Abstract
This chapter discusses hypercalcemia of malignancy which is the commonest biochemical complication of cancer and recognized as a medical emergency. Hypercalcemia presents with a wide range of clinical symptoms which in some cases can be severe and life-threatening. It is essential for clinicians to consider hypercalcemia as a differential diagnosis in patients with nonspecific symptoms, as hypercalcemia is potentially reversible. The following sections will review normal calcium homeostasis and discuss the mechanisms of how cancer disrupts this tightly regulated system. It is recognized that hypercalcemia is normally associated with advanced disease and, unless antineoplastic treatments are available, is a poor prognostic sign. It is therefore important to consider the individual clinical situation before deciding on an appropriate management plan. Hypercalcemia results in hypovolemia, and the initial management should consist of rehydration. Following this, specific calcium-lowering treatment should be considered. Following rehydration, bisphosphonates have been the treatment of choice for the last 20 years and are effective in the initial treatment for the majority of cases. Unfortunately, it is common for hypercalcemia to relapse, and the best approach to treatment of recurrent and refractory hypercalcemia is not clear. Denosumab is an emerging option, and the initial evidence appears favorable. Further research regarding the use of denosumab for hypercalcemia of malignancy is warranted.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
Malignant hypercalcemia is a common metabolic complication of cancer seen in oncology and palliative care. It is important for clinicians to remain vigilant as its presentation can often be insidious and mimic general disease progression. It may cause significant symptoms that can often be reversed, resulting in major improvements in quality of life. The occurrence of malignant hypercalcemia is a poor prognostic marker with or without treatment. Despite a number of anti-hypercalcemic agents available, bisphosphonates are currently considered the mainstay of treatment.
2 Epidemiology
Hypercalcemia is a common presentation seen both in primary care and in the emergency department. Ninety percent of cases are due to either primary hyperparathyroidism or malignancy (Lafferty 1991). Hypercalcemia due to malignancy typically evolves rapidly and often leads to significant symptoms and therefore acute clinical presentation. Patients admitted to the hospital with hypercalcemia have an almost 50% chance of having a malignancy, compared with those that present to primary care where hyperparathyroidism is the most likely cause (Lindner et al. 2013).
Malignant hypercalcemia is reported to develop in up to 40% of all cancer patients, although incidence varies quite widely depending on the literature (Burt and Brennan 1980; Vassilopoulou-Sellin et al. 1993; Alsirafy et al. 2009). This may be in part due to the variation in defining hypercalcemia. It is also dependent upon which patient group is included, as it is more common in advanced disease and in certain malignancies. The most common solid malignancies associated with malignant hypercalcemia are breast, renal, lung, and squamous cell cancers where the incidence may be close to 50% (Alsirafy et al. 2009). Multiple myeloma, leukemia, and non-Hodgkin’s lymphoma are the most common hematological malignancies associated with malignant hypercalcemia (Burt and Brennan 1980; Vassilopoulou-Sellin et al. 1993).
The presence of malignant hypercalcemia is recognized as a marker of advanced disease and is a poor prognostic sign (Stewart 2005; Rosner and Dalkin 2012). Up to 50% of patients with treated hypercalcemia will have died within 1 month and 75% within 3 months (Ralston et al. 1990; Stewart 2005). Treating hypercalcemia alone has a limited impact on the overall prognosis as it does not modify the underlying advanced malignancy. Antineoplastic treatments together with anti-hypercalcemia management offer the best chance of a longer survival time (Ralston et al. 1990; Kristensen et al. 1998).
3 Pathophysiology
3.1 Normal Homeostasis of Calcium
Calcium is an essential element that is important in maintaining normal cellular function and signalling and maintaining physiological processes, e.g., neuromuscular signalling, hormonal secretion, and blood coagulation (Kasper et al. 2015). As a result, calcium homeostasis to maintain the extracellular calcium ions (ca2+) is tightly regulated. Broadly, calcium levels are controlled by four organs: small intestine, bones, kidneys, and the parathyroid glands. About 10–20% of dietary calcium is absorbed by the small intestine, and the rest is excreted in feces.
Over 90% of the calcium in the body is stored as hydroxyapatite in the skeleton, acting as a reservoir. The remaining 10% is present in the plasma via two forms: the physiologically active calcium ions (ca2+) and calcium bound to carriers, particularly albumin.
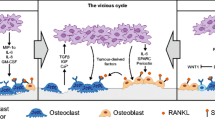
Several hormonal systems are involved in controlling the level of calcium ions in the plasma. The main systems affect the bone remodeling process to increase or decrease the release of calcium from skeletal stores, as well as affect the kidneys to increase or decrease renal calcium excretion. Fig. 1 shows the basic mechanism.
The most significant system is a negative feedback mechanism regulated by two main hormones, parathyroid hormone (PTH) and the active vitamin D metabolite 1,25-dihydroxyvitamin D [Calcitriol] (Kasper et al. 2015).
Calcium sensors on the parathyroid glands activate the release of parathyroid hormone (PTH) when the levels of extracellular calcium ion are low. PTH acts on the kidneys and bones to increase the extracellular calcium levels. PTH affects the kidneys in two ways to increase calcium levels: the reduction of calcium excretion and the production of Calcitriol. The renal production of Calcitriol assists the PTH in mobilizing calcium release from bones and also stimulates the small intestine to increase calcium absorption.
The release of PTH triggers calcium release from the bones by altering bone remodeling, a process that is complex and involves bone-forming cells (osteoblasts) and bone-resorbing cells (osteoclasts). It is the balance between osteoblasts and osteoclasts that controls the rate of bone turnover and calcium release. An important element of this process involves the receptor activator of nuclear factor kappa-B (RANK). This receptor is carried on the osteoclast precursor cells and when stimulated by a ligand (RANKL), which is expressed on the osteoblast, results in the formation of mature osteoclasts leading to increased bone resorption and calcium release (Kasper et al. 2015).
