Abstract
The Peruvian Amazon basin is home to communities with some of the highest primate species richness found anywhere in the world. While the Amazon basin is a mosaic of terra firme, igapó, and várzea forests, with primates occupying all of these, there is surprisingly little comprehensive research comparing primate species richness among these forest types. The few studies that have looked at this found lower primate species richness in flooded forests compared to terra firme, presumably due to lower plant species diversity in flooded forests. Most of these studies, however, concentrated on comparisons between terra firme and várzea forests; until recently little work has been done on primates in igapó forests. Over a period of 12 years, beginning in 2001, we sampled primate species richness in igapó and terra firme habitats in the Área de Conservación Regional Comunal Tamshiyacu Tahuayo (ACRCTT), a communal reserve located in northeastern Peru. We found an equal number of primate species in both igapó and terra firme habitats (11 sympatric primate species in each). Our findings of high primate species richness in both igapó and terra firme forests are likely explained by multiple factors, including the unique characteristics of Peruvian igapó which, perhaps as a result of geographic proximity to the Andes, more closely resembles várzea, with more nutrient-rich soils than is typical of black-water forests farther east in Amazonia. In addition, our findings support earlier suggestions that some primate species’ use of igapó habitat is seasonal. These species may be moving into igapó during periods when fruit, especially that of palms like Mauritia flexuosa, is superabundant there although scarce in terra firme. Thus, during critical times of the year, igapó forests may be critical to maintaining sustainable primate populations and primate species richness in areas with flooded and unflooded forests.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Primate species richness
- Área de Conservación Regional Comunal Tamshiyacu Tahuayo
- Pithecia
- Seasonal habitat use
1 Introduction
1.1 Peruvian Amazonia Forest Habitats
The Amazon River basin in Peru contains approximately 600,000 km2 of tropical lowland rain forest of which approximately 20% is subjected to annual monomodal flooding (Kvist and Nebel 2001). This tropical lowland rain forest can be subdivided into three major types of forest: terra firme forest, which is above the floodplain and does not experience seasonal inundation, and two types of flooded forests, typically characterized based on the type of river they border.
Várzea forests are seasonally flooded by white-water rivers , which are turbid, are close to a neutral pH, and carry a nutrient-rich alluvial suspension from the Andes (Prance 1979; Kvist and Nebel 2001). These white-water rivers dominate the Peruvian Amazon basin. Nutrient-rich várzea forests have the highest floral species richness of any floodplain forest in the world (Whitmann et al. 2010).
Igapó forests are seasonally flooded by black-water rivers , which are clear, dark brown and acidic due to the colloidal suspension of plant compounds, originate in sandy areas, and are nutrient-poor compared to white-water rivers (Prance 1979; Kvist and Nebel 2001). Tree species richness is poorer in igapó forest compared to várzea forest (Whitmann et al. 2010).
Terra firme forest comprises the remaining 80% of tropical lowland rain forest in the Peruvian Amazon basin and is not subject to annual flooding (Kvist and Nebel 2001). As terra firme forest does not benefit from the annual deposition of alluvial sediments, the soil of terra firme is typically nutrient-poor from leaching (Haugaasen and Peres 2005). Even though the soil of terra firme forest is nutrient-poor compared to both várzea and igapó forest, tree species richness is higher than in the flooded forests, and this is considered to be because the soil in flooded forest is subjected to periodic lack of oxygen during times of water inundation (Whitman et al. 2010).
Western Amazonia has some of the highest primate species-rich communities found anywhere in the world (Peres and Janson 1999). Primate communities with 14 sympatric species have been found in Brazil on the Juruá River (Peres 1997) and in Peru within the ACRCTT (Aquino and Encarnación 1994; Puertas and Bodmer 1993). Thirteen sympatric primate species have been observed near the headwaters of the Urucu River in Brazil (Peres 1993) and inhabiting the Ponta da Castanha, Brazil (Johns 1985). Twelve sympatric primate species were detected in the Purús region of Brazil (Haugaasen and Peres 2005).
