Abstract
Metal oxide nanomaterials have been attracting growing interest for large domains applications such as gas sensors, photocatalysts, solar cell and UV photodetectors. In this works, the undoped and F, Al, Zn (3 at%) doped SnO2 thin films were successfully deposited by sol-gel dip coating technique and characterized by X-ray diffraction (XRD), UV–Visible spectroscopy and photoconductivity study. Structural analysis showed that all films are polycrystalline with tetragonal rutile structure where the crystallite size is calculated by the debye Scherer’s formula and it was obtained in the range of 6.49 to 9.33. Optical transmittance spectra of the films showed high transparency (>80%) in the visible range and gap energy values were obtained in the range 3.93 to 3.99. Finally, the variation of photocurrent with voltage and with time has been studied under UV illumination (λ = 365 nm). The high photocurrent is observed using F-doped SnO2 sample while the film doped with Al had the highest photosensitivity. Current-Voltage characteristics of dark and photo current exhibited linear behavior. The phenomena of photoconductivity in SnO2 thin films were interpreted by chemisorptions of oxygen molecules on the surface.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent years, the transparent conducting oxide (TCO) such as zinc oxide, indium oxide, cadmium oxide, titanium oxide and tin oxide have attracted much attention because of their unique combination of low electrical resistivity and high optical transparency.
Tin oxide is IV VI2 compound semiconductors with rutile structure. Thin films of SnO2 are being studied for several applications such as gas sensor [1], solar cells [2], thin films transistors [3] and UV photodetectors [4]. SnO2 thin film are attractive for UV photodetectors application because of their unique properties including large band gap (3.6 a 4 eV) [5, 6] the character n-type conduction [7], large excitonic binding energy of 130 meV [8], stoichiometric structure [9], and high UV absorption rate [10].
UV photodetectors have several applications in the field of biological analysis, environmental, optical communication, flam detection, astronomy lithography and detection of missile [11, 12]. A number of earlier reports showed the performance of UV photodetectors in SnO2 can be dependent by time and temperature of annealing [4], method deposition and element dopant [13,14,15].
For enhancing the optoelectronic properties of SnO2 some elements of group IIB, IIIB, and VIIB of the periodic table are being used. Among these elements, Zinc, aluminium and fluorine. Numbers of research groups elaborated and studied structural, optical, and morphological proprieties of Zn, Al, and F doped SnO2 thin films [16,17,18]. However, to the best of our knowledge, there are no reported works on SnO2 thin films based photo-sensor and photoconductor doped with Zn, Al, and F.
In this works, our objective is to determine the influence of Zn, Al, and F atoms, in the constant doping ration, on the structural, morphological, optical properties and the performance of UV photodetectors. Sol-gel dip-coating technique was used to deposit the SnO2 thin films on cleaned glass.
2 Experiment
2.1 Materials
Tin (II) chloride dihydrate 98% (SnCl2:2H2O), Aluminium trichloride hexahydrate (AlCl3:6H2O), zinc (II) chloride (ZnCl2), fluorine ammonium (NH4F), absolute ethanol 99.8% (C2H6O) and hydrochloride acid (HCl) were used to preparing undoped, Zn, Al and F doped thin films.
2.2 Preparation and Characterization
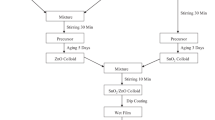
The films were prepared by sol-gel dip coating technique on ultrasonically cleaned substrates. The glass substrate was cleaned by acetone, ethanol and deionized water for 10 min in ultrasonic bath and then dried at room temperature. In another hand, the coating solution for undoped SnO2 thin film was prepared by dissolving Tin (II) chloride dihydrate 98% (SnCl2:2H2O) (0.2 M) in 30 ml of ethanol absolute (C2H6 O), then a few drops of HCl was added to solution for accelerating the hydrolysis reaction between the precursor and solvent. The homogenous solution was obtained after stirring at 70 ℃ for 120 min and was kept in a covered for 24 h at room temperature. The film was deposited by the dip-coating technique with speed 60 mm/min. After dip coating process the film was dried 200 ℃ for 10 min. In order to increase the thickness of the film, the procedure was repeated 10 times and this film was annealed at 550 ℃ for 2 h. 3% wt Al, Zn and F doped SnO2 thin films were elaborated under similar experimental conditions with adding (AlCl3:6H2O), (ZnCl2) and (NH4F) as a source of Al, Zn and F respectively.