The final important hormone of note is calcitonin, which is produced by thyroid C cells in response to increased calcium levels. The effect of calcitonin reduces bone turnover and calcium reabsorption in the kidneys, which in turn reduce serum calcium.
Understanding this complex relationship between calcium levels, PTH, active vitamin D, and bone cells have allowed effective treatments to be developed that modify this system.
3.2 Mechanism of Malignant Hypercalcemia
The role of bone remodeling and PTH in calcium homeostasis is important in the understanding of the pathophysiology of hypercalcemia in malignancy. Normal calcium homeostasis is disrupted in advanced cancer through two main mechanisms described below.
3.2.1 Humoral Hypercalcemia of Malignancy
The humoral mechanism is responsible for approximately 80% of hypercalcemia related to malignancy, via an increased release of parathyroid hormone-related protein (PTHrP) from the tumor. PTHrP is structurally similar to PTH and initiates calcium release from the bones through increased osteoclastic activity, as well as reduced excretion from the kidneys, causing increased extracellular calcium levels. Unlike PTH, it does not influence the production of Calcitriol and hence has minimal influence on small bowel absorption of calcium (Horwitz et al. 2005). PTHrP acts on osteoblasts, which in turn increase the production of RANKL, and activates osteoclasts and bone resorption. The humoral mechanism does not depend upon the presence or absence of bone metastases and is most commonly seen in breast cancer, squamous cell cancers (e.g., head and neck, esophagus, cervix, or lung), and endometrial and renal cell cancers (Stewart 2005).
3.2.2 Local Osteolytic Hypercalcemia
Patients who have high volume metastatic, osteolytic bone involvement may develop hypercalcemia, via the production of local cytokines from increased osteoclastic activity (Francini et al. 1993). Osteoblastic metastatic disease, such as those typically seen in prostate cancer, is not associated with increased risk of hypercalcemia of malignancy. PTHrP is also a likely mediator of this mechanism, acting on the microenvironment within the bone; hence systemic PTHrP levels may not be raised (Rosner and Dalkin 2012). Metastatic breast and lung cancers, as well as myeloma, commonly involve an osteolytic mechanism which is causing hypercalcemia.
Other rare causes of malignant hypercalcemia include those mediated by increased active vitamin D production, seen most commonly in lymphoma (Seymour and Gagel 1993). In addition, parathyroid carcinoma can cause ectopic PTH production leading to hypercalcemia (VanHouten et al. 2006). A combination of mechanisms may occur simultaneously. In one study of 443 patients with cancer and hypercalcemia, 53% of patients had osteolytic hypercalcemia, 35% had humoral hypercalcemia, and 12% had both osteolytic and humoral factors (Soyfoo et al. 2013).
4 Clinical Presentation
Clinical presentation and development of symptoms in hypercalcemia are related to the rate of increase in serum calcium, rather than simply the absolute value. Hypercalcemia may be asymptomatic in severe levels if it has evolved slowly (Stewart 2005). This is particularly true in younger patients that have no pre-existing comorbidities. Hypercalcemia may therefore be only diagnosed due to an incidental finding on a blood test. It can however also present with very severe symptoms requiring urgent treatment.
The well-known mnemonic often associated with hypercalcemia of “painful bones, renal stones, abdominal groans, and psychic moans” is typically associated with the presentation of primary hyperparathyroidism. Hypercalcemia due to primary hyperparathyroidism often develops over a longer period, allowing for a patient to remain relatively well, but resulting in the development of complications such as renal stones and peptic ulceration.
In hypercalcemia of malignancy, the rapid rise in calcium level results in a patient becoming more constitutionally unwell. A combination of neurological and gastrointestinal symptoms is most common, especially confusion, somnolence, nausea, and constipation. Patients are often significantly dehydrated due to many factors: reduced oral intake, vomiting, and polyuria. In the most severe cases, cardiac complications and seizures may occur. Table 1 details the mild and severe symptoms and signs of hypercalcemia.
5 Diagnosis and Investigation
As the symptoms can be varied and nonspecific, often the most important element of diagnosis is in ensuring that it is part of the differential diagnosis. A patient’s corrected calcium levels should be checked if there is a clinical suspicion that it may be raised. Serum calcium is present in two forms: calcium that is bound to protein, predominantly albumin, and ionized calcium. The physiologically active form is the ionized calcium, and this is maintained despite fluctuations in albumin levels. Laboratories routinely test total serum calcium levels. In healthy individuals, 45% of total calcium is in the active ionized form and 55% bound to carriers. Reference ranges for normal total calcium levels are made assuming an albumin level of 40 g/L (4 g/dL). In patients where albumin levels are low, this ratio is disrupted, and therefore a total serum calcium level will not reflect the active ionized calcium level. Some laboratories can directly measure ionized calcium; however in most cases, it is calculated from the total serum calcium level using the following formula to give a corrected calcium level:
As patients with advanced malignancy commonly have low albumin levels, it is important to ensure that the corrected calcium level is known before a diagnosis of malignant hypercalcemia is made or excluded.
There can be variation in the diagnostic level of hypercalcemia, depending on local guidelines, but it is often classified as mild, moderate, or severe based on the serum ionized calcium level below (Stewart 2005). See Table 2.
