Abstract
A patient with acute hemiparesis on the left-hand side and acute dysarthria and suffering from headaches for the past week presented at the emergency department of the referring hospital. Based on a non-contrast CT examination within normal limits, his symptoms were misdiagnosed as an acute ischemic stroke with an assumed large vessel occlusion. As a result of the stroke work-up in our hospital, however, a profound vasospasm and a large PcomA aneurysm on the right-hand side were identified. The aneurysmal subarachnoid hemorrhage (SAH) was responsible for the post-hemorrhagic vasospasm, which in turn caused the clinical signs and symptoms. The aneurysm was subsequently treated by balloon-assisted coil occlusion, and the patient underwent multiple endovascular treatments with intra-arterial vasospasmolysis during the following days. The patient’s outcome was acceptable although minor infarction caused by the vasospasm could not be prevented. The sufficient occlusion of the aneurysm was confirmed by a follow-up DSA after 3 months. This case history illustrates that the clinical symptoms and the imaging results of a patient with an SAH are sometimes misleading. Despite rapid and uneventful treatment of the ruptured aneurysm, post-hemorrhagic vasospasm is frequently the key reason for a poor clinical outcome.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Post-hemorrhagic vasospasm
- Negative CT
- PcomA aneurysm
- Balloon-assisted coil occlusion
- Vasospasmolysis
- Intra-arterial short-term nimodipine infusion
Patient
A 56-year-old, male, presenting with acute ischemic stroke symptoms due to massive vasospasm after aneurysmal SAH 1 week prior.
Diagnostic Imaging
This patient presented at the referring hospital having suffered from severe headaches for the last week and with an acute onset of a hemiparesis on the left-hand side and dysarthria on that day. A non-contrast CT (NCCT) examination showed no signs of an SAH or an acute infarction, however did pick up a hyperdense structure adjacent to the right cavernous sinus. The patient was transferred to our hospital with the diagnosis of an acute ischemic stroke, most likely due to a proximal large vessel occlusion.
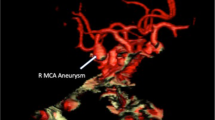
As part of our standard ischemic stroke work-up, a CT angiography (CTA) was performed which ruled out any major vessel occlusion. Multiple arterial caliber irregularities were found in the course of the internal carotid artery (ICA) termination, of the middle cerebral artery (MCA) and the anterior cerebral artery (ACA) on both sides, most pronounced on the right-hand side. Furthermore, the hyperdense structure seen in the NCCT examination turned out to be a large PcomA aneurysm (fundus depth 9.5 mm, fundus width 8 mm, neck width 4 mm). Due to the new diagnosis of a PcomA aneurysm having ruptured 1 week ago, now having led to a massive symptomatic vasospasm, the patient was immediately transferred to the angiography suite for endovascular vasospasmolysis and coil occlusion of the aneurysm. The results of the diagnostic imaging are summarized in Fig. 1.
Diagnostic work-up of a patient with a suspected ischemic stroke which turned out to be due to post-hemorrhagic vasospasm resulting from the rupture of a large PcomA aneurysm. The initial NCCT examination, carried out at the referring hospital, showed a hyperdense lesion adjacent to the right cavernous sinus and no signs of an SAH (a). CTA revealed no proximal large vessel occlusion, but multiple caliber irregularities in the course of the ICA termination and the MCA and the ACA on both sides. CTA furthermore identified the hyperdense structure seen in the NCCT as a large right-hand PcomA aneurysm (b, c)
Treatment Strategy
There were two equally important goals in the treatment: (1) improvement of brain perfusion by reducing the diffuse vasospasm using nimodipine IA infusion and (2) urgent exclusion of the aneurysm from the blood circulation by endovascular coil occlusion. Due to the post-hemorrhagic vasospasm, microsurgical clipping of the aneurysm was not considered the best treatment in this case.
