Abstract
RNA viruses are a large group of widespread and extremely prevalent pathogens capable of eliciting a broad spectrum of innate and adaptive immune responses. Additionally, persistent infection by some RNA viruses can induce or enhance accelerated immune activation. The hypothesis that molecular mimicry is implicated in autoimmunity was first proposed in 1987. Since then, a growing evidence from medical literature of a possible role for viral infection in autoimmunity has risen. In particular, enteroviruses have been investigated as possible causes of type 1 diabetes. Some hypotheses have put forward the possible implication of hepatitis A virus in autoimmune phenomena. The two most prevalent RNA viruses causing chronic infection, hepatitis C virus and human immunodeficiency virus type 1 (HIV-1), are both associated with autoimmune disorders. HCV can trigger and sustain a clonal B-cell expansion which causes a wide spectrum of autoimmune/lymphoproliferative disorders, through a multistep process. Similarly, HIV is responsible for derangement of the immune regulation and is associated with some autoimmune disorders, such as autoimmune thrombocytopenia. Nevertheless, a formal scientific demonstration of an etiological relationship between any RNA virus and a major autoimmune disease has not yet been obtained.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Enterovirus and Autoimmunity
Enteroviruses belong to the Picornaviridae family, comprising non-enveloped, small, single-stranded positive-sense RNA genome viruses. On the basis of their pathogenesis, enteroviruses were originally classified into four groups: polioviruses, coxsackie A viruses (CA), coxsackie B viruses (CB), and echoviruses. Due to the significant overlaps in such taxonomy, enteroviruses (EV) isolated more recently are named with a system of consecutive numbers: EV68, EV69, EV70, EV71, etc. [1]. Enteroviruses are named after their transmission route which is mainly fecal-oral (despite other routes of transmission being present for some species).
The main clinical entities caused almost exclusively by enteroviruses are poliomyelitis, herpangina, hand-foot-and-mouth disease, and epidemic pleurodynia. Other enterovirus-related disorders, such as aseptic meningitis and myopericarditis, may also be caused by other etiological agents.
Several picornavirus infections showed a strong association with autoimmunity. This is especially true in type 1 diabetes (T1D) and myocarditis. The exact mechanisms inducing autoimmunity remain controversial. Antigenic mimicry , i.e., the shared sequence or tertiary structure between foreign and self-antigens, is the most well-established theory for enterovirus- induced autoimmunity. For example, in a subpopulation of patients with T1D, a molecular mimicry has been found between glutamic acid decarboxylase (GAD65) and protein 2C of coxsackie B-like enteroviruses, suggesting a possible cross-reaction involved in the pathogenesis of the disease [2]. Alternatively, cytopathic infectious agents can cause the release of either sequestered or intracellular autoantigens inducing dual TCR expression (i.e., the induction of T cells bearing receptors potentially responding also against self-tissues) [3].
Viruses have been suggested as a potential environmental trigger of T1D; a disease having a well-documented genetic basis, whose etiology remains to be elucidated. The body of evidence supporting a relationship between viral infections and initiation or acceleration of islet autoimmunity remains largely circumstantial. Among the different possible candidates, the most robust association was established with some enterovirus strains inducing or accelerating the disease in animal models [4].
Higher coxsackievirus-neutralizing antibody titers in serum were reported from recent-onset T1D patients as compared to nondiabetic subjects, and a possible relationship with these viruses was later confirmed using polymerase chain reaction testing, particularly with coxsackie B virus (B4 in particular) [5]. Cross-sectional studies have focused predominantly on recent-onset individuals with T1D, although enterovirus was also identified as a risk factor in prediabetic children and pregnant women . There is still a lack of large prospective studies that establish a clear temporal relation between enterovirus infection and the development of islet autoimmunity.
The possibility of a viral infection specifically affecting pancreatic endocrine cells constitutes a straightforward explanation for the selective demise of beta cells, either through lysis induced by cytopathic viruses or immune-mediated destruction of infected beta cells. Coxsackievirus, however, displays pancreas tropism rather than a preference for islet beta cells; furthermore, direct studies on the pancreas in vivo in order to search for viral infection signatures are limited by the organ’s relative inaccessibility [4].
