Abstract
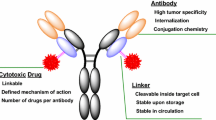
Antibody-drug conjugates (ADCs) have been used for more than two decades as tools for the selective delivery of cytotoxic agents to the tumor site, with the aim to increase anti-cancer activity and spare normal tissues from undesired toxicity. Until recently, most ADC development activities have focused on the use of monoclonal antibodies, capable of selective binding and internalization into the target tumor cells. However, in principle, it would be conceivable to develop non-internalizing ADC products, which liberate their toxic payload in the extracellular environment. In this Chapter, we review previous work performed on non-internalizing ADC products, with a special emphasis on drug conjugates which selectively localize to the modified extracellular matrix in the neoplastic mass.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Conventional pharmacological approaches for the chemotherapy of cancer are mostly based on the administration of cytotoxic agents, which promote cell death by blocking biological pathways that are essential for cell proliferation. The efficacy of this class of antitumor agents is often limited by their inability to preferentially accumulate at the tumor site, as demonstrated both in preclinical biodistribution studies and in positron-emission tomography (PET) imaging of cancer patients [1]. Drug accumulation in healthy tissues may give rise to side effects, which prevent dose escalation to therapeutically active regimens. For this reason, the covalent conjugation of potent cytotoxic payloads to suitable vehicles (e.g., antibodies), capable of binding to tumor-associated antigens (e.g., receptors or other proteins that are overexpressed at the site of disease), has been proposed as a general strategy to improve the therapeutic index of cancer chemotherapy [2].
ADC products result from the conjugation of a cytotoxic agent and a monoclonal antibody (mAb), using a suitable linker. Most of the antibodies that have been used for ADC development display insufficient anti-tumor activity, when administered as “naked” immunoglobulins [3]. On the other hand, the role of the mAb moiety in ADC products mainly consists in the selective delivery of a cytotoxic compound at the tumor site, where the latter is released and acts on cellular targets causing direct damage. According to this mechanism of action, ADCs can be considered as pro-drugs, for which the release of the cargo is of fundamental importance for therapeutic activity.
While ADC products specific to internalizing receptors have shown encouraging clinical responses in patients bearing non-solid tumors, the therapeutic activity against the most frequent solid malignancies (e.g., tumors of breast, lung and colon) is still far from optimal [4]. The emerging clinical results are often less favorable than the preclinical data obtained in tumor-bearing mice, where several internalizing ADC products have led to cancer cures. The higher permeability of interstitial tissues in mice xenografts, compared to solid malignancies in human patients, may partially account for this observed discrepancy [5]. A suboptimal penetration of ADC products within the tumor mass may result in an insufficient delivery of suitable payload concentrations. The limited diffusion properties of monoclonal antibodies emerged from immunofluorescence detection studies, which revealed the striking accumulation of mAbs in IgG formats on perivascular tumor cells, with a substantial inability to penetrate the tumor mass and to reach the majority of neoplastic cells [6].
Since the birth of the ADC technology, it has commonly been assumed that the mAb should preferably be directed against tumor-associated antigens expressed on the surface of cancer cells. Ideally, the ADC would internalize upon binding to its cognate target, thus facilitating the delivery and release of the cytotoxic cargo inside the malignant cell. This receptor-mediated endocytosis represents the most exploited mechanism for ADC activation, as discussed extensively in different reviews [7].
In principle, it is conceivable that also ADC products, based on internalizing antibodies, may display at least part of their activity through drug release in the extracellular space. The internalization efficiency is typically variable and rarely reaches 100%. Furthermore, while antibody internalization can be easily studied in vitro, an in vivo characterization of the process is hindered by many technical limitations, associated with the processing of the tumor mass and with the specific detection of individual antibody, linker and payload components. As a result, the need to use internalizing mAbs for ADC development has recently been questioned and the availability of novel antibodies, with exquisite tumor-targeting properties, has prompted the investigation of ADC products based on non-internalizing ligands.
In the following sections, the development and in vivo testing of non-internalizing ADC products is described, with a special focus on molecules targeting the modified extracellular matrix within tumor lesions.
Non-internalizing ADC Products: Mechanism of Action
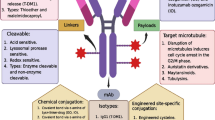
As an alternative to the traditional receptor-mediated endocytic process, the drug release from tumor-targeting devices could ideally take place in the tumor microenvironment, allowing the subsequent diffusion of the active payload and its internalization into neighboring neoplastic cells. Since passive diffusion is a non-specific process, the cytotoxic agent has the potential to reach antigen-negative cancerous cells within the tumor mass (a mechanism often referred to as “bystander effect”). In principle, non-internalizing ADCs may display potent therapeutic activity against tumors with high mutation rates or characterized by antigen loss, where certain cell populations can develop resistance to conventional internalizing ADCs. Potentially, the bystander killing effect could also impair structures which support tumor growth, such as stromal cells, leukocytes and tumor blood vessels, thus enhancing the anti-tumor effect of the product [8].
Ideally, this alternative strategy could be potentially pursued using monoclonal antibodies specific to both tumor-specific extracellular structures and poorly/non-internalizing transmembrane antigens. However, due to the identical localization of the target protein, mAbs targeting non-internalizing transmembrane receptors would show similar features in terms of tumor accumulation, as compared to antibodies specific to internalizing antigens. Together with the mAb development and the choice of a suitable payload, the design of a proper linker is crucially important for the generation of efficacious and well-tolerated ADCs. While both cleavable and non-cleavable linkers have found application in internalizing ADC products, only cleavable bonds have been so far used as linkers of choice, for the development of non-internalizing drug delivery systems. This can be easily explained by the intrinsic nature of the endocytic process, which leads to the proteolytic degradation of the antibody structure in the intracellular compartments, followed by the release of an active drug metabolite. Various cleavable linkers have been proposed for the preferential drug release in the tumor interstitium. A main requirement to prevent premature drug release and the related side effects is a high linker stability in plasma, after ADC administration. Provided that a sufficient amount of the ADC reaches intact the tumor microenvironment, a second key attribute of the linker is the ability to efficiently release the payload at the tumor site. Glutathione (GSH) represents the most abundant thiol and reducing agent in the intracellular space, both in normal cells and in tumors, which often contain higher concentrations of this species [9, 10]. While disulfide-based linkers have been designed for the intracellular release of anti-cancer drugs, the same chemical structures can be considered for the extracellular drug release, as a consequence of tumor cell death and increased GSH concentration. Disulfides are typically stable in the absence of free thiols at physiological pH, with a serum half-life that can be longer than 1 week. In vivo, certain disulfide-based ADCs have exhibited stability in blood for 2–4 days. Moreover, this stability can be dramatically improved by increasing the steric hindrance of substituents at the cleavage site [11]. Several ADCs and small molecule-targeted cytotoxics that incorporate reducible linker systems such as disulfide bridges have been considered for clinical development [2, 12]. Most of these new products have been designed to release the payload through receptor-mediated endocytosis. However, it is conceivable to assume that the tumor environment in vivo is a more complex scenario, in which dying cells are constantly releasing reducing agents to the surrounding areas. Therefore, non-internalizing ADC products based on disulfide bonds can potentially be cleaved in the tumor extracellular milieu, releasing the payload and promote apoptosis in cancer cells. The release of GSH to the extracellular environment may generate a self-amplifying cycle of cell death and subsequent drug release (Fig. 1).
