phytochemical class, it can be expected that different gut microbiota members may be differently involved in their degradation, with the consequent production of a wide range of bioactive metabolites.
Gut microbiota can also produce detrimental metabolites (Fig. 6.1). High-protein intake results in an increase in degradation of proteins in the colon and consequently higher levels of fecal amino acid-derived products, such as branched-chain fatty acids and phenylacetic acid (Ou et al. 2013; Russell et al. 2011). High levels of branched-chain amino acids in the blood have been associated with insulin resistance and development of type 2 diabetes (Pedersen et al. 2016). In addition, some bacteria, such as Bacteroides spp., can ferment aromatic amino acids to produce phenylacetic acid, phenols, indoles, and p-cresol, associated with a pro-inflammatory and carcinogenic effect (Louis et al. 2014). Sulfate-reducing bacteria (e.g., Desulfovibrio spp.) produce sulfides through the catabolism of sulfur amino acids and taurine. Sulfides are pro-inflammatory and toxic for colonocytes (O’Keefe 2016). Moreover, amine derived from microbial fermentation of proteins in the colon can be nitrosated to produce N-nitroso compounds (NOCs), which exert a carcinogenic and mutagenic effect and are correlated to the incidence of colorectal cancer (Loh et al. 2011). Indeed, increased fecal NOCs and phenylacetic acid and decreased SCFA levels can be found after administration of a high-protein/low-carbohydrate diet (Russell et al. 2011). In addition, high-fat diet leads to an increase in bile secretion and consequent higher quantity of bile acids in the colon. Bile salt hydrolases can cleave glycine and taurine residues from the primary bile acids, converting them into several secondary bile acids, mainly deoxycholic and lithocholic acids. Ou et al. (2013) observed that microbial genes encoding for secondary bile acid production and fecal secondary bile acid concentration were more abundant in African Americans compared to rural native Africans, also showing lower colorectal cancer risk. Accordingly, when African Americans and rural Africans switched their diets for 2 weeks, a reduction in fecal secondary bile acids and in colonic mucosal inflammation markers was observed in African Americans, associated with the decrease in the abundance of Bilophila wadsworthia. On the contrary, the high-fat intervention in rural Africans was linked to an increase in Fusobacterium nucleatum, previously found in human colon cancer tissues (O’Keefe et al. 2015).
Another important microbial metabolite associated with a detrimental effect for host health is trimethylamine-N-oxide (TMAO; Fig. 6.1). Intestinal microbiota catabolism of choline, phosphatidylcholine, and l-carnitine produces trimethylamine (TMA), which is further oxidized in the liver resulting in TMAO. The latter considered a risk factor in the development of cardiovascular diseases (CVDs) and atherosclerosis (Tang et al. 2013; Wang et al. 2011).
6.4 Gut Microbiome and Dietary Habits in the Western World
Western diets are not necessarily detrimental for the gut microbiome. In Westernized countries, healthier dietary patterns such as vegetarian and vegan diets are becoming increasingly popular. According to an American poll in 2016, approximately 3.3% of American adults are vegetarians or vegans. This percentage increases to 6% when considering only young adults (18–34 years), while only 2% of people 65 years or older are vegetarians. Accordingly, sales of alternative meat products reached $553 million in 2012, an 8% increase in 2 years (Melina et al. 2016). A well-planned vegetarian diet contains vegetables, fruits, whole grains, legumes, nuts, and seeds, besides some products of animal origin, such as eggs, milk, and derivates thereof. Both vegetarian and vegan diets are devoid of flesh foods (such as meat, poultry, wild game, seafood, and their products). In addition, vegans do not consume any food of animal origin. The adoption of a vegetarian diet may cause a reduced intake of certain nutrients; however, deficiencies can be readily avoided by appropriate dietary planning and, if necessary, the consumption of supplements (Melina et al. 2016). Although the spread of these dietary patterns is growing, only few studies addressed the question if these diets select for distinctive traits in the gut microbiome. Recently, gut microbiome and metabolome were studied in a cohort of 153 vegetarian, vegan, and omnivore Italians (De Filippis et al. 2016). Vegans and vegetarians showed higher abundance of plant-degrading bacteria, such as Lachnospira and Prevotella. Moreover, these genera were positively correlated to the fecal levels of the three main SCFAs and negatively correlated with the urinary concentration of TMAO, with the former being higher and the latter being lower in vegetarians/vegans compared to omnivores (De Filippis et al. 2016). Moreover, increased plasma levels of metabolites likely derived from microbial catabolism of plant polyphenolic compounds as well as increased abundance of equol were found in a small cohort of vegans (Wu et al. 2016). However, differences in equol concentration were not linked to the consumption of foods rich in equol precursors (such as soy-based products). Indeed, only about 30–40% of Western adults are likely able to convert isoflavones to equol, regardless the dietary intake, against about the 70% of Asians (Magee 2011). Thus, these data emphasize that both the consumption of the right substrates and the presence of specific microbial metabolic capacity jointly determine the potential development of a health-promoting metabolome.
