Abstract
Potato is one of the most important food crops contributing to nutritional and food security in the world. It is grown as a summer crop in temperate areas of the world and as a winter crop in the subtropics of India and China. Potato crop is damaged by numerous pests and diseases of which aphid transmitted viruses are the major concern for healthy seed potato production. Since potato is a vegetatively propagated crop, the viral diseases lead to an ongoing decline in health of the propagating material i.e., seed degeneration. Hence, the management of aphids and aphid transmitted viruses is the first and foremost requirement for seed potato production. Potatoes are infested by a large number of aphid species very few of them actually able to colonise the crop. Most of the aphids are non-specific to the crop and are cosmopolitan and polyphagous. The common colonising aphid species are Myzus persicae, Macrosiphum euphorbiae, Aulcorthum solani, Aphis gossypii, A. fabae, A. spiraecola etc.; while as more than 100 species of aphids are reported to transiently visit potato crop. Of these, more than 65 species are known to transmit one or more potato viruses. Aphids because of their cyclic parthenogenesis and short life cycle can assume epic proportions while on the other hand, their host selection and feeding behaviour predisposes them to being the most effective virus vectors. The host rang and life cycle characteristics of aphids are also key in determining the rate of spread of the viruses. Keeping in view the importance of life cycle variation and population ecology of aphids for virus transmission in potato crop, information is compiled and analysed to identify the gaps in knowledge and help determine the direction of future research, with special emphasis on the subtropics.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Migration
- Myzus persicae
- Non-persistent transmission
- Parthenogenesis
- Potato virus Y
- Primary host
- Seed potato
5.1 Introduction
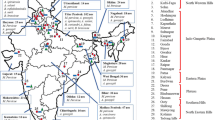
Potato (Solanum tuberosum L.) is a major world food crop, exceeded only by maize, rice and wheat in world production. Over three-fourth of the potato area is in Europe and Asia and the remainder is in Africa, North-Central America, South America and Oceania. Major areas are located between 45°N and 57°N that represent potato production zones in the temperate climate where potato is a summer crop (Fig. 5.1). Another potato concentration area is between 23°N and 34°N that mainly represents potato area in Gangetic plains, southern China and Egypt, where potato is grown as a winter crop. This belt that goes from south-west to south China and continues into the plains of Ganges River dominates potato production in Asia (Khurana and Naik 2003).
In India, potato is cultivated in almost all states under very diverse agro-climatic conditions (Pandey and Kang 2003). More than 85% of India’s potatoes are grown in the vast Indo-Gangetic plains of north India (subtropics) during short winter days from October to March. The states of Punjab, Uttar Pradesh, West Bengal, and Bihar account for more than 75% of the potato-growing area in India and for about 80% of total production. Hilly areas, where the crop is grown during the summer from April to September (temperate), account for less than 5% of production. In the plateau regions of south-eastern, central, and peninsular India (tropics), which constitute about 6% of the potato-growing area, potato is mainly a rain-fed crop or is irrigated as winter crop. In the Nilgiri and Palni hills of Tamil Nadu, the crop is grown year-round under both irrigated and rain-fed conditions (Chandel et al. 2013).
Among the various biotic and abiotic stresses that constrain potato production, viruses are of highest importance for quality seed production. A series of mosaic causing potyviruses like potato virus Y (PVY) and the potato leaf roll virus (PLRV) are of utmost concern (Fig. 5.2). These viruses are transmitted by aphids in non-persistent and persistent manner, respectively. Aphids rarely cause a direct damage as a pest by feeding on the plants but they are important for potato being the natural vectors that transmit a large number of viruses responsible for progressive degeneration of the seed stock. Among the aphids, Myzus persicae (Sulzer) is the most efficient and most common vector of potato viruses. The main aphid species associated with potatoes worldwide are non-specific to this crop. Most of them are cosmopolitan and polyphagous. Apart from the aphid species that colonise potato crop, hundreds of non-colonising species are important to various extents. In this chapter, we first present attributes common to most aphid species and then we discuss characteristics that are specific to the most important aphid species of potato. In this chapter, emphasis is laid on the population ecology of aphids, mainly their life cycle and host range that are of potential importance in subtropical and tropical areas of potential production mainly so in India. An attempt has been made to bring out the knowledge gaps so that investigations could be taken up for the better understanding of the biology and ecology of the concerned species for the purpose of modelling, forecasting and hence better management.
5.2 Aphids Infesting Potato; Fundamentals of Population Ecology
Aphids have complex life cycles, and their classification depends on host alternation and on their mode of reproduction (Blackman and Eastop 2000; Williams and Dixon 2007). Different morphs are associated with these life cycles. Heteroecy and monoecy refer to the status of aphids regarding their host plant. Aphids that practice host alternation are heteroecious. They live on a primary host during the winter and colonize secondary hosts during the rest of the year before coming back to their primary host . Heteroecy occurs in only 10% of aphid species that generally colonize herbaceous plants, including economically important crop species such as potatoes. In contrast, a majority of aphid species live on the same plant throughout the year, do not have host plant alternation, and are classified as monoecious species. Some of these species are monophagous. Other species are oligo- or polyphagous and may migrate between plant species. However, they do not have regular alteration of primary and secondary hosts in their life cycles (Williams and Dixon 2007).
Holocycly and anholocycly refer to the ability of aphids to reproduce using parthenogenesis alone or in combination with sexual reproduction (Blackman and Eastop 2000). Most aphid species alternate parthenogenesis and sexual reproduction and are holocyclic. In this case, parthenogenesis occurs from the first generation in spring to the appearance of sexual morphs in autumn. The appearance of sexual individuals is triggered by seasonal changes in temperature and photoperiod. In contrast, some species are anholocyclic; they do not produce sexual morphs or eggs, and only reproduce by parthenogenesis (Williams and Dixon 2007). Anholocyclic life cycles may occur when climatic conditions are favourable for aphids to maintain populations on various plants during winter. Depending on the region, certain populations in some holocyclic aphid species can lose their sexual phase and become anholocyclic or generate only male populations (androcycly) during winter (Blackman 1971; Fenton et al. 1998; Williams and Dixon 2007). Aphids that colonize potato are mainly heteroecious and holocyclic. Their life cycles include an overwintering phase, during which fertilized eggs constitute the resistant form during periods of cold temperatures. Because potato is an annual plant, its colonizing aphids are heteroecious.
The viviparous mode of reproduction in aphids confers a rapid reproduction rate with short developmental times, resulting in population growth that is atypically high, even for insects. For instance, Dixon (1971) estimated that aphid populations in potato fields can reach densities of 2 × 109 individuals per hectare. Douglas (2003) suggested that such rates of population increase reflect nutrient allocation to the reproductive system. Energy is preferentially invested in embryo biomass and larval development rather than in maternal tissues. Aphids have telescoping generations—i.e., ovarian development and embryo formation start at the same time in embryonic mothers (Powell et al. 2006). Parthenogenetic reproduction results in clonal aphid colonies that have the same genotype. With this reproduction mode, an atypical characteristic can be amplified and become predominant in a given population after several generations. This can explain why aphids are able to adapt quickly to disturbances in their environment. Aphid populations may crash depending on the weather (Barlow and Dixon 1980), deteriorating resources, or pesticide treatments. However, parthenogenesis rapidly generates new populations that are adapted to their environment and, in some cases, resistant to pesticides . Parthenogenesis generally occurs during the warmer months of the year and maximizes offspring production. In fall, it is interrupted and followed by sexual reproduction that produces overwintering eggs.
Aphids produce both apterous (wingless) and alate (winged) morphs. Production of alate morphs is energetically costly (Dixon et al. 1993). Alates appear at different times during the year. They are considered to be colonizers, and use winds to disperse and locate new hosts. Wingless fundatrices emerge from eggs laid on the primary host . Their alate progeny are the spring migrants. Alate production is completed within a 2-week period (Radcliffe 1982). These individuals fly to secondary hosts (e.g., potato) and, when conditions are favourable, generate apterous and parthenogenetic populations. During summer, overpopulation of aphids, degradation of host plant nutritional suitability, or variations in light intensity, temperature, and precipitation induce the decline in aphid populations and the appearance of winged morphs that move to more suitable host habitats.
