Abstract
Heat shock proteins (HSP) constitute a large family of proteins involved in protein folding and maturation and the expressions of HSP are induced by heat shock or other stressors. The major groups, which are classified based on their molecular weight, include HSP27, HSP40, HSP60, HSP70, HSP90, and large HSP (HSP110 and glucose-regulated protein 170). The human HSP70 family is consists of 13 members and five of them have a strong association with cancer. HSP play a significant role in cellular proliferation, differentiation, survival, apoptosis, and carcinogenesis. In this chapter, we thoroughly discussed the roles of HSP70s in cancer biology and pharmacology. The HSP70 proteins have important functions in the molecular mechanisms leading to cancer development, progression, and metastasis. They may also have potential clinical use as biomarkers for cancer diagnosis or assessing disease progression, and as therapeutic targets for cancer therapy. Understanding of the functions and molecular mechanisms of HSP70 proteins is critical for enhancing the accuracy of cancer diagnosis as well as for developing more effective and less toxic chemotherapeutic agents.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Heat shock proteins (HSP) are a group of proteins that function to reverse or inhibit denaturation or unfolding of cellular proteins in response to stress or high temperature. Traditionally, HSP have also been known as molecular chaperones because of their physiological and protective roles in cells. They facilitate protein folding and maintenance of natural structure and function when cells are exposed to homeostatic challenges such as extreme temperature, anoxia, hypoxia, heavy metals, drugs, or other chemical agents that may induce stress or protein denaturation (Macario and Conway de Macario 2007; Liu et al. 2012). HSP are generally classified according to their molecular weights with the majority of them belonging to the groups of HSP27(HSPB1), HSP40, HSP60, HSP70, HSP90, and large HSP [HSP110 and glucose-regulated protein 170 (GRP170)] (Ciocca and Calderwood 2005). Heat shock factors (HSF) act as inducible transcriptional regulators of HSP and they are required for the expression of the majority of HSP. Heat shock elements (HSE) are cis-acting sequences located upstream of HSP genes where HSF bind to and induce HSP gene expression (Akerfelt et al. 2010). Except for the small HSP group, other HSP family members including HSP70 proteins are ATP-dependent proteins with adenosine triphosphatase (ATPase) activity (Bepperling et al. 2012). The HSP70 proteins are an ATP binding chaperones with intrinsic ATPase activity which hydrolyzes ATP into ADP. Hydrolysis of ATP initiates the conformational change of HSP70 proteins and further causes substrate binding to them (Fig. 1) (Jakob et al. 1996; Sullivan and Pipas 2002).
The model of HSC70 binds to the substrates and releasing cycle. HSP70 has a low affinity with the substrates in the ATP-bound state. After hydrolysis of ATP by ATPase, HSP70 binds to the substrates with a high affinity in the ADP bound state. Some co-chaperones such as Dna J homologues enhance the ATPase activity of HSP70. Nucleotide exchange factors such as GrpE enhance the dissociation of bound ADP from HSP70 to allow the binding of ATP and reset the cycle
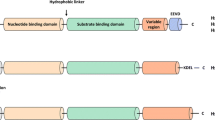
The nomenclature of human HSP is based on the system assigned by the Human Genome Organization (HUGO) Gene Nomenclature Committee and uses the Entrez Gene database from the National Center of Biotechnology Information. Specifically, as shown in Table 1, the human HSP70 family consists of thirteen members which are encoded by the HSPA genes (Kampinga et al. 2009). HSP70 proteins have a highly conserved domain structure including a ~44 kDa amino-terminal ATPase domain, a ~18 kDa substrate binding domain, and a ~10 kDa carboxyl-terminal domain (Liu et al. 2012). The chaperone activity of HSP is regulated by their co-chaperones including HSP40, Bcl-2 associated athanogene 1 (BAG-1), and carboxyl-terminus of HSP70-interacting protein (CHIP) (Wang et al. 2014a, b). In general, HSP70 proteins play an important role in cancer development, progression, and metastasis and they are often observed at abnormally high expression in cancer cells. HSP70 proteins promote carcinogenesis as a survival factor due to their tumor-associated expression and anti-apoptotic effect (Rérole et al. 2011). HSP70 proteins can protect cancer cells from tumor necrosis factor (TNF)-induced cytotoxicity, indicating that HSP70s may increase the oncogenic potential in certain cancer cells by an escaping mechanism from the immune system (Jäättelä et al. 1992). The proposed roles of HSP70s in cancer development and as potential therapeutic targets for anti-cancer agents are illustrated in Fig. 2 (Wu et al. 2017).
Proposed roles of HSP70 in regulation of cancer development and as the potential therapeutic targets for anticancer drugs. (a) Inhibition of both intrinsic and extrinsic apoptotic pathways by HSP70. HSP70 inhibits apoptosis through (1) binding to and inhibition of translation of BAX from cytosol to mitochondria, (2) prevention of the recruitment and transportation of APAF-1 to apoptosome, (3) binding to and inhibition of the activity of kinases involved in stress signaling, and (4) binding to and disruption of the sensitivity and function of AIF. (b) Control of cell senescence by HSP70. HSP70 inhibits cell senescence through (1) interruption of p53-dependent regulation and (2) antagonization of p53-independent regulation. (c) Stabilization of lysosome function and regulation of autophagy by HSP70. HSP70 promotes cell survival through (1) stabilization of lysosomes by binding to endo-lysosmal lipid bisphosphate, (2) inhibition of lysosomal membrane permeabilization and (3) stimulation of autophagy. (d) Regulation of HSP90 client proteins by HSP70. HSP70 works as a co-chaperone of HSP90 via delivery of client proteins to HSP90. The HSP90 client proteins delivered by HSP70 include HER2, CDK4, AKT and C-RAF
HSP70 proteins have crucial functions in mediation of protein folding, maintenance of protein homeostasis, and enhancement of cell survival following a multitude of stresses (Murphy 2013). Five important and well studied members in the HSP70 family have a strong association with cancer: the stress inducible HSP70s include HSPA1A (also known as HSP72, mainly located in the cytosol, ~72 kDa) and HSPA6 (also known as HSP70B’, mainly located in the cytosol, ~71 kDa); the constitutively expressed HSP70s include HSPA5 (also known as GRP78 or BIP, mainly located in the endoplasmic reticulum (ER), ~78 kDa), HSPA8 (also known as HSC70, mainly located in the cytosol, ~73 kDa), and HSPA9 (also known as mortalin, GRP75 or mtHSP70, mainly located in the mitochondria, ~75 kDa) (Arispe and De Maio 2000; Kampinga et al. 2009). In this chapter, we will focus on discussing the five major HSP70 members of HSPA1A/HSP72, HSPA5/GRP78, HSPA6/HSP70B’, HSPA8/HSC70, and HSPA9/mortalin. Their roles related to cancer characters are summarized in Table 2.