Treatment
Procedure, 02.10.2015: balloon-assisted coil occlusion of a ruptured PcomA aneurysm
Anesthesia: general anesthesia, 1× 5000 IU heparin (Heparin-Natrium, Rotexmedica) IV
Premedication: none
Access: right femoral artery, 6F sheath (Cordis); guide catheter: 6F Neuron MAX 088 90 cm sheath (Penumbra); microcatheter: PX Slim (Penumbra) for the coils; balloon: Scepter C 4/15 mm (MicroVention); and microguidewires: Synchro2 0.014 “200 cm (Stryker), Transend 0.014” (Stryker)
Implants: 5 volume coils: complex extra soft 2 mm/3 cm, complex soft 8 mm/20 cm, complex soft 5 mm/9 cm, complex extra soft 4 mm/8 cm, and complex extra soft 3 mm/8 cm (all Penumbra)
Course of treatment: The right common carotid artery was catheterized with a 6 F Simmons 2 catheter to reach the right internal carotid artery with a Terumo 0.035″ 260 cm guidewire which was then swapped for a 6F Neuron MAX guiding sheath. DSA runs in standard projections showed a high-grade diffuse vasospasm. Firstly, 6 mg nimodipine (Nimodipin IV, Carinopharm) IA was administered over the next 60 minutes. Then, a rotational angiography with a 3D reconstruction was performed. Based on these images, a working projection for the subsequent treatment was selected. The microcatheter and the balloon catheter were inserted simultaneously. The Scepter C balloon was positioned in the right ICA to cover the neck of the aneurysm. Then, the aneurysm was carefully catheterized with the PX Slim microcatheter. A small coil was put in place in order to protect the perforation site. Then, the microcatheter was gently pulled back, and the tip was positioned in the proximal part of the aneurysm. A large complex soft coil was used to form a cage, and afterward three more coils were inserted and detached. After achieving a complete occlusion of the aneurysm with no visible inflow of contrast, the PX Slim microcatheter was slowly withdrawn.
The final DSA run confirmed an occlusion of the aneurysm and a normal opacification of the ICA. The endovascular treatment is summarized in Fig. 2.
DSA of the right ICA after insertion of the guiding sheath, showing a massive vasospasm of the termination of the right ICA and in the entire territory of the right MCA and A1 segment (a). Rotational DSA with 3D reconstruction shows the PcomA aneurysm (b). On a 2D DSA in a straight lateral view, the aneurysm neck appears narrow and well defined (c). The rupture site can be identified on the posterior-inferior aspect of the aneurysm sac (c, arrow). Under the protection of a remodeling balloon catheter, the endovascular treatment started with a coil occlusion of the rupture site using a soft coil (d). Subsequently, the aneurysm was filled with the coils listed above. A final DSA run in posterior-anterior (e) and lateral projection (f) confirmed the complete occlusion of the aneurysm. Due to the intra-arterial nimodipine infusion, the vasospasm of the proximal MCA and ACA had meanwhile receded. The cortical branches of the right MCA, however, are still narrow
Duration: 1st–18th DSA run: 135 minutes; fluoroscopy time: 68 minutes
Complications: none
Postmedication: nimodipine IV was given for 10 days at a dosage of 2 mg/h; vasospasmolysis was performed with nimodipine IA infusion ahead of the intervention and on the following 6 days with 6 mg nimodipine IA, using a 4F sheath and a diagnostic 4F catheter with daily injections of both ICAs. Each treatment resulted in a significant reduction of the vasospasm, also confirmed by daily transcranial Doppler examinations.
Clinical Outcome
The clinical outcome was acceptable. At a 3-month follow-up visit, the patient complained of a marginal hypesthesia and a monoparesis in his left leg (mRS 1).
Follow–Up Examination
The first follow-up DSA after 3 months showed minor coil compaction with a neck remnant, but that the aneurysm had been sufficiently occluded. For further follow-up, we also performed a baseline MRI/MRA examination. T2WI MRI revealed a right parietal chronic stage ischemic lesion as a result of the previous vasospasm (Fig. 3). Further non-invasive follow-up are scheduled MRI/MRA examinations once a year.
Discussion
This is an example of a patient with an SAH , which initially only caused a sudden onset of mild headaches. One week after the underlying aneurysm rupture, and with an ongoing headache, the patient developed a delayed ischemic neurological deficit due to post-hemorrhagic vasospasm. At this time, a NCCT examination showed no signs of an SAH. As a result, the new symptoms of dysarthria and hemiparesis were misdiagnosed as being related to an acute ischemic stroke due to a large vessel occlusion. The results of a second – now contrast-enhanced – CT examination with CTA and perfusion imaging revealed a restriction of the brain perfusion and a PcomA aneurysm. This led to the diagnosis of a ruptured aneurysm, causing an SAH with symptomatic post-hemorrhagic vasospasm and a delayed ischemic neurological deficit. This case history illustrates how misleading clinical signs and symptoms can be and how important comprehensive diagnostic imaging is. We should keep in mind that not long ago, the indication for intravenous thrombolysis from an acute ischemic stroke was based on clinical criteria and NCCT only. Nowadays, non-invasive vascular imaging by CTA or MRA for all stroke patients is considered to be standard care.
