Abstract
Foot-and-mouth disease remains one of the most feared viral diseases affecting cloven-hoofed animals such as cattle, pigs, sheep and goats. The disease has been successfully eradicated from some regions like North America, Western Europe and Australia, but it is still endemic in most of the world. Although mortality is generally low in adult animals, outbreaks result in devastating economic consequences due to production losses and a major constraint to international trade of live animals and their products. Immunization with the current inactivated vaccine has been successfully used in many parts of the world. However, its production process requires the growth of large amounts of infectious virus in high-level bio-containment facilities, which is not only very expensive but carries the risk of escape of live virus during vaccine manufacture and/or incomplete inactivation. Because of these hazards and other limitations, such as thermal instability, short duration of immunity and lack of cross protection, intense research focused on the design of improved vaccines, has been developed. Important issues concerning foot-and-mouth disease occurrence, pathogenesis and vaccine development, are reviewed in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Disease symptoms and occurrence
1.1 Foot and Mouth Disease and geographical distribution
Foot-and-mouth disease (FMD) has been recognized as one of the most important contagious viral diseases of cloven-hoofed livestock. Although mortality associated with FMD is usually low, this severe disease poses a significant economic impact worldwide, due to both direct losses because of reduced milk and meat production, and indirect losses caused by costs of disease control and poor access to markets (Knight-Jones and Rushton 2013).
The disease was present in almost every livestock-containing region worldwide until the early 20th century, when it was progressively eradicated from the developed world. Since then, various major outbreaks have occurred in south-east Asia (Japan, South Korea and Taiwan), South America (Paraguay, Argentina, Uruguay and Brazil) and Western Europe (UK, The Netherlands, France and Ireland) (Knowles et al. 2001; Thomson et al. 2003; Brito et al. 2015). Nowadays, North America, most European countries, Australia, New Zealand and many island states, are recognized as FMD-free; the disease persists in South America, most African countries, the Middle East, and many parts of Asia, where the disease is endemic (Thomson et al. 2003). Updated information on the incidence and distribution of FMD as well as epidemiological reports, can be obtained from the Office International des Epizooties (OIE) website at http://www.oie.int/en/animal-health-in-the-world/official-disease-status, or the website of the World Reference Laboratory for FMD at http://www.wrlfmd.org.
Currently, the OIE recognizes countries and zones to be in one of three disease states with regard to FMD: FMD present with or without vaccination, FMD-free with vaccination and FMD-free without vaccination (Fig. 1). It has been recently estimated that outbreaks in FMD free countries and zones cause losses of more than US$1.5 billion a year (Knight-Jones and Rushton 2013). Therefore, many of the FMD-free countries, including Canada, the United States, and the UK, work hard to maintain their current status, focusing their control policy on minimizing the risk of virus incursion and the impact of an outbreak should one occur. In South America, after the regional eradication plan implemented in 1988, at present most of the countries/regions have their status recognized by the OIE as FMD-free either with or without vaccination.
FMD status recognized by the OIE. With the kind authorization of the World Organization for Animal Health (OIE), www.oie.int. Map extracted from OIE website on December 2017, available at http://www.oie.int/en/animal-health-in-the-world/official-disease-status/fmd/en-fmd-carte
The disease affects a wide variety of species, but cloven-hoofed animals (order: Artiodactyla) have a critical epidemiological role in maintaining the virus in the environment. Cattle, pigs, sheep, goats and water buffalo (Bubalus bubalis) are susceptible to viral infection and can spread the disease (Alexandersen and Mowat 2005). African buffalo (Syncerus caffer) play an important role as the natural maintenance host in Africa (Thomson et al. 2003). They are mainly thought to maintain the South African Territories (SAT) types of the virus, although antibodies to other serotypes have been found in buffalo populations of west and central Africa (Di Nardo et al. 2015).
Other animals may contribute to viral transmission under certain conditions including Indian elephants and any animal of the order Artiodactyla, like deer, camels, llamas, and alpaca. These species do not appear to play an important role in the wild, but they have to be considered as a potential risk when they are kept under farmed or crowded conditions (Thomson et al. 2003; Alexandersen and Mowat 2005). Although laboratory mice, guinea pigs and rabbits are susceptible to infection with foot-and-mouth disease virus (FMDV) under experimental conditions, there is no evidence of such animals being involved in the spread of FMD in the field (Alexandersen and Mowat 2005).
Foot-and-mouth disease virus is the aetiological agent of this infectious disease and it was the first virus of vertebrates to be discovered (Loeffler and Frosch 1897). Seven major viral serotypes have been described, termed O, A, C, Asia 1 and South African Territories (SAT) 1, 2 and 3 (Grubman and Baxt 2004), and each contains several, constantly-evolving subtype strains (Bachrach 1968). These serological types were assigned on the basis of lack of cross-protection after infection or vaccination. Viruses showing partial cross-protection were assigned to the same serotype but to a different subtype (Domingo et al. 2002).
FMDV serotypes are not distributed uniformly across the world. Six of these serotypes (O, A, C, SAT-1, SAT-2, SAT-3) have occurred in Africa, four in Asia (O, A, C, Asia 1), and only three in South America (O, A, C) (Rweyemamu et al. 2008). A particular strain of serotype O, named PanAsia, was responsible for the explosive pandemic across Asia, Africa and Europe from 1998 to 2001. The PanAsia strain caused outbreaks in the Republic of Korea, Japan, Russia, Mongolia, South Africa, the United Kingdom, Republic of Ireland, France, and the Netherlands, countries which had last experienced FMD outbreaks decades before (Knowles et al. 2005). There have been sporadic incursions of serotypes SAT-1 and SAT-2 from Africa into the Middle East, at the crossroad between Africa, Europe and Asia (Valarcher et al. 2008). Serotype C was last detected in Kenya and Brazil in 2004 (Sangula et al. 2011), and in Ethiopia in 2005 (Rweyemamu et al. 2008).
1.1.1 Disease symptoms
The incubation period for FMD is highly variable since it depends to a high degree on the dose of virus received, the route of transmission and the specific strain of FMDV (Gailiunas and Cottral 1966; Arzt et al. 2011). In cattle, the first symptoms are high fever, anorexia, and lesions that initially present as blanched areas. These lesions progress into vesicles, which increase in size and rupture, generating areas of epithelial damage. They are typically located on the tongue, hard palate, dental pad, lips, gums, muzzle, coronary band and interdigital spaces (Fig. 2), and may also be present on teats, particularly of lactating cows (Kitching 2002). According to the location of these vesicles, animals can present abundant secretion of foamy saliva, drooling and lameness (Kitching 2002). Consequently, feeding, milking and suckling become difficult, resulting in a rapid weight loss and a marked decrease in milk production (Shahan 1962).
The disease in sheep and goats is in general clinically milder than in cattle, with lameness being the first indication of FMD, since pain may be detected in the feet for 1–2 days before vesicular lesions appear. Vesicles may develop in the interdigital cleft, on the heel bulbs, on the coronary band and in the mouth (Kitching and Hughes 2002; Hughes et al. 2002). It has been reported that up to 25% of infected sheep may fail to develop lesions, and an additional 20% may form only one lesion (Hughes et al. 2002).
In pigs, clinical disease is usually severe, and the early signs include acute lameness, reluctance to stand, depression, loss of appetite and fever (Alexandersen et al. 2003). Vesicles around the coronary bands are the most consistent finding in pigs, while lesions at other sites like the bulb of the heel, the interdigital space, the snout, lower jaw and tongue, may be found less regularly depending on environmental and other factors (Fig. 3) (Kitching and Alexandersen 2002; Stenfeldt et al. 2014b; Stenfeldt et al. 2016a).
FMD in pigs. a unruptured vesicles in the coronary bands, b lesion on the snout (reproduced from Alexandersen and Mowat 2005)
Although most animals eventually recover from FMD, the disease can lead to myocarditis and death, especially in young calves, piglets and lambs (Kitching and Hughes 2002; Kitching 2002; Alexandersen et al. 2003; Grubman and Baxt 2004).
2 Mechanism of infection
2.1 The virus
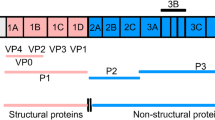
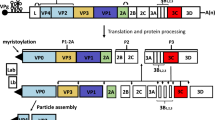
FMDV is a single-stranded positive-sense RNA virus belonging to the genus Aphthovirus in the family Picornaviridae (Bachrach 1968). The viral genome of about 8,500 nucleotides contains a single long open reading frame (ORF) flanked by heavily structured 5´- and 3´-untraslated regions (UTR) (Fig. 4). The 5´UTR consists of a short (S) fragment, a poly (C) tract, repeated pseudoknots (PKs), a cis-acting replication element (cre), and an internal ribosome entry site (IRES), which is required for viral replication and translation (Belsham 2005). Viral mRNA translation begins at two in-frame AUG codons, rendering a polyprotein precursor which is co-and post-translationally proteolytically processed to generate both the intermediate and mature structural and non-structural proteins. The first mature product of viral translation is Lpro, a papain-like protease of fundamental role in virulence (Kleina and Grubman 1992). The capsid precursor P1 is cleaved by the 3C protease to produce the structural proteins VP0, VP3, and VP1. The most immunogenic epitope in all FMDV serotypes is located within a highly variable G-H loop (residues 141-160) in the VP1 protein (Borrego et al. 1995; Mateu et al. 1995). The P2 and P3 regions encode non-structural proteins (NSP), which play critical roles in RNA replication, translation, viral assembly and maturation, and virulence (Grubman and Baxt 2004; Belsham 2005). At the end of the ORF there is a relatively short 3´UTR composed of two stem–loops and a poly (A) tail, both required for viral infectivity and known to stimulate IRES activity (Rohll et al. 1995; Serrano et al. 2006). One copy of the structural proteins VP0, VP3 and VP1 spontaneously assemble and form the 5S protomer, which subsequently assembles into the 12S pentameric subunit. Twelve of these pentameric subunits associate to form the icosahedral empty capsid-like particle (75S) (Belsham 1993). Encapsidation of viral RNA to produce mature virion (146S) is accompanied by the cleavage of VP0 into VP2 and VP4. This non-enveloped icosahedral particle is about 25-30 nm in diameter (Racaniello 2001).
Schematic diagram of FMDV genome, processing of viral polyprotein and capsid assembly. The 5ʼUTR, including the S-fragment, poly(C), pseudoknot structures (PKs), cis-acting replication element (cre), and IRES, is adapted from Mason et al. (2003). The intermediate and mature structural and non-structural proteins are named according to the nomenclature of Rueckert and Wimmer (Rueckert and Wimmer 1984). The sites of the primary cleavages and the proteases responsible are indicated
2.1.1 Pathogenesis
Receptors are the major determinant factors for the tropism and pathogenesis of viruses. Different integrin proteins have been shown to be receptors for FMDV: αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8; yet, αvβ6 has been identified as one of the most efficient receptors for all FMDV serotypes and its expression is associated with FMDV tropism (Jackson et al. 2000; Jackson et al. 2002; Jackson et al. 2004; Monaghan 2005; Dicara et al. 2008). The integrin receptor recognition site involves a highly conserve Arg-Gly-Asp (RGD) amino acid sequence motif present on the G-H loop of the VP1 capsid protein (Fox et al. 1989; Baxt and Becker 1990; Verdaguer et al. 1995; McKenna et al. 1995). In addition to cellular integrins, FMDV is also able to utilize alternative membrane molecules as receptors (reviewed in Ruiz-Sáenz et al. 2009 and Wang et al. 2015). It has been demonstrated that in vitro cultivation of FMDV selects viruses that bind to heparan sulfate, resulting in the attenuation of virulence in host species (Sa-Carvalho et al. 1997).
