Abstract
Ascidians form a widespread marine invertebrate group and are heterogeneous in terms of the taxonomic groups’ evolutionary lineages. The ascidian genomes lack significant homologies for rearranging genes of the vertebrate adoptive immunity. Genome analysis, gene sequencing, and transcriptional profiling have allowed us to disclose upregulation of innate immunity genes and cell labeling with riboprobes and antibodies has identified hemocyte types in tunic and pharynx inflammatory responses. Lymphocyte-like cells are stem cells and their immunocompetence has been proposed. Granulocyte types (compartment/morula cells) and hemocytes with large granules/vacuoles (compartment/morula cells) are mature cells expressing and releasing inflammatory components. LPS stimulates gene families of innate immune receptor homologs of the mammalian counterparts, as well as immune regulatory genes, during inflammatory responses. Proinflammatory components are involved in allogeneic reactions, and nonself and missing-self recognitions may be proposed. The findings on Ig-like domains contained in chitin-binding proteins (VCBPs) indicate the ancestral origin of vertebrate adaptive immunity and show that relevant genetic circuitry was already in place in the common ancestor of the protochordates and vertebrates. On the other hand, ascidians share with the other invertebrates the prophenoloxidase system that produces melanin and is involved in the inflammatory cytotoxic mechanism. The peroxinectin gene is also upregulated. Damage signals could be proinflammatory, but there are difficulties in assessing this that presumably could be examined during larva metamorphosis.
Findings indicate that genetic circuitries relevant for vertebrate innate immunity were already in place in the common ancestor of the protochordates and vertebrates.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Ascidians
- Tunic
- Pharynx
- Hemocytes
- Evolution
- Toll-like receptors
- Galectins
- RBLs
- Collectins
- Complement
- Phenoloxidase
- Cytokines
- Allorecognition
Introduction
The Ascidians—New Insights into an Old Problem
Tunicata (phylum Chordata) are filter-feeding marine invertebrate protochordates that occupy a key phylogenetic position in chordate evolution, representing modern-day descendants of the chordate progenitor. At the larval stage, most of them present temporary chordate characters including a notochord and dorsal nerve cord . In addition, the adults are provided with a wide respiratory pharynx, equipped with a ventral ciliated channel, structurally distinguishable (endostyle ), for collecting food particles. The endostyle is also provided with a glandular thyroid-like structure secreting iodoproteins (Burighel and Cloney 1997). According to genome-wide sequence information, Tunicata are considered the sister group of Vertebrata (Delsuc et al. 2006, 2008; Swalla and Smith 2008), thus assuming a deep meaning in the study of the evolutionary biology (Fig. 1).
Ascidiacea (sea squirts) are a representative class of the Tunicata subphylum. They are sessile and include both solitary and colonial organisms widespread all over the seas (about 3000 species). This class includes the most common and favored model species studied for developmental biology as well as for immune-related gene annotation; comparative analysis of conserved protein sequences such as domains, modules, or motifs; and the upregulation of gene transcription challenged by harmful agents. Genome-wide surveying and evolutionary history disclose—besides conservation of genes across metazoans —genes shared with vertebrates and ascidian/tunicate–specific genes that diverge between orders, while polymorphism can characterize distinct populations. In tunicate species, the rates and patterns of molecular evolution are peculiar and they appear to be fast evolving (Berná and Alvarez-Valin 2014). The debate on the phylogenesis of the ascidian orders and families remains open.
The findings reported here mainly result from species belonging to different orders, different families of a same order, and species within the same family, as well as from ascidians with opposite lifestyles (solitary/colonial). Therefore, differences between evolutionary lineages can be expected.
The different species here that are mainly mentioned include Ciona intestinalis, Ciona savigny, and Phallusia mammillata, which belong to two distinct families of the order Phlebobranchiata; Styela plicata, Styela clava, Molgula manhattensis, the colonial Botryllus schlosseri, Botrylloides leachi, and the budding Polyandrocarpa misakiensis, which belong to the same family (styelids) of stolidobranchs, whereas another family of this order includes Halocynthia roretzi, Halocynthia papillosa, and Pyura stolonifera. The colonial lifestyle has been independently acquired; many colonial species are Aplousobranchiata , while solitary forms prevail in Phlebobranchiata and Stolidobranchiata. They differ in the type of budding and colony structure. A molecular study of styelids indicates several independent acquisitions of coloniality (e.g., Botryllus, Botrylloides) (Pérez-Portela et al. 2009).
C. intestinalis and B. schlosseri have been preferably chosen as model species to study immunoevolution.
In spite of the established classification, care must be taken with ascidian specific status, which could affect the homogeneity of the results from geographically distinct populations. On the basis of genetic divergence and the geographic distribution , the C. intestinalis populations have been temporarily named as type A (Mediterranean, Pacific, and Southern Atlantic coast of Europe) or type B (North Atlantic) (Suzuki et al. 2005; Caputi et al. 2007; Nydam and Harrison 2007; Sato et al. 2012). These types have also been regarded as taxonomically different species (Pennati et al. 2015). Nonetheless, evidence of incomplete reproductive isolation in the wild populations, as well as laboratory hybridization experiments (Nydam and Harrison 2011), have raised the taxonomic issue.
Similar phylogenetic and population genetic data have been reported for the colonial B. schlosseri. Mitochondrial and nuclear genes, as well as polymorphic microsatellites for colonies sampled from the southern and northern coasts of Europe and the eastern–western coasts of North America, have shown that this well-known model organism comprises three highly divergent and probably reproductively isolated cryptic species. Among these, the “type A” recovered in all of the surveyed regions is by far the most common and widespread (Bock et al. 2012).
Anyway, on the basis of the collection sites, most published reports on innate immunity mainly refer to C. intestinalis populations designed as type A, as well as to B. schlosseri designated as type A. Therefore, while waiting for the taxonomic status to be precisely defined and taking into account the sampling geographic area, in the present work both ascidians are referred as belonging to type A.
Genome sequencing analyses (Dehal et al. 2002; Voskoboynik et al. 2013a, b) revealed that ascidians have a basic, nonduplicated set of a chordate-type genome. In several species (mainly C. intestinalis and B. schlosseri), gene sequencing and transcriptional profiling significantly contribute in disclosing gene upregulation; meanwhile cell labeling with riboprobes and immunohistochemistry performed with specific or cross-reactive antibodies identify cell types and indicate their functions. A comprehensive picture of immune-related genes and their phylogenetic lineages helps to clarify the evolution of a system pivotal for survival, also supporting the evolutionary meaning of multifunctional genes.
Some Topics Relevant to the Subject
Inflammation is the first nonspecific response for innate self-protection and tissue repair, triggered when tissues are injured by harmful stimuli including mechanical stress and intrusion of invasive agents (or their products) (Janeway et al. 2001; Medzhitov 2008; Ashley et al. 2012). It is a vital basic part of the immune system; the initial cause is cleared out and tissue repair initiated. The response, largely based on the extent and size of the injuring and/or invading agents, underlies a wide variety of physiological and pathological processes. Among vertebrates, the inflammatory cascade is a complex network of immunological, physiological, and behavioral events which, starting from self or nonself recognition, are coordinated by signaling and production of bioactive molecules. Mediators act as autocrine and paracrine, and interact with various cell types to amplify the inflammatory response.
In mammals, the mononuclear phagocyte system (monocytes, tissue macrophages, and dendritic cells), and the polymorphonuclear cell family (neutrophils, eosinophils, and basophils) are the main cells involved in the nonspecific innate immunity. The inflammatory response proceeds with the recruitment of leukocytes and degranulation (delivering of secretory vesicles/granules) of neutrophils, mast cells, and eosinophils, and with an orchestrated reciprocal functional regulation with macrophages (Guilliams et al. 2014). The permeability of the involved vasculature increases, and neutrophils and monocytes detecting gradients of chemokines (chemotaxis ) migrate (transendothelial and transepithelial migration) to the site of inflammation. Concurrently, proinflammatory and anti-inflammatory cytokines and effector molecules are produced. Some stimuli evoke a fast (occurring within minutes or hours), acute, and short-lived inflammation that may switch to a long-term chronic phase.
Upon exposure to proinflammatory cytokines , LPS or other microbial products, heat shock proteins, and molecular fragments of the extracellular matrix, macrophages acquire a proinflammatory “classically activated phenotype,” act as phagocytes, mediate cytotoxic activity, and produce a large number of mediators including complement components and several other factors. Macrophages that are “alternatively activated” or with a “reparative phenotype” function in resolution of inflammation and wound tissue repair (Koh and Di Pietro 2011; Mantovani et al. 2013; Wynn and Vannella 2016). In chronic inflammation, macrophages can collect in layers surrounding the foreign material and form a compact structure (granuloma) with a significant protective function such as efficient intracellular bactericidal activity and prevention of microbe dissemination.
Neutrophils and macrophages (originating from monocytes) are professional phagocytes that recognize and engulf pathogens and have a role in the removal of apoptotic corpses. Also dendritic cells are phagocytes, and sets of them act as peripheral sentinels. They detect signals displayed by foreign agents and, after intake and processing, they present antigenic determinants (antigen-presenting cells (APCs)) to T lymphocytes through a process that is MHC dependent. After phagocytosis, macrophages can also be APCs. Thereby a linkage between innate and adaptive immune systems occurs; the innate immune response traced back to invertebrates has evolved into a more complex system interacting with the adaptive immunity that in jawed vertebrates responds to different and various environmental stimuli in their habitats.
Ascidian Tissues Involved in Inflammatory Responses
The Tunic
The tunic , of epidermal origin, is the physical barrier against intruders. The tissue matrix is made up of an amorphous ground substance containing fibrous components (“tunicin”: cellulose-like polysaccharide filaments associated with collagen, elastin, and mucopolysaccharides) (Endean 1961; Deck et al. 1966). The tunic external margin is bordered by a thin layer (cuticle, containing keratin), and the inner border is lined by a monolayered epidermis, in turn enveloped in a connective tissue that forms a lacunar network. Tunic cells, scattered in the matrix, and the epidermis produce the tunic matrix (Burighel and Cloney 1997; Di Bella et al. 1998, 2009). In vascularized tunics, the cells can directly derive from tunic vessels, otherwise they—crossing the epidermis—come from the connective tissue and the circulating hemolymph. Cells also derive from the proliferating activity of the epidermis (Di Bella et al. 2005; Hirose 2009). The body contractions are due to longitudinal and circular muscles. In C. intestinalis, tunic cells express a type IX collagen α-chain (cloned and sequenced), with structural features of fibril-associated collagens with interrupted triple helices (FACIT ) (Vizzini et al. 2008). In addition, antibodies specific for mammalian collagen have identified a type I–like collagen (Vizzini et al. 2001) that, with the type IX, may stabilize the matrix (Shaw and Olsen 1991).
The Circulatory System
The circulatory system consists of a tubular heart, enclosed in a pericardium, that pumps the hemolymph by means of peristaltic contractions regulated by two pacemakers, one at each end of the heart. The peristalsis originates at one end of the heart and the direction reverses periodically. The hemolymph flows from each end of the heart, through a single vessel lined by monolayered epithelium. Sinuses or lacunae in the connective tissue are the terminal of the system.
In the tunic of many solitary ascidians (e.g., C. intestinalis), vessels are absent, whereas in other species (e.g., Phallusia mammillata (Endean 1961) and B. schlosseri (Burighel and Cloney 1997)), vessels delimited by epithelium ramify through the tunic and terminate in knob-like bulbils.
In colonial ascidians, the individuals (zooids) are embedded in a common tunic and each of them has a complete body plan (heart, gastrointestinal tract, nervous system). In the tunic matrix, an extracorporeal common vascular system is interconnected by a network of vessels joined to the unique marginal vessel that runs along the contour of the colony. The vessels give rise to many finger-like blind endings (ampullae) bordered by columnar epithelial cells.
According to Konrad (2016) the ascidian circulatory system shows structural characteristics that allow to define it as “closed.” The epithelial wall of vessels or ducts, as well as the lacunar network of the connective tissue, prevent the hemolymph from percolating around the cells of the body tissue. Here, the term “hemolymph,” instead of “blood,” is used to distinguish vertebrate blood from the ascidian circulatory tissue.
The Pharynx
The pharynx , which usually is anterior to the visceral organs, extends in the greatest part of the body. It consists of two epithelial monolayers perforated by rows of ciliated stigmata. The hemolymph flows inside a mesh of vessels called transversal and longitudinal bars, delimited by monolayered epithelium. In the lumen, abundant mature and immature hemocyte types are contained (Konrad 2016). In the bars, clusters of stationary cells called hemopoietic lymph nodules consist of dividing hemoblasts collected in groups surrounded by maturing hemocytes (Ermak 1976, 1982). Besides respiration and food particle collection, this organ is retained as the main organ of immunity, in which stem cells proliferate (Giacomelli et al. 2012).
The inflammatory components mainly originate from the pharynx. When soluble harmful agents (including LPS preparation) are locally inoculated into the tunic, they permeate the underlying tissues, reach the pharynx, and stimulate the response.
The findings here reported support the concept that the pharynx is directly involved in immune responses.
Hemocytes
Internal defense of ascidians mainly relies on hemocytes circulating in the hemolymph and therefore in the pharynx , which reach the lacunar connective network and infiltrate the tissues including the tunic. Several hemocyte populations can be inflammatory cells synthesizing and releasing bioactive proteins, carrying out phagocytosis, cytotoxicity, and encapsulation (De Leo 1992; Arizza and Parrinello 2009; Cima et al. 2016). In a cDNA/EST study to identify the genes expressed in hemocytes from C. intestinalis, 62 out of 530 of the obtained clusters had significant homology with vertebrate innate defense mechanisms (Shida et al. 2003).
The hemocytes show distinct morphological and functional features. In different species, light and electron microscopy observations distinguish various cell types that cannot not take into account hemocyte differentiation stages. In addition, seasons and/or mutable environmental conditions can affect the frequency of cell types in wild specimens. Nevertheless, basic hemocyte types can be distinguished as follows (Arizza and Parrinello 2009; Wright and Cooper 1983): (i) undifferentiated stem cells (hemoblasts/lymphocyte-like); (ii) agranular (hyaline/vacuolated) amoebocytes; and (iii) granular amoebocytes. Here, pigmented cells are disregarded. Agranular and granular hemocyte populations can be inflammatory cells (Fig. 2): (1) hyaline amoebocytes with fine granules and small vacuoles; (2) vacuolated cells including “signet ring cells” (SRCs) with a single very large vacuole, containing electron-transparent material, and compartment cells (CCs) in which vacuoles of medium size fill the cytoplasm and contain electron-transparent material and small granules; (3) granulocytes with small granules; and (4) granulocytes with large granules, including “morula cells” (MCs) in which large granules in the cytoplasm give them a raspberry-like shape. A possible characterization of MCs concerns their phenoloxidase (PO) content . In several botryllids, differences in frequency, morphology, PO level, and amoeboid behavior, have been reported (Shirae and Saito 2000). In B. scalaris and C. intestinalis, amoebocytes with granules varying in size also show weak PO activity (Shirae and Saito 2000; Parrinello et al. 2001). When activated, granulocytes can degranulate hyaline and granular amoebocytes (small granules) can be phagocytes. In C. intestinalis a particular granulocyte (URG) contains a single and large electron-dense granule that fills the whole cytoplasm.
The Ciona intestinalis pharynx inflammatory response : hemocytes involved, products, and activities. CC: compartment cell, GLG: granulocyte with large granule, GSG: granulocyte with small granules, HA: hyaline amoebocytes, hemoblast, LLC: lymphocyte-like cell, SRC: signet ring cell, URG: granulocyte with a sole large granule
Activated vacuolated cells display various features originated by a vacuolization process leading to vacuoles varied in size and content. Similarly, granules of granulocytes undergo processing phases in their content before being released, and their features (as seen by TEM) change, assuming the appearance of vacuolated cells (De Leo 1992). MCs and CCs with granules or vacuoles containing small electron-dense granules could be interchangeable with each other in terminology (here called MCs/CCs) (Fig. 2). In a B. schlosseri nonfusion reaction, a macrophage-like cell type (MLC) originating from granulocytes has also been described (Ballarin et al. 2013).
Various models, often confusing, have been proposed for hemocyte differentiation lineages and functional maturation. The hemocyte renewal mainly occurs in the pharynx and connective lymph nodules, as well as in nodules associated with the postbranchial digestive tract (Ermak 1975a). Hemoblasts (mainly in nodules) and circulating lymphocyte-like cells (LLCs ; bigger in size than hemoblasts, with a nucleolus) are retained stem cells that give rise to the hemocyte types (Fig. 2). Normally, in the hemolymph, LLCs occur at low frequency.
It is presumable that each hemocyte type may include distinct populations with morphological and functional peculiarity. In a recent paper, hemocyte types from H. roretzi were examined using flow cytometry and morpho-functional parameters (Donaghy et al. 2017). The following hemocyte populations were identified: (i) one of the three granulocyte populations is deeply involved in phagocytosis; (ii) one of the two main hyaline amoebocyte populations, provided with lysosomal content, inducible oxidative activity, and no proteases, does not show phagocytic activity; (iii) the second hyalinocyte population mainly contains proteases; LLCs and a population of hyalinocytes present with different sizes and complexity but similar profiles, suggesting that they may be intermediate/maturation stages. These findings suggest that several morpho-functional characters of ascidian hemocyte populations remain to be clarified.