In addition to disulfides, certain peptide sequences have been used as linkers for the generation of ADC products. These functional groups combine a high systemic stability with a rapid release of the drug at the site of disease. Indeed, proteolytic enzymes such as cathepsin B, urokinase-type plasminogen activator and matrix metalloproteinases (MMPs), which are involved in cancer progression features like angiogenesis, invasion and metastasis [13], may be over-expressed at the tumor site, both in intra- and extra-cellular compartments [14]. In particular, the Valine-Citrulline (Val-Cit) dipeptide had shown promising features for the development of internalizing ADCs. This line of research led to the use of a Val-Cit-containing linker in the marketed Adcetris™ product and in other clinical-stage candidates [15, 16]. Similarly to the cleavage of disulfide bonds, proteolytically-cleavable linkers could be exploited also for the release of drugs in the extracellular tumor microenvironment. Indeed, the protease-mediated release of payloads from non-internalizing ADCs can be amplified by tumor cell death, which sheds a large number of proteolytic activities into the cancer microenvironment (Fig. 1).
Non-internalizing ADC Products: Early Evidence of Biological Activity
Studies on non-internalizing (or poorly internalizing) ADC products have been performed both against targets expressed on the cell membrane (such as CD20, CD21, CAIX and FAP) and against components of the modified extracellular matrix in the neoplastic mass (e.g., splice variants of fibronectin and tenascin-C, fibrin and collagen IV). CD20 and CD21 are well-known cell-surface markers of B-cell derived non-Hodgkin’s Lymphomas (NHLs) and have been intensively investigated as antigens for ADC products [17, 18]. NHLs have been extensively studied as targets for ADC development, providing insights into the mechanism of action, the anti-tumor potential and limiting toxicities. NHLs are often successfully treated with a combination of chemotherapeutic agents and antibody-based products [19]. However, there is a need for improved medications, especially for patients who relapse from previous pharmacological interventions. CD19 and CD22 have been described as internalizing NHL antigens, while anti-CD20 and anti-CD21 antibodies typically remain on the membrane of B cells and lymphoma cells [20]. Polson and coworkers generated ADCs against different NHL antigens (i.e. CD19, CD20, CD21, CD22, CD72, CD79b, and CD180), in which potent anti-tubulin agents (DM1 or MMAE) were conjugated to the parental antibodies through either cleavable (disulfide or peptide bonds) or non-cleavable linkers [17]. Therapy experiments performed in tumor-bearing mice showed that all ADCs featuring cleavable linkers (i.e., both products based on internalizing and on non-internalizing antibodies) exhibited a therapeutic effect in vivo. By contrast, when non-cleavable linkers were used, only the products directed against internalizing antigens showed a therapeutic activity. Similar results were reported for an anti-CD20 antibody coupled to calicheamicin through both cleavable and non-cleavable linkers [21]. These observations are compatible with the assumption that the cleavable linker is processed in the tumor extracellular space after ADC localization. Subsequently, the drug may diffuse through the cell membrane, reaching its biochemical target. This mechanism of action was reinforced by the observation that the substitution of MMAE with its charged analogue MMAF (i.e., membrane-impermeable) led to a lower antitumor activity, for ADC products directed against non-internalizing antigens [22].
Investigated as tumor marker for the development of targeted cytotoxics, Carbonic Anhydrase IX (CAIX) has long been considered to be an internalizing antigen. However, recent studies have clearly shown that CAIX displays extremely poor internalization properties and resides virtually exclusively on the cell membrane. Carbonic Anhydrases are metalloenzymes that can be found in most of living organisms, where they catalyze the hydration of carbon dioxide to bicarbonate. Among all the CA isoforms, CAIX (formerly referred to as MN antigen) is a transmembrane homodimeric enzyme overexpressed in more than 90% of clear cell renal cell carcinoma (ccRCC) subtypes [23]. In addition, CAIX is one of the best markers of hypoxia and, as such, can be found in many tumor types, especially those characterized by a low oxygen concentration [24]. The pattern of expression of CAIX in healthy organs is limited on the first portion of the gastro-intestinal tract (e.g., stomach, duodenum and gallbladder) [25], These encouraging immunohistochemical results stimulated the investigation of CAIX as a target for ADC products. Although in the early development anti-CAIX therapeutics were designed to be internalized by tumor cells, our group recently reported the inefficient internalization of the protein upon ligand binding [26, 27]. Petrul and coworkers explored the conjugation of an anti-CAIX mAb to MMAE, through the cleavable Val-Cit linker, to generate the ADC BAY 79–4620 [28]. The group demonstrated the selective affinity of this product to the CAIX isoform and its ability selectively kill CAIX-positive cancer cells in vitro, by tubulin disruption. BAY 79–4620 was also shown to be effective in vivo in mice grafted with HT-29 and Colo205 colorectal tumors or with cervix carcinoma HeLa-MaTu tumors, at doses between 5 and 10 mg/kg. A modest anticancer activity was reported against other cancer models, albeit at higher doses (30–60 mg/kg). However, while free MMAE (0.2 mg/kg, equivalent to 10 mg/kg of ADC) was less effective than BAY 79–4620, the efficacy of paclitaxel administered at the dose of 15 mg/kg was comparable to the one described for the ADC. In 2014, BAY 79–4620 entered a phase I dose-escalation clinical study with 12 patients, bearing histologically or cytologically confirmed solid tumors [29]. The product was administered at doses ranging from 0.3 to 4.6 mg/kg. While no complete or partial response were reported, treatment-related side effect occurred in the majority of patients and the highest dose led to patient death due to cardiac arrest and pancreatitis. This tragic event underlined the importance of an accurate preliminary evaluation of the antigen expression in patients, since data of CAIX expression in the studied tumors were available for only 50% of patients, among whom only 2 showed more than 10% antigen-positive cells in the tumor mass.