Role of HSPA1A/HSP72 in Cancer Development and Diagnosis
The HSP72 protein is coded by the HSPA1A gene and has malignant behaviors as evidenced by the decrease of cell proliferation, migration and invasion, increase of apoptosis after knockdown of HSP72 in cervical squamous cell carcinoma cells (Yoshidomi et al. 2014). HSP72 promotes the survival of glioblastoma cells through the inhibition of the degradation of activating transcription factor 5 (ATF5) (Li et al. 2011). HSP72 also inhibits oncogene-induced senescence pathways in either a p53-depdendent (via PI3K) or a p53-independent (via Ras/ERK) manner in multiple types of cancer cells including breast, colon, lung, ovarian and pancreatic cancers (Gabai et al. 2009). In addition, the study of HSP72 in autophagy and cancer showed that HSP72 functions as a survival protein by stabilizing the integrity of lysosomes in cancer cells (Nylandsted et al. 2004).
HSP72 is overexpressed in various cancers and the high expression is correlated with increased tumor grade and poor prognosis. The overexpression of HSP72 is observed in oral cancer, liver cancer, prostate cancer, colorectal cancer, lung cancer, uterine cervical cancer, and melanoma (Murphy 2013; Wu et al. 2017). Cai et al. studied tumor tissues of 507 patients with nasopharyngeal cancer and they found that the expression patterns of HSP72 are correlated with the outcomes of the patients as: high levels of HSP72 in the membranes and cytoplasm are associated with increased survival, while high levels of HSP72 in the nuclei are correlated with poor survival (Cai et al. 2012). The study by Wang et al. showed that the expression of HSP72 in the cytoplasm of esophageal cancer cells was significantly associated with remote metastasis (Wang et al. 2010). However, HSP72 was found to be under-expressed in renal cancer cells (Ramp et al. 2007). HSP72 is a prognostic marker for cholangiocarcinoma, chondroscarcoma, bladder cancer, acute myelogenous leukemia (AML), breast cancer, endometrial cancer, cervix/uterus cancer, pancreatic cancer, heat and neck cancer and colorectal cancer (Kocsis et al. 2011; Boonjaraspinyo et al. 2012; Bayer et al. 2014; Trieb et al. 2016; Wu et al. 2017).
Role of HSPA5/GRP78 in Cancer Development and Diagnosis
The HSPA5 protein, commonly known as GRP78, is coded by the HSPA5 gene. It is the HSP70 family protein primarily presented in the ER, thus it is important in regulation of the cellular response to ER stress. GRP78 is responsible for maintaining the normal function of ER during protein translocation, assisting protein folding and assembly, and targeting misfolded proteins for degradation (Ma and Hendershot 2004]. Cancer cells are subject to ER stress and GRP78 is a survival factor in cancer development. GRP78 plays an important role in initiation of cancinogenesis, prevention of apoptosis and autophagy, and enhancement of drug resistance (Lee 2007). GRP78 is a co-factor for Cripto signaling via both TGF-β and Src/MAPK/PI3K pathways to promote oncogenesis in somatic stem cells (Gray and Vale 2012). GRP78 binds to prostate-specific antigen (PSA) and upregulates the expression of PSA in prostate cancer cells, promoting cell survival via the activation of ERK, p38 MAPK, and PI3K pathways (Misra et al. 2011). GRP78 prevents ER-stress induced apoptosis in normal and cancer cells. GRP78 interacts to and inhibits pro-apoptotic BIK and BAX proteins in ER to protect human breast cancer cells from estrogen starvation-induced apoptosis (Fu et al. 2007). GRP78 also protects cancer cells from topoisomerase inhibitors-induced apoptosis via binding to and suppression of caspase-7 (Reddy et al. 2003). Inhibition of GRP78 by polyclonal antibodies up-regulates p53 activity and promotes apoptosis, via the inhibition of Ras/MAPK and PIK3/AKT pathways and activation of NF-κB (Misra et al. 2009). Another important function of GRP78 in cancer development is the regulation of ER stress-related autophagy in cancer cells (Li et al. 2008). For examples, GRP78 level is elevated during ER-stress mediated autography, which is induced by drug and irritation treatments in nasopharyngeal carcinoma cells (Song et al. 2013). The polymethoxyflavone derivative nobiletin displays anticancer effect by inducing apoptosis, however, the apoptotic effect is compromised by protective autophagy induced by GRP78 in human gastric cancer cells SNU-16 (Moon and Cho 2016). Studies also have showed that treatment of serine/threonine-protein kinase B-RAF inhibitors vemurafenib and debrafenib activated GRP78 regulated ER stress response to induce protective autophagy and further induced drug resistance, while autophagy inhibitor hydroxychloroquine can overcome the resistance to B-RAF inhibitors in melanoma cells and tumor xenografts in animal model (Ma et al. 2014).
GRP78 is overexpressed in multiple cancers including breast cancer, lung cancer, prostate cancer, ovarian cancer, gastric cancer, liver cancer, esophageal cancer, renal cancer, endometrial cancer, melanoma, glioma, and fibrosarcoma. Overexpression of GRP78 is a negative predictor of survival in high-risk patients with endometrial cancer (Ulianich and Insabato 2014). In addition, cell surface GRP78 may be a potential marker for good prognosis and response to chemotherapy in breast cancer (Yerushalmi et al. 2015).
Role of HSPA6/HSP70B′ in Cancer Development and Diagnosis
The HSPA6 gene is located in chromosome 1q in the human genome but not presents in the genomes of rat and mouse (Khalouei et al. 2014). It encodes a 71-kDa HSP70 family protein, also known as HSP70B’, with the characteristics of unique induction (Khalouei et al. 2014). The expression of HSPA6 is low to undetectable under normal physiological condition but it is highly stress induced in most cancer cells and tissues (Leung et al. 1990). Ramired et al. reported that the expressions of HSP including HSPA6, HSP72, and HSP40s were reduced following overexpression of TNF-α-induced protein 3-interacting protein 1 (TNIP1) in human epidermal keratinocytes, suggesting the potential role of HSPA6 in TNF-mediated inflammation and apoptosis (Ramired et al. 2015). The study by Smith showed that the expression of HSPA6 can be induced by oxidative modified low density lipoprotein (oxLDL) in the human promonocytic lymphoma cells U937 which originated from resident macrophages. Knockdown of HSPA6 by small interfering RNA resulted in a decrease of the level of mRNA and phosphorylation of sphingosine kinase 1 (SK1), as well as an increase in the production of IL-10 in U937 cells. The results indicate that HSPA6 has functions in activation and stimulation of macrophage survival via the regulation of SK1 activity and IL-10 production (Smith 2010). A study by Regeling et al. also showed that the extract from cigarette induced HSPA6 expression in human intestinal epithelial cells DLD-1. HSPA6 interacts with and stabilizes the anti-apoptotic protein BCL-XL in DLD-1 cells, suggesting the anti-apoptotic role of HSPA6 (Regeling et al. 2016). The expression of HSPA6 is higher in the tumor tissues of liver cancer compared to normal tissues and it is associated with earlier recurrence and poor outcome in the patients with HBV-related early-stage hepatocellular carcinoma (Yang et al. 2015).