As an effect of the inflammatory stimulus and cytokine network, stem cells proliferate and differentiate, enhancing the frequency of mature cells. Granulocytes degranulate and release signaling molecules. Complement cascades (alternative and/or lectin-dependent), phagocytosis, cytotoxicity, and encapsulation are activated, and finally the possible wound in the tissues is repaired.
In the hemolymph plasma, bioactive substances could contribute to the inflammatory process. In S. plicata, heparin, sulfated heteropolysaccharides (glucose and galactose), and sulfated disaccharides have been found in the hemolymph. Heparin and histamine colocalize in the intracellular granules of granulocytes. In mammals, histamine is associated with heparin in the granules of mast cells and basophils; therefore, this hemocyte type (or a granulocyte population) appears to be circulating basophil-like cells. Finally, histamine-containing cells have been also detected in the pharynx . The possibility exists that heparin- and histamine-containing granulocytes may be presumptive counterparts of mammalian basophils. They could perform immunological functions and tissue regeneration (de Barros et al. 2007).
A search of the C. intestinalis genome identified no reliable orthologs of vertebrate blood coagulation factors, although paralogs and/or constituent domains were evident (Jiang and Doolittle 2003). The findings concern plasminogen-like carboxyl-terminal domains of fibrinogen, a scaffold conceivably related to factors V and VIII, a number of serpins that do not match with antithrombin, and a carboxypeptidase paralogous to thrombin-activated fibrinolysis inhibitor, as well as numerous domains that are similar to those identified in tissue factor, tissue factor inhibitor, and thrombomodulin.
Phagocytes
Phagocytosis is the most phylogenetically ancient process. First observed by Elie Metchnikoff (1887) in amoeboid cells from marine invertebrates, phagocytosis has a pivotal role in internal defense of invertebrates and vertebrates.
In vertebrates, antimicrobial proteins (e.g., lysozyme), peptides (e.g., defensins), binding proteins (e.g., lactoferrin ), reactive oxygen species (ROS) (respiratory burst), and reactive nitrogen species (RNS) are the main phagolysosome effectors. These toxic molecules can also damage host tissues when inflammatory cells are inappropriately activated. Germ line –encoded receptors discriminate potential pathogens, enabling phagocytes to internalize and kill an array of pathogens (phagosomes mature into phagolysosomes) without the need for opsonization (Di Meo et al. 2016; Robinson 2008). In general, these receptors are called “pattern recognition receptors” (PRRs ) and their ligands are “pathogen-associated molecular patterns” (PAMPs ) on the surface of Gram-negative and Gram-positive bacteria (e.g., mannans, peptides, lipopolysaccharides, and lipoteichoic acids). PAMPs bind to PRRs and initiate signaling cascades leading to cell activation. The key elements of this framework can be found in ascidians. Hyaline amoebocytes, granulocyte populations, and their transition types can be retained functional analogs of neutrophils and macrophages. They are recruited, cross the vessel epithelium to reach the injured tissue, represent the dominant cells in the earliest inflammatory stages, and can exert phagocytic activity.
In B. schlosseri, circulating professional phagocytes are represented by hyaline amoebocytes and macrophage-like cells, which may be transition stages; the former is the active phagocyte that upon ingestion takes the globular form of a macrophage-like cell (Voskoboynik et al. 2004; Ballarin 2008; Cima et al. 2016). Both cells have similar cytochemical properties and common content of lysosomal enzymes (such as phosphatases, 5′-nucleotidase, β-glucuronidase, and esterases), share the same surface glycans, and cross-react with anti-CD39 antibody (a tool used in mammals for monitoring immune activation) (Ballarin and Cima 2005). During the phagosome formation, reactive oxygen metabolite production, nitrite ion release, and acid phosphatase secretion increase. A comparison of the major hemocyte types reported in several botryllid species showed that SRCs can also be equipped with the phagocyte enzymatic apparatus, and they have been retained to belong to the same cell lineage.
In B. schlosseri, phagocytosis is modulated by cross talk with MCs that, when activated, release IL-1α-like and TNFα-like factors that enhance the phagocytic activity (Menin et al. 2005; Menin and Ballarin 2010). In the colonial Aplidium yamazii, phagocytic activity of tunic cells containing phagosomes has also been shown. Presumably these phagocytic cells engulf extraneous substances (including bacteria) and also function as scavengers to keep the tunic free of discarded tunic cells and other debris (Hirose et al. 1994).
Phagocytosis can be facilitated by opsonins such as lectins and complement pathway products, which bind to the target and enhance the phagocyte activity. In H. roretzi, products of C3 complement cascade are opsonins (Nonaka and Azumi 1999).
LLCs as Stem Cells
In general, stem cells have been defined as clonogenic cells capable of self-renewal and multilineage differentiation. These cells, provided with physical and cell surface characteristics, give rise to renewal of lineage progenitors, from which progeny more restricted in their differentiating potential originate, and finally mature cells are formed (Weissman 2000). The role of ascidian LLCs/hemoblasts is intriguing (Fig. 2); they are a retained primordial form of vertebrate lymphocyte/stem cells (Peddie and Smith 1995; Cooper and Parrinello 2001; Cooper 2009).
The topic has been mainly examined in colonial ascidians , and the question as to whether hemoblasts are stem cells or tissue-restricted progenitor cells has been posed (Kawamura and Sunanaga 2010).
In B. schlosseri, somatic stem cell populations exist in “niches” in the anterior ventral region of the endostyle and in the vasculature, where they proliferate in developing buds and migrate to regenerate organs (Voskoboynik et al. 2008). X-ray treatments of primary hemocyte cultures from B. primigenius and B. schlosseri colonies decrease the LLC proliferative response to mitogeneic factors (Rinkevich and Rabinowitz 1993).
Homologous genes predominantly expressed in human hematopoietic stem cells, myeloid populations, and early lymphoid populations have been identified in the B. schlosseri genome (Voskoboynik et al. 2008, 2013a, b). The findings indicate that at least some genetic circuitry relevant for vertebrate immunity appeared to be already in place in the protochordates’ and vertebrates’ common ancestor. However, the meaning of CD34 epitopes identified by immunocytochemical assay in LLCs remains to be established. CD34, first identified in mammalian hematopoietic stem and progenitor cells, is expressed by a multitude of other nonhematopoietic cell types and identifies progenitor cells from many tissue types (Sidney et al. 2014). In Botrylloides at least two LLC differentiation pathways have been proposed, and phagocytes (hyaline amoebocytes ) also show the CD34 marker (Cima et al. 2001; Ballarin and Cima 2005).
In solitary ascidians, electron micrographs of lymph nodules indicate hemocyte differentiation from hemoblasts (Ermak 1975a, b, 1976, 1982). In circulating hemolymph, hemoblasts are rarely distinguished, and LLCs can have the potential for differentiation into hemocyte lineages (Donaghy et al. 2017).
Recombinant human IL-2 and phytohemoagglutinin (PHA) stimulation increases the LLC proliferative activity in S. clava pharynx explants (Raftos et al. 1991a, b). PHA binds glycan components of the cell surface glycome; human IL-2 interacts with specific receptors and exhibits a variety of affinity states depending on the subunit composition (Wang et al. 2000). Therefore, a cross-linking with hemocyte receptor–like can be expected, whereas notable differences, including a low level of stimulation in pharyngeal cultures, have been reported.
Histological observations do not show LLCs directly involved in B. schlosseri nonfusion reaction, and only a few LLCs have been observed in C. intestinalis inflammatory response. The immunocompetence potential is indicated by significantly greater proliferative activity among individuals immunized with allogeneic tissues (Raftos and Cooper 1991; Cooper 1992, 2009). The enhanced proliferation was restricted to discrete crypts of dividing cells within the body wall of the recipients, and in S. plicata allograft rejection, adoptive transfer of alloimmune memory has also been reported (Raftos et al. 1988; Raftos 1996a, b).
The Ascidian Inflammatory Response Is Orchestrated
Ascidians have evolved complex inflammatory reactions characterized by molecular and functional homologies with mammals (Azumi et al. 2003; Cha et al. 2001; Voskoboynik et al. 2013a, b). The basic value of the inflammation in ascidian innate immunity is emphasized by the absence of vertebrate-type adaptive immunity (Cooper 2016). C. intestinalis and B. schlosseri genome-wide sequence analyses have provided a comprehensive picture of immunity-related genes (Azumi et al. 2003; Satoh et al. 2003; Voskoboynik et al. 2013a,b).
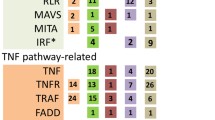
The genomes lack significant homologies to genes known to play a pivotal role in the vertebrate adaptive immune system, including assembled MHC genes; dimeric immunoglobulin molecules; genes with homology to RAG1/RAG2, which are involved in Ig and TCR rearrangements; terminal deoxynucleotidyl transferase, which adds nucleotides to the rearrangement; VDJ elements to create receptor diversity, V-region subgenic elements encoding T cell and Ig antigen receptor domains; or VLR-like immune receptor elements. Nevertheless, outside the jawed vertebrate lineage, a RAG1/2–like gene pair in the purple sea urchin has been identified. An evolutionary scenario of significant gene loss from the highly compacted genome of the ascidian lineage, or horizontal gene transfer, may be suggested (Fugmann et al. 2006; Fugmann 2010). In this respect, it is intriguing that proto-MHC regions, Ig-like domains and transcripts, and activating and inhibitory receptors with MHC-independent functions have been reliably traced throughout ascidian genomes (Du Pasquier 2004; Satake et al. 2003; Azumi et al. 2003; Voskoboynik et al. 2013a, b). A number of genes predict integral membrane proteins with extracellular C-type lectin or Ig-like domains, intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs ), and immunoreceptor tyrosine-based activation motifs (ITAMs ) (plus their associated signal transduction molecules). C. intestinalis expresses immunoglobulin variable region–containing chitin-binding proteins (VCBPs) , which are not found in vertebrates (Cannon et al. 2002, 2004; Dishaw et al. 2016). Unlike V-region-containing antibodies and T cell antigen receptors, the VCBPs do not undergo somatic rearrangement, but some exhibit regionalized hyperpolymorphism due to haplotypically variable alleles. The variable region consists of two variable (V) Ig domains and a single chitin-binding domain. These domains bind and promote the opsonization of bacteria, and the distinctive C-terminal chitin-binding domain (CBD) likely is also integral to overall function. The expression of CiVCBP genes is confined largely to the gut epithelium (stomach and intestine); the protein is secreted into the lumen where they bind bacteria. The VCBPs, through association with an extensive network of chitin fibrils, an integral component of the gut-specific mucus, may also influence settlement of bacterial communities by modulating adherent biofilms on epithelial surfaces. In addition, hemocytes (granular amoebocyte population) scattered within the lamina propria and in the circulatory system express the VCBPs. These localizations are significant because the gut is an entry portal for pathogens and a site of complex microbial communities, including commensals (Dishaw et al. 2011). Thus, before the evolutionary emergence of adaptive immunity, soluble immune mediators encoding V-type Ig domains likely served a role in the establishment and maintenance of gut homeostasis.
The B. schlosseri genome encodes homologs of Foxn1, the thymus epithelial gene marker of the thymopoietic microenvironment in vertebrates, and a polymorphic Hsp is involved in allorecognition (see the section: Inflammatory Events Characterize Colonial Ascidian Take Over and Allorecognition). In addition, genes homologous for complement components, Toll-like receptors (TLRs) , and genes involved in intracellular signal transduction of immune responses have been identified.
These data indicate that genetic circuitries relevant for vertebrate immunity were already in place in the common ancestor of the protochordates and vertebrates.
PRRs
Response and effector mechanisms start from direct hemocyte/tissue receptor–ligand interaction. Hemocytes (phagocytes, cytotoxic cells) are recruited crossing the epithelium, innate immunity genes are upregulated, and a network of inflammatory factors is produced.
In all invertebrates, a key facet of defense responses lies in the ability to recognize and respond to invading microbes and cell disturbance through a set of germ line–encoded pattern recognition receptors (PRRs ), which detect invariant pathogen motifs (PAMPs ) and put in place a variety of cellular and molecular inflammatory responses, including phagocytosis, pathogen killing, nodule formation, and encapsulation. PRRs comprise an array of sensors whose basic characteristics include a protein domain for detection coupled to a protein domain that interacts with downstream signaling molecules. The ligand-bound PRR delivers a signal that activates specific transcription factors and creates a network of cross talk by which they regulate multiple host proinflammatory genes and coordinate an appropriate immune response toward the detected pathogen (Hansen et al. 2011; Mogensen 2009; Amparyup et al. 2012). PRRs are expressed by the first responder cells (in mammals: monocytes, macrophages, dendritic cells, and neutrophils), as well as by tissue-specific epithelial and endothelial cells. They include complement receptors, C-type lectin family members, and members of galectin and TLR families. Scavenger receptors, which are structurally heterogeneous, recognize several ligands and structures (including glycans) and function directly as phagocytic receptors. Soluble PRRs (e.g., C-type lectins, pentraxins, and galectins) mediate the binding signaling for cellular responses and can opsonize pathogens, facilitating recognition and ingestion. Structural properties allow a single PRR to recognize a wide range of microbial agents (Silva and Correia-Neves 2012).
Endogenous nonmicrobial signals, termed “damage-associated molecular patterns” (DAMPs), could be involved in stimulating inflammatory responses (Matzinger 1994, 2002). In this respect, Matzinger’s DAMPs cannot be retained as an alternative to self/nonself recognition, but they may be additional signals from distressed or damaged cells that could share the same receptors with PAMPs (Pradeu and Cooper 2012).
TLRs
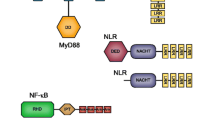
Toll-like receptor (TLR ) genes that initiate defensive responses against a wide variety of pathogens have been identified throughout the animal kingdom (Voogdt and van Putten 2016). At first, the Toll gene was discovered in Drosophila to control dorsal–ventral patterning during embryonic development. The protein product was then identified as a transmembrane receptor important for antifungal immunity in the adult fly. The genome sequencing of Drosophila showed that there are eight Toll-like receptors and these may also function in innate immunity (Parker et al. 2001; Valanne et al. 2011).
In mammals, TLR genes encode 10–12 membrane molecules with diverse specificities for extracellular and endosomal ligands. They are expressed by lymphocyte populations, macrophages, and dendritic cells (Schmitz et al. 2004; Takeda and Akira 2005). Acting as transmembrane receptors, they recognize PAMPs and express signaling pathways leading to cell activation for appropriate responses to various classes of pathogens. Different TLRs activate distinct patterns of gene expression and instruct the development of antigen-specific acquired immunity participating in activation of antigen-presenting cells (APCs) . TLRs recruit adapters to initiate a proinflammatory signaling cascade culminating in the activation of several transcription factor families, also promoting T helper–dependent inflammation. At the cellular level, TLR signals affect many aspects of the cellular response, including cell survival, proliferation, and regulation of the proinflammatory response (Akira and Takeda 2004; Billack 2006; Reynold and Dong 2013). In macrophages and neutrophils, TLR activation enhances phagocytosis and increases the oxidative burst, while resident macrophages secrete proinflammatory cytokines.
They are type I integral membrane receptors, each with an N-terminal ligand recognition domain, a single transmembrane helix, and a C-terminal cytoplasmic signaling (TIR) domain. The solenoid-like ectodomain, made up of leucine-rich repeat (LRR) motifs , shows variations in structure and organization, and mediates recognition and signaling to activate transcription factors. On the basis of sequence homologies, vertebrate TLRs have been grouped into six subfamilies, and not all vertebrate species express all TLR paralogs (Botos et al. 2011).
LPS is a potent activator that involves both TLR4 and the CD14 protein required for LPS-induced TLR4 endocytosis, LPS transport to the receptor, and delivery of the TLR to the endosomal signaling machinery (Zanoni et al. 2011; Liu et al. 2001). TLR4 and TLR2 can also respond to endogenous molecules from traumatic tissue injury. The stress-induced heat shock proteins Hsp60 and Hsp70 released from dying cells are recognized by both TLR4 and TLR2, and a form of fibronectin, expressed in situations of tissue injury, binds to TLR4. Engagement of the TLRs leads to NF-κB activation and production of the proinflammatory cytokines IL-1, IL-6, IL-8, IL-12, and TNF, as well as stimulating inducible nitric oxide synthase (iNOS) and the production of reactive nitrogen intermediates by macrophages (Mak and Saunders 2006).
Genome-wide analyses have shown that TLRs or related genes, essentially conserved in the genome of nonmammalian organisms, diverge in number, structural organization, and biological roles (Satake and Sasaki 2010; Satake and Sekiguchi 2012). In invertebrate deuterostomes, TLR-like genes are paralogous, and the expansion of TLR-related genes may occur in a species-specific manner, whereas in vertebrates, the number of TLRs does not significantly differ among species (Coscia et al. 2011).