In autumn, as day length and temperature decrease, the quality of secondary host plants is altered. These factors generate the appearance of a new generation of virginoparous alates that migrate to the primary host . After the second generation on the primary host, oviparous females appear and are fertilized by winged males (Radcliffe 1982). After reproduction, oviparous females lay their eggs on the primary host for overwintering (Powell et al. 2006). Timing of flight and the number of migrants are important for colonization, clonal fitness, and overwintering success.
5.3 Biodiversity of Economically Important Aphid Species on Potato
Aphids belong to the Stenorrhyncha (Hemiptera). The most important genera found on potato crops, i.e. Myzus spp., Aulacorthum spp., Aphis spp. and Macrosiphum spp. belong to the family Aphididae. Aphids are characterized by high polymorphism. The colour of the individuals can also be highly variable within a given population and can be influenced by the symbiotic bacteria they host (Tsuchida et al. 2010). Morphology is influenced by several factors, such as environmental, climatic, and seasonal conditions; quality of the host plants and population densities. Blackman and Eastop (1994, 2000) listed more than 22 species of aphids that commonly infest potato plants (Table 5.1), and provided an identification key using morpho-anatomic criteria such as body colour, length, shape, and segmentation; antennal tubercles, head, siphunculi, legs and femurs, cauda and anal plate; and hairs on these structures. Verma and Chandla (1990) described five major species ingesting potato under Indian conditions viz., Myzus persicae , Aphis gossypii, A. fabae, Rhopalosiphoninus latysiphon and Rhopalosiphum rufiabdominalis with notes on biology, life cycle, migration and management, in addition to two minor species Rhopalosiphum nymphaeae and Tetraneura nigriabdominalis. Later, Shridhar et al. (2015) compiled information on 13 species of aphids recorded on potato viz., M. persicae, A. gossypii, A. fabae, A. phis spiraecola, A. nerii, A. craciivora, Macrosiphum euphorbiae, Brevicoryne brassicae, Aulacorthum solani, Lipaphis erysimi, Hydaphis coriandari, Rhopalosiphum rufiabdominalis and Rhopalosiphum maidis.
Although green peach aphid is the most efficient vector of potato virus Y (PVY) in potato (van Hoof 1980; Sigvald 1984; Singh et al. 1996; Fernandez-Calvino et al. 2006), several non-colonizing species also contribute to PVY prevalence (Ragsdale et al. 2001). Non-colonizing species do not reproduce on potato, but may transiently visit potato plants. Such species can occur in very large numbers, making their effect on virus spread large despite their lower virus transmission efficiency (Halbert et al. 2003). Hundreds of non-colonising aphid species have been reported from potato fields and tested for virus transmission ability. In Table 5.2, a consensus list of non-colonising aphid species is given most of which have been implicated with virus transmission albeit with low efficiency. In addition, notes on common hosts are also given which are indicative of the probable host plants from which they originate or are oriented to while crossing through potato fields.
Next, we will briefly introduce the main species of aphids infesting potato from ecological point of view and the variation they exhibit round the world.
5.3.1 Acyrthosiphon pisum (Harris) (Pea Aphid)
Acyrthosiphon pisum is a complex of races and subspecies with different host ranges and preferences. This species feeds mostly on Leguminosae. It is worldwide in distribution. Biology is holocyclic on various leguminous hosts in temperate regions; in warmer climates there is presumably facultative anholocycly (Blackman and Eastop 2000).
Not much is known about the biology of the aphid in India where both adult apterous and alate viviparous females reproduce parthenogenetically. One generation apparently takes about a week for completion. The colony of green or pink aphids is found round the stems, young shoots and the underside of leaves, they drop to the ground at the slight disturbance. It is sporadically serious pest of peas and causes cupping and distortion of leaves. It’s distributed throughout the country and has wide host range. It happens to be potential vector of more than 30 viruses, including Potato virus Y (Misra 2002).
5.3.2 Aphis craccivora Koch (Cowpea Aphid; Groundnut Aphid; Black Legume Aphid) (Fig. 5.3)
Aphis craccivora Koch, commonly known as black aphid, attacks many plants and most often leguminous crops. It is a major pest of bean and cowpea. The aphid species is known to infest at least 90 plant species belonging to 23 plant families in India (Raychaudhuri 1983; Chakrabarti and Sarkar 2001). The species acts as a vector of numerous viral diseases.
Both apterous and alate viviparous females reproduce asexually and both breed throughout the year (Talati and Butani 1980). Reproduction is the highest in February and lowest in June. The species completes 31 overlapping generations in a year (Bakhetia and Sidhu 1977). This species is anholocyclic almost everywhere, but monoecious holocyclic populations are recorded from Germany and India; sexuales on leguminous plants in Germany (Falk 1957/58) and on Tinospora cordifolia (Family Leguminosae) in Calcutta. Verma and Khurana (1974) reported males and oviparous females on green gram during December, 1973 at Haryana (India). Sometimes alatae of A. craccivora and A. gossypii are confused particularly if small, lightly coloured specimens are involved. However, both can be separated by the relative lengths of u.r.s. and h.t.2. Also, it differs from gossypii in the transverse sclerites on the individual tergites being much longer, particularly on the posterior segments, when they often extend along the entire width of the tergites, uniting with the postsiphuncular and marginal sclerites (Cottier 1953).
5.3.3 Aphis fabae Scopoli (Black Bean Aphid) (Fig. 5.4)
Aphis fabae causes curling of the leaves of Euonymus europaeus in spring and migrates to a wide range of secondary hosts, including young growth of some trees, and many crops. It has one of the broadest host ranges, having been recorded from nearly 120 plant families. It is particularly important on beans, peas, beets, crucifers, cucurbits, potato, tobacco, tomato, and tulip (Blackman and Eastop 2007). Anholocyclic populations of aphids of the A. fabae group occur on secondary hosts in southern Europe, south-west Asia, Africa, Indian subcontinent, Korea (Kim et al. 2006), South America, Hawaii and Auckland Isles. Over much of Europe, A. fabae is heteroecious holocyclic, alternating between Euonymous and a wide range of secondary host plants.
Aphis fabae is a bewildering complex of species, at least some of which also have wide host ranges. Favret (2014) has recognised five subspecies of Aphis fabae viz. cirsiiacanthoidis Scopoli, 1763, eryngii Blanchard, 1923, evonymi Fabricius, 1775, fabae Scopoli, 1763, mordvilkoi Mordvilko, 1923, in addition to two species viz. Aphis solanella Theobold, 1914 and Aphis euonymi Gmelin in the context of the species complex.
In India, adults of both apterae and alatae reproduce parthenogenetically throughout the year. The life cycle is rather very complicated. The female deposits about 100 nymphs which become adults in about 10 days, passing through four moults. When the population is overcrowded and food source is hampered, the number of adult winged viviparous females increases so that they migrate from one plant to another. However, sexual forms of both apterous oviparous females as well as alate males are on record (Raychaudhuri et al. 1980a) from India. This suggests that the pest species enjoys both anhlolocyclic and holocyclic life cycle in the Indian conditions.
5.3.4 Aphis gossypii Glover (Melon Aphid; Cotton Aphid) (Fig. 5.5)
Aphis gossypii occurs on a very wide range of host plants, its polyphagy being particularly evident during the dry season in hot countries. It’s a major pest of cotton and cucurbits, and in glasshouses in cold temperate regions (Blackman and Eastop 2000). Distributed almost worldwide, A. gossypii is particularly abundant and well-distributed in the tropics. Life cycle is anholocyclic in warm climates, and mainly so in Europe. Host alternation and a sexual phase occur more regularly in parts of east Asia (e.g. Japan, Takada 1988; China, Zhang and Zhong 1982) and also in North America (Kring 1959), with several unrelated plants utilised as primary hosts (such as Catalpa bignonioides, Hibiscus syriacus, Celastrus orbiculatus, Rhamnus spp. and Punica granatum). Apterae of spring populations on primary hosts are usually greenish. Males are always alate.