While the Amazon basin is a mosaic of terra firme, igapó, and várzea forests, primate species richness is reported to be higher in terra firme than either of the flooded forests (Peres 1993, 1997; Haugaasen and Peres 2005, 2009). Haugaasen and Peres speculate that lower species richness in várzea and igapó forest is due to their lower floral diversity compared to terra firme as a result of the soil composition and impact of seasonal flooding on the flora (2005).
1.2 Primates in Neotropical Flooded Forests
As a radiation, South American (Neotropical) primates, the Platyrrhini, have traditionally been considered to be overwhelmingly forest-dwelling and arboreal in their habitat use (Sussman 2003; Rosenberger et al. 2009). This contrasts markedly with primate radiations in Africa and Asia where species invaded and successfully occupied more open habitats (woodlands, savannahs, and even steppe areas) and which have given rise to many terrestrial species (Lynch Alfaro et al. 2015). Since across their biogeographic range with rare exceptions, Neotropical primates spend relatively little time on the ground, no matter the forest type, until recently, not much attention has been paid to their use of flooded forests, other than as an impediment to studying them (Boyle et al. in press). The assumption appears to have been that, as primarily arboreal species, primates would be relatively unaffected by forest flooding. As recent work on the evolution of Platyrrhines makes clear, however, the Amazon basin with its complex and constantly shifting river drainages, including its flooded forests, has been a major generator of species diversity for South American primates (Lynch Alfaro et al. 2015). The debate as to whether Amazonian rivers should be regarded mainly as barriers to dispersal or as generators of new species (and therefore diversity for monkeys) goes back to Alfred Russel Wallace (1852) and continues to be actively debated. Differences center on the weighting of effects of geographic isolation leading to vicariance as opposed to those of species’ adaptation to the complexly changing Amazonian environment which occurred over the late Miocene–Pliocene, including the emergence of forest types discussed here (e.g., Boubli et al. 2015, Jameson Kiesling et al. 2015). A study on the distribution of small Amazonian mammals and frogs found that habitat and geographic distance were better predictors of species distribution than riverbank side supporting the view that rivers were not acting as insurmountable barriers to dispersal (Gascon et al. 2000). This finding is significant considering that the species surveyed by Gascon and colleagues are less able to cross rivers than most primates. Perhaps the most nuanced view of the role of these rivers, their flooding cycles, and their propensity to meander is that they act to separate populations of monkeys at least temporarily and that these episodes of isolation can, if they persist, generate diversity (e.g., Lynch Alfaro et al. 2015). However, all Platyrrhines do not respond identically to these dynamic riverine environments as comprehensive study of diversification of primates in the Rio Negro and Rio Branco regions of Brazil demonstrated (Boubli et al. 2015). Some species’ distribution did indeed appear strongly limited by rivers (e.g., titi monkeys, genus Callicebus), while others did not including howlers (Alouatta spp.) and especially squirrel monkeys (Saimiri spp.), which are apparently competent swimmers. Field primatologists are occasionally dramatically reminded that monkeys can and do cross streams that appear to be too wide for them to easily jump. At our study site we have seen a red howler monkey (Alouatta seniculus) successfully swim across the Tahuayo River and an adult male saki monkey ( Pithecia monachus ) leap across that same river where it was 15 m wide. However, the monkey actually jumped about 3 m between the overhanging branches of trees on each side (Jackson 2016).
The importance of Amazonia forests to South American primates is indicated by Peres’ (1997) calculation that for this taxonomic group, 66% of species and 88% of genera occur in these forests. Worldwide, primate species richness is highest in the Neotropics, and a recent analysis attributes this to vertical structure of the forests, measured using canopy height as a proxy, rather than to rainfall as has been previously supposed (Gouveia et al. 2014).