Antigens that localize in the tumor microenvironment, or on the surface of stromal cells have also been studied as targets for non-internalizing ADCs. The extravascular deposition of fibrin has been described in different human solid tumors as a consequence of the disruption of vascular barriers, which allows the extravasation of fibrinogen and other substrates of the coagulation cascade [30]. Indeed, after tumor transplantation in animal models of cancer, fibrin deposition is one of the first morphological changes that can be observed [31]. While in wound healing processes fibrin is progressively replaced by collagen fibers in few weeks, fibrin clot formation persists in cancer until living tumor cells are present [32]. Yasunaga and coworkers exploited this tumor-specific pathophysiological feature to develop the first fibrin-specific ADC [33]. This immunotoxin comprised a chimeric IgG1 mAb coupled to the active metabolite of Irinotecan (SN-38) as payload. Cysteine residues of the immunoglobulin were coupled to dendrimeric structures bearing 3 SN-38 molecules, individually bound to a PEG spacer via ester linkers. Such a complex design allowed a heavy functionalization of the mAb scaffold, achieving a drug/antibody ratio (DAR) of approximately 24. The resulting anti-fibrin immunoconjugate was stable at acidic pH values, but released gradually and effectively SN-38 at physiological pH in saline buffer and in mouse serum. As expected, this so-called AFCA-branched-PEG-(SN-38)3 ADC acted as a pro-drug in vitro, displaying no substantial direct activity against tumor cells. By contrast, four injections per week of the product into tumor-bearing mice at a dose of 13.3 mg/kg were able to suppress tumor growth for more than 1 month. This potent anti-tumor activity was remarkable, especially when compared to the administration of Irinotecan (injected daily at the MTD) which was largely inefficacious. A long-term observation of side-effects revealed that the ADC product was well tolerated in mice, with no signs of bone marrow, liver or kidney dysfunction at the recommended dose. Immunohistochemistry and in vivo fluorescence endomicroscopy indicated that the antitumoral activity was mainly due to tumor vessel disruption.
The same group working on anti-fibrin ADCs also reported activity for products directed against murine collagen IV [34]. The naked antibody was coupled to eight molar equivalents of SN-38 cytotoxic agent via a PEG spacer and a cleavable ester linker. The group compared the anti-collagen-4 conjugate and another ADC product, targeting the EpCAM, an antigen expressed on the cancer cell membrane. The comparative evaluation of the two products highlighted the potential of ADCs targeting the tumor stroma to localize and efficiently release their toxic cargo within the tumor microenvironment. Indeed, some cell-targeting products are hindered by stromal barriers, preventing access to the antigen on the cell membrane. When comparing the two products, the anti-collagen IV ADC was found to be superior in two different EpCAM-positive murine models of carcinoma. Since the treatment with the collagen-targeted ADC resulted in a higher SN-38 concentration in the tumor and with the death of vascular endothelial cells, the authors concluded that the uneven distribution of the anti-EpCAM product within the tumor mass may have led to an inferior performance and lower efficacy.
The work by Yasunaga and colleagues highlighted the potential of stromal-targeting ADCs. Metastasis and tumor invasion are usually linked to adaptation of mesenchyme-derived stromal cells (fibroblasts, myofibroblasts, endothelial cells, pericytes, smooth muscle, and hematopoietic cells) of the neighboring healthy organs [35, 36]. Fibroblasts respond to cancer progression producing Fibroblast Activation Protein α (FAPα), a serine protease involved in tissue remodeling and wound healing [37]. This antigen has been initially proposed as a possible target for cancer therapy with unconjugated antibodies in colorectal cancer patients. Sibrotuzumab, a humanized anti-FAPα antibody, was found to be well tolerated and to exhibit a selective tumor uptake 24–48 h after i.v. administration. No anticancer activity, however, was detected in patients [38]. Ostermann and colleagues conjugated an anti-FAPα mAb to different maytansinoid payloads using both cleavable and non-cleavable linkers [39]. The internalizing behavior of the FAPα antigen was demonstrated by cell antiproliferative assays in vitro, where all the ADC products, including the ones featuring non-cleavable linkers, were found to be active against FAPα-transfected cells. However, the in vivo administration of the ADCs in mice bearing a panel of FAPα-positive tumors (i.e., pancreatic, a non-small cell lung, a head and neck squamous cell and a colorectal carcinoma) revealed that cleavable linkers are required to induce a potent anticancer effect. These data suggested that the efficacy of the anti-FAP ADC products was due to a bystander effect, associated with the diffusion of the active payload within the tumor microenvironment. In line with this proposed mechanism of action, histological analysis and biomarker studies identified the death of malignant cells surrounding stromal cells as an early therapeutic event.
Another example of a tumor-associated antigen expressed on fibroblasts in the tumor stromal environment is LRRC15 (i.e., leucine rich repeat containing 15). Also known as Lib, this protein is a transmembrane member of the leucine-rich repeat superfamily, which are involved in cell-cell and cell-ECM interactions. Showing weak expression in healthy tissues, LLRC15 was initially detected in astrocytes in response to pro-inflammatory cytokines [40]. It was then found to be frequently overexpressed in many solid tumors, such as aggressive breast cancer [41] and prostate tumors [42]. The anti-LRRC15 ADC product ABBV-085, based on the linker-toxin combination ValCit-MMAE, has recently entered Phase I clinical trial, after showing promising activity against different murine tumor models, administered both as single agent and in combination with chemotherapy, radiation or checkpoint inhibitors [43].
In general, it is technically challenging to quantify internalization rates in vivo, even for products directed against cell surface targets. While the examples reviewed below relate to non-internalizing ADC targets (e.g., extracellular matrix antigens), it is reasonable to assume that a substantial portion of putative internalizing ADCs may not reach the corresponding intracellular compartments in vivo as intact conjugates.
Targeting the Modified Extracellular Matrix in Tumors
Among the clinically-validated tumor markers, specific isoforms of ECM proteins represent ideal targets for biopharmaceutical intervention. The generation of these tumor-specific proteins can be considered as an end-product of the abnormal proliferative rates of cancer cells, which is not only sustained by several defections in fundamental inhibitory functions of neoplastic cells (e.g. contact inhibition, apoptosis, autophagy, cellular homeostasis) but it is also favored by substantial alterations of the extracellular environment [44]. In particular, the high proliferation rate and the irregular vascularization of a fast-growing tumor mass lead to inadequate oxygen supply to the tumor tissue. It is now well established that cancer cells modify their metabolism to adapt to hypoxia: cellular respiration runs under anaerobic conditions, causing high glucose consumption and production of large quantities of respiratory end-products (i.e. CO2 and H+-lactate) [45]. The latter are released in the extracellular environment, resulting in a substantial acidification of the tumor interstitium (the pH can shift from the usual values of 6.5–7.0 to values as low as 6.0). While this phenomenon may lead to apoptosis in normal cells, it acts as a Darwinian selection process for cancer cells, which eventually develop resistance to the altered environmental conditions. For instance, the enzymes carbonic anhydrase IX and XII are over-expressed in many tumors to catalyze the CO2 hydration in the extracellular environment. This process minimizes the passive diffusion of CO2 through the membrane, thus allowing the cell to maintain a slightly alkaline intracellular pH (pH 7.2–7.4), which results from increased metabolism and supports cell proliferation [46].
