Abstract
Lyme disease (LD) is one of the most frequently diagnosed tick-borne bacterial diseases worldwide. It is caused by species within the Borrelia genus, spread by the Ixodes ticks. The initial sign of the disease is a ring-shaped rash with occasional central clearing, called erythema migrans (EM). If untreated, LD can result in early disseminated manifestations including generalized EM, carditis and central nervous system involvement, and late manifestations, including arthritis. LD generally responds well to a short course of antibiotics. However, about 10–15% of patients with Lyme arthritis fail the initial course of antibiotics, and some of those develop a chronic inflammatory arthritis optimally treated with local or systemic immunosuppressive agents. This is likely to be an autoimmune process, and these patients have similar human leukocyte antigen types as observed in patients with rheumatoid arthritis. Lessons learned from this illness may help us better understand the pathophysiology of postinfectious and possibly other forms of idiopathic arthritis as well.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
History
In the mid-1970s, there was a peculiar cluster of what appeared to be cases of juvenile idiopathic arthritis (JIA) occurring in the city of Lyme, Connecticut, as well as in two nearby communities. What set off alarm bells was the large number of cases occurring in a limited geographic distribution in a town with a population at the time of 5000, with several cases occurring on the same city block and even the same household [1]. These cases were brought to the attention of a postdoctoral fellow in rheumatology named Allen Steere, who suspected an infectious etiology, although the causative organism was not identified in this original report. Since 25% of the cases also presented with a preceding rash consistent with erythema migrans (EM), already known to be associated with the Ixodes tick , an association between these two entities was postulated. Thus, the term Lyme disease (LD) was introduced in 1977 [1]. Four years later, Willy Burgdorfer analyzed the midguts of several Ixodes dammini ticks, finding spirochetes in several of them; he also reported that patients with LD had antibodies against them. Thus, the bacteria were given the name Borrelia burgdorferi, and it was correctly identified as the causative agent of LD [2]. Since then, substantial progress has been made toward the recognition, diagnosis, and management of this disorder.
Epidemiology
LD is the most common tick-borne infection in the United States. The numbers reported to the Centers for Disease Control and Prevention (CDC) have remained fairly constant over the past few years at about 25–30,000 per annum (CDC. Reported cases of Lyme disease by year, United States, 2003–2012. 2013. www.cdc.gov/lyme/stats/chartstables/casesbyyear.html, accessed 12/18/2017). However, a CDC analysis of medical claim information from a large insurance database suggests that physician diagnosed LD yearly may be up to ten times that reported to the CDC [3]. In the United States, the prevalence remains highest in the Northeast, in the mid-Atlantic region, in the upper Midwest around the Great Lakes, and in the Pacific Northwest, with a small number of states—Connecticut, Massachusetts, Rhode Island, New York, New Jersey, Pennsylvania, Delaware, Maryland, Virginia, Wisconsin, and Minnesota—accounting for about 90% of reported cases. As in the United States, certain areas in Europe such as Sweden [4], Austria, Estonia, Lithuania, the Netherlands, and Slovenia are at higher risk than others [5], although the European literature also postulates under-reporting [6]. There appears to be a bimodal age distribution, with peaks around 5–10 years of age and another from 35–55 years, likely reflecting ages in which humans are most likely to be outdoors [7]. There is a fairly even sex distribution of cases.
Borrelia burgdorferi spp. and Its Transmission
Ixodes ticks transmit Borrelia spp. wherever in the world Lyme disease is found. This includes I. scapularis in the Northeast, mid-Atlantic, and upper Midwest and I. pacificus in the Western United States. Animal reservoir species include small mammals, particularly the white-footed mouse Peromyscus leucopus [8].