Among main Amazonian forest habitat types (terra firme, várzea, and igapó), igapó forests have typically been regarded as the least productive due to their nutrient-poor soils and typically show less species diversity in both plants and animals (Wittman et al. 2010; Myster this volume). Myster (2009) reported that in Western Amazonia the number of tree species was lower in flooded than in terra firme forests and trees in flood plains are shorter. While there has been no systematic sampling of primate diversity in these three main forest types across Amazonia, the few comprehensive studies which have been carried out found lower species diversity of primates in flooded than in unflooded forests (e.g., Peres 1997; Palminteri et al. 2011). These findings agreed with the general pattern of lower faunal species diversity in flooded forests (Haugaasen and Peres 2005). This lower primate species diversity in flooded forests has been attributed to two factors. First, the lower floral species diversity may result in fewer potential food sources for primates which are heavily dependent for food on plants (fruit and leaves primarily) or the insects attracted to or living in them. The second factor is tree height, which is reduced in flooded forests (Gouveia et al. 2014). Since Neotropical primate species have long been described as having diversified niches based at least partly on vertical stratification (e.g., Terborgh 1983), if flooded forests are shorter than unflooded ones, they may also provide less complex structures and therefore less opportunity for such niche differentiation based on canopy or understory structure.
However, despite reduced plant species richness in flooded forests compared to unflooded ones, primates do occupy flooded forests, and some species even appear to specialize in them (e.g., howlers, Alouatta and squirrel monkeys, Saimiri species, in várzea forests in Brazil, Peres 1997). In fact, primate species biomass (as opposed to species richness) is higher in some of these flooded forests (Haugaasen and Peres 2005) suggesting that while niches for primates may be fewer, at least at some times of the year resources can be superabundant. If the seasonal flooding also produces marked seasonality in fruit abundance, some primate species may be based predominantly in terra firme forests and only shift to flooded forests when resources there (presumably fruit) are abundant. Conversely, some flooded forest-specialist species may temporarily abandon these forests for unflooded ones, a pattern suggested for sites in Brazil (Peres 1997; Haugaasen and Peres 2005).
2 Primates in Igapó Forests
There have been few studies of primates in igapó habitats. In a recent review of studies conducted at sites in flooded forests, just 7 of 53 sites identified igapó as a habitat (Boyle et al. in press). Further, even fewer studies have compared primates’ use of all three forest types, igapó, várzea, and terra firme, in the same area. One of the most comprehensive of these, conducted in Brazil, found that while primate species richness was lower in igapó than in terra firme habitats, the difference was much less than between várzea and terra firme (Haugaasen and Peres 2005). This was somewhat surprising since igapó forests are considered to be nutrient-poor and less floristically diverse than other flooded and unflooded forests (Wittman et al. 2010). However, if these forests are flooded for a limited time (just a few months rather than most of the year) and if the flooding is predictable, trees and the animals which depend on them for food appear to be able to adapt to these conditions. For animals like primates, which do not need to come to the ground to move about, the key to successfully using these habitats may be the ability to move in and out of nearby terra firme habitats when resources in the flooded forest are scarce, as Peres and colleagues described for their sites in Brazil (Peres 1997; Haugaasen and Peres 2005).
2.1 Primates’ Use of Igapó Forest in Peru
In Peru , species classified in 12 genera of primates have been reported to use igapó forests (Boyle et al. in press). These include species of the genera Alouatta, Ateles, Lagothrix, Aotus, Cebuella, Saguinus, Cebus, Saimiri, Sapajus, Cacajao, Callicebus, and Pithecia. These primates range from the largest New World primates (Ateles, spider monkeys) to the smallest (Cebuella, pygmy marmosets) and represent the full range of dietary adaptations for South American species including strongly folivorous species such as howler monkeys, gummivores such as the marmosets, and the strongly frugivorous species such as spider monkeys. While some of these species are definitely known to use both igapó and other habitats (terra firme, várzea), so far none have been definitively found to be igapó specialists.