Beyond the strict vegetarian or vegan regimes, the consumption of a healthy and diverse dietary pattern, such as that based on the Mediterranean-style, can help to develop a health-associated microbiome and metabolome, without completely banning meat and other products of animal origin. The Mediterranean diet has been recognized by UNESCO as intangible cultural heritage (http://www.unesco.org/culture/ich/RL/00884). The Mediterranean dietary pattern is characterized by high intake of fruit, vegetables, legumes, nuts, and whole grains, moderate consumption of fish, and low intake of saturated fat, meat, and dairy products (Trichopoulou et al. 1995). It has been demonstrated to be beneficial for the treatment of obesity, type 2 diabetes, and inflammatory and cardiovascular diseases (Santoro et al. 2014; Estruch et al. 2013; Salas-Salvadó et al. 2011). Studying the gut microbiota and metabolome in an Italian cohort of adults with different diary habits, De Filippis et al. (2016) also evaluated the adherence level to the Mediterranean diet, calculating the Mediterranean dietary score (Agnoli et al. 2011). They observed a progressive increase in the concentration of fecal SCFA going from low-adherence to high-adherence subjects (Fig. 6.2a). Intriguingly, even when considering the omnivore group alone, the higher the adherence to the Mediterranean diet, the higher the concentration of fecal SCFAs (Fig. 6.2b). Therefore, a healthy dietary pattern, such as the Mediterranean-style diet, can provide appropriate substrates and shape the gut microbiome to promote the production of health-related metabolites even in an overall omnivore-type diet.
Box plots showing the fecal concentrations of butyrate, propionate, and acetate in Italian omnivores, vegetarians, and vegans analyzed in De Filippis et al. (2016). Panel a shows the data for the subjects grouped according to adherence level to the Mediterranean diet, while panel b shows the data for the omnivore subjects. (* p < 0.05 and ** p < 0.01). Reproduced from “High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome.”, De Filippis F. et al. 2016. Gut, 65:1812–1821 with permission from BMJ Publishing Group Ltd
6.5 Modulation of Gut Microbiome Through Diet: Possible Implications for Human Health
Several studies tried to address the question whether we can modulate the microbiome through diet. Indeed, the presence/abundance of specific gut microbiota members was shown to be adjustable through a specific dietary intervention. However, the consumption of Westernized diets may induce changes in the gut microbiota composition that are not reversible. Indeed, experiments on humanized mice showed that the consumption of a diet low in microbiota-accessible carbohydrates (low-MACs) over several generations results in a progressive loss of microbial diversity in the offspring, which is not recoverable after the reintroduction of dietary MACs (Sonnenburg et al. 2016). However, response to dietary intervention is person-specific and has been shown to be strongly linked to the gut microbiota composition. Indeed, Kovatcheva-Datchary and co-workers (2015) demonstrated that a dietary intervention with barley kernel fiber may induce changes in the gut microbiome which are associated with improved glucose metabolism. Nevertheless, the supplementation did not have the same effect on all the subjects, and the authors suggested that responders have peculiar traits in their gut microbiota, such as higher Prevotella abundance, and that this microbe may be responsible for increased glycogen storage in the liver. On the contrary, subjects showing lower abundance of P. copri did not show a metabolic response to the fiber supplementation. Accordingly, people eating identical meals showed different metabolic responses, consistently associated with gut microbiota features. Integration of blood parameters, dietary habits, and anthropometric and gut microbiota features in a complex algorithm enables an accurate prediction of postprandial glycemic response, linked to type 2 diabetes development (Zeevi et al. 2015). Moreover, such algorithm may be successfully used in setting up personalized dietary intervention to lower postprandial blood glucose.