In patients who have advanced cancer, it may not be necessary or appropriate to investigate the mechanism of hypercalcemia once it has been diagnosed, particularly as the treatment in most situations is the same regardless of cause. There are occasional situations where there is uncertainty or there is a suspicion of simultaneous mechanisms occurring. Primary hyperparathyroidism is a relatively common diagnosis across the general population with around 20 cases per 100,000 (Ayuk et al. n.d.). Therefore, primary hyperparathyroidism may occur concurrently with hypercalcemia of malignancy, and there is evidence that it may be more common in certain tumor types than the general population (Fierabracci et al. 2001). Primary hyperparathyroidism can be successfully treated with surgical resection of the parathyroid glands. Therefore, in a patient who has a low disease burden and a favorable prognosis, it may be of benefit to confirm the underlying cause of the hypercalcemia. Fig. 2 represents an approach to the diagnosis. In summary, serum PTH level will be raised in primary hyperparathyroidism and suppressed in hypercalcemia of malignancy. It is also possible to check PTHrP levels which can help confirm the underlying mechanism involved in those who have hypercalcemia of malignancy.
While PTH or PTHrP is not routinely requested for a patient with advanced cancer, one study suggests that the level of PTHrP may inform prognostication and predict likely response to the common treatment of bisphosphonates (Wimalawansa 1994b). In this study it was suggested that higher PTHrP levels would result in a poorer response to bisphosphonates treatment possibly due to the nonskeletal effects of PTHrP, such as the renal response, which bisphosphonates will not modify.
6 Treatment
6.1 General Approach
Multiple factors should be considered before deciding on a treatment plan. These include goals of care, severity of hypercalcemia, symptomatology, previous episodes of hypercalcemia (including response to treatment), and finally patient wishes.
Firstly, it is important to establish whether treatment is appropriate. If a patient is moribund due to their advanced malignancy, then treatment is likely to be futile. The difficulty can be distinguishing between disease progression and reversible symptoms secondary to hypercalcemia. Treatment may also not be appropriate in patients who have recently been treated for hypercalcemia and have either rapidly relapsed or been refractory to treatment. In these uncertain situations, a frank discussion about the limitations about efficacious treatments with a patient and their family is required.
When the treatment of the hypercalcemia is considered appropriate, the severity of the hypercalcemia and the symptom burden should be considered next. In asymptomatic patients with mildly raised hypercalcemia, conservative measures can be taken. These measures may include ensuring sufficient parenteral hydration, as well as stopping any contributory medications, e.g., calcium, vitamin D supplements, and thiazide diuretics.
In symptomatic patients with calcium levels greater than 3.0 mmol/L (12 mg/dL), further management is indicated. This initially involves parenteral rehydration, followed by specific anti-hypercalcemic treatments. The most commonly used agents are the bisphosphates and more recently denosumab. Calcitonin is frequently given, although it has limited benefits due to its short-acting effects. There are a number of other medications that are mentioned in the literature, including loop diuretics, gallium nitrate, and octreotide (Stewart 2005; Mirrakhimov 2015; Rosner and Dalkin 2012).
If a more active management approach is warranted, then antineoplastic treatments should also be considered to maintain normocalcemia after hypercalcemia is treated. Antineoplastic treatments such as chemotherapy provide optimal long-term treatment of hypercalcemia and offer the best prognosis (Ralston et al. 1990; Kristensen et al. 1998).
Regardless of the decisions pertaining to the goals of care, the occurrence of hypercalcemia of malignancy is a marker of poor prognosis and a harbinger of death within a few months in the majority of patients. Clear and sensitive information regarding the patient’s advanced illness should be communicated to the patient and their family. It is important to explain to them that the correcting of the calcium levels is a temporizing measure, with the management and control of their underlying malignancy offering the best chance in prolonging survival. Patients and their family are also often fearful of the symptoms caused by hypercalcemia. Irrespective of the decision to treat the hypercalcemia, reassurance should be provided to the patient and the family that the treating team will endeavor to ensure the patient’s comfort by managing the patient’s symptoms utilizing other medications.
6.2 Intravenous Hydration and Role of Loop Diuretics
Hypercalcemia causes significant hypovolemia through a combination of mechanisms. Firstly, raised calcium results in an acquired nephrogenic diabetes insipidus cause polyuria. Secondly, gastrointestinal symptoms may result in nausea, reduced fluid intake, and vomiting. Finally, the hypovolemia itself results in a reduced glomerular filtration rate and therefore reduces the kidney’s ability to excrete calcium. In all cases of symptomatic hypercalcemia, intravenous fluid hydration should be given to correct the volume deficit and to treat the hypercalcemia. The volume and rate of fluid replacement administered should be considered according to the clinical picture, severity of hypercalcemia, renal dysfunction, and cardiac insufficiency. In severe hypercalcemia, the fluid deficit can be profound, and aggressive fluid replacement is required. Current evidence recommends the use of intravenous normal saline at a rate of 200–300 mls/h (Mirrakhimov 2015). The total volume required may be as much as 4–6 l; however caution must be given to avoid fluid overload, particularly in the elderly. Historically, loop diuretics have been used to treat hypercalcemia to promote renal calcium loss. With the availability of more effective treatments, loop diuretics are now only indicated in situations of fluid overload following rehydration (Stewart 2005; Mirrakhimov 2015).
All current evidence recommends the intravenous route for rehydration. In the palliative care population, intravenous access can often be challenging, and the administration of fluids via the subcutaneous route is commonly utilized. The evidence regarding the benefits of administration of subcutaneous fluids is limited, and there is no research available at present assessing the use of subcutaneous fluids in the treatment of hypercalcemia. As most calcium-lowering treatments are given intravenously, using the same route for rehydration would be a sensible option, particularly as aggressive fluid replacement is often required. In situations where intravenous access is challenging, 2 l of normal saline can be administered subcutaneously in 24 h (Barton et al. 2004).
6.3 Specific Calcium-Lowering Treatments
6.3.1 Bisphosphonates
Bisphosphonates have been the mainstay in the treatment of malignant hypercalcemia for over 20 years (Saunders et al. 2004). As pyrophosphate analogs, bisphosphonates inhibit intracellular osteoclast activity, as well as bind to hydroxyapatite and stabilize the bone matrix (Rogers et al. 2000). Following administration, about 50% of the drug is selectively retained in the skeleton, and the remainder is eliminated in the urine without being metabolized. Skeletal uptake and retention are dependent on bisphosphonate potency for bone matrix, as well as patient factors including renal function, rate of bone turnover, and binding site availability. This adhesion to the bone matrix results in a prolonged half-life and mechanism of action. It is this enduring effect that has made bisphosphonates so important in the management of hypercalcemia of malignancy.