Anomalies in pH values at both side of the cell membrane have been associated with the expression of proteins in mutated isoforms, generated by alternative splicing of their primary RNA transcript. Although the latter is a fundamental process in many physiological functions (e.g. in tissue and organ development) [47], the understanding of alternative splicing in cancer is a field of growing interest in oncology, to such an extent that aberrant alternative splicing is now commonly included in the list of the hallmarks of cancer [48, 49]. Alternative splicing events can generate protein isoforms that help tumors to acquire therapeutic resistance. Moreover, protein splice variants have been associated with particular diagnostic and prognostic features for certain types of cancers, even though their functional/mechanistic role is often not understood [50]. For instance, acidification of the tumor microenvironment have been shown to influence the alternative splicing of vascular endothelial growth factor (VEGF-A) in by endometrial cancer cells [51]. The produced isoforms are known to activate signaling pathways that stimulate tumor progression (e.g. angiogenesis and metastasis) and thus represent a mechanism of tumor cells adaptation to the acidic stress.
A basic intracellular pH may lead to the modulation of splice variants for certain extracellular matrix (ECM) components, such as fibronectin and tenascin C [52, 53]. Fibronectins (FNs) are glycoproteins, which are present either in soluble form in plasma and other body fluids or in cellular form in the ECM and basement membranes of tissues. Acting as a bridge between the cell surface and the extracellular material, FN is involved in various cell-ECM interactions, such as adhesion, cell migration, hemostasis, thrombosis and wound healing [54]. FN is secreted from cells as a dimer consisting of two 250 kDa subunits covalently linked by two disulfide bonds near their C-termini. Although FN is encoded by a single gene, it exists in multiple isoforms which result from the combination of three alternatively-spliced domains: EDA, EDB and IIICS (see Fig. 2) [55]. While EDA and EDB show constant structures, composed respectively by 90 and 91 amino acids, the extra domain IIICS can be expressed in multiple variants in humans, ranging from 64 to 120 amino acids [56]. The group of Luciano Zardi firstly reported the over-expression of FN extra-domains in tumor-derived or SM40-transformed human cells, compared to normal human fibroblasts [57].
This discovery stimulated an intense research activity around FN splice variants, aimed at understanding their expression pattern and pathological role. In particular, the EDB of FN was found to be virtually absent in all normal adult tissues, but abundantly expressed in the proximity of angiogenic blood vessels and in the stroma of various types of aggressive tumors, including brain, lung, skin, kidney and bladder [58, 59]. Similarly to EDB, the EDA domain was found to be expressed in subendothelial ECM of proliferating tumors, while being undetectable in human plasma and healthy tissues [60]. The singular expression profile of EDA and EDB led to the identification of these markers of angiogenesis as “oncofetal” domains of fibronectin [61, 62].
Tenascin C (TnC) is another cell-binding, large oligomeric glycoprotein of the ECM, composed by 240 kDa subunits that assembly in oligomers (mainly hexamers) through disulfide bonds [63]. A functional antagonism between TnC and FN have emerged from different observations: (i) TnC shows poor binding affinity of to ECM components (FN, collagen, laminin), thus supporting only a weak cell attachment to ECM; (ii) TnC promotes cell rounding and detachment, whereas FN promotes cell-substrate adhesion; (iii) FN is ubiquitously distributed while TnC expression is restricted to morphogenesis and remodeling events [64]. Two main human TnC isoforms are generated by alternative splicing of the single TnC primary mRNA, resulting in the inclusion (or omission) of eight extra domains (Fig. 2) in the final transcript. The expression of these two isoforms was proposed to be dependent to intracellular pH, as a result of adaptation to environmental conditions. In particular, while TnC alternative splicing in normal cultured fibroblasts showed a sensitivity towards small variation of extracellular pH [65], malignantly-transformed cells mainly expressed the large TnC variant (i.e., bearing the 8 extra domains). This observation was explained by the ability of malignant cells to maintain a basic intracellular pH even in an acidic environment, which promotes the alternative splicing event [53].
The abundant and tumor-specific expression of oncofetal FN and TnC stimulated the investigation of these proteins as ideal targets for biomolecular intervention. For instance, 131I-labeled murine and chimeric antibodies specific to A1 and D domains of TnC have been evaluated in the clinic for the treatment of glioma and lymphoma [66, 67]. Moreover, the human recombinant antibodies L19 and F16 were generated upon selections of a phage display library against the EDB and A1 antigens [68, 69]. The two antibodies have been produced in different formats (scFv, diabody, SIP, IgG) and their tumor-targeting properties were studied by quantitative biodistribution analysis, revealing promising in vivo tumor targeting performances [69, 70]. Importantly, quantitative biodistribution data are available for L19 and F16 both in mice and in man. The 131I-L19 and 131I-F16 antibodies in SIP format have been evaluated for radio-immunotherapy applications in patients bearing Hodgkin’s lymphoma [71, 72] and head and neck cancer [73]. In addition to radiopharmaceutical applications, a variety of immunocytokines composed by the L19 and F16 antibodies fused with either interleukin 2 or TNF are currently evaluated in the clinic for the treatment of different solid tumors (i.e. melanoma, soft tissue sarcoma, diffuse large B-cell lymphoma, oligometastatic solid tumor, Merkel cell carcinoma, acute myeloid leukemia and non-small cell lung cancer) in combination with chemotherapy. Similarly to L19 and F16, also the F8 antibody (specific to the EDA domain of FN) may be considered as a delivery vehicle for pharmaceutical applications. F8 displayed encouraging tumor-targeting properties in mouse models and a characteristic ability to stain neo-vascular structures not only in aggressive solid tumors, but also of solid masses of hematological malignancies [74]. EDA is expressed not only in cancer, but also in other pathological conditions, characterized by extensive tissue remodeling. The observation of an intense and diffuse staining pattern of F8 in synovial tissue biopsies obtained from rheumatoid arthritis patients led to the development of the immunocytokine F8-IL10, which is currently evaluated in the clinic [75].