In India, A. gossypii is highly polyphagous and infests more than 500 species of plants in 76 families both cultivated as well as wild. On cotton it inflicts appreciable damage to the crop and hence it is commonly known as cotton aphid. Both adults and nymphs suck plant sap of many ornamental plants like Hibiscus rosa-sinensis Linn., Cassia glauca Linn., Tecoma capensis Lindl. and Rosa spp. from September to April in northern India. The maximum population is observed in H. rosa-sinensis during March-April. On Rosa spp., it is also observed in September to October and on C. glauca in March-April. The aphid is present throughout the year in the plains. In Punjab it remains very active from 4th week of July to third week of October on okra, from first week of August to 4th week of November on brinjal and from second week of August to fourth week of December on chilli (Jamwal and Kandoria 1990). In western Uttar Pradesh it is found in early, main and late potato crops while in Bihar its population is heavy on the seed crop (Verma and Parihar 1991). In Bangalore, the population of A. gossypii remains high from 3rd week of October to 1st week of January, and from last week of June to the last week of July, respectively. Aphids are observed in more numbers in August planted cotton crop than the March planted crop (Jalali et al. 2000).
The life cycle of this cotton aphid is very complicated. It is polymorphic and adults of both apterae and alatae are viviparous and reproduce parthenogenetically. The female deposits 80–100 nymphs which become adults in 7–9 days on cotton after passing through four moults. When the population is overcrowded, the number of winged adults increases so that they migrate from one plant to others. Both males and oviparous females have been reported from India (Ghosh 1970; Basu and Raychaudhuri 1980). The complete life-cycle is still unknown under Indian conditions.
5.3.5 Aphis nasturtii Kaltenbach (Buckthorn Aphid; Buckthorn-Potato Aphid)
It is found on a wide range of herbaceous plants in summer, including Nasturtium officinale, Solanum tuberosum, Veronica beccabunga, Drosera rotundifolia (Müller 1978) and Rumex spp. In India, the species infests nearly 74 species belonging to 64 genera under 37 plant families (Ghosh 1990). Severe infestation results in curling of leaves, started growth and gradual drying and death of young plants. Adults of both apterae and alatae are viviparous and reproduce by parthenogenesis . However, sexual morphs of both apterous oviparous females and alate males are known to occur in India. This hints at the possibility of holocyclic life cycle of the species in the Indian conditions. Life cycle is heteroecious holocyclic worldwide, with a sexual phase on Rhamnus cathartica (Blackman and Eastop 2006).
5.3.6 Aphis spiraecola Patch (Spiraea Aphid; Green Citrus Aphid) (Fig. 5.6)
Highly polyphagous species, usually colonises the under surface of leaves and tender buds of the host plants chiefly Citrus group of fruit trees. The species is however, not host specific to Citrus plants; instead it has wide preference of alternate hosts. Emigrant alatae normally initiate colonies on the tender leaves and shoots during late monsoon and sizeable population is produced on apical twigs, branches and sometimes even on young fruits. Increase in population is accompanied by the production of alatae and a large part of the aphid infestation migrates to other plants. Citrus plants support moderate to heavy aphid population for 2–3 months and it is preceded and succeeded by poor colonization for 15–20 days respectively. The species usually reproduces anholocyclically. However, Ghosh et al. (1972) reported sexual female of the species for the first time in India. Life cycle is holocyclic in North America and Brazil where Spiraea is the primary host . In Japan both Spiraea and Citrus serve as primary hosts; differences are indicative of either separate host races or species occur between the forms colonising these two plants (Komakazi et al. 1979).
5.3.7 Aulacorthum solani (Kaltenbach) (Glasshouse-Potato Aphid; Foxglove Aphid) (Fig. 5.7)
Aulacorthum solani is extremely polyphagous, colonising nearly 40 species belonging to 21 plant families. The important families are Asteraceae, Brassicaceae, Leguminasae, Poaceae, Polygonaceae, Solanaceae etc. Probably of European origin, now almost world-wide in distribution. Life cycle is monoecious holocyclic with apterous or alate males, and with the unusual ability to go through the sexual phase on many different plant species. Also, anholocycly is commonly reported in mild climates and glasshouses (Blackman and Eastop 2006).
Aulacorthum solani was reported by David (1958) for the first time from India. Apterous and alate viviparous females were found on the leaves of Digitalis in the Nilgiris. David (1958) believed that the aphid was introduced around the mid-twentieth century into the country. Basu (1967) recorded apterous and alate viviparous females on potato in West Bengal. In Indian Conditions, this aphid reproduces parthenogenetically throughout the year. Several generations are completed in a year. One generation takes about two weeks in favourable conditions. The biology of the species is not properly known.
The brownish aphids usually infest young shoots and the undersides of young leaves of the host plants. As a result, leaves are cupped or otherwise distorted and become yellowish. Drops of sticky honey dew or patches of sooty mould on the upper sides of leaves. According to Misra (2002) this aphid is a vector of more than 30 viruses and is known to be present throughout the country.
5.3.8 Brachycaudus helichrysi (Kaltenbach) (Leaf-Curling Plum Aphid)
Brachycaudus helichrysi is extremely polyphagous, infesting nearly 185 species belonging to 115 genera under 49 plant families. Primary host plants are various Prunus species, notably domestica, insititia, spinosa. Secondary hosts are numerous species of Compositae (e.g. Achillea, Chrysenthemum, Erigeron, Ageratum) and Boraginaceae (Myosotis, Cyanoglossum) and sometimes other plants e.g., Cucurbita, Rumex, Veronica etc. life cycle is heteroecious holocyclic, but with widespread anholocycly in mild climates and in glasshouses (Blackman and Eastop 2000). The damage is caused by nymphs and females (apterous and alate) which are confined to the growing shoots and leaves, from where they suck the cell sap. It also sucks the sap from buds, blossoms, petioles, tender fruits and leaves. Excess feeding causes ventral curling of peach leaves which adversely affects the yield. On sprouting the leaves emerge curled while the fruits either do not set or falloff prematurely. The infestation starts with the commencement of bud swelling and continues during and after flowering.
In India, the pest species is active from February to March on temperate fruits. During the winter, it is found only in the egg stage at the base of the buds. During spring the eggs hatch and nymphs move on to the young leaves. Here they start sucking the plant sap. In about 4 weeks the nymphs become apterous viviparous female adults. The viviparous females give birth to young ones. 3 or 4 generations are completed on the fruit plants. With the warming up winged females and probably males are also produced. They migrate to other alternative host plants such as golden-rod and start reproducing parthenogenetically again. 4 or 5 generations are completed on the said plant from June to October. Early in November, the alate viviparous females are produced again. They migrate back to peach, plum and other fruit trees. Later, the females lay eggs at the base of the buds. Egg laying is completed by the middle of December. In Kumaon hills, this peach leaf curl aphid infests peach, plum and almond from November to May and from May to October it spends on an alternate host Erigeron Canadensis and other Asteraceae. On both the fruit trees and E. canadenses reproduction is normally through parthenogenesis . Basu et al. (1970) reported ovipara from Prunus sp. and alate males at Shillong. Alate males were also recorded (Ghosh et al. 1971) from Prunus sp. at Kalimpong (N-E Himalaya). Ghosh (1986) reported both oviparous female and alate males from N.W. Himalaya. Thus, it is a holocyclic and heteroecious species alternating between Prunus spp. (primary host) and a number of heterogenous secondary host plants including Anaphalis sp., Ageratus conyzydes, Chrysanthemum sp., Eupatorium sp., Erigeron canadensis (Raychaudhuri 1983)
It is widely distributed in North India, South India including Nilgiris and Coimbatore and also Eastern India including Assam, Meghalaya, Manipur, Sikkim and West Bengal.
5.3.9 Brevicoryne brassicae (L.) (Cabbage Aphid; Mealy Cabbage Aphid)
Brevicoryne brassicae is virtually restricted to members of the Cruciferae and is common in all temperate and warm temperate parts of the world. Life cycle is monoecious holocyclic in colder regions, anholocyclic where winter climate is mild (Blackman and Eastop 2000).