Most of the current knowledge about the pathogenesis of FMDV has been achieved by experimental studies in cattle and pigs. Although there are numerous reports on the subject, there is still no consensus regarding the early stages of infection. This may be due to the fact that the efficiency of transmission under experimental conditions varies between different serotypes and subtypes of FMDV, which may have considerable differences in tissue tropism and virulence. Furthermore, other factors, such as the inoculation system, the duration of exposure, housing density and differences in sensitivity and/or specificity of virus detection methods, may directly influence the results of experimental transmission studies (Quan et al. 2004; Quan et al. 2009; Pacheco et al. 2012; Stenfeldt et al. 2014b; Pacheco et al. 2016).
As has been recently reported by Stenfeldt and co-workers (2016a), although cattle and pigs may be similarly susceptible to FMDV infection under most circumstances, there are critical differences between these two animals in FMD pathogenesis and infection dynamics. These include variations in susceptibility of infection by different routes of virus exposure, which lead to differences in the mechanisms of virus transmission; variations in the amount of virus shed by aerogenous routes, and variations in the capability of clearing infectious virus from all tissues. Additionally, species-specific variation has been also demonstrated, in both systemic and cellular responses to infection (Toka and Golde 2013). Therefore, the pathogenesis of FMDV will be analyzed in each of these species separately.
2.1.1.1 FMD in cattle
Susceptible cattle in contact with an infected animal are most likely to be infected via the respiratory route by aerosolized virus (Sellers and Parker 1969). Infection can also occur through abrasions on the skin or mucous membranes, although it is less efficient requiring almost 10,000 times more virus (Donaldson 1987). Indirect contact with FMDV may also occur via contaminated persons, vehicles, fomites, and wild animals. Since the virus is excreted into the milk of dairy cattle, calves can become infected by insufflation of milk droplets as they drink (Donaldson 1997). Under appropriate environmental conditions, spread by airborne carriage on the wind is likely to occur, with some isolates potentially spreading up to about 300 km by the wind (Gloster et al. 1982; Sørensen et al. 2000).
Numerous studies have investigated the events occurring during and immediately following the host’s initial exposure to the virus. In cattle, FMD has been experimentally reproduced by simulated natural methods (direct or indirect contact with infected animals or virus aerosols from such animals), or by artificial methods like parenteral inoculation of virus (intradermal, intravenous, subcutaneous, or intramuscular), intranasal instillation, pulmonary inoculation or exposure to artificially created aerosols. Of these, direct introduction of virus to the respiratory tract has been widely used since it has the advantage of simulating the natural route of transmission, allowing the study of early events in pathogenesis (Alexandersen et al. 2003; Sellers and Gloster 2008).
By using a controlled aerosol inoculation system, recent reports demonstrated that after aerolization of cattle with FMDV O1-Manisa and A24-Cruzeiro, there is a primary replication in epithelial crypts of the nasopharynx, followed by extensive replication in pneumocytes in the lungs and the establishment of viremia and generalized disease (Arzt et al. 2010; Pacheco et al. 2010; Arzt et al. 2014). These reports have changed the original notion that after initial replication in the pharynx, FMDV is spread through the regional lymph nodes (Henderson 1948; Burrows et al. 1981).
Once viremia is established, the virus spreads to different tissues and organs, especially the skin and the epithelia of the tongue and mouth, where the main viral amplification occurs. This generates the characteristic vesicles that are also present in feet, teats and prepuce (Alexandersen and Mowat 2005; Arzt et al. 2009). During the viraemic and clinical phase of the disease, all excretions and secretions (saliva, nasal and lachrymal fluid, milk and expired breath) become infectious and can contain significant titres of virus. Urine and faeces also contain virus but to a lesser extent (Alexandersen et al. 2003).
Following the acute phase of FMDV infection, some ruminants may experience a long asymptomatic persistent infection. This persistent infection can occur either after a clinical or a subclinical FMD infection, and occurs in vaccinated and in non-vaccinated animals (Doel et al. 1994). Carrier animals are defined as those from which infectious FMDV can be recovered from oropharyngeal fluid for more than 28 days after infection (Van Bekkum et al. 1959). The prevalence rate of carriers depends on the species, the incidence of disease (or infection) and the immune status of the population (Alexandersen et al. 2003). In cattle, approximately 50% of infected animals become carriers, irrespective of their vaccination status (Moonen and Schrijver 2000). Studies on persistently infected animals have reported that FMDV may persist in the dorsal surface of the soft palate and the pharynx (Burrows 1966), more specifically in epithelial cells of the pharyngeal area (Zhang and Kitching 2001), and also in follicular dendritic cells within the germinal centres of lymphoid follicles (Juleff et al. 2008). The role of carrier animals in the epidemiology of the virus is still controversial, though there is evidence of transmission from buffalo to cattle both experimentally (Dawe et al. 1994b) and under natural conditions (Dawe et al. 1994a) in Africa.
2.1.1.2 FMD in pigs
Of the domestic species, pigs liberate the largest quantities of aerosolized FMDV, reaching values up to 105.6 to 108.6 TCID50 per pig per day, whereas ruminants excrete about 104–105 TCID50 per day. However, pigs are less susceptible to airborne infection when compared to ruminants, since it has been demonstrated that the latter can be infected by airborne exposure with only 10 TCID50, while more than 103 TCID50 are needed to infect pigs by this same route (Sellers and Parker 1969; Donaldson et al. 1970; Donaldson and Alexandersen 2001; Alexandersen et al. 2002a; Alexandersen and Donaldson 2002).
Recent investigations have confirmed that pigs are more susceptible to FMDV infection via exposure of the upper gastrointestinal tract (oropharynx) than through inhalation of virus (Stenfeldt et al. 2014a; Fukai et al. 2015). By using a simulated natural experimental system that has been previously demonstrated to efficiently infect pigs, Stenfeldt and co-workers have identified as the primary site of FMDV replication, segments of reticular epithelium within the paraepiglottic tonsils (Stenfeldt et al. 2014b).
After 24 h of natural or experimental exposure, pigs become viraemic and this is associated with increased shedding of virus via the oropharyngeal route (Murphy et al. 2010; Stenfeldt et al. 2014b; Stenfeldt et al. 2014c; Stenfeldt et al. 2016b). As has been recently demonstrated, the tonsil of the soft palate is the only tissue in the respiratory or gastrointestinal tract that supports substantial levels of FMDV replication (Alexandersen et al. 2001; Stenfeldt et al. 2014b), suggesting that this would be the best candidate as the source of aerosolized virus derived from pigs. Since these clinically infected pigs constitute an important source of contagion for exposed ruminants, movement of live pigs or associated products can have substantial impact on disease spread. Therefore, FMD control policies should be based on species-specific data and should be adapted to account for the animal population in each region.
Recent studies performed in domestic pigs indicate that there is a high prevalence of FMDV RNA and capsid antigen in lymphoid tissues for 30–60 days after infection (Stenfeldt et al. 2014c), indicating transient persistence of FMDV in these animals. However, the virus could not be isolated from a large number of porcine tissues processed, confirming that domestic pigs are not competent long-term carriers of FMDV (Arzt et al. 2011; Stenfeldt et al. 2014c).
3 Plant-made vaccines candidates
Vaccines play a vital role in FMD control, since they are used both to limit the spread of the virus during an outbreak in FMD-free countries and to control disease in endemic regions. Current FMD vaccine consists of binary ethyleneimine-inactivated whole virus preparation, formulated with an oil-based adjuvant (Doel 2003). Systematic vaccination programs with this inactivated vaccine have successfully reduced the number of disease outbreaks in enzootic areas, including Western Europe and parts of South America where FMDV has been eliminated with the use of this vaccine (Brown 2003). However, since there are a number of concerns about its use (see “Inactivated FMD vaccine”), there are several strategies that have been explored with the aim of developing recombinant FMDV vaccines that are safer, more effective, more stable and more economical. One of these strategies is the use of plants as bioreactors.
Most of the attempts to develop plant-based anti-FMDV vaccines have concentrated on the expression of capsid protein VP1 either in transgenic plants or transiently, since this protein contains the immunodominant epitope from the virus (summarized in Table 1) (Ruiz et al. 2015). First reports were based on the use of chimeric plant viruses engineered to display short viral sequences of FMDV on its surface as a safe way to produce vaccines. Cowpea mosaic virus (CPMV) was the first plant virus used to express a VP1 epitope of FMDV on the surface of the virus and used to infect cowpea plants, raising the possibility of producing vaccines in plants (Usha et al. 1993). However, subsequent studies revealed that the chimera did not spread systematically in cowpea, could not be purified in significant quantities, and the inserted sequence was rapidly lost during serial passages in plants, probably by a process of homologous recombination (Porta et al. 1994). Further investigation showed that both length and isoelectric point (pI) of the inserted sequence have profound effects on the growth of chimeras, indicating that chimeras with inserts that are not significantly greater than 30 amino acids and have a pI below 8.0 should give virus yields similar to those obtained with wild-type virus (up to 1 mg per gram of infected leave tissue) (Porta et al. 2003; Montague et al. 2011). When the pI of the FMDV insert was adjusted to be close to 7 by the addition of acidic residues at either or both sides of the insert, the infection could be passaged to further plants, and sequence analysis of RNA isolated from purified virus particles indicated that no reversion or mutations had taken place following serial virus passaging (Montague et al. 2011). A few years later, Wigdorovitz and co-workers reported the expression of the complete open reading frame of VP1 using a recombinant Tobacco mosaic virus (TMV). Crude extracts of Nicotiana benthamiana plants transiently infected with the recombinant virus were injected intraperitoneally into mice. Animals received 5 doses of leaf extract containing approximately 0.5–1 µg of recombinant VP1per dose. All immunized animals developed a significant and specific immune response and were protected from the experimental challenge with virulent FMDV. Additionally, the recombinant VP1 was synthesized in comparable quantities in both inoculated and systemic leaves and its concentration was maintained at detectable levels for an extensive period during the replication of the TMV vector (Wigdorovitz et al. 1999b). It is worth noting that both guinea pigs and mice have been used extensively to study FMDV pathogenesis and vaccine efficacy trials, and that the mouse model has successfully predicted immune responses to FMDV in cattle and pigs (Habiela et al. 2014).
Using the same strategy, Tobacco mosaic virus (TMV)-based expression of short VP1 antigenic peptides of 11 or 14 amino acids, fused to the open reading frame of TMV coat protein (CP), has also been described (Wu et al. 2003a). Both recombinant viruses (TMVF11 and TMVF14) systemically infected tobacco plants, producing stable progeny particles as efficiently as wtTMV (about 5 g of purified particles per kilo of infected leaves). Moreover, TMVF11 and TMVF14 were quite stable and no loss of the peptide insert or degradation of the viruses was detected even after three sequential passages in tobacco plants by mechanical transmission. When the mixture of recombinant viruses was parenterally injected in guinea pigs (0.6 mg total in one dose), all the animals were protected against challenge. Preliminary tests in swine, showed that 9 pigs immunized once by parenteral injection of 3 mg of TMVF11 were protected against FMDV challenge (Wu et al. 2003a). The same group also developed an improved TMV-based vector in which up to six C-terminal amino acid residues could be deleted from the coat protein subunit, allowing the expression of a longer peptide of 25 amino acids, containing two fused epitopes (F14 and F11). Although yields of the recombinant viruses were higher, immunity in guinea pigs and swine appears to be less efficient as previously reported, possibly due to lack of a spacer arm between the two fused epitopes (Jiang et al. 2006).