2.2 Primates in the Igapó Forest Habitats of the Área de Conservación Regional Comunal Tamshiyacu Tahuayo, Northeastern Peruvian Amazon
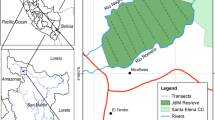
The Área de Conservación Regional Comunal Tamshiyacu Tahuayo (ACRCTT) is a communal reserve located in Loreto Department in northeastern Peru covering 420,080 hectares (Penn 2009). The reserve was established in 1991 in response to combined efforts by local inhabitants, conservationists, and researchers to prevent loggers and hunters coming into the area from outside and extracting resources and for the protection of the endangered red uakaris (Cacajao calvus ucayalii) (Meyer and Penn 2003; Newing and Wahl 2004). The predominant habitat in the reserve is terra firme, but it includes both várzea and igapó flooded forests along its smaller white and black-water rivers. One of the rivers that defines the reserve, the Tahuayo, is black water at its source and upper reaches and then becomes a mix of black and white water after its confluence with the Río Blanco. This reserve has among the highest primate species diversity reported for any site in South America (Puertas and Bodmer 1993; Peres 1997), with at least 14 species. Indeed due to continuing uncertainty about the taxonomic status of several of these species, there may be as many as 16 or 17 present in the area (Chism and Jackson in prep.).
3 Census of Primates in the ACRCTT
Here we summarize information on primate use of habitat use collected during fieldwork conducted in the ACRCTT with several graduate students in 12 years beginning in 2001. We report data for primates seen in igapó habitats and compare these with observations of species in terra firme habitats in the same area. This fieldwork included methodical censuses using transects in terra firme and várzea habitats along the Río Blanco (in 2005, 2006) and systematic but less formal censuses by boat and on foot in igapó habitats along the upper Tahuayo River and its tributary streams during and outside flood periods (in 2001, 2003, 2004, 2008, 2010, 2011, 2012, 2014, 2015, 2016).
Data presented here derive from the formal transect surveys carried out to assess the effects of hunting on primate species richness and abundance (Matthews 2006; Chism, Guinan, and Seidewand unpublished) and from ad libitum observations collected during several field projects which were focused on surveying for red uacaris (Cacajao calvus ucayalii) (Ward and Chism 2003) and the behavioral ecology, including habitat use , of saki monkeys (Pithecia spp.) (Frisoli and Chism 2009; Chism and Kieran 2008, Chism unpublished data) (see Fig. 10.1). During these later studies, we made systematic surveys along the Tahuayo River covering distances of approximately 5 km up- and downriver from the Tahuayo River Amazon Research Center (ARC) (S 04° 23.334′, W 073° 15.438′), a research station located on the black-water portion of the Tahuayo River on the northwest border of the ACRCTT. The main goal of these surveys was to search for groups of uakaris or saki monkeys, but we systematically recorded the presence of all primates encountered during these searches. In addition, in 2008, 2010, 2012, 2015, and 2016, we also searched for and followed groups of saki monkeys in igapó forest directly behind the ARC on a 2 km × 2 km trail grid consisting of 42 intersecting trails spaced approximately 100 m apart, oriented southeast of the Tahuayo River. Again during this fieldwork, we systematically recorded the identity of all primates encountered in the area. While the majority of this fieldwork occurred in the period just after the peak of the flood season to dry season (mid-May to mid-August), in two years surveys were conducted during February (2011) and late February to mid-April (Lehtonen 2016). Thus observations reported here include periods of maximum flooding, falling water, and when forests were unflooded.
Systematic census data in terra firme were collected during two study periods. The first occurred during June–July 2005 at two different sites about 5 km apart in forest along the Río Blanco (S40 25.98′, W730 7.63′ and W40 23.14′, W730 9.93′) where we cut four transects ranging in length from 2 to 4 km following a protocol described by Peres (1999). Transects were cut by hand with machetes and marked every 50 m. Transects were walked at a pace of approximately 1.00–1.50 km per hour by teams of one to three researchers. When a primate group was located, we recorded the species, time detected, number of individuals, location, and behavior. During this study we accumulated 279 km of transect effort. In addition, a second study in this same area collected approximately the same amount of census data over 43 days in May–July 2006 (Chism, Guinan, and Seidewand unpublished data).