There are two groups of bisphosphonates: first-generation, non-nitrogen-containing bisphosphonates which include etidronate and clodronate; and the second-generation, nitrogen-containing bisphosphonates which include pamidronate, ibandronate, and, most recently, zoledronate. The second-generation bisphosphonates are considered more potent. There are both oral and parenteral bisphosphonates available. In the treatment of hypercalcemia, they are always given parenterally to ensure absorption and to avoid gastrointestinal side effects often seen with oral preparations. While most parenteral bisphosphonates can only be given intravenously, clodronate can be given intravenously or subcutaneously (Roemer-Bécuwe et al. 2003). The subcutaneous route may be useful in cases where intravenous access is difficult or when the patient is seen in the community setting.
Although extremely well-tolerated, bisphosphonates do have potential adverse effects. The most significant adverse effect is the risk of renal injury with possible nephrotic syndrome. To prevent renal toxicity, intravenous rehydration prior to the administration of bisphosphonates is always recommended. Where renal impairment also exists, a dose reduction may be considered to reduce the risk of further renal damage. A rare, but significant, adverse effect of bisphosphonates is osteonecrosis of the jaw. This is typically associated with prolonged and repeated use of bisphosphates (greater than 4 months). In the acute management of hypercalcemia of malignancy, the risk of osteonecrosis of the jaw is low (Saad et al. 2012). It may be worthwhile assessing the dentition of the patient prior to administration; however there is no evidence to support this approach when bisphosphonates are being used in the treatment of hypercalcemia. Other reported adverse effects include drug-related induced fevers, hypophosphatemia, and hypocalcaemia (Major et al. 2001). The drug-related induced fever is part of an acute phase reaction that causes transient flu-like symptoms. The true incidence of hypocalcaemia associated with bisphosphonate use in the treatment of hypercalcemia is unknown due to underreporting of cases. However, in clinical trials comparing zoledronate versus denosumab in the prevention of skeletal-related events in cancer patients, hypocalcaemia occurred in about 3.4–5.8% of patients treated with zoledronate (Body et al. 2015; Dranitsaris and Hatzimichael 2012). Although the frequency of bisphosphonate administration in the prevention of skeletal-related events is different compared to the treatment of hypercalcemia, clinicians should be vigilant of the possible complications of bisphosphonate-related symptomatic hypocalcaemia if using a bisphosphonate.
When selecting a bisphosphonate , the systematic review by Saunders et al. provides some limited guidance. The review showed that all bisphosphonates are effective when compared with placebo, with normal calcium being achieved in at least 70% of cases, regardless of which drug was used (Saunders et al. 2004). Table 3 details typical dose and administration regimes.
Pamidronate and zoledronate are the most commonly used bisphosphonates in the treatment of hypercalcemia. Although both drugs are effective in achieving normocalcemia, zoledronate tends to be favored for its ease in administration (15 min for zoledronate versus 2 h for pamidronate), potency, and efficacy (Major et al. 2001). In a pooled analysis of two randomized controlled trials involving 275 patients with hypercalcemia of malignancy, 87–88% of patients achieved normocalcemia after a single dose of zoledronate (4 mg or 8 mg) compared to 70% of patients who were treated with pamidronate. The mean duration of normocalcemia in patients who had received zoledronate was 32–43 days, compared to 18 days in patients who had received pamidronate (Major et al. 2001).
Although pamidronate and zoledronate have been shown to have a similar side effect profile, the 8 mg dose of zoledronate has shown an increased risk of causing renal injury compared to the 4 mg zoledronate dose and pamidronate (Major et al. 2001; Saunders et al. 2004). It is generally not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) to receive bisphosphonates. However, in some clinical situations where patients have limited effective options, the use of bisphosphonates may be indicated. Limited data suggests that ibandronate may be the safest option (Jackson 2005). Dose reduction, slowing the rate of the infusion, and the addition of increased hydration therapy can also be considered; however there is minimal literature to support this (Conte and Guarneri 2004; Kyle et al. 2007). Denosumab may potentially be an option in this scenario, and this will be discussed later.
Manufacturers suggest that the dose of pamidronate administered should depend on the severity of hypercalcemia. However, a systematic review from 2004 suggests that higher doses of bisphosphonates correlate with increased efficacy and therefore recommend use of the highest dose irrespective of the calcium level (Saunders et al. 2004). Given this review, pamidronate 90 mg or zoledronate 4 mg are appropriate first-line options in the treatment of malignant hypercalcemia.
Ibandronate and etidronate are less commonly used bisphosphonates. Ibandronate is a second-generation bisphosphonate and has been shown to be as effective as pamidronate. It appears to have a lower risk of renal injury; however there is limited data (Jackson 2005). Etidronate is a first-generation bisphosphonate and one of the first bisphosphonates to show efficacy in the treatment of hypercalcemia of malignancy. As it is administered via a 2-h intravenous infusion on 3 consecutive days, it has been superseded by newer more potent drugs that can be administered over a shorter time frame.
Regardless of which bisphosphonate is used, the reduction in calcium levels takes approximately 2–4 days to occur with the maximum effect between 4 and 7 days (Major et al. 2001). It is recommended that the serum-corrected calcium is rechecked 5–7 days following treatment with a bisphosphonate (Fleisch 1998). In most cases, intravenous rehydration given prior to bisphosphonate reduces the calcium level sufficiently while waiting for the bisphosphonates to act. In patients who have severe symptoms, needing immediate calcium reduction, calcitonin may be used. (See section below for further details.)