Chimeric Bamboo mosaic virus (BaMV) expressing T and B-cell epitopes of VP1 was produced in C. quinoa and N. benthamiana plants, and proved to induce not only strong humoral (as indicated by neutralizing antibodies) and cell-mediated immune responses (as indicated by VP1-specific IFN-γ production), but also full protection against FMDV in swine even after just one inoculation of 1 mg of the chimeric virus (Yang et al. 2007). Recently, the same group reported the innovative development of transgenic cell-suspension cultures from N. benthamiana leaves carrying wild-type or chimeric BaMV expression constructs encoding VP1 epitopes (Muthamilselvan et al. 2016), providing a cost-effective and efficacious means of producing vaccine candidates. These purified chimeric virus particles triggered the production of specific antibodies in guinea pigs.
Tobacco necrosis virus-A (TNV-A) was engineered as a vector to express different peptides from FMDV VP1. Most of the obtained chimaeras contained unmodified foreign peptides even after six successive passages in Chenopodium amaranticolor and three passages in N. benthamiana, suggesting long-lasting stability. The purified chimeric virus particles could induce a strong immune response against VP1 in mice immunized intramuscularly with three doses of 0.2 mg. Mice immunized intranasally with 5 doses of 0.1 mg of chimeric virus particles developed both systemic and mucosal immune responses against FMDV VP1(Zhang et al. 2010). More recently, Li et al. showed that a nine amino acid sequence from VP1 could be expressed on the surface of apple latent spherical virus (ALSV) and that this chimeric virus can infect N. benthamiana and Chenopodium quinoa plants. However, the immunogenicity of this virus particles was not evaluated (Li et al. 2014a).
The expression of VP1 in transgenic plants has also been used in the development of experimental vaccines, some of them oriented to the development of edible vaccines. First attempts have successfully demonstrated that VP1 of FMDV can be expressed in Arabidopsis thaliana, alfalfa and potato plants (Carrillo et al. 1998; Wigdorovitz et al. 1999a; Carrillo et al. 2001). In these studies a binary vector containing the Cauliflower Mosaic Virus (CaMV35S) promoter was used to direct constitutive expression of the foreign gene. Mice parenterally immunized with 4 or 3 doses of 15-20 mg of fresh leaf tissue presented a FMDV VP1 specific antibody response that provided 77-100% protection against challenge (Carrillo et al. 1998; Wigdorovitz et al. 1999a; Carrillo et al. 2001). Moreover, 0.3 g of fresh leaves from alfalfa plants were used to feed mice three times a week for 2 months. All orally immunized mice developed a specific antibody response, and 66 to 75% of the animals showed protection after the experimental challenge (Wigdorovitz et al. 1999a). However, since the concentration of the expressed protein was low in all those cases (approximately, 0.005–0.01% of the total soluble protein) (Carrillo et al. 2001), the same group developed an experimental immunogen based on the expression of VP1 fused to the glucuronidase (gus A) reporter gene, which allowed the selection of transgenic plants expressing high levels of the recombinant protein by the β-glucuronidase (βGUS) enzymatic activity (Dus Santos et al. 2002). By using this alternative, authors were able to select transgenic plants of alfalfa with expression levels 10 times higher than those observed in transgenic plants previously developed (Carrillo et al. 2001). Mice immunized intraperitoneally with the transgenic selected plants (100 mg of fresh leaf tissue) developed a strong and protective antibody response against virulent FMDV.
The use of transgenic plants expressing the antigen protein of FMDV as feedstuff additives was first reported by Wang and co-workers (Wang et al. 2008). They produced transgenic Stylosanthes guianensis cv. Reyan II plants expressing FMDV VP1 protein, to levels of 0.1–0.5% total soluble protein (TSP). Mice that were orally immunized using the transgenic hay meal developed a specific immune response. A few years later, Rao et al. developed transgenic forage crop Crotalaria juncea expressing FMDV VP1 proteins of serotypes O and A linked in tandem, and tested them as bivalent FMD vaccine in guinea pigs (Rao et al. 2012). Animals were immunized twice with leaf-extracted proteins corresponding to 1 g of the leaf material or oral fed with 1 g of leaves of the transgenic plants. Guinea pigs reacted to orally or parenterally applied vaccine, by humoral as well as cell-mediated immune responses. Two of three animals (66%) were protected against challenge with the virus of both serotypes. Guinea pigs immunized with the conventional inactivated vaccine were fully protected against challenge, and the authors suggest that this could be due to the presence of conformational epitopes of the capsid proteins which can induce better immune response, than only 2 sequential epitopes of VP1 protein.
In another approach, transgenic rice expressing the capsid precursor polypeptide (P1) of FMDV was generated by Agrobacterium-mediated transformation. Expression levels of the recombinant P1 protein ranged from 0.6 to 1.3 mg/g of TSP in transgenic rice leaves, which was sufficient to induce a protective immune response in mice after four intraperitoneal immunizations with transformed plant extracts (containing approximately 10 µg of P1 protein). In addition, when mice were fed with 100 µl of transformed plant extract (containing approximately 10 µg P1 protein) five times per week for one month, FMDV-specific systemic and mucosal immune responses were detected, as well as partial virus clearance after challenge (Wang et al. 2012).
In order to achieve high accumulation of recombinant proteins in plants, transplastomic technology has also been attempted. In this regard, VP1 recombinant protein accumulated in tobacco chloroplasts to 3% of TSP (Li et al. 2006b). Similarly, when a highly immunogenic epitope of VP1 was fused to the ßGUS protein, high accumulation of the recombinant protein was produced in tobacco transplastomic plants, representing 51% of the soluble proteins in mature leaves (Lentz et al. 2010). This protein was also found to be immunogenic in mice.
Generally, vaccines based on individual viral proteins rarely present epitopes in their native conformation, making them less effective than whole virus preparations. Therefore, this kind of subunit vaccines often require more doses of antigen as well as adjuvanted delivery systems for immune response stimulation. In order to improve immunogenicity of subunit vaccines, alternative strategies have been explored, including the production of polyepitope and empty capsids. In this regard, an anti-FMD vaccine based on a recombinant protein formed by a set of well-studied B- and T-cell viral epitopes was developed (Andrianova et al. 2011). For this purpose, codon-optimized genes encoding B-cell epitopes of the structural proteins VP4 and VP1, and T-cell epitopes of nonstructural proteins 2C and 3D were generated and cloned together divided by ‘flexible’ glycine-rich linkers G4S2 to avoid potential problems of protein folding. This recombinant polyepitope protein (H-PE) was expressed in N. benthamiana plants using a phytovirus expression system. A single intramuscular injection of guinea pigs with an oil emulsion containing 120 µg of the purified protein induced the formation of virus-neutralizing antibodies to the homologous FMD virus and no evidence of infection was seen after challenge.
The production of recombinant FMDV empty capsids or virus-like particles (VLPs) requires the simultaneous expression of the capsid precursor P12A and the protease 3C. This viral protease processes the P12A precursor to generate structural proteins (VP0, VP3 and VP1), which then self-assemble to form the viral capsid. The first attempt towards the production of plant-based FMDV VLPs has been the expression of the precursor P12A and the viral protease 3C in alfalfa plants (Dus Santos et al. 2005). Although the formation of FMD VLPs could not be reliably demonstrated, the administration of 4 doses of 15–20 mg of fresh leaf tissue from transgenic plants was able to evoke a strong antibody response in mice. In addition, all animals were protected from FMDV challenge. Likewise, transgenic tomato plants expressing P12A and 3C were produced (Pan et al. 2008). Although the expression and processing of the capsid precursor was demonstrated, the authors could not conclusively determine whether the capsid proteins assembled into VLPs since an electron microscopy analysis was not performed. However, all guinea pigs intramuscularly immunized with 3 doses of 50 mg fresh leaf tissue from transgenic tomato plants developed a virus-specific antibody response and were protected against challenge infection. More recently, it has been reported the expression of the P1 precursor modified to contain a CPMV 24K protease recognition site instead of the native 3C protease, as this is less toxic than 3C when expressed in plants (Saunders et al. 2009). When the modified P1 precursor was co-expressed with a CPMV 24K-containig construct in N. benthamiana leaves, the individual capsid proteins were identified, suggesting the correct cleavage by the CPMV 24k protease. However, no VLPs were observed by electron microscopy, indicating that either assembly or stability of FMDV capsids may not be optimal in plant cells (Thuenemann et al. 2013). Although these reports could not conclusively demonstrate the formation of VLPs, it is possible that other subviral structures were present. As mentioned in sect. 2.1, capsid proteins spontaneously assemble to form the protomer (5S), five of which subsequently form the pentamer (12S), and finally twelve pentamers assemble into the empty capsid (75S). A critical threshold concentration of pentamers is required before capsid formation can occur (Zlotnick and Stray 2003; Goodwin et al. 2009). Therefore, it is likely that recombinant pentamer protein yield was below this threshold, or that the VLPs were actually assembled, but due to instability problems would subsequent dissociate into more stable 12S pentameric capsid subunits.
4 Established vaccine and alternatives
4.1 Inactivated FMD vaccine
Foot-and-mouth disease vaccines represent the largest share of sales in the veterinary vaccine market worldwide, meaning 26.4% of the entire livestock biological business (Gay et al. 2003). Routine vaccination against FMD is often applied in countries or zones recognized as “FMD-free with vaccination” to maintain FMD-free status, and in endemic countries to control the virus.
Currently, all FMD vaccines are produced by infecting baby hamster kidney-21 (BHK-21) cells with virulent FMDV in roller bottles or in suspension under biosecure conditions in large volumes. The virus is harvested, chemically inactivated with binary ethyleneimine (BEI), concentrated by polyethylene glycol (PEG) precipitation, and purified by ultrafiltration. Some vaccine manufacturers use industrial scale chromatography to purify the antigens previously concentrated by ultrafiltration (Doel 2003). For vaccine formulation, the purified antigen is diluted with buffers and mixed with either oil or aluminum hydroxide/saponin adjuvants. The oil-emulsion vaccines are preferred for FMD prevention as they can be used to protect all susceptible species and are ideal for emergency vaccination, whereas aluminum hydroxide/saponin-adjuvanted vaccines are not recommended in pigs due to low efficacy in this species (Cao 2014).
Considering that vaccination with one serotype does not protect against other serotypes, the antigenic composition of FMD vaccines must represent the epidemiological situation of the particular country or region. This is especially important for endemic areas where several FMD viral subtypes may be circulating and where vaccination with trivalent vaccines is needed (Parida 2009). This highlights the importance of surveillance plans in order to know the local strains of FMD virus circulating in a region.
As mentioned before, the use of this inactivated vaccine has proven to be a critical component of control and eradication programs worldwide. However, there are still some concerns and shortcomings, most of them related with the vaccine production process. Since this process requires the growth of large volumes of virulent virus, expensive facilities for high biological containment are required for production. Additionally, there is a constant risk of live virus release from the manufacturing sites or inadequate inactivation of the virus. A further problem is that vaccines may contain traces of contaminating viral NSP; these vaccines will therefore induce the production of antibodies against NSP in the same way as natural infection, interfering with the NSP-based serological differentiation between infected and vaccinated animals (DIVA). Furthermore, while inactivated FMD vaccines are effective in preventing clinical disease, they do not necessarily prevent viral replication in the epithelial surface of vaccinated animals, which can result in persistent infection (Alexandersen et al. 2002b), a situation which can be very costly in lost trade if vaccination is included in the control policy of a country or a region normally free of FMD. Therefore, although there is no evidence that these vaccinated carrier animals can transmit virus, their occurrence is one of the main barriers to implement vaccination in control and eradication of disease outbreaks in FMD-free countries(Rodriguez and Gay 2011).