For the river surveys and for searching for monkeys during periods when the forest was flooded, we used small motorboats or canoes (see Fig. 10.2). When using motorboats, we motored slowly upriver then turned off the motor and floated back down the river. Teams of two to three observers in the boats were able to visually search forest on both sides of the river for signs of primates. While primates were often detected first by their vocalizations, species identity was always confirmed visually except for howler monkeys (Alouatta seniculus) whose calls are distinct and definitive for identification. While searching for saki monkeys on the ARC trail grid, teams of one to three observers walked at a pace of approximately 1.00–1.50 km per hour, and visual identification was required for confirmation of a species’ presence. Grid location and habitat type were recorded for every primate sighting (Frisoli 2008; Jackson 2016).
4 Habitat Use by ACRCTT Primates
Of the minimum of 14 securely identified primate species reported to occur in the ACRCTT (Puertas and Bodmer 1993; Peres 1997), we observed 13 during our field studies in the area. The only species we have never observed in the study area in any habitat is the spider monkey Ateles chamek. This species, among the largest Neotropical primates in body size, is classified by IUCN as endangered and hunting, especially market hunting, and is considered its main threat (Matthews 2006; Wallace et al. 2008). After no sightings in the ACRCTT for at least 15 years, this species may be locally extinct. The species we observed and the habitats we found them in are presented in Table 10.1.
Of the 13 species we observed over the period from 2001 to 2016, 11 species were confirmed to occur in terra firme habitats, while 11 were confirmed in igapó habitats. Eight species (red howlers, red uakaris, coppery titis, white-fronted capuchins, burnished sakis, squirrel monkeys, both species of tamarins and large-headed capuchins) were observed in both habitats and on multiple occasions making it highly probable that these species are habitat generalists able to occupy flooded and unflooded forests. While we did not have any confirmed sightings of the nocturnal owl monkey in igapó, diurnal censuses in any habitat are highly likely to miss this species which is rarely active during the day. Experienced guides who regularly camp in igapó tell us that it is present in this habitat, however. Only one species, the pygmy marmoset, appeared to be a habitat specialist for flooded forests since it was never observed in terra firme. We did have one possible observation of these tiny monkeys in várzea. We had no confirmed observations of the largest surviving primate species in the area, the woolly monkey, in igapó forests during our studies but again experienced guides report seeing it on the research trail grid. Red uacaris enter igapó areas adjacent to terra firme forests at the edge of the ARC trail grid at some times of the year.
5 Interpreting These Patterns of Primate Use of Igapó Forests
Our finding that the number of primate species did not differ between the igapó and terra firme habitats we surveyed in the ACRCTT appears to contradict the findings of other studies comparing primates’ use of Amazonian habitats that primate species richness was higher in terra firme habitats than in flooded forests (e.g., Peres 1997; Haugaasen and Peres 2005; Palminteri et al. 2011). What factors might explain the species richness of the igapó forest in the ACRCTT of the northeastern Peruvian Amazon, then?
In the ACRCTT, igapó forests visually closely resemble várzea forests which typically grow on younger, more nutrient-rich soils. While igapó forests in other regions of Amazonia are described as having large areas of open, sandy soil and being almost desert-like when they dry out, this is not true of igapó in Peru which grows on richer soil, similar to that of várzea perhaps because of the proximity of this area to the Andes (Prance 1979). Thus, even though igapó typically has fewer tree species (Wittman et al. 2010), it may produce abundant food for primates, especially at certain times of the year. Some igapó tree species pursue a strategy of investing in nutrient-rich seed masses (Parolin 2001), a food source that especially pitheciin primates, such as sakis, seem to be well-adapted to exploit (Kay et al. 2013; Norconk and Veres 2011; Norconk et al. 2013).
Interestingly, the number and identity of species we observed in ACRCTT igapó match that reported by Bennett et al. (2001) for floodplain forest seasonally inundated by black and white-water rivers in the Río Tapiche area of northeastern Peru.