Role of HSPA8/HSC70 in Cancer Development and Diagnosis
The HSC70 (also called HSP73) protein is a 73 kDa heat shock cognate protein which is coded by the HSPA8 gene. The basic structure of human HSC70 has three parts: a 44 kDa amino-terminal ATPase domain (1–384 residues), also known as the ATP-binding domain, a 18 kDa peptide (substrate) binding domain (385–543 residues), and a 10 kDa carboxyl-terminal domain (544–646 residues), also known as the variable or “lid” domain (Liu et al. 2012). Studies have found that the concentrations of HSC70 were much higher in the cells during proliferation, particularly in S-phase, compared to differentiated cells in rat glioma C6 cells, suggesting the role of HSC70 in promotion of cell proliferation (Helmbrecht and Rensing 1999). HSC70 regulates the functions of tumor-related genes and proteins. It binds and links to the non-phosphorylated tumor suppressor retinoblastoma (pRb) protein and mutant form of p53 and p73 for degradation (Liu et al. 2012). More recent studies showed that HSC70 promoted cell survival by inhibition of the degradation of Ras-related protein Rab-1A under stress conditions in colon adenocarcinoma cells (Tanaka et al. 2014).
HSC70 is overexpressed in colon cancer and esophageal cancer (Kubota et al. 2010; Moghanibashi et al. 2013). Mutation or deletion of HSC70 is detected in the tissues from patients with breast cancer, suggesting HSC70 is a target of somatic mutation and deletion in breast cancer (Bakkenist et al. 1999). The 1541–1542 delGT heterozygous genotype of HSC70 is associated with decreased risk in lung cancer (Rusin et al. 2004). In addition, HSC70 has been identified in the neuroblastoma cells cultured in conditioned media, suggesting it may be a potential tumor marker (Sandoval et al. 2006).
Role of HSPA9/Mortalin in Cancer Development and Diagnosis
The HSPA9 gene is located in the human chromosome 5q31 interval coding for mortalin. It is involved in myeloid malignancies and is commonly deleted in patients with myelodysplasticsyndrome (MDS) and AML (Chen et al. 2011). Mortalin (HSPA9) binds to and sequesters p53 in the cytoplasm to prevent the translocation of p53 into nucleus in human colorectal adenocarcinoma cells, indicating its role in regulation of cell cycle and apoptosis (Gestl and Anne Böttger 2012). Inhibition of mortalin induces apoptosis in hematopoietic progenitor cells and cancer cells (Chen et al. 2011; Lu et al. 2011; Liu et al. 2017). Mortalin contributes to carcinogenesis by activation of MAPK/MEK/ERK pathway in ovarian cancer and medullary thyroid cancer cells (Starenki et al. 2015; Hu et al. 2016). Overexpression of mortalin increases the migration, invasion, epithelial–mesenchymal transition (EMT), and metastasis in breast cancer cells, in which the process is regulated by PI3/AKT and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathways (Na et al. 2016). Mortalin is overexpressed in breast cancer and liver cancer (Chen et al. 2014; Jin et al. 2016). The overexpression of mortalin is associated with early tumor recurrence and metastasis in liver cancer (Yi et al. 2008). Elevated levels of mortalin can be used as a prognostic marker for worse prognosis and poor survival in patients with gastric cancer and colorectal cancer (Rozenberg et al. 2013; Ando et al. 2014).
Role of HSP70 Proteins as the Therapeutic Targets for Anticancer Drug Discovery and Development
HSP70 proteins promote tumorigenesis and their expressions are elevated in various cancers. HSP70 proteins are also involved in mediating drug resistance in cancer therapy. Due to their important roles in cancer biology, the HSP70 molecular chaperones, especially HSP72 and GRP78, are promising drug targets for cancer therapy. Therefore, identification, characterization and development of HSP70 inhibitors are of high interest for novel anticancer drug discovery and development.
Some compounds target and inhibit general HSP70 family proteins. Kim and colleagues reported that fisetin, a dietary flavonoid, can induce apoptosis in colon cancer HCT-116 cells by inhibition of the activity of HSF1 via blocking the binding of HSP72 promoter. Fisetin down-regulates HSP72/BAG3 and induces apoptosis via decreasing the levels of BCL-2, BCL-XL and myeloid cell leukemia 1 (MCL-1) proteins in human colon cancer HCT-116 cells (Kim et al. 2015). The same group also found that the natural compound cantharidin induces cancer cell death through the inhibition of HSP72 and BAG3 by binding of HSF1 to the promoter of HSP72 (Kim et al. 2013). Apoptozole, a small molecule inhibitor of the ATPase region of HSP72, induces apoptosis in cancer cells and displays antitumor activities against tumor xenografts in mouse models, through disruption of the interaction between HSP70 and APAF-1 (Ko et al. 2015). MKT-077, as an analog of the allosteric HSP70 inhibitor, is effective against human breast cancer MDA-MB-231 and MCF-7 cells (Li et al. 2013). Pifithrin-μ (PFT-μ), also known as 2-phenylethynesulfonamide (PES) inhibits the activity of HSP72 via binding to the substrate-binding domain of HSP70 (Balaburski et al. 2013). As a potent and selective HSP72 inhibitor, PFT-μ has been proved to have anti-tumor activities against multiple cancers including AML, acute lymphoid leukemia (ALL), and prostate cancer (Kaiser et al. 2011; Sekihara et al. 2013). Quercetin is a HSP72 inhibitor (also inhibiting HSP27 and HSP90) and inhibits the proliferation of human cervical cancer Hela cells via down-regulation of AMP-activated protein kinase (AMPK)-induced HSP72 and epidermal growth factor receptor (EGFR) (Jung et al. 2010). Demethoxycurcumin (DMC) is a kind of curcumin. Similar to quercetin, DMC also inhibits cell proliferation via AMPK-induced down-regulation of HSP72 and EGFR in prostate cancer cells (Hung et al. 2012). Apoptin is a nonstructural viral protein encoded by the VP3 gene of chicken anemia virus. Apoptin inhibits HSP72 transcription and induces apoptosis in a HSP70-dependent manner in liver cancer HepG2 cells. Inhibition of HSP72 expression is regulated by the ability of apoptin to promote HSF1 trimer depolymerization and inhibits HSF1-mediated HSP70 transcription (Yuan et al. 2013).