In ascidians, TLR-like receptors have been identified. C. intestinalis possesses only two authentic TLR-like genes (CiTLR1 and CiTLR2), expressed in the hemocytes and gut (Nonaka and Satake 2010; Sasaki et al. 2009). This finding contrasts with the large number of TLR genes found in the echinoderm Strongylocentrotus purpuratus, in which more than 200 gene models have been classified into a number of distinct subgroups (Rast et al. 2006; Tu et al. 2012; Hibino et al. 2006). Most sea urchin TLR genes display greater similarity to each other than to TLRs of other species, and they are encoded in tandem arrays, suggesting an enormous gene expansion. The amphioxus genome has numerous predicted TLR complete gene models; 72 TLR or TLR-related genes have been detected in the genome of Branchiostoma floridae (Satake and Sekiguchi 2012; Huang et al. 2008). Most of these genes seem to have been generated via species-specific gene duplication. The presumptive evolutionary scenario indicates that only a few TLRs or their related genes might have existed in a common deuterostome ancestor. In this case, C. intestinalis conserves the ancestral characteristics, whereas sea urchins and amphioxus have expanded their gene paralogs during their divergence in concert with variations in their lifetimes, life cycles, and environments.
In C. intestinalis the putative amino acid sequence has a unique structural organization with similarity to mammalian TLRs. However, the CiTLRs are localized in both the cell plasma membrane and endosomes, providing evidence that the CiTLRs are functionally “hybrids” of the vertebrate TLRs that are located on either the cell surface or endosomes (Sasaki et al. 2009; Satake and Sekiguchi 2012). CiTLRs have more extensive binding affinity for PAMPs (CiTLR1 and CiTLR2 bind multiple ligands triggering signal transduction), whereas in mammals different TLRs are necessary. Genes expressed by hemocytes in the hemolymph and pharynx, or associated with the gut, respond to the pathogenic ligands, and this supports the view that TLR-mediated innate immune functions are conserved in ascidian tissues.
CiTLR–ligand interaction elicits a dose-dependent induction of NF-χB transcription factor that upregulates cytokine-like genes. In the anterior and middle intestine, where both CiTLRs are abundantly expressed, ligands differentially upregulate CiTNFα gene expression. In this ascidian, LPS activates pharynx inflammatory responses including CiTNFα production and lectin complement activation. However, neither CiTLR1 nor CiTLR2 recognizes LPS (Sasaki et al. 2009; Satake and Sekiguchi 2012); therefore, the possibility exists that other receptors could be involved in the induction of CiTNFα or, as in mammals, Ciona TLRs could utilize accessory molecule(s). Interestingly, according to Sasaki et al. (2009), the expression profiles for CiTLRs may be implicated in recognition of endogenous ligands.
Lectins
Glycans are components of the outer surface of all cells and form large parts of the extracellular matrices. They have extraordinary structural diversity, biochemical specificity, and regulatory flexibility. The diversity of the glycome, including considerable intra- and interspecies variations, reflects the central role played by oligosaccharides, glycoproteins, and glycolipids in numerous biological systems and evolutionary machinery (Springer and Gagneux 2013; Cummings et al. 2017; Bianchet et al. 2008). The enormous combinatorial possibility of glycan presentation is manifested during immune cell activation, differentiation, and signaling, as well as in their aberrant expression in inflamed or neoplastic tissue. Glycans have a prominent role as PAMPs and DAMPs and are crucial for self/nonself discrimination (Varki et al. 2009; Rabinovich and Croci 2012).
Lectins are proteins or glycoproteins that mainly bind glycans (including glycoproteins and glycolipids) with weak bonds forming three-dimensional arrangements of multivalent lectins and glycans. Most of them are oligomers of subunits covalently or noncovalently bound, thus determining the avidity of lectin–glycan interactions and amplifying both recognition and effector capabilities.
They are soluble or integral membrane components and act as PRRs characterized by the carbohydrate recognition domain (CRD) . Integral membrane lectins are mostly type II transmembrane proteins with a short hydrophobic domain and an extracellular C-terminal region that carries the CRD. Soluble lectins agglutinate a wide variety of erythrocytes and, at first, they were identified by hemagglutination assays. Aside from the CRD, the lectins exhibit domains with variable structures. The presence of conserved or variant residues within the CRD, the structure of the other domains, the Ca2+-dependence/independence, and glycan specificity or protein binding distinguish several lectin families with intrafamily variations, representing a very heterogeneous group of proteins.
The lectin domains are functionally connected with inflammatory reactions , as supported by gene upregulation and tissue localization compatible with internal defense roles. In ascidian hemolymph, several humoral and cellular lectins have been reported; they can display opsonic activity and mediate inflammatory responses (Parrinello 1995; Vasta et al. 2004; Quenseberry et al. 2003).
Galectins
The galectin molecular family, formerly named S-lectins, is defined by the evolutionarily conserved CRD and Ca+2-independent binding to β-galactoside-containing glycans (such as lactose and N-acetyllactosamine). They are nonglycosylated proteins with a wide taxonomic distribution and structural conservation in vertebrates, invertebrates, protists, and fungi (Houzelstein et al. 2004; Yu et al. 2007; Vasta et al. 2012). The conserved β-sandwich structure is formed by six strands with the CRD and five distinct strand sheets.
In the cytoplasm, they bind endogenous ligands performing several intracellular functions, and can be translocated into the nucleus. Missing a secretion signal peptide, they are released into the extracellular matrix by direct translocation across the plasma membrane. Once released, galectins bind glycoproteins or other glycoconjugate ligands on target cell surfaces or in the extracellular environment, recognizing exogenous ligands such as glycans and LPS (Rabinovich and Gruppi 2005; Rabinovich et al. 2002; Vasta 2012; Vasta et al. 2012).
Their binding capacity, functional multivalence, and cellular effects are improved by oligomerization. Some galectins have diverged to bind ligands in a carbohydrate-independent manner (Nesmelova et al. 2008). Galectins are involved in acute and chronic inflammation (Liu et al. 2008, 2012).
In mammals, more than 15 galectins have been identified and structurally classified into three groups: (i) prototype galectin monomers with a single CRD, which are noncovalently linked in dimers for effective binding and signaling; (ii) tandem galectins, with two distinct but homologous CRDs per monomer in which the flexible linker domain allows formation of dimers that increase their potency; and (iii) chimera-type galactins, in which the oligomerization results in multivalent carbohydrate ligand binding. In mammals, the chimera-type Gal-3 is a multifunctional lectin with proinflammatory activity, inducing migration of monocytes and macrophages involved in endocytosis and antigen presentation (Norling et al. 2009; Sano et al. 2003).
In C. intestinalis, two galectins—CiLgals-a and CiLgals-b—form distinct oligomers (Vizzini et al. 2012; Ballarin et al. 2013). The galectin genes recorded in the genome (Dehal et al. 2002) are organized into three exons with two subtypes: N-terminal F4 subtype CRD and C-terminal F3 subtype CRD (F4-CRDs and F3-CRDs). A similar exon/intron organization has been found in echinoderm orthologs (Houzelstein et al. 2004). CiLgals-a exhibits the F4-CRD-liker-F3-CRD gene organization; CiLgals-b shows an F4-CRD-linker-F4-CRD structure not known in vertebrate genes.
Comparative analysis of the CiCRD deduced amino acid sequences showed that the N-CRD and C-CRD , like vertebrate CRDs, are included in two distinct clusters, suggesting a domain duplication model and an early domain divergence. The divergence between the vertebrate N-CRD and C-CRD was greater than that between invertebrate deuterostomes (Shida et al. 2003; Azumi et al. 2007; Terajima et al. 2003; Vasta et al. 2004). The vertebrate galectin signature sequence, directly involved in galactoside binding, is conserved in the N-CRD and C-CRD of CiLgals-a and in the N-CRD of CiLgals-b. CiLgals-a is considered orthologous in the deuterostome galectin lineages. On the contrary, the CiLgals-b C-CRD is so divergent that the signature sequence could not be suitable as a sugar-binding motif and has been related to a distinct functional role.
The homology molecular modeling (human Gal-3-C-CRD, Gal-9 N-CRD, Gal-4-C-CRD superimposition) shows a CiLgals-a common structural model that includes two antiparallel β-sheets composed of five and six β-strands, respectively, with a CRD suitable for binding to β-galactosides. The divergent sequence of the CiLgals-b C-CRD lacks superimposition. Both galectins are constitutively expressed by hemocytes as well as by the stomach epithelium, where they can interact with environmental microorganisms (Parrinello et al. 2017). According to Houzelstein et al. (2004), although CiLgals-b is outside the CiLgals-a group, it is orthologous to the S. clava mono-CRD galectin supporting tandem duplication events from a mono-CRD galectin to bi-CRD galectins. A prototype galectin was also found in the colonial ascidian D. candidum, in which multiple members of the galectin family have been identified (Vasta et al. 1986).
Galectins Participate in the Inflammatory Response
In mammals, pathogens upregulate the expression of galectin genes and participate in the inflammatory response (Rubinstein et al. 2004; Klyosov 2008). In C. intestinalis pharynx hemocytes, the LPS stimulus significantly upregulates the transcription of the CiLgals-a and -b genes (Vizzini et al. 2012). In this respect, since the two CiTLRs do not bind to LPS, the possibility exists that galectins are involved directly or as TLR-associated molecules (Sasaki et al. 2009). The Gal-3 discriminates Saccharomyces cerevisiae and Candida albicans in association with TLR2 for signaling (Jouault et al. 2006; Martchenko et al. 2007). More generally, the triggering via the galectin-mediated signal transduction pathway depends on cross-linking with β-galactoside glycojugate or glycoprotein receptors. The amphioxus Branchiostoma belcheri tsingtauense galectins (BbtGals, F4-CRD-linker-F3-CRD-type bi-CRD) may function like their vertebrate homologs, directly binding to bacteria, and so the transcription of BbtGal-L mRNA is increased (Yu et al. 2007). In mammals, galectins upregulated in infections are required for the specific recognition of fungi.
In comparison with the mRNA expression profiles of the other inflammatory components (see below), the perceptible beginning of the transcription is delayed and the maximum level was reached at 24 h post-inoculation (p.i.). An increased number of riboprobe-labeled hemocytes are engaged inside the vessels, and CCs and SRCs express both CiLgals. The riboprobes are localized in the nucleus and in the surrounding cytoplasm, and specific antibodies label the proteins mainly associated with granules and the nuclear envelope. Galectins expressing cells migrate into the tunic, while both galectins outline the endothelium basal membrane. Functions can be deduced from domain organization and amino acid sequence homologies. Structural differences and the highest CiLgal-a transcription level suggest that CiLgals-a has a more major role than CiLgals-b in the LPS-challenged pharynx response. Findings on galectin-like molecules released by cultured C. intestinalis and B. schlosseri hemocytes also suggest an opsonic role (Parrinello et al. 2007; Ballarin 2008).
Galectins can also sense damage signals by transmission of the information to effector cells (Sato and Nieminen 2004).
RBLs
Rhamnose-binding lectins (RBLs) are Ca2+-independent lectins, specific for rhamnose and galactosides, which have been found in marine invertebrates and fish (Jimbo et al. 2007; Terada et al. 2007; Ogawa et al. 2011; Cammarata et al. 2014; Ballarin et al. 2013). RBLs share one or multiple CRDs with a unique α/β fold, eight highly conserved Cys residues engaged in four disulfide bridges, and conserved motifs (YGR, DPC, and KYL). They are involved in glycan metabolism regulation, cell proliferation, phagocytosis, and cytotoxicity.
The hemolymph of B. schlosseri contains soluble RBLs, and sequences of five isoforms have been identified. The predicted proteins contain a single CRD, Cys and characteristic motifs, a signal peptide, and no glycosylation sites. A phylogenetic tree, built with the RBL sequences in databases, clearly shows that BsRBLs are located within the protochordate cluster (Gasparini et al. 2008). Specific antibodies and riboprobes label BsRBLs expressed by professional phagocytes, whereas MCs do not express them. BsRBLs exert multiple roles in immunosurveillance and immunomodulation, acting as opsonins, stimulating the respiratory burst and ROI production, exerting chemotactic activity, and challenging MCs to release cytokine-like molecules. During the allogeneic immune response, activated MCs release BsIL1α and BsTNFα (Menin and Ballarin 2010). The BsTNFα further induces the synthesis of BsRBL by a limited number of phagocytes, thus additional phagocytes become activated and migrate toward the inflamed tissue. The released BsRBLs are involved in MC degranulation and act as opsonins favoring clearance and encapsulation, and potentiate positive feedback with a progressive increase in the local concentration (Ballarin et al. 2013).
C-Type Lectins
These lectins form a large protein superfamily sharing a CRD basic structure in which a fold shows highly variable amino acid sequences. They are Ca2+ dependent or independent and can bind ligands other than glycans, thereby the typical CRD has been designed as CTLD (Zelensky and Gready 2005; Cummings and McEver 2009; Drickamer and Taylor 2015). The CTLD structure is characterized by a double loop (loop in a loop) stabilized by two highly conserved disulfide bridges at the base of the loops, and a set of conserved hydrophobic and polar interactions. The second long loop is structurally and evolutionarily flexible, and it is involved in glycan binding and interactions with diverse ligands. Generally, the structural diversity between the different C-type lectins is higher in the loop regions, mainly because of amino acid insertions or deletions. The diversity within families is amplified by subunit oligomerization that affects the avidity for multivalent ligands. Multiple gene copies, allelic variation, posttranscriptional and posttranslational modifications produce multiple isoforms that further expand the lectin recognition capabilities, providing wider recognition and effector capacity and functions (Kerrigan and Brown 2009; Gijtenbeel and Inghuis 2009; Drickamer and Fadden 2002).
Serving as PRRs , they are transmembrane or soluble proteins (glycoproteins). As signaling receptors they have diverse functions depending on the motifs in their cytoplasmic domain, and are crucial in shaping immune responses. They induce endocytic, phagocytic, antimicrobial, proinflammatory, or anti-inflammatory responses (Hoving et al. 2014). On the basis of molecular phylogeny and domain organization, various families have been distinguished, including collectins, selectins, and pentraxins.
C-type lectin genes have radiated independently in each animal lineage (mammals, ascidians, flies, nematodes), and they have diverged in chordate lectin families.
In ascidians, despite the literature description of “bona fide” mammalian homologs, the multifunctional roles of C-type-like lectins—including immune responses and regulation of cell growth, adhesion, and differentiation—have been widely recognized (Matsumoto et al. 2001). The structure of a C-type lectin (TC14) isolated from the budding ascidian P. misakiensis, specific for D-galactose and related monosaccharides, has been resolved in detail (Poget et al. 1999). This lectin is a dimer that adopts a typical CTLD fold with differences in the loop regions and in the second α-helix involved in the formation of a dimeric interface. The binding site, coordinated by a calcium ion per monomer, is quite exposed and located on the surface of the loop region. The TC14 lectin plays a role in generalized defense mechanisms, such as strong antibacterial activity.
In C. intestinalis, C-type lectin genes have been recorded in the genome, and soluble lectins are contained in the hemolymph. In S. plicata, they are components of the acute-phase response (Green et al. 2003; Raftos et al. 2001).
A putative C-type lectin with CTDL and an Ig domain (BsCLT) has been cloned from the B. schlosseri genome; the deduced amino acid sequence features three building blocks: (i) a Greek-key motif signature (a class of β-sheet) at the N terminus; (ii) a CTDL domain signature; and (iii) an immunoglobulin (Ig) domain at the C terminus. The nonpolymorphic Ig domain has been classified as an intermediate-type Ig domain. Antibodies raised against recombinant BsCLT cross-reacted with a polypeptide in tunicate crude extract, suggesting that they may play a systemic defense role (Pancer et al. 1997).
CTLD Lectins that Bind Protein Targets
In mammals, Natural Killer (NK) cells are lymphocytes classified as “innate” lymphocytes that respond quickly to a variety of pathological challenges through a distinct repertoire generated by the combinatorial assortment of germ line–encoded activating and inhibitory receptors expressed on their surface (Kelly et al. 2015; Bartel et al. 2013). One of two classes of NK receptors is the C-type lectin–like superfamily encoded in the natural killer gene complex (NKC) . In this respect, the divergent evolution of ancient C-type lectins, acting on the CTDL fold that loses the Ca-dependent sugar binding capacity and binds proteins or lipids, and the components of the NKC are expressed by natural killer (NK) cells. Most NK cell–associated CTLRs are known to bind glycoproteins with an MHC class I–like fold: these include classical and nonclassical MHC class I molecules and MHC class I–like molecules. A prominent member of this group is NKG2D, an activating receptor that binds to several MHC class I–like molecules induced by various forms of cellular stress such as viral infection, tumor formation, tissue damage, and heat shock protein expression. In distinct mammal orders, the NKCs diverged in their binding affinity; thereby, in the mouse, Ly49 receptors detect allelic variants of MHC I molecules and CD94/NKG2x receptors interact with a nonclassical MHC class I molecule presenting signal peptides of MHC class I molecules.
The receptors are type II transmembrane glycoproteins with an N-terminal cytoplasmic domain and a single transmembrane domain, followed by a stalk region and a single extracellular C-type lectin–like domain (CTLD) at the C terminal. The receptors are basically built up by two α-helices and two antiparallel β-sheets forming a compact homoheterodimer structure stabilized by two or (mostly) three conserved intramolecular disulfide bonds (Bartel et al. 2013; Wada et al. 2004; López-Botet et al. 1997). The mammalian NKC encodes for several dozen CTLRs. These sensors allow the release of NK cell cytotoxicity toward self-MHC-deficient cells (viral infections or tumor cell lines) and hence represent the molecular substrates of the “missing-self” recognition mode.