Viral infections are presumed to represent the most common causes of myocarditis in North America and Europe [6]. Viral genomes are detected in the myocardium of a variable proportion of patients with myocarditis and dilated cardiomyopathy (DCM) using molecular techniques. Several lines of evidence support the involvement of autoimmunity in myocarditis. These include the production of antibodies against self-antigens, improvement of myocarditis symptoms with immunosuppressive therapy, and a co-occurrence of myocarditis with other autoimmune diseases [7]. In genetically predisposed mouse strains, viral RNA and inflammation can persist in heart cells for several weeks, triggering myocardial autoimmune reactions [8]. However, there is no direct evidence that this can occur in humans.
Is Hepatitis A Virus a Trigger of Chronic Autoimmune Phenomena?
Like enteroviruses, hepatitis A virus (HAV) is a single-stranded RNA virus belonging to the Picornaviridae family. It is transmitted through the fecal-oral route and is the most common cause of acute viral hepatitis, particularly common among children and young adults. Different from other major hepatotropic viruses, it does not sustain a chronic viral hepatitis, as it is systematically cleared after the acute phase [9].
Patients with HAV infection occasionally manifest symptoms consistent with circulating immune complex formation. These include cutaneous vasculitis, arthritis, antinuclear antibody (ANA) production, and cryoglobulinemia. Either IgM or IgG anti-HAV is detected in the cryoglobulins [10]. The symptoms resolve spontaneously with resolution of hepatitis A.
A possible role for HAV as an autoimmune hepatitis trigger has been proposed from a study on relatives of patients with autoimmune chronic active hepatitis [11] and described later in some case reports [12, 13]. The finding of molecular mimicry (cross-reactivity between epitopes of viruses and certain liver antigens) may support the hypothesis of a role for HAV as a trigger of autoimmunity, although this should concern a minority of individuals genetically predisposed, and is influenced by unknown cofactors. In particular, the development of chronic autoimmune hepatitis has been described in patients with a defect in suppressor-inducer T lymphocytes specifically controlling immune responses to the asialoglycoprotein receptor. These predisposed patients might develop specific antibodies directed to the asialoglycoprotein receptor after HAV infection [11]. However, these reports have remained anecdotal.
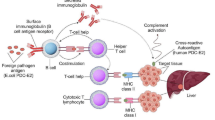
A graphic representation of autoimmune and rheumatic diseases associated with HCV chronic infection, on a gradient based on the association strength, according to literature [15,16,17,18,19,20]. The left box includes conditions with a definite association with HCV, a high percentage of which occurs in HCV-positive patients. For such diseases, a pathogenic mechanism involving HCV has been described. The middle box encompasses conditions which have some association with HCV chronic infection but have low overall HCV positivity prevalence and/or lack a definite pathogenic link. The right box includes conditions with weak or anecdotal association to HCV
Hepatitis C Virus and Autoimmunity
HCV is a small, enveloped, positive-sense single-stranded RNA virus of the Flaviviridae family. As many as 80–90% of HCV-infected patients have chronic infection defined by persistent serum HCV RNA despite humoral and cellular immune responses [14].
Persistent HCV infection leads to the development of chronic liver disease, cirrhosis, hepatocellular carcinoma, and also a broad spectrum of extrahepatic diseases. HCV infection can in fact subvert the immune system in several ways, ranging from the expansion of selective B-cell subsets to tolerance induction and to the reaction of T cells against apoptosis-derived self-antigens. Cryoglobulins; rheumatoid factor; ANA; and anticardiolipin, antithyroid, and anti-liver/kidney microsomal antibodies (anti-LKM), as well as HCV/anti-HCV immune complex formation and deposition, can be found in infected patients [15]. An association with HCV has been established with cryoglobulinemic vasculitis (CV), membranoproliferative glomerulonephritis (MGN), and porphyria cutanea tarda and also suggested with thyroiditis, Sjogren’s syndrome, idiopathic pulmonary fibrosis, polyarthralgias in the setting of positive rheumatoid factor, and some cases of polymyositis/dermatomyositis (PM/DM) (Fig. 12.1) [16].
Cryoglobulins are anti-immunoglobulin immunoglobulins that reversibly precipitate at reduced temperatures. Mixed cryoglobulins usually contain IgM and IgG immunoglobulins, with the IgM having rheumatoid factor activity directed against IgG molecules. This leads to immune complex formation and cryoprecipitation . The presence of a monoclonal IgM component (type 2 cryoglobulin) may prelude to a progression to frank lymphoma.