Studies have also shown that HSP72 is a therapeutic target for certain anti-cancer drugs and regulates drug resistance. For example, knockdown of HSP72 enhances the sensitivity to cisplatin treatment in cervical cancer cells (Yoshidomi et al. 2014). HSP72 protects human gastric cancer cells from oxalipatin induced apoptosis (Takahashi et al. 2016). Overexpression of HSP72 promotes bortezomib resistance, and inhibition of HSP72 enhances bortezomib-induced cell death in human bladder cancer cells (Qi et al. 2013). Howe et al. identified HS-72 as an allosteric selective inhibitor of HSP72. HS-72 reduces the ATP affinity of HSP72 to inhibit tumor growth and prolong the survival of tumor-bearing mice in an animal model of HER2+ breast cancer (Howe et al. 2014). Study by Zhang et al. showed that HSP72 significantly promoted the proliferation of human gammasigma T cells in vitro, indicating that HSP72 could be a potential target for adjuvant therapy of gammasigma T cells in cancer immunotherapy (Zhang et al. 2005).
GRP78 is involved in mediation of drug resistance and may be a potential therapeutic target for anticancer drugs. GRP78 and its downstream target AKT are important in regulation of cisplatin resistance in ER stress-tolerant human lung cancer cells (Lin et al. 2011). Inhibition of GRP78 by small-interference RNA sensitizes cisplatin- and doxorubicin-induced apoptosis in drug-resistant human melanoma cells (Jiang et al. 2009). GRP78 was identified as a positive regulator for sorafenib resistance acquisition in liver cancer cells (Chiou et al. 2010). The anti-HIV drug nelfinavir enhances the cytotoxicity of doxorubicin in vitro and potentiates the antitumor efficacy of doxorubicin in vivo via up-regulation of GRP78 in doxorubicin-resistant breast cancer MCF-7 cells, suggesting a role of GRP78 in mediation of doxorubicin resistance (Chakravarty et al. 2016). In addition, GRP78 is also a therapeutic target for the exertion of anti-tumor activity of anticancer drugs. Nanoparticles conjugated with GRP78 antibody inhibit carcinogenesis in liver cancer cells and promote the delivery of 5-fluorouracil (5-FU) to the cell surface with higher expression of GRP78 to enhance the anti-tumor efficiency (Zhao et al. 2014). GRP78 is identified as a specific and direct target of natural compound isoliquirtigenin which inhibits the proliferation of breast cancer cells via the blockage of β-catenin signaling (Wang et al. 2014a, b). HKH40A belongs to bisimidazoacridones and has antitumor activity. The mechanisms of action for its antitumor activity include downregulation of GRP78 and upregulation of ATF6 in different types of cancer cells (Kosakowska-Cholody et al. 2014). OSU-CG-5 is a novel energy restriction mimetic agent. It inhibits the proliferation of human colorectal cancer cells via up-regulation of GRP78 and inhibition of p-mTOR and p-p70S6 kinase (Arafa et al. 2014). Combination of carfilzomib (a proteasome inhibitor) and suberanilohydroxamic acid (SAHA, an HDAC inhibitor) synergistically promotes ER stress with up-regulation of GRP78 in non-small cell lung cancer (NSCLC), suggesting GRP78 may be a potential therapeutic target for the combination therapy in the treatment of NSCLC (Hanke et al. 2016). The combination treatment of gefitinib (an EGFR inhibitor) and clarithromycin (a macrolide antibiotic) effectively enhances cytotoxity via up-regulation of GRP78 in NSCLC cells (Sugita et al. 2015). The COX-2 inhibitor celecoxib can inhibit cell growth and induce apoptosis via down-regulation of GRP78 expression in human leukemia cells (Sobolewski et al. 2015). Some important GRP78 inhibitors including antibodies, natural compounds and peptides are listed in a review article by Roller and Maddalo (Roller and Maddalo 2013). We modified and updated them in Table 2.
The expression of HSPA6 can be induced by anticancer agents. MG-132 is an inhibitor of proteasome used for the treatments of multiple myeloma and lymphoma clinically. Treatment of MG-132 can induce the expression of HSPA6 on the surface of human colon cancer HT-29 and CRL-1809 cells (Noonan et al. 2008). HSP90 inhibitors tanespimycin and radicicol significantly induced the expression of HSPA6 in breast cancer MCF-7 cells, suggesting that HSPA6 may be a specific target for HSP90 inhibition (Kuballa et al. 2015).
Liu et al found that HSC70 located in the cell membrane works as a transporter of methotrexate (MTX) in L1210 leukemia cells, and abnormal tyrosine phosphorylation of HSC70 leads to the occurrence of MTX resistance in the cells (Liu et al. 2015). Significant upregulation of HSC70 is observed in CD34+ chronic myeloid leukemia (CML) cells and treatment of HSC70-specific inhibitor 15-deoxyspergualin decreases cell proliferation, suggesting HSC70 can be a target for cancer therapy (Liu et al. 2012).
Mortalin mediates drug resistance in cancer therapy. For instance, inhibition of the expression of mortalin inhibits cell growth and reverses cisplatin resistance in ovarian cancer cells (Yang et al. 2013). MKT-077 shows anti-tumor efficacy through binding to mortalin and interruption of the interaction between mortalin and p53. MKT-077 also reverses the resistance and sensitizes the cellular response to complement-dependent cytotoxicity in human leukemia K562 cells and human colon cancer HCT-116 cells (Wadhwa et al. 2000; Pilzer et al. 2010). Embelin, a natural quinone, is found in the fruits of Embelia ribes. It inhibits the transcription of mortalin and abrogates the interactions of mortalin-p53 in cancer cells. Embelin targets mortalin leading to the activation of p53 and inhibition of the metastatic signaling in human breast cancer cells (Nigam et al. 2015). Recent studies have showed that UBXN2A, a UBX-domain containing protein, is a potential mortalin inhibitor and can sensitize cancer cell response to chemotherapeutic agents such as 5-FU. UBXN2A also promotes cell death by interfering with the interaction of mortalin-p53 in colon cancer cells (Sane et al. 2016).