The human invariant CD94 glycoprotein covalently assembles with different C-type lectins of the NKG2 family and forms disulfide-linked heterodimers. Five different molecular species of NKG2 (NKG2A, B, C, E and H) have been reported. NKG2A and B, produced by alternative splicing, have two receptor tyrosine-based inhibitory motifs in their cytoplasmic domains and form inhibitory receptors complexed with CD94. CD94 forms heterodimers with NKG2 family molecules and, with CD94/NKG2A binding to the specific ligand, suppresses activation signaling processes; thus, the NK cytotoxic activity toward “self” is inhibited, whereas it is displayed when a missing-self target is met (Borrego et al. 2005).
In both B. schlosseri and C. intestinalis a CD94-like gene (CD94/NKR–like) provided with a CTLD that recognizes proteins and a homolog of the vertebrate NK receptor have been reported (Zucchetti et al. 2008; Khalturin et al. 2003; Boyington et al. 1999). Both deduced amino acid sequences share structural features that recognize proteins, connecting them to human CD94 functionality.
The comparative analysis of CiCD94 displays 50/66% identity/similarity with BsCD94 and 30/49% with H. sapiens CD94 (Zucchetti et al. 2008). The deduced amino acid sequence discloses that the receptor is provided with a single CTDL, a transmembrane sequence, and a short cytoplasmic tail at the N terminus that is a typical feature of type II C-type lectin, and contains three possible sites for glycosylation. Four cysteines form two of the four intrachain disulfide bonds, and hydrophobic residues are involved in the dimerization. The CTLD of the CiCD94-1 lacks Ca2+-binding sites. The CiCD94-1 receptor shares structural features with the CTLDs that recognize proteins; the amino acids that in human CD94 are involved in the interaction with peptides presented by the MHC class I molecules are conserved (Cambi and Figdor 2003; Brown and Gordon 2001).
Unlike mammalian CD94, the identified BsCD94-1 presents a short cytoplasmic domain; therefore it is presumable that it requires a partner chain to become functional NKR (Khalturin et al. 2003). However, the extent of structural conservation between the Botryllus BsCD94-1 molecule and the vertebrate orthologs strongly implies functional conservation. Specific antibodies raised toward the recombinant BsCD94-1 protein label three groups of hemocyte types: granulocytes with a relatively small nucleus and small cytoplasmic granules, granulocytes with a large nucleus and a small cytoplasm to nucleus ratio, and SRCs. The label was limited to the cell surface, confirming the transmembrane localization of the BsCD94-1. MCs do not show epitopes of the protein.
In C. intestinalis, the gene is upregulated by LPS stimulus, and both mRNA and protein are expressed in the majority of granular amoebocytes that populate the tunic and the hemolymph following the LPS stimulation. These hemocytes are phagocytes and their activity is inhibited by specific anti-CiCD94 protein , suggesting that the receptor is involved in phagocytosis (Zucchetti et al. 2008).
The deduced molecular characters of BsCD94/NKR and CiCD94/NKR receptors forecast a cell lineage with the NK receptors functioning in the missing-self model.
Collectins
In mammals, Ca2+-dependent collectins recognize PAMPs ; activate the lectin complement cascade counteracting bacteria, parasites, and transformed cells; and are relevant in triggering effector responses. The structure is characterized by a mannose- or N-actylglucosamine (GlcNAc) -specific CTDL, a coiled neck region joining the CTDL to a large collagen domain, and a short N-terminal tail region. Subunits assemble in large oligomeric complexes via interactions by the collagenous tail. As a type I transmembrane protein, collectins are expressed by macrophages and several tissues, and their expression is upregulated by several cytokines (Marshall and Gordon 2004; East and Isacke 2002; Turner 2003). In soluble form, collectins facilitate in vitro phagocytosis, promote chemotaxis, stimulate the production of cytokines and ROIs by inflammatory cells, and are implicated in the phagocytic uptake of apoptotic corpses. Collectins, such as MBL and ficolins with associated serine proteases (MASPs) , have a pivotal role in activating the lectin complement pathway. MBL recognizes mannose and mannans, and appears to be a spatially coordinated TLR coreceptor increasing the microbial uptake. It is also localized in the phagosome (Ip et al. 2009). The ficolins are a group of GlcNAc-specific proteins containing collagen-like and fibrinogen-like (FBG) sequences; they can be secreted or function as PRR cellular receptors. They have overall collectin structure and activity similar to those of MBL, but in contrast to MBL, it is the FBG domain that binds GlcNAc (Matsushita et al. 2000; Gupta and Surolia 2007; Sim and Laich 2000).
In ascidians, collectin-like lectins show the typical CTDL and variations in the remaining structure and in glycan specificity. The ascidian MBL-like and ficolin-like lectins, complexed with MASPs, activate C3, leading to the complement cascade (Sekine et al. 2001; Nair et al. 2005; Fujita et al. 2004a, b; Raftos et al. 2001; Skjoedt et al. 2010).
In H. roretzi, the complement activating Hr-collectin binds to glucose (thereby designated GBL) but not to mannose or GlcNAc (Ji et al. 1997; Kenjo et al. 2001; Nonaka and Azumi 1999). The GBL C-terminal half contains the CTDL, but the collagen domain is replaced by another sequence with an α-helix structure similar to the configuration of Gly-X-Y repeats.
Phylogenetic analysis showed that CiMBL clusters with vertebrate MBL, indicating a common ancestor, while CiMBL and HrMBL form separate clusters, supporting a common ancestor before the divergence of the two taxonomic orders.
In S. plicata, the subunit of the dimeric collectin-like (TC14) includes a collagenous domain and a short, cysteine-bearing N-terminal domain; it is specific for D-galactose and related monosaccharides. TC14 is expressed by circulating hemocytes, and the expression increases following challenges by an inflammatory agent (LPS, carrageenans) (Green et al. 2003). In activated circulating hemocytes (presumably hyaline amoebocytes) a C3 homolog is promptly upregulated and exocytosed (Raftos et al. 2001, 2002, 2003, 2004).
Transcripts for MBL-like proteins have been identified in several ascidian species (Franchi and Ballarin 2017; Vasta et al. 1999). Transcripts for ficolins are present in H. roretzi (Kenjo et al. 2001), B. leachii (Rinkevich et al. 2007), and B. schlosseri (Franchi and Ballarin 2017). The transcription of the H. roretzi ficolin-3 gene is significantly impaired in organisms with soft tunic disease (Cha et al. 2011), and the collectin-like (GBL) involved in the recognition of microbes interacts with MASP and leads to HrC3 activation (Sekine et al. 2001). In S. plicata, increased collectin secretion has been related to the inflammatory response (Nair et al. 2000; Green et al. 2003). In colonial ascidians the MBL-like pathway has been identified; genes for MASPs and ficolins are upregulated in MCs during the B. schlosseri nonfusion reaction (Oren et al. 2007; Franchi and Ballarin 2017).
In C. intestinalis, which expresses the complete lectin-triggered complement activation pathway, a CiMBL has been cloned and sequenced (see below). The CiMBL initiates the lectin pathway of the complement and promotes phagocytosis, killing of pathogens, and induction of other cellular responses. In addition, two CiMBLs and a CiMBL-associated serine protease are expressed in the gut epithelia (Skjoedt et al. 2009). The deduced CiMBL amino acid sequence shows a protein structure that includes a Cys-rich N-terminal domain, presumably engaged in disulfide bridges between monomers, two collagen-like domains, one α-helix domain, and one CTLD that binds mannose/glucose residues. The CiMBL mRNA transcription profile, after LPS stimulation, shows the rapid expression and the enhanced level of the transcript: at 1 h p.i. the CiMBL level is sixfold increased, then it decreases (2–4 h p.i.) and after a further increase it reaches its maximum peak at 24 h p.i. The CiMBL is mainly expressed by amoeboid granulocytes in the tunic matrix and CCs in the pharynx bars and connective tissue (Bonura et al. 2009).
Complement
Complement pathways and their products are pivotal in inflammation and largely detectable in invertebrates.
The complement system is a complex innate immune surveillance system. Complement components are activated in a cascade fashion after a triggering event; each step of the pathway results in conformational changes or cleavage of the downstream components, which become activated and gain the capacity to activate the subsequent cascade components (Merle et al. 2015a; Nesargikar et al. 2012). Proteolytic cleavage is, in part, performed by enzymatic complexes originating from association of products from the same complement cascade. The complement core components are named with a simple number designation in the order of their discovery, while the sequence of the cascade reactions is C1, C4, C2, C3, C5, C6, C7, C8 and C9. In ongoing inflammatory reaction, the level of the complement components increases—contributing to acute and chronic inflammation, immunostimulation, lysis of bacteria and foreign cells, and opsonization and chemotaxis—and moreover it participates in B cell activation.
C3 is the central component shared by three routes: antibody-dependent, lectin-dependent (detailed below), and alternative.
Alternative and lectin-dependent pathways can be traced back in invertebrates; the lectin pathway has been described in deuterostome invertebrates, including ascidians (Nonaka 2014; Smith et al. 1999; Fujita et al. 2004a, b; Nonaka and Kimura 2006; Nonaka and Yoshizaki 2004; Nair et al. 2005). In this pathway, MBL or ficolin lectins form a complex with proenzymes, i.e., MBL-associated serine proteases (MASPs) that are provided with a modular structure including a serine protease C-terminal domain (Matsushita and Fujita 2001; Dinasarapu et al. 2013). Upon binding of the MBL– or Ficolin–MASP complex to pathogens, the MASP zymogen is converted into the active form that cleaves C3, C2 and C4, leading to fragments, some of which are necessary for continuing the pathway. Several MASPs have been identified and each of them has a defined role (Bobó et al. 2016). At the end, the triggered lectin-dependent downstream cascade (as well as the alternative pathway) merges into the classical pathway involving terminal complement components (C6, C7, C8, and C9) that form the C7–C9 membrane attack complex perforin domain (MAC). This complex causes pores to appear on the plasma membrane of the target cells, leading to their lysis (Kondos et al. 2010). Proteins containing the MACF domain, but lacking the other terminal complement components, have been found in organisms in a broad range of phyla (Nonaka 2014).
One of the major consequences of complement activation is the generation of three small cationic peptides (C3a, C4a and C5a) usually referred to as complement anaphylatoxins. They are mainly involved in several activities: (1) attraction of phagocytes by chemotaxis (mainly C3a and C5a) and promotion of extravasion of leukocytes (that bear the specific receptors C3aR and C5aR) into the injured site; (2) upregulation of adhesion molecule expression by neutrophils and endothelium, increasing (mainly C5a) vascular permeability and “leukocyte rolling”; (3) opsonization of potential pathogens for facilitating phagocytes in recognition and ingestion of targets; (4) induction of the oxidative burst by macrophages and neutrophils; (5) induction of C3 receptor expression; and (6) C5a stimulation of the secretion of proinflammatory cytokines such as IL-1 and IL-6, which can also stimulate the proliferation of T cells, and modulation of dendritic cells (APCs) influencing the adaptive immune response. The functional profile of C4a, usually included among the anaphylatoxins, is questionable (Barnum 2015; Merle et al. 2015a, b). Complement components and their receptors are expressed by activated leukocytes, macrophages, dendritic cells, mast cells, and NK cells. Production of receptors for C3, C3- and C5-peptides, and products of C3b degradation are upregulated during inflammation (Lubbers et al. 2017; Li et al. 2011; Futosi et al. 2013; Van Lookeren Campagne et al. 2007).
Genome analysis of many representative species has allowed us to trace the evolutionary route of the complement system on the basis of the presence or absence of each complement gene (Nonaka and Kimura 2006). The genomes of invertebrates, including cnidarians, contain homologs of C3 and other components of the complement alternative and/or lectin-dependent pathways. However, many of them are merely predicted genes from the draft genome; their functions are not wholly known or can be suggested by structural homologies with mammals. The absence of complement genes in some species (Drosophila melanogaster and Coenorhabditis elegans) could be the effect of a secondary loss, presumably due to their short generation time.
The structural features shared between vertebrate C3, C4, and C5, and the similarity with the protease inhibitor α2-macroglobulin, have suggested that two lineages could have evolved from a common ancestor by gene duplication and divergence (Levasseur and Pontarotti 2011). Anyway, individual domains of complement components have been found in both protostomes and deuterostomes; in the latter the complement components appear to be established as a combination of pre-existing domains.
Ascidian Complement Lectin Pathway
Although both the alternative and lectin complement activation pathways are present in ascidians, here only the established lectin pathway is described (Fujita et al. 2004a, b; Nonaka 2014). In this pathway, the MBL/Ficolin-like-MASP complex bound to target cells directly activates the cascade, which can serve several functions, including agglutination, opsonization of cellular agents, activation of phagocytes, inhibition of microbial growth, cytotoxicity, and modulation of the inflammatory response (Raftos et al. 2001; Franchi and Ballarin 2017; Nonaka and Yoshizaki 2004; Nonaka and Satake 2010).
Homologs of the pathway key components (MBL, MASP, C3) have been identified in several ascidians (Vasta et al. 1999). C3 is a heterodimer made up of α- and β-C3-like chains. Collectins mediate recognition of PAMPs and stimulate the activation of the α-chain thiolester bonds that can directly bind to bacteria. MASPs cleave C3 into two fragments. The large C3b peptide mediates opsonization and the small C3a-like peptide is akin to the corresponding vertebrate anaphylatoxin (Marino et al. 2002; Pinto et al. 2003; Matsushita et al. 1998).
In the colonial B. schlosseri, complement component (C3-like, MBL-like, and Bf-like) genes are transcribed by hemocytes (mainly MCs) and the lectin activation pathway has been identified (Franchi and Ballarin 2016). The C3b receptor and the ficolin-like lectin associated with two MASPs were also found (Corey et al. 2016). The BsMBL gene is upregulated by zymosan, and opsonic activity appears to be C3 dependent (Franchi and Ballarin 2017).
C. intestinalis genome analysis has provided the most comprehensive picture of an almost complete set of genes homologous to the mammalian lectin complement pathway: MBL, ficolin, four MASP genes, two C3s (CiC3-1 and CiC3-2), three Bf/C2s, two α2-macroglobulin-like, and genes for C6/C7/C8/C9 proteins containing MAC/perforin domains (Azumi et al. 2003). The Ci-C6/C7/C8/C9 components exhibit protein structures similar to those of human late components (MAC); however, the activity of a cytolytic pathway needs to be established (Skjoedt et al. 2010; Marino et al. 2002; Nonaka and Satake 2010; Giacomelli et al. 2012). The deduced amino acid sequence of the CiC3-1 protein exhibits the above-reported structure and the thioester site is provided with a catalytic histidine and a convertase cleavage site. The anaphylatoxin-like CiC3a peptide is generated by MASP proteolytic cleavage of the CiC3α-chain N-terminus. As for mammalian C3a, the chemotactic function of CiC3a is localized at the C terminus, but the terminal Arg is not critical for the activity. The C3a fragment, which in ascidians may be chemotactic or opsonic, in C. intestinalis exerts chemotactic activity toward hemocytes, as shown by the attractive effect of the recombinant CiC3a. The inhibition with pertussis toxin also suggests that the receptor molecule mediating the chemotactic effect is the G protein–coupled receptor(s) (GPCRs) belonging to the rhodopsin family (Pinto et al. 2003; Melillo et al. 2006). GPCR-based signal transduction is ubiquitous in eukaryotic genomes. There is a highly compact set of GPCRs in the Ciona genome, and a wide survey refers to the presence of 169 putative receptors homologous with human GPCRs, indicating that they serve several functions shared with vertebrate signaling biology (Kamesh et al. 2008; Prasobh and Manoj 2009). Many Ciona GPCR receptors are highly divergent homologs of the chemokine receptor cluster genes.
The domain amino acid sequence of the cloned CiC3aR shows high homology with mammalian C3aR (Melillo et al. 2006). Differences concern the carboxyl-terminal tail and the third cytoplasmic loop; both are longer than the corresponding regions of C3aRs, and the shorter extracellular N-terminal sequence lacks the presumptive N-glycosylation site. As in mammals, the C-terminal end of the cytosolic tail contains many serine and threonine residues that represent presumptive phosphorylation sites.
In the C. intestinalis complement pathway, CiMBL, CiC3a, and CiC3a-R have a role in the proinflammatory process. Real-time PCR analysis showed that C3, constitutively expressed in the pharynx , is upregulated by LPS stimulation, while specific anti-CiC3 antibodies showed that the gene and lectin pathway are localized in hemocytes (granular amoebocytes) of the pharyngeal bars and in stigmata ciliated cells. CiC3a and CiC3b are present in the pharynx, and the amount of the CiC3a fragment increases following the LPS challenge . CiC3a-R is constitutively expressed only in granular and hyaline amoebocytes which, in chemotaxis and inhibition experiments, migrate in a directional way (Pinto et al. 2003; Melillo et al. 2006; Giacomelli et al. 2012).
The two CiC3-like genes seem to be diverged from a common ancestor of the vertebrate C3/C4/C5, and then duplicated into CiC3-1 and CiC3-2 in the Ciona lineage. Independent gene duplication and various diversification events have occurred in distinct ascidian orders or cognate families. The phylogenetic analysis of the CiC3a-R amino acid sequence indicates that it does not cluster with any of the vertebrate C3aR and C5aR clades (Marino et al. 2002). The phylogenetic tree, based on the alignment of CiC3-1 and CiC3-2, including molecules of the α2-macroglobulin superfamily, shows that the Ciona C3s form a cluster with H. roretzi C3. Thereby, the duplication event from which the CiC3-1 and CiC3-2 genes arose would have happened after the separation of the Halocynthia and Ciona ancestor.