Since the application of generalized medical practices did not always ensure consistent results among subjects, the possibility of a personalized nutrition strategy recently emerged. The knowledge acquired in the past decades about gut microbiome composition and functionality and the presence of diet-responsive members will be used in the near future in personalized therapeutic approaches based on a targeted modulation of intestinal microbial communities.
Controversy
Recent studies highlighted the possibility of boosting changes in the gut microbiome through specific and appropriate dietary interventions. Although this opportunity is tantalizing, there are several issues to consider. All intervention studies must face the presence of many confounding factors, e.g., different physical activity or lifestyle and consumption of alcohol, drugs, or medications in the cohort studied, all of which may obscure the effect of the treatment. Thus, controlling for external factors in microbiome studies is extremely important, disentangling the effect of the treatment from the effects of confounders. Moreover, high intersubject variability in the gut microbiome composition exists. Interindividual differences in the gut microbiome at baseline may lead to subject-specific responses to the same treatment. Indeed, results reported in literature are often contrasting, and even in the same study, different subjects in the cohort may show distinct outcomes in response to the same dietary intervention. Integration of blood parameters, dietary habits, and anthropometric and gut microbiota features in complex algorithms can help to accurately predict metabolic response to different meals and to formulate subject-specific dietary regimes. Personalized nutrition strategies based on individual gut microbiome features are recently emerging and in a next future will allow developing new therapeutic or disease-preventive approaches based on a targeted modulation of gut microbiome through diet.
History
Gut microbiota plays an important role in regulating human health/disease status, affecting the development of the immune system, metabolizing dietary nutrients and drugs, and synthesizing vitamins, bioactive molecules, or other beneficial or detrimental metabolites. Several factors may influence gut microbiome, leading to gut dysbiosis, which was linked to the development of several diseases. Diet may be considered as the primary factor influencing gut microbiota composition and functionality. In a recent past, dietary habits in the Western world have strongly changed, with increased consumption of animal origin fat/proteins and reduced intake of complex polysaccharides. Comparison of the gut microbiome of urbanized, Western subjects with traditional, rural populations living in Africa and South America highlighted several differences, suggesting that our microbiome coevolved with humans, shaped by diet and lifestyle. Westernized subjects show lower microbial diversity and lose specific fiber-degrading bacteria in their gut microbiome. Differences in gut microbiota composition reflect its functionality. Indeed, gut microbiome produces several health-promoting (e.g., short-chain fatty acids, equol) or detrimental (e.g., sulfides, N-nitroso compounds, trimethylamine) metabolites, depending on the substrates that are made available through the diet. Specific dietary interventions may induce change in the gut microbiome. However, recent studies highlighted subject-specific responses to the same intervention, possibly due to specific features of the gut microbiome.
Highlights
-
Gut microbiome may influence host health through the production of beneficial or detrimental metabolites, depending on the type of substrates provided by diet.
-
What is food for us is also food for our microbial symbionts; thus, gut microbiome is strongly influenced by the habitual diet.
-
Changes in dietary habits and lifestyles in Western populations may have affected their gut microbiome.
-
Short dietary intervention may induce changes in the gut microbiome.
-
Recent research provides the bases for the development of new therapeutic strategies based on a targeted modulation of the gut microbiome through diet.