Additionally, the FMD vaccine does not induce long-term protective immunity, requiring multiple vaccinations to control the disease (Rodriguez and Gay 2011). Other important shortcomings of the inactivated vaccine include the short shelf life, the need of cold chain from production to delivery, and the difficulty of some serotypes or subtypes to adapt to cell culture, hindering their production.
4.1.1 Alternative vaccines
Intensive research has been carried out to achieve the production of alternative and improved FMD vaccines (Robinson et al. 2016). Different approaches have been successfully developed, based on attenuated and/or marker (DIVA) inactivated vaccines, recombinant protein vaccines, synthetic peptide vaccines and empty capsid vaccines. For a more in-depth review of these novel technologies, see Cao et al. (2016) and Diaz-San Segundo et al. (2016). The most relevant advances are summarized in this section.
In general, vaccines based in synthetic peptides or recombinant proteins with a limited number of antigenic sites have proven poor protection against challenge in host animals (DiMarchi et al. 1986; Taboga et al. 1997; Sobrino et al. 1999; Rodriguez et al. 2003). However, promising results were obtained when using dendrimeric peptides or multi-epitope proteins. A synthetic dendrimeric peptide containing one copy of a T-cell epitope and branching out into four copies of a B-cell epitope, elicited B- and T- cell specific immune responses and solid protection in pigs after two inoculations with 1.4 mg. Interestingly, despite the parental administration of the peptide, immunized pigs also developed a potent anti-FMDV immunoglobulin A response (Cubillos et al. 2008). Recently, Blanco and co-workers reported that a reduced version of the dendrimeric peptide, bearing two copies of a B-cell epitope from a type O isolate, induced in swine similar or higher B- and T-cell specific responses than the tetravalent peptide (Blanco et al. 2016). The bivalent version conferred full protection and entirely prevented virus shedding.
The commercial company UBI has developed a commercial peptide vaccine (UBITh® vaccine) for the prevention FMD in pigs, and licensed for use in Taiwan (www.unitedbiomedical.com). The UBI peptide spans the entire G-H loop and flanking sequences (amino acid positions 129–169) of VP1, has a unique consensus sequence to confront the hypervariability of serotype O viruses, and includes an artificial T helper (Th) site. This design, intended to improve and broaden VP1 G-H loop peptide immunogenicity, included disulphide bonds between the cysteine residues at positions 134 and 158 that stabilised the presentation of a flexible G–H loop-like structure providing a better immunogen than the linear equivalent. This peptide induced protective immunity against a Taiwanese isolate of FMDV O1(Wang et al. 2002). Although this vaccine is now widely used on pig farms in China, its composition must be adjusted to extend the antigenic spectrum. Moreover, a subsequent study in cattle revealed that neutralizing antibodies titres induced by the UBI peptide were relatively low and failed to protect cattle following challenge with a serotype-O strain of FMDV at 3 weeks post-vaccination (Rodriguez et al. 2003).
Multi-epitope vaccines have also been evaluated. Cao and co-workers developed a series of multi-epitope proteins containing the G–H loops of three topotypes of FMDV serotype O and promiscuous artificial Th sites. One of these proteins (B4) showed optimal immunogenicity and cross-reactivity in a mouse model. When this protein was co-administered with polyriboinosinic-polyribocytidylic acid [poly(I:C)], this vaccine elicited FMDV-specific neutralizing antibodies, IFN-α/β as well as IFN-γ, and offered a cross-protection against three topotypes of FMDV serotype O in pigs (Cao et al. 2013; Cao et al. 2014). Similar results were recently reported for a multi-epitope vaccine containing epitopes of currently circulating strains of FMDV serotype A, using cytidine-phosphate-guanosine (CpG) DNA as adjuvant (Cao et al. 2017). In cattle, both peptide and multi-epitope protein vaccines have shown limited efficacy, providing 60 % protection after a single immunization (Zhang et al. 2015), and 80% protection when CpG DNA and Montanide ISA-206 were used as adjuvant (Ren et al. 2011).
Protein/peptide vaccines pose no risk as they do not involve infectious virus, are highly stable at room temperature, can be easily stored and may function as DIVA vaccines. However, in most cases they are poorly immunogenic, especially for T cells, requiring additional adjuvants to induce protective immune response based both on antibodies and effector T cells.
The use of genetically engineered attenuated FMDV viruses to prepare inactivated vaccines was also considered as a safer alternative. Taking into account that viruses lacking the leader protease coding region (leaderless) are attenuated in vivo, and that the use of these viruses after chemical inactivation proved to be as effective as the wild-type inactivated antigen (Mason et al. 1997; Chinsangaram et al. 1998), Uddowla et al. have reported the design of a vaccine candidate that included the deletion of the Lpro, and one of the three 3B coding sequences, rendering a virus that was attenuated in both cattle and swine. Moreover, this recombinant virus harbours negative markers for potential DIVA capabilities, encoded in 3D alone or in 3B and 3D (Uddowla et al. 2012). This attenuated, antigenically marked virus was chemically inactivated and used to immunize cattle, providing 100% protection from challenge with parental wild-type virus. Similarly, a marker vaccine with a deletion within FMDV 3A was constructed using an infectious cDNA clone and proved to protect pigs against challenge with homologous wild-type FMDV (Li et al. 2014b). These attenuated viruses might provide a safer antigen for inactivated FMDV vaccine production, but they can still produce persistent viral infections in ruminants, and there is also the possibility of recombination of field and vaccine viruses due to virus escape or incomplete inactivation (Park 2013). This would be more likely if the attenuation is not stable and complete in all susceptible species. The use of FMDV empty capsids or VLPs is a very promising alternative that has been extensively explored. These immunogens have the entire repertoire of immunogenic epitopes displayed in the correct conformation and in a highly repetitive manner, enabling the induction of strong humoral and cellular immune responses (Noad and Roy 2003; Kushnir et al. 2012). In addition, the lack of genomic material and NSP makes them safe marker vaccines. As mentioned before, for the production of recombinant FMD VLPs, it is necessary to express the viral capsid proteins VP0, VP3 and VP1. This can be achieved by expressing these proteins individually, or co-expressing the capsid precursor (P12A) and protease 3C, which cleaves this precursor to generate the capsid proteins.
FMD VLPs can be produced in vitro by different expression systems and used as a subunit vaccine, or they can be produced in vivo following immunization with a DNA vaccine or a viral vector vaccine with the genetic material needed for capsid formation.
Many heterologous expression systems have been used for the production of recombinant FMD VLPs. Although prokaryotic expression system would not be the best choice since the correct assembly of FMDV empty capsids requires a post-translational modification (myristoylation), the SUMO fusion system for the expression of VP0, VP3 and VP1 in E. coli has been reported by different authors (Guo et al. 2013; Xiao et al. 2016). Expression of recombinant proteins as fusions with SUMO (small ubiquitin-like modifier) protein has significantly increased the yield of difficult-to-express proteins in E, coli, since SUMO usually promotes correct folding and structural stability of the fusion proteins (Lee et al. 2008). The co-expression of VP0, VP3, and VP1 assembled successfully into VLPs in vitro, and provided protection to both swine and cattle (Guo et al. 2013; Xiao et al. 2016). Since the issue of myristoylation was not addressed in those reports it is unclear how VLPs have assembled. The SUMO-modified E. coli expression system is relatively simple and economical, but requires further steps of chromatographic purification and proteolytic cleavage of the SUMO protein tag to ensure that structural proteins assembled into particulate structures in vitro (Xiao et al. 2016). These additional steps significantly reduce final yield and counteract the simplicity of the bacterial expression system.
The baculovirus expression vector system has been extensively explored for the production of recombinant FMD VLPs. Li and co-workers reported the use of recombinant baculoviruses containing P12A-3C coding sequences from serotypes Asia 1 and A, which produced VLPs in silkworm larvae (Li et al. 2008b) and pupae (Li et al. 2012), respectively. Cattle vaccinated with these VLPs developed high titres of FMDV-specific antibodies and were completely protected against virulent homologous virus challenge. FMD VLPs were also expressed in recombinant baculovirus-infected insect cells, but protein expression levels and efficiency of capsid assembly have been highly variable in initial reports (Roosien et al. 1990; Grubman et al. 1993; Oem et al. 2007; Li et al. 2008b). However, subsequent studies demonstrated that the down regulation of 3C protease activity, which is known to be toxic to cells, improved capsid protein expression levels (Porta et al. 2013b; Vivek Srinivas et al. 2016). Moreover, by mutating a single histidine in VP2, Porta et al. were able to produce stable empty capsids of FMDV A22, that resisted both heat and acid treatments (Porta et al. 2013a). Cattle vaccinated with these recombinant capsids showed sustained virus neutralisation titres and protection from challenge.
Mammalian cell expression systems are the ideal choice to produce recombinant eukaryotic proteins as they are able to introduce post-translational modifications, proper protein folding and product assembly, which are essential for complete biological activity. However, initial attempts to constitutively express the capsid precursor P12A with 3C protease were unsuccessful (Abrams et al. 1995), probably due to protease 3C toxicity. Indeed, using a vaccinia virus-based transient expression system, it has been shown that optimal production of the processed capsid proteins from P12A precursor (from serotypes O and A) is achieved when expression levels of 3C protease or its activity are reduced (Polacek et al. 2013; Gullberg et al. 2013). Transient gene expression in serum-free suspension-growing cells for the production of FMDV empty capsids has been recently reported (Mignaqui et al. 2013). For this purpose, a plasmid encoding the complete cassette P12A3C from serotype A was transiently transfected into 293-6E cells. The recombinant proteins were expressed at levels similar to the ones achieved in the vaccine facilities after BHK-21 infection (approximately 3 µg/ml), and assembled into VLPs that induced protective immune response against viral challenge in mice. The absence of serum in the cell culture diminishes the cost of the whole process, which can be easily scaled up.
Recombinant FMD VLPs as subunit vaccines represent the alternative vaccine most similar to the current inactivated virus, with the advantage of safe production and DIVA characteristics.