Conclusions
Here we have provided an overview of the complex relationship between cancer and the major HSP70 genes/proteins including HSPA1A/HSP72, HSPA5/GRP78, HSPA6/HSP70B’, HSPA8/HSC70, and HSPA9/mortalin. As summarized in Table 2, HSP70 proteins play an important role in tumorigenesis and may be used as potential clinical biomarkers for the diagnosis and predicting prognostic outcome of the patients with cancer. HSP70 proteins are molecular chaperones and their expressions and functions are activated during stress stimulation. Most of the HSP70 proteins have similar functions in carcinogenesis, prevention of apoptosis and conferring of drug resistance. In addition, HSP70 proteins may be used as therapeutic targets for cancer therapy, prompting discovery and development of novel chemotherapeutic agents. Among HSP70 proteins, GRP78 is the target of most of the novelly developed anti-cancer drugs. These drugs have proven to be effective in cancer cells in vitro and tumor xenografts in animal models in vivo, and some of them are being tested in clinical trials. A variety of anticancer drugs with HSP targets have been approved by the USA FDA for cancer treatment. For example, sorafenib (Nexavar®), a kinase inhibitor that reduces the expression of GRP78 in cancer cells, was approved by the USA FDA for treatment of renal cell carcinoma (2005), hepatocellular carcinoma (2007) and locally recurrent or metastatic, progressive, differentiated thyroid carcinoma (2013) (Roberts et al. 2015). Ruxolitinib (Jakafi®) is a JAK inhibitor that also decreases the expression of HSP70 and HSP90 in cancer cells and animal models. It was approved by the USA FDA for the treatment of intermediate or high-risk myelofibrosis (2011) and polycythemia vera (2014) (Tavallai et al. 2016). However, none of the specific HSP inhibitors has been approved by the USA FDA for the treatment of patients with cancer so far. We propose three major possible reasons for why no HSP inhibitors have been approved by the USA FDA in clinical use: (1) HSP are very critical for the survival of the cells. Once the activities of HSP are inhibited, the basic functions of cancer cells as well as normal cells will be severely affected. We still do not fully understand the different requirement in the amount of HSP between cancer cells and normal cells, which may provide a necessary therapeutic window for discovery and development of more efficacious and less toxic novel anticancer HSP inhibitors. (2) HSP inhibitors can cause severe organ-specific toxicities (liver or ocular toxicity) which are not easy to be managed. The functions of HSP are essential for both normal and tumor cells. It is difficult to identify the functions of HSP that are only presented in cancer cells but not in normal cells. To identify the functions of HSP only being specific for cancer cells may overcome HSP inhibitors induced organ-specific toxicities. (3) Some HSP inhibitors may lack convincing anticancer activity. Different HSP family members interact and collaborate with each other in the signaling network to regulate cellular functions in the cells. Inhibition of one HSP can stimulate the overexpressions of other HSP members, which compensates the inhibitory effects caused by a single HSP inhibitor. Therefore, combination of different HSP inhibitor may be a good strategy to enhance the anticancer efficacy in cancer therapy. In conclusion, HSP70 proteins have a significant role in cancer development and progress. Understanding of the functions and molecular mechanisms of HSP70 proteins is critical for enhancing the accuracy of cancer diagnosis and for the development of more effective chemotherapeutic agents.
Abbreviations
- 5-FU:
-
fluorouracil
- ADP:
-
adenosine diphosphate
- AIF:
-
apoptosis-inducing factor
- AKT:
-
protein kinase B
- ALL:
-
acute lymphoid leukemia
- AML:
-
acute myelogenous leukemia
- AMPK:
-
AMP-activated protein kinase
- APAF-1:
-
apoptotic protease activating factor 1
- ATF:
-
activating transcription factor
- ATP:
-
adenosine triphosphate
- ATPase:
-
adenosine triphosphatase
- BAG-1:
-
Bcl-2 associated athanogene 1
- BAX:
-
Bcl-2 associated X
- Bcl-2:
-
B-cell lymphoma-2
- Bcl-xL:
-
B-cell lymphoma-extra-large
- BIK:
-
Bcl-2 interacting killer
- BIP:
-
binding immunoglobulin protein
- B-RAF:
-
v-raf murine sarcoma viral oncogene homolog B
- CDK:
-
cyclin-dependent kinase
- CHIP:
-
carboxyl-terminus of HSP70-interacting protein
- CML:
-
chronic myeloid leukemia
- C-RAF:
-
v-raf murine sarcoma viral oncogene homolog C
- DMC:
-
demethoxycurcumin
- EGFR:
-
epidermal growth factor receptor
- EMT:
-
epithelial-mesenchymal transition
- ER:
-
endoplasmic reticulum
- ERK:
-
Ras/extracellular signal-regulated kinase
- FDA:
-
Food and Drug Administration
- GRP:
-
glucose-regulated protein
- HBV:
-
hepatitis B virus
- HDAC:
-
histone deacetylases
- HER2:
-
human epidermal growth factor receptor 2
- HIV:
-
human immunodeficiency virus
- HSC:
-
heat-shock cognate protein
- HSE:
-
heat shock element
- HSF:
-
heat shock factor
- HSP:
-
heat shock protein
- HUGO:
-
Human Genome Organization
- IL:
-
interleukin
- JAK:
-
Janus kinase
- MAPK:
-
mitogen-activated protein kinase
- MCL-1:
-
myeloid cell leukemia 1
- MEK:
-
mitogen-activated protein kinase kinase
- mTOR:
-
mechanistic target of rapamycin
- MTX:
-
methotrexate
- NF-κB:
-
nuclear factor NF-κB
- NSCLC:
-
non-small cell lung cancer;
- oxLDL:
-
oxidative modified low density lipoprotein
- PES:
-
phenylethynesulfonamide
- PFT:
-
pifithrin
- PI3K:
-
phosphatidylinositol-4, 5-bisphosphate 3-kinase
- PSA:
-
prostate-specific antigen
- SAHA:
-
suberanilohydroxamic acid
- SK1:
-
sphingosine kinase 1
- STAT:
-
signal transducer and activator of transcription
- TGF:
-
transforming growth factor
- TNF:
-
tumor necrosis factor;
- TNIP1:
-
TNF-α-induced protein 3-interacting protein 1
- UBXN2A:
-
UBX Domain Protein 2A
References
Akerfelt, M., Morimoto, R. I., & Sistonen, L. (2010). Heat shock factors: Integrators of cell stress, development and lifespan. Nature Reviews. Molecular Cell Biology, 11(8), 545–533.
Ando, K., Oki, Z., Zhao, Y., et al. (2014). Mortalin is a prognostic factor of gastric cancer with normal p53 function. Gastric Cancer, 17(2), 255–262.
Arafa, e.-S. A., Abdelazeem, A. H., Arab, H. H., et al. (2014). OSU-CG5, a novel energy restriction mimetic agent, targets human colorectal cancer cells in vitro. Acta Pharmacologica Sinica, 35(3), 394–400.
Arispe, N., & De Maio, A. (2000). ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. The Journal of Biological Chemistry, 275(40), 30839–30843.
Bakkenist, C. J., Koreth, J., Williams, C. S., et al. (1999). Heat shock cognate 70 mutations in sporadic breast carcinoma. Cancer Research, 59(17), 4219–4221.
Balaburski, G. M., Leu, J. J., Beeharry, N., et al. (2013). A modified HSP70 inhibitor shows broad activity as an anticancer agent. Molecular Cancer Research, 11(3), 219–229.