The C3-like protein from S. plicata is closely related to H. roretzi C3 and Ciona C3s. Similar results have been obtained in P. stolonifera. Activation by LPS and zymosan in this species generates an 8.5-kDa proteolytic fragment that confers chemotactic activity toward hemocytes , as demonstrated by in vitro chemotaxis experiments. The C3 expression by P. stolonifera hemocytes is coincident with chemotactic activity (Raftos et al. 2002, 2003). A C3-like transcript has been found in S. plicata hemocytes challenged with LPS or carrageneen; the transcription was upregulated and the protein contained in vesicles promptly exocytosed. C3-containing granulocytes actively infiltrate the injured tissue and degranulate (Raftos et al. 2001, 2002). LPS treatment also stimulates the expression of a C3-like protein by H. roretzi cells in the stomach wall, which is engaged in antibacterial activity (Nonaka et al. 1999).
Even ascidian C3 receptors, homologous to mammalian complement receptors (integrin family), have been identified. In H. roretzi, two integrin-like proteins (αHr1 and αHr2 and two βHr1 and βHr2) have been found on the surface of a granulocyte population, and specific antibodies against a recombinant protein, reproducing the extracellular region of αHr1, inhibited C3-dependent phagocytosis, suggesting that in this ascidian, an ancestral form of CR3 and CR4 mediates phagocytosis (Miyazawa et al. 2001; Miyazawa and Nonaka 2004; Ewan et al. 2005). Both the βHr1 and βHr2 subunits are associated with the αHr1 subunit. The type of pairing found in ascidians, namely the same integrin α subunit (αHr1) paired with different integrin β subunits (βHr1 and βHr2), is different from the mammalian CR3 and CR4 pairing pattern and refers to ancestral forms of complement receptors.
Also, a C1q-like gene is expressed in ascidians; it is constitutively transcribed in the C. intestinalis pharynx , in B. schlosseri (Oren et al. 2013), and in D. candidum (Iwanaga and Lee 2005; Azumi et al. 2003, 2007). In mammals, C1q, as a complement recognition subcomponent, binds to a wide variety of targets (microorganisms, apoptotic and necrotic cells ); the protein initiates C1r and C1s activation, and also forms a weak complex with MASP (Wallis 2007). In addition, it can bind pentraxins (CRP and SAP in humans), which belong to an ancient lectin family characterized by a unique structure of a disk-shaped cyclic pentamer of noncovalently bound identical subunits. Pentraxins function as soluble Ca2+-dependent PRRs . They bind a variety of microbes, facilitate phagocytosis, and regulate the inflammatory response (Du Closs 2013). In addition, pentraxins share with collectins and ficolins the property of activating complement. A galactose-binding pentraxin-like protein has been isolated from the colonial D. candidum (Vasta et al. 1986; Quesenberry et al. 2003).
Genes for putative complement-control protein (CCP) featuring a mammalian CCP domain and genes for α2-macroglobulin (MASP-inhibitor) that are regulators of the complement activity have been identified (Pancer et al. 1995; Azumi et al. 2003). The α2-macroglobulin is one of the founding members of the larger thiol-ester protein superfamily, which includes a variety of similar protease-binding proteins with a wide phylogenetic distribution and shares a defined suite of structural and functional characters (Armstrong 2010).
PO and ProPO
Melanin is a pigment found in almost all animals; its role varies depending on the organism, and it is critical for survival. In vertebrates, the tyrosinase plays a crucial role in melanin biosynthesis, and components of the evolutionarily conserved tyrosinase family serve different functions (Cammarata and Parrinello 2009; Esposito et al. 2012). The survey of the C. intestinalis genome revealed one ortholog to human TYRP1, expressed in developmental stages.
Invertebrates, including ascidians, use phenoloxidase (PO) in place of tyrosinase for melanin biosynthesis, and the enzyme activity concurs with wound healing, sclerotization, pigmentation, and defense. Phenoloxidase and tyrosinase share two active sites that are copper dependent but vary in their remaining sequence and oligomeric organization. Phylogenetic analysis shows that the POs belong to the arthropod hemocyanin superfamily, and evolutionary origin from hemocyanin ancestors with enzymatic function has been suggested (Cerenius et al. 2008). Unlike arthropod PO, which is monophenoloxidase, ascidian PO is a bifunctional redox enzyme (o-diphenol:O2 oxidoreductase) that catalyzes the ortho-hydroxylation of monophenol (i.e., tyrosine) forming o-diphenol and, then, the dehydrogenation of diphenols into o-quinones, which can polymerize producing melanin (Cammarata and Parrinello 2009).
PO and phenolic compounds have been identified in ascidian tunic cells, participating in tunic formation; in some species, melanin is also produced (Chaga 1980). In ascidians, as in arthropods, concomitant with prophenoloxidase (proPO) activation many immune reactions are performed, such as the generation of factors with antimicrobial, cytotoxic, opsonic, or encapsulation-promoting activities (Söderhäll and Cerenius 1998; Cerenius et al. 2008).
Ascidian hemocytes contain the proenzyme proPO , which is activated to PO by a serine protease cascade, in turn activated by PRRs after their binding to ligands (peptidoglycans, LPSs, bacterial carbohydrates, fungal β-glucans) (Jackson et al. 1993; Immesberger and Burmester 2004; Hata et al. 1998; Parrinello et al. 2003; Amparyup et al. 2012). Generally, the ascidian proPO is contained in MCs, although the activity has also been reported in other granulocyte types. The mechanism can be regulated by protease inhibitors, e.g., α2-macroglobulin, thereby avoiding overproduction of melanin, phenolic substances and ROIs that could lead to the disruption of self-tissues.
In B. schlosseri, soluble factors potentiate the activity of MCs and induce their degranulation. The MC vacuoles and granules contain both proPO and PO substrates (polyphenol tunichromes, quinones, DOPA-containing proteins), and the enzyme pathway produces quinones and cytotoxic ROIs (Nappi and Ottaviani 2000).
Among the ascidian PO enzymes, differences have been observed. Divalent cations are requested to enhance the L-dopa oxidation by B. schlosseri PO, whereas in C. intestinalis and S. plicata, the PO activity is not cation dependent. Although the ascidian PRRs involved in proPO activation are poorly known, there are differences regarding the ligands; β-1,3-glucans and oligosaccharides are active in C. intestinalis whereas they are inactive in S. plicata and B. schlosseri (Ballarin et al. 1994; Jackson et al. 1993; Arizza et al. 1995; Cammarata et al. 1999). In addition, following recognition of a harmful agent by hemocytes, molecular cross talk can be involved in the proenzyme activation (Lemaitre and Hoffmann, 2007; Cerenius et al. 2008).
Two C. intestinalis PO genes, CiPO-1 and CiPO-2, have been identified and cloned. They encode putative proteins clearly distinct from tyrosinase, lack a signal peptide, and display variations in their amino acid sequences (Immesberger and Burmester 2004; Cammarata and Parrinello 2009).
Upregulation of the ProPO System
The prophenoloxidase (proPO) pathway, fated to produce melanin, also releases intermediates involved in the inflammatory responses, in which melanin may not be the final product and the pathway is mainly related to the production of intermediate active factors, i.e., quinones or oxygen radicals.
The PO activity is expressed by activated S. plicata MCs and C. intestinalis URGs, and quinones that lyse in vitro erythrocytes and tumor cell lines are produced (Cammarata et al. 1997; Parrinello et al. 2003). Differently, in B. schlosseri nonfusion alloreaction, the cytotoxic activity is dependent on ROIs (Ballarin and Cima 1998; Ballarin et al. 2002). Indeed, in vivo, the enzymatic reduction of quinones forms toxic ROIs (Nappi and Vass 1993).
In several species, MCs are retained as the main proPO-expressing cell type, which contain substrate molecules and activating proteases, and are the effectors of the nonfusion reaction (Ballarin 2008; Smith and Söderhäll 1991). However, among botryllids, other cell types appear to be involved, such as diverse granulocytes, SRCs, and CCs (Scofield and Nagashima 1983; Shirae and Saito 2000).
In C. intestinalis, strong PO activity was found in cytotoxic URGs, whereas morula cells do not show any cytotoxic activity, although both are involved in inflammatory reactions (Parrinello 1996; Peddie and Smith 1993; Cammarata et al. 2008). In Ph. mamillata and S. plicata “hemocytes with large granules” display low PO activity whereas the typical morula cells do not show any PO-related activity (Cammarata et al. 1997; Parrinello et al. 2003). Although the activating PRR in C. intestinalis has not been identified, in crustaceans the proPO system is activated upon recognition of pathogens by pattern recognition proteins, including LPS- and β-glucan-binding proteins (LGBPs) . In the shrimp (Paeneus monodon) proPO system, a PmLGBP functions as a PRP for both LPS and β-glucan, and the transcription in hemocytes is enhanced by the microbial challenge (Amparyup et al. 2012).
In C. intestinalis, as an effect of LPS intratunic inoculation, the CiPO1 and CiPO2 transcription and the enzyme activity are enhanced by LPS in circulating hemocytes and in the tunic (Vizzini et al. 2015a). In the tunic matrix, the proPO activation pathway could depend on unknown proteases diverse from serine proteases; in fact the PO activity enhanced by LPS challenge could not be inhibited by trypsin inhibitors (Cammarata et al. 2008). A high proportion of hemocytes (CCs and URGs) express both POs in the pharynx and are spread in the connective tissue lining the epidermis. In addition, POs of different sizes are modulated in the tunic and pharynx , indicating that LPS inoculation challenges the proenzyme production by tunic cells and hemocytes, as well as the activation of the serine protease pathway (Trapani et al. 2015).
Differences have been found in the CiPOs transcription time courses and in the transcription level profiles of the two identified genes. The transcription of CiPO-1 is faster (1-4 h p.i.) and it is mainly expressed in two waves (the second at 24 h p.i.); CiPO-2 upregulation occurs later, reaching the maximum at 8 h p.i. These profiles could be indicative of distinct hemocyte populations (CCs, URGs) engaged in CiPO1 or CiPO2 production. The inflammatory role of PO is also indicated by the increased number of PO-containing URGs that populate the tunic matrix after the LPS stimulus (Cammarata et al. 2008). Phenoloxidase is also involved in the H. roretzi “contact reaction” in which hemocytes were mixed in vitro with allogeneic or xenogeneic hemocytes (Fuke 1980).
Peroxinectin
Peroxinectin is a component of the peroxidase–cyclooxygenase superfamily characterized by a C-terminal peroxidase domain and an integrin-binding motif (KGD: Lys-Gly-Asp) that can participate in cell–cell and cell–extracellular matrix interactions . This protein is involved in adhesion and migration mechanisms essential for immunity (Dong et al. 2009), granulocyte degranulation, microorganism immobilization, phagocytosis, encapsulation, and nodule formation (Johansson and Söderhäll 1989; Cerenius et al. 2008; Johansson et al. 1995; Johansson 1999; Kobayashi et al. 1990; Thornqvist et al. 1994; Hsu et al. 2006). Peroxiditic heme protein genes appeared very early in evolution, presumably recruited upon pathogen invasion to develop enzyme-dependent unspecific antimicrobial defense. To exert this activity, the enzyme requires the heme group linked in a suitable cavity, and oxidation products are responsible for killing microorganisms (Zederbauer et al. 2007a, b).
A C. intestinalis peroxinectin (CiPxt ) has been cloned and sequenced from the pharynx, and the entire CiPxt sequence has been analyzed (Vizzini et al. 2013a). The peroxidase domain contains two His; one of them may be the proton acceptor involved in the peroxidase catalytic function, and the second one could be a heme binding site, while four cysteines indicate intrachain disulfide linkages. At the C-terminal sequence, the putative KGD integrin–binding motif, the peptide signal at the N-terminal, and highly probable trypsin and chimotrypsin cleavage sites can be predicted. The three-dimensional model, which in part overlaps with human myeloperoxidase, shows the KGD in a loop in an external position. The secondary structure is mainly α-helical and each monomer has a central heme-containing core. The comparative analysis of the peroxidase domains showed significant homologies with peroxidase–cyclooxygenase superfamily members, including mammalian myeloperoxidase (MPO) , eosinophil peroxidase (EPO) , thyroid peroxidase (TPO) and invertebrate thyroid peroxidase (TPO) (C. intestinalis and H. roretzi), and peroxinectins (Pxt) from insects, crustaceans, and echinoderms. In vertebrates, the entire sequence of Pxt was not found. An increasing phylogenetic distance separates the peroxidase domain from chordata MPO, TPO, and EPO, and echinoid and crustacean Pxts. Although the C. intestinalis domain is included in the invertebrate Pxt subfamily, it forms, together with the echinozoa, a deuterostome cluster distinct from the arthropod group which, in turn, is distinguished into crustacean and insect clades; it is the closest to the mammalian group.
In ascidians, as in crustaceans, the Ptx may be involved in adhesion and migration mechanisms essential for immunity. The activity could be generated by the proteolysis associated with concomitant activation of the proPO system (Sritunyalucksana et al. 2001).
Peroxinectin Upregulation
In crustaceans, the Pxt gene can be upregulated by LPS or β-1,3-glucans and the transduction pathway regulating the expression of antimicrobial peptide genes (Dong et al. 2009; Liu et al. 2005, 2007).
The LPS inoculation enhances the level of CiPxt mRNA; the gene transcription is promptly and significantly boosted (4–8 h p.i.) reaching a maximum value at 12 h p.i., depending on the increased frequency of activated cells in the pharynx vessels and tunic, then it decreases (Vizzini et al. 2013a). CCs/MCs and SRCs inside the pharynx vessels appear to be the CiPtx–producing cells, and the riboprobe also labels the cytoplasm rim of URGs in the inflamed tunic matrix.
As reported above, LPS activates the proPO system through proteolytic cleavage that, as in other invertebrates, could activate properoxinectin into peroxinectin.
An increased number of CiPxt-positive compartment/morula cells and signet ring cells in the vessels can be related to LPS inoculation. Furthermore, a large number of CiPxt-expressing URGs intensely populate the inflamed tunic matrix (Vizzini et al. 2013a). URGs also express proPO, which is activated by proteolysis, thereby the same enzymatic cascade could cleave pro-Pxt, producing active Pxt.
The potential involvement of CiPxt in the C. intestinalis inflammatory response may be related to the defense role of mammalian myeloperoxidase and peroxidase of neutrophils, monocytes, and eosinophils (Klebanoff 2005; Wang and Slungaard 2006).
Cytokines
In mammals, cytokines form a large family of low-weight proteins including interleukins (ILs) and tumor necrosis factors (TNFs). They are mainly produced and secreted by endothelial cells, epithelial cells, granulocytes, resident macrophages, and NK cells in response to various harmful stimuli, including LPS. The same cytokine can be secreted by different cell types, can stimulate the production of additional cytokines, or can be pleiotropic; similar functions can be stimulated by different cytokines; and they can act synergically and regulate various and contrasting actions. Being proinflammatory , they are involved in cell proliferation, cell differentiation and activation, cell motility, chemotaxis, phagocytosis, apoptosis, and necrosis, as well as stimulating collagen synthesis in wound healing and tissue repair (Dinarello 2007; Meager and Wadhwa 2013). Cytokine-like activities have been reported in several invertebrate species, playing an essential role in defense (Ottaviani et al. 1995, 1996; Buchmann 2014; Hughes et al. 1990).
Many cytokines signal via the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway, which is also frequently altered and constitutively active in a broad range of tumor cells.
TNFα-Like
Tumor necrosis factor-α (TNFα) is a major proinflammatory cytokine with pleiotropic and multifunctional properties (Goetz et al. 2004; Idriss and Naismith 2000; Bodmer et al. 2002). The signaling function of TNF receptor is also mediated by adapter protein factors (TRAFs) (Inoue et al. 2000).
In C. intestinalis , cDNA/EST derived from hemocytes and the draft genome sequence disclosed an ectodysplasin/TNF-like multigene family (Terajima et al. 2003; Shida et al. 2003) and in C. savigny a TNF factor ligand superfamily member (CsTL) has been identified (Zhang et al. 2008). A BLAST search revealed four C. intestinalis predicted genes related to TNF family members, including a TRAIL-like protein (TNF-related apoptosis-inducing ligand) of D. rerio, the bovine ectodysplasin A, the rabbit lymphotoxin-α precursor, and the feline Fas ligand. Since in mammals these members are type II membrane glycoproteins with limited homology to TNFα in the extracellular region, they have not been studied further. The CiTNFα gene with significant predicted similarity and identity to TNFα of the monkey Aotes vociferans and human TNFα was cloned and sequenced (Parrinello et al. 2008).
The CiTNFα deduced amino acid sequence shows that it is a type II transmembrane protein with an extracellular C-terminal domain containing sites for proteolytic cleavage, two conserved Cys residues that can form an intrachain disulfide bridge, and two potential N-glycosylation sites. The domain structure is similar to that of mammal and fish TNFα, supporting that CiTNFα is a component of the protein family. The putative molecular homology modeling process disclosed a conserved “jellyroll” structure consisting of two β-antiparallel sheets building a sandwich. Minor amino acid replacements are scattered, whereas major differences (10 aa) form a very short α-helix. Frequent “hot” mutation points characterize the C-terminal sequence that, with the N-terminal sequence, forms the first two strands of the inner sheet. A high degree of CiTNFα genetic variability has been found in a C. intestinalis wild population, and mutation points with effects on the amino acid sequence are more numerous within the C-terminal region (Vizzini et al. 2017).