The Borrelia genus are spirochetal bacteria (phylum: Spirochaetales) that comprises approximately 20 different species, three of which are most responsible for clinical manifestations of infection: B. burgdorferi, B. afzelii, and B. garinii; the former is mostly responsible for the disease in the United States, while the latter two are largely responsible for the disease in Europe [9]. These bacteria do not directly infect humans but rather can only do so following the bite of the Ixodes tick. Despite generally being referred to as a “deer tick ,” Ixodes does not actually feed on deer until its adult life; however, the deer is essential for its life cycle, as this is where mating takes place [10]. As reviewed [10], there are three stages to the Ixodes life cycle, with one meal per stage. Adult ticks mate while attached to the deer and then drop to the ground to release their eggs. The eggs themselves are not infected with Borrelia, so they hatch into uninfected larvae (first part of life cycle). The larvae feed upon a variety of small animals, including mice, squirrels, and birds. If the host animal happens to be infected with Borrelia, the larva will then acquire these bacteria as well. After its meal, regardless of whether it has acquired Borrelia, the larva drops to the ground and molts into a nymph (second part of life cycle). The nymph will retain any Borrelia acquired as a larva. Like its larva predecessor, the nymph will feed on small animals, the same type of animals infected by the larva. In this manner, the nymph can transmit the bacteria to these animals, thus ensuring reservoirs of infection for the next generation of larva. Nymphs can also feed on humans (and dogs), thus transmitting the infection. The nymph part of the life cycle generally takes place from May through early July, which therefore represents the most likely time for humans to be infected with Borrelia [11, 12]. Following their blood meal, nymphs molt into adults (third part of life cycle), which generally feed on large animals such as deer. Adult ticks are generally not considered to be infectious, as deer do not maintain Borrelia as efficiently as do the smaller animals. However, as noted above, they are essential for the life cycle, as this is where mating takes place.
Genospecies and Virulence
There are numerous borrelial genospecies, but the most important for the transmission of clinical disease to humans are the under the B. burgdorferi sensu lato complex. This includes B. burgdorferi sensu stricto, the important genospecies in the United States and Europe, as well as two other species which also cause disease in Europe—B. afzelii and B. garinii—commonly associated with rashes and neurologic involvement, respectively. In addition, a provisionally named species that causes Lyme borreliosis, candidatus B. mayonii, has been recently described from Minnesota in the United States [13]. Borrelia spp. are spirochetes with a complex genomic structure consisting of linear chromosomes and linear and circular plasmids [14]. This genetic heterogeneity contributes to antigenic heterogeneity that changes with initial infection and subsequent dissemination. It also likely accounts for virulence factors which lead to differing genospecific invasive potentials and thus differences in disease expression [15, 16].
Mechanism of Transmission
Borrelia spirochetes are resident in the tick midgut. With tick attachment to the host skin and ingestion of a blood meal, the bacteria replicate and migrate through the tick wall and disseminate into the hemocoele and eventually to the salivary glands from which site they are injected into the skin. This process is consequential since it takes 48–72 h for its completion [17, 18]. This is also the time frame for tick engorgement with blood. Therefore, early removal of unengorged ticks is much less likely to result in clinical disease although infection has been occasionally described with shorter duration of attachment.
Potential Role of the Tick Microbiome
Researchers at Yale University have investigated the role of gut microbiota of I. scapularis ticks in the efficiency of B. burgdorferi to colonize the tick gut epithelium. Perturbation of the larval tick microbiome resulted in decreased B. burgdorferi colonization possibly through modulation and decrease of tick transcription factor signal transducer and activator of transcription (STAT) levels and alteration of the gut barrier integrity [19, 20]. The potential clinical or therapeutic implications of these observations are uncertain.
Infectious Features of Early Lyme Disease
Because nymphal ticks are the most common transmission vector, the early features of LD occur most frequently in late spring through the summer and early fall when nymphs are most actively seeking a blood meal. In most cases, borrelial organisms have been detected by culture or borrelial DNA/RNA by polymerase chain reaction (PCR) in blood or affected tissue/fluid, supporting the infectious nature of these manifestations [21,22,23]. However, clinical diagnosis is generally made without microbiological support as described in section “Lyme Disease Diagnosis”.