Our observations strongly support the idea that at least some primate species’ use of igapó has a seasonal component as suggested by several other authors (e.g., Peres 1997; Haugaasen and Peres 2005; Bowler and Bodmer 2011). Some species seem to be mostly based in igapó but may move into terra firme areas at some times of the year when their preferred foods are scarce (e.g., sakis), while others may make brief seasonal incursions into igapó when fruits there are superabundant. This later scenario may be the case with uacaris and woolly monkeys and may explain their occasional appearances at the edge of the trail grid in igapó in our study area at ARC. It is likely that capuchins may also be moving widely and using both igapó and terra firme habitats in this area.
It has been suggested that small-bodied primates such as tamarins are less abundant or absent from flooded forests because these species occupy lower levels of the forest, descending into the shrub layer to forage on arthropod prey (Peres 1997). Thus, when forests are flooded, their available habitat and an important food source would be curtailed. If these species could not compensate by switching to alternate food sources, they would be disadvantaged in this type of habitat. However, tamarins and other small-bodied primates (pygmy marmosets and titis) were abundant in ACRCTT igapó even when flooding was at its maximum. These primates must be able to find some compensatory food resources during several weeks of flooding they experience each year in our study area, because they do not leave the forest.
An important factor in this unexpected species richness in igapó at least at our site may be the presence of extensive aguajales (palm swamps) dominated by Mauritia flexuosa (aguaje palms). These palms fruit abundantly at exactly the time of year when fruit is otherwise scarce in these forests (Bowler and Bodmer 2011). When an aguaje tree is fruiting, it becomes a mecca for most primates in the area as well as for other nonprimate animals, confirming its reputation as a keystone species. Our observations of saki monkeys suggest that groups shift their ranges during these periods to allow them to use this superabundant resource and that the groups within whose range these palms occur furiously attempt to repel encroachment from nonresident saki groups (Frisoli and Chism 2009).
Our observations of primates in igapó habitat in northeastern Peru show that while these flooded forests may be less diverse in tree species, they harbor as much primate richness as nearby terra firme forests. This is true, even though these flooded forests have been subjected to higher human disturbance and hunting pressure because of the easier access to them than to terra firme forests in the same area. (Matthews 2006; Porter et al. 2013). For example, Alouatta, which is abundant in flooded forests elsewhere (Haugaasen and Peres 1997), is now rarely encountered at our site in igapó. Alouatta is at the top end of the size range for primates surviving in these forests since Ateles appears to be locally extinct now and the other large-bodied primates, uacaris and woolly monkeys, are becoming rare. So while flooded forests represent only about 20% of Peruvian Amazonian forests (e.g., Kvist and Nebel 2001), they harbor a significant amount of primate species diversity. If, as many primatologists working in these forests suspect, the ability of at least some of these species to move back and forth between flooded and unflooded forest is critical at some times of the year, these flooded forests may be key to maintaining sustainable populations. This may be particularly true for the endangered and rare species such as spider monkeys, uakaris, and woolly monkeys.
