Abstract
-
Heart transplantation has become an increasingly common and effective therapy for adults with end-stage congenital heart disease (ACHD) because of advances in patient selection and surgical technique. Indications for transplantation in ACHD are similar to other forms of heart failure. Timing of referral, pretransplant assessment of ACHD patients, wait-list outcomes and opportunities for decreasing wait-list mortality as well as outcome, alternatives to transplantation, and peri- and postoperative management are discussed in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Transplantation waiting list
- Sensitization
- Assist devices
- Extracorporeal membrane oxygenation
- Transplantation outcomes
1 Introduction
As transplant centers become more familiar with heart transplantation (HTx) in adult congenital heart disease (ACHD) patients, short- and long-term outcomes have improved. Long-term survival conditional on 1-year survival is now better for ACHD patients undergoing HTX than for non-ACHD patients [1]. Despite the perception that the risks associated with HTx are often too high, many ACHD patients have acceptable risk profiles, and all can benefit from review in an advanced heart failure (HF) program. This chapter is dedicated to breaking down barriers to advanced HF care for patients with ACHD-related HF. This chapter has six sections:
-
Timing of referral for HTx assessment
-
Risks and contraindications to HTx
-
Wait-list outcomes and opportunities for decreasing wait-list mortality
-
HTx outcomes
-
Alternatives to transplantation
-
Peri-/postoperative management
2 Timing of Referral for HTx Assessment
Predicting progression from stable to advanced and/or decompensated HF in ACHD-related HF is challenging, even more so than in acquired HF. Declining NYHA class, progressive ventricular dysfunction, elevated serum BNP [2, 3], reduced peak oxygen uptake (VO2 max), low BMI [4], hyponatremia [5], anemia [6], renal dysfunction [7], elevated troponin [8], pulmonary hypertension (PH), and arrhythmias [9] have each been associated with increased ACHD mortality. More important than any single variable is the overall clinical picture. In particular, the emergence of clinical features of advanced HF portends adverse health outcomes (summarized in Table 13.1). Emphasized here is a history of repeated HF hospitalizations or emergency room visits, escalating diuretic dose, deteriorating kidney function, unexplained weight loss, and intolerance to ACE inhibitors or beta-blockers. Early referral to a is recommended for all ACHD patients with features of advanced HF. Advanced HF review is also recommended in those with idiosyncratic complications suggesting increased short-term mortality risk such as protein-losing enteropathy (PLE) in Fontan patients [10, 11].
An advanced HF service comprises an interdisciplinary team with expertise and formal training in advanced HF treatment strategies. Early referral does not equate to early listing for HTx. Nor does it imply transfer of care from the ACHD to the acquired HF team. Instead, the purpose of early assessment is to facilitate collaboration between the ACHD and HF programs and to afford the team sufficient time to understand each patient’s anatomy, hemodynamics, and clinical course. The team should include clinicians with both traditional and advanced HF expertise but also ACHD clinicians. In addition to discussing advanced HF strategies, the team should assess each patient’s understanding of mortality risk with and without treatment and advanced care preferences including resuscitation. Research into care preferences show most ACHD patients prefer to discuss life expectancy and advanced directives before their condition becomes life-threatening [12].
Figure 13.1 provides a stepwise approach to evaluation and treatment of ACHD patients with advanced HF [13]. Worsening symptoms, biomarkers, or exercise intolerance prompts review of medications and candidacy for percutaneous/surgical intervention and device therapies. Multimodality cardiac imaging (echocardiogram, cardiac MRI, cardiac CT, and/or vascular ultrasound) provides a detailed assessment of anatomy and hemodynamics. In addition to quantifying ventricular dimensions and function, attention is paid to the position of the heart and coronary vessels in relation to the sternum, arterial and venous connections, peripheral vascular access, and collateral vessels. Invasive hemodynamic evaluation by a CHD specialist is recommended for ACHD patients with newly diagnosed HF. In addition to guiding medical HF therapy, hemodynamic assessment identifies residual lesions contributing to HF pathophysiology. Advanced therapies (HTx and mechanical circulatory support) should be considered in any ACHD patient who is not responding to medical or device therapy or in whom repeat cardiac surgery is deemed too high risk or unlikely to reset the clinical course. Indications for HTx are summarized in Table 13.2.
3 Risks and Contraindications to HTx
Contraindications to HTx in ACHD patients are based on ISHLT guidelines [14] while also taking into account ACHD-related issues. ACHD-related factors such as complex anatomy, the presence of multiple congenital anomalies, or neurocognitive impairment rarely serve as absolute contraindications in isolation. More frequently it is these issues in combination that lead to determination of excessive risk although risk thresholds vary significantly by transplant and ACHD center volume and expertise. Over time thresholds of risk in ACHD patients are being challenged. A good example is a history of multiple sternotomies which despite being associated with increased HTx risk can generally be managed with anticipatory measures (i.e., peripheral cannulation and bypass, careful dissection of adhesions) [15]. Absolute, relative, and ACHD-related contraindications are summarized in Table 13.3. For this review we have chosen to focus on three issues of major concern—liver disease, PH, and sensitization.