It forms colony of soft mealy-grey nymphs and apterous viviparous females that are found feeding in clusters on leaves, stems and flowers. White cast skins and drops of sticky honeydew and sooty mould growing on the honeydew are often noticed on the leaves. In India, both apterous and alate viviparous females are reported besides the sexuales; apterous oviparous females and alate males. In most of the cases, the aphid reproduces parthenogenetically both in the plains and altitudes (Debraj et al. 1995). Although anholocycly takes place in most of the cases of this aphid, sexual reproduction plays significant role in the biology of the aphid occurring at the higher elevations where cold climate prevails and day length is short. David (1958) reported the occurrence of only apterous oviparous females on Brassica oleracea from Shimla. Banerjee et al. (1969) reported males (alate) from Kuti valley (Uttar Pradesh) and Ghosh et al. (1969) reported alate male on B. oleracea in colony with viviparae at Shimla (Himachal Pradesh). All the above records of the aphid are from the plants of Brassicaceae growing at an elevation of above c 5,000 feet in the Western Himalaya. This suggests that Brevicoryne brassicae (L.) reproduces both anholocyclically and holocyclically at higher elevations. It is known that the species is more common above ca. 3,000 feet in Indian conditions where there is a chance of overlapping of mustard aphid Lipaphis erysini (Kaltenbach) and cabbage aphid Brevicoryne brassicae (L.)
5.3.10 Macrosiphum euphorbiae (Thomas) (Potato Aphid) (Fig. 5.8)
The potato aphid is a common and highly polyphagous species. Primary host plant is Rosa spp., and the species is hoghly polyphagous on secondary hosts feeding on over 200 plant species. It is often a pest on various crops such as potato (Solanum tuberosum), lettuce (Lactuca sativa) and beets (Beta vulgaris) as well as on numerous garden ornamentals. Macrosiphum euphorbiae is a vector of about one hundred plant viruses. The species originates from the north-eastern USA where it produces sexual forms and host alternates with rose (Rosa) as its primary host. Elsewhere it usually overwinters as viviparae. Aphid numbers increase rapidly from early spring, and alatae spread infestations to other plants. It is an especial problem in unheated greenhouses (Blackman and Eastop 2000).
Life cycle is heteroecious holocyclic with a sexual phase on Rosa in north-eastern USA, but elsewhere probably mainly or entirely anholocyclic on secondary hosts in more than 20 different plant families.
5.3.11 Myzus antirrhinii (Macchiati)
These aphids occur on leaves and young growth of numerous plants, on which it may be confused with M. persicae (Blackman and Paterson 1986). It often forms large, dense colonies, and only produces alatae rather sporadically. This species enjoys anholocycly almost everywhere, and produces alatae only sporadically, so it is most often found on perennial plants. However, there is now evidence of a possible sexual phase in Japan (Shigehara et al. 2006). Separation from M. persicae is difficult except using enzyme or molecular analysis (Hales et al. 2000).
5.3.12 Myzus ascalonicus Doncaster (Shallot Aphid)
Myzus ascalonicus is extremely polyphagous, with hosts in more than 20 families, but particularly Alliaceae, Caryophyllaceae, Compositae, Brassicaceae, Liliaceae and Rosaceae (Blackman and Eastop 2000). This species of aphids is apparently completely anholocyclic everywhere. During winter, it is frequently found on stored bulbs, potatoes or root vegetables, in glasshouses and on potted plants (Müller and Möller 1968).
5.3.13 Myzus ornatus Laing Violet Aphid
Myzus ornatus is extremely polyphagous, infesting nearly 180 species of plants. They live singly on the leaves of host plants in many different plant families including especially Bignonaceae, Compositae, Lamiaceae, Polygonaceae, Primulaceae, Rosaceae, and Violaceae. Anholocyclic populations occur throughout the world, and in colder climates they probably overwinter in glasshouses, on pot plants, or in sheltered situations. Both alate males and oviparaous females (Maity and Chakrabarti 1981) are known from India. This suggests that the species enjoys holocycly besides usual anholocyclic life cycle in the Indian conditions.
5.3.14 Myzus persicae (Sulzer) (Peach Potato Aphid; Green Peach Aphid) (Fig. 5.9)
The peach potato or green peach aphid, Myzus persicae is the most economically important aphid crop pest worldwide (van Emden and Harrington 2007). It is a notable example of a heteroecious aphid species. As the day length drops below a critical level in the autumn, apterous holocyclic viviparae produce gynoparae and males on secondary (herbaceous) hosts which migrate to the primary host , peach, Prunus persica L. (Rosaceae). The gynoparae then give birth to oviparae that lay the overwintering eggs after mating with males (van Emden et al. 1969). However, the life cycle of M. persicae appears to be polymorphic. The life cycle categories have been described in relation to the photoperiodic response and temperature regime, i.e. holocyclic, anholocyclic, androcyclic and intermediate. In temperate regions clones with different reproductive strategies could coexist. The relative frequencies of holocyclic and anholocyclic populations in the spring depend on the severity of the previous winter. Anholocyclic clones are unable to produce any sexual morph and overwinter as parthenogenetic females on weeds or winter crops. Other genotypes are able to invest in both reproductive and overwintering strategies. Androcyclic clones, under short day conditions, produce parthenogenetic morphs and males, which are able to mate with oviparae of holocyclic or intermediate clones. Intermediate genotypes produce many apterous and alate virginoparae, some males and alate females which give birth both to virginoparae and oviparae (Blackman 1971, 1972).
Blackman (1974) reviewed the life cycle variation of M. persicae in different parts of the world and propounded that in tropics, where some varieties of peach are grown the mean monthly temperature never falls below 20 °C except at high altitudes, so that sexual morph production may be directly inhibited by high temperature all the year round. Parthenogenesis may continue uninterrupted for a long period of time, and although the holocyclic character could not be selected against, because it is not phenotypically expressed, one might expect loss of sexual viability due to the accumulation of chromosomal and genetic mutations. Apparently distinct anholocyclic biotypes of M. persicae such as that on tobacco may have originated in the tropics in this way. The extent to which splitting of the species occurs in tropical conditions must be largely governed by the frequency and degree of mixing between populations and integration of genotypes due to migrations between host-plants (Shaposhnikov 1966). The tropics cannot however be considered in isolation from the rest of the world, as the extent of frequently of long-range displacements into and out of the tropics of M. persicae from other latitudes is not known. In subtropical zone temperature is not low enough to permit the production of sexual morphs by October north of the equator, and by April south of the equator, then it will be too late for migration to Prunus and maturation of the oviparae before leaf fall. Therefore an induced holocycle in these regions is likely to be abortive. During the winter months temperature ceases to be inhibitory to sexual morph determination, and in winter or spring gynoparae and males may migrate to primary hosts (Bodenheimer and Swirski 1957). Mating and oviposition on peach trees in February and March have been recorded at localities in Egypt, Pakistan and India. As far as is known, any eggs laid in spring do not hatch. Where an abortive holocycle persists in climatic conditions which strongly favour anholocycly this implies immigration of holocyclic genotypes from other regions. This situation warrants further investigation. Winters are so mild in this subtropical zone that they present no obstacle to continuous parthenogenesis , and anholocycly predominates. It is significant that male M. persicae were caught from June to September in all-year-round traps for flying aphids in the region of Sao Paulo, Brazil, where the holocycle has not yet been recorded (Costa 1970). Males have also been collected from Solanaceae in Hong Kong in January and in Taiwan in March (Takahashi 1923). It is likely that the life cycle is androcyclic under such conditions.
Myzus persicae is a notorious polyphagous pest infesting nearly 250 species belonging to 77 genera in India, inflicting heavy losses to variety of crops and is also an important vector of many plant virus diseases (Raychaudhuri 1983; Chakrabarti and Sarkar 2001). It is universally distributed present in all ecological conditions prevailing in the country. The pest species usually appears on potato crop in the field from mid November onwards in most parts of Indo-Gangetic plains and does not migrate to the primary host plant for egg laying as in other temperate countries. Its population goes on increasing up to the end of February and early March (Chauhaf et al. 1975). However, by the end of March many alatae are formed and migrate to mid and then to higher hill where the climate is mild and suitable and a number of secondary host plants are available. The aphids keep on multiplying till November-December on high hills and thereafter, its return migration starts from hills to plains and vice versa. It is, thus, clear that M. persicae is present on the secondary host plants throughout the year either in the plains or hills (Nirula and Pushkarnath 1970). It can also overwinter in the hills, in green houses, sprouts of stored tuber and even in the fields (Lal and Verma 1987).