CiTNFα domain organization and 3′UTR comparative analysis display a relatively complex gene organization with seven exons and six introns. The gene organization shares regions with vertebrates, such as an interferon-γ-activated inhibitor of translation (GAIT) and a Musashi binding element (MBE) that may be sufficient for temporal regulation and translational silencing. The gene transcription occurs in different tissues (pharynx , ovary, stomach, intestine), but the expression is higher in the pharynx and intestine, which are mainly exposed to environmental challenge.
The phylogenetic tree unveils that CiTNFα protein and two predicted C. savigny TNFs form a cluster related to the vertebrate cluster, suggesting that the TNFα family domain and cytokine-like activities were present in ascidians. In this respect, it is noteworthy that D. melanogaster TNFα homologs, the Eiger A and B isoforms (Moreno et al. 2002), appear to be distant from the chordate group, and the earthworm CCF, reported as a functional analog (Silerova et al. 2006), also lies far from the TNF family.
In addition, the Ci-TNFα and Cs-TNF deduced amino acid sequences form a cluster with sea urchin TNF1A, separated from other invertebrates. Therefore, an evolutionary lineage traced back to invertebrate deuterostomes appears to diverge at the ascidian–vertebrate node.
Upregulation of the CiTNFα Gene
In mammals, activated neutrophils produce proinflammatory and immunoregulatory cytokines, including interleukins and TNF, and chemokines belonging to distinct protein families. Many germ line–encoded PRRs , colony-stimulating factors, cytokine receptors, and complement receptors have been shown to trigger cytokine production (Tecchio et al. 2014).
Ciona intestinalis CiTNFα is constitutively expressed and upregulated in vivo by LPS. In the pharynx , the CiTNFα gene is promptly upregulated, with an expression profile similar to that of mouse and fish TNFα (Parrinello et al. 2008, 2010; MacKenzie et al. 2003). The upregulation was already evident at 2–4 h p.i., decreased at 8 h p.i., then increased again until 24 h p.i. In spite of the subsequent decline, a low mRNA level was maintained, probably as an effect of the protracted inflammatory action differently exerted by LPS on the ascidian body wall tissues. The mRNA is transcribed by GAs and CC/MCs that during the inflammation enrich the tunic matrix, pharynx vessels, and the lacunar connective tissue. The nucleus of these cells was marked by the riboprobe, and a specific antibody localized the protein in the cytoplasm. URGs and various granulocyte transition forms, abundant in the inflamed tunic matrix, do not present any transcription signal. In the circulating hemolymph, also HAs are CiTNFα-expressing cells. Hemocyte-lysate and hemolymph plasma analysis unveiled that, just after the stimulation, the CiTNFα produced as hemocyte membrane-bound protein (43 kDa) is cleaved into a mature soluble form (15 kDa). These findings further suggest that the CiTNFα exerts an important role in both local and systemic responses as a proinflammatory cytokine. In addition, the vascular epithelium is committed as shown by TNFα protein in the basal membrane lining vessel walls.
Surprisingly, some recognizable LLCs, collected in nodules associated with the vessel epithelium, appeared to express the cytokine; the mRNA was found in the large nucleus and the protein in the cytoplasm rim. Taking into account that activated LLCs did not appear to be directly involved in the inflammatory reaction, this finding leaves open the question on the LLC function and the possibility of an autocrine signaling.
In mammals, TNF receptors expressed by leukocytes are primarily involved in apoptosis and inflammation, but they can also take part in proliferation and differentiation (Ward-Kavanagh et al. 2016; Locksley et al. 2001). The Ciona genome contains eight TNFR-associated factor (TRAF)-related genes, which are the major signal transducers for the TNFR superfamily (Terajima et al. 2003), and three possible CiTNF receptors are capable of initiating signal transduction that culminates in caspase activation and programmed cell death (Chambon et al. 2007).
In botryllid ascidians, during the allorejection reaction, MCs produce and release molecules reacting with anti-TNFα antibodies. Indirect evidence also suggests that this cytokine is involved in the recruitment of MCs in the ampullae involved in allogeneic contacts (Cima et al. 2004; Ballarin 2008).
IL-1- and IL-17-Like Interleukins
Interleukins are a group of cytokines that function in the immune system; the majority of them are synthesized by a T lymphocyte population, monocytes, macrophages, and endothelial cells. They promote the development and differentiation of T and B lymphocytes and hemopoietic cells (Brocker et al. 2010). Interleukin-1 (IL-1) is a major immunoregulatory protein released by macrophages with many host defense–related properties.
Interleukin (IL-1)-like activities have been identified in a number of invertebrate species (Ottaviani et al. 1995, 1996; Beck et al. 1989b; Beck and Habicht 1991; Beck et al. 1993). In ascidian hemolymph, proteins sharing a number of IL-1-like physicochemical characteristics have been reported (Beck et al. 1989a, b, 1993). A fraction isolated from the S. clava hemolymph contains an IL-1β-like component that stimulates in a dose-dependent fashion the proliferative activity of granular amoebocytes, LLCs, and mouse thymocytes (Raftos et al. 1991b). An immunohistochemical study showed that molecules containing interleukin-1-like epitopes are expressed by endothelial tissue lining the pharyngeal wall. An IL-1-like functional activity may be indicated by the increased number of hemocytes in the vascular lacunae as a result of the cell proliferation response of challenged specimens. Accordingly, IL-1-receptor epitopes in cell nodules of the pharyngeal bar ansae have been found. Although the C. intestinalis genome does not reveal IL-1-like genes, human IL-1 molecular traits can be observed and an IL-1-receptor identified.
In botryllid ascidians, during the allorejection reaction, MCs produce and release molecules that cross-react with antihuman-IL-1α antibodies; in the nonfusion reaction this IL-1-like is involved in the MC recruitment into the ampullae (Ballarin 2008).
In mammals, interleukin-17 (IL-17) is a T cell–derived cytokine characterized by a Cys knot fold formed by two sets of paired β-strands stabilized by three disulfide interactions (Pappu et al. 2010). Several IL-17 family members are produced by NK cells and neutrophils (Michel et al. 2008; Weaver et al. 2007). The IL-17 induces proinflammatory effectors, neutrophil infiltration, and clearance of microorganisms; synergizes with other cytokines such as TNFα, may render cells cytotoxic, and participates in tissue injury (Tecchio et al. 2014; Gu et al. 2013).
Candidate IL-17 genes have been identified in invertebrate genomes (Roberts et al. 2008; Wu et al. 2013). In C. intestinalis, three IL-17-like genes (CiIL17-1, CiIL17-2, and CiIL17-3) have been found to be vertebrate orthologs (Vizzini et al. 2015b). Sequence and structural analysis of CiIL-17s show the same gene organization as the human IL-17A/F, formed with two introns and three exons, differing in the length of the introns which are longer in the human IL-17A/F. Four cysteines are strictly conserved in regions that correspond to functionally and structurally essential motifs. The three-dimensional model displays the Cys localization in β-sheets and supports the preservation of the disulfide linkage position in all of the IL-17 homologs. The sequence of the C-terminal region is critical for receptor binding in accordance with the presence, in the genome, of an IL-17 receptor homologous.
The phylogenetic tree unveils that the CiIL-17 genes share a common ancestor in the chordate lineages.
CiL-17 Gene Upregulation
In the pharynx from LPS-treated specimens, the transcription of the three CiIL-17-like mRNAs is upregulated in a short time (4-8 h p.i.) but their transcriptional profiles are slightly different (Vizzini et al. 2015b). CiL-17-1 and CiL17-3 are mostly upregulated at 4 h p.i., whereas the CiL-17-2 expression is challenged at 8 h p.i. These profiles indicate differential transcriptional activity of distinct hemocyte populations in the pharynx vessels. Riboprobes are contained in GAs with large granules, in CCs, and in the cytoplasm of a cell type provided with a large vacuole resembling the SRC or URG transition stage.
The riboprobe-containing hemocyte populations increase in density following the LPS stimulus, while the relative numbers of hemocytes that express each transcript vary in accordance with the time courses, further suggesting a modulated response in distinct cell populations.
The role of the CiIL-17 has not been ascertained, and hypotheses concern the known activity in the mammalian inflammatory reaction and findings from other ascidians. The recombinant Botryllus IL-17 significantly enhances, in a dose-dependent manner, the cellular cytotoxicity of allogeneic effector cells (Cima et al. 2016; Corey et al. 2016). In the colony, during the generation change, the gene for an IL-17 ortholog is overtranscribed and probably involved in modulation of the cellular events occurring during the take-over phase of the life cycle (see below). The IL-17-like could mediate cross talk between MCs and phagocytes.
TGF-β (CiTGF-β)
Transforming growth factor-β (TGF-β) belongs to a family of regulatory cytokines that have pleiotropic functions in a broad range of cell lineages involved in numerous physiological and pathological processes and immune responses (Li et al. 2006). TGF-β signaling elicits diverse cellular responses that are primarily mediated through Smad transcription factors, the key of cytokine signaling pathways (Shi and Massagué 2003; Massagué and Gomis 2006).
In C. intestinalis, the CiTGF-β is structurally related to the protein family, synthesized as a long proprotein composed of a hydrophobic signal peptide, an N-terminal prodomain, and a C-terminal active peptide with a cleavage site for generating the C-terminal active fragment (Vizzini et al. 2016a). The tridimensional model shows a cysteine knot motif, and the secondary structure is characterized by two α-helices and seven β-sheets. In addition, an RGD motif (tripeptide Arg-Gly-Asp) may be a potential binding site. Like other family members, the prodomain shows a low degree of conservation for correct processing and secretion of the mature dimeric complex. The active peptide exhibits conserved cysteine residues engaged in intrachain disulfide bonds.
Comparative analysis of the TGF-β genes discloses an ancestral bilateria repertoire consisting of two TGF-β type II and three type I receptors (Huminiecki et al. 2009). In ascidians, the ancestral TGF receptor repertoire includes three type II receptors and at least two R-Smad.
CiTGF Upregulation
In the LPS-induced C. intestinalis inflammatory reaction, the CiTGF-β gene is promptly upregulated (1–4 h p.i.), suggesting its engagement in the first phase of the response (1–4 h), then a second transcription wave (48 h p.i.) seems to correspond to time-elapsed activation of distinct cell populations. This transcription profile foreshadows the CiTGF-β potential function as a proinflammatory cytokine. Within the vessel lumen, tightly packed hemocyte clusters, mainly formed by granulocytes and URGs, express the mRNA. It is of interest to mention that in mammals, TGF-β has a role in differentiation of T helper 17 cells and in IL-17 production (Lohr et al. 2006), and some functional relationship between CiTGF-β and CiIL-17 in the early inflammatory reaction may be hypothesized (Vizzini et al. 2016a).
LPS Challenges Gene Upregulation in the Vessel Epithelium and Epidermis
In mammals, the vessel endothelium functions as an interactive barrier between blood and tissue, and it is the primary target for inflammatory agents. As a response, the endothelial cells express chemokines that initiate recruitment at sites of tissue inflammation and activation of circulating leukocytes (Trepels et al. 2006; Bierhaus et al. 2000). LPS induces gene upregulation, producing adhesion molecules, while the endothelial permeability is enhanced and proinflammatory mediators are secreted.
In C. intestinalis, LPS challenges the pharynx epithelium as well as the epidermis lining the tunic (Vizzini et al. 2008, 2013a; Parrinello et al. 2015a, b). Although a systematic histological study, taking into account the various timing phases, has not been carried out, at an early stage (a few hours p.i.) of the response, galectins (CiLgals-a and CiLgals-b), CiTNFα, CiPO2, CiPxt, and CiMBL, as well as the CiCAP modulatory factor, are upregulated to various extents in traits of the vessel epithelium.
Also, the transcription of the Ci–type IX collagen α-chain is upregulated by LPS inoculation. Hemocytes circulating, scattered in the connective tissue under the epidermis and in the tunic matrix, produce the mRNA within 4 h p.i. Morula cells, amoebocytes (granular/hyaline), and URGs express the collagen. In the URGs the protein is confined in the cytoplasmic rim lining the granule. In a slower time (about 24 h p.i.), the epithelial cells of the epidermis that outline the tunic are mainly involved in collagen expression. The epidermis and infiltrating hemocytes could have an active role in tunic-repairing processes. In addition, the proliferative activity of the epidermis for the renewal of the tunic cells (Di Bella et al. 2005, 2015) could be stimulated by the cytokine-like enhanced expression. In this respect, traits of the epidermis usually monolayered in naïve ascidians appeared to be multilayered during the encapsulation process (Di Bella et al. 2005; Parrinello and Patricolo 1984).
As in vertebrates, the C. intestinalis inflammatory reaction results in a regulated pattern of proinflammatory factors and tissue remodeling.
CiLgals, CiPOs, and Pxt Are Upregulated in the Endostyle
The ascidian endostyle is a glandular ciliated groove that lies along the middle ventral wall of the pharynx. It is the first trait of the digestive system and extends to the esophagus, and its function concerns feeding as well as a thyroid-like activity. Histologically, it has been divided into nine functional units called “zones” numbered bilaterally from midventral to dorsolateral. Zones 1, 3, and 5 are supporting elements involved in catching and transporting food. Zones 1–4 produce the mucus, a complex of mucoproteins and mucopolysaccharides that capture food particles, including bacteria, conveyed from the pharynx ciliated epithelium (Petersen 2007; Parrinello et al. 2017). The bottom of the groove (zone 1) is lined with a longitudinal row of very long cilia that move the mucus to the sides of the endostyle and then lateral cilia push it toward the gut.
Endostyle zones express both CiLgals. Specific antibodies and riboprobes showed that cells of zones 2 and 3 constitutively produce CiLgals-a and CiLgals-b. Both zones are further activated by LPS inoculation, and the CiLgals-a and -b production is enhanced. In zone 4, the LPS stimulus induces CiLgals-b mRNA transcription and the protein is localized in taller epithelial cells, suggesting differential zone expression and function (Parrinello et al. 2015a). Vesicles are involved in galectin production and transport. Although data on the galectins function in the endostyle are not available, it is reasonable to suggest that, as in the stomach, they could be involved in activity related to microorganisms with which this structure is in continuous contact.
Other zones appear to be challenged by LPS. The CiPO1, CiPO2, and CiPxt riboprobe signals were found in zones 7, 8, and 9 (Vizzini et al. 2013a, 2015a). These zones consist of low epithelial cells similar in their features. They are the thyroid-equivalent components of the endostyle that contain iodoproteins and express thyroid-specific genes that are developmentally regulated. In these zones, peroxidase activity has been reported, while a specific thyroid peroxidase (TPO) has been identified in zone 7 (Fujita and Sawano 1979; Ogasawara et al. 1999). A relationship between the hemocyte CiPOs and CiPxt activities and the peroxidase of thyroid-like tissues may be only hypothesized. However, it is known that in humans, proinflammatory cytokines cause thyroid inflammatory disorders (Ajjan et al. 1996). Furthermore, thyroid cells can express functional sensors for exogenous and endogenous damage signals , and they are also capable of launching innate immune responses without the assistance of immune cells (Kawashima et al. 2013). These findings open a new front in ascidian biology.
In the Pharynx , LPS Challenges Immune Regulatory Mechanisms
Posttranscriptional regulation of mRNA processing is well known to play a fundamental role in determining the outcome of gene upregulation, also affecting initiation and resolution of the inflammatory reaction (Anderson 2010). Furthermore, in almost all eukaryotes, a polyadenylation process intervenes just after the gene transcription, affecting qualitatively and quantitatively the dynamic of mature mRNA for translation. In vertebrates, an alternative polyadenylation (APA) mechanism is operational in inflammation. The mRNA metabolism is controlled and protein isoforms with distinct functions, or mRNAs differing in the length of their 3′ untranslated regions (3′UTR), are produced (Di Giammartino et al. 2011). The 3′UTRs serve as traits for binding to factors (i.e., microRNA, RNA-binding proteins) that control the regulation. Through variations of the 3′UTRs, APA regulates the stability, tissue localization, and translation efficiency of the target mRNAs. In the CR-APA (coding region-APA-alternative polyA), sites are located in internal intron/exon organization and APA events can produce different protein isoforms. Alternatively, APA sites are located in the 3′ untranslated region (3′UTR, UTR-APA), resulting in transcripts with 3′UTRs of different lengths but encoding the same protein. CR-APA can affect gene expression qualitatively (Elkon et al. 2013), whereas UTR-APA has the potential to quantitatively affect the expression (Fox 2015).
In C. intestinalis, LPS could stimulate a CR-APA event in the CAP gene (Bonura et al. 2010; Vizzini et al. 2016b; Parrinello et al. 2016). The catabolite activator protein (CAP) that belongs to the superfamily of cysteine-rich secretory protein (antigen 5 and pathogenesis-related1), is a transcriptional activator (dimeric) with a DNA-binding domain at the C terminus that modulates immune responses (Gibbs et al. 2008). Two cAMP molecules bind dimeric CAP and function as allosteric effectors by increasing the affinity for DNA.