Up to 30% of cases of hypercalcemia are refractory to treatments with a bisphosphonate (Major et al. 2001; Saunders et al. 2004). There is limited evidence about which drug should be used in these cases. In the pooled analysis of the two randomized controlled trials by Major et al., patients who were refractory to zoledronate (4 or 8 mg) or pamidronate were retreated with 8 mg of zoledronate. Up to 55% of patients with refractory hypercalcemia responded to retreatment with zoledronate (Major et al. 2001). It is important to note that with time, hypercalcemia will usually become more difficult to treat and eventually may become resistant to bisphosphonate treatment. It is uncertain exactly why this occurs, but it is thought most likely related to the advancing underlying disease. In situations of refractory hypercalcemia, there is emerging evidence that the use of denosumab may be effective and is discussed further below. In patients with refractory hypercalcemia, zoledronate 8 mg may be trialled following initial treatment (Major et al. 2001).
Despite relapse being common in those who achieve normal calcium levels, there are no clear guidelines regarding how often serum calcium levels should be checked. However, given that the median time for relapse is between 2 and 4 weeks (Major et al. 2001; Wimalawansa 1994a), calcium levels could be checked 2–4 weeks posttreatment. A more conservative option would be to retest only if symptoms reoccur.
Finally, there is limited evidence to support the regular administration of bisphosphonates rather than waiting for relapse. One small study with 34 patients, investigating optimal frequency of pamidronate in the treatment of hypercalcemia, showed that a regular infusion every 2 weeks decreased the incidence of symptomatic hypercalcemia and prolonged survival compared to the regular infusion every 3 weeks (Wimalawansa 1994a). Until further evidence becomes available, the decisions regarding follow-up and the best drug to use in retreatment should be determined on an individual basis.
6.3.2 Denosumab
Denosumab is the latest treatment option in the management of hypercalcemia of malignancy. It is a human monoclonal antibody that specifically binds human RANKL. Denosumab inhibits osteoclast activity resulting in reduced bone resorption. Originally developed as an alternative option in the prevention and treatment of osteoporosis, it was subsequently used in the management and prevention of skeletal complications in cancer.
Currently, there are no randomized controlled trials comparing denosumab and bisphosphonates as first-line therapy for the management of hypercalcemia of malignancy. There is one single-arm study carried out by Hu et al. involving 33 patients who had bisphosphonate refractory hypercalcemia. The patients in this study had to have a corrected serum calcium level of >3.1 mmol/L (12.5 mg/dL) despite intravenous bisphosphonate treatment within 7–30 days. In this study, 64% of patients had serum calcium levels below 3.0 mmol/L (11.5 mg/dL) by day 10 after receiving denosumab. An improvement in symptoms was observed in over 50% of patients. The treatment effects were durable with an estimated median duration for compete response being 34 days (Hu et al. 2014). In this study the dose used was 120 mg given subcutaneously, every 4 weeks, with additional loading doses of 120 mg on days 8 and 15 of the first month. A repeat dose of denosumab was given successfully to patients who had relapsed. About 80% of the patients responded to the repeat dose. Therefore, a repeat dose of denosumab treatment on day 8 and 15 after initial treatment could be considered if calcium levels have not previously responded.
A retrospective case series of seven patients treated with single doses of denosumab for the management of hypercalcemia was described. In this small study, six of the seven patients had received bisphosphonates prior to treatment with denosumab. The mean corrected calcium levels were 3.06 mmol/L (12.24 mg/dL) on the day of the denosumab administration, and the last mean corrected calcium while in the hospital was 2.48 mmol/L (9.92 mg/dL) (Dietzek et al. 2015).
With the exception of the study by Hu et al. and Dietzek et al., the vast majority of the research performed utilizing denosumab is in the context of the management or prevention of skeletal-related events, and these results have been extrapolated to the management of hypercalcemia. Although the administration and dose of the drug are similar in both clinical scenarios, and the patient population appears similar, one must exercise caution in presuming that the use of denosumab in both clinical situations are identical. Patients with hypercalcemia typically have advanced disease, a different calcium metabolism profile and a poor prognosis, and may be different from patients who only have metastases to bones.
However, because of the scarcity of studies with the primary purpose of determining the role of denosumab in the management of hypercalcemia, understanding the effects of denosumab in the management and prevention of skeletal-related events will inform clinicians about the issues to be aware of when using denosumab for management of hypercalcemia.
In studies comparing denosumab and zoledronate in the prevention of skeletal complications in advanced cancer, the denosumab arm had fewer episodes of hypercalcemia compared to the zoledronate arm. Furthermore, the time to hypercalcemia was also delayed with the use of denosumab compared to zoledronate (Martin et al. 2012; Stopeck et al. 2010; Diel et al. 2015; Henry et al. 2011).
In regard to its safety and adverse effects, denosumab was well-tolerated in the management of osteoporosis and the skeletal complications of cancer. The most serious risk is that of osteonecrosis of the jaw; however this is rare, and rates appear similar to that of bisphosphonates (Stopeck et al. 2010; Fizazi et al. 2011; Henry et al. 2011; Martin et al. 2012; Dranitsaris and Hatzimichael 2012). The most clinically relevant risk is hypocalcaemia, extrapolated from studies where denosumab has been used in management of malignant bone disease and not in hypercalcemia. Up to 12.8% of patients treated with denosumab for skeletal complications develop significant hypocalcaemia, compared with 1–5% of those treated with zoledronate (Henry et al. 2011; Fizazi et al. 2011; Body et al. 2015; Dranitsaris and Hatzimichael 2012). In one study in patients with skeletal-related events, the median time to first occurrence of hypocalcaemia was 3.8 months with denosumab and 6.5 months with zoledronate (Body et al. 2015). It is worth noting that the highest incidence of hypocalcaemia was in the treatment of metastatic prostate cancer. As discussed, prostate cancer is associated with osteoblastic bone metastases, which in themselves may contribute to development of hypocalcaemia (Henry et al. 2011; Fizazi et al. 2011). In the context of the management of hypercalcemia, it is unclear what the clinical impact of denosumab-induced hypocalcaemia has. In a case series where denosumab was used for the management of hypercalcemia, one of seven patients developed symptomatic hypocalcaemia (Dietzek et al. 2015).