As mentioned above, DNA vaccines and viral vector vaccines can also be used to deliver genetic information to express FMD VLPs inside the host cells and potentially induce both humoral and cell-mediated immune responses. So far, DNA vaccines have not been very efficient in inducing protective immune responses, requiring multiple immunizations, the addition of adjuvants and cytokines [e.g., granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 18 (IL-18)] (Cedillo-Barrón et al. 2001; Li et al. 2006a; Mingxiao et al. 2007; Fowler et al. 2011; Ganges et al. 2011; Borrego et al. 2011) or a prime-boost regime, using a DNA vaccine for priming and the commercial vaccine for boosting (Li et al. 2008a). Conversely, several viral vectors have been successfully used to produce FMD VLPs, including herpesvirus, poxvirus, alphavirus and adenovirus. The most effective and extensively tested platform uses a replication-defective human adenovirus 5 (hAd5) (Mayr et al. 1999; Mayr et al. 2001; Moraes et al. 2002; Wu et al. 2003b; Moraes et al. 2003; Santos et al. 2006; Pena et al. 2008). The use of hAd5 containing the P1 coding region of FMDV A24 and the 3C coding region of A12 (Ad5-24) was evaluated in both swine and cattle. Animals vaccinated with a single dose of this vaccine were protected from clinical disease after direct inoculation challenge or contact exposure as early as 7 days post-vaccination (Moraes et al. 2002; Pacheco et al. 2005). During 2012, the Ad5-FMD A24 Cruzeiro (Ad5-24) vaccine was granted a conditional license for use in cattle in emergency situations in the United States (Grubman et al. 2012). Since the vaccine lacks the coding regions of most of NS proteins, vaccinated animals can be distinguished from infected animals. Moreover, its production does not require high-level biocontainment facilities, allowing its production in biosafety level-2 laboratories in the United States. The Ad5-based FMD vaccine has been developed for other serotypes and subtypes, and several of these candidates have successfully completed the efficacy studies (Grubman et al. 2010). The first generation of the Ad5-O1Campos (Ad5-O1C) vaccine induced lower levels of neutralizing antibodies than Ad5-A24 in swine, and did not protect animals against homologous challenge, even with the co-administration of porcine GM-CSF as an adjuvant (Caron et al. 2005). A second generation of Ad5-FMD vectors, containing the full-length 2B coding region (Ad5-FMD-2B) was constructed for O1C and A24 subtypes, in order to enhance the synthesis of viral capsid proteins and the efficiency and/or stability of capsid assembly. These vaccines have shown to improve vaccine efficacy both in cattle and swine (Moraes et al. 2011; Grubman et al. 2012).
5 Pathway to commercialization
Many efforts have been made in order to develop new FMD vaccines that meet the requirements that would be expected for an ideal vaccine, such as safety production, induction of rapid and long-lasting protective immunity after a single inoculation, compatibility with DIVA principle, and cost-effectiveness. However, it is unlikely that a single vaccine would meet all these requirements, and different strategies could be implemented for different particular cases. For example, emergency vaccines should be produced with simple and versatile technologies able to match the field strain responsible for an outbreak as fast as possible, while vaccines for endemic regions should emphasize cost-effective technologies.
In order to accelerate progress, the Global FMD Research Alliance (GFRA) was established in 2003. The core of the GFRA is a consortium of research institutions with the aim of developing a new generation of accessible and efficacious vaccines, diagnostics and antiviral agents to successfully prevent, control and eradicate FMD.
It is evident that FMD remains a severe threat to the livestock industry worldwide. Recently, it has been reported that the annual impact of this disease in terms of production losses and vaccination in endemic regions alone amount to between USD 6,5 and 21 billion (Knight-Jones and Rushton 2013). It has been estimated that 2.35 billion doses of FMD vaccine are administered annually in the world, with the main regions being China (1600 million doses), South America (500 million doses) and India (150 million doses) (Knight-Jones and Rushton 2013).
In the past few years, favourable government initiatives to control FMD outbreaks have significantly increased the growth of the global FMD vaccines market. Additionally, the rising consumption of meat and dairy products across the globe has further powered the need for FMD vaccines. According to a recent report published by the Transparency Market Research (2015), the global foot and mouth disease vaccines market is expected to rise from a valuation of USD 0.51 billion in 2013 to USD 0.95 billion by 2020 (http://www.transparencymarketresearch.com/foot-mouth-disease-vaccines.html).
There is a general interest for global eradication of FMD, and vaccine-based eradication has demonstrated to be a feasible strategy regionally that could be expanded to global application. However, in order to achieve this goal, significant challenges must be overcome, especially in developing countries. For example, the need of sustainable and competent veterinary service trained to monitor vaccination and detect outbreaks; the development of effective surveillance methods to rapidly confirm clinical suspicions, preferably performed on-site; and vaccine production technologies significantly less expensive and more flexible (Smith et al. 2014). In addition, actual FMD vaccine production has several drawbacks, specially associated with the large amount of infectious virus that must be manipulated, the existence of different serotypes and strains that complicates the maintenance of vaccine stocks and the instability of the formulated vaccine (12-18 months). This thermal instability is also problematic when vaccines are formulated from frozen antigen concentrates in vaccine banks, since it is difficult to keep them refrigerated, especially in poorer parts of the world. The need of expensive high-level bio-containment facilities and the constant risk of viral escape are undoubtedly other important disadvantages of the current vaccine production system that have prompted different countries to prohibit the production of this vaccine in their mainland.
In this scenario, the implementation of molecular farming for FMD vaccine production could be easily introduced in the market since it may not require bio-containment facilities, or these would be less stringent or less expensive. Moreover, it favors a faster design of new immunogens in case of an eventual introduction of exotic strains, potentially conceding the optimization of the time for supplying the market with a product that enables the control of the outbreak. Additionally, like any other recombinant vaccine would allow the accurate differentiation between vaccinated and infected animals, guaranteeing the control of outbreaks through sero-epidemiological surveillance programs.
Taking in mind that the immunogenicity of recombinant FMD VLPs has already been demonstrated, this approach should be the focus in the development of a novel vaccine against FMD. However, there is still a need to improve expression levels to move the production of FMDV vaccines in plants closer to the development phase. Successful commercialization of recombinant veterinary vaccines will require cooperation with business associates, the creation of a suitable business plan, and multiple stages of financing. Since the process from research to product registration can take up to 7 years, the target market should be predicted well in advance, and the ability for economical scale-up must be assessed to determine whether the product can be produced cost-effectively at consistent quality. In the field of plant-based vaccines, intellectual property protection and freedom to operate are more complicated by the intricacies of the technology, and their requirements are continually changing. While there are variations between countries in terms of how plant-based vaccines are regulated, in general, they must be shown to be safe, efficacious, and environmentally benign in order to gain approval (Macdonald et al. 2015).
Even when considering what was just said, the achievements and ripeness of molecular farming, especially when large doses of vaccine are required, certainly makes this production platform one of the most interesting options as an alternative to the conventional FMDV vaccine.
References
Abrams CC, King AM, Belsham GJ (1995) Assembly of foot-and-mouth disease virus empty capsids synthesized by a vaccinia virus expression system. J Gen Virol 76:3089–3098
Alexandersen S, Brotherhood I, Donaldson AI (2002a) Natural aerosol transmission of foot-and-mouth disease virus to pigs: minimal infectious dose for strain O-1 Lausanne. Epidemiol Infect 128:301–312. https://doi.org/10.1017/s095026880100646x
Alexandersen S, Donaldson AI (2002) Further studies to quantify the dose of natural aerosols of foot-and-mouth disease virus for pigs. Epidemiol Infect 128:313–323. https://doi.org/10.1016/S0034-5288(02)90037-8
Alexandersen S, Mowat N (2005) Foot-and-mouth disease virus: host range and pathogenesis. Curr Top Microbiol Immunol 288:9–42. https://doi.org/10.1007/b138628
Alexandersen S, Oleksiewicz MB, Donaldson AI (2001) The early pathogenesis of foot-and-mouth disease in pigs infected by contact: A quantitative time-course study using TaqMan RT-PCR. J Gen Virol 82:747–755. https://doi.org/10.1099/0022-1317-82-4-747
Alexandersen S, Zhang Z, Donaldson AI (2002b) Aspects of the persistence of foot-and-mouth disease virus in animals—the carrier problem. Microbes Infect 4:1099–1110. https://doi.org/10.1016/S1286-4579(02)01634-9
Alexandersen S, Zhang Z, Donaldson AI, Garland AJM (2003) The Pathogenesis and Diagnosis of Foot-and-Mouth Disease. J Comp Pathol 129:1–36. https://doi.org/10.1016/S0021-9975(03)00041-0
Andrianova EP, Krementsugskaia SR, Lugovskaia NN, et al (2011) Foot and mouth disease virus polyepitope protein produced in bacteria and plants induces protective immunity in guinea pigs. Biochem Biokhimii͡a 76:339–46.
Arzt J, Baxt B, Grubman MJ et al (2011) The Pathogenesis of Foot-and-Mouth Disease II: Viral Pathways in Swine, Small Ruminants, and Wildlife; Myotropism, Chronic Syndromes, and Molecular Virus-Host Interactions. Transbound Emerg Dis 58:305–326. https://doi.org/10.1111/j.1865-1682.2011.01236.x
Arzt J, Gregg DA, Clavijo A, Rodriguez LL (2009) Optimization of immunohistochemical and fluorescent antibody techniques for localization of Foot-and-mouth disease virus in animal tissues. J Vet Diagnostic Investig 21:779–792. https://doi.org/10.1177/104063870902100604
Arzt J, Pacheco JM, Rodriguez LL (2010) The early pathogenesis of foot-and-mouth disease in cattle after aerosol inoculation. Identification of the nasopharynx as the primary site of infection. Vet Pathol 47:1048–1063. https://doi.org/10.1177/0300985810372509
Arzt J, Pacheco JM, Smoliga GR et al (2014) Foot-and-mouth disease virus virulence in cattle is co-determined by viral replication dynamics and route of infection. Virology 452:12–22. https://doi.org/10.1016/j.virol.2014.01.001
Bachrach HL (1968) Foot-And-Mouth Disease. Annu Rev Microbiol 22:201–244. https://doi.org/10.1146/annurev.mi.22.100168.001221
Baxt B, Becker Y (1990) The effect of peptides containing the arginine-glycine-aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes 4:73–83
Belsham G (1993) Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Prog Biophys Mol Biol 60:241–260
Belsham GJ (2005) Translation and replication of FMDV RNA. Curr Top Microbiol Immunol 288:43–70
Blanco E, Guerra B, De La Torre BG et al (2016) Full protection of swine against foot-and-mouth disease by a bivalent B-cell epitope dendrimer peptide. Antiviral Res 129:74–80. https://doi.org/10.1016/j.antiviral.2016.03.005
Borrego B, Argilaguet JM, Pérez-Martín E et al (2011) A DNA vaccine encoding foot-and-mouth disease virus B and T-cell epitopes targeted to class II swine leukocyte antigens protects pigs against viral challenge. Antiviral Res. https://doi.org/10.1016/j.antiviral.2011.07.017
Borrego B, Camarero JA, Mateu MG, Domingo E (1995) A highly divergent antigenic site of foot-and-mouth disease virus retains its immunodominance. Viral Immunol 8:11–8. https://doi.org/10.1089/vim.1995.8.11
Brito BP, Rodriguez LL, Hammond JM, et al (2015) Review of the Global Distribution of Foot-and-Mouth Disease Virus from 2007 to 2014. Transbound Emerg Dis 1–17. doi: 10.1111/tbed.12373
Brown F (2003) The history of research in foot-and-mouth disease. Virus Res 91:3–7
Burrows R (1966) Studies on the carrier state of cattle exposed to Foot-and-mouth disease virus. J Hyg (Lond) 64:81–90. https://doi.org/10.1017/S0022172400040365
Burrows R, Mann JA, Garland AJM et al (1981) The pathogenesis of natural and simulated natural Foot-and-Mouth disease infection in cattle. J Comp Pathol 91:599–609
Cao Y (2014) Adjuvants for foot-and-mouth disease virus vaccines: recent progress. Expert Rev Vaccines 13:1377–1385. https://doi.org/10.1586/14760584.2014.963562
Cao Y, Li D, Fu Y et al (2017) Rational design and efficacy of a multi-epitope recombinant protein vaccine against foot-and-mouth disease virus serotype A in pigs. Antiviral Res 140:133–141. https://doi.org/10.1016/j.antiviral.2017.01.023
Cao Y, Lu Z, Li D et al (2014) Evaluation of cross-protection against three topotypes of serotype O foot-and-mouth disease virus in pigs vaccinated with multi-epitope protein vaccine incorporated with poly(I:C). Vet Microbiol 168:294–301. https://doi.org/10.1016/j.vetmic.2013.11.023
Cao Y, Lu Z, Li Y et al (2013) Poly(I:C) combined with multi-epitope protein vaccine completely protects against virulent foot-and-mouth disease virus challenge in pigs. Antiviral Res 97:145–153. https://doi.org/10.1016/j.antiviral.2012.11.009
Cao Y, Lu Z, Liu Z (2016) Foot-and-mouth disease vaccines: progress and problems. Expert Rev Vaccines 584(14760584):1140042. https://doi.org/10.1586/14760584.2016.1140042
Caron L, Brum MCS, Moraes MP et al (2005) Granulocyte-macrophage colony-stimulating factor does not increase the potency or efficacy of a foot-and-mouth disease virus subunit vaccine. Pesqui Vet Bras 25:150–158
Carrillo C, Wigdorovitz A, Oliveros JC, et al (1998) Protective Immune Response to Foot-and-Mouth Disease Virus with VP1 Expressed in Transgenic Plants. 72:1688–1690.