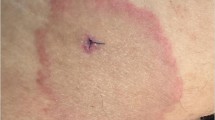
Erythema Migrans (EM) and Other Rashes
EM is the commonest clinical feature of LD affecting about 70% of reported cases [8, 24]. The true incidence of EM may be significantly higher based on cohort and epidemiological studies. One reason for this is that tick attachment and EM are usually asymptomatic, except for mild pruritus in some cases, and bites may occur at sites not easily visible such as the back or back of the leg. It begins as an erythematous macule or papule within 2 weeks at the site of tick attachment. A cardinal feature of EM is erythematous expansion within days to weeks with or without central clearing. Rarely a vesicular center or ulceration may be seen. The rash generally fades without sequelae. Secondary lesions (disseminated EM) are found in about 20% of patients and are due to hematogenous dissemination of the spirochete. Skin culture positivity and detection of B. burgdorferi DNA by PCR at the margin of EM lesions support the infectious nature of this clinical presentation [21]. EM-like lesions not caused by Borrelia have been described in the United States. Specifically, in the Southern United States, where LD is rare, the Amblyomma americanum tick can spread unknown bacteria that results in a rash clinically indistinguishable from EM called Southern Tick-Associated Rash Illness (STARI) , which does not appear to respond well to anti-borrelial therapy [25], but which does not necessarily require specific therapy. Other cutaneous lesions including borrelial lymphocytoma (early) and acrodermatitis chronica atrophicans (late) are seen in Europe [26, 27]. The latter is usually caused by B. afzelii and is thus more commonly seen in Europe than in the United States; it is characterized by a slowly progressive rash on the extensor surfaces of the extremities frequently occurring at a site of a previous EM rash [9]. At its onset, it typically manifests as bluish-red discoloration with swelling and can enlarge over months to years, eventually resolving with atrophy [6, 8]. In some cases, morphea-like lesions can also occur; this as well appears to be more common in Europe than in the United States [28].
Lyme Neuroborreliosis (LN)
Neurologic features occur with disseminated LD and are seen within weeks to months after a tick bite, especially in patients who were not treated at an earlier stage because EM was not present or not noted or because of misdiagnosis. LN occurs in about 15% of patients with LD and is even commoner in Europe because of the neurotropism of B. garinii [8, 24, 29]. The commonest manifestation is cranial neuritis, especially 7th nerve palsy, mimicking Bell’s palsy of viral origin. Bilateral facial involvement may occur and is a hallmark of LN. Other neurological features, which may occur simultaneously or separately, include lymphocytic meningitis and painful radiculoneuropathies, motor or sensory. The triad of these three features was described by Bannwarth in the 1940s long before the etiology became known [30].
Other Clinical Features
Lyme carditis is another well-recognized feature of early bacterial dissemination but is now an unusual clinical feature, accounting for only 1% of reported cases. High-grade atrioventricular nodal block (second and third degree) may be seen with accompanying symptoms, even fatality. Acute myopericarditis may rarely occur [8].
Acutely, flu-like constitutional symptoms with predominate headache, myalgias, and arthralgias may occur especially with dissemination. This may be the only early feature of borrelial infection, seen in about 15% of patients, and the presence of these symptoms in a Lyme endemic area in the summertime should raise the suspicion of LD [31].
While LA is considered a late feature of LD when presenting as a monoarthritis , it often begins as an early feature of disseminated infection with intermittent and migratory joint pains, with or without signs of inflammation. During this phase, unusual musculoskeletal features may be seen, including bursitis, tendinitis, temporomandibular joint involvement, and carpal tunnel syndrome. Left untreated, this will eventually evolve to classical LA [32, 33].
Numerous other clinical features have been attributed to LD for which there is incomplete or unconvincing microbiological support. However, ocular complications, including uveitis and keratitis, may rarely occur with borrelial infection.
Infectious Features of Late Lyme Disease
These complications generally occur in borrelial infected individuals who have never received appropriate antibiotic therapy for early LD, often because the early features were unrecognized or misdiagnosed. Appropriate treatment of early LD almost invariably prevents these later features. Microbiological support for many of the later features is often wanting, and even positive PCR results for borrelial DNA in synovial fluid of patients with LA may represent nonviable organisms [34]. Despite this, the diagnosis can be made with a good degree of accuracy by clinical features and laboratory testing for antibodies to borrelial antigens as described below.