HCV infection is the cause of more than 90% of the diagnosed cases of CV, which is a small vessel vasculitis involving mainly the skin, the joints, the peripheral nervous system, and the kidneys. The drivers of B-cell dysregulation during the course of chronic HCV infection are still to be fully characterized [17]. The disease expression is variable, ranging from mild symptoms (purpura, arthralgia) to fulminant life-threatening complications (glomerulonephritis, widespread vasculitis). The prevalent type of glomerulonephritis associated with mixed cryoglobulinemia is membranoproliferative glomerulonephritis (see Chap. 26).
HCV may interfere with the functions and mechanisms of self-recognition both on the immune system and thyroid cells, where HCV may directly destroy thyroid tissue or mimic the structure of some components of thyroid gland, igniting the autoimmune disease. In the course of HCV infection, both hypothyroidism and hyperthyroidism may emerge, Hashimoto’s thyroiditis being the most common thyroid disorder observed in patients with HCV infection. Interferon, which has been the mainstay of chronic HCV infection treatment until recently, can be an additional risk factor for the development of thyroid complications. It was advisable for clinicians to monitor thyroid function regularly in patients with chronic HCV and, in particular during treatment, with interferon-based regimens [18].
It has been reported that more than 60% of patients from the Mediterranean area presenting with type 2 autoimmune hepatitis carry anti-HCV antibodies along with the typical anti-LKM autoantibody pattern. However, a very limited proportion of HCV-positive patients have positive anti-LKM [15]. It appears that primary Sjogren’s syndrome may only be sporadically associated with HCV infection, and definitive evidence that HCV infection may trigger Sjogren’s syndrome is still lacking. Conversely, chronic HCV infection is associated frequently with sialoadenitis and occasionally with sicca syndrome. However, the pathogenic overlap of the increased risk of B-cell malignancies in Sjogren’s syndrome and the emergence of an association between HCV infection and monoclonal gammopathies and lymphoproliferative disorders are worth mentioning [19].
Arthritis can be observed along the course of HCV infection and in some cases is associated with mixed cryoglobulinemia. These patients typically present an anti-CCP antibody-negative, nonerosive intermittent oligoarticular arthritis [20].
Non-cryoglobulinemic glomerulonephritis has been associated with HCV, especially in children, and immune-mediated skin diseases, especially oral lichen planus, have been linked to HCV. Neurologic autoimmune diseases, including myelitis and encephalomyelitis, as well as several neuromuscular diseases, have also been reported to occur in HCV infection. The virus might be involved in the pathogenesis of other hematologic entity subsets, such as immune thrombocytopenic purpura (ITP) and autoimmune hemolytic anemia (AHA). Conversely, autoimmune mechanisms have been implicated in thrombocytopenia associated with chronic HCV [19].
HIV and Autoimmunity Before and After Combined Antiretroviral Treatment Introduction
HIV, a member of the genus Lentivirus, which is part of the family Retroviridae, is the causative agent of acquired immunodeficiency syndrome (AIDS). HIV is a double-stranded RNA virus that infects vital cells of the immune system such as helper T lymphocytes (CD4+ T cells), dendritic cells, and macrophages. HIV infection leads to a progressive decline in the CD4+ T cell count producing a dysregulation in the balance between CD4 and CD8 cells. The use of combined antiretroviral therapy (cART ) has revolutionized the life expectancy of infected persons, leading to a growing number of controlled chronic infections. A link between HIV and rheumatological diseases appeared soon after the appearance of AIDS with the description of painful, disabling asymmetrical inflammatory arthropathy in some AIDS patients [21]. Following the first reports, there have been case series and epidemiological studies describing different clinical manifestations affecting the musculoskeletal system [22]. In the early stage of the HIV infection, when the immune system is only partially impaired, rare cases of autoimmune diseases may develop as in the general population. On the contrary, the loss of competence of the immune system, due to the CD4 cell depletion in the late phases of the disease, leads to an increased incidence of predominantly CD8 T-cell-driven autoimmune diseases, such as psoriasis and diffuse infiltrative lymphocytosis (SjÖgren-like) syndrome [22]. Moreover, B cells are continuously stimulated since the early phases of infection, causing the frequent production of autoantibodies (which are present in up to 23% of HIV-infected patients, often without any clinical manifestation) (Fig. 12.2a) [23]. The immune restoration inflammatory syndrome (IRIS ) in HIV-infected patients initiating cART is characterized by a paradoxical clinical worsening of a previously known opportunistic disease or the appearance of a new condition after initiating cART. The overall incidence of IRIS is dependent on the population studied and its underlying opportunistic infection burden (Fig. 12.2b) [24].