References
Aquino R, Encarnación F (1994) Primates of Peru Primate Report 40:1–127
Bennett C, Leonard S, Carter S (2001) Abundance, diversity, and patterns of distribution of primates on the Tapiche River in Amazonian Peru. Am J Primatol 54:119–126
Boubli J-P, Ribas C, Lynch Alfaro J, Alfaro M, da Silva M, Pinho G, Farias I (2015) Spatial and temporal patterns of diversification on the Amazon: a test of the riverine hypothesis for all diurnal primates of Rio Negro and Rio Branco in Brazil. Mol Phylogenet Evol 82:400–412
Bowler M, Bodmer RE (2011) Diet and food choice in Peruvian red uakaris (Cacajao calvus ucayalii): selective or opportunistic seed predators. Int J Primatol 32:1109–1122
Boyle S, Alho C, Chism J, Defler T, Palacios E, Santos R, Wallace R, Wright B, Wright K, Barnett A (in press) Conservation of primates and their flooded habitats in the Neotropics. In: Barnett A et al (eds) Primates in flooded forests. Cambridge University Press, Cambridge
Chism J, Kieran T. (2008) Vocal communication of sympatric equatorial and monk sakis (Pithecia aequatorialis and Pithecia monachus) in Northeastern Peruvian Amazon. Int. Congress of Primatol XXV, Hanoi, Vietnam
Frisoli L (2008) A behavioral investigation of the Pithecia niche in Northeastern Peru. MS thesis Winthrop University, Rock Hill, SC, USA
Frisoli L, Chism J (2009) Habitat and resource use by saki monkeys (Pithecia spp.) in Amazonian Peru. Annual Meetings of the American Society of Primatology, San Diego, CA
Gascon C, Malcolm J, Patton J, da Silva M, Boragt J, Loughheed S, Peres C, Neckel S, Boag P (2000) Riverine barriers and the geographic distribution of Amazonian species. PNAS 97:13672–13677
Gouveia S, Villalobos F, Dobrovolski R, Beltrão-Mendes R, Ferrari S (2014) Forest structure drives global diversity of primates. J Anim Ecol 83:1523–1530
Haugaasen T, Peres C (2005) Primate assemblage structure in Amazonian flooded and unflooded forests. Am J Primatol 67:243–258
Haugaasen T, Peres C (2009) Interspecific primate associations in Amazonian flooded and unflooded forests. Primates 50:239–251
Jackson RL Jr (2016) Habitat stratification of Pithecia Species in the Área de Concervación Regional Comunal Tamshiyacu Tahuayo in the Northeastern Peruvian Amazon. MS thesis. Winthrop University, Rock Hill SC, USA
Jameson Kiesling N, Yi S, Sperone FG, Wildman D (2015) The tempo and mode of new world monkey evolution and biogeography in the context of phylogenetic analysis. Mol Phylogenet Evol 82:386–399
Johns A (1985) Primates and forest exploitation at Tefé. Brazilian Amazonia Primate Conservation No 6:27–29
Kay RF, Meldrum DJ, Taki M (2013) Pitheciidae and other platyrrhine seed predators. Pp 3-12. In: Viega LM, Barnett AA, Ferrari SF, Norconk MA (eds) Evolutionary biology and conservation of Titis, Sakis and Uakaris. Cambridge University Press, New York
Kvist LP, Nebel G (2001) A review of Peruvian flood plain forests: ecosystems, inhabitants and resource use. For Ecol Manag 150:3–26
Lehtonen E (2016) The behavioural ecology of a potentially undescribed morph of saki monkey (genus Pithecia) in a highly diverse primate community. MS thesis. Uppsala University, Sweden
Lynch Alfaro J, Cortés-Ortiz L, Di Fiore A, Boubli J-P (2015) Special issue: comparative biogeography of Neotropical primates. Mol Phylogenet Evol 82:518–529
Matthews, H (2006) The effects of hunting on an primate community in the Peruvian Amazon. MS thesis. Winthrop University, Rock Hill, SC, USA
Meyer D, Penn J (2003) An overview of the Tamshiyacu-Tahuayo communal reserve. In: Pitman N, Vriesendorp C, Moskovits D (eds) Perú: Yavarí: Rapid Biological Inventories: 11. The Field Museum, Chicago, pp 176–177
Myster RW (2009) Plant communities of western Amazonia. Bot Rev 75:271–291
Myster RW (this volume) Introduction. In: Myster RW (ed) Igapó (black-water flooded forests) of the Amazon Basin. Springer, Berlin, p xx
Newing H, Wahl L (2004) Benefiting local populations? Communal reserves in Peru. Cultural Survival. 28.1. www.culturalsurvival.org