Lyme Arthritis
If early borrelial infection is left untreated, about 60% of patients in the United States will develop LA [33]. This late feature usually presents many months after the initial infection and thus may occur in the winter, when early Lyme disease diagnosis is distinctly unusual. Most patients have an inflammatory arthritis affecting one or both knees. The synovial fluid is inflammatory. If the features of early Lyme disease were not present and if a prior history of migrating joint pains was not elicited, then the diagnosis of Lyme arthritis might be missed and other causes of infectious arthritis, idiopathic inflammatory arthritis (including JIA in children), and even crystal arthropathy in the elderly will be considered. Although the pathogenesis is clearly infectious with most patients responding to oral or parenteral antibiotics, it is of interest that viable borrelial organisms are not cultivable from the synovial fluid. Rarely spirochetes may be found in the synovium or enmeshed in a fibrinous synovial exudate. There is evidence that borrelial-triggered inflammations, involving both the innate and adaptive immune systems, are key elements in the pathogenesis of the inflammatory arthritis [33]. Clinically, the onset is often acute, with a marked knee effusion and elevated acute phase reactants. Symptomatic response to antibiotic therapy is seen to occur slowly over weeks to months since the large effusion has resulted in a mechanically disadvantaged knee joint. Physical therapy with quadriceps muscle strengthening can help with rehabilitation.
Late Lyme Neuroborreliosis
True late LN is unusual in the United States, and in fact there is considerable skepticism regarding the diagnosis without adherence to diagnostic guidelines, which include the appropriate clinical picture, often encephalomyelitis with cognitive dysfunction, sleep disturbances, and mood changes, and demonstration of positive intrathecal anti-Bb antibody index, often with cerebrospinal fluid (CSF) pleocytosis and/or increased CSF protein [35, 36]. Of course, most patients also have high titers of serum antibodies to B. burgdorferi. A chronic distal axonal neuropathy has also been described leading mainly to sensory paresthesias. A much broader variety of more severe neurological complications has been described in Europe, including cranial nerve palsies and paraparesis because of the neurotropism of B. garinii. Parenteral antibiotics are recommended for LN, early and late [37].
Noninfectious Features of Lyme Disease
These clinical conditions are associated with prior Lyme disease infection. The causes are not confirmed although it is highly likely that immunological mechanisms play a role in antibiotic-refractory Lyme arthritis.
Recurrent Arthritis: Antibiotic-Refractory Lyme Arthritis
While LA may take many months to resolve after antibiotic therapy, it resolves completely in about 90% of patients. However, in about 10% of patients, joint swelling and pain will recur or persist for months to years. This usually involves the same joint(s) as the original LA, that is, one or both knees [32]. Viable organisms are not found in the synovium or synovial fluid from these joints, and PCR for borrelial DNA is generally negative. Patients with this syndrome have an increased carriage of certain rheumatoid arthritis-associated human leukocyte antigen (HLA) alleles, including HLA-DRB1*0401 and *0101, as well as others, supporting an immunological predisposition [38]. Furthermore, these HLA alleles demonstrated greater capacity to present Borrelia-associated peptides, as compared to HLA alleles not associated with chronic LA, thereby suggesting a mechanism by which infection may progress to chronic arthritis [38]. Autoreactive B and T cell responses can be detected in both antibiotic-responsive and antibiotic-refractory Lyme arthritis, but in antibiotic-refractory Lyme arthritis, there are a number of other immunological features including Th1 inflammatory responses, altered regulatory T cell numbers, and possibly autoimmunity to endothelial cell growth factor [39, 40]. This condition does not respond to repeated or prolonged courses of oral or parenteral antibiotics. Patients can respond to local treatment, including intra-articular corticosteroid injections or synovectomy, or to systemic antirheumatic drugs such as hydroxychloroquine, sulfasalazine, and methotrexate. Eventual resolution is the rule after many months or years [41].
Other Systemic Rheumatic Syndromes Following Lyme Disease
Some patients have been characterized as developing rheumatoid arthritis (RA), psoriatic arthritis, or peripheral spondyloarthropathy months after antibiotic-treated Lyme disease infection [42]. These patients, while clinically resembling autoimmune arthritis, had some serological features that were atypical, including a lack of rheumatoid factor and antibodies to citrullinated proteins in most of the RA patients. Furthermore, four subjects have obtained a drug-free remission. This raises the possibility that at least some of these patients may have an illness in the spectrum of antibiotic-refractory Lyme arthritis.