In a study by Body et al. (2015), the pooled results of three randomized controlled trial comparing the efficacy and safety of denosumab versus zoledronate in the prevention of skeletal-related events in metastatic bone disease showed that patients who took calcium and/or vitamin D supplements had a lower incidence of hypocalcaemia. This may suggest that adequate supplementation of both vitamin D and calcium reduced the risk of hypocalcaemia in patients treated with either denosumab or zoledronate. Patients with skeletal-related events have a different calcium profile compared with patients with hypercalcemia. There is no evidence for the routine monitoring of vitamin D levels and its replacement in the patients with hypercalcemia treated with denosumab. Indeed, the replacement of vitamin D has the potential to exacerbate hypercalcemia by mobilizing calcium release from bones and also stimulating the small intestine to increase calcium absorption.
In addition, the study found that patients who were at risk of developing hypocalcaemia include patients with prostate cancer or small cell lung cancer, reduced creatinine clearance (30 to <60 mL/min), and higher baseline values of urinary N-telopeptide of type 1 collagen and bone-specific alkaline phosphatase (Body et al. 2015). Given these findings, it would be prudent to monitor the calcium levels in patients who have these risk factors who are treated with denosumab regardless of reason.
Despite the limited information about the use of denosumab, there are some definite advantages identified. Denosumab is less likely to cause the acute phase reactions that are commonly seen with bisphosphonates (Henry et al. 2011; Fizazi et al. 2011; Stopeck et al. 2010; Dranitsaris and Hatzimichael 2012). It is also safer in renal impairment and not associated with renal injury (Henry et al. 2011; Stopeck et al. 2010; Martin et al. 2012; Dranitsaris and Hatzimichael 2012). In addition, it is administered via the subcutaneous route which may facilitate the use of denosumab in the community. Despite denosumab being more expensive compared to zoledronate, the ability to administer it at home subcutaneously may save on hospitalization costs.
6.3.3 Calcitonin
Calcitonin is a hormone produced by the parafollicular C cells of the thyroid gland. It inhibits the resorption of the bone by reducing both the number and activity of osteoclasts. Calcitonin also acts on the kidneys to reduce calcium reabsorption and inhibits intestinal calcium absorption. Administration of calcitonin occurs subcutaneously or intramuscularly every 12 h, with an initial dose of 4 international units/kg that can be increased up to 8 international units/kg every 6 h. As calcitonin works rapidly within 4–6 h (Vaughn and Vaitkevicius 1974), it may be used in combination with another anti-hypercalcemic agent such as bisphosphonates or glucocorticoids (Binstock and Mundy 1980; Sekine and Takami 1998).
Tachyphylaxis, the rapid reduction in the efficacy of a drug with repeated doses, seems to occur, therefore limiting long-term use after approximately 48–72 h (Vaughn and Vaitkevicius 1974). The reasons for tachyphylaxis are unclear and controversial but thought to be due to the formation of antibodies against heterologous calcitonins like salmon calcitonin (Grauer et al. 1995). The co-administration of glucocorticosteroids may prevent tachyphylaxis (Binstock and Mundy 1980). The main side effects of calcitonin include flushing, nausea, and vomiting.
6.3.4 Corticosteroids
Corticosteroids are most likely to benefit patients who have hypercalcemia as a result of increased Calcitriol production, as seen in some patients with lymphoma or chronic granulomatous disease. Steroids inhibit 1-alpha-hydroxylase conversion of 25-hydroxyvitamin D into Calcitriol, where reduced Calcitriol levels cause a decrease in intestinal absorption of calcium. In patients with hypercalcemia due to granulomatous diseases, prednisolone 20–40 orally daily would be a reasonable starting dose. The calcium levels should decrease within 3–5 days (Sharma 1996).
6.3.5 Gallium Nitrate
Gallium nitrate was initially developed because of its anticancer effect but was observed to cause a transient hypocalcaemia. Gallium nitrate works by inhibiting the release of calcium from the bone, but the mechanisms by which gallium nitrate exerts its effects are unclear (Warrell et al. 1984). It appears to have multiple effects such as the inhibition of osteoclast-mediated bone resorption, stimulation of bone formation, and alteration of the mineral composition and properties of bone.
The usual dose of gallium nitrate is a 5-day continuous intravenous infusion of 200 mg/m2 per day. It is the long duration of treatment that limits its clinical use. There have been three randomized controlled trials comparing gallium nitrate and pamidronate, etidronate, and calcitonin. Gallium nitrate was effective in achieving normocalcemia and appeared to have a longer duration of normocalcemia compared to the bisphosphonates (Cvitkovic et al. 2006; Warrell et al. 1991). In a phase two randomized, double-blind trial of gallium nitrate versus pamidronate, 69% of the patients treated with gallium nitrate achieved normocalcemia compared with 56% of patients who were treated with pamidronate. The duration of normocalcemia was 14 days in patients who responded to gallium nitrate compared to 10 days in patients who responded to pamidronate (Cvitkovic et al. 2006). Gallium nitrate is generally well-tolerated, with the main side effects being asymptomatic hypophosphatemia (Warrell et al. 1991).