Carrillo C, Wigdorovitz A, Trono K et al (2001) Induction of a virus-specific antibody response to foot and mouth disease virus using the structural protein VP1 expressed in transgenic potato plants. Viral Immunol 14:49–57
Cedillo-Barrón L, Foster-Cuevas M, Belsham GJ et al (2001) Induction of a protective response in swine vaccinated with DNA encoding foot-and-mouth disease virus empty capsid proteins and the 3D RNA polymerase. J Gen Virol 82:1713–1724
Chinsangaram J, Mason PW, Grubman MJ (1998) Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine 16:1516–1522. https://doi.org/10.1016/S0264-410X(98)00029-2
Cubillos C, de la Torre BG, Jakab A et al (2008) Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J Virol 82:7223–30. https://doi.org/10.1128/JVI.00401-08
Dawe PS, Flanagan FO, Madekurozwa RL et al (1994a) Natural transmission of foot-and-mouth disease virus from African buffalo (Syncerus caffer) to cattle in a wildlife area of Zimbabwe. Vet Rec 134:230–232
Dawe PS, Sorensen K, Ferris NP et al (1994b) Experimental transmission of foot-and-mouth disease virus from carrier African buffalo (Syncerus caffer) to cattle in Zimbabwe. Vet Rec 134:211–5
Di Nardo A, Libeau G, Chardonnet B et al (2015) Serological profile of foot-and-mouth disease in wildlife populations of West and Central Africa with special reference to Syncerus caffer subspecies. Vet Res 46:77. https://doi.org/10.1186/s13567-015-0213-0
Diaz-San Segundo F, Medina GN, Stenfeldt C et al (2016) Foot-and-mouth disease vaccines. Vet Microbiol. https://doi.org/10.1016/j.vetmic.2016.12.018
Dicara D, Burman A, Clark S et al (2008) Foot-and-mouth disease virus forms a highly stable, EDTA-resistant complex with its principal receptor, integrin alphavbeta6: implications for infectiousness. J Virol 82:1537–46. https://doi.org/10.1128/JVI.01480-07
DiMarchi R, Brooke G, Gale C, et al (1986) Protection of cattle against foot-and-mouth disease by a synthetic peptide.
Doel TR (2003) FMD vaccines. Virus Res 91:81–99
Doel TR, Williams L, Barnett PV (1994) Emergency vaccination against foot-and-mouth disease: Rate of development of immunity and its implications for the carrier state. Vaccine 12:592–600. https://doi.org/10.1016/0264-410X(94)90262-3
Domingo E, Baranowski E, Escarmı́s C, Sobrino F (2002) Foot-and-mouth disease virus. Comp Immunol Microbiol Infect Dis 25:297–308. doi: 10.1016/S0147-9571(02)00027-9
Donaldson AI (1987) Foot-and-mouth disease: the principal features. Irish Vet J 41:325–327
Donaldson AI (1997) Risks of spreading foot and mouth disease through milk and dairy products. Rev Sci Tech 16:117–24
Donaldson AI, Alexandersen S (2001) Relative resistance of pigs to infection by natural aerosols of FMD virus. Vet Rec 148:600–2
Donaldson AI, Herniman KAJ, Parker J, Sellers RF (1970) Further investigations on the airborne excretion of foot and mouth disease virus. J Hyg, Camb 68:557–564. https://doi.org/10.1016/B978-0-12-382219-2.00490-7
Dus Santos MJ, Carrillo C, Ardila F et al (2005) Development of transgenic alfalfa plants containing the foot and mouth disease virus structural polyprotein gene P1 and its utilization as an experimental immunogen. Vaccine 23:1838–43. https://doi.org/10.1016/j.vaccine.2004.11.014
Dus Santos MJ, Wigdorovitz A, Trono K et al (2002) A novel methodology to develop a foot and mouth disease virus (FMDV) peptide-based vaccine in transgenic plants. Vaccine 20:1141–1147
Fowler VL, Bashiruddin JB, Maree FF et al (2011) Foot-and-mouth disease marker vaccine: Cattle protection with a partial VP1 G-H loop deleted virus antigen. Vaccine 29:8405–8411. https://doi.org/10.1016/j.vaccine.2011.08.035
Fox G, Parry NR, Barnett PV et al (1989) The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J Gen Virol 70(Pt 3):625–37. https://doi.org/10.1099/0022-1317-70-3-625
Fukai K, Yamada M, Morioka K et al (2015) Dose-dependent responses of pigs infected with foot-and-mouth disease virus O/JPN/2010 by the intranasal and intraoral routes. Arch Virol 160:129–139. https://doi.org/10.1007/s00705-014-2239-4
Gailiunas P, Cottral GE (1966) Presence and persistence of foot-and-mouth disease virus in bovine skin. J Bacteriol 91:2333–2338
Ganges L, Borrego B, Fernández-Pacheco P, et al (2011) DNA immunization of pigs with foot-and-mouth disease virus minigenes: From partial protection to disease exacerbation.
Gay CG, Salt J, Balaski C (2003) Challenges and opportunities in developing and marketing vaccines for OIE List A and emerging animal diseases. Dev Biol (Basel) 114:243–50
Gloster J, Sellers RF, Donaldson AI (1982) Long distance transport of foot-and-mouth disease virus over the sea. Vet Rec 110:47–52
Goodwin S, Tuthill TJ, Arias A et al (2009) Foot-and-mouth disease virus assembly: processing of recombinant capsid precursor by exogenous protease induces self-assembly of pentamers in vitro in a myristoylation-dependent manner. J Virol 83:11275–11282. https://doi.org/10.1128/JVI.01263-09
Grubman M, Moraes M, Schutta C et al (2010) Adenovirus serotype 5-vectored foot-and-mouth disease subunit vaccines: the first decade. Future Virol 5:51–64
Grubman MJ, Baxt B (2004) Foot-and-Mouth Disease. 17:465–493. https://doi.org/10.1128/CMR.17.2.465
Grubman MJ, Lewis S a, Morgan DO (1993) Protection of swine against foot-and-mouth disease with viral capsid proteins expressed in heterologous systems. Vaccine 11:825–9
Grubman MJ, Segundo FD, Perez-martin E, Service I (2012) Use of replication-defective adenoviruses to develop vaccines and biotherapeutics against foot-and-mouth disease. Future Virol 7:767–778
Gullberg M, Muszynski B, Organtini LJ et al (2013) Assembly and characterization of foot-and-mouth disease virus empty capsid particles expressed within mammalian cells. J Gen Virol 94:1769–1779. https://doi.org/10.1099/vir.0.054122-0
Guo H-C, Sun S-Q, Jin Y et al (2013) Foot-and-mouth disease virus-like particles produced by a SUMO fusion protein system in Escherichia coli induce potent protective immune responses in guinea pigs, swine and cattle. Vet Res 44:48. https://doi.org/10.1186/1297-9716-44-48
Habiela M, Seago J, Perez-Martin E et al (2014) Laboratory animal models to study foot-and-mouth disease: a review with emphasis on natural and vaccine induced immunity. J Gen Virol 95:2329–2345. https://doi.org/10.1099/vir.0.068270-0
Henderson WM (1948) A consideration of some of the factors concerned in intracutaneous injection of cattle. J Pathol Bacteriol 60:137–139
Hughes GJ, Mioulet V, Kitching RP et al (2002) Foot-and-mouth disease virus infection of sheep: implications for diagnosis and control. Vet Rec 150:724–7
Jackson T, Clark S, Berryman S et al (2004) Integrin alphavbeta 8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J Virol 78:4533–40
Jackson T, Mould AP, Sheppard D, King AMQ (2002) Integrin alphavbeta1 is a receptor for foot-and-mouth disease virus. J Virol 76:935–41
Jackson T, Sheppard D, Denyer M et al (2000) The epithelial integrin alphavbeta6 is a receptor for foot-and-mouth disease virus. J Virol 74:4949–56
Jiang L, Li Q, Li M et al (2006) A modified TMV-based vector facilitates the expression of longer foreign epitopes in tobacco. Vaccine 24:109–115. https://doi.org/10.1016/j.vaccine.2005.09.060
Juleff N, Windsor M, Reid E et al (2008) Foot-and-mouth disease virus persists in the light zone of germinal centres. PLoS One 3:e3434. https://doi.org/10.1371/journal.pone.0003434
Kitching RP (2002) Clinical variation in foot and mouth disease: cattle. Rev Sci Tech 21:499–504. https://doi.org/10.20506/rst.21.3.1367
Kitching RP, Alexandersen S (2002) Clinical variation in foot and mouth disease: pigs. Rev Sci Tech 21:513–8
Kitching RP, Hughes GJ (2002) Clinical variation in foot and mouth disease: sheep and goats. Rev Sci Tech 21:505–512
Kleina LG, Grubman MJ (1992) Antiviral effects of a thiol protease inhibitor on foot-and-mouth disease virus. J Virol 66:7168–75
Knight-Jones TJD, Rushton J (2013) The economic impacts of foot and mouth disease - What are they, how big are they and where do they occur? Prev Vet Med 112:162–173. https://doi.org/10.1016/j.prevetmed.2013.07.013
Knowles NJ, Samuel AR, Davies PR et al (2001) Outbreak of foot-and-mouth disease virus serotype O in the UK caused by a pandemic strain. Vet Rec 148:258–9
Knowles NJ, Samuel AR, Davies PR et al (2005) Pandemic strain of foot-and-mouth disease virus serotype O. Emerg Infect Dis 11:1887–1893. https://doi.org/10.3201/eid1112.050908
Kushnir N, Streatfield SJ, Yusibov V (2012) Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 31:58–83. https://doi.org/10.1016/j.vaccine.2012.10.083
Lee C-D, Sun H-C, Hu S-M et al (2008) An improved SUMO fusion protein system for effective production of native proteins. Protein Sci 17:1241–8. https://doi.org/10.1110/ps.035188.108
Lentz EM, Segretin ME, Morgenfeld MM et al (2010) High expression level of a foot and mouth disease virus epitope in tobacco transplastomic plants. Planta 231:387–395. https://doi.org/10.1007/s00425-009-1058-4
Li C, Yamagishi N, Kaido M, Yoshikawa N (2014a) Presentation of epitope sequences from foreign viruses on the surface of apple latent spherical virus particles. Virus Res 190:118–126. https://doi.org/10.1016/j.virusres.2014.07.013
Li P, Lu Z, Bai X et al (2014b) Evaluation of a 3A-truncated foot-and-mouth disease virus in pigs for its potential as a marker vaccine. Vet Res 45:1–11. https://doi.org/10.1186/1297-9716-45-51
Li Y, Aggarwal N, Takamatsu H et al (2006a) Enhancing immune responses against a plasmid DNA vaccine encoding a FMDV empty capsid from serotype O. Vaccine 24:4602–4606
Li Y, Stirling CMA, Denyer MS et al (2008a) Dramatic improvement in FMD DNA vaccine efficacy and cross-serotype antibody induction in pigs following a protein boost. Vaccine 26:2647–2656. https://doi.org/10.1016/j.vaccine.2008.01.037
Li Y, Sun M, Liu J et al (2006b) High expression of foot-and-mouth disease virus structural protein VP1 in tobacco chloroplasts. Plant Cell Rep 25:329–333. https://doi.org/10.1007/s00299-005-0074-5
Li Z, Yi Y, Yin X et al (2008b) Expression of foot-and-mouth disease virus capsid proteins in silkworm-baculovirus expression system and its utilization as a subunit vaccine. PLoS One 3:1–7. https://doi.org/10.1371/journal.pone.0002273
Li Z, Yi Y, Yin X et al (2012) Development of a Foot-and-Mouth Disease Virus Serotype A Empty Capsid Subunit Vaccine Using Silkworm (Bombyx mori) Pupae. PLoS One 7:e43849. https://doi.org/10.1371/journal.pone.0043849
Loeffler F, Frosch P (1897) Summarischer bericht ueber der ergebnisse der untersuchungen zur erforschung der maul- und klauenseuche. Zentbl Bakteriol Parasitenkd Infekt Hyg 22:257–259