If the patient is already in the vasospasm phase, endovascular coil occlusion appears to be the better choice of treatment because we expect fewer complications compared to microsurgical clipping (Molyneux et al. 2005; Yu et al. 2007). Another advantage is the possibility of treating the vasospasm with nimodipine IA infusion ahead of coiling the aneurysm. It is important to monitor and treat the vasospasm in the days following the coiling to prevent further complications such as ischemic brain damage. In our case, a minor infarction caused by the vasospasms could not be prevented despite the rapid treatment of both vasospasm and ruptured aneurysm.
Our routine strategy for all patients presenting with aneurysmal SAH is the monitoring of the patient on the ICU for about 14 days with daily transcranial Doppler examinations. We attempt to prevent vasospasm by administering a continuous nimodipine (2 mg/h for 10 days) IV infusion. The mean arterial blood pressure is maintained at a target value of 80–90 mmHg, and the liquid balance should be equalized.
In patients with symptomatic post-hemorrhagic vasospasm , despite this regimen our treatment of choice is the short-term IA administration of nimodipine, usually at a maximum dose of 6 mg per day applied over 30 minutes via a diagnostic 4F catheter. In most patients this therapy works well and is sufficient to avoid further ischemic brain damage. If the IA infusion of nimodipine is not successful, the next therapy step is the use of a remodeling balloon or the use of a small stent retriever to reconstitute the vessel lumen of the affected segment. Like in the case report presented here, post-hemorrhagic vasospasm is often the determining factor for the clinical outcome after the survival of an intracranial aneurysm rupture and treatment (Jabbarli et al. 2016).
The use of volume coils is a treatment option especially for large aneurysms and has the benefit of filling the aneurysm nearly four times faster than using conventional coils (Berge et al. 2016; Kaesmacher et al. 2016). For this coil line, it is necessary to use a 0.025” ID microcatheter, which might be a minor disadvantage, especially in arteries affected by vasospasm.
Therapeutic Alternatives
-
Balloon Angioplasty
-
Continuous Local Intra-Arterial Nimodipine Administration
-
Milrinone
-
pRELAX
-
Stellate Ganglion Block
-
Stent Implantation
-
Stent-Retriever Angioplasty
References
Berge J, Gariel F, Marnat G, Dousset V. PC400 volumetric coils minimize radiation, reduce procedure time and optimize packing density during endovascular treatment in medium sized cerebral aneurysms. J Neuroradiol. 2016;43(1):37–42. https://doi.org/10.1016/j.neurad.2015.10.002.
Jabbarli R, Reinhard M, Shah M, Roelz R, Niesen WD, Kaier K, Taschner C, Weyerbrock A, Van Velthoven V. Early vasospasm after aneurysmal subarachnoid hemorrhage predicts the occurrence and severity of symptomatic vasospasm and delayed cerebral ischemia. Cerebrovasc Dis. 2016;41(5–6):265–72. https://doi.org/10.1159/000443744.
Kaesmacher J, Müller-Leisse C, Huber T, Boeckh-Behrens T, Haller B, Shiban E, Friedrich B, Zimmer C, Dorn F, Prothmann S. Volume versus standard coils in the treatment of intracranial aneurysms. J Neurointerv Surg. 2016;8(10):1034–40. https://doi.org/10.1136/neurintsurg-2015-012014.
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–17.
Yu SC, Wong GK, Wong JK, Poon WS. Endovascular coiling versus neurosurgical clipping for ruptured intracranial aneurysms: significant benefits in clinical outcome and reduced consumption of hospital resources in Hong Kong Chinese patients. Hong Kong Med J. 2007;13(4):271–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Loehr, C., Kuhnt, J.O. (2020). Posterior Communicating Artery Aneurysm: Unrecognized Previous Aneurysm Rupture, Acute Ischemic Stroke Symptoms Due to Post-Hemorrhagic Vasospasm, Emergent Balloon-Assisted Coil Occlusion, and Repeated Intra-arterial Vasospasmolysis with Acceptable Clinical Outcome. In: Henkes, H., Lylyk, P., Ganslandt, O. (eds) The Aneurysm Casebook. Springer, Cham. https://doi.org/10.1007/978-3-319-77827-3_35
Download citation
DOI: https://doi.org/10.1007/978-3-319-77827-3_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77826-6
Online ISBN: 978-3-319-77827-3
eBook Packages: MedicineReference Module Medicine