6.3.6 Mithramycin
Mithramycin is an antineoplastic antibiotic used as a chemotherapy agent. It is works by reducing both bone resorption and renal tubular calcium reabsorption (Ralston et al. 1985). It is usually administered as a single intravenous injection of 25 mcg/kg in 500 ml dextrose and can be repeated after 2 days. The serum calcium levels fall within 24–48 h of administration with a maximal effect at 2–4 days and a duration of action of 9–10 days (Godfrey 1971). The side effects of mithramycin include nausea, vomiting, fatigue, thrombocytopenia, and worsening liver function (Ralston et al. 1985). As the bisphosphonates are more efficacious and safer, mithramycin is rarely used in practice today.
6.3.7 Ocreotide
The evidence supporting the use of octreotide for the management of hypercalcemia is weak. Most of the evidence in the literature is based on single case reports (Mantzoros et al. 1997; Shiba et al. 1996).
6.3.8 Dialysis
Dialysis is effective in reducing serum calcium levels by hemodialysis with little or no calcium in the dialysate fluid. It is usually only used if no other options are available and has to be considered in the context of the clinical goals of treatment. Dialysis is likely to be considered when a patient has renal impairment or cardiac failure and where aggressive fluid hydration may be challenging.
7 Conclusion and Summary
Hypercalcemia of malignancy is a common condition and must be considered in a patient who presents with nonspecific symptoms and functional deterioration. The symptoms of hypercalcemia may be reversible with a number of treatments. Initial treatment should include intravenous hydration, followed by bisphosphonates. If urgent reduction of calcium levels is required and the patient is distressed by the symptoms, commencing calcitonin could be considered. Bisphosphonates such as zoledronate 4 mg or pamidronate 90 mg are currently the main medications of choice in the management of hypercalcemia of malignancy. The evidence for the use of denosumab is limited but can be considered if the hypercalcemia is refractory to bisphosphonates or if the patient has renal impairment. Hypercalcemia signifies a poor prognosis and antineoplastic treatments to manage the underlying cancer which has the best chance of improving survival where appropriate.
References
Alsirafy SA, Sroor MY, Al-Shahri MZ. Hypercalcemia in advanced head and neck squamous cell carcinoma: prevalence and potential impact on palliative care. J Support Oncol. 2009;7(5):154–7.
Ayuk J, Gittoes N, Acknowledgements. Primary hyperparathyroidism. http://bestpractice.bmj.com/best-practice/monograph/133/basics/epidemiology.html. (n.d.). Accessed 28 Feb 2017.
Barton A, Fuller R, Dudley N. Using subcutaneous fluids to rehydrate older people: current practices and future challenges. QJM Int J Med. 2004;97(11):765–8. https://doi.org/10.1093/qjmed/hch119.
Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269–72.
Body J-J, Bone HG, de Boer RH, Stopeck A, Van Poznak C, Damião R, Fizazi K, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer (Oxford, England: 1990). 2015;51(13):1812–21. https://doi.org/10.1016/j.ejca.2015.05.016.
Burt ME, Brennan MF. Incidence of hypercalcemia and malignant neoplasm. Arch Surg (Chicago, Ill.: 1960). 1980;115(6):704–7.
Conte PF, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist. 2004;9(Suppl 4):28–37. https://doi.org/10.1634/theoncologist.9-90004-28.
Cvitkovic F, Armand J-P, Tubiana-Hulin M, Rossi J-F, Warrell RP. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J (Sudbury, Mass). 2006;12(1):47–53.
Diel IJ, Body J-J, Stopeck AT, Vadhan-Raj S, Spencer A, Steger G, von Moos R, Goldwasser F, Feng A, Braun A. The role of denosumab in the prevention of hypercalcaemia of malignancy in cancer patients with metastatic bone disease. Eur J Cancer. 2015;51(11):1467–75.
Dietzek A, Connelly K, Cotugno M, Bartel S, McDonnell AM. Denosumab in hypercalcemia of malignancy: a case series. J Oncol Pharm Pract. 2015;21(2):143–7. https://doi.org/10.1177/1078155213518361.
Dranitsaris G, Hatzimichael E. Interpreting results from oncology clinical trials: a comparison of denosumab to zoledronic acid for the prevention of skeletal-related events in cancer patients. Support Care Cancer. 2012;20(7):1353–60. https://doi.org/10.1007/s00520-012-1461-4.
Fierabracci P, Pinchera A, Miccoli P, Conte PF, Vignali E, Zaccagnini M, Marcocci C, Giani C. Increased prevalence of primary hyperparathyroidism in treated breast cancer. J Endocrinol Investig. 2001;24(5):315–20. https://doi.org/10.1007/BF03343867.
Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. https://doi.org/10.1016/S0140-6736(10)62344-6.
Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19(1):80–100. https://doi.org/10.1210/edrv.19.1.0325.
Francini G, Petrioli R, Maioli E, Gonnelli S, Marsili S, Aquino A, Bruni S. Hypercalcemia in breast cancer. Clin Exp Metastasis. 1993;11(5):359–67. https://doi.org/10.1007/BF00132979.
Godfrey TE. Mithramycin for hypercalcemia of malignant disease. Calif Med. 1971;115(4):1–4.
Grauer A, Ziegler R, Raue F. Clinical significance of antibodies against calcitonin. Exp Clin Endocrinol Diabetes. 1995;103(6):345–51. https://doi.org/10.1055/s-0029-1211376.
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32. https://doi.org/10.1200/JCO.2010.31.3304.
Horwitz MJ, Tedesco MB, Sereika SM, Syed MA, Garcia-Ocaña A, Bisello A, Hollis BW, et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 Vitamin D. J Bone Miner Res. 2005;20(10):1792–803. https://doi.org/10.1359/JBMR.050602.
Hu MI, Glezerman IG, Leboulleux S, Insogna K, Gucalp R, Misiorowski W, Yu B, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metabol. 2014;99(9):3144–52. https://doi.org/10.1210/jc.2014-1001.
Jackson GH. Renal safety of ibandronate. Oncologist. 2005;10(Suppl 1):14–8. https://doi.org/10.1634/theoncologist.10-90001-14.