Spectrum of rheumatic diseases in the natural history of HIV infection. (a) Rheumatic disease incidence related to CD4 cells declines without treatment intervention. (b) Occurrence of rheumatic diseases after cART introduction. ITP could be observed during all the stages of HIV infection, but the introduction of cART has a favorable impact on platelet count. Diseases like SLE and RA seem to improve with uncontrolled HIV infection and could restart when cART leads to immunological recovery. IRIS occurs exclusively after cART introduction manifesting with different phenotypes due to the underlying triggering condition. SLE systemic lupus erythematosus, RA rheumatoid arthritis, ITP immune thrombocytopenic purpura, APS antiphospholipid syndrome, PsA psoriatic arthritis, DILS diffuse infiltrative lymphocytosis syndrome, AHA autoimmune hemolytic anemia, cART combined antiretroviral therapy, IRIS immune restoration inflammatory syndrome, LCV leukocytoclastic cutaneous vasculitis
When immune competence is restored by cART, new onset of autoimmune diseases can occur. Several pathophysiologic hypotheses could explain this phenomenon, including the direct role of viral particles, immune complex-mediated diseases, dysregulation of the B/T lymphocyte interaction [25], molecular mimicry [26], and polyclonal B lymphocyte activation [23]. On the other hand, the partially rescued immune activation control might reduce the autoantibody production. Among the rheumatic manifestations, arthralgia has a prevalence estimates varying widely between 1 and 79%, regardless of whether the studies were carried out in the pre-cART (1.6–45%) or post-cART era [25, 27]. In HIV patients in the pre-cART era, myalgia was reported in 1.7–11% HIV patients [28]. Since the advent of cART, prevalence estimates between 0 and 77% have been reported, while rates of prevalence between 1 and 17% have been reported for fibromyalgia [23, 29]. Thus, the epidemiological data of pre- and post-cART do not allow us to draw clear conclusions on the relationship between actively replicating HIV infection and reactive arthritis. In the studies where HLA data were available, those HIV-infected patients with overlapping features of psoriatic arthritis (PsA) and reactive arthritis were often positive for the HLA-B27 allele [30]. Further confounding factors were derived from the emergence of reactive arthritis reported as a manifestation of IRIS after the introduction of cART [24]. The estimated prevalence rates of PsA among HIV-infected patients have ranged between 0.02% and 5.7% but most commonly were found between 0.02 and 2% [31]. Interestingly, the reports of ankylosing spondylitis, the most common form of seronegative spondyloarthropathy in the Western world, were very few in the pre-cART era [30]. Since cART introduction, when reactive and PsA became less frequent, diseases such as rheumatoid arthritis (RA), osteoporosis, or aseptic bone necrosis became more frequent. The earliest mention of RA and HIV came from pre-cART case reports describing patients with established RA who experienced clinical improvement or remission after the development of HIV [32]. This could be explained by the reduction of the immunogenic autoimmune activity due to the HIV-associated depletion of CD4 lymphocytes, which led to the conclusion that HIV and RA were mutually exclusive diagnoses [32]. ANA is present in up to 23% of HIV-infected persons; nonetheless, few cases of systemic lupus erythematosus (SLE) were described. In the advanced stage of HIV, the severely induced immune depression makes SLE incidence less frequent, probably because CD4 T lymphocytes play a crucial role in SLE pathogenesis [33]. On the contrary, the restoration of the normal immune function could lead to SLE flare. Anticardiolipin antibodies are found in 36–67% of HIV-infected patients; their level is associated with HIV viral load and the degree of immune activation, and cART reduces the probability of detecting anticardiolipin antibodies [34].
The diffuse infiltrative lymphocytosis syndrome is a rheumatic condition mimicking a Sjogren-like multisystem disease, typically causing salivary gland swelling and chronic sicca syndrome. The commonest presentation (88–100% of cases) is bilateral parotid gland enlargement. Its incidence declined following introduction of cART. Antiretroviral treatment improves the symptoms, with adjunctive glucocorticoids required in a minority of cases [35, 36].