X-ray diffraction (XRD, Bruker AXS-8D) with Cu Kα radiation (Cu Kα = 0.1541 nm) was used to study the structural properties, the surface morphology of the films was analysed with atomic force microscopy (A 100-AFM). The optical band gap of samples was performed by UV-Visible spectrophotometer (jasko V-630) in the range (200–1100).
2.3 Measurement of Photoconductivity
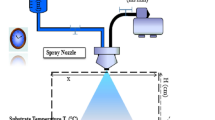
SnO2-based UV photodetectors were fabricated with planar interdigital Ag electrodes. The Keithley source meter was used to measure UV photoresponse under illumination with 4 W, UV lamp available in tow wavelengths: 254 nm, 365 nm (VL- 4LC, vilber lourmat) and equipped. The Keithley was used to measure current-voltage (I-V) characteristics in dark and under UV source of 365 nm wavelength and intensity 350 µW/cm2.
3 Results
3.1 Structural Analysis
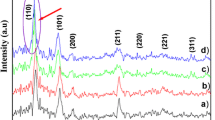
Figure 1 shows the XRD patterns of the undoped, Zn, Al and F doped SnO2 thin films. As can be seen five diffraction peaks corresponding to (110), (101), (200), (210), (220) direction without any impurity and secondary phase. These spectra confirm that all films have polycrystalline nature with tetragonal rutile structure with a preferred orientation along (110) plane. All diffraction peaks intensity varies with a dopant. The crystallite size (D) can be calculated from the Scherer’s equation [19].
Where \( \lambda \) is the X-ray wavelength, \( \beta \) is the full width at half maximum of the XRD peak, \( \theta \) is the Bragg diffraction angle.
The (110) peak was utilized to estimate the crystallite sizes (Table 1). It can be noticed from Table 1 that the crystallite size of the films decreases from 9.33 to 6.49 nm.
3.2 Optical Properties
The optical transmission spectra of un-doped, fluorine, aluminium and zinc doped tin oxide thin films prepared and deposited by sol-gel dip coating technique on a lime glass substrate and annealed at 550 ℃ are plotted in Fig. 2a. All films showed high transparency (>80%) in the visible range.
The band gap energy values of different type doped tin oxide thin films were calculated by using the tuc equation [20].
Where \( \alpha \) The absorption coefficients, \( A \) is constant, \( h\upsilon \) is photon energy and \( E_{g} \) is the band gap energy.
Figure 2b shows \( \left( {\alpha h\upsilon } \right)^{2} \) variation as a function of photon energy \( \left( {h\upsilon } \right) \). The values of optical energy band gap, which was, be estimated from the extrapolating the linear part of \( \left( {\alpha h\upsilon } \right)^{2} \) versus \( \left( {h\upsilon } \right) \) plot to \( \left( {\alpha h\upsilon } \right)^{2} \) shown in Table 1. It can be seen that the band-gap energy increased for the all thin films doped with Al, Zn and F. These results are very similar to some reports [21, 22].
3.3 Photoconductivity Study
3.3.1 Voltage Dependence of Dark and Photocurrent
Figure 3 shows a variation of photocurrent and dark current with an applied voltage obtained from undoped as well as Al, Zn and F doped SnO2 on a log-log scale. From the I-V measurements, it can be seen that the photocurrent increase of the applied bias voltage because of the augmentation of the carrier drift velocity [23]. The ln (I) vs. ln (V) curves are straight lines having different slopes with respect to varying voltage according to the power law relation I α Vr where r is the slope of a line. In the dark current and photocurrent, the linear behavior of curves is indicated the ohmic conduction. The ohmic behavior is very important to the photo sensing because the sensitivity of photodetector can be maximized when the metal-semiconductor junction is ohmic [24].
3.3.2 UV Detection Mechanism of SnO2 Thin Films
The photoresponse mechanism of SnO2 thin films to UV light can be explained in Fig. 4 with according to the following equations:
The process of oxygen chemisorptions plays a vital role in UV photoconduction mechanism. Under dark condition, the oxygen molecule O2 (g) is absorbed on the surface of as negatively charged ions by capturing free electron.