Posttreatment Lyme Disease Symptoms and Syndrome
A significant minority of patients, from 10% to 15% in clinical trials, have reported residual subjective symptoms following treatment for Lyme disease [43, 44]. In many, these symptoms eventually resolve spontaneously, although some patients have persistent complaints (>6 months). These symptoms are some combination of fatigue, musculoskeletal pain (myalgia and arthralgia), headache, difficulty with concentration and memory, and paresthesias. Although subjective and without physical findings or testing that support structural abnormalities, these complaints can result in significant disability. Since these symptoms are common in the general population, it remains uncertain whether they are truly reflective of prior Lyme disease with some studies supporting that hypothesis [45] and others not [43, 46]. There is no convincing evidence in controlled trials that patients with post-Lyme disease syndrome have ongoing borrelial infection, and they do not respond to aggressive and prolonged antibiotic therapy [45,46,49]. The cause remains unknown, the symptoms tend to wax and wane chronically, and treatment has been symptomatic.
Controversies Surrounding Chronic Lyme Disease
This widely used term is poorly defined [50, 51]. It should be distinguished from late Lyme disease manifestations. While sometimes referring to posttreatment Lyme disease syndrome as described above, many patients with these clinical symptoms have no clear-cut evidence of prior borrelial infection. The belief that LD is the cause of these symptoms despite evidence to the contrary has been perpetuated by scientific misconceptions and distortions including the propensity of LD to cause disability in the absence of objective clinical signs, the insensitivity of currently accepted diagnostic tests for even late features of Lyme disease, the persistence of B. burgdorferi within cells or in hidden sites, and the effectiveness of prolonged (months to years) treatments with combinations of antibiotics or alternative and sometimes harmful therapies [52]. Patient advocacy groups, physician proponents, the media (both traditional and social), and even well-intentioned but misled politicians have contributed to these unfortunate pseudoscientific beliefs [53].
Lyme Disease Diagnosis
Principles of Diagnosis
In early LD, positive bacterial cultures can be obtained from the skin, the cerebrospinal spinal fluid, and the blood. However, the yield is low, and cultivation takes many weeks making it an impractical method of diagnosis. The use of PCR to detect the DNA or RNA of viable organisms has proven disappointing because of low yield (blood, spinal fluid) or positivity in the presence of nonviable Borrelia (synovial fluid) [21,22,23, 34].
Therefore, the laboratory methods to support the clinical diagnosis of Lyme disease are indirect and employ serological testing of the immune response to the Borrelia. Since antibody responses to an infecting organism may take days to weeks to develop, may not develop at all if early effective treatment is instituted, and may persist for many years after the infection is eradicated, this must be kept in mind when interpreting the “Lyme test” results. Another important feature of the antibody response to Borrelia, similar to other infections, is that IgM responses are seen first acutely and IgG antibodies appear weeks to months later with persisting, untreated infection and most commonly with simultaneous diminution of the IgM antibodies.
The standard way to screen for antibodies to Borrelia is by a sensitive enzyme-linked immunosorbent assay (ELISA) , which detects both IgM and IgG antibodies. Because of the poor specificity of this test, any positive or equivocal result should be followed by the more specific Western blot assay according to the guidelines of the Centers for Disease Control and Prevention [54]. Specifically, false-positive results can result from a variety of causes, including infections with other members of the Spirochaete phyla, the Borrelia genus, and even unrelated infections and normal host microbiota [55, 56], as well as from patients with autoimmune diseases such as lupus and rheumatoid arthritis [57]. This two-tiered testing approach has been studied and used for over two decades, for the most part with good success. In these guidelines, an IgM Western blot is positive when any two of the 23 kD, 39 kD, or 41 kD bands are present. An IgG Western blot is positive when any five of the 18 kD, 23 kD, 28 kD, 30 kD, 39 kD, 41 kD, 45 kD, 60 kD, 66 kD, or 93 kD bands are present. Since the criteria for a positive IgG Western blot are much more stringent than for a positive IgM Western blot, it is a much more specific result indicating prior or current exposure to the borrelial organism. Patients with late Lyme arthritis almost always exhibit strongly positive IgG Western blots [58].
Single-tiered testing using the C6 peptide antigen of the VlsE borrelial protein has proven to be sensitive and specific for the diagnosis of Lyme disease, reduces the problem of misinterpretation of IgM Western blot results (see below), and is commercially available [59, 60]. It may be particularly advantageous in Europe, as the two-tiered approach appears to be somewhat less sensitive for the detection of the European strains B. garinii and B. afzelii as compared to B. burgdorferi [61].