Ghosh and Raychaudhuri (1962) recorded sexual male from Delhi while sexual female was reported by Verma and Ghosh (1990) from northern part of the country such as Nainital (Uttarakhand) and Shillong (Meghalaya), but also from plains like Modipuram and Meerut (Uttar Pradesh). Verma and Chauhan (1993) reported that in the plains a few gynoparae (alatae) which produce oviparae, start arriving on peach trees by the end of January and February, the nymphs laid by these alatae mature into oviparous females by the middle of February. The males also start arriving during this period and mating takes place which lasts for about 2–5 min. The eggs are laid in the crevices of auxillary buds in clusters. Some eggs are also laid on the twigs. The eggs are first greenish in colour which later turn shining black. During this period, the temperature and day length go on rising and most of the eggs die and are also preyed on by the predators. It seems that these conditions are not suitable for egg hatching. Based on these observations, Singh and Ghosh (2012) presumed that it enjoys both asexual and sexual life cycle in northern India which however, does not seem to be the case as this type of abortive life-cycle is known in many subtropical conditions and is referred to as androcycly (Margaritopoulos et al. 2002). The oviparae and eggs do not contribute to and nor continue the life cycle instead are a dead end to it. The life cycle continues through migration of alate virginoparae out of the subtropics to mild climate areas and then back as the temperature becomes suitable.
5.3.15 Neomyzus circumflexus (Buckton) Mottled Arum Aphid
It is extremely polyphagous, feeding on numerous species of both monocots and dicots, and even ferns and gymnosperms. In temperate climates N. circumflexus is found especially in glasshouses and on house plants (e.g. Cineraria, Cyclamen, Fuschia, Zantedeschia). Distribution is virtually world-wide. Apparently it is entirely anholocyclic; no sexual morphs have been recorded.
The crescent-marked lily aphid is entirely parthenogenetic with no sexual stage in the life cycle. In temperate climates it is primarily a pest of glasshouse crops where it attacks Asparagus, Begonia, Fuchsia and many others. Heavy infestations cause direct harm to many ornamental plants, and the aphids also transmit viruses (Blackman and Eastop 2000).
5.3.16 Pseudomegoura magnoliae (= Aulacorthum magnoliae) (Essig and Kuwana)
Polyphagous, feeding mainly on leaves of plants in over 20 different families, including Citrus and many ornamental shrubs and trees. Indian records are mainly from Cucurbitaceae (Raychaudhuri et al. 1980a). Life cycle is mainly anholocyclic, with a “relict” holocycle on Sambucus in Japan (Blackman and Eastop 2000). Matsuka and Imanishi (1982) studied its life cycle on Sambucus sieboldiana near Tokyo, where populations overwinter both as viviparae and, less commonly, as eggs. Clones descended from fundatrices produced males and viviparous females, but very few oviparae, so the sexual phase was almost non-existent in that population. Males are also recorded from India (Raychaudhuri et al. 1980b).
5.3.17 Rhopalosiphoninus latysiphon (Davidson) Bulb and Potato Aphid
It colonizes bulbs (Tulipa, Gladiolus) and potato tubers in store, and the roots of many plants, especially in clay soils (e.g. potato crops), or on etiolated stems or runners growing in darkness under stones (e.g. Bromus sterilis, Convolvulus arvensis, Potentilla anserina, Vinca major, Urtica spp.). It is a recorded vector of PVY and also has the ability to tranamit potato leaf roll virus (Bell 1988). Anholocyclic, overwintering on stored bulbs and potatoes in cold temperate regions; sexual morphs are not recorded. However, it is possible that Rhopalosiphoninus deutzifoliae (q.v.) on Hydrangaceae in Japan and east Siberia is the primary host form. Rhopalosiphoninus latysiphon ssp. panaxis Zhang, described from Panax quinquefolium in China (Qiao and Zhang 1999) appears to be a synonym (Blackman and Eastop 2006).
5.3.18 Rhopalosiphum padi (L.) Bird Cherry-Oat Aphid
Common primary hosts are Prunus padus in Europe and P. virginiana in North America. Secondary hosts are numerous species of Graminae, including all major cereal and pasture grasses. Also it has been found overwintering on dicotyledonous weeds (Capsella, Stellaria). Life cycle is heteroecious holocyclic between Prunus padus and Graminae in Europe, or anholocyclic on Graminae where winter conditions permit and in many part of the world where P. padus or alternative primary hosts are not available.
In India, Rhopalosiphum padi is known to infest about 30 species of plants belonging to many families Severe infestation is observed in the poaceous plants (November–March). From centres of infestation, it spreads in ever-widening circles. The aphid is responsible for Barley yellow dwarf (Nagaich and Vashisth 1963) and Wheat streak mosaic (Raychaudhuri and Ganguli 1968).
The species apparently reproduces parthenogenetically throughout the year in India. However, oviparae of this species are recorded from India (Raychaudhuri 1980a, b). This hints at the possibility that it may have sexual life cycle in the altitudinal areas where day length is short and temperature is low which may initiate the production of the sexuales. According to Richards (1960) the number of generations of alienicolae produced is unknown, but certainly several are produced. Fall migrant alate viviparous females resemble spring migrants but are produced by alienicolae and sexuales occur on the winter host from the middle of September to the end of October.
5.3.19 Rhopalosiphum rufiabdominale (Sasaki) Rice Root Aphid
Apterae in spring colonise on young leaves, stems and suckers of Prunus spp. (e.g. mume, salicina, yedoensis) (Moritsu 1983). Rhodotypos scandens may also be used as a primary host (Torikura 1991). Heteroecious holocyclic (Tanaka 1961); alatae migrate in May-June to form colonies on underground parts of numerous species of Poaceae, Cyperaceae and some dicots, particularly Solanaceae (potato, tomato, capsicums). Alatae on secondary hosts normally have 5-segmented antennae. It is a major pest of rice (Yano et al. 1983; Blackman and Eastop 2000). Anholocyclic populations occur throughout most of the world on secondary hosts, particularly in warmer climates and in glasshouses. However, early spring populations have recently been found on Prunus spp. (armeniaca, domestica) in Italy (Rakauskas et al. 2015), indicating that a holocycle may now have been established in Europe.
In India, R. rufiabdominalis has been found to attack roots and aerial parts of potato. Heavy infestations have been recorded on potato roots (Chahal et al. 1974) but the exact role the species plays is still unknown. The adults reproduce parthenogenetically and produce nymphs throughout the year. The alate forms that are produced after 2–3 generations of the apterous forms are responsible for dispersal of this aphid. They are carried to the potato fields by wind and subsequently nymphs are produced on the leaves, which move towards the roots at soil level. In India, alate male was reported and described for the first time from snow at Naini peak (Ca. 8,563 ft) in Uttar Pradesh by Ghosh (1969). Later, Verma (1988) reported apterous oviparous females collected on Peach. The finding of both sexual male and female from the same geographical range hints at the possibility that the species breeds holocyclically at least in the northern part of India.
Young et al. (1971) reported the aphid species from roots and underground stems of barley in Delhi. The aphid colonies were present on the crop from the 1st week of January to the end of February.
5.3.20 Smynthurodes betae Westwood (Bean Root Aphid)
Primary hosts are Pistacia spp. (atlantica, mutica and, rarely, vera). The galls on Pistacia spp. are yellow-green or red, spindle-shaped, about 20 mm long, formed by rolling of the edge of the leaflet near its base. These are secondary galls, produced by the progeny of the fundatrix, which lives in a small red mid-rib gall (Burstein and Wool 1991). Smynthurodes betae is heteroecious holocyclic with a two-year cycle; alatae emerge in September-November and migrate to roots of numerous, mostly dicotyledonous, plants. Secondary hosts are particularly Compositae/Asteraceae (Artemisia, Arctium), Leguminosae/Fabaceae (Phaseolus, Vicia, Trifolium), and Solanaceae (Solanum tuberosum, S. nigrum, Lycopersicon esculentum); also sometimes on Beta, Brassica, Capsella, Gossypium, Heliotropum, Rumex, etc. Only rarely is it found on monocots (Poaceae, Cyperaceae). The holocycle is recorded throughout the range of the primary hosts; Algeria, Morocco, Israel, Syria, Iran, southern Crimea, Transcaucasus and Pakistan. Anholocyclic populations occur commonly on secondary hosts in other parts of the world (Blackman and Eastop 2000).