References
Agnoli, C., Krogh, V., Grioni, S., et al. (2011). A priori–defined dietary patterns are associated with reduced risk of stroke in a large Italian cohort. The Journal of Nutrition, 141, 1552–1558.
Arumungam, M., Raes, J., Pelletier, E., et al. (2011). Enterotypes of the human gut microbiome. Nature, 473(7346), 174–180.
Breton, J., Tennoune, N., Lucas, N., et al. (2016). Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metabolism, 23(2), 324–334.
Cardona, F., Andrés-Lacueva, C., Tulipani, S., et al. (2013). Benefits of polyphenols on gut microbiota and implications in human health. The Journal of Nutritional Biochemistry, 24, 1415–1422.
Clemente, J. C., Pehrsson, E. C., Blaser, M. J., et al. (2015). The microbiome of uncontacted Amerindians. Science Advances, 1(3), e1500183. https://doi.org/10.1126/sciadv.1500183.
David, L. A., Maurice, C. F., Carmody, R. N., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563.
De Filippis, F., Pellegrini, N., Vannini, L., et al. (2016). High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut, 65(11), 1812–1821.
De Filippo, C., Cavalieri, D., Di Paola, M., et al. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14691–14696.
Estruch, R., Ros, E., Salas-Salvadó, J., et al. (2013). Primary prevention of cardiovascular disease with a Mediterranean diet. The New England Journal of Medicine, 368(14), 1279–1290.
Flint, H. J., Scott, K. P., Duncan, S. H., et al. (2012). Microbial degradation of complex carbohydrates in the gut. Gut Microbes, 3(4), 289–306.
Furness, J. B., Rivera, L. R., Cho, H.-J., et al. (2013). The gut as a sensory organ. Nature Reviews Gastroenterology & Hepatology, 10, 729–740.
Girard, C., Tromas, N., Amyot, M., et al. (2017). Gut microbiome of the Canadian arctic Inuit. mSphere, 2(1), e00297-16.
Gomez, A., Petrzelkova, K. J., Burns, M. B., et al. (2016a). Gut microbiome of coexisting BaAka pygmies and Bantu reflects gradients of traditional subsistence patterns. Cell Reports, 14(9), 2142–2153.
Gomez, A., Rothman, J. M., Petrzelkova, K., et al. (2016b). Temporal variation selects for diet–microbe co-metabolic traits in the gut of Gorilla spp. The ISME Journal, 10, 514–526.
Jeffery, I. B., Claesson, M. J., O’Toole, P. W., & Shanahan, F. (2012). Categorization of the gut microbiota: Enterotypes or gradients? Nature Reviews Microbiology, 10(9), 591–592.
Knights, D., Ward, T. L., McKinlay, C. E., et al. (2014). Rethinking “enterotypes”. Cell Host & Microbe, 16(4), 433–437.
Koeth, R. A., Wang, Z., Levison, B. S., et al. (2013). Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine, 19(5), 576–585.
Kovatcheva-Datchary, P., Nilsson, A., Akrami, R., et al. (2015). Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metabolism, 22(6), 971–982.
Le Chatelier, E., Nielsen, T., Qin, J., et al. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature, 500(7464), 541–546.
Ley, R. E., Hamady, M., Lozupone, C., et al. (2008). Evolution of mammals and their gut microbes. Science, 320, 1647–1651.
Loh, Y. H., Jakszyn, P., Luben, R. N., et al. (2011). N-nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. The American Journal of Clinical Nutrition, 93(5), 1053–1061.
Louis, P., Hold, G. L., & Flint, H. J. (2014). The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology, 12(10), 661–672.
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., et al. (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489(7415), 220–230.
Magee, P. J. (2011). Is equol production beneficial to health? The Proceedings of the Nutrition Society, 70(1), 10–18.
Marchesi, J. R., Adams, D. H., Fava, F., et al. (2016). The gut microbiota and host health: A new clinical frontier. Gut, 65, 330–339.
Martínez, I., Stegen, J. C., Maldonado-Gómez, M. X., et al. (2015). The gut microbiota of rural Papua New Guineans: Composition, diversity patterns, and ecological processes. Cell Reports, 11(4), 527–538.