3.1 Liver Disease
Liver disease is common in ACHD patients due to the high prevalence of right heart disease, passive liver congestion and hepatitis C [16]. Fontan patients are at especially high risk due to systemic venous hypertension, hepatic congestion, and low cardiac output leading to cirrhosis and, in some patients, hepatocellular carcinoma [17]. Whether Fontan patients should undergo isolated heart versus combined heart-liver transplantation continues to be hotly debated with the current approach to assessment and treatment varying across transplant centers. The Hospital of the University of Pennsylvania reported outcomes in eight Fontan patients who underwent combined heart-liver transplantation for biopsy-proven advanced fibrosis or cirrhosis; 100% survival was achieved at 30 days and 1 year [18]. It has been postulated that the addition of liver transplant reduces bleeding risk in Fontan patients [19]. In addition, there is increasing evidence that cardiac cellular and antibody-mediated rejection are less frequent after combined heart-liver transplant [20] [21]. Despite these potential advantages, it remains unclear whether combined heart-liver transplant should be recommended in all Fontan patients. A recent UNOS study reported equivalent survival in CHD patients undergoing isolated heart versus combined heart-liver transplantation [15].
The model for end-stage liver disease excluding international normalized ratio (MELD-XI) score may be helpful for stratifying risk. A MELD-XI score >18 has been associated with increased ACHD recipient mortality after HTx [22]. A second study reported significantly lower survival after HTx in Fontan patients when more than one of the following findings was identified: elevated MELD-XI, MELD-XI >19, EF <20%, moderate or severe AV valve regurgitation, Fontan pressure >16.5 mmHg, and need for dialysis and ECMO support [23]. Validation of these risk scores in larger patient populations will be important before these criteria, and cutoffs for liver disease can be integrated into transplant selection criteria.
Based on the existing data, a case-by-case approach is recommended with combined heart-liver transplant being an important consideration in those with high-risk features as described but particularly once cirrhosis is confirmed on liver biopsy.
3.2 Pulmonary Hypertension
Assessment of PH in ACHD patients is made more challenging by the presence of complex anatomy, passive pulmonary blood flow, imbalanced flow to the right and left lung, and alternative sources of pulmonary blood flow in the form of collateral vessels. Based upon current ISHLT HTx guidelines, a transpulmonary gradient (TPG) <15 mmHg and pulmonary vascular resistance (PVR) <3 Wood units are considered acceptable for HTx [14]. Vasodilator challenge should be undertaken if PH is confirmed at catheterization. Inotropes and diuretics are important in patients with pulmonary venous HT arising secondary to a failing systemic ventricle. Reversible PH has been associated with good outcomes after isolated HTx [24, 25] despite an increased risk of donor right HF during the first 30 days after HTx [26].
Although PVR and TPG are commonly used to differentiate HF patients with pulmonary vascular disease from those with passive PH, elevations in TPG and PVR do not always reflect precapillary PH. As such more sensitive measures of PH continue to be sought. An elevated diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient (DPG) has been proposed as a better indicator of pulmonary vascular remodeling. However, in a study of over 5800 patients with PH, there was no difference in post-HTx survival in those with high versus low DPG indicating a limited prognostic role for this measure [27]. Others have shown the hemodynamic response to nitroprusside at the time of right heart catheterization to be useful for differentiating risk in PH patients with non-ACHD-related HF. Short-term mortality was significantly higher in patients with fixed PVR above 2.5 Wood units and those whose PVR could be reduced but only at the expense of systemic hypotension (SBP <85 mmHg) [28]. These findings are more applicable to patients with biventricular anatomy and are as yet unproven in ACHD.
Overall, these studies underscore the fact that reliance on one static measure to characterize PH type and severity prior to HTx is inadequate and that serial monitoring, provocative maneuvers, and integration of several indices are important at the time of ACHD HTx assessment.
3.3 Sensitization
Sensitization, the process by which preformed antibodies to human leukocyte antigens (HLA) are developed, remains an important barrier to HTx. Patients with panel reactive antibodies (PRA) greater than 10% are considered sensitized. Sensitization risk factors include prior blood transfusions, pregnancies, homografts, tissue allografts, and mechanical circulatory support [29]. HTx recipients with high degrees of sensitization have increased wait-list times and are at a higher risk of posttransplant rejection, cardiac transplant vasculopathy, graft failure, and mortality [30,31,32,33]. Treatment options for sensitization include plasmapheresis/intravenous immunoglobulin/rituximab (pre-/posttransplant) and thymoglobulin (posttransplant) (IVIg). By decreasing circulating antibodies, desensitization can allow patients to undergo HTx with a negative prospective donor-specific crossmatch. Surprisingly, equivalent 5-year survival has been reported in sensitized patients undergoing HTx with and without prior desensitization [34]. These findings suggest desensitization increases the chance of sensitized patients proceeding to HTx with a negative crossmatch but without clear evidence for improved long-term survival.