5.3.21 Uroleucon compositae (Theobald)
On flower stems, and in low numbers along the mid-ribs of the leaves, of a wide range of Compositae/Asteraceae in tropical and subtropical climates, particularly plants growing in moist or shady situations at the end of the dry season. It is a pest of Carthamus tinctoria (safflower) in India (Blackman and Eastop 2000), and is common on herbaceous Vernonia after the rains in Africa (Eastop 1958). Sometimes it is found on non-composite plants such as Malva and Morus. The aphid species is widely distributed in Africa, South America and on the Indian subcontinent. Apparently it is anholocyclic everywhere; no sexual morphs have been recorded. U. compositae is difficult to distinguish from the East Asian species U. gobonis, and it could even possibly be an anholocyclic form of that species. Early records of U. jaceae or U. solidaginis on safflower in Africa or India probably all refer to U. compositae (Blackman and Eastop 2000).
5.4 Summary
Various aphid species such as M. persicae, A. gossypii etc. exhibit huge variation in life cycle. It appears that in tropical and subtropical zones the aphid life cycle gets modified given the exorbitantly high temperature in summer and round the year availability of favourable temperature in tropics and absence of preferred primary hosts. The survival and life cycle variation of aphids in areas other than temperate zone needs thorough exploration.
A section of the population of M. persicae migrates from temperate to tropical and subtropical areas as was identified by Blackman (1974). However the factors inducing production of such migratory clones and their subsequent routes and behaviour in immigration zone needs further exploration. It is of paramount importance keeping in view the impact of M. persicae for the healthy seed production in subtropics like India.
It has been identified that there is influx of various species of aphids from various Poaceous plants into potato bringing viruses along. In this connection the importance of cropping pattern vis-a-vis healthy seed production needs exploration. Since the occasional visitor aphids can contribute to virus transmission and spread in an appreciable manner, the possibility of feeding repellents and physical barriers needs to be re-explored.
References
Bakhetia DRC, Sidhu AC (1977) Biology and seasonal activity of the groundnut aphid Aphis craccivora Koch. J Res Punjab Agric Univ 14(3):299–303. AGRIS record ID = US201302431594
Banerjee H, Ghosh AK, Raychaudhuri DN (1969) On a collection of aphids (Homoptera) from Kutivalley West Himalaya. Orient Ins 3(3):255–264. https://doi.org/10.1080/00305316.1969.10433914
Barlow ND, Dixon AFG (1980) Simulation of lime aphid population dynamics. Pudoc, Wageningen, 165 pp. ISBN: 9022007065
Basu AN (1967) One new genus and seven new species of aphids from Darjeeling district, West Bengal (Homoptera: Aphididae). Bull Ent 8(2):143–157
Basu RC, Raychaudhuri DN (1980) A study on the sexuales of aphids (Homoptera: Aphididae) in India. Records of Zoological Survey of India, Occasional Paper No. 18, 54 pp. ISSN: 0375-1511
Basu RC, Ghosh AK, Raychaudhuri DN (1970) A new genus and records of some sexual forms from Assam. Proc Zool Soc Calcutta 23:83–91
Bell AC (1988) The efficiency of the bulb and potato aphid Rhopalosiphoninus latysiphon (Davidson) as a vector of potato virus V. Potato Res 31(4):691–694. https://doi.org/10.1007/BF02361862
Blackman RL (1971) Variation in the photoperiodic response within natural populations of Myzus persicae (Sulz.). Bull Ent Res 60:533–546. https://doi.org/10.1017/S0007485300042292
Blackman RL (1972) The inheritance of life-cycle differences in Myzus persicae (Sulz.) (Hem., Aphididae). Bull Ent Res 62:281–294. https://doi.org/10.1017/S0007485300047726
Blackman RL (1974) Life-cycle variation of Myzus persicae (Sulz.) (Horn., Aphididae) in different parts of the world, in relation to genotype and environment. Bull Ent Res 63:595–607. https://doi.org/10.1017/S0007485300047830
Blackman RL, Eastop VF (1994) Aphids on the World’s trees. CAB International, Wallingford, 987 pp. ISBN: 0851988776
Blackman RL, Eastop VF (2000) Aphids on the world’s crops: An identification and information guide, 2nd edition. Wiley, Chichester, UK. 466 pp. ISBN: 978-0-471-85191-2
Blackman RL, Eastop VF (2006) Aphids on the world’s herbaceous plants and shrubs. (2 vols) Wiley, Chichester, 1439 pp. ISBN: 978-0-471-48973-3
Blackman RL, Eastop VF (2007) Taxonomic Issues. In: van Emden, HF, Harrington R (eds), Aphids as Crop Pests. CABI, Wallingford, UK., pp 1–29. https://doi.org/10.1079/9780851998190.0001
Blackman RL, Paterson AJC (1986) Separation of Myzus (Nectarosiphon) antirrhinii from M. (N.) persicae and related species in Europe. Syst Ent 11:267–276. https://doi.org/10.1111/j.1365-3113.1986.tb00181.x
Bodenheimer FS, Swirski E (1957) The Aphidoidea of the Middle East. Weizmann Science Press, Jerusalem, p 378
Burstein M, Wool D (1991) A galling aphid with extra life-cycle complexity: population ecology and evolutionary considerations. Res Pop Ecol 33:307–322. https://doi.org/10.1007/BF02513556
Chahal BS, Sekhon SS, Sandhu MS (1974) Rhopalosiphum rufiabdominalis recorded on roots of potato in the Punjab. In: Symposium on problems in potato production. CPRI, Shimla, India
Chakrabarti S, Sarkar A (2001) A supplement to the’ food plant catalogue of Indian Aphididae. J Aphidol 15:9–62
Chandel RS, Chandla VK, Verma KS, Pathania M (2013) Insect pests of potato in india: biology and management In: Giordanengo P, Vincent C, Alyokhin A (eds) Insect pests of potato. Global Perspectives on Biology and Management Elsevier Inc, USA, pp 227–270. http://dx.doi.org/10.1016/B978-0-12-386895-4.00008-9
Chauhaf BS, Sekhon SS, Bindra OS (1975) The incidence and the time of appearence of Myzus persicae in autumn potato crop under different agroclimatic conditions in the Punjab. Indian J Ecol 2:155–162. AGRIS record ID = IN19760083833
Costa CL (1970) Variacoes sazonais da migracao de Myzus persicae em Campinas nos anos de 1967 a 1969. Bragantia 29:347–359. http://repositorio.unb.br/handle/10482/15811
Cottier W (1953) Aphids of New Zealand. Bull N Z Dept Sci Ind Res 106:1–368
David SK (1958) Some rare Indian aphids. J Bombay Nat Hist Soc 55(1):110–116
De Bokx JA, Piron PGM (1990) Relative efficiency of a number of aphid species in the transmission of potato virus YN in the Netherlands. Ned J Pl Path 96(4):237–246. https://doi.org/10.1007/BF01974261
Debraj Y, Singh SL, Shantibala K, Singh TK (1995) Comparative biology of the cabbage aphid, Breuicoryne brassicae (L.) on six cruciferous hosts. J Aphidol 9:30–35
DiFonzo CD, Ragsdale DW, Radcliffe EB, Gudmestad NC, Secor GA (1997) Seasonal abundance of aphid vectors of potato virus Y in the Red River Valley of Minnesota and North Dakota. J Econ Ento 90:824–831. https://doi.org/10.1093/jee/90.3.824