Macdonald J, Doshi K, Dussault M et al (2015) Bringing plant-based veterinary vaccines to market: Managing regulatory and commercial hurdles ☆. Biotechnol Adv 33:1572–1581. https://doi.org/10.1016/j.biotechadv.2015.07.007
Mason PW, Grubman MJ, Baxt B (2003) Molecular basis of pathogenesis of FMDV. Virus Res 91:9–32. https://doi.org/10.1016/S0168-1702(02)00257-5
Mason PW, Piccone ME, McKenna TSC et al (1997) Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology 227:96–102. https://doi.org/10.1006/viro.1996.8309
Mateu MG, Camarero JA, Giralt E et al (1995) Direct evaluation of the immunodominance of a major antigenic site of foot-and-mouth disease virus in a natural host. Virology 206:298–306. https://doi.org/10.1016/S0042-6822(95)80045-X
Mayr GA, Chinsangaram J, Grubman MJ (1999) Development of replication-defective adenovirus serotype 5 containing the capsid and 3C protease coding regions of foot-and-mouth disease virus as a vaccine candidate. Virology 263:496–506
Mayr GA, O’Donnell V, Chinsangaram J et al (2001) Immune responses and protection against foot-and-mouth disease virus (FMDV) challenge in swine vaccinated with adenovirus-FMDV constructs. Vaccine 19:2152–62
McKenna TS, Lubroth J, Rieder E et al (1995) Receptor binding site-deleted foot-and-mouth disease (FMD) virus protects cattle from FMD. J Virol 69:5787–90
Mignaqui AC, Ruiz V, Perret S et al (2013) Transient Gene Expression in Serum-Free Suspension-Growing Mammalian Cells for the Production of Foot-and-Mouth Disease Virus Empty Capsids. PLoS One 8:e72800. https://doi.org/10.1371/journal.pone.0072800
Mingxiao M, Ningyi J, Jaun L et al (2007) Immunogenicity of plasmids encoding P12A and 3C of FMDV and swine IL-18. Antiviral Res 76:59–67. https://doi.org/10.1016/j.antiviral.2007.05.003
Monaghan P (2005) The alfa(v)beta6 integrin receptor for Foot-and-mouth disease virus is expressed constitutively on the epithelial cells targeted in cattle. J Gen Virol 86:2769–2780. https://doi.org/10.1099/vir.0.81172-0
Montague NP, Thuenemann EC, Saxena P et al (2011) Recent advances of Cowpea mosaic virus-based particle technology. Hum Vaccin 7:383–390. https://doi.org/10.4161/hv.7.3.14989
Moonen P, Schrijver R (2000) Carriers of foot-and-mouth disease virus: A review. Vet Q 22:193–197. https://doi.org/10.1080/01652176.2000.9695056
Moraes M, Mayr G, Mason P, Grubman M (2002) Early protection against homologous challenge after a single dose of replication-defective human adenovirus type 5 expressing capsid proteins of foot-and-mouth disease virus (FMDV) strain A24. Vaccine 20:1631–1639. https://doi.org/10.1016/S0264-410X(01)00483-2
Moraes MP, Chinsangaram J, Brum MC, Grubman MJ (2003) Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine 22:268–279. https://doi.org/10.1016/S0264-410X(03)00560-7
Moraes MP, Segundo FD-S, Dias CC et al (2011) Increased efficacy of an adenovirus-vectored foot-and-mouth disease capsid subunit vaccine expressing nonstructural protein 2B is associated with a specific T cell response. Vaccine 29:9431–40. https://doi.org/10.1016/j.vaccine.2011.10.037
Murphy C, Bashiruddin JB, Quan M et al (2010) Foot-and-mouth disease viral loads in pigs in the early, acute stage of disease. Vet Rec 166:10–4. https://doi.org/10.1136/vr.b5583
Muthamilselvan T, Lee CW, Cho YH et al (2016) A transgenic plant cell-suspension system for expression of epitopes on chimeric Bamboo mosaic virus particles. Plant Biotechnol J 14:231–239. https://doi.org/10.1111/pbi.12377
Noad R, Roy P (2003) Virus-like particles as immunogens. Trends Microbiol 11:438–444. https://doi.org/10.1016/S0966-842X(03)00208-7
Oem J-K, Park J-H, Lee K-N et al (2007) Characterization of recombinant foot-and-mouth disease virus pentamer-like structures expressed by baculovirus and their use as diagnostic antigens in a blocking ELISA. Vaccine 25:4112–4121
Pacheco J, Brum M, Moraes M et al (2005) Rapid protection of cattle from direct challenge with foot-and-mouth disease virus (FMDV) by a single inoculation with an adenovirus-vectored FMDV subunit vaccine. Virology 337:205–209
Pacheco JM, Arzt J, Rodriguez LL (2010) Early events in the pathogenesis of foot-and-mouth disease in cattle after controlled aerosol exposure. Vet J 183:46–53. https://doi.org/10.1016/j.tvjl.2008.08.023
Pacheco JM, Lee K-N, Eschbaumer M et al (2016) Evaluation of Infectivity, Virulence and Transmission of FDMV Field Strains of Serotypes O and A Isolated In 2010 from Outbreaks in the Republic of Korea. PLoS One 11:e0146445. https://doi.org/10.1371/journal.pone.0146445
Pacheco JM, Tucker M, Hartwig E et al (2012) Direct contact transmission of three different foot-and-mouth disease virus strains in swine demonstrates important strain-specific differences. Vet J 193:456–463. https://doi.org/10.1016/j.tvjl.2012.01.012
Pan L, Zhang Y, Wang Y et al (2008) Foliar extracts from transgenic tomato plants expressing the structural polyprotein, P1-2A, and protease, 3C, from foot-and-mouth disease virus elicit a protective response in guinea pigs. Vet Immunol Immunopathol 121:83–90
Parida S (2009) Vaccination against foot-and-mouth disease virus: strategies and effectiveness. Expert Rev Vaccines 8:347–365. https://doi.org/10.1586/14760584.8.3.347
Park J (2013) Requirements for improved vaccines against foot-and-mouth disease epidemics. Clin Exp Vaccine Res 2:8–18. https://doi.org/10.7774/cevr.2013.2.1.8
Pena L, Moraes MP, Koster M et al (2008) Delivery of a foot-and-mouth disease virus empty capsid subunit antigen with nonstructural protein 2B improves protection of swine. Vaccine 26:5689–5699. https://doi.org/10.1016/j.vaccine.2008.08.022
Polacek C, Gullberg M, Li J, Belsham GJ (2013) Low levels of foot-and-mouth disease virus 3C protease expression are required to achieve optimal capsid protein expression and processing in mammalian cells. J Gen Virol 94:1249–1258. https://doi.org/10.1099/vir.0.050492-0
Porta C, Kotecha A, Burman A et al (2013a) Rational Engineering of Recombinant Picornavirus Capsids to Produce Safe. Protective Vaccine Antigen. PLoS Pathog 9:e1003255. https://doi.org/10.1371/journal.ppat.1003255
Porta C, Spall VE, Findlay KC et al (2003) Cowpea mosaic virus-based chimaeras: Effects of inserted peptides on the phenotype, host range, and transmissibility of the modified viruses. Virology 310:50–63. https://doi.org/10.1016/S0042-6822(03)00140-5
Porta C, Spall VE, Loveland J et al (1994) Development of Cowpea Mosaic Virus as a High-Yielding System for the Presentation of Foreign Peptides. Virology 202:949–955. https://doi.org/10.1006/viro.1994.1417
Porta C, Xu X, Loureiro S et al (2013b) Efficient production of foot-and-mouth disease virus empty capsids in insect cells following down regulation of 3C protease activity. J Virol Methods 187:406–412. https://doi.org/10.1016/j.jviromet.2012.11.011
Quan M, Murphy CM, Zhang Z et al (2009) Influence of Exposure Intensity on the Efficiency and Speed of Transmission of Foot-and-Mouth Disease. J Comp Pathol 140:225–237. https://doi.org/10.1016/j.jcpa.2008.12.002
Quan M, Murphy CM, Zhang Z, Alexandersen S (2004) Determinants of Early Foot-and-Mouth Disease Virus Dynamics in Pigs. J Comp Pathol 131:294–307. https://doi.org/10.1016/j.jcpa.2004.05.002
Racaniello V (2001) Picornaviridae: the viruses and their replication. Fields Virology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 685–722
Rao JP, Agrawal P, Mohammad R et al (2012) Expression of VP1 protein of serotype A and O of foot-and-mouth disease virus in transgenic sunnhemp plants and its immunogenicity for guinea pigs. Acta Virol 56:91–99. https://doi.org/10.4149/av_2012_02_91
Ren J, Yang L, Xu H et al (2011) CpG oligodeoxynucleotide and montanide ISA 206 adjuvant combination augments the immune responses of a recombinant FMDV vaccine in cattle. Vaccine 29:7960–7965. https://doi.org/10.1016/j.vaccine.2011.08.072
Robinson L, Knight-Jones TJD, Charleston B et al (2016) Global Foot-and-Mouth Disease Research Update and Gap Analysis: 3 - Vaccines. Transbound Emerg Dis 63:30–41. https://doi.org/10.1111/tbed.12521
Rodriguez LL, Barrera J, Kramer E et al (2003) A synthetic peptide containing the consensus sequence of the G-H loop region of foot-and-mouth disease virus type-O VP1 and a promiscuous T-helper epitope induces peptide-specific antibodies but fails to protect cattle against viral challenge. Vaccine 21:3751–3756. https://doi.org/10.1016/S0264-410X(03)00364-5
Rodriguez LL, Gay CG (2011) Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert Rev Vaccines 10:377–87. https://doi.org/10.1586/erv.11.4
Rohll JB, Moon DH, Evans DJ, Almond JW (1995) The 3’ untranslated region of picornavirus RNA: features required for efficient genome replication. J Virol 69:7835–44
Roosien J, Belsham GJ, Ryan MD et al (1990) Synthesis of foot-and-mouth disease virus capsid proteins in insect cells using baculovirus expression vectors. J Gen Virol 71:1703–1711
Rueckert RR, Wimmer E (1984) Systematic nomenclature of picornavirus proteins. J Virol 50:957–9
Ruiz-Sáenz J, Goez Y, Tabares W, López-Herrera A (2009) Cellular Receptors for Foot and Mouth Disease Virus. Intervirology 52:201–212. https://doi.org/10.1159/000226121
Ruiz V, Mozgovoj MV, Dus Santos MJ, Wigdorovitz A (2015) Plant-produced viral bovine vaccines: What happened during the last 10 years? Plant Biotechnol J 13:1071–1077. https://doi.org/10.1111/pbi.12440
Rweyemamu M, Roeder P, MacKay D et al (2008) Epidemiological patterns of foot-and-mouth disease worldwide. Transbound Emerg Dis 55:57–72. https://doi.org/10.1111/j.1865-1682.2007.01013.x
Sa-Carvalho D, Rieder E, Baxt B et al (1997) Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol 71:5115–23
Sangula AK, Siegismund HR, BelshamGJ, et al (2011) Low diversity of foot-and-mouth disease serotype C virus in Kenya: evidence for probable vaccine strain re-introductions in the field. Epidemiol Infect 139:189–196. doi: 10.1017/S0950268810000580
Santos TDL, Botton SDA, Weiblen R, Grubman MJ (2006) The Leader Proteinase of Foot-and-Mouth Disease Virus Inhibits the Induction of Beta Interferon mRNA and Blocks the Host Innate Immune Response. 80:1906–1914. https://doi.org/10.1128/JVI.80.4.1906
Saunders K, Sainsbury F, Lomonossoff GP (2009) Efficient generation of cowpea mosaic virus empty virus-like particles by the proteolytic processing of precursors in insect cells and plants. Virology 393:329–37. https://doi.org/10.1016/j.virol.2009.08.023
Sellers R, Gloster J (2008) Foot-and-mouth disease: A review of intranasal infection of cattle, sheep and pigs. Vet J 177:159–168. https://doi.org/10.1016/j.tvjl.2007.03.009
Sellers RF, Parker J (1969) Airborne excretion of foot-and-mouth disease virus.