4 Wait-List Outcomes and Opportunities for Decreasing Wait-List Mortality
The decision to proceed with listing for HTx is based on an estimated 1-year survival of less than 80%. Estimating survival in ACHD HF patients is challenging since patients are younger and often appear compensated despite significant circulatory dysfunction and end-organ disease. In a multicenter study of 45 ACHD patients undergoing HTx evaluation between 2000 and 2012, 13 were deemed too high risk, 6 because of liver cirrhosis, and 1 due to irreversible pulmonary HT. Five were considered too well [35]. As observed elsewhere [18], those declined were more likely to have single-ventricle physiology. No difference in survival was observed between those listed versus not listed for HTx. Among patients who underwent HTx, 1-year survival was 85% with four ACHD deaths occurring within 30 days of HTx. Of the 13 patients not listed due to high risk, 5 (38%) died secondary to progressive HF. Of the five patients considered too well for HTx, one died from a hemorrhagic stroke, and the other four continued to be managed medically without need for HTx. In reviewing these outcomes, it is clear that morbidity and mortality are high in ACHD HF patients, both with and without HTx.
Once listed, ACHD patients spend longer on the HTx wait list [36, 37]. Wait-list times in ACHD recipients are increased for several reasons including being listed at lower urgency, being more highly sensitized, and the need for non-lung donors to enable vascular reconstruction in some recipients [37]. Since 1996, the number of ACHD patients listed status 2 has remained constant, whereas for non-ACHD patients, this percentage has fallen likely due to a relative increase in the number of non-ACHD patients being upgraded to status 1 [38]. Changes in the organ allocation policies, including a provision that patients with a ventricular assist device (VAD) be listed as status 1B, have influenced this shift. VAD patients also receive 30 days of discriminatory 1A time as well as 1A status for VAD complications leaving ACHD patients, many of whom do not require or qualify for mechanical circulatory support, at a significant disadvantage.
Wait-list mortality of 10% in ACHD patients is comparable to others listed for HTx although ACHD patients are significantly more likely to die secondary to cardiac causes, most commonly sudden cardiac death [37]. Highlighting a need for improved arrhythmia risk stratification and treatment, ACHD patients are less likely to be protected with an implantable cardiac defibrillator while on the HTx wait list (44% of ACHD patients listed for transplantation have an ICD vs. 75% of non-ACHD patients; p < 0.001) [37].
5 HTx Outcomes
ACHD patients comprise approximately 3% of the total HTX population, although this number is growing rapidly with a 40% increase in ACHD HTx over the last two decades [36, 39]. Multicenter registries provide a broad overview of ACHD outcomes after HTx (Table 13.4). Single center studies report outcomes by congenital subtype and therefore enable comparison between ACHD subgroups and transplant center [18, 40,41,42,43,44,45,46,47].
ACHD is an independent predictor of increased mortality with a two- to threefold increase in the relative risk of 1-year posttransplant mortality [30, 36, 48]. Of the ACHD subgroups, evaluated mortality is highest in the Fontan population with posttransplant mortality of 25–30% [41, 44,45,46]. The main causes of increased mortality in Fontan recipients are primary graft failure (reflecting longer ischemic times), early rejection (due to sensitization), and the combined effect of comorbid disease (hypoalbuminemia, immune dysfunction, liver disease, coagulopathy, renal dysfunction, and occult PH).
Technical complications are important with death secondary to bleeding and other surgical factors accounting for 10% of ACHD HTx recipient deaths vs. 4% of others (p < 0.0001) [1]. Ischemic times are significantly longer due to the need for reconstructive surgery, required in up to 75% of CHD patients undergoing HTx [49]. Bleeding risks are especially high in patients with single-ventricle anatomy following reconstruction of the aortic arch, vena cavae, or pulmonary arteries and those with dense sternal adhesions from previous sternotomies [50]. For these reasons congenital surgical expertise at the time of HTx in CHD recipients is essential. Excellent outcomes have been reported with congenital heart and adult HTx surgeons working in collaboration, not only at the time of surgery but also in the assessment and planning stages [18, 40]. Current guidelines recommend all ACHD patients undergo transplantation in high-volume centers with combined expertise in congenital heart disease and HTx [14].