Melina, V., Craig, W., & Levin, S. (2016). Position of the academy of nutrition and dietetics: Vegetarian diets. Journal of the Academy of Nutrition and Dietetics, 116(12), 1970–1980.
O’Keefe, S. J. (2016). Diet, microorganisms and their metabolites, and colon cancer. Nature Reviews Gastroenterology & Hepatology, 13(12), 691–706.
O’Keefe, S. J., Li, J. V., Lahti, L., et al. (2015). Fat, fibre and cancer risk in African Americans and rural Africans. Nature Communications, 6, 6342. https://doi.org/10.1038/ncomms7342.
Obregon-Tito, A. J., Tito, R. Y., Metcalf, J., et al. (2015). Subsistence strategies in traditional societies distinguish gut microbiomes. Nature Communications, 6, 6505. https://doi.org/10.1038/ncomms7505.
Ochman, H., Worobey, M., Kuo, C. H., et al. (2010). Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology, 8(11), e10000546. https://doi.org/10.1371/journal.pbio.1000546.
Ou, J., Carbonero, F., Zoetendal, E. G., et al. (2013). Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. The American Journal of Clinical Nutrition, 98(1), 111–120.
Pedersen, H. K., Gudmundsdottir, V., Nielsen, H. B., et al. (2016). Human gut microbes impact host serum metabolome and insulin sensitivity. Nature, 535(7612), 376–381.
Qin, J., Li, Y., Cai, Z., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 490(7418), 55–60.
Rampelli, S., Schnorr, S. L., Consolandi, C., et al. (2015). Metagenome sequencing of the Hadza hunter-gatherer gut microbiota. Current Biology, 25(13), 1682–1693.
Russell, W. R., Gratz, S. W., Duncan, S. H., et al. (2011). High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. The American Journal of Clinical Nutrition, 93(5), 1062–1072.
Salas-Salvadó, J., Bulló, M., Babio, N., et al. (2011). Reduction in the incidence of type 2 diabetes with the Mediterranean diet results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care, 34(1), 14–19.
Santoro, A., Pini, E., Scurti, M., et al. (2014). Combating inflammaging through a Mediterranean whole diet approach: The NU-AGE project’s conceptual framework and design. Mechanisms of Ageing and Development, 136-137, 3–13.
Schnorr, S. L., Candela, M., Rampelli, S., et al. (2014). Gut microbiome of the Hadza hunter-gatherers. Nature Communications, 5, 3654. https://doi.org/10.1038/ncomms4654.
Sonnenburg, E. D., Smits, S. A., Tikhonov, M., et al. (2016). Diet-induced extinctions in the gut microbiota compound over generations. Nature, 529(7585), 212–215.
Tang, W. H. W., Wang, Z., Levison, B. S., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England Journal of Medicine, 368, 1575–1584.
Trichopoulou, A., Kouris-Blazos, A., Wahlqvist, M. L., et al. (1995). Diet and overall survival in elderly people. BMJ, 311, 1457–1460.
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., et al. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 444(7122), 1027–1031.
Wang, Z., Klipfell, E., Bennett, B. J., et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 472(7341), 57–63.
Wu, G. D., Chen, J., Hoffmann, C., et al. (2011). Linking long term dietary patterns with gut microbial enterotypes. Science, 334(6052), 105–108.
Wu, G. D., Compher, C., Chen, E. Z., et al. (2016). Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut, 65, 63–72.
Yatsunenko, T., Rey, F. E., Manary, M. J., et al. (2012). Human gut microbiome viewed across age and geography. Nature, 486(7402), 222–227.
Zeevi, D., Korem, T., Zmora, N., et al. (2015). Personalized nutrition by prediction of glycemic responses. Cell, 163(5), 1079–1094.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
De Filippis, F., Ercolini, D. (2018). Microbiome and Diet. In: Haller, D. (eds) The Gut Microbiome in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-90545-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-90545-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90544-0
Online ISBN: 978-3-319-90545-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)