Vasculitis is present in up to 1% of HIV-infected people, most usually affecting small- to medium-sized vessels. Etiopathogenetic mechanisms proposed combined immune complex formation and viral tropism for endothelial cells, the latter potentially offering a mechanism by which cART therapy is usually beneficial. Polyarteritis nodosa without concurrent HBV infection may occur at a moderate to advanced level of immunosuppression, and its clinical course seems to be less severe than in non-HIV-infected people, with a favorable evolution on corticosteroids. Henoch-Schonlein purpura , improving with cART introduction, was also described. ANCA-associated vasculitides are extremely rare, notwithstanding ANCA being detected in up to 8% of the patients at an advanced stage of the disease. Leukocytoclastic cutaneous vasculitis , either secondary to the HIV infection itself or caused by direct or immune-mediated damage to the vessel walls, has also been described [37].
Despite a high prevalence of positive direct antiglobulin test (up to 34% of cases), autoimmune hemolytic anemia (AHA ) rarely occurs in HIV-infected people; when it does, it usually occurs at an advanced stage of the disease. Here, cART is usually beneficial. On the contrary, ITP was frequently described in HIV-positive patients at all stages of the disease, while decreased platelet production favored by possible viral infection of megakaryocytes is observed in the advanced stage of the disease [38, 39]. Molecular mimicry plays an important role, since cross-reactivity was observed between antibodies directed to GPIIIa platelet surface antigen and an epitope of the Nef viral protein, as well as between antibodies directed to GPIIb/IIIa platelet surface glycoprotein and a particular form of glycosylated viral gp160/120. An increase in platelet count is observed within 3 months of treatment with cART , which is independent of the CD4 cell count but directly correlates with the decrease of the HIV plasma viral load [40].
Since cART introduction, cases of sarcoidosis were described as delayed IRIS manifestation (occurring with a median time of 9 months after cART introduction). In pulmonary forms, alveolitis is usually of CD4 type, with higher CD4/CD8 ratio in the bronchoalveolar lavage compared to blood. The usual evolution is spontaneously favorable or after administration of corticosteroids [41]. Graves’ disease and Hashimoto’s thyroiditis were also described as a manifestation of delayed IRIS (median time of 17 months from cART introduction) [42]. Autoimmune hepatitis was rarely reported, mainly in IRIS cases requiring temporary cART interruption and immunosuppressive treatment (with corticosteroids and/or azathioprine) [43].
Regarding drugs used to relieve symptoms in rheumatic patients, nonsteroidal anti-inflammatory drugs can be used according to the same guidelines for HIV-negative patients. Methotrexate may be used with careful monitoring of HIV viral loads and CD4 counts. Hydroxychloroquine has been effectively used in HIV-associated arthropathies. The literature on the use of biologic therapies in HIV-infected populations is at the moment limited to case reports and small case series and to the use of rituximab in hematological malignancies [42,43,46].
Conclusion
RNA viruses are able to elicit autoimmune reactions during acute or chronic infection (Table 12.1). The great majority of these phenomena are transient and strictly related to the acute phase of the disease. In type 1 diabetes, a clear demonstration of a causative role of RNA viruses in triggering autoimmune responses against pancreatic islets of Langerhans has not been demonstrated. Cryoglobulinemic syndrome during chronic HCV infection is the most extensively studied autoimmune disease and is the only autoimmune manifestation with a clear relation to a viral trigger. In HIV infection, immune system dysregulation is the primary cause of autoimmune disorders. The partial restoration of the immune system after the introduction of antiretroviral therapy could also play a role in the development of autoimmune diseases. In conclusion, a causative role of RNA viruses in the development of major autoimmune conditions has not yet been demonstrated.
Colored Plate “Take-Home Message”
-
HIV infection can underlie autoimmune diseases.
-
Autoimmune diseases occur in HIV-infected people, most often in a context of good immunological control (except essentially for autoimmune hemolytic anemia) or during IRIS (vasculitis, sarcoidosis, thyroid diseases).
-
By improving immune status, cART might favor autoimmune disease onset.
-
When necessary, immunosuppressant treatments may be used in this context with good tolerance.
-
A close link has been described between Enterovirus and autoimmunity, in particular regarding type 1 diabetes.
-
Chronic autoimmune liver disease may follow acute hepatitis A infection.
-
As a consequence of its lymphotropic nature, hepatitis C virus can trigger and sustain a clonal B-cell expansion which causes a wide spectrum of autoimmune/lymphoproliferative disorders, through a multistep process.