A survival paradox exists among ACHD recipients whose high early mortality is balanced by better long-term survival. Compared to other HF subgroups, ACHD patients who survive the early period after HTx have better long-term survival due to lower infection and malignancy-related mortality as well as decreased incidence of coronary allograft vasculopathy. Lower rates of infection and malignancy are likely due to ACHD patient’s younger age and a more robust immune system but with a converse increased risk of graft failure and rejection. The clinical implication of this finding is that immunosuppression protocols need to be carefully adjusted in ACHD recipients to maintain the balance between the competing risks of rejection, infection, and malignancy.
With appropriate risk stratification, patient selection, planning, and team-based care, ACHD patients can successfully undergo transplantation in high-volume centers with combined expertise in congenital heart disease and HTx [14]. An interdisciplinary team providing subspecialist expertise at the time of ACHD patient evaluation/listing, HTx, and perioperative and postoperative care is recommended [18] (Fig. 13.2).
6 Alternatives to Transplantation
6.1 Mechanical Circulatory Support
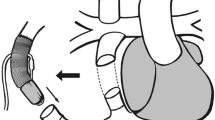
A recent analysis of the INTERMACS data demonstrated that VAD use is limited overall in the ACHD population [51]. ACHD patients frequently require alternative positioning and surgical implantation techniques that vary by anatomy and device (Fig. 13.3) [52,53,54,55,56].
Illustration detailing the orientation of VAD placement and the direction of blood flow in a patient with congenitally corrected transposition of the great arteries. AO aorta, CS coronary sinus, IVC inferior vena cava, LA left atrium, LV left ventricle, PA pulmonary artery, RA right atrium, RV right ventricle. Illustration used with permission and taken from Menachem Congenital Heart Disease 2015
Outcomes in ACHD patients with biventricular anatomy supported by LVAD alone are equivalent to non-ACHD HF patients [51]. However, for those requiring biventricular assist device or total artificial heart, outcomes are poor with low short-term survival and a high incidence of stroke and thromboembolic complications [55]. For most ACHD patients, VAD is used as a bridge to HTx (rather than destination therapy) although little is known about outcomes after HTx in this population. For non-ACHD HF patients bridged to HTx with a VAD, 60-day survival is significantly lower [57]. Future research into outcomes in ACHD patients being bridged to HTx on VADs is important given the potential for higher early mortality related to complex anatomy, multiple sternal reentries, dense scarring, longer ischemic times, and higher sensitization [58].
Mechanical support has been used in patients with a systemic right ventricle (RV) [52, 59] to support the failing RV and to reverse pulmonary (venous) HT. Practical considerations in Mustard/Senning patients include the integrity of the atrial baffles which must be assessed prior to RVAD implant. Baffle stenosis interferes with VAD flow and may contribute to persistent PH in the case of pulmonary baffle stenosis. Baffle leaks increase the risk of thromboembolism, cyanosis, and ventricular volume overload. Preemptive treatment of arrhythmia substrate should also be considered prior to VAD implant given the impact of uncontrolled atrial and ventricular arrhythmias on VAD flows and ventricular function. There is a perception that the subpulmonic LV is less prone to failing after systemic RV implantation [52]. However, failure of the subpulmonic LV is not uncommon and requires preemptive planning at the time of RVAD implant. For those not responding to inotropes and volume removal with ultrafiltration/dialysis, temporary LVAD support may be needed.
6.2 Extracorporeal Membrane Oxygenation (ECMO)
Extracorporeal membrane oxygenation (ECMO) is arguably the most commonly used mechanical support platform for ACHD patients. Most commonly ECMO is used to support ACHD patients who develop acute HF in the postoperative period [60] witnessed as failure to wean from cardiopulmonary bypass. Among 77 CHD patients undergoing HTx between 1988 and 2014, postoperative ECMO was required in 14 patients [61]. Eleven of these patients were placed on ECMO in the operating room due to failure to wean from cardiopulmonary bypass. The remaining three were placed on ECMO within 48 h of surgery due to low cardiac output. Of these 14 patients, 7 died in the hospital. In addition to highlighting poor outcomes with ECMO support, these findings emphasize the importance of ACHD patient selection for cardiac surgeries. ACHD HF patients with significantly reduced ventricular systolic function are especially high risk for acute HF, and candidacy for “exit strategies” (i.e., ECMO transitioning to VAD as a bridge to HTx) is important to consider before surgery is undertaken.
6.3 Palliative Care
Palliative care improves quality of life, anxiety, depression, and spiritual well-being in patients with HF [62]. Increasingly, advanced HF teams incorporate palliative care specialists with expertise in symptom management and end-of-life therapies in the care of all patients with HF requiring advanced therapies. Palliative care can be offered in addition to advanced HF therapies or as the mainstay of treatment in those with contraindications to transplant and mechanical circulatory support.