Bayer, C., Liebhardt, M. E., Schmid, T. E., et al. (2014). Validation of heat shock protein 70 as a tumor-specific biomarker for monitoring the outcome of radiation therapy in tumor mouse models. International Journal of Radiation Oncology, Biology, Physics, 88(3), 694–700.
Bepperling, A., Alte, F., Kriehuber, T., et al. (2012). Alternative bacterial two-component small heat shock protein systems. Proceedings of the National Academy of Sciences of the United States of America, 109(50), 20407–20412.
Boonjaraspinyo, S., Boonmars, T., Kaewkes, S., et al. (2012). Down-regulated expression of HSP70 in correlation with clinicopathology of cholangiocarcinoma. Pathology Oncology Research, 18(2), 227–237.
Cai, M. B., Wang, X. P., Zhang, J. X., et al. (2012). Expression of heat shock protein 70 in nasopharyngeal carcinomas: Different expression patterns correlate with distinct clinical prognosis. Journal of Translational Medicine, 10, 96.
Chakravarty, G., Mathur, A., Mallade, P., et al. (2016). Nelfinavir targets multiple drug resistance mechanisms to increase the efficacy of doxorubicin in MCF-7/Dox breast cancer cells. Biochimie, 124, 53–64.
Chen, T. H., Kambal, A., Krysiak, K., et al. (2011). Knockdown of Hspa9, a del(5q31.2) gene, results in a decrease in hematopoietic progenitors in mice. Blood, 117(5), 1530–1539.
Chen, J., Liu, W. B., Jia, W. D., et al. (2014). Overexpression of Mortalin in hepatocellular carcinoma and its relationship with angiogenesis and epithelial to mesenchymal transition. International Journal of Oncology, 44(1), 247–255.
Chiou, J. F., Tai, C. J., Huang, M. T., et al. (2010). Glucose-regulated protein 78 is a novel contributor to acquisition of resistance to sorafenib in hepatocellular carcinoma. Annals of Surgical Oncology, 17(2), 603–612.
Ciocca, D. R., & Calderwood, S. K. (2005). Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress & Chaperones, 10(2), 86–103.
Fu, Y., Li, J., & Lee, A. S. (2007). GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Research, 67(8), 3734–3740.
Gabai, V. L., Yaglom, J. A., Waldman, T., & Sherman, M. Y. (2009). Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Molecular and Cellular Biology, 29(2), 559–569.
Gestl, E. E., & Anne Böttger, S. (2012). Cytoplasmic sequestration of the tumor suppressor p53 by a heat shock protein 70 family member, mortalin, in human colorectal adenocarcinoma cell lines. Biochemical and Biophysical Research Communications, 423(2), 411–416.
Gray, P. C., & Vale, W. (2012). Cripto/GRP78 modulation of the TGF-β pathway in development and oncogenesis. FEBS Letters, 586(4), 1836–1845.
Hanke, N. T., Garland, L. L., & Baker, A. F. (2016). Carfilzomib combined with suberanilohydroxamic acid (SAHA) synergistically promotes endoplasmic reticulum stress in non-small cell lung cancer cell lines. Journal of Cancer Research and Clinical Oncology, 142(3), 549–560.
Helmbrecht, K., & Rensing, L. (1999). Different constitutive heat shock protein 70 expression during proliferation and differentiation of rat C6 glioma cells. Neurochemical Research, 24(10), 1293–1299.
Howe, M. K., Bodoor, K., Carlson, D. A., et al. (2014). Identification of an allosteric small-molecule inhibitor selective for the inducible form of heat shock protein 70. Chemistry & Biology, 21(12), 1648–1659.
Hu, Y., Yang, L., Yang, Y., et al. (2016). Oncogenic role of mortalin contributes to ovarian tumorigenesis by activating the MAPK-ERK pathway. Journal of Cellular and Molecular Medicine, 20(11), 2111–2121.
Hung, C. M., Su, Y. H., Lin, J. N., et al. (2012). Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR. Journal of Agricultural and Food Chemistry, 60(34), 8427–8434.
Jäättelä, M., Wissing, D., Bauer, P. A., et al. (1992). Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. The EMBO Journal, 11(10), 3507–3512.
Jakob, U., Scheibel, T., Bose, S., Reinstein, J., et al. (1996). Assessment of the ATP binding properties of Hsp90. The Journal of Biological Chemistry, 271(17), 10035–10041.
Jiang, C. C., Mao, Z. G., Avery-Kiejda, K. A., et al. (2009). Glucose-regulated protein 78 antagonizes cisplatin and adriamycin in human melanoma cells. Carcinogenesis, 30(2), 197–204.
Jin, H., Ji, M., Chen, L., et al. (2016). The clinicopathological significance of Mortalin overexpression in invasive ductal carcinoma of breast. Journal of Experimental & Clinical Cancer Research, 35, 42.
Jung, J. H., Lee, J. O., Kim, J. H., et al. (2010). Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. Journal of Cellular Physiology, 223(2), 408–414.
Kaiser, M., Lee, J. O., Kim, J. H., et al. (2011). Antileukemic activity of the HSP70 inhibitor pifithrin-μ in acute leukemia. Blood Cancer Journal, 1(7), e28.
Kampinga, H. H., Hageman, J., Vos, M. J., et al. (2009). Guidelines for the nomenclature of the human heat shock proteins. Cell Stress & Chaperones, 14(1), 105–111.
Khalouei, S., Chow, A. M., Brown, I. R. (2014). Localization of heat shock protein HSPA6 (HSP70B′) to sites of transcription in cultured differentiated human neuronal cells following thermal stress. Journal of Neurochemistry, 131(6), 743–754.
Kim, J. A., Kim, Y., Kwon, B. M., et al. (2013). The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. The Journal of Biological Chemistry, 288(40), 28713–28726.
Kim, J. A., Lee, S., Kim, D. E., et al. (2015). Fisetin, a dietary flavonoid, induces apoptosis of cancer cells by inhibiting HSF1 activity through blocking its binding to the hsp70 promoter. Carcinogenesis, 36(6), 696–706.
Ko, S. K., Kim, J., Na, D. C., et al. (2015). A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chemistry & Biology, 22(3), 391–403.
Kocsis, J., Mészáros, T., Madaras, B., et al. (2011). High levels of acute phase proteins and soluble 70 kDa heat shock proteins are independent and additive risk factors for mortality in colorectal cancer. Cell Stress & Chaperones, 16(1), 49–55.
Kosakowska-Cholody, T., Lin, J., Srideshikan, S. M., et al. (2014). HKH40A downregulates GRP78/BiP expression in cancer cells. Cell Death & Disease, 5, e1240.