In silico analysis showed that CiCAP is characterized by the CAP superfamily motifs (CiCAP1, CiCAP2, and CiGLIPR1). The CiCAP2 deduced amino acid sequence shows an N-terminal domain with high homology to that of vertebrates, a C terminus homologous to the collagen-binding adhesion of Streptococcus mutans, and glycosylation sites. In the phylogenetic tree, the CiCAPs appear to be closely related to the human GLIPR1 protein (glioma pathogenesis-related protein1). A GAIT element (gamma interferon inhibitor of translation element) was found in the 3′UTR of CiCAP-2 mRNA. The GAIT is a cis-acting RNA element found in several immune-related mRNAs, involved in specific translation control (Vyas et al. 2009).
The CiCAP-2 gene is challenged by LPS through a CR-APA site. In the pharynx , it is rapidly upregulated (1–4 h p.i.), and the mRNA level is in agreement with the increased hemocyte populations in the pharyngeal vessels. Genes expressing cytokines are regulated at transcriptional, posttranscriptional, and translational levels, and in humans, the biosynthesis of TNFα is mainly regulated at the posttranscriptional level (Jensen and Whitehead 2001; Karpova et al. 2001). A computational analysis of C. intestinalis revealed several post-transcriptional CiTNFα regulatory elements (Vizzini et al. 2013b, 2016b).
Overall, these findings suggest that the initiation phase of the ascidian inflammatory reaction can be controlled by both transcriptional and posttranscriptional regulation.
Various Agents Challenge Encapsulation
Inflammatory responses can be caused by irritant agents and tunic injury. The typology of the cellular reaction, the cell types involved, and the resulting effects can depend on the nature and size of the injuring agent, as well as on the interactions between the tunic and vascular system. Small particles (e.g., carmine, colloidal carbon, trypan blue, colloidal thorium dioxide) injected into the tunic or the vascular system are phagocytized and cleared, whereas a capsule surrounds larger objects. Tissue damage accompanies the inflammatory response and damage signals could be involved in encapsulation responses, and their potential role in the modulation of inflammation cannot be excluded (Kaczmarek et al. 2013; Pradeu and Cooper 2012). However, since invertebrate experimental procedures can hardly be performed in aseptic conditions, contamination with microbial molecules cannot be excluded. In addition, following tissue stress or cell death, different cellular stimuli (e.g., TNF, Fas ligand, TRAIL ligand, dsRNA), IFN-g, ATP depletion, and pathogens) have been shown to induce necrosis (Vanlangenakker et al. 2012).
Glass fragments that injure the M. manhattensis tunic and branchial tissues are enveloped by infiltrating hemocyte populations. MCs migrate from the hemolymph and collect in the wound edges to form a capsular multilayered structure containing strands of tunicin (Anderson 1971). The tunic matrix becomes filled with material released from degranulating MCs, and fibrous material envelops the wound. A similar response was observed in P. stolonifera injured tunic (Wright and Cooper 1983). It has been suggested that in naïve ascidians, MC intracellular content contributes to tunic matrix formation.
In C. intestinalis the tunic inflammatory response has been stimulated by inoculating particulate materials or soluble proteins (erythrocytes, colloidal carbon, bovine serum albumin, limpet hemocyanin). High concentrations of various erythrocyte types form an agglutinated mass and a large capsule becomes visible to the naked eye (from hours to days) through the transparent tunic (Fig. 3: 1, 2). The inoculation of different erythrocyte types did not show any specificity of the response. Although a similar reaction has been observed by inoculating proteins, the erythrocyte mass entrapped in the tunic matrix stimulates the strongest reaction. Histochemical analysis of capsules identified abundant neutral polysaccharides and proteins in the tunic matrix (Parrinello 1984, unpublished data). Just after the inoculation of any agent type, the tunic matrix is enriched by a massive hemocyte infiltration, mainly granulocytes, CCs, and URGs (Fig. 3: 4), while univacuolated cells release their vacuolar content to form the capsular barrier (Fig. 3: 1, 6). Because of the absence of vessels in the Ciona tunic, the recruited activated hemocytes come from the hemolymph in the pharynx vessels and connective tissue, crossing the epidermis (Di Bella and De Leo 2000). As an effect of the LPS-induced activation, URGs and CCs express the CiC3a-1 chemotactic fragment as a product of proteolytic cleavage of CiC3-1 (Pinto et al. 2003).
Encapsulation in the tunic of Ciona intestinalis inoculated with erythrocytes. 1 Histological section of the capsule, in which infiltrated hemocytes (granulocytes and vacuolated cells) construct the capsule (Mallory’s stain). 2 Outside view of the capsule at 12–24 h post-inoculation. 3 Enlarged view of granulocytes and vacuolated cells. 4 Granulocyte. 5 Compartment/morula cells labeled with anti-CiTNFα-specific antibody. 6 Vacuolated hemocytes (SRCs) that release unstained (Mallory’s stain) material (v). 7 Activated epidermis cells, which contribute to the reaction by exocytosis of material from large vacuoles (e) (Mallory’s stain). 8 Vacuolated hemocyte labeled with anti-CiPO2 antibody. 9–12 Transition stages of URGs (9) and compartment/morula cells (10, 11) containing various PO localizations and levels (DOPA-MBTH cytochemical stain). 13 Granulocytes envelop the erythrocyte mass (E). 14 Compartment/morula cells labeled with anti-CiLgals-a antibody. Bars 5 μm: 4, 5, 6, 9, 10, 12, 14; bar 20 μm: 3, 11; Bar 25 μm: 13; bar 50 μm: 1
Granulocytes with small granules degranulate while URGs and vacuolated cells release their contents, encasing the inflamed tissue (Parrinello 1981; Parrinello et al. 1984; Parrinello and Patricolo 1984) (Fig. 3: 9–11). Electron microscopy observations showed granulocytes with large granules and vacuolated cells with various granules and vacuolization features (Parrinello et al. 1990; De Leo et al. 1996, 1997). The identification of the classic raspberry-like MCs was doubtful, conversely vacuoles and granule contents disclosed various features in their electron transparency or density, indicating maturation pathways before the release. In this respect, antimicrobial peptides may be released by URGs and CCs/MCs. In C. intestinalis, two putative gene families coding for antimicrobial peptides (Cimam-A and Cipap-A) have been identified (Fedder and Leippe 2008). Two synthetic peptides, representing the cationic core region of AMPs, displayed in vitro potent antibacterial and antifungal activity permeabilizing the target plasma membrane. Immunogold electron microscopy showed that the Cimam-A AMP is contained in the URG granule and among the vacuoles/granules, or at the cytoplasmic periphery of CCs/MCs.
During the inflammatory response challenged by erythrocytes, an extraordinary number of URGs and CC/MCs populate the tunic matrix along with several cells that appear to be advanced inflammatory stages of granular or vacuolated cells. In particular, part or all of the electron-dense content of the URGs becomes electron transparent, and vacuoles differing in size contain both electron-transparent and/or electron-opaque materials. Although direct evidence is lacking, the antimicrobial peptide release could be associated with the degranulation and vacuolization processes (Di Bella et al. 2011). These finding further suggest that microbial stimuli could be involved. Also the epidermis was challenged and epithelial cells presented large vacuoles (Fig. 3: 7), the larger size and vacuolization are indicative of intensive releasing activity implementing the capsule structure. In addition, as an effect of proliferative activity, traits of the monolayered epidermis became multilayered, and activated cells could separate from the tissue to reach the tunic matrix (Di Bella et al. 2005).
Production of collagen could also contribute to capsule structure. CCs scattered in the tunic and contained in the hemolymph, as well as the epidermis cells, express the Ci–type IX collagen 1α-chain, and the gene is promptly upregulated by LPS. This discovery, associated with type I collagen-like identified by cross-reacting antibodies, suggest that a collagen-rich granulation tissue is formed (Vizzini et al. 2001, 2002, 2008).
In specimens from some batches, after a prolonged period (many days) of unresolved inflammation, the capsule degenerates, a gelatinous blister is formed, and a large wound in the reacting area is formed. Although no data have been reported, in this reaction, environmental microbia could affect the wound, further challenging the degenerative response. Findings on tunic cell behaviors and fate were obtained by TEM observations. Numerous cell populations around the irritant agents formed a carpet in the tunic matrix sections, and they displayed various levels of membrane dissolution, while granules and vacuoles were spread in the extracellular matrix (Fig. 3: 1, 11, 13) (Parrinello 1981; De Leo 1992; De Leo et al. 1996, 1997; Parrinello et al. 1990). Both URGs and vacuolated cells undergo cell wounding, and their content could be involved in the capsule degeneration and/or in reparative processes (Fig. 3: 9–12). URGs, from circulating hemolymph of naïve ascidians, are known to release in vitro hemolytic substances, and displayed contact-dependent lytic activity toward various cell targets independently of their origin (sheep, rabbit, human erythrocytes, and tumor cell lines) that could indicate a missing-self recognition mechanism (Parrinello et al. 1995; Parrinello et al. 1996; Parrinello 1996). This activity, also related to sphingomyelin as a target, appeared to be dependent on phospholipase A2 (Arizza et al. 2011). In addition, activated URGs are PO-expressing cells and an excess of cytotoxic products from the enzyme activity could contribute to the tunic lysis and cell necrosis. In S. plicata the phenoloxidase-dependent cytotoxic activity of granulocytes with large granules (which may be morula cells) against erythrocytes and a tumor cell line has been demonstrated (Cammarata et al. 1997).
The causes of the necrotic events have not been ascertained; however, loss of membrane integrity and release of intracellular components can challenge and amplify the inflammatory response with the possible involvement of damage signals (Kaczmarek et al. 2013).
Inflammatory Events Characterize Colonial Ascidian Take-Over and Alloreactions
The ability for intraspecific self/nonself recognition is manifest in colony fusion or nonfusion reaction of genetically compatible or incompatible colonies, respectively, that come in contact at their growing edges. These responses occur in several botryllid species. In the Japanese species Botryllus primigenus and the cosmopolitan species B. schlosseri, the outcome of the contact is genetically controlled by a highly polymorphic (fusibility/histocompatibility) FuHc locus, the alleles of which are codominantly expressed (see references in Ballarin (2008)). In B. schlosseri , this locus is responsible for colony fusion/rejection in a simple Mendelian ratio. The great majority of botryllid colonies in nature are heterozygous and fusion occurs when at least one allele is shared by two contacting colonies that fuse their tunics and anastomize their vascular systems (chimera). In B. schlosseri chimerae, one of the partners reabsorbs the other one (losing partner) during one of the blastogenetic cycles (take-over) mimicking the zooid resorption that cyclically occurs in a normal colony. Zooids of a precedent generation undergo apoptosis and are cleared by phagocytosis while buds produce a new generation (Rinkevich 2002, 2005). As mainly shown in B. primigenus and B. schlosseri, nonfusion of incompatible colonies is initiated by a partial fusion of the tunics followed by a rejection reaction at the facing marginal ampullae (vascular endings) where points of rejection are formed along the contact borders. Active, soluble histocompatibility factors diffuse between both the facing partners.
Cell recruitment by chemotaxis, extravasation, cell degranulation, and induction of cytotoxicity—characteristic mechanisms of the inflammatory reaction—lead to necrosis and colony separation. MCs are the effector cells; they migrate through the ampullar epithelium into the tunic, degranulate, releasing polyphenols, PO, and intermediate products of the enzymatic pathway, NOIs and ROIs, and so cause cytotoxicity.
There are no unique results for genomic characterization of the B. schlosseri FuHc locus and for the allorecognition factor candidates (McKitrick and De Tomaso 2010; Voskoboynik et al. 2013a, b; Rinkevich et al. 2012). In any case, none of the genes with potential roles in the allorecognition response are directly related to the MHC gene. Among the candidates, the polymorfic Hsp40-L gene has been included. The Hsp40-L deduced amino acid sequence shows that most of the polymorphisms of the protein occur in the last 100 residues of the C terminus (client-binding domain), which presumably provides specificity for target proteins. Polymorphism of Hsp40-L correlates with fusion/rejection outcomes and a transcript was detected in the epithelial cell layer of the ampullae (Nydam et al. 2013). Several Hsps have been found to function like MHC molecules by binding antigenic peptides, being expressed within cells and on cell surfaces, and mediate T cell activation (see references in Bartl et al. 2003). Evidence from a set of chaperones, besides processing of antigen, indicates that an Hsp might have been a MHC predecessor.
Although the mechanism of recognition remains to be elucidated, potential functional relationships between disparate allorecognition systems have been proposed (Taketa and De Tomaso 2014). From different approaches it emerges that the candidate FuHc gene (also termed “Botryllus Histocompatibility Factor” (BHF)) (Voskoboynik et al. 2013a, b) is upregulated in opposing colonies and the transcript associated with the rejection response. The enhanced expression was found in hemolymph, ampullae, buds, and endostyle, as well as in larva and sperm, supporting the histocompatibility-related function, and it is intriguing that separate lineages of somatic and germ line stem cells or pluripotent stem cells differentiate according to the niche in which they land (Laird et al. 2005).
The complexity of the colonial ascidian recognition system is further increased by the mechanism reminiscent of the missing-self effector arm of vertebrate NK cells. Screening for genes differentially expressed during allorecognition in B. schlosseri indicates the involvement of the CD94/NKR–like gene (McKitrick and de Tomaso 2010; Taketa and De Tomaso 2014; Voskoboynik et al. 2013a, b), which may be one of multiple genes within the FuHc locus.
The Take-Over
Inflammatory components are involved during the B. schlosseri blastogenetic cycle.
Each colony is a clone derived from a founder “oozoid,” which is the outcome of the metamorphosis of tadpole-like and swimming larvae. The founding oozoid begins an asexual budding process forming the fertile hermaphrodite colony, and cyclical budding produces blastogenetic generations (Brown et al. 2009). In B. schlosseri, zooids connected by a common vasculature have a short life-span (about 1 week), then cease their main physiological activity and die in a massive wave of apoptosis (take-over) (Lauzon et al. 1992). In the colony, the zooids are joined peripherally by “primary buds,” which migrate into the vacated region left by the resorbed individuals. Secondary buds are connected to the new zooids, and so multiple individual generations can develop.
The resorbed zooids are selectively removed via phagocytosis in a programmed cell clearance homeostatic process (Cima et al. 2010; Lauzon et al. 2013; Elliot and Ravochandran 2010). The recognition of apoptotic cells involves both phosphatidylserine (eat me signal) and CD36 (Cima et al. 2003). In mammals, CD36 is a membrane glycoprotein present on mononuclear phagocytes that functions as a scavenger receptor and participates in internalization of apoptotic corpses, thus contributing to the inflammatory reaction (Moodley et al. 2003). On the basis of morphological and histoenzymatic properties, two distinct phagocytic cell populations (amoebocytes and macrophage-like cells) are the effector cells (Ballarin and Cima 2005; Voskoboynik et al. 2004). MCs, which are effector cells in allorejection, are not involved. Circulating professional phagocytes producing BsRBL are actively involved; they produce the lectin that interacts with the surface of the apoptotic cells and facilitate the corpses’ removal. The rise in the frequency of phagocytes is related to the massive apoptosis and to the enhanced mRNA transcription during the blastogenetic cycle (Ballarin et al. 2013).
Chimerae
Vertebrates do not undergo transplantation reactions naturally. Compound ascidians are the phylogenetically closest group in which transplantation reactions can occur in nature, even if durable and lasting success is very rare (Rinkevich 2002).
Botryllid colonies that share one FuHc allele can fuse, the ampullae undergo anastomosis, and a single chimeric colony is formed (Ballarin 2008). Following a fusion, one chimeric partner can be eliminated in a process of allogeneic “resorption” in which an inflammatory cell-based rejection is mainly mediated by cytotoxic MCs and phagocytes that remove the remains of the “losing” partner. This reaction is a delayed allogeneic response put into effect to prevent the risk of somatic/germ cell parasitism in genetically nonhomozygote partners (Rinkevich and Weissman 1987).
The resorption is similar to the nonfusion reaction of allogeneic incompatible colonials (see below), including recruitment by chemotaxis, extravasation, cell degranulation, and activation of phagocytic and cytotoxic programs in which the BsRBLs are presumably involved (Corey et al. 2016; Rinkevich and Weissman 1992; Rinkevich 2005 ; Ballarin and Zaniolo 2007; Ballarin et al. 2013). In B. schlosseri, the resorption is due to PO-dependent cytotoxicity by MCs and phagocytosis by HAs and MLCs, which infiltrate the regressing zooids and cause a necrotic lesion in the ampullar epithelium, whereas LLC populations do not appear to be directly involved (Rinkevich et al. 1998).
The allogeneic resorption process and the blastogenetic take-over share the massive phagocytosis, but in a chimera, losing partner elimination is an integrated function of cytotoxic (MCs) and phagocytic programs.
Interestingly, there is a relationship between the blastogenetic cycle and the cytotoxic program. Microinjections for cell transfer showed that the maximal effector response of MCs occurs when these cells are isolated during the developmental period of take-over, indicating a presumptive licensing effect on MCs (Corey et al. 2016). Comparative transcriptome analysis disclosed that pathways induced during take-over are also used in the allogeneic resorption setting. The transcriptome profiles showed a significant overlap at the intersection of the transcription profiles of take-over and chimera resorption. Several genes that express mediators, known to play a role in acute inflammation, were significantly upregulated in both processes, such as the transcription factor NF-ΚB, a member of the Janus kinase (JAK) family, two members of the TNF receptor–associated signal transduction family, and a member of the interferon regulatory transcription factor family. In both processes, genes usually activated during the inflammatory response are upregulated, including components of the lectin complement activation pathway (C3, CFB), the C3b receptor CR1, MBL-like lectin, MASP1 and MASP2, thrombin factor VIII, factor XI, plasminogen, and kallikrein family proteins. CFB is a component of the alternative pathway that binds C3b; the kallikreins belong to a group of serine proteases involved in plasminogen proteolysis, producing plasmin (Yousef and Diamandis 2003). Furthermore, a network of genes involved in phagocytosis is shared, including tyrosine kinase and phosphatidylserine receptors (Linger et al. 2008). Others genes are related to proteolysis, programmed cell death, and phagocytic clearance.