7 Peri-/Postoperative Management
The perioperative period has been described as the “Achilles’ heel” for transplantation in ACHD patients [48]. In an evaluation of UNOS from 2000 to 2014, graft and cardiovascular dysfunction were found to be the top two reasons for early mortality and were most likely due to the elevated PVR, allosensitization, and longer donor ischemic times. The management of PH in the perioperative setting is paramount to a successful outcome. Following congenital heart surgery, the incidence of perioperative pulmonary hypertensive crisis is in the range of 2–5% [63]. Pulmonary vasoconstriction leads to right HF and systemic hypotension. Treatment options include inhaled nitric oxide, prostacyclins, endothelin receptor antagonists, phosphodiesterase inhibitors, inotropes, and pressors.
Conclusion
Both HF and ACHD are rapidly growing subspecialties of cardiology, but it is only relatively recently that ACHD HF is being recognized as complication requiring particular expertise. As the number of ACHD patients grows, there will be a shortage of appropriately trained providers to care for them. Over time, advanced fellowships will need to collaborate to adequately train both specialties to handle the vast number of patients with ACHD-related HF [64].
In conclusion, HTx is an effective treatment strategy for selected ACHD patients with end-stage cardiac disease. Diagnosis and treatment of ACHD-related HF rely heavily on the art rather than science of medical practice. A number of opportunities for improving outcomes in patients are identified including:
(1) Earlier collaboration with advanced HF teams
(2) Mitigation of risk through team-based evaluation
(3) Recognition that liver disease, PH, and sensitization play important roles in the risk profile of patients
(4) Assessment of sudden cardiac death risk while awaiting transplantation
(5) Multicenter studies to better define guidelines for transplant
(6) Expert surgical planning involving congenital and transplant surgeons to reduce ischemic time and bleeding risk
(7) Tailored immunosuppression protocols in the posttransplant period
(8) Identification of centers of excellence specializing in transplant ACHD patients
(9) The use of VAD as a bridge to HTX
(10) Palliative care as a vital component of care irrespective of the decision to proceed with HTx or not
References
Burchill LJ. Heart transplantation in adult congenital heart disease. Heart. 2016;102:1871–7.
Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M, de Waart M, van Schaik RH, Zijlstra F, Boersma E, Roos-Hesselink JW. Prognostic value of N-terminal Pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation. 2017;135:264–79.
Giannakoulas G, Dimopoulos K, Bolger AP, Tay EL, Inuzuka R, Bedard E, Davos C, Swan L, Gatzoulis MA. Usefulness of natriuretic peptide levels to predict mortality in adults with congenital heart disease. Am J Cardiol. 2010;105:869–73.
Brida M, Dimopoulos K, Kempny A, Liodakis E, Alonso-Gonzalez R, Swan L, Uebing A, Baumgartner H, Gatzoulis MA, Diller GP. Body mass index in adult congenital heart disease. Heart. 2017;103:1250–7.
Dimopoulos K, Diller GP, Petraco R, Koltsida E, Giannakoulas G, Tay EL, Best N, Piepoli MF, Francis DP, Poole-Wilson PA, Gatzoulis MA. Hyponatraemia: a strong predictor of mortality in adults with congenital heart disease. Eur Heart J. 2010;31:595–601.
Dimopoulos K, Diller GP, Giannakoulas G, Petraco R, Chamaidi A, Karaoli E, Mullen M, Swan L, Piepoli MF, Poole-Wilson PA, Francis DP, Gatzoulis MA. Anemia in adults with congenital heart disease relates to adverse outcome. J Am Coll Cardiol. 2009;54:2093–100.
Dimopoulos K, Diller GP, Koltsida E, Pijuan-Domenech A, Papadopoulou SA, Babu-Narayan SV, Salukhe TV, Piepoli MF, Poole-Wilson PA, Best N, Francis DP, Gatzoulis MA. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–8.
Schuuring MJ, van Riel AC, Vis JC, Duffels MG, van Straalen JP, Boekholdt SM, Tijssen JG, Mulder BJ, Bouma BJ. High-sensitivity troponin T is associated with poor outcome in adults with pulmonary arterial hypertension due to congenital heart disease. Congenit Heart Dis. 2013;8:520–6.
Rodriguez FH 3rd, Moodie DS, Parekh DR, Franklin WJ, Morales DL, Zafar F, Adams GJ, Friedman RA, Rossano JW. Outcomes of heart failure-related hospitalization in adults with congenital heart disease in the United States. Congenit Heart Dis. 2013;8:513–9.
John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014;64:54–62.
Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg. 1998;115:1063–73.
Tobler D, Greutmann M, Colman JM, Greutmann-Yantiri M, Librach LS, Kovacs AH. End-of-life care in hospitalized adults with complex congenital heart disease: care delayed, care denied. Palliat Med. 2012;26:72–9.