Kuballa, P., Baumann, A. L., Mayer, K., et al. (2015). Induction of heat shock protein HSPA6 (HSP70B′) upon HSP90 inhibition in cancer cell lines. FEBS Letters, 589(13):1450–1458.
Kubota, H., Yamamoto, S., Itoh, E., et al. (2010). Increased expression of co-chaperone HOP with HSP90 and HSC70 and complex formation in human colonic carcinoma. Cell Stress & Chaperones, 15(6), 1003–1011.
Lee, A. S. (2007). GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Research, 67(8), 3496–3499.
Leung, T. K., Rajendran, M. Y., Monfries, C., et al. (1990). The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B′) and isolation of its cDNA and genomic DNA. The Biochemical Journal, 267: 125–132.
Li, J., Ni, M., Lee, B., et al. (2008). The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death & Disease, 15(9), 1460–1471.
Li, G., Xu, Y., Guan, D., et al. (2011). HSP70 protein promotes survival of C6 and U87 glioma cells by inhibition of ATF5 degradation. The Journal of Biological Chemistry, 286(23), 20251–20259.
Li, X., Srinivasan, S. R., Connarn, J., et al. (2013). Analogs of the allosteric heat shock protein 70 (Hsp70) inhibitor, MKT-077, as anti-cancer agents. ACS Medicinal Chemistry Letters, 4(11), 1042–1047.
Lin, Y., Wang, Z., Liu, L., & Chen, L. (2011). Akt is the downstream target of GRP78 in mediating cisplatin resistance in ER stress-tolerant human lung cancer cells. Lung Cancer, 71(3), 291–297.
Liu, T., Daniels, C. K., & Cao, S. (2012). Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacology & Therapeutics, 136(3), 354–374.
Liu, T., Singh, R., Rios, Z., et al. (2015). Tyrosine phosphorylation of HSC70 and its interaction with RFC mediates methotrexate resistance in murine L1210 leukemia cells. Cancer Letters, 357(1), 231–241.
Liu, T., Krysiak, K., Shirai, C. L., et al. (2017). Knockdown of HSPA9 induces TP53-dependent apoptosis in human hematopoietic progenitor cells. PLoS One, 12(2), e0170470.
Lu, W. J., Lee, N. P., Kaul, S. C., et al. (2011). Induction of mutant p53-dependent apoptosis in human hepatocellular carcinoma by targeting stress protein mortalin. International Journal of Cancer, 129(8), 1806–1814.
Ma, Y., & Hendershot, L. M. (2004). The role of the unfolded protein response in tumor development: Friend or foe? Nature Reviews. Cancer, 4(12), 966–967.
Ma, X. H., Piao, S. F., Dey, S., et al. (2014). Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. The Journal of Clinical Investigation, 124(3), 1406–1417.
Macario, A. J., & Conway de Macario, E. (2007). Molecular chaperones: multiple functions, pathologies, and potential applications. Frontiers in Bioscience, 1(12), 2588–2600.
Misra, U. K., Mowery, Y., Kaczowka, S., et al. (2009). Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Molecular Cancer Therapeutics, 8(5), 1350–1362.
Misra, U. K., Payne, S., & Pizzo, S. (2011). Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: A role for secreted prostate-specific antigen. The Journal of Biological Chemistry, 286(2), 1248–1259.
Moghanibashi, M., Rastgar-Jazii, F., Soheili, Z. S., et al. (2013). Esophageal cancer alters the expression of nuclear pore complex binding protein Hsc70 and eIF5A-1. Functional & Integrative Genomics, 13(2), 253–260.
Moon, J. Y., & Cho, S. K. (2016). Nobiletin induces protective autophagy accompanied by ER-stress mediated apoptosis in human gastric cancer SNU-16 cells. Molecules, 21(7), 914.
Murphy, M. E. (2013). The HSP70 family and cancer. Carcinogenesis, 34(6), 1181–1188.
Na, Y., Kaul, S. C., Ryu, J., et al. (2016). Stress chaperone mortalin contributes to epithelial-mesenchymal transition and cancer metastasis. Cancer Research, 76(9), 2764–2765.
Nigam, N., Grover, A., Goyal, S., et al. (2015). Targeting mortalin by embelin causes activation of tumor suppressor p53 and deactivation of metastatic signaling in human breast cancer cells. PLoS One, 10(9), e0138192.
Noonan, E. J., Fournier, G., Hightower, L. E. (2008). Surface expression of Hsp70B′ in response to proteasome inhibition in human colon cells. Cell Stress & Chaperones, 13(1), 105–110.
Nylandsted, J., Gyrd-Hansen, M., Danielewicz, A., et al. (2004). Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. The Journal of Experimental Medicine, 200(4), 425–435.
Pilzer, D., Saar, M., Koya, K., et al. (2010). Mortalin inhibitors sensitize K562 leukemia cells to complement-dependent cytotoxicity. International Journal of Cancer, 126(6), 1428–1435.
Qi, W., White, M. C., Choi, W., et al. (2013). Inhibition of inducible heat shock protein-70 (hsp72) enhances bortezomib-induced cell death in human bladder cancer cells. PLoS One, 8(7), e69509.
Ramired, V. P., Krueger, W., & Aneskievich, B. J. (2015). TNIP1 reduction of HSPA6 gene expression occurs in promoter regions lacking binding sites for known TNIP1-repressed transcription factors. Gene, 555(2), 430–437.
Ramp, U., Mahotka, C., Heikaus, S., et al. (2007). Expression of heat shock protein 70 in renal cell carcinoma and its relation to tumor progression and prognosis. Histology and Histopathology, 22(10), 1099–1107.
Reddy, R. K., Mao, C., Baumeister, P., et al. (2003). Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: Role of ATP binding site in suppression of caspase-7 activation. The Journal of Biological Chemistry, 278(23), 20915–20924.
Regeling, A., Imhann, F., Volders, H. H., et al. (2016). HSPA6 is an ulcerative colitis susceptibility factor that is induced by cigarette smoke and protects intestinal epithelial cells by stabilizing anti-apoptotic Bcl-XL. Biochimica et Biophysica Acta, 1862, 788–796.
Rérole, A. L., Jego, G., & Garrido, C. (2011). Hsp70: Anti-apoptotic and tumorigenic protein. Methods in Molecular Biology, 787, 205–230.
Roberts, J. L., Tavallai, M., Nourbakhsh, A., et al. (2015). GRP78/Dna K is a target for nexavar/stivarga/votrient in the treatment of human malignancies, viral infections and bacterial diseases. Journal of Cellular Physiology, 230(10), 2552–2578.
Roller, C., & Maddalo, D. (2013). The Molecular chaperone GRP78/BiP in the development of chemoresistance: Mechanism and possible treatment. Frontiers in Pharmacology, 4, 10.