Serrano P, Pulido MR, Sáiz M, Martínez-Salas E (2006) The 3’ end of the foot-and-mouth disease virus genome establishes two distinct long-range RNA-RNA interactions with the 5’ end region. J Gen Virol 87:3013–3022. https://doi.org/10.1099/vir.0.82059-0
Shahan M (1962) Shahan : Virus of Foot-and-Mouth Disease. Ann N Y Acad Sci 101:444–454
Smith MT, Bennett AM, Grubman MJ, Bundy BC (2014) Foot-and-mouth disease: Technical and political challenges to eradication. Vaccine
Sobrino F, Blanco E, García-Briones M, Ley V (1999) Synthetic peptide vaccines: foot-and-mouth disease virus as a model. Dev Biol Stand 101:39–43
Sørensen JH, Mackay DK, Jensen CO, Donaldson AI (2000) An integrated model to predict the atmospheric spread of foot-and-mouth disease virus. Epidemiol Infect 124:577–90
Stenfeldt C, Diaz-San Segundo F, de Los Santos T et al (2016a) The Pathogenesis of Foot-and-Mouth Disease in Pigs. Front Vet Sci 3:41. https://doi.org/10.3389/fvets.2016.00041
Stenfeldt C, Pacheco JM, Brito BP et al (2016b) Transmission of Foot-and-Mouth Disease Virus during the Incubation Period in Pigs. Front Vet Sci 3:105. https://doi.org/10.3389/fvets.2016.00105
Stenfeldt C, Pacheco JM, Rodriguez LL, Arzt J (2014a) Infection dynamics of foot-and-mouth disease virus in pigs using two novel simulated-natural inoculation methods. Res Vet Sci 96:396–405. https://doi.org/10.1016/j.rvsc.2014.01.009
Stenfeldt C, Pacheco JM, Rodriguez LL, Arzt J (2014b) Early Events in the Pathogenesis of Foot-and-Mouth Disease in Pigs; Identification of Oropharyngeal Tonsils as Sites of Primary and Sustained Viral Replication. PLoS One 9:e106859. https://doi.org/10.1371/journal.pone.0106859
Stenfeldt C, Pacheco JM, Smoliga GR et al (2014c) Detection of Foot-and-mouth Disease Virus RNA and Capsid Protein in Lymphoid Tissues of Convalescent Pigs Does Not Indicate Existence of a Carrier State. Transbound Emerg Dis 63:152–164. https://doi.org/10.1111/tbed.12235
Taboga O, Tami C, Carrillo E et al (1997) A large-scale evaluation of peptide vaccines against foot-and-mouth disease: lack of solid protection in cattle and isolation of escape mutants. J Virol 71:2606–14
Thomson G, Vosloo W, Bastos AD (2003) Foot and mouth disease in wildlife. Virus Res 91:145–161. https://doi.org/10.1016/S0168-1702(02)00263-0
Thuenemann EC, Lenzi P, Love AJ, et al (2013) The Use of Transient Expression Systems for the Rapid Production of Virus-like Par- ticles in Plants.
Toka FN, Golde WT (2013) Cell mediated innate responses of cattle and swine are diverse during foot-and-mouth disease virus (FMDV) infection: a unique landscape of innate immunity. Immunol Lett 152:135–43. https://doi.org/10.1016/j.imlet.2013.05.007
Transparency Market Research (2015) In: Foot Mouth Dis. Vaccines Mark. http://www.transparencymarketresearch.com/foot-mouth-disease-vaccines.html. Accessed 1 Mar 2017
Uddowla S, Hollister J, Pacheco JM et al (2012) A safe foot-and-mouth disease vaccine platform with two negative markers for differentiating infected from vaccinated animals. J Virol 86:11675–85. https://doi.org/10.1128/JVI.01254-12
Usha R, Rohll J, Spall V et al (1993) Expression of an animal virus antigenic site on the surface of a plant virus particle.pdf. Virology 197:366–374
Valarcher J-F, Leforban Y, Rweyemamu M et al (2008) Incursions of foot-and-mouth disease virus into Europe between 1985 and 2006. Transbound Emerg Dis 55:14–34. https://doi.org/10.1111/j.1865-1682.2007.01010.x
Van Bekkum J, Frenkel H, Frederiks H, Frenkel S (1959) Observations on the carrier state of cattle exposed to foot- and-mouth disease virus. Tijdschriſt voor Diergeneeskd 84:1159–1164
Verdaguer N, Mateu MG, Andreu D et al (1995) Structure of the major antigenic loop of foot-and-mouth disease virus complexed with a neutralizing antibody: direct involvement of the Arg-Gly-Asp motif in the interaction. EMBO J 14:1690–6
Vivek Srinivas VM, Basagoudanavar SH, Hosamani M (2016) IRES mediated expression of viral 3C protease for enhancing the yield of FMDV empty capsids using baculovirus system. Biologicals 4:1–5. https://doi.org/10.1016/j.biologicals.2015.12.002
Wang CY, Chang TY, Walfield AM et al (2002) Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine 20:2603–2610. https://doi.org/10.1016/S0264-410X(02)00148-2
Wang DM, Zhu JB, Peng M, Zhou P (2008) Induction of a protective antibody response to FMDV in mice following oral immunization with transgenic Stylosanthes spp. as a feedstuff additive. Transgenic Res 17:1163–1170. https://doi.org/10.1007/s11248-008-9188-1
Wang G, Wang Y, Shang Y et al (2015) How foot-and-mouth disease virus receptor mediates foot-and-mouth disease virus infection. Virol J 12:9. https://doi.org/10.1186/s12985-015-0246-z
Wang Y, Shen Q, Jiang Y et al (2012) Immunogenicity of foot-and-mouth disease virus structural polyprotein P1 expressed in transgenic rice. J Virol Methods 181:12–17. https://doi.org/10.1016/j.jviromet.2012.01.004
Wigdorovitz A, Carrillo C, Dus Santos M et al (1999a) Induction of a Protective Antibody Response to Foot and Mouth Disease Virus in Mice Following Oral or Parenteral Immunization with Alfalfa Transgenic Plants Expressing the Viral Structural Protein VP1 1. Virology 255:347–353
Wigdorovitz A, Pérez Filgueira DM, Robertson N et al (1999b) Protection of Mice against Challenge with Foot and Mouth Disease Virus (FMDV) by Immunization with Foliar Extracts from Plants Infected with Recombinant Tobacco Mosaic Virus Expressing the FMDV Structural Protein VP1. Virology 264:85–91. https://doi.org/10.1006/viro.1999.9923
Wu L, Jiang L, Zhou Z et al (2003a) Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine 21:4390–4398. https://doi.org/10.1016/S0264-410X(03)00428-6
Wu Q, Moraes MP, Grubman MJ (2003b) Recombinant adenovirus co-expressing capsid proteins of two serotypes of foot-and-mouth disease virus (FMDV): in vitro characterization and induction of neutralizing antibodies against FMDV in swine. Virus Res 93:211–219. https://doi.org/10.1016/S0168-1702(03)00116-3
Xiao Y, Chen H-Y, Wang Y et al (2016) Large-scale production of foot-and-mouth disease virus (serotype Asia1) VLP vaccine in Escherichia coli and protection potency evaluation in cattle. BMC Biotechnol 16:56. https://doi.org/10.1186/s12896-016-0285-6
Yang C-D, Liao J-T, Lai C-Y et al (2007) Induction of protective immunity in swine by recombinant bamboo mosaic virus expressing foot-and-mouth disease virus epitopes. BMC Biotechnol 7:62. https://doi.org/10.1186/1472-6750-7-62
Zhang Y, Li J, Pu H et al (2010) Development of Tobacco necrosis virus A as a vector for efficient and stable expression of FMDV VP1 peptides. Plant Biotechnol J 8:506–523. https://doi.org/10.1111/j.1467-7652.2010.00500.x
Zhang Z, Pan L, Ding Y et al (2015) Efficacy of synthetic peptide candidate vaccines against serotype-A foot-and-mouth disease virus in cattle. Appl Microbiol Biotechnol 99:1389–98. https://doi.org/10.1007/s00253-014-6129-1
Zhang ZD, Kitching RP (2001) The localization of persistent foot and mouth disease virus in the epithelial cells of the soft palate and pharynx. J Comp Pathol 124:89–94. https://doi.org/10.1053/jcpa.2000.0431
Zlotnick A, Stray SJ (2003) How does your virus grow? Understanding and interfering with virus assembly. Trends Biotechnol 21:536–542. https://doi.org/10.1016/j.tibtech.2003.09.012
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ruiz, V., Wigdorovitz, A. (2018). Foot-and-mouth Disease. In: MacDonald, J. (eds) Prospects of Plant-Based Vaccines in Veterinary Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-90137-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-90137-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90136-7
Online ISBN: 978-3-319-90137-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)