This process leads to the formation of depletion layer near the surface resulting in the bending of the conduction band and valence band, as well as trap center-related band or trap depth. Formation of a large number of ionized oxygen on the thin films surface enhanced the band bending, resulting in a very low conductivity. When the sample is illuminated with UV light, the electron-hole pair is generated by light absorption [25].
The photo-generated hole oxidizes negatively, charged oxygen ions adsorbed on the surface \( O_{2}^{ - } \) (ads). While the remaining unpaired electron in the conduction band increases the conductivity.
3.3.3 Rise and Decay of Photocurrent
The effect of dopant material on the photoconductivity response of SnO2 thin films under 2 V bias voltages is plotted in Fig. 5, when UV illumination is on, a large increase in the current result and when the illumination is off the current increase gradually, indicating that the films are highly UV sensitive. This figure clearly shows that the SnO2 thin film based UV photodetector doped with fluorine displayed the maximum photocurrent value.
The highest on/off ratio was recorded by Al-doped SnO2 about 5000.
Rise and decay time are estimated by the fitting curve using the exponential functions.
The times constants values are noted in Table 2, the minimum values of the rise time and the decay time constants were obtained for the sample doped with aluminum. On other hand, samples doped with fluorine which present a high photocurrent compared to other samples show high rise time and decay time constants.
4 Conclusions
In summary, we have successfully fabricated undoped as well as Zn, Al and F doped SnO2 UV photodetectors on glass substrates by sol-gel method using low cost dip coating technique. XRD and UV-visible transmission were used to characterize the elaborated films. The results indicated that our thin films show a polycrystalline nature with rutile structure and optical transmittance spectra of the films showed high transparency (>80%) in the visible range and gap energy values were obtained in the range 3.93 to 3.99. In photoconductivity study, the low dark current and high \( {{I_{ON} } \mathord{\left/ {\vphantom {{I_{ON} } {I_{OFF} }}} \right. \kern-0pt} {I_{OFF} }} \) current ration with rise time constant \( \tau_{r} \) and decay time constants \( \tau_{d1} \) and \( \tau_{d2} \) of about 24.39, 4.51 and 27.63 respectively have been obtained for the sample doped with aluminium while the highest \( I_{ON} \) current has been obtained for F doped SnO2 thin films. In this study, it was concluded that the kind of dopant atoms change strongly the performance of UV photodetectors based on SnO2 thin films.
References
Selma, M.H.: Influence of multilayer deposition on characteristics of nanocrystalline SnO2 thin films produce by Sol-Gel Technique for gas sensor application. Optic 146, 17–26 (2017)
Bu, I.Y.Y.: Sol-gel deposition of fluorine-doped tin oxide glasses for dye sensitized solar cell. Ceram. Int. 40, 417–422 (2014)
Priyadarshini, D.M., Ramanjaneyulu, M., Ramachandra, M.S., Nandita, D.G.: Effect of annealing ambient on SnO2 thin film transistors. Appl. Surf. Sci. 418, 414–417 (2016)
Rechem, D., Khaial, A., Souifi, A., Djeffal, F.: Effect of annealing time on the performance of tin oxide thin films ultraviolet photodetectors. Thin Solid Films 623, 1–7 (2017)
Khan, A.F., Mazhar, M., Aslam, M., Ashraf, M.: Characterisatics of electron beam evaporated nanocrystalline SnO2 thin films annealed in air. Appl. Surf. Sci. 256, 2252–2258 (2010)
Kumaril, N., Ghosh, A., Tewari, S., Bhattacharjeel, A.: Synthesis, structural and optical properties of Al doped SnO2 nanoparticles. Indian J. Phys. 88, 65–70 (2014)
Kucheyev, S.O., Baumann, T.F., Sterne, P.A., Wang, Y.M., Buuren, T., Hamza, A.V., Terminello, L.J., Willey, T.M.: Surface electronic state in three-dimensional SnO2 nanostructures. Phys. Rev. B 72(035404), 1–5 (2005)
Reddy, A.S., Figueiredo, N.M., Cavaleiro, A.: Nanocrystalline Au: Ag SnO2 films prepared by pulsed magnetron sputtering. J. Phys. Chem. Solids 74, 825–829 (2013)
Kilic, C., Zunger, A.: Origine of coexistence of conductivity and transparency in SnO2. Phys. Rev. Lett. 88(095501), 1–4 (2002)
Venkateswara Reddy, P., Venkatramana Reddy, S., Sankra Reddy, B.: Synthesis and properties of (Ni, Al) co-doped nanoparticles. J. Mater. Sci.: Mater. Electron. 27, 10712–10719 (2016)
Liao, M.Y., Sang, L.W., Teraji, T., Imura, M., Alvarez, J., Koide, Y.: Comprehensive investigation of single crystal diamond deep-ultraviolet detectors. J. Appl. Phys. 51, 090115:1–090115:7 (2012)
Sandvik, P., Mi, K., Shahedipour, F., McClintock, R., Yasan, A., Kung, P., Razeghi, M.: AlxGa1-xN for solar-blind UV detectors. J. Cryst. Growth 231, 366–370 (2001)
Wu, J.M., Kuo, C.H.: Ultaviolet photodetectors made from SnO2 nanowires. Thin Solid Films 517, 3870–3873 (2009)
Huang, S., Matsubara, K., Cheng, J., Li, H., Pani, W.: Highly enhanced ultraviolet photosensitivity and recovery speed in electrspun Ni-doped SnO2 nanobelts. Appl. Phys. Lett. 103(141108), 1–5 (2013)
Deng, K., Lu, H., Shi, Z., Liu, Q., Li, L.: Flexible three-dimensional SnO2 nanowire arrays: atomic layer deposition-assisted synthesis, excellent photodetectors, and field emitters. Appl. Mater. Interfaces 5, 7845–7851 (2013)
Xu, B., Ren, X.R., Gu, G.R., Lan, L.L., Wu, B.J.: Structural and optical properties of Zn-doped SnO2 films prepared by DC and RF magnetron co-sputtering. Superlattices Microstruct. 89, 34–42 (2016)
Benouis, C.E., Benhaliba, M., Mouffak, Z., Avila-Garcia, A., Tiburcio-Silver, A., Ortega Lopez, M., Romano Trujillo, R., Ocak, Y.S.: The low resistive and transparent Al-doped SnO2 films: P-type conductivity, nanostructures and photoluminescence. J. Alloys Compd. 603, 213–223 (2014)
Tran, Q.P., Fang, J.S., Chin, T.S.: Properties of fluorine-doped SnO2 thin films by agreen sol–gel method. Mater. Sci. Semicond. Process. 40, 664–669 (2015)
Anandhi, R., Mohan, R., Swaminathan, K., Ravichandran, K.: Influence of aging time of the starting solution on the physical properties of fluorine doped zinc oxide films deposited by a simplified spray pyrolysis technique. Superlattices Microstruct. 51, 680–689 (2012)
Tauc, J., Grigorovici, R., Vancu, A.: Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 15, 627–637 (1966)
Ahmed, S.K.F., Khan, S., Ghosh, P.K., Mitra, M.K., Chattopadhyay, K.K.: Effect of Al doping on the conductivity type inversion and electro-optical properties of SnO2 thin films synthesized by sol-gel technique. Sol-gel Science Technology 39, 241–247 (2006)
Pacheco, A.P., Acosta, D.R., Magana, C.: Effect of the amount of the starting solution on physical properties of SnO2: F thin films. Surf. Interfaces 6, 85–90 (2017)
Soci, C., Zhang, A., Xiang, B., Dayeh, S.A., Aplin, D.P.R., Park, J., Bao, X.Y., Lo, Y.H., Wang, D.: ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 7, 1003–1009 (2007)
Yoon, Y.J., Park, K.S., Heo, J.H., Park, J.G., Nahm, S., choi, K.J.: Synthesis of ZnXCd1-XSe (06X61) alloyed nanowires for variable wave length photodetectors. Mater. Chem. 20, 2386–2390 (2010)
Kim, J.M., Lim, S.J., Nam, T., Kim, D., Kim, H.: The effects of exposure on the device characteristics of atomic layer deposited-ZnO: N thin films transistors. Electrochem. Soc. 158, 150–154 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Selma, K., Djamil, R. (2018). Effect of Dopant Material on the Performance UV Photodetector Based SnO2 Thin Films Deposited by Sol-Gel Dip-Coating Method. In: Abdelbaki, B., Safi, B., Saidi, M. (eds) Proceedings of the Third International Symposium on Materials and Sustainable Development. SMSD 2017. Springer, Cham. https://doi.org/10.1007/978-3-319-89707-3_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-89707-3_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89706-6
Online ISBN: 978-3-319-89707-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)