Rozenberg, P., Kocsis, J., Saar, M., et al. (2013). Elevated levels of mitochondrial mortalin and cytosolic HSP70 in blood as risk factors in patients with colorectal cancer. International Journal of Cancer, 133(2), 514–518.
Rusin, M., Zientek, H., Krześniak, M., et al. (2004). Intronic polymorphism (1541-1542delGT) of the constitutive heat shock protein 70 gene has functional significance and shows evidence of association with lung cancer risk. Molecular Carcinogenesis, 39(3), 155–163.
Sandoval, J. A., Hoelz, D. J., Woodruff, H. A., et al. (2006). Novel peptides secreted from human neuroblastoma: Useful clinical tools? Journal of Pediatric Surgery, 41(1), 245–251.
Sane, S., Abdullah, A., Nelson, M. E., et al. (2016). Structural studies of UBXN2A and mortalin interaction and the putative role of silenced UBXN2A in preventing response to chemotherapy. Cell Stress & Chaperones, 21(2), 313–326.
Sekihara, K., Harashima, N., Tongu, M., et al. (2013). Pifithrin-μ, an inhibitor of heat-shock protein 70, can increase the antitumor effects of hyperthermia against human prostate cancer cells. PLoS One, 8(11), e78772.
Smith, K. J. (2010). Heat shock protein 70B′ (HSP70B′) expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. The Journal of Biological Chemistry, 285(21), 15985–15993.
Sobolewski, C., Rhim, J., Legrand, N., et al. (2015). 2,5-Dimethyl-celecoxib inhibits cell cycle progression and induces apoptosis in human leukemia cells. The Journal of Pharmacology and Experimental Therapeutics, 355(2), 308–328.
Song, L., Liu, H., Ma, L., et al. (2013). Inhibition of autophagy by 3-MA enhances endoplasmic reticulum stress-induced apoptosis in human nasopharyngeal carcinoma cells. Oncology Letters, 6(4), 1031–1038.
Starenki, D., Hong, S. K., Lloyd, R. V., et al. (2015). Mortalin (GRP75/HSPA9) upregulation promotes survival and proliferation of medullary thyroid carcinoma cells. Oncogene, 34(35), 4624–4634.
Sugita, S., Ito, K., Yamashiro, Y., et al. (2015). EGFR-independent autophagy induction with gefitinib and enhancement of its cytotoxic effect by targeting autophagy with clarithromycin in non-small cell lung cancer cells. Biochemical and Biophysical Research Communications, 461(1), 28–34.
Sullivan, C. S., & Pipas, J. M. (2002). T antigens of simian virus 40: Molecular chaperones for viral replication and tumorigenesis. Microbiology and Molecular Biology Reviews, 66(2), 179–202.
Takahashi, K., Tanaka, M., Yashiro, M., et al. (2016). Protection of stromal cell-derived factor 2 by heat shock protein 72 prevents oxaliplatin-induced cell death in oxaliplatin-resistant human gastric cancer cells. Cancer Letters, 378(1), 8–15.
Tanaka, M., Mun, S., Harada, A., et al. (2014). Hsc70 contributes to cancer cell survival by preventing Rab1A degradation under stress conditions. PLoS One, 9(5), e96785.
Tavallai, M., Booth, L., Roberts, J. L., et al. (2016). Rationally repurposing Ruxolitinib (Jakafi (®)) as a solid tumor therapeutic. Frontiers in Oncology, 6, 142.
Trieb, K., Sulzbacher, I., & Kubista, B. (2016). Recurrence rate and progression of chondrosarcoma is correlated with heat shock protein expression. Oncology Letters, 11(1), 521–524.
Ulianich, L., & Insabato, L. (2014). Endoplasmic reticulum stress in endometrial cancer. Frontiers of Medical (Lausanne), 1, 55.
Wadhwa, R., Sugihara, T., Yoshida, A., et al. (2000). Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Research, 60(24), 6818–6821.
Wang, X., Wang, Q., & Lin, H. (2010). Correlation between clinicopathology and expression of heat shock protein 72 and glycoprotein 96 in human esophageal squamous cell carcinoma. Clinical & Developmental Immunology, 2010, 212537.
Wang, N., Wang, Z., Peng, C., et al. (2014a). Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via β-catenin/ABCG2 signaling. Carcinogenesis, 35(11), 2544–2554.
Wang, X., Chen, M., Zhou, J., et al. (2014b). HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). International Journal of Oncology, 45(1), 18–30.
Wu, J., Liu, T., Rios, Z., et al. (2017). Heat shock proteins and cancer. Trends in Pharmacological Sciences, 38(3), 226–256.
Yang, L., Li, H., Jiang, Y., et al. (2013). Inhibition of mortalin expression reverses cisplatin resistance and attenuates growth of ovarian cancer cells. Cancer Letters, 336(1), 213–221.
Yang, Z., Zhuang, L., Szatmary, P., et al. (2015). Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. International Journal of Medical Sciences, 12(3), 256–263.
Yerushalmi, R., Raiter, A., Nalbandvan, K., & Hardy, B. (2015). Cell surface GRP78: A potential marker of good prognosis and response to chemotherapy in breast cancer. Oncology Letters, 10(4), 2149–2155.
Yi, X., Luk, J. M., Lee, N. P., et al. (2008). Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Molecular & Cellular Proteomics, 7(2), 315–325.
Yoshidomi, K., Murakami, A., Yakabe, K., et al. (2014). Heat shock protein 70 is involved in malignant behaviors and chemosensitivities to cisplatin in cervical squamous cell carcinoma cells. The Journal of Obstetrics and Gynaecology Research, 40(5), 1188–1196.
Yuan, L., Zhang, L., Dong, X., et al. (2013). Apoptin selectively induces the apoptosis of tumor cells by suppressing the transcription of HSP70. Tumour Biology, 34(1), 577–585.
Zhang, H., Hu, H., Jiang, X., et al. (2005). Membrane HSP70: The molecule triggering gammadelta T cells in the early stage of tumorigenesis. Immunological Investigations, 34(4), 453–468.
Zhao, L., Li, H., Shi, Y., et al. (2014). Nanoparticles inhibit cancer cell invasion and enhance antitumor efficiency by targeted drug delivery via cell surface-related GRP78. International Journal of Nanomedicine, 10, 245–256.
Acknowledgements
This work was supported by the West Virginia School of Osteopathic Medicine faculty startup funding (T. Liu); and the Distinguished Professor Research Startup Funding (S. Cao) from Southwest Medical University, Lanzhou, Sichuan, China.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Liu, T., Cao, S. (2018). Heat Shock Protein 70 and Cancer. In: Asea, A., Kaur, P. (eds) HSP70 in Human Diseases and Disorders. Heat Shock Proteins, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-319-89551-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-89551-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89550-5
Online ISBN: 978-3-319-89551-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)