The CSF is the only body fluid in addition to serum where antibody testing is of proven value. The finding of a high Lyme antibody index, based on the ratio of antibodies in the CSF to serum, can support the diagnosis of neuroborreliosis [35].
Diagnostic Pitfalls
Because antibodies may not be detected in the blood for days to weeks after initial borrelial infection, a negative Lyme ELISA in patients with very early symptoms does not rule out Lyme disease as a cause. Therefore, a patient who presents with classical symptoms beginning with EM in a Lyme disease endemic area in the spring and summer months should be treated for Lyme disease, even without serological testing. On the other hand, since antibodies will eventually appear in untreated patients and even in many patients who have received antibiotics, it may be worthwhile to repeat the test 3–4 weeks later in patients with atypical symptoms such as summer flu or facial palsy without EM to determine if seroconversion has occurred.
A major diagnostic problem is the misuse and misinterpretation of the IgM Western blot result. The criteria were devised for sensitivity for early Lyme disease when the IgG antibodies may not have yet appeared, but not for specificity. Thus, a positive IgM result may indicate early Lyme disease when used in the first 4–6 weeks of symptom onset. In patients with prolonged symptoms, a positive IgM Western blot in the absence of a positive IgG Western blot is more likely to be a false positive and does not indicate Lyme disease infection [62]. Many patients with chronic nonspecific symptoms or symptoms of an alternative diagnosis have been labeled as chronic Lyme disease because of a false-positive IgM Western blot result.
Treatment and Prevention of Lyme Disease
Since the first treatment trials of Lyme borreliosis, the organism has not developed resistance to doxycycline or amoxicillin, which remain the mainstays of treatment in adults and young children, respectively. Extensive treatment guidelines have been published by the Infectious Disease Society of North America [63]. In general, one course of oral antibiotics from 2 to 4 weeks is usually sufficient to cure early Lyme disease and prevent later complications [64]. Neuroborreliosis may require intravenous ceftriaxone therapy. Late LA usually responds to one 4-week course of antibiotics, but a second course is sometimes required, e.g., parenteral ceftriaxone. Non-responsiveness to more than two courses of antibiotics suggests alternative diagnoses such as antibiotic-refractory LA or post-Lyme disease syndrome. Rarely, a confounding factor in treatment may be related to coinfection with other organisms such as Anaplasma phagocytophilum (which responds to doxycycline), Babesia microti, and Borrelia miyamotoi or a Powassan virus, all carried by the Ixodes tick [63, 65].
Prevention of LD has many approaches, including wearing protective clothing as well as the use of tick repellants. However, most effective is undergoing inspection for ticks and removal when found after being in grassy areas in a Lyme disease endemic region. Removal of ticks prior to their engorgement markedly reduces the likelihood of transmission of B. burgdorferi to the skin [66]. Attempted removal of the tick should be done with caution, since the application of torque to the offending tick may result in its decapitation, while nevertheless enabling its mouth to remain embedded in the skin and transmit the disease [67]; crushing the tick may also allow infective agents in its body to enter the bloodstream [67]. Furthermore, one dose of doxycycline 200 mg within 72 h of tick attachment greatly reduces the chance of developing Lyme disease [68]. Although vaccines were developed for Lyme disease and were shown to be relatively effective and safe, none is currently on the market [69, 70].
Conclusions
LD is a tick-borne bacterial infection largely limited to endemic areas in the Northeastern United States and in parts of Europe. The manifestations are protean, and in the absence of the distinctive rash, the diagnosis can be missed. The causative bacteria are highly sensitive to several antibiotics, resulting in generally good outcomes in patients treated during the early localized or early disseminated phase [12]. LD has the potential to develop into an arthritic process, which appears to begin as an infected joint but can evolve into a chronic reactive process. Lessons learned from this illness may help us better understand the pathophysiology of postinfectious and possibly other forms of idiopathic arthritis.