Dixon AFG (1971) The role of intra-specific mechanisms and predation in regulating the numbers of the lime aphid Eucallipterus tiliae L. Oecol 8:179–193. https://doi.org/10.1007/BF00345812
Dixon AFG, Horth S, Kindlmann P (1993) Migration in Insects: cost and strategies. J Animal Ecol 62:182–190. https://doi.org/10.2307/5492
Douglas AE (2003) Nutritional physiology of aphids. Adv Insect Physiol 31:73–140. https://doi.org/10.1016/S0065-2806(03)31002-1
Eastop VF (1958) A study of the Aphididae of East Africa. Colonial Research Publication, H.M.S.O, London, 126 pp
Falk U (1957/58) Biologie and Taxonomic der Schwarzen Blattlause der Leguminosen. Wiss. Z. Uniu Rostock 7(4):616–634
Favret C (2014) Aphid Species File. Version 5.0/5.0. [30-12-2014]. http://Aphid.SpeciesFile.org
Fenton B, Woodford JAT, Malloch G (1998) Analysis of clonal diversity of the peach–potato aphid, Myzus persicae (Sulzer), in Scotland, UK and evidence for the existence of a predominant clone. Mol Ecol 7:1475–1487. http://doi.org/:10.1046/j.1365-294x.1998.00479.x
Fernandez-Calvino L, Lopez-Abella D, Lopez-Moya JJ, Fereres A (2006) Comparison of Potato virus Y and Plum pox virus transmission by two aphid species in relation to their probing behavior. Phytoparasitica 34:315–324. https://doi.org/10.1007/BF02980959
Ghosh LK (1969) Notes on the male of Rhopolosiphum rufiabdominalis (Sasaki) (Homoptera: Aphididae) from Uttar Pradesh, India. Indian J Sci Indust 3(4):215–217
Ghosh LK (1970) On a collection of aphids (Homoptera: Aphididae) from Rajasthan, India. Indian J Sci Indust (B) 4(2):85–89
Ghosh LK (1986) A conspectus of Aphididae (Homoptera) of Himachal Pradesh in Northwest Himalaya, India. Technical Monograph No. 16. Zoological Survey of India, 282 pp
Ghosh LK (1990) A taxonomic review of the genus Aphis Linnaeus (Homoptera: Aphididae) in India. Mem Zool Surv India 17(3):45–48
Ghosh AK, Chakrabarti S, Chowdhuri AN, Raychaudhuri DN (1969) Aphids (Homoptera) of Himachal Pradesh, India-II. Orient Ins 3(4):327–334
Ghosh AK, Raychaudhuri DN (1962) A preliminary account of bionomics and taxonomy of aphids from Assam. J Bombay Nat Hist Soc 59:238–253
Ghosh AK, Ghosh MR, Raychaudhuri DN (1971) Studies on the aphids (Homoptera: Aphididae) from Eastern India. VII. New species and new records from West Bengal. Oriental Ins 5(2):209–221. http://dx.doi.org/10.1080/00305316.1971.10434009
Ghosh AK, Ghosh MR, Raychaudhuri DN (1972) Studies on the aphids (Homoptera: Aphididae) from eastern India XI: Descriptions of hitherto unknown or newly recorded sexual morphs of some species from West Bengal. Oriental Ins 6(3):333–341. http://dx.doi.org/10.1080/00305316.1972.10434083
Halbert SE, Corsini DL, Wiebe MA (2003) Potato Virus Y transmission efficiency for some common aphids in Idaho. Am J Potato Res 80:87–91. http://doi.org/10.1007/BF02870-207
Hales DH, Wilson ACC, Spence JM, Blackman RL (2000) Confirmation that Myzus antirrhinii (Macchiati) (Hemiptera: Aphididae) occurs in Australia, using morphometrics, microsatellite typing and analysis of novel karyotypes by fluorescence in situ hybridisation. Austr J Entomol 39:123–129. https://doi.org/10.1046/j.1440-6055.2000.00160.x
Harrington R, Gibson RW (1989) Transmission of potato virus Y by aphids trapped in potato crops in southern England. Potato Res 32(2):167–174
Harrington R, Katis N, Gibson RW (1986) Field assessment of the relative importance of different aphid species in the transmission of potato virus Y. Potato Res 29(1):67–76. https://doi.org/10.1007/BF02361982
Jalali SK, Singh SR, Biswas SR (2000) Population dynamics of Aphis gossypii Glover (Homoptera: Aphididae) as its natural enemies in the cotton ecosystem. J Aphidol 14:25–32
Jamwal R, Kandoria JL (1990) Appearance and build up of Aphis gossypii (Homoptera: Aphididae) on chilli, brinjal and okra in Punjab. J Aphidol 4:49–52
Katis N, Gibson RW (1985) Transmission of potato virus Y by cereal aphids. Potato Res 28:65–70. https://doi.org/10.1007/BF02357571
Khurana SMP, Naik PS (2003) The potato: an overview. In: Khurana SMP, Minhas JS, Pandey SK (eds) The potato—production and utilization in subtropics. Mehta Publishers, New Delhi, India, pp 1–14
Kim H, Lee W, Lee S (2006) Three new records of the genus Aphis (Hemiptera: Aphididae) from Korea. J Asia-Pacific Ent 9:301–312. https://doi.org/10.1016/S1226-8615(08)60307-6
Komakazi S, Sakagami Y, Korenaga R (1979) Overwintering of aphids on citrus trees. Jap J Appl Ent Zool 23: 246–250. https://doi.org/10.1303/jjaez.23.246
Kring JB (1959) The life cycle of the melon aphid, Aphis gossypii Glover, an example of facultative migration. Ann Ent Soc Am 52:284–286. https://doi.org/10.1093/aesa/52.3.284
Lal L, Verma KD (1987) Seasonal incidence and over seasoning of Myzus persicae (Sulzer) on potato in Meghalaya. Indian J Hill Frmg 1:35–39
Maity SP, Chakrabarti S (1981) On poplar inhabiting aphids (Homoptera: Aphididae) of India and adjoining countries with notes on some species. Entomon 6(4):297–305. AGRIS record no. 19820591768
Margaritopoulos JT, Tsitsipis JA, Goudoudaki S, Blackman RL (2002) Life cycle variation of Myzus persicae (Hemiptera: Aphididae) in Greece. Bull Entomol Res 92:309–319. https://doi.org/10.1079/BER2002167
Matsuka M, Imanishi M (1982) Life cycle of an aphid, Acyrthosiphon magnoliae (Essig et Kuwana) observed near Tokyo and in a laboratory under controlled condition. Bull Fac Agric Tamagawa Univ 22:56–66 (in Japanese)
Misra SS (2002) Aphids as Vector of plant diseases and their chemical control. Abs.: Nat. Seminar on Ecology and Diversity of aphids and aphidophaga complex, Tripura University May 4–5, 2002, p 26
Mondal S, Wenninger EJ, Hutchinson PJS, Weibe MA, Eigenbrode SD, Bosque-Pérez NA (2016a) Contribution of noncolonizing aphids to potato virus y prevalence in potato in Idaho. Env Ent pii: nvw131. http://doi.org/10.1093/ee/nvw131
Mondal S, Wenninger EJ, Hutchinson PJS, Whitworth JL, Shrestha D, Eigenbrode SD, Bosque-Pérez NA (2016b) Comparison of transmission efficiency of various isolates of Potato virus Y among three aphid vectors. Ent Exp et App 158(3):258–268. https://doi.org/10.1111/eea.12404
Moritsu M (1983) Aphids of Japan in colours. Zenkoku Noson, Tokyo, 545 pp (ISBN 4- 88137-017-0)
Müller FP (1978) Untersuchungen über Blattläuse mecklenburgischer Hochmoore. Arch Freunde Naturg Mecklenb 18:31–41
Müller FP, Möller FW (1968) Ein bermerkenswerters Massenauftreten von Myzus ascalonicus Doncaster (Homoptera: Aphididae) in Freiland. Archiv der Freunde der Naturgeschichte in Mecklenburg 14:44–55
Nagaich BB, Vashisth KS (1963) Barley yellow dwarf, a new virus disease for India. Indian Phytopath 16:318–319
Pande SK, Kang GS (2003) Ecological and varietal improvement. In: Khurana SMP, Minhas JS, Pandey SK (eds) The Potato—Production and Utilization in Subtropics. Mehta Publishers, New Delhi, India, pp 48–60
Piron PGM (1986) New aphid vectors of potato virus YN. Ned J Pl Path 92(5):223–229. https://doi.org/10.1007/BF01977688