Noconck MA, Veres M (2011) Physical properties of fruit and seeds ingested by primate seed predators with emphasis on sakis and bearded sakis. The Anat Rec 294:2092–2111
Norconck MA, Grafton BW, McGraw WS (2013) Morphological and ecological adaptations to seed predation – a primate-wide perspective. In: Viega LM, Barnett AA, Ferrari SF, Norconk MA (eds) Evolutionary biology and conservation of Titis, Sakis and Uakaris. Cambridge University Press, New York, pp 55–71
Palminteri S, Powell G, Peres CA (2011) Regional-scale heterogeneity in primate community structure at multiple undisturbed forest sites across South-Eastern Peru. J Trop Ecol 27:181–194
Parolin P (2001) Seed germination and early establishment of 12 tree species from nutrient-rich and nutrient-poor central Amazonian floodplains. Aquat Bot 70:89–103
Penn J (2009) RCF update. Rainforest conservation fund: July, 2009. http://www.rainforestconservation.org/archives/1
Peres CA (1993) Structure and spatial organization of an Amazonian terra firme forest primate community. J Trop Ecol 9:259–276
Peres CA (1997) Primate community structure at twenty western Amazonian flooded and unflooded forests. J Trop Ecol 13:381–405
Peres CA (1999) General guidelines for standardizing line-transect surveys of tropical forest primates. Neotropical Primates 7:11–16
Peres CA, Janson CH (1999) Species coexistence, distribution, and environmental determinants of neotropical primate richness: a community- level zoogeographic analysis. In: Fleagle JG, Janson C, Reed KE (eds) Primate communities. Cambridge University Press, Cambridge, pp 55–74
Porter L, Chism J, Defler T, Marsh L, Martinez J, Matthews H, McBride W, Tirira D, Velilla M, Wallace R (2013) Pitheciid conservation in Ecuador, Colombia, Peru, Bolivia and Paraguay. In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA (eds) Evolutionary biology and conservation of Titis, Sakis and Uacaris. Cambridge University Press, New York, pp 320–333
Prance GT (1979) Notes on the vegetation of Amazonia III. The terminology of Amazonian forest types subject to inundation. Brittonia 31:26–38
Puertas P, Bodmer RE (1993) Conservation of a high diversity primate assemblage. Biodivers Conserv 2:586–593
Rosenberger A, Tejedo M, Cooke S, Pekar S (2009) Platyrrhine ecophylogenetics in space and time. In: Garber P, Estrada A, Bicca-Marques J, Heymann E, Strier K (eds) South American Primates. Springer, New York, p 113
Sussman R (2003) Primate ecology and social structure, vol 2. Pearson, Boston, MA
Wallace AR (1852) On the monkeys of the Amazon. Proc Zool Soc Lond 20:107–110
Wallace RB, Mittermeier RA, Cornejo F, Boubli J-P (2008) Ateles chamek. The IUCN Red List of Threatened Species 2008
Whitman F, Schöngart J, Junk WJ (2010) Phytogeography, species diversity community structure and dynamics of central Amazonian floodplain forests. In: Junk WJ, Pidade MTF, Wittman F, Schöngart J, Parolin P (eds) Amazonian floodplain forests. Springer, New York, pp 61–102
Acknowledgments
The authors wish to thank Hope Matthews, Sean Guinan, Amy Seidewand, Lauren Frisoli Brasington, Troy Kieran, and Candace Stenzel for their work and companionship in the field. We thank Dr. Paul and Dolly Beaver and Alfredo Dosantos for their support and enthusiasm over more years than seems possible. JC thanks Dr. Bill Rogers for years of support and holding down the fort at home while she wandered the Amazon and Will and Ali Rogers for pretending to listen with interest when she insisted on talking about monkeys again at dinner. We thank our many Peruvian guides and field assistants without whose help we would never have emerged from the igapó.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chism, J., Jackson, R.L. (2018). Primates’ Use of Flooded and Unflooded Forests in Peruvian Amazonia. In: Myster, R. (eds) Igapó (Black-water flooded forests) of the Amazon Basin. Springer, Cham. https://doi.org/10.1007/978-3-319-90122-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-90122-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90121-3
Online ISBN: 978-3-319-90122-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)