Abbreviations
- CDC:
-
US Centers for Disease Control and Prevention
- ELISA:
-
Enzyme-linked immunosorbent assay
- EM:
-
Erythema migrans
- HLA:
-
Human leukocyte antigen
- JIA:
-
Juvenile idiopathic arthritis
- LA:
-
Lyme arthritis
- LD:
-
Lyme disease
- LN:
-
Lyme neuroborreliosis
- PCR:
-
Polymerase chain reaction
- RA:
-
Rheumatoid arthritis
References
Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977;20:7–17.
Burgdorfer W. How the discovery of Borrelia burgdorferi came about. Clin Dermatol. 1993;11:335–8.
Kuehn BM. CDC estimates 300,000 US cases of Lyme disease annually. JAMA. 2013;310:1110.
Orczyk K, Swidrowska-Jaros J, Smolewska E. When a patient suspected with juvenile idiopathic arthritis turns out to be diagnosed with an infectious disease – a review of Lyme arthritis in children. Pediatr Rheumatol Online J. 2017;15:35.
Mead PS. Epidemiology of Lyme disease. Infect Dis Clin N Am. 2015;29:187–210.
Hofmann H, Fingerle V, Hunfeld KP, Huppertz HI, Krause A, Rauer S, et al. Cutaneous Lyme borreliosis: guideline of the German Dermatology Society. Ger Med Sci. 2017;15:Doc14.
Bacon RM, Kugeler KJ, Mead PS, Centers for Disease C, Prevention. Surveillance for Lyme disease – United States, 1992-2006. MMWR Surveill Summ. 2008;57:1–9.
Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–47.
Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090.
Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99.
Anderson JF. Ecology of Lyme disease. Conn Med. 1989;53:343–6.
Kowalski TJ, Tata S, Berth W, Mathiason MA, Agger WA. Antibiotic treatment duration and long-term outcomes of patients with early Lyme disease from a Lyme disease-hyperendemic area. Clin Infect Dis. 2010;50:512–20.
Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556–64.
Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;46:515–36.
Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64.
Baranton G, De Martino SJ. Borrelia burgdorferi sensu lato diversity and its influence on pathogenicity in humans. Curr Probl Dermatol. 2009;37:1–17.
Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129(Suppl):S191–220.
Des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC 3rd, Fish D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis. 2001;183:773–8.
Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe. 2014;15:58–71.
Narasimhan S, Fikrig E. Tick microbiome: the force within. Trends Parasitol. 2015;31:315–23.
Liveris D, Wang G, Girao G, Byrne DW, Nowakowski J, McKenna D, et al. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J Clin Microbiol. 2002;40:1249–53.
Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142:751–5.
Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–34.
Borchers AT, Keen CL, Huntley AC, Gershwin ME. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82–115.
Goddard J. Not all erythema migrans lesions are Lyme disease. Am J Med. 2017;130:231–3.
Asbrink E, Hovmark A, Olsson I. Clinical manifestations of acrodermatitis chronica atrophicans in 50 Swedish patients. Zentralbl Bakteriol Mikrobiol HygA. 1986;263:253–61.
Berglund J, Eitrem R, Ornstein K, Lindberg A, Ringer A, Elmrud H, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–27.
Moreno C, Kutzner H, Palmedo G, Goerttler E, Carrasco L, Requena L. Interstitial granulomatous dermatitis with histiocytic pseudorosettes: a new histopathologic pattern in cutaneous borreliosis. Detection of Borrelia burgdorferi DNA sequences by a highly sensitive PCR-ELISA. J Am Acad Dermatol. 2003;48:376–84.
Hildenbrand P, Craven DE, Jones R, Nemeskal P. Lyme neuroborreliosis: manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol. 2009;30:1079–87.
Bannwarth A. Chronische lymphocytare meningitis, entzundliche polyneuritis und “rheumatismus” [Chronic lymphocytic meningitis, inflammatory polyneuritis and ‘rheumatism’]. Arch Psychiatr Nervenkr. 1941;113:284–376.
Aucott J, Morrison C, Munoz B, Rowe PC, Schwarzwalder A, West SK. Diagnostic challenges of early Lyme disease: lessons from a community case series. BMC Infect Dis. 2009;9:79.
Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31.
Bockenstedt LK, Wormser GP. Review: unraveling Lyme disease. Arthritis Rheumatol. 2014;66:2313–23.
Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63:2238–47.