The L19, F8 and F16 antibodies, specific to non-internalizing ECM antigens, have been instrumental for the selective delivery of cytotoxic compounds to the tumor environment. Initial studies involved the functionalization of the anti-EDA F8 antibody with cemadotin, a tubulin inhibitor [76]. A thiol derivative of this dolastatin analogue, with low-nanomolar cytotoxic activity, was coupled in a site-specific manner to two C-terminal Cys residues of the F8 antibody in SIP format. The resulting ADC showed a drug-antibody ratio (DAR) of 2 and the disulfide linker displayed acceptable stability in mouse plasma (half-life of approximately 48 h). On the other hand, the ADC incubation with glutathione resulted in a fast and “traceless” release of the drug in its active thiol form. Therapy experiments performed in immunocompetent mice, subcutaneously grafted with F9 teratocarcinoma cells, showed a substantial tumor growth inhibition. Most probably, the disulfide linker can be cleaved by glutathione [77], which is released from apoptotic cells, promoting an exponential increase of the free payload concentration in the tumor environment. However, despite the high dose (43 mg/kg) and the frequent administration schedule, no complete responses were observed, suggesting that more potent cytotoxic payloads should be used. Indeed, the maytansinoid DM1 payload led to the generation of more potent ADC products based on the F8 antibody [78]. As for cemadotin, DM1 was connected to the SIP(F8) antibody through a cleavable disulfide linker and administered to immunocompetent mice bearing different cancer models (e.g., F9 teratocarcinomas and CT26 colon carcinoma). When administered in three doses of 7 mg/kg, the SIP(F8)-SS-DM1 ADC cured 60% of the treated mice bearing F9 tumors, but not mice bearing the CT26 carcinoma model. These data reflected the 100-fold higher in vitro cytotoxicity of free DM1 against F9 cells, as compared to the CT26 cell line. This correlation between the in vitro and in vivo observations suggested that the tumor cells, rather than the endothelial cells, may be the primary target for the activity of the ADC, despite the selective expression of the EDA antigen around tumor blood vessels.
Coupling of the DM1 payload to F8 did not alter biodistribution profiles when the antibody was used in IgG or SIP format. However, the stability of disulfide linkers was substantially longer for ADC products based on the IgG format [79]. The longer residence time of IgG(F8)-SS-DM1 in the tumor did not result in better anticancer properties. A comparative evaluation of IgG(F8)-SS-DM1 and SIP(F8)-SS-DM1, administered to tumor-bearing mice in equimolar doses, revealed a more potent anti-cancer activity for the ADC product in SIP format, even though the IgG product exhibited a slower clearance and a higher tumor accumulation. These data suggest that a suitable (i.e., not too slow) rate of drug release in the tumor environment may be beneficial, in order to expose malignant cells to sufficiently high concentrations of the cytotoxic agent.
The F16 antibody, specific to the A1 extra-domain of tenascin C, has been also investigated as vehicle for cytotoxic agents in both IgG and SIP format. In particular, the antibodies were equipped with the microtubule-disrupting agent monomethyl auristatin E (MMAE) and the protease-sensitive linker Val-Cit [80]. Also in this case, the IgG antibody showed higher absolute accumulation in three different tumor models (A431, U87 and MDA MB 231) as compared to its SIP counterpart. The latter product, however, displayed better tumor/organ ratios, as a result of an efficient tumor uptake combined with a rapid clearance from blood and normal tissues. The administration of IgG(F16)-Val-Cit-MMAE led to complete tumor eradication in mice, bearing either A431 or U87 human tumors. Mice treated with SIP(F16)-Val-Cit-MMAE experienced a significant and prolonged tumor regression, but tumors eventually started growing again. The different anticancer properties of the ADC products based on the two formats may be explained by considering that: (i) the highly stabile peptide linker is compatible with the long half-life in circulation of the IgG-formatted ADC; (ii) the IgG shows a higher absolute accumulation in the tumor than the SIP analogue (i.e., %ID/g of ca. 30 and 10 at 24 h, respectively) indicating that large quantities of payload are necessary to achieve complete response. More recently, other peptide linkers have been investigated for the delivery of MMAE from the IgG(F16) antibody, with the Val-Ala sequence showing similar anticancer activity and in vivo metabolic profile to the Val-Cit counterpart [81]. The Val-Cit-MMAE module represents the linker-payload combination used in the approved pharmaceutical product brentuximab vedotin (Adcetris™) and in many others ADCs which are currently in clinical development [16]. Historically, the Val-Cit peptide had been designed as a protease-sensitive linker for products based on internalizing antibodies [82]. The linker should be sufficiently stable in circulation, while being efficiently cleaved by certain intracellular proteases (in particular, cathepsin B) after receptor-mediated endocytosis. This mechanism is supported by in vitro cytotoxicity data, whereby only antigen-positive cell lines were efficiently killed by the cognate ADC product [83]. However, the evaluation of the F16-Val-Cit-MMAE product revealed that a more complex series of events may occur in vivo, involving an extracellular cleavage and release of the linker-payload combinations.
Improving the Potency and Selectivity of Non-internalizing ADC Products
All three moieties in ADC products (antibody, linker and payload) contribute to activity and selectivity. When non-internalizing antibodies are used, lipophilic payloads capable of rapid diffusion through the cell membrane may be preferred. While proteolytic degradation of the antibody moiety may be a release mechanism for internalizing products with non-cleavable linkers, this option does not apply for agents with long residence in the extracellular space [84]. Non-cleavable linkers have gained increasing research interest in the recent past, also in light of the approval of T-DM1 (Kadcyla™), a product that relies on this technology. The use of non-cleavable linkers is, in principle, attractive for very hydrophilic payloads, as one would expect to confine the cytotoxic agent either to the extracellular space (in which it would not be toxic) or to those cells capable of target-based antibody internalization. Unfortunately, in vitro experiments provide insufficient information regarding antigen accessibility and accumulation at the tumor site in vivo. Quantitative biodistribution experiments may be combined with other investigations (e.g., plasma stability and immunohistochemistry), in order to gain a detailed information regarding the mechanism of action of ADC products. Other structural innovations in the ADC field, such as the use of polymeric linkers, may result compatible with the use of non-internalizing mAbs. These highly functionalized linkers allow the macromolecule labelling with a large number of cytotoxic payloads (DAR > 10). While high DARs have often been associated to poor pharmacokinetic properties [85], the hydrophilic nature of these biodegradable polymers has shown favorable plasma PK profiles [86]. However, the potential immunogenicity of these highly functionalized structures may represent an important aspect during clinical investigations.
The use of ADC products may benefit from combination with immunostimulatory drugs. In the recent past, immune-mediated cancer treatment has become an important area of pharmaceutical oncology, thanks to the clinical advance of immunological checkpoint inhibitors, immunocytokines, bispecific antibodies, CAR T cells, vaccines and other products. There are different pathways that may lead to cancer cell death, upon exposure to different types of cytotoxic agents. Some drugs are particularly active for dendritic cell activation and in promoting immunogenic cell death. It is still not clear how tubulin drugs promote direct activation of dendritic cells. Anthracyclines and other DNA-targeting cytotoxics have been found to promote the expression of the so-called damage-associated molecular patterns (DAMPs) [87, 88]. When certain markers (e.g., calreticulin, HMGB1, ATP and type I interferon) are released into the extracellular environment by dying cells, they may stimulate dendritic cell maturation and activation, leading to an increased infiltration of CD8+ T cells into the tumor mass, followed by cytotoxic activity. In many instances, immunological check-point inhibitors (e.g., anti-PD-1 antibodies) are used in patients that had progressed after treatment with conventional anti-cancer drugs, but use of pembrolizumab or nivolumab in first line is becoming more and more frequent [89, 90]. The use of ADC products in combination with certain immunotherapeutic agents can lead to synergistic activity, as damage to cancer cells may result in improved antigen presentation (with subsequent recognition by CD8+ T cells) or surface expression of proteins such as MIC-A, which trigger NK cell activation through NKG2D receptors [91, 92]. Specifically, it would be interesting to understand whether non-internalizing ADC products could give significant advantages over internalizing analogues in enhancing the activity of the immunotherapeutic partner. Indeed, considering the more widespread cytotoxic action that non-internalizing ADCs could promote in the tumor microenvironment, a more heterogeneous area of the solid mass could efficiently lead to inflammation and to an increase of the population of infiltrating lymphocytes in the tumor mass. It is now becoming increasingly evident that this process, often described as the conversion of “immunologically cold” tumors into “hot”, is a key parameter to extend the efficacy of immunotherapy to a larger number of patients and indications [93].