Ross HJ, Law Y, Book WM, Broberg CS, Burchill L, Cecchin F, Chen JM, Delgado D, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Martin CM, Murphy AM, Singh G, Spray TL, Stout KK. American Heart Association Adults With Congenital Heart Disease Committee of the Council on Clinical C, Council on Cardiovascular Disease in the Young tCoCR, Intervention, the Council on Functional G and Translational B. Transplantation and mechanical circulatory support in congenital heart disease: a scientific statement From the American Heart Association. Circulation. 2016;133:802–20.
Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A. International Society for Heart Lung Transplantation Infectious Diseases P, Heart F and Transplantation C. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35:1–23.
Bradley EA, Pinyoluksana KO, Moore-Clingenpeel M, Miao Y, Daniels C. Isolated heart transplant and combined heart-liver transplant in adult congenital heart disease patients: insights from the united network of organ sharing. Int J Cardiol. 2017;228:790–5.
Wang A, Book WM, McConnell M, Lyle T, Rodby K, Mahle WT. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2007;100:1307–9.
Josephus Jitta D, Wagenaar LJ, Mulder BJ, Guichelaar M, Bouman D, van Melle JP. Three cases of hepatocellular carcinoma in Fontan patients: review of the literature and suggestions for hepatic screening. Int J Cardiol. 2016;206:21–6.
Menachem JN, Golbus JR, Molina M, Mazurek JA, Hornsby N, Atluri P, Fuller S, Birati EY, Kim YY, Goldberg LR, Wald JW. Successful cardiac transplantation outcomes in patients with adult congenital heart disease. Heart. 2017. https://doi.org/10.1136/heartjnl-2016-310933.
Greenway SC, Crossland DS, Hudson M, Martin SR, Myers RP, Prieur T, Hasan A, Kirk R. Fontan-associated liver disease: implications for heart transplantation. J Heart Lung Transplant. 2016;35:26–33.
Reich HJ, Awad M, Ruzza A, De Robertis MA, Ramzy D, Nissen N, Colquhoun S, Esmailian F, Trento A, Kobashigawa J, Czer LS. Combined heart and liver transplantation: the Cedars-Sinai experience. Transplant Proc. 2015;47:2722–6.
Padegimas A, Molina M, Korwin A, Birati E, Kamoun M. Successful long-term outcomes in combined heart-liver transplants across pre-formed high levels of donor-specific antibodies in highly sensitized patients. J Heart Lung Transplant. 2016;35:1382–4.
Lewis M, Ginns J, Schulze C, Lippel M, Chai P, Bacha E, Mancini D, Rosenbaum M, Farr M. Outcomes of adult patients with congenital heart disease after heart transplantation: impact of disease type, previous thoracic surgeries, and bystander organ dysfunction. J Card Fail. 2015;22:578–82.
Berg CJ, Bauer BS, Hageman A, Aboulhosn JA, Reardon LC. Mortality Risk Stratification in Fontan Patients Who Underwent Heart Transplantation. Am J Cardiol. 2017;119:1675–9.
Drakos SG, Kfoury AG, Gilbert EM, Horne BD, Long JW, Stringham JC, Campbell BA, Renlund DG. Effect of reversible pulmonary hypertension on outcomes after heart transplantation. J Heart Lung Transplant. 2007;26:319–23.
Goland S, Czer LS, Kass RM, De Robertis MA, Mirocha J, Coleman B, Capelli C, Raissi S, Cheng W, Fontana G, Trento A. Pre-existing pulmonary hypertension in patients with end-stage heart failure: impact on clinical outcome and hemodynamic follow-up after orthotopic heart transplantation. J Heart Lung Transplant. 2007;26:312–8.
Klotz S, Wenzelburger F, Stypmann J, Welp H, Drees G, Schmid C, Scheld HH. Reversible pulmonary hypertension in heart transplant candidates: to transplant or not to transplant. Ann Thorac Surg. 2006;82:1770–3.
Tedford RJ, Beaty CA, Mathai SC, Kolb TM, Damico R, Hassoun PM, Leary PJ, Kass DA, Shah AS. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant. 2014;33:289–97.
Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54.
Geft D, Kobashigawa J. Current concepts for sensitized patients before transplantation. Curr Opin Organ Transplant. 2017;22:236–41.
Patel ND, Weiss ES, Allen JG, Russell SD, Shah AS, Vricella LA, Conte JV. Heart transplantation for adults with congenital heart disease: analysis of the United network for organ sharing database. Ann Thorac Surg. 2009;88:814–21. discussion 821–2
Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B, Spannagl M, Ueberfuhr P, Donor-specific HLA. alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6:229–35.
Kobashigawa J, Mehra M, West L, Kerman R, George J, Rose M, Zeevi A, Reinsmoen N, Patel J, Reed EF, Consensus Conference P. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213–25.
Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84:1556–62. discussion 1562–3
Kobashigawa J, Crespo-Leiro MG, Ensminger SM, Reichenspurner H, Angelini A, Berry G, Burke M, Czer L, Hiemann N, Kfoury AG, Mancini D, Mohacsi P, Patel J, Pereira N, Platt JL, Reed EF, Reinsmoen N, Rodriguez ER, Rose ML, Russell SD, Starling R, Suciu-Foca N, Tallaj J, Taylor DO, Van Bakel A, West L, Zeevi A, Zuckermann A, Consensus Conference P. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2011;30:252–69.
Boucek D, Yetman AT, Yeung E, Miyamoto SD, Stehlik J, Kfoury AG, Kaza AK, Weng C, Everitt MD. Survival based on patient selection for heart transplant in adults with congenital heart disease: a multi-institutional study. Int J Cardiol. 2014;172:e89–90.
Davies RR, Russo MJ, Yang J, Quaegebeur JM, Mosca RS, Chen JM. Listing and transplanting adults with congenital heart disease. Circulation. 2011;123:759–67.
Everitt MD, Donaldson AE, Stehlik J, Kaza AK, Budge D, Alharethi R, Bullock EA, Kfoury AG, Yetman AT. Would access to device therapies improve transplant outcomes for adults with congenital heart disease? Analysis of the United Network for Organ Sharing (UNOS). J Heart Lung Transplant. 2011;30:395–401.
Gelow JM, Song HK, Weiss JB, Mudd JO, Broberg CS. Organ allocation in adults with congenital heart disease listed for heart transplant: impact of ventricular assist devices. J Heart Lung Transplant. 2013;32:1059–64.
Burchill LJ, Edwards LB, Dipchand AI, Stehlik J, Ross HJ. Impact of adult congenital heart disease on survival and mortality after heart transplantation. J Heart Lung Transplant. 2014;33:1157–63.
Mori M, Vega D, Book W, Kogon BE. Heart transplantation in adults with congenital heart disease: 100% survival with operations performed by a surgeon specializing in congenital heart disease in an adult hospital. Ann Thorac Surg. 2015;99:2173–8.
Chen JM, Davies RR, Mital SR, Mercando ML, Addonizio LJ, Pinney SP, Hsu DT, Lamour JM, Quaegebeur JM, Mosca RS. Trends and outcomes in transplantation for complex congenital heart disease: 1984 to 2004. Ann Thorac Surg. 2004;78:1352–61. discussion 1352–61
Coskun O, Coskun T, El-Arousy M, Parsa MA, Reiss N, Blanz U, Von Knyphausen E, Sandica E, Schulz U, Knobl H, Tenderich G, Bairaktaris A, Kececioglu D, Korfer R. Heart transplantation in adults with congenital heart disease: experience with 15 patients. ASAIO J. 2007;53:103–6.
Greutmann M, Pretre R, Furrer L, Bauersfeld U, Turina M, Noll G, Luescher TF, Trindade PT. Heart transplantation in adolescent and adult patients with congenital heart disease: a case-control study. Transplant Proc. 2009;41:3821–6.
Irving C, Parry G, O’Sullivan J, Dark JH, Kirk R, Crossland DS, Chaudhari M, Griselli M, Hamilton JR, Hasan A. Cardiac transplantation in adults with congenital heart disease. Heart. 2010;96:1217–22.
Izquierdo MT, Almenar L, Martinez-Dolz L, Moro J, Aguero J, Sanchez-Lazaro I, Cano O, Ortiz V, Sanchez R, Salvador A. Mortality after heart transplantation in adults with congenital heart disease: a single-center experience. Transplant Proc. 2007;39:2357–9.
Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM, Hsu DT. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol. 2004;44:2065–72.
Seddio F, Gorislavets N, Iacovoni A, Cugola D, Fontana A, Galletti L, Terzi A, Ferrazzi P. Is heart transplantation for complex congenital heart disease a good option? A 25-year single centre experience. Eur J Cardiothoracic Surg. 2013;43:605–11. discussion 611
Shah DK, Deo SV, Althouse AD, Teuteberg JJ, Park SJ, Kormos RL, Burkhart HM, Morell VO. Perioperative mortality is the Achilles heel for cardiac transplantation in adults with congenital heart disease: evidence from analysis of the UNOS registry. J Card Surg. 2016;31:755–64.
Lamour JM, Addonizio LJ, Galantowicz ME, Quaegebeur JM, Mancini DM, Kichuk MR, Beniaminovitz A, Michler RE, Weinberg A, Hsu DT. Outcome after orthotopic cardiac transplantation in adults with congenital heart disease. Circulation. 1999;100:II200–5.
Doumouras BS, Alba AC, Foroutan F, Burchill LJ, Dipchand AI, Ross HJ. Outcomes in adult congenital heart disease patients undergoing heart transplantation: a systematic review and meta-analysis. J Heart Lung Transplant. 2016;35:1337–47.
VanderPluym CJ, Cedars A, Eghtesady P, Maxwell BG, Gelow JM, Burchill LJ, Maltais S, Koehl DA, Cantor RS, Blume ED. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J Heart Lung Transplant. 2017;37:88–99.
Schweiger M, Falk V, Biry M, Hubler M, Wilhelm MJ. Biventricular failure in dextro-transposition of the great arteries corrected with the Mustard procedure: VAD support of the systemic ventricle is enough. Int J Artif Organs. 2015;38:233–5.
Maly J, Netuka I, Besik J, Dorazilova Z, Pirk J, Szarszoi O. Bridge to transplantation with long-term mechanical assist device in adults after the Mustard procedure. J Heart Lung Transplant. 2015;34:1177–81.
Peng E, O'Sullivan JJ, Griselli M, Roysam C, Crossland D, Chaudhari M, Wrightson N, Butt T, Parry G, MacGowan GA, Schueler S, Hasan A. Durable ventricular assist device support for failing systemic morphologic right ventricle: early results. Ann Thorac Surg. 2014;98:2122–9.
Poh CL, Chiletti R, Zannino D, Brizard C, Konstantinov IE, Horton S, Millar J, d’Udekem Y. Ventricular assist device support in patients with single ventricles: the Melbourne experience dagger. Interact Cardiovasc Thorac Surg. 2017;25:310–6.
Stokes MB, Saxena P, McGiffin DC, Marasco S, Leet AS, Bergin P. Successful bridge to orthotopic cardiac transplantation with implantation of a HeartWare HVAD in management of systemic right ventricular failure in a patient with transposition of the great arteries and previous atrial switch procedure. Heart Lung Circ. 2016;25:e69–71.
Awad M, Czer LS, De Robertis MA, Mirocha J, Ruzza A, Rafiei M, Reich H, Trento A, Moriguchi J, Kobashigawa J, Esmailian F, Arabia F, Ramzy D. Adult heart transplantation following ventricular assist device implantation: early and late outcomes. Transplant Proc. 2016;48:158–66.
Ko BS, Drakos S, Kfoury AG, Hurst D, Stoddard GJ, Willis CA, Delgado JC, Hammond EH, Gilbert EM, Alharethi R, Revelo MP, Nativi-Nicolau J, Reid BB, McKellar SH, Wever-Pinzon O, Miller DV, Eckels DD, Fang JC, Selzman CH, Stehlik J, Utah Transplant Affiliated Hospitals Cardiac Transplant P. Immunologic effects of continuous-flow left ventricular assist devices before and after heart transplant. J Heart Lung Transplant. 2016;35:1024–30.
Menachem JN, Swaminathan AC, Bashore TM, Ward CC, Rogers JG, Milano CA, Patel CB. Initial experience of left ventricular assist device support for adult patients with transposition of the great vessels. Congenit Heart Dis. 2015;10:382–6.
Clark JB, Pauliks LB, Myers JL, Undar A. Mechanical circulatory support for end-stage heart failure in repaired and palliated congenital heart disease. Curr Cardiol Rev. 2011;7:102–9.
Shi WY, Saxena P, Yong MS, Marasco SF, McGiffin DC, Shipp A, Weintraub RG, d'Udekem Y, Brizard CP, Konstantinov IE. Increasing complexity of heart transplantation in patients with congenital heart disease. Semin Thorac Cardiovasc Surg. 2016;28:487–97.
Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, Adams PA, Speck A, Johnson KS, Krishnamoorthy A, Yang H, Anstrom KJ, Dodson GC, Taylor DH Jr, Kirchner JL, Mark DB, O'Connor CM, Tulsky JA. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol. 2017;70:331–41.
Brunner N, de Jesus Perez VA, Richter A, Haddad F, Denault A, Rojas V, Yuan K, Orcholski M, Liao X. Perioperative pharmacological management of pulmonary hypertensive crisis during congenital heart surgery. Pulm Circ. 2014;4:10–24.
Menachem JN. Advanced heart failure in the ACHD population: finding the fellows’ role in a growing field. J Am Coll Cardiol. 2017;69:1986–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Menachem, J.N., Burchill, L.J. (2018). Transplantation and Mechanical Circulatory Support in Adult Congenital Heart Disease-Related Advanced Heart Failure. In: Swan, L., Frogoudaki, A. (eds) Heart Failure in Adult Congenital Heart Disease. Congenital Heart Disease in Adolescents and Adults. Springer, Cham. https://doi.org/10.1007/978-3-319-77803-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-77803-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77802-0
Online ISBN: 978-3-319-77803-7
eBook Packages: MedicineMedicine (R0)