The upregulation of a member of the IL-17 family (BsIL-17), related to the significant augmentation of the MC cytolytic activity, suggests that during the “take-over,” cell death programs render MCs cytotoxic, eliminating the chimeric partner in collaboration with activated phagocytes (Corey et al. 2016). In mammals, macrophages stimulated by IL-17 express many proinflammatory cytokines and chemokines (Zhang et al. 2011). Also, BsRBLs expressed by activated phagocytes are involved in the take-over, contributing in enhancing MC and phagocyte activities (Ballarin et al. 2013).
According to Corey et al. (2016), the disparate immunogenic pathways induced during take-over may be also used in the setting of allogeneic resorption.
As reported above, self-renewing stem cells with competitive phenotypes may originate cell lineages involved in chimerae rejection. In this respect, the cell death program that render MCs effector cells may be traceable to a distinct cell lineage. Finally, the effector system is reminiscent of missing-self recognition that involves differential expression of cell surface germ line–encoded receptors (Taketa and De Tomaso 2015).
Inflammatory Events in Nonfusion Allogeneic Rejection
Allorecognition, rejection, and the FuHc locus form a complex system that has been well studied for B. schlosseri. When allogeneic intraspecific lab-reared colonies are artificially brought in contact at their cut surfaces, the initial fusion of the tunic occurs, soluble histocompatibility factors diffuse, and then allorecognition and consequent irreversible rejection are manifested (Ballarin 2008). Following the initial tunic fusion of the partners, the epithelium permeability of the facing ampullae increases, the MC frequency inside the contacting ampullae is enhanced, and the MCs migrate into the tunic through the epithelium of the ampullar tips and degranulate, releasing their contents, including the PO (Oren et al. 2008). Among botryllids there are differences in the mechanism of nonfusion reaction, and variations in allorejection reactions include the ratio of MCs to total hemocytes, and the levels of PO activity, which varies among the examined species (Shirae and Saito 2000). In B. scalaris, phagocytes crowd inside the fused ampullae and stimulate the aggregation of hemocytes into large clusters, which are encapsulated by other phagocytes, plugging the lumen of the fused ampullae, and blood flow is interrupted. In contrast to other botryllid species, granular amoebocytes contain low levels of PO, and no signs of selective recruitment or degranulation of MCs were observed (Shirae et al. 1999).
The missing-self model could involve the BsCD94-1. In the allorejection, a population of granulocytes bears this receptor but no MCs express the receptor. The labeled granulocytes were found inside the vessels, ampullae, and tunic, and, according to the previous findings, a large number of MCs accumulate at the tip of the interacting ampullae, but none of them express BsCD94-1 (Khalturin et al. 2003). The mechanism of allorecognition and the precise roles of the competent cells, including cooperative effects, remain to be defined.
In the B. schlosseri nonfusion reaction, melanin, the end product of the PO pathway, is synthesized and accumulated as brownish color dots between the interacting ampullae where a clot is formed by fibers and clumped dead cells (mainly MCs; points of reactions, PORs). In this botryllid the cytotoxicity has been related to the induction of oxidative stress, such as with superoxide dismutase and NOS, while the PO pathway has a role in the formation of the melanized necrotic mass. Finally, the interacting ampullae are destroyed, the vascular continuity between the two partners is interrupted, and the allogeneic colonies separate.
Upon MC activation, proinflammatory cytokines (IL-1α-like, TNFα-like, IL-17-like) are released, and complement-like components (BsC3, BsBf, ficolins, MASPs) are expressed. The cytokine-like molecules contribute to cell recruitment and modulate cellular events, inducing phagocytes to produce BsRBLs with opsonic activity, while the BsC3 pathway can be opsonic. Molecular and morphological studies substantiate the existence and activation of vertebrate-like blood-based coagulation components that are retained to be homologs to proteolytic coagulation factors (Oren et al. 2007). High similarity with thrombin, coagulation factors V and IX, lower similarity with fibrinogen/fibronectin, and a plasminogen were found. EGF-like and von Willebrand factor–like domains, serine proteases, and protease inhibitors were also identified. These coagulation-related genes are differentially upregulated during the allorejection processes and a role in clot formation has been suggested. The fibrinogen-like is confined to vacuoles within a specific SRC population circulating in the interacting ampullae and in CCs scattered inside the vasculature. Most of these reacting cells appeared to be attached to each other, forming small aggregates (Oren et al. 2008). The expression of a von Willebrand factor was exclusive of a macrophage-like cell population, located mainly within the vasculature and ampullae, thus indicating a systemic response.
In mammals, the hemostatic feature of coagulation is activated immediately upon injury. Platelets bind to collagen exposed by a damaged blood vessel, strengthened by the von Willebrand factor, ensuring the formation of primary hemostatic plugs.
Inflammatory Arm in Solitary Ascidian Tissue Transplantation
In solitary ascidians, naturally occurring fusion or allorejection similar to that described in colonial organisms cannot be hypothesized. Transplantation experiments provide results that are not always homogeneous, probably related to the ascidian species and experimental procedure differences.
In M. manhattensis, pharynx auto- and allogeneic grafts do not fuse with host tissues, and both present characters of an inflammatory reaction (Anderson 1971). The implant becomes infiltrated with morula cells that degranulate, causing graft necrosis without any encapsulation.
In S. plicata, most first-set integumentary allografts are rejected whereas the majority of autografts remain viable (Raftos et al. 1987a, b, 1988). The late phase of the tunic allograft rejection present cellular and molecular components of the host inflammatory response. Hemocytes actively infiltrate the grafted tissue and aggregate around it, then dense boundaries of extracellular material are formed between the graft and the surrounding tunic matrix; thereby the transplanted tissue appears to be encapsulated. Phagocytes undertake phagocytosis, and degranulating granulocytes may be cytotoxic. Vascular components within allografts are destroyed; the allograft undergoes nonspecific gradual necrosis and it detaches.
Among the cell types observed in first-set and secondary allografts, LLCs are implicated. They invade incompatible allografts and surround the tissue prior to rejection. This influx coincides with the destruction of the grafted tissues, while the other cell types undertake nonspecific responses, presumably stimulated as an inflammatory reaction to the injury. According to Raftos et al. (1987a, b), the allograft response of S. plicata exhibits a memory, with a second allograft being lost far more rapidly than the first-set allograft, while a third-party grafting indicates specificity. This graft rejection has been imputed to the role played by the LLCs. However, the identification of specific markers on LLCs could contribute to better clarification of the dynamics and mechanisms of the response, also taking into account the differences among species and the limited role exerted by these cells in inflammation.
In Styela clava, in vitro allogeneic cytotoxicity has been reported (Kelly et al. 1992).
In C. intestinalis allogeneic tunic transplants, a persistent inflammatory response is characterized by granulocytes and phagocytes, whereas morula cells were not observed (Reddy et al. 1975). Because of the high mortality of the treated ascidian specimens, the secondary immune response was not examined. The direct involvement of LLCs as effector cells, based on morphological observations of the reacting tissues, remains unclear, while the involvement of URGs and the proPO system activation may be suggested.
While more detailed insight is awaited, these findings suggest that independently from the involvement of LLCs as initiators of the allorejection and being eventually responsible for specific recognition, the effector mechanisms seem to be associated with the inflammatory reactions.
Anyway, the responses of the urochordate S. plicata to tunic grafts and B. schlosseri allorejection confirm the existence of a histocompatibility system and suggest that ascidian innate immunity may have adaptive features (Kvell et al. 2007).
Are Damage Signals Involved in Inflammatory Reactions ?
The “danger theory” (Matzinger 1994; Gallucci and Matzinger 2001; Matzinger 2002), by which intracellular molecules (damage-associated molecular patterns (DAMPs)) released by injured tissues can activate the immune system, comes from the observation that a systemic inflammatory response syndrome can be caused by a sterile injury. Many reports on vertebrates, mainly mammals, have identified danger signals and their presumptive receptors, while DAMP molecules have also been associated with tissue repair (Pandolfi et al. 2016; Hirsiger et al. 2012). The real meaning of the theory, in explaining several responses of the innate and adaptive immune systems, has been debated and basic search criteria have been indicated (Kono and Rock 2008).
The initial danger theory was not intended to account for innate immunity that is the natural, nonspecific, nonanticipatory, and nonclonal but germ line–encoded response by invertebrates. Several observations suggest that in invertebrates, the inflammatory response may be triggered by damage done to the host; however, there are difficulties in examining the experimental models, including wild-type ascidian individuals. Except for lab-reared organisms (maybe botryllids), results exclusively due to endogenous damage signals from a trauma excluding contamination with bacterial products may be doubtful, and in all cases the requested criteria cannot usually be respected.
Therefore, while taking into account these restrictions and waiting for them to be overcome, the evaluation of the danger signals and their effects in ascidian organisms is mainly based on the knowledge of the already identified DAMPs, their presumptive receptors, the signaling pathways involved, and their potential effects, aided by genome and transcriptome analyses.
In addition, according to Pradeu and Cooper (2012), the more appropriate term “damage signal” (not opposed but associated with self/nonself recognition) can be used.
The endogenous stress signals can be released in response to a variety of tissue trauma resulting from environmental temperature, chemicals, xenobiotics, radiation, oxygen deprivation, and food constraints (Bianchi 2007). Molecules released immediately after nonprogrammed cell death or secreted from immune cells without them dying are retained DAMPs that can recruit and activate innate immune cells. Heat shock proteins (Hsps) are perhaps the most diverse DAMPs (Calderwood et al. 2012); they reside in several cellular compartments and can exert functions with regard to immunity and inflammation. In addition to their roles in promoting correct protein folding, Hsps are involved in initiating innate immune responses to cellular stress. These proteins, lacking a signal peptide, are released from damaged cells outside the classical ER–Golgi system and probably bind to one or more of the Toll-like receptors (TLRs) inducing (Hps60/70) the production of NO, TNFα, and IL-12 via TLR4 (Asea et al. 2002; Vabulas et al. 2002).
A conserved Hsp70 chaperone system (with eight members) has been identified in the Ciona genome (Wada et al. 2006); they are similar to but simpler than those in humans. In addition, the genome contains 36 genes for J-proteins, a gene for a J-like protein, and three genes for BAG multifunctional (including apoptosis) family proteins. Several reports have disclosed that C. intestinalis Hsps are stress inducible, and the degree of the induction was different from gene to gene. Under heat stress (a 10 °C upshift), the transcriptional profiles showed that the expression of six Hsp70 genes, eight J-protein family genes, and two BAG family genes were enhanced. Endoplasmic reticulum (ER) stress (brefeldin A treatment) increases the mRNA levels of four Hsp70 genes and four J-proteins (Wada et al. 2006; Fujikawa et al. 2010). The J-protein and BAG families are major groups of co-chaperones of the HSP70 proteins and are responsible for the functional diversity and modulation of the chaperone system.
In S. plicata the Hsp70 gene has been characterized and amplified; the examined deduced amino acid sequence is part of a large clade including C. intestinalis Hsp70, and the transcriptional profile has been performed from specimens living in environmentally stressing conditions (Pineda et al. 2012). The results showed that the protein expression increases with higher seasonal stress levels, as did monitoring of stress responses in a salt marsh population exposed to wide temperature and salinity fluctuations. The Hsp70 expression varied over time, with higher stress levels recorded in summer and winter. In addition the interaction between temperature and salinity was significant in enhancing Hsp70 expression, and mortality events were related to drastic changes in abiotic factors that overwhelmed the observed stress response mechanisms.
Although no data on possible modulation of the innate immune response by environmental factors have been reported, these findings suggest that also in ascidians, Hsp70 may be a DAMP candidate.
Some insights into endogenous DAMPs that challenge the immune system come from the ascidian larva and metamorphosis that may be retained as a model for inflammatory gene expression in the presumable absence of sepsis. The nonfeeding “urodele” larva that characterizes most ascidian species, after a very short swimming period (from a few minutes to many hours, according to the species) in which it becomes “competent” (able to initiate metamorphosis), settles onto the substrate and the papillae used for the adhesion and the tail are rapidly resorbed. According to Cloney (1982), in many ascidians, within a few minutes following onset of the metamorphosis phase, the sensory vesicle is withdrawn, and the axial complex sensory organ and visceral ganglion are destroyed by phagocytes over a period of a few days.
In nature, during the short swimming period in the large seawater volume, bacterial product contamination is unlikely. Nevertheless, several components of the inflammatory machinery appear to be activated. In the competent larva, hemocytes and mesenchymal cells undergo a variety of targeted migrations across the epidermis into the larval tunic, and after settlement, they reach the expanding tunic (Cloney 1982; Cloney and Grimm 1970). Furthermore, cells migrate across the epidermis in both the trunk and the anterior papillary region.
In Boltenia villosa (Davidson and Swalla 2002) and C. intestinalis (Chambon et al. 2007) precompetent and competent larvae, genes are activated and multiple transcripts for each gene that match proteins involved in immunity have been found. In addition, similarity between two species belonging to different orders can be observed.
In B. villosa, two selectins, hemocytin, pentraxin, Bv-LRR (leucine-rich repeat domain), von Willebrand factors (Bv-vWa1), Bv-Sccp2 (selectin 2), and Bv-MASP were first detected in precompetent larvae and were distinctly upregulated during larval competence. Bv-Ccp2 complement factor (complement control domain) is first detected in competent larvae and is then highly upregulated within 1 h after settlement. Bv-Ptx (pentraxin) and Bv-Ccp3 are mainly upregulated just after settlement. A trypsin-like serine protease is also transcribed with differently modulated expression patterns. The majority of the immune-related transcripts display dynamic patterns of temporal expression. Bv-VWa1, Bv-Ptx, and Bv-Ccp3 show distinct peaks of expression followed by declining levels. The expression patterns of immune-related transcripts is modulated in the main development stages. Bv-LRR and Bv-Ccp2 genes are upregulated during larval or postlarval development, whereas Bv-MASP and Bv-Sccp2 show increasing transcription during larval competence and then remain relatively stable. Bv-Ptx is also expressed in the area of the resorbing sensory vesicle, indicating that this lectin could be involved in phagocytosis of the vesicle. In the precompetent larvae, none of the immune-related transcripts were detected, whereas transcripts are often expressed in the mesenchyme of precompetent larvae, as shown for Bv-Crn (coronin), which is known as actin-binding protein, widespread in eukaryotes, that can be associated with phagocytic activity (Rybakin and Clemen 2005). Bv-Sccp2 and Bv-Ccp3 transcripts were localized in both the epidermis and in nearby hemocytes. Interestingly, although the Bv-HspBP2 (Hsp-70 binding protein) gene expression was used for housekeeping, the expression is not observed in the ampullae and displays a distinctive pattern concentrated around the bases of the ampullae, suggesting that this stress-related gene is modulated during metamorphosis.
According to Davidson and Swalla (2002), akin to the extravasation of leukocytes across endothelia, complement signaling and selectins may be involved in the cell migration and initial adherence crossing the epithelia, while innate immune signaling may coordinate the resorption of larval tissues (Vestweber and Blanks 1999). The expression of the transcripts in the migrating hemocytes, and in the areas of the epidermis across which they migrate, support this hypothesis. In addition, these activities can be associated with differential transcription of putative extracellular matrix–modifying genes such as tenascin-c-like, thrombospondin-like, arylsulfatase-like, and tenascin-x-like. These gene transcriptions indicate that, in parallel with vertebrate processes, their products could be involved in the restructuring and repair of transforming tissues during metamorphosis.
In the Ciona intestinalis larva, programmed cell death during metamorphosis correlates with Ci-ERK and Ci-JNK activation, which plays a proapoptotic role. In the tail, this activation precedes the wave of apoptosis, suggesting that the phosphorylated form of Ci-ERK transduces the death-activating signal in tail tissues (Chambon et al. 2002, 2007; Tarallo and Sordino 2004). The screening of genes regulated by Ci-ERK and Ci-JNK identified gene transcripts known to be involved in innate immunity localized in the papillae, nerve cord, visceral ganglion, and sensory vesicle (Chambon et al. 2007). In particular, seven genes (including CiFicolin and Ci-von Willebrand factors ) controlled by Ci-JNK could be involved in phagocytosis of the visceral ganglion and sensory organs. The modulation of gene expression such as that of Ci-Sccp (complement control domain) could also enhance cell–cell communication. Complement control proteins regulate the complement system activation and are also involved in directing the complement toward unwanted material such as cell debris (Kirkitadze and Barlow 2001).
In addition, within a few hours from hatching, the mesenchyme cells of C. intestinalis competent larvae produce (transcript and protein) CiTNFα (Parrinello et al. 2010). These cells, coming from mesenchyme pockets located in the posterior part of the trunk, migrate along the epidermis to reach the papillae, as well as crossing the epidermis, and populate the matrix of the larval tunic. The cytokine-producing cells are compartment/morula cell-like in accordance with the producing hemocytes identified in the pharynx challenged by LPS. The CiTNFα protein was also found along the sensory vesicles, where it may be involved in the