Steere AC, Berardi VP, Weeks KE, Logigian EL, Ackermann R. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J Infect Dis. 1990;161:1203–9.
Hansen K, Lebech AM. The clinical and epidemiological profile of Lyme neuroborreliosis in Denmark 1985-1990. A prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody production. Brain. 1992;115(Pt 2):399–423.
Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol. 2015;11:446–56.
Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–71.
Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–35.
Vudattu NK, Strle K, Steere AC, Drouin EE. Dysregulation of CD4+CD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2013;65:1643–53.
Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86.
Arvikar SL, Crowley JT, Sulka KB, Steere AC. Autoimmune arthritides, rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthritis following Lyme disease. ArthritisRheumatol. 2017;69:194–202.
Cerar D, Cerar T, Ruzic-Sabljic E, Wormser GP, Strle F. Subjective symptoms after treatment of early Lyme disease. Am J Med. 2010;123:79–86.
Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, et al. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med. 1994;121:560–7.
Cairns V, Godwin J. Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms. Int J Epidemiol. 2005;34:1340–5.
Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Farber S, Pakash P, Ash J, Nowakowski J. Long-term assessment of fibromyalgia in patients with cultureconfirmed Lyme disease. Arthritis Rheumatol. 2015;67:837–9.
Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92.
Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60:1923–30.
Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003.
Feder HM Jr, Johnson BJ, O’Connell S, Shapiro ED, Steere AC, Wormser GP, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–30.
Lantos PM. Chronic Lyme disease: the controversies and the science. Expert Rev Anti-Infect Ther. 2011;9:787–97.
Marzec NS, Nelson C, Waldron PR, Blackburn BG, Hosain S, Greenhow T, et al. Serious bacterial infections acquired during treatment of patients given a diagnosis of chronic Lyme disease – United States. MMWR Morb Mortal Wkly Rep. 2017;66:607–9.
Auwaerter PG, Bakken JS, Dattwyler RJ, Dumler JS, Halperin JJ, McSweegan E, et al. Antiscience and ethical concerns associated with advocacy of Lyme disease. Lancet Infect Dis. 2011;11(9):713.
Centers for Disease C, Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1.
Magnarelli LA, Anderson JF. Enzyme-linked immunosorbent assays for the detection of class-specific immunoglobulins to Borrelia burgdorferi. Am J Epidemiol. 1988;127:818–25.
Magnarelli LA, Miller JN, Anderson JF, Riviere GR. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990;28:1276–9.
Weiss NL, Sadock VA, Sigal LH, Phillips M, Merryman PF, Abramson SB. False positive seroreactivity to Borrelia burgdorferi in systemic lupus erythematosus: the value of immunoblot analysis. Lupus. 1995;4:131–7.
Kowal K, Weinstein A. Western blot band intensity analysis. Application to the diagnosis of Lyme arthritis. Arthritis Rheum. 1994;37:1206–11.
Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, Johnson BJ, Wormser GP, Steere AC. 2-tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin Infect Dis. 2010;50:20–6.
Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis. 2013;75:9–15.
Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767–77.
Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect. 2012;18:1236–40.
Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134.
Wormser GP, Ramanathan R, Nowakowski J, McKenna D, Holmgren D, Visintainer P, et al. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:697–704.
Hermance ME, Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis. 2017;17:453–62.
Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424–30.
Needham GR. Evaluation of five popular methods for tick removal. Pediatrics. 1985;75:997–1002.
Warshafsky S, Lee DH, Francois LK, Nowakowski J, Nadelman RB, Wormser GP. Efficacy of antibiotic prophylaxis for the prevention of Lyme disease: an updated systematic review and meta-analysis. J Antimicrob Chemother. 2010;65:1137–44.
Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med. 1998;339:209–15.
Plotkin SA. Need for a new Lyme disease vaccine. N Engl J Med. 2016;375:911–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Weinstein, A. (2018). Lyme Disease: Infectious and Noninfectious Features. In: Ragab, G., Atkinson, T., Stoll, M. (eds) The Microbiome in Rheumatic Diseases and Infection. Springer, Cham. https://doi.org/10.1007/978-3-319-79026-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-79026-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-79025-1
Online ISBN: 978-3-319-79026-8
eBook Packages: MedicineMedicine (R0)