Conclusions and Outlook
The possibility to develop non-internalizing ADCs is, by now, firmly established, at least at the preclinical level. Splice isoforms of tenascin-C and of fibronectin represent ideal targets for pharmacodelivery applications, but it is possible that other tumor-associated antigens may be considered [94]. ECM components offer unique opportunities for pharmacodelivery applications, as these targets are often abundant and stable, thus allowing a long residence time of ADC products at the tumor site. The field of ADC research, both for internalizing and non-internalizing products, will continue to face an important scientific challenge, namely the translation of preclinical data into a prediction of efficacy in patients.
In this context, the therapeutic widows of marketed ADC products were found to be much smaller in human patients than in rodents. For instance, while early clinical studies of MMAE-based ADCs reported MTD values between 1.2 and 2.4 mg/kg [95], administrations of Tenfold higher doses are commonly well tolerated in mice. This important aspect is due to several factors (e.g., the different tumor size in mice and humans, the number of antigen copies in the tumor and their accessibility by ADCs, etc.) and it limits the progression of promising ADC candidates through the clinical stages. The pharmaceutical relevance of this “bottleneck” is reflected in the fact that only two ADC products are currently available on the market, whereas more than 40 ADC candidates are currently being investigated in clinical trials [96]. The use of tumor-associated ECM proteins as targets for ADC development takes the internalization process out of the mechanism of action, thus potentially promoting an easier and more rational design of future ADC products.
One of the most challenging issues for future developments in the ADC field relates to the quantification of product uptake in mouse and man, as well as to the comparative evaluation of drug release kinetics in different species. In particular, a quantitative evaluation of the targeting properties of ADCs in human patients is often missing, which negatively impacts on the clinical development of drug candidates. In principle, initial information about antibody biodistribution, pharmacokinetics, tumor targeting properties and interpatient variability could be obtained from microdosing (phase 0) PET clinical studies [73]. However, these trials are normally performed with drug dosages that are substantially lower than the ones used for therapy purposes. A more systematic and accurate use of imaging techniques for the analysis of antibody performances in patients, as well as the real-time monitoring of ADC fragments at preclinical level (e.g. through the labeling of drug and antibody with different radioisotopes) [97], may provide important insights for the optimal pharmaceutical development of targeted cytotoxics.
In summary, while most academic and industrial efforts have so far been devoted to the development of internalizing ADC products, there is a strong rationale for the design and optimization of antibody-drug conjugates, which do not directly internalize into the target cells. ADC products directed against splice isoforms of fibronectin and tenascin-C are particularly attractive, as those targets are abundantly expressed in the majority of solid tumors and lymphomas, while being virtually undetectable in the majority of normal adult tissues.
References
(a) van der Veldt AA, Hendrikse NH, Smit EF, Mooijer MP, Rijnders AY, Gerritsen WR, et al (2010) Biodistribution and radiation dosimetry of 11C-labelled docetaxel in cancer patients. Eur J Nucl Med Mol Imaging 37:1950–1958; (b) van der Veldt AA, Lubberink M, Mathijssen RH, Loos WJ, Herder GJ, Greuter HN, et al (2013) Toward prediction of efficacy of chemotherapy: a proof of concept study in lung cancer patients using 11C-docetaxel and positron emission tomography. Clin Canc Res 19:4163–4173; (c) Kesner AL, Hsueh WA, Htet NL, Pio BS, Czernin J, Pegram MD, et al (2007) Biodistribution and predictive value of 18F-fluorocyclophosphamide in mice bearing human breast cancer xenografts. J Nucl Med 48:2021–2027; (d) Abe Y, Fukuda H, Ishiwata K, Yoshioka S, Yamada K, Endo S, et al (1983) Studies on 18F-labeled pyrimidines. Tumor uptakes of 18F-5-fluorouracil, 18F-5-fluorouridine, and 18F-5-fluorodeoxyuridine in animals. Eur J Nucl Med 8:258–261; (e) Kuchar M, Oliveira MC, Gano L, Santos I, Kniess T (2012) Radioiodinated sunitinib as a potential radiotracer for imaging angiogenesis-radiosynthesis and first radiopharmacological evaluation of 5-[125I]Iodo-sunitinib. Bioorg Med Chem Lett 22:2850–2855
(a) Senter PD (2009) Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol 13:235–244; (b) Srinivasarao M, Galliford CV, Low PS (2015) Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Disc 14:203–219; (c) Krall N, Scheuermann J, Neri D (2013) Small targeted cytotoxics: current state and promises from DNA-encoded chemical libraries. Angew Chem Int Ed 52:1384–1402
(a) Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR (2009) A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 9:10–25; (b) Wu AM, Senter PD (2005) Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 23:1137–1146; (c) Carter P (2001) Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer 1:118–129
Kim EG, Kim KM (2015) Strategies and advancement in antibody-drug conjugate optimization for targeted Cancer therapeutics. Biomol Ther 23:493–509
Ricart AD, Tolcher AW (2007) Technology insight: cytotoxic drug immunoconjugates for cancer therapy. Nat Clin Pract Oncol 4:245–255
Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A et al (2007) Imaging tumors with an albumin-binding fab, a novel tumor-targeting agent. Cancer Res 67:254–261
(a) Gerber HP, Senter PD, Grewal IS (2009) Antibody drug-conjugates targeting the tumor vasculature: Current and future developments. mAbs 1:247–253;(b) Teicher BA, Chari RV (2011) Antibody conjugate therapeutics: challenges and potential. Clin. Canc. Res. 7:6389-6397; (c) Sievers EL, Senter PD (2013) Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 64:15-29; d) Chari RV, Miller ML, Widdison WC (2014) Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed 53:3796–3827
Lambert JM (2013) Drug-conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol 76:248–262
Gamcsik MP, Kasibhatla MS, Teeter SD, Colvin OM (2012) Glutathione levels in human tumors. Biomarkers 17:671–691
Mills BJ, Lang CA (1992) Differential distribution of free and bound glutathione and cysteine in human blood. Biochem Pharmacol 52:401–406
(a) Thorpe PE, Wallace PM, Knowles PP, Relf MG, Brown AN, Watson GJ, Knyba RE, Wawrzynczak EJ, Blakey DC (1987) New coupling agents for the synthesis of immunotoxins containing a hindered disulfide bond with improved stability in vivo. Cancer Res 47:5924–5931;(b) Kellogg BA, Garrett L, Kovtun Y, Lai KC, Leece B, Miller M, et al (2011) Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjugate Chem 22:717–727
Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK et al (2010) Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res 70:2528–2537
Zucker S (1988) A critical appraisal of the role of proteolytic enzymes in Cancer invasion: emphasis on tumor surface proteinases. Cancer Investig 6:219–231
Choi KY, Swierczewska M, Lee S, Chen X (2012) Protease-activated drug development. Theranostics 2:156–178
Senter PD, Sievers EL (2012) The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol 30:631–637
Jain N, Smith SW, Ghone S, Tomczuk B (2015) Current ADC linker chemistry. Pharm Res 32:3526–3540
Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S et al (2009) Antibody-drug conjugates for the treatment of non-Hodgkin's lymphoma: target and linker-drug selection. Cancer Res 69:2358–2364
Hong EE, Erickson H, Lutz RJ, Whiteman KR, Jones G, Kovtun Y et al (2015) Design of Coltuximab Ravtansine, a CD19-targeting antibody-drug conjugate (ADC) for the treatment of B-cell malignancies: structure-activity relationships and preclinical evaluation. Mol Pharm 12:1703–1716
Armitage JO, Gascoyne RD, Lunning MA, Cavalli F (2017) Non-Hodgkin lymphoma. Lancet 390:298
Press OW, Farr AG, Borroz KI, Anderson SK, Martin PJ (1989) Endocytosis and degradation of monoclonal antibodies targeting human B-cell malignancies. Cancer Res 49:4906–4912
Dijoseph JF, Dougher MM, Armellino DC, Kalyandrug L, Kunz A, Boghaert ER et al (2007) CD20-specific antibody-targeted chemotherapy of non-Hodgkin's B-cell lymphoma using calicheamicin-conjugated rituximab. Cancer Immunol Immunother 56:1107–1117
Li F, Emmerton KK, Jonas M, Zhang X, Miyamoto JB et al (2016) Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res 76:2710–2719
Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y et al (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9:802–811
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60:7075–7083
Thiry A, Dogne JM, Masereel B, Supuran CT (2006) Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 27:566–573
Krall N, Pretto F, Decurtins W, Bernardes GJ, Supuran CT, Neri D (2014) A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed 53:4231–4235
(a) Cazzamalli S, Dal Corso A, Neri D (2016) Acetazolamide serves as selective delivery vehicle for dipeptide-linked drugs to renal cell carcinoma. Mol Cancer Ther 15:2926-2935; (b) Cazzamalli S, Dal Corso A, Neri D (2017) Linker stability influences the anti-tumor activity of acetazolamide-drug conjugates for the therapy of renal cell carcinoma. J Control Release 246:39–45
Petrul HM, Schatz CA, Kopitz CC, Adnane L, TJ MC, Trail P et al (2012) Therapeutic mechanism and efficacy of the antibody-drug conjugate BAY 79-4620 targeting human carbonic anhydrase 9. Mol Cancer Ther 11:340–349
Clinical Study Report No. PH-37705, BAY 79-4620 / 12671: Bayer HealthCare (2014)
Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM et al (1989) Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta 948:305–326
Dvorak HF, Dvorak AM, Manseau EJ, Wiberg L, Churchill WH (1979) Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in Guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst 62:1459–1472
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Yasunaga M, Manabe S, Matsumura Y (2011) New concept of cytotoxic immunoconjugate therapy targeting cancer-induced fibrin clots. Cancer Sci 102:1396–1402
Yasunaga M, Manabe S, Tarin D, Matsumura Y (2011) Cancer-stroma targeting therapy by cytotoxic immunoconjugate bound to the collagen 4 network in the tumor tissue. Bioconjug Chem 22:1776–1783
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Joyce JA (2005) Therapeutic targeting of the tumor microenvironment. Cancer Cell 7:513–520
(a) Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al (1994) Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA. 91:5657–5661;(b) Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. (1988) Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA 85:3110–3114; (c) Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Ozer HL, Schwab M, et al (1993) Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Res 53:3327–3335
(a) Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, et al (1994) Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol 12:1193–1203; (b) Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jager D, Renner C, et al (2003) Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26:44–48; (c) Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, et al (2003) A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 9:1639–1647
Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C et al (2008) Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res 14:4584–4592
Satoh K, Hata M, Yokota H (2002) A novel member of the leucine-rich repeat superfamily induced in rat astrocytes by beta-amyloid. Biochem Biophys Res Commun 290:756–762
Satoh K, Hata M, Yokota H (2004) High lib mRNA expression in breast carcinomas. DNA Res 11:199–203
Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP (2006) Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 66:2815–2825
Gish KC, Hickson JA, Purcell JW, Morgan-Lappe SE (2015) Anti-huLRRC15 Antibody Drug Conjugates and methods for their use. WO2017095805A1
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A (2014) The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc B 369:–20130099
(a) Yamada K, Nomura N, Yamano A, Yamada Y, Wakamatsu N (2012) Identification and characterization of splicing variants of PLEKHA5 (Plekha5) during brain development. Gene 492:270–275;(b) Yousaf N, Deng Y, Kang Y, Riede H (2001) Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J Biol Chem 276:40940–40948
Ladomery M (2013) Aberrant alternative splicing is another hallmark of cancer. Int J Cell Biol 2013:463786
Venables JP (2004) Aberrant and alternative splicing in cancer. Cancer Res 64:7647–7654
Oltean S, Bates DO (2014) Hallmarks of alternative splicing in cancer. Oncogene 33:5311–5318
Elias AP, Dias S (2008) Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron 1:131–139
Gaus G, Demir-Weusten AY, Schmitz U, Bose P, Kaufmann P, Huppertz B, Frank HG (2002) Extracellular pH modulates the secretion of fibronectin isoforms by human trophoblast. Acta Histochem 104:51–63
Borsi L, Allemanni G, Gaggero B, Zardi L (1996) Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int J Cancer 66:632–635
Hynes RO, Yamada KM (1982) Fibronectins: multifunctional modular glycoproteins. J Cell Biol 95:369–377
Colombi M, Barlati S, Kornblihtt A, Baralle FE, Vaheri A (1986) A family of fibronectin mRNAs in human normal and transformed cells. Biochim Biophys Acta 868:207–214
Paul JI, Schwarzbauer JE, Tamkun JW, Hynes RO (1986) Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem 261:12258–12265