Powell G, Tosh CR, Hardie J (2006) Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Ann Rev Entomol 51:309–330. https://doi.org/10.1146/annurev.ento.51.110104.151107
Pushkarnath Nirula KK (1970) Aphid-warning for production of seed potato in subtropical plains of India. India J Agr. Sci 40(12):1061–1070
Qiao G, Zhang G (1999) Five new species and one new subspecies of Macrosiphinae from Fujian province, China. Acta Zootaxonomica Sinica 24:304–314
Radcliffe EB (1982) Insect pests of potato. Ann Rev Entomol 127:173–204. https://doi.org/10.1146/annurev.en.27.010182.001133
Ragsdale D, Radcliffe E, diFonzo CD (2001) Epidemiology and field control of PVY and PLRV. In: Lobenstein G, Berger, PH, Brunt AA, Lawson R (eds), Virus and virus-like diseases of potatoes and production of seed potatoes. Kluwar Academic Publishers, Dordrecht, Netherlands. pp 237–70. https://doi.org/10.1007/978-94-007-0842-6_22
Rakauskas R, Bašilova J, Bernotienė R (2015) Aphis pomi and Aphis spiraecola (Hemiptera: Sternorrhyncha: Aphididae) in Europe—new information on their distribution, molecular and morphological peculiarities. Eur J Ent 112:270–280. https://doi.org/10.14411/eje.2015.043
Raychaudhuri DN (1983) Food plant catalogue of Indian Aphididae. Grafic Print All, Calcutta, p 181
Raychaudhuri SP, Ganguly B (1968) A mosaic streak of wheat. Phytopathologische Zeitschrift 59:385–389
Raychaudhuri DN, Pal PK, Ghosh AK (1980a) Subfamily Pemphiginae. In: Raychaudhuri DN (ed) Aphids of North East India and Bhutan. The Zoological Society, Calcutta, pp 409–433
Raychaudhuri DN, Ghosh MR, Basu, RC (1980b) Subfamily Aphidinae. In: Raychaudhuri DN (ed) Aphids of North East India and Bhutan. The Zoological Society, Calcutta, pp 47–275
Richards WR (1960) A synopsis of the genus Rhopalosiphum in Canada. Memoirs Ent Soc Canada 92(S13):5–51. https://doi.org/10.4039/entm9213fv
Shaposhnikov GK (1966) Origin and breakdown of reproductive isolation and the criterion of the species. Ent Rev 45:1–18
Shigehara T, Komazaki S, Takada H (2006) Detection and characterization of new genotypes of Myzus antirrhinii in Japan, with evidence for production of sexual morphs. Bull Ent Res 96:605–611. https://doi.org/10.1079/BER2006460
Shridhar J, Venkateshwarlu, Nagesh M (2015) Aphids. In: Singh BP, Nagesh M, Sharma S, Sagar, Jeevalatha A, Shridhar J (eds) A manual on diseases and pests of potato. Technical Bulletin No. 101. ICAR-central Potato Research Institute Shimla, pp 56–61
Sigvald R (1984) The relative efficiency of some aphid species as vectors of potato virus Yo (PVYo). Potato Res 27(3):285–290. https://doi.org/10.1007/BF02357636
Sigvald R (1987) Aphid migration and the importance of some aphid species as vectors of potato virus YO (PVYO) in Sweden. Potato Res 30:267–283. https://doi.org/10.1007/BF02357668
Sigvald R (1989) Relationship between aphid occurrence and spread of potato virus Yo (PVYO) in field experiments in southern Sweden. J App Ent 108:35–43. https://doi.org/10.1111/j.1439-0418.1989.tb00430.x
Sigvald R (1990) Aphids on potato foliage in Sweden and their importance as vectors of potato virus Yo. Acta Agric Scand 40(1):53–58. https://doi.org/10.1080/0001512900943-8547
Singh R and Ghosh S. (2012) Sexuales of Aphids (Insecta: Homoptera: Aphididae) in India. Lambert Academic Publishing Gmbh & CO KG Germany, pp 402
Singh RP, Kurz J, Boiteau G (1996) Detection of stylet-borne and circulative potato viruses in aphids by duplex reverse transcription polymerase chain reaction. J Virol Methods 59:189–196. https://doi.org/10.1016/0166-0934(96)02043-5
Takada H (1988) Interclonal variability in the photoperiodic response for sexual morph production of Japanese Aphis gossypii Glover (Hom., Aphididae). J Appl Ent 106:188–197. https://doi.org/10.1111/j.1439-0418.1988.tb00582.x
Takahashi R (1923) Aphididae of Formosa Part 2. Rep. Govt Res. lnst. Dep. Agric. Formosa No. 4, 173 pp
Talati GM, Bhutami PG (1980) Reproduction and population dynamics of groundnut aphids. Gujarat Agric Univ J Res 5:54–56
Tanaka T (1961) The rice root aphids, their ecology and control. Spec Bull Coll Agric Utsunomiya 10:1–83
Torikura H (1991) Revisional notes on Japanese Rhopalosiphum, with keys to species based on the morphs on the primary host. Jpn J Ent 59:257–273
Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon JC, Fukatsu T (2010) Symbiotic bacterium modifies aphid body color. Science 330:1102–1104. https://doi.org/10.1126/science.1195463
van Emden HF, Harrington R (2007) Aphids as crop pests, 1st edn. CAB International, Willingford, United Kingdom
van Emden HF, Eastop VF, Hughes RD, Way MJ (1969) The Ecology of Myzus persicae. Ann Rev Entomol 14:197–270. https://doi.org/10.1146/annurev.en.14.010169.001213
van Hoof HA (1977) Determination of the infection pressure of potato virus YN. Ned J Pl Path 83:123–127. https://doi.org/10.1007/BF01981557
van Hoof HA (1980) Aphid vectors of potato virus YN. Eur J Pl Path 86(3):159–162. https://doi.org/10.1007/BF01989708
Verma KD (1988) First record of apterous oviparous females of potato root aphid from India. J Indian Potato Assoc 15:192
Verma KD, Chandla VK (1990) Potato aphids and their management. Technical Bulletin No. 26 (Revised). ICAR-Central Potato Research Institute, Shimla, 34 pp
Verma KD, Chauhan RS (1993) The life cycle of potato vector, Myzus persieae (Sulzer). Curr Sci 65:488–489
Verma KD, Ghosh LK (1990) Discovery of sexual female of Myzus persicae (Sulzer) with redescription of its alate male from India. J Aphidol 4:30–35
Verma KD, Khurana AD (1974) Sexuals of Aphis craccivora Koch, on green gram in India. Entomol News l 4:53
Verma KD, Parihar SBS (1991) Build up of the vector Aphis gossypii (Glover) on potato. J Aphidol 5:16–18
Williams IS, Dixon AFG (2007) Life cycles and polymorphism. In: van Emden H, Harrington R (eds) Aphids as crop pests. CAB International, Wallingford, UK, pp 69–86
Yano K, Miyake T, Eastop VF (1983) The biology and economic importance of rice aphids (Hemiptera: Aphididae): a review. Bull Ent Res 73:539–566. https://doi.org/10.1017/S0007485300009160
Young WR, Bhatia SK, Phadke KG (1971) Rice Root Aphid observed on Barley at Delhi. Entomol Newsl 1:53
Zhang G, Zhong T (1982) Experimental studies on some aphid life-cycle patterns. Sinozoologia 2:7–17
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Shah, M.A., Jandrajupalli, S., Venkateshwarlu, V., Malik, K., Bhatnagar, A., Sharma, S. (2018). Population Ecology of Aphid Pests Infesting Potato. In: Gaba, S., Smith, B., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 28. Sustainable Agriculture Reviews, vol 28. Springer, Cham. https://doi.org/10.1007/978-3-319-90309-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-90309-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90308-8
Online ISBN: 978-3-319-90309-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)