Abbreviations
- AHA:
-
Autoimmune hemolytic anemia
- AIDS:
-
Acquired immunodeficiency syndrome
- ANA:
-
Antinuclear antibody
- Anti-LKM:
-
Anti-liver/kidney microsomal antibodies
- CA:
-
Coxsackie A viruses
- cART:
-
Combined antiretroviral therapy
- CB:
-
Coxsackie B viruses
- CD4+ T cells:
-
Helper T lymphocytes
- CV:
-
Cryoglobulinemic vasculitis
- DCM:
-
Dilated cardiomyopathy
- DM:
-
Dermatomyositis
- GAD65:
-
Glutamic acid decarboxylase autoantigen
- MGN:
-
Membranoproliferative glomerulonephritis
- HAV:
-
Hepatitis A virus
- HCV:
-
Hepatitis C virus
- HIV-1:
-
Human immunodeficiency virus type 1
- IRIS:
-
Immune restoration inflammatory syndrome
- PM:
-
Polymyositis
- PsA:
-
Psoriatic arthritis
- RA:
-
Rheumatoid arthritis
- RNA:
-
Ribonucleic acid
- SLE:
-
Systemic lupus erythematosus
- T1D:
-
Type 1 diabetes
- TCR:
-
T-cell receptor
References
Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73(3):1941–8.
Vreugdenhil GR, Geluk A, Ottenhoff TH, Melchers WJ, Roep BO, Galama JM. Molecular mimicry in diabetes mellitus: the homologous domain in coxsackie B virus protein 2C and islet autoantigen GAD65 is highly conserved in the coxsackie B-like enteroviruses and binds to the diabetes associated HLA-DR3 molecule. Diabetologia. 1998;41(1):40–6.
Massilamany C, Koenig A, Reddy J, Huber S, Buskiewicz I. Autoimmunity in picornavirus infections. Curr Opin Virol. 2016;16:8–14.
Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1):a007682.
Jaïdane H, Hober D. Role of coxsackievirus B4 in the pathogenesis of type 1 diabetes. Diabetes Metab. 2008;34(6 Pt 1):537–48.
Huber SA. Viral myocarditis and dilated cardiomyopathy: etiology and pathogenesis. Curr Pharm Des. 2016;22(4):408–26.
Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114.
Rose NR. Myocarditis: infection versus autoimmunity. J Clin Immunol. 2009;29(6):730–7.
Trepo C, Zoulim F, Pradat P. Viral hepatitis. Curr Opin Infect Dis. 1999;12:481–90.
Shalit M, Wollner S, Levo Y. Cryoglobulinemia in acute type-A hepatitis. Clin Exp Immunol. 1982;47:613–6.
Vento S, Garofano T, Di Perri G, et al. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991;337:1183–7.
Singh G, Palaniappan S, Rotimi O, Hamlin PJ. Autoimmune hepatitis triggered by hepatitis A. Gut. 2007;56:304.
Cuthbert JA, Hepatitis A. Old and new. Clin Microbiol Rev. 2001;14(1):38–58. Review. Erratum in: Clin Microbiol Rev 2001;14(3):642.
Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin N Am. 2015;44(4):717–34. https://doi.org/10.1016/j.gtc.2015.07.003.
McMurray RW, Elbourne K. Hepatitis C virus infection and autoimmunity. Semin Arthritis Rheum. 1997;26(4):689–701.
Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76(8):818–24.
Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46(Suppl 5):S165–73.
Paroli M, Iannucci G, Accapezzato D. Hepatitis C virus infection and autoimmune diseases. Int J Gen Med. 2012;5:903–7.
Jadali Z, Alavian SM. Autoimmune diseases co-existing with hepatitis C virus infection. Iran J Allergy Asthma Immunol. 2010;9(4):191–206.
Zuckerman E, Yeshurun D, Rosner I. Management of hepatitis C virus-related arthritis. BioDrugs. 2001;15(9):573–84.
Winchester R, Bernstein DH, Fisher HD, et al. The co-occurrence of Reiter’s syndrome and acquired immunodeficiency. Ann Intern Med. 1987;106:19–26.
Fox C, Walker-Bone K. Evolving spectrum of HIV-associated rheumatic syndromes. Best Pract Res Clin Rheumatol. 2015;29(2):244–58. https://doi.org/10.1016/j.berh.2015.04.019.
Marquez J, Restrepo CS, Candia L, et al. Human immunodeficiency virus-associated rheumatic disorders in the HAART era. J Rheumatol. 2004;31:741–6.
Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9.
Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. J Int AIDS Soc. 2014;17(1):18719.
Oldstone MB, et al. Cell. 1987;50(6):819–20. Erratum in Cell 1987;51(5):878.
Medina-Rodriguez F, Guzman C, Jara LJ, et al. Rheumatic manifestations in human immunodeficiency virus positive and negative individuals: a study of 2 populations with similar risk factors. J Rheumatol. 1993;20:1880–4.
Berman A, Espinoza LR, Diaz JD, et al. Rheumatic manifestations of human immunodeficiency virus infection. Am J Med. 1988;85:59–64.
Kole AK, Roy R, Kole DC. Musculoskeletal and rheumatological disorders in HIV infection: experience in a tertiary referral center. Indian J Sex Transm Dis. 2013;34(2):107–12.
Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622–32.
Achuthan K, Uppal SS. Rheumatological manifestations in 102 cases of HIV infection. J Ind Rheum Ass. 1996;3:43–7.
Bijlsma JWJ, Derksen RHWM, Huber-Brunning O, Borleffs JCC. Does AIDS ‘cure’ rheumatoid arthritis? Ann Rheum Dis. 1988;47:350–1.
Rigante D, Mazzoni MB, Esposito S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev. 2014;13:96–102.
Martinez V, Diemert MC, Braibant M, ALT ANRS CO15 Study Group, et al. Anticardiolipin antibodies in HIV infection are independently associated with antibodies to the membrane proximal external region of gp41 and with cell-associated HIV DNA and immune activation. Clin Infect Dis. 2009;48(1):123–32. https://doi.org/10.1086/595013.
Itescu S, Brancato LJ, Winchester R. A sicca syndrome in HIV infection: association with HLA-DR5 and CD8 lymphocytosis. Lancet. 1989;2(8661):466–8.
Basu D, Williams F, Ahn C, Reveille J. Changing spectrum of the diffuse infiltrative lymphocytosis syndrome. Arthritis Rheum. 2006;55(3):466–72.
Guillevin L. Vasculitides in the context of HIV infection. AIDS. 2008;22(Suppl. 3):S27–33.
Galli M, Musicco M, Gervasoni C, et al. No evidence of a higher risk of progression to AIDS in patients with HIV-1-related severe thrombocytopenia. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(3):268–75.
Franzetti M, Adorni F, Oreni L, et al. Changes in the incidence of severe thrombocytopenia and its predisposing conditions in HIV-infected patients since the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;67(5):493–8. https://doi.org/10.1097/QAI.0000000000000347.
Ambler KL, Vickars LM, Leger CS, et al. Clinical features, treatment, and outcome of HIV-associated immune thrombocytopenia in the HAART era. Adv Hematol. 2012;2012:910954. https://doi.org/10.1155/2012/910954.
Lenner R, Bregman Z, Teirstein AS, DePalo L. Recurrent pulmonary sarcoidosis in HIV-infected patients receiving highly active antiretroviral therapy. Chest. 2001;119(3):978–81.
Chen F, Day SL, Metcalfe RA, Sethi G, Kapembwa MS, Brook MG, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine (Baltimore). 2005;84(2):98–106.
Murunga E, Andersson M, Rensburg C. Autoimmune hepatitis: manifestation of immune reconstitution inflammatory syndrome in HIV infected patients? Scand J Gastroenterol. 2016;51(7):814–8. https://doi.org/10.3109/00365521.2016.1157888.
Maurer TA, Zackheim HS, Tuffanelli L, Berger TG. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372–5.
Sperber K, Kalb TH, Stecher VJ, Banerjee R, Mayer L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res Hum Retrovir. 1993;9:91–8.
Castillo JJ, Echenique IA. Rituximab in combination with chemotherapy versus chemotherapy alone in HIV associated non-Hodgkin lymphoma: a pooled analysis of 15 prospective studies. Am J Hematol. 2012;87:330–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Galli, M., Bozzi, G., Giacomelli, A. (2018). RNA Viruses and Autoimmunity: A Short Overview. In: Ragab, G., Atkinson, T., Stoll, M. (eds) The Microbiome in Rheumatic Diseases and Infection. Springer, Cham. https://doi.org/10.1007/978-3-319-79026-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-79026-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-79025-1
Online ISBN: 978-3-319-79026-8
eBook Packages: MedicineMedicine (R0)