(a) Borsi L, Carnemolla B, Castellani P, Rosellini C, et al (1987) Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol 104:595–600;(b) Zardi L1, Carnemolla B, Siri A, Petersen TE, et al (1987) Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J 6:2337–2342
Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, Zardi L (1994) The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer 5:612–618
Carnemolla B, Balza E, Siri A, Zardi L, Nicotra MR, Bigotti A, Natali PG (1989) A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol 108:1139–1148
Borsi L, Castellani P, Allemanni G, Neri D, Zardi L (1998) Preparation of phage antibodies to the ED-A domain of human fibronectin. Exp Cell Res 240:244–251
Matsuura H, Hakomori S (1985) The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc Natl Acad Sci U S A 82:6517–6521
Kumra H, Reinhardt DP (2016) Fibronectin-targeted drug delivery in cancer. Adv Drug Deliv Rev 97:101–110
Erickson HP (1989) Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol 5:71–92
Sage EH, Bornstein P (1991) Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem 23:14831–14834
Borsi L, Balza E, Gaggero B, Allemagni I, Zardi L (1995) The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J Biol Chem 270:6243–6245
Reardon DA, Akabani G, Coleman RE, Friedman AH et al (2002) Phase II trial of murine (131)I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J Clin Oncol 20:1389–1397
Rizzieri DA, Akabani G, Zalutsky MR, Coleman RE et al (2004) Phase 1 trial study of 131I-labeled chimeric 81C6 monoclonal antibody for the treatment of patients with non-Hodgkin lymphoma. Blood 104:642–648
Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, Neri D (1998) Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem 21:21769–21776
Brack SS, Silacci M, Birchler M, Neri D (2006) Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res 12:3200–3208
Borsi L, Balza E, Bestagno M, Castellani P, Carnemolla B et al (2002) Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer 102:75–85
Sauer S, Erba PA, Petrini M, Menrad A et al (eds) (2009) Expression of the oncofetal ED-B-containing fibronectin isoform in hematologic tumors enables ED-B-targeted 131I-L19SIP radioimmunotherapy in Hodgkin lymphoma patients. Blood 113:2265–2274
Aloj L, D'Ambrosio L, Aurilio M, Morisco A et al (2014) Radioimmunotherapy with Tenarad, a 131I-labelled antibody fragment targeting the extra-domain A1 of tenascin-C, in patients with refractory Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging 41:867–877
Heuveling DA, de Bree R, Vugts DJ, Huisman MC, Giovannoni L, Hoekstra OS, Leemans CR, Neri D, van Dongen GA (2013) Phase 0 microdosing PET study using the human mini antibody F16SIP in head and neck cancer patients. J Nucl Med 54:397–401
Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, Rösli C, Borsi L, Neri D (2008) A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer 122:2405–2413
Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, Trachsel E (2009) Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther 11:R142
Bernardes GJ, Casi G, Trüssel S, Hartmann I, Schwager K, Scheuermann J, Neri D (2012) A traceless vascular-targeting antibody-drug conjugate for cancer therapy. Angew Chem Int Ed 51:941–944
Austin CD, Wen X, Gazzard L, Nelson C, Scheller RH, Scales SJ (2005) Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci U S A 102:17987–17992
Perrino E, Steiner M, Krall N, Bernardes GJ, Pretto F, Casi G, Neri D (2014) Curative properties of noninternalizing antibody-drug conjugates based on maytansinoids. Cancer Res 74:2569–2578
Gébleux R, Wulhfard S, Casi G, Neri D (2015) Antibody format and drug release rate determine the therapeutic activity of noninternalizing antibody-drug conjugates. Mol Cancer Ther 14:2606–2612
Gébleux R, Stringhini M, Casanova R, Soltermann A, Neri D (2016) Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int J Cancer 140:1670–1679
Dal Corso A, Cazzamalli S, Gébleux R, Mattarella M, Neri D (2017) Protease-cleavable linkers modulate the anticancer activity of noninternalizing antibody-drug conjugates. Bioconjug Chem 28:1826–1833
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG et al (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21:778–784
Dal Corso A, Pignataro L, Belvisi L, Gennari C (2016) αvβ3 integrin-targeted peptide/Peptidomimetic-drug conjugates: in-depth analysis of the linker technology. Curr Top Med Chem 16:314–329
Verma VA, Pillow TH, DePalatis L, Li G, Phillips GL, Polson AG, Raab HE, Spencer S, Zheng B (2015) The cryptophycins as potent payloads for antibody drug conjugates. Bioorg Med Chem Lett 25:864–868
Sun X, Ponte JF, Yoder NC, Laleau R, Coccia J et al (2017) Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-Maytansinoid conjugates. Bioconjug Chem 28:1371–1381
Yurkovetskiy AV, Yin M, Bodyak N, Stevenson CA, Thomas JD et al (2015) A polymer-based antibody-Vinca drug conjugate platform: characterization and preclinical efficacy. Cancer Res 75:3365–3372
Bianchi ME (2014) Killing cancer cells, twice with one shot. Cell Death Differ 21:1–2
Gerber HP, Sapra P, Loganzo F, May C (2016) Combining antibody-drug conjugates and immune-mediated cancer therapy: what to expect? Biochem Pharmacol 12:1–6
Litterman AJ, Zellmer DM, Grinnen KL, Hunt MA, Dudek AZ, Salazar AM, Ohlfest JR (2013) Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol 190:6259–6268
Mahoney KM, Rennert PD, Freeman GJ (2015) Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 14:561–584
List T, Casi G, Neri D (2014) A chemically defined Trifunctional antibody-cytokine-drug conjugate with potent antitumor activity. Mol Cancer Ther 13:2641–2652
Gutbrodt KL, Schliemann C, Giovannoni L, Frey K, Pabst T, Klapper W, Berdel WE, Neri D (2013) Antibody-based delivery of Interleukin-2 to Neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med 5:201ra118
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to Cancer immunotherapy. Cell 168:707–723
Hynes RO (2014) Stretching the boundaries of extracellular matrix research. Nat Rev Mol Cell Biol 15:761–763
Donaghy H (2016) Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs 8:659–671
Bakhtiar R (2016) Antibody drug conjugates. Biotechnol Lett 38:1655–1664
Cohen R, Vugts DJ, Visser GW, Stigter-van Walsum M et al (2014) Development of novel ADCs: conjugation of tubulysin analogues to trastuzumab monitored by dual radiolabeling. Cancer Res 74:5700–5710
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Dal Corso, A., Cazzamalli, S., Neri, D. (2018). Antibody-Drug Conjugates: Targeting the Tumor Microenvironment. In: Damelin, M. (eds) Innovations for Next-Generation Antibody-Drug Conjugates. Cancer Drug Discovery and Development. Humana Press, Cham. https://doi.org/10.1007/978-3-319-78154-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-78154-9_13
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-78153-2
Online ISBN: 978-3-319-78154-9
eBook Packages: MedicineMedicine (R0)