Kasper D, Fauci A, Hauser S, Longo D, Jameson J. Harrison’s principles of internal medicine. New York: McGraw-Hill Education; 2015.
Kristensen B, Ejlertsen B, Mouridsen HT, Loft H. Survival in breast cancer patients after the first episode of hypercalcaemia. J Intern Med. 1998;244(3):189–98. https://doi.org/10.1046/j.1365-2796.1998.00355.x.
Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, Orlowski RZ, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25(17):2464–72. https://doi.org/10.1200/JCO.2007.12.1269.
Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(Suppl 2):S51–9; discussion S61. https://doi.org/10.1002/jbmr.5650061413.
Lindner G, Felber R, Schwarz C, Marti G, Leichtle AB, Fiedler G-M, Zimmermann H, Arampatzis S, Exadaktylos AK. Hypercalcemia in the ED: prevalence, etiology, and outcome. Am J Emerg Med. 2013;31(4):657–60. https://doi.org/10.1016/j.ajem.2012.11.010.
Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, Yunus F, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558–67.
Mantzoros CS, Suva LJ, Moses AC, Spark R. Intractable hypercalcaemia due to parathyroid hormone-related peptide secretion by a carcinoid tumour. Clin Endocrinol. 1997;46(3):373–5.
Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, Fallowfield L, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18(17): 4841–9. https://doi.org/10.1158/1078-0432.CCR-11-3310.
Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci. 2015;7(11):483–93. https://doi.org/10.4103/1947-2714.170600.
Ralston SH, Gardner MD, Dryburgh FJ, Jenkins AS, Cowan RA, Boyle IT. Comparison of aminohydroxypropylidene diphosphonate, mithramycin, and corticosteroids/calcitonin in treatment of cancer-associated hypercalcaemia. Lancet (London, England). 1985;2(8461):907–10.
Ralston SH, Gallacher SJ, Patel U, Campbell J, Boyle IT. Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112(7):499–504.
Roemer-Bécuwe C, Vigano A, Romano F, Neumann C, Hanson J, Quan HK, Walker P. Safety of subcutaneous clodronate and efficacy in hypercalcemia of malignancy: a novel route of administration. J Pain Symptom Manag. 2003;26(3):843–8. https://doi.org/10.1016/S0885-3924(03)00252-5.
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(Suppl 12):2961–78.
Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol. 2012;7:1722. https://doi.org/10.2215/CJN.02470312.
Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, Diel IJ, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341–7. https://doi.org/10.1093/annonc/mdr435.
Saunders Y, Ross JR, Broadley KE, Edmonds PM, Patel S, Steering Group. Systematic review of bisphosphonates for hypercalcaemia of malignancy. Palliat Med. 2004;18(5):418–31.
Sekine M, Takami H. Combination of calcitonin and pamidronate for emergency treatment of malignant hypercalcemia. Oncol Rep. 1998;5(1):197–9.
Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood. 1993;82(5):1383–94.
Sharma OP. Vitamin D, calcium, and sarcoidosis. Chest. 1996;109(2):535–9.
Shiba E, Inoue T, Akazawa K, Takai S. Somatostatin analogue treatment for malignant hypercalcemia associated with advanced breast cancer. Gan to Kagaku Ryoho Cancer Chemother. 1996;23(3):343–7.
Soyfoo MS, Brenner K, Paesmans M, Body JJ. Non-malignant causes of hypercalcemia in cancer patients: a frequent and neglected occurrence. Support Care Cancer. 2013;21(5):1415–9. https://doi.org/10.1007/s00520-012-1683-5.
Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373–9. https://doi.org/10.1056/NEJMcp042806.
Stopeck AT, Lipton A, Body J-J, Steger GG, Tonkin K, de Boer RH, Lichinitser M, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. https://doi.org/10.1200/JCO.2010.29.7101.
VanHouten JN, Yu N, Rimm D, Dotto J, Arnold A, Wysolmerski JJ, Udelsman R. Hypercalcemia of malignancy due to ectopic transactivation of the parathyroid hormone gene. J Clin Endocrinol Metab. 2006;91(2): 580–3. https://doi.org/10.1210/jc.2005-2095.
Vassilopoulou-Sellin R, Newman BM, Taylor SH, Guinee VF. Incidence of hypercalcemia in patients with malignancy referred to a comprehensive cancer center. Cancer. 1993;71(4):1309–12.
Vaughn CB, Vaitkevicius VK. The effects of calcitonin in hypercalcemia in patients with malignancy. Cancer. 1974;34(4):1268–71.
Warrell RP, Bockman RS, Coonley CJ, Isaacs M, Staszewski H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J Clin Invest. 1984;73(5): 1487–90. https://doi.org/10.1172/JCI111353.
Warrell RP, Murphy WK, Schulman P, O’Dwyer PJ, Heller G. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8): 1467–75. https://doi.org/10.1200/JCO.1991.9.8.1467.
Wimalawansa SJ. Optimal frequency of administration of pamidronate in patients with hypercalcaemia of malignancy. Clin Endocrinol. 1994a;41(5):591–5.
Wimalawansa SJ. Significance of plasma PTH-Rp in patients with hypercalcemia of malignancy treated with bisphosphonate. Cancer. 1994b;73(8):2223–30. https://doi.org/10.1002/1097-0142(19940415)73:8<2223::AID-CNCR2820730831>3.0.CO;2-C.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Tham, K.A., Seah, D.S.E. (2019). Hypercalcemia of Malignancy. In: MacLeod, R., Van den Block, L. (eds) Textbook of Palliative Care. Springer, Cham. https://doi.org/10.1007/978-3-319-77740-5_70
Download citation
DOI: https://doi.org/10.1007/978-3-319-77740-5_70
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77738-2
Online ISBN: 978-3-319-77740-5
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences