Abstract
Aerogels of pure and aluminium doped ZnO (Al-ZnO) were synthesized by sol-gel route followed by supercritical drying in ethanol. The atomic percentage of the dopant is fixed at 1, 3, 5 and 10 at.%. The elaborated aerogels were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray energy dispersive analysis (EDAX) and Fourier transform infrared spectroscopy (FTIR). The DRX results revealed good crystalline quality of the synthesized samples with hexagonal wurtzite crystallographic structure. No crystalline phase related to aluminum ions or aluminum based compounds has been detected. With increasing Al content in aerogels, the crystallinity deteriorates and the cell parameters are found to be slightly changed. SEM images showed that the presence of Al atoms in the ZnO lattice strongly alters grain morphology, especially for higher doping levels (≥ 5 at.%). The EDAX spectra showed well-defined peaks related only to Zn, O and Al and confirmed the absence of peaks related to other impurities. FTIR measurements have shown that the introduction of the A1 ions in the aerogels greatly reduces the intensity of the Zn-O bond-vibrating band and shifts it towards low wavenumbers.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Zinc oxide (ZnO) attracts a great deal of attention from researchers in laboratories all over the world (Chen et al. 2014). It is a most attractive field of research, in view of these qualities either in optoelectronics and electroluminescence diode (LED) (Saleem et al. 2016), varistors (Liu et al. 2007), gaz sensors (Ryzhikov et al. 2015) or in the biomedical (Tallósy et al. 2014, Gopikrishnan et al. 2010, Madhumitha et al. 2016). It is also known for its large optical gap (3.37 eV) and its high exciton binding energy (60 meV) at room temperature.
ZnO can be synthesized as either a nanoscale powder, thin layers or monoliths. Several methods have been used to elaborate ZnO powders, such as spray pyrolysis (Dounia et al. 2016), radiofrequency sputtering (Bidmeshkipourand and Shahtahmasebi 2013), chemical vapor deposition (CVD) (Singh et al. 2008), and sol-gel (Kwon et al. 2013, Meddouri et al. 2016, Djouadi et al. 2014). The sol-gel method is a method, which has several advantages, because of its simplicity and low cost, and the possibility to obtain various sample forms with good crystalline qualities. In recent years, research has focused on doping semiconductors to improve their optical, electronic and structural properties (Panda and Tseng 2013; Narula et al. 2015). The dopant atoms generally used are transition metals as Al, Ga, Cd, Cu ... etc (Cunha et al. 2013, Slimi et al. 2018).
2 Experimental Procedures
Pure ZnO and Al-doped ZnO aerogels were obtained by mixing, in adequate volume and mass proportions, zinc acetate dihydrate (Zn(C2H3O2)2, 2H2O), methanol and aluminum nitrates \( ({\text{Al}}({\text{NO}}_{3} )_{3} , \, 9{\text{H}}_{2} {\text{O}}) \) under continuous magnetic stirring. The obtained solution underwent a drying at supercritical conditions of ethanol (critical temperature Tc = 243 °C and critical pressure Pc = 63.6 bars) in a one-liter reactor (autoclave), in order to obtain pure and Al-doped ZnO nanoparticles in the form of powder aerogels. Once the supercritical conditions reached, the solvent was removed and the autoclave was allowed to cool spontaneously to room temperature. The obtained aerogels were characterized without any chemical or heating treatments.
The crystalline structure of the as-prepared aerogels was studied using a PanAlytical diffractometer where the X-rays are produced from a radiation source CuKα (λ = 0.154 nm), with an acceleration voltage of 40 kV and a current of 30 mA. Infrared spectra (FT-IR) were recorded with a Shimadzu’s IRAffinity-1 spectrometer by KBr pellet technique. SEM micrographs were recorded by a Quanta 2000 microscope with energy dispersive X-ray (EDS) spectroscope. EDS was used to identify the composition of the obtained products.
3 Results and Discussion
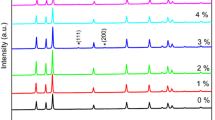
Figure 1 shows the XRD patterns of pure and Al-doped ZnO aerogels powders synthesized in supercritical ethanol with different Al contents. All the observed peaks [(100), (002), (101), (102), (110), (103) and (112)] are those of the ZnO hexagonal wurtzite structure (JCPDS 36-1451). Peaks have been found to be quite sharp and intense which implies high crystallinity of the aerogels. No crystalline phases related to Al or Al based compounds have been detected by the XRD measurements which indicates that the doping atoms are introduced in ZnO crystal lattice. The most important XRD peaks of the aerogels are shown in Fig. 2 to investigate the effect of Al atoms on the structural properties of the samples. It is observable that the most intense peaks are those of pure ZnO. This means that the introduction of Al atoms in ZnO lattice deteriores the crystalline quality of the samples. Compared to the undoped aerogel peaks, it can be noted that the peaks position of the aerogels doped with 1 and 10 at.% shifts towards lower diffraction angles more than those of the aerogels doped with 3 and 5 at.%. In addition, it can be show that the peak (101) of ZnO doped with 5 at.% is the most intense one and indicate that the introduction of Al atoms ameliorates the crystalline quality in the direction (101).
XRD patterns of the most important peaks of the Fig. 1.
In addition, compared to the pure ZnO peaks, the peaks of the doped aerogels shift toward low angle indicating the existence of tensile stress introduced by the lattice distortion. This result confirms the largening of the lattice parameters and the peaks moving to small-angles. Normally, due to the smaller radius of Al3+ ions compared to Zn2+ ions, the incorporation of Al3+ will lead to the decrease of lattice constants (Serier et al. 2011).
The lattice parameters of ZnO cell a and c were calculated respectively from the positions of (101) and (002) using the following equations:
In these equations, θ is the diffraction angle and λ is the X rays wavelength. The average crystallites size of pure and aluminum doped ZnO powders is calculated using the well-known Scherrer formula:
Where \( \Delta \theta \) is the is the full width at half maximum (in radians) of Miller’s planes. The calculated lattice parameters and the average crystallites sizes of the aerogels are resumed in Table 1. The crystallites size has been found to be between 36 and 50 nm in all the prepared aerogels (as shown in Table 1).
The SEM micrographs of the pure and Al-doped ZnO with 3, 5 and 10 at. % are shown in Fig. 3. They show that the aerogels are formed by crystallites which agglomerate to form grains with various geometries and morphologies. The very small grains are attached on the surfaces of the big grains and randomly increase their sizes. For the 5 and 10 at.% Al-doped ZnO the small grains agglomerate to form big grains with torus-like and cauliflowers-like forms morphologies. These changes in the morphologies are probably due to the Al concentration in the ZnO lattice.
The X-ray energy dispersive analysis (EDAX) has been used to characterize the different chemical elements composed the aerogels. The EDAX spectra are shown in Fig. 4. The EDAX data of these aerogels show the presence only O, Zn, Al and C atoms. The C atoms come from the grid used in the experimentation. The results indicate that the percentage of Al in the samples did not strongly deviates from their stoichiometry.
Recorded FT-IR spectra of pure and Al-doped ZnO aerogels are shown in Fig. 5. The vibrational bands with different intensities are observed in all spectra approximately at the same positions (3429, 2370, 1624, 1416 and 1030 cm−1). No bonds related to Al or Al-based compounds are found in the FT-IR curves. The small intensities of the lines testify the high chemical purity of the elaborated aerogels. The band at 2370 cm−1 can be assigned to CO2 in trace and the one at 1416 cm−1 is assigned to C-C asymmetric stretching bond vibration. The peak at 1036 cm−1can be attributed to aromatic C = C stretching mode. The absorption bands at 3429 cm−1 is due to the stretching mode of O-H group which reveals the existence of small amount of water absorbed by the ZnO aerogels (Djouadi et al. 2012). The band at 1624 cm−1 is due to the vibration of H-O-H bond (Vigneshwaran et al. 2006). A very strong band between 400 and 460 cm−1 due to the vibration of the Zn-O bond characterizes the spectra. The intensity of the band related to Zn-O bond decreases after the introduction of Al atoms in the aerogels. It can be noted that the presence of Al atoms in ZnO lattice shifts the absorption band of Zn-O.
4 Conclusion
Undoped and Al-doped ZnO aerogels were successfully synthesized in supercritical ethanol. [Al]/[Zn] atomic ratios was fixed to 1, 3, 5 and 10% in all solutions. The Al-doping caused the changes in the crystallite size and lattice parameters of ZnO lattice. XRD characterizations manifest that the aerogels have high crystallinity and shared the presence of wurtzite structure. Al-doping strongly deteriorates the crystalline quality of the samples. EDAX studies confirm the high purity of the aerogels and the incorporation of Al atoms in ZnO lattice. FTIR spectra confirm that the presence of Al ions in ZnO lattice induces a shift of Zn-O bond vibration band toward low wavenumbers and strongly decreases its intensity. The introduction of dopant elements in the ZnO lattice shifts the absorption The morphology of the aerogels grains was found to be strongly dependent on the Al concentration.
References
Bidmeshkipourand, S., Shahtahmasebi, N.: Different properties of aluminum doped zinc oxide nanostructured thin films prepared by radio frequency magnetron sputtering. Semiconductors 47, 787–790 (2013)
Chen, J., Pan, J.Y., Qingguo, D., Alagappan, G., Lei, W., Li, Q., Xia, J.: Highly efficient white quantum dot light-emitting diode based on ZnO quantum dot. Appl. Phys. A 117, 589–591 (2014)
Cunha, D.M., Ito, N.M., Xavier, A.M., Arantes, J.T., Souza, F.L.: Zinc oxide flower-like synthesized under hydrothermal conditions. Thin Solid Films 537, 97–101 (2013)
Djouadi, D., Meddouri, M., Chelouche, A.: Structural and optical characterizations of ZnO aerogel nanopowder synthesized from zinc acetate ethalonic solution. Opt. Mater. 37, 567–571 (2014)
Djouadi, D., Chelouche, A., Aksas, A.: Amplification of the UV emission of ZnO: Al thin films prepared by Sol-Gel Method. J. Mater. Envir. Sci. 3, 585–590 (2012)
Dounia, R., Migalska-Zalas, A., Addou, M., Bernede, J.C., Outzourhit, A., Benbrahim, M.: Preparation and characterization of highly transparent and conductive indium-zinc oxide thin films deposited by pyrolysis spray technique. Opt. Quantum Electron. 48, 339 (2016)
Gopikrishnan, R., Zhang, K., Ravichandran, P., Baluchamy, S., Ramesh, V., Biradar, S., Ramesh, P., Pradhan, J., Hall, J.C., Pradhan, A.K., Ramesh, G.T.: Synthesis, characterization and biocompatibility studies of zinc oxide (ZnO) nanorods for biomedical application. Nano-Micro Lett. 2, 31–36 (2010)
Kwon, B.J., Woo, H.J., Park, J.Y., Jang, K.: Optical properties of ZnO powder prepared by using a proteic sol-gel process. J. Korean Phys. Soc. 62, 739–742 (2013)
Liu, H.Y., Kong, H., Ma, X.M., Shi, W.Z.: Microstructure and electrical properties of ZnO-based varistors prepared by high-energy ball milling. J. Mater. Sci. 42, 2637–2645 (2007)
Madhumitha, G., Elango, G., Roopan, S.M.: Biotechnological aspects of ZnO nanoparticles: overview on synthesis and its applications. Appl. Microbiol. Biotechnol. 100, 571–581 (2016)
Meddouri, M., Hammiche, L., Slimi, O., Djouadi, D., Chelouche, A.: Effect of cerium on structural and optical properties of ZnO aerogel synthesized in supercritical methanol. Mater. Sci. Pol. 34, 659–664 (2016)
Narula, C., Kaur, I., Kaur, N.: Investigation of optical properties of mixed ligand directed ZnO luminescent nanoparticles for application in light emitting diodes. J. Mater. Sci. Mater. Electr. 26, 8167–8175 (2015)
Panda, D., Tseng, T.Y.: One-dimensional ZnO nanostructures: fabrication, optoelectronic properties, and device applications. J. Mater. Sci. 48, 6849–6877 (2013)
Ryzhikov, A., Jonca, J., Kahn, M., Fajerwerg, K., Chaudret, B., Chapelle, A., Menini, P., Shim, C.H., Gaudon, A., Fau, P.: Organometallic synthesis of ZnO nanoparticles for gas sensing: towards selectivity through nanoparticles morphology. J. Nanoparticles Res. 17, 280 (2015)
Saleem, M., Manzoor, A., Zaffar, M., Hussain, S.Z., Anwar, M.S.: Tailoring of ZnO with selected group-II elements for LED materials. Appl. Phys. A 122, 589–595 (2016)
Serier, H., Demourgues, A., Majimel, J., Gaudon, M.: Infrared absorptive properties of Al-doped ZnO divided powder. J. Solid State Chem. 184, 1523–1529 (2011)
Singh, P., Kumar, A., Kaushal, A., Kaur, D., Pandey, A., Goyal, R.N.: In situ high temperature XRD studies of ZnO nanopowder prepared via cost effective ultrasonic mist chemical vapour deposition. Bull. Mater. Sci. 31, 573–577 (2008)
Slimi, O., Djouadi, D., Hammiche, L., Chelouche, A., Touam, T.: Structural and optical properties of Cu doped ZnO aerogels synthesized in supercritical ethanol. J. Porous Mater. 25, 595–601 (2018)
Tallósy, S.P., Janovák, L., Ménesi, J., Nagy, E., Juhász, A., Balázs, L., Deme, I., Buzás, N., Dékány, I.: Investigation of the antibacterial effects of silver-modified TiO2 And ZnO plasmonic photocatalysts embedded in polymer thin films. Environ. Sci. Pollut. Res. 21, 11155–11167 (2014)
Vigneshwaran, N., Kumar, S., Kathe, A.A., Varadarajan, P.V., Prasad, V.: Functional finishing of cotton fabrics using zinc oxide-soluble starch nanocomposites. Nanotechnology 17, 5087–5095 (2006)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Mouzaia, F., Djouadi, D., Chelouche, A., Hammiche, L. (2018). Al-Doping Effects on Structural and Morphological Properties of ZnO Aerogels Synthesized in Supercritical Ethanol. In: Abdelbaki, B., Safi, B., Saidi, M. (eds) Proceedings of the Third International Symposium on Materials and Sustainable Development. SMSD 2017. Springer, Cham. https://doi.org/10.1007/978-3-319-89707-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-89707-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89706-6
Online ISBN: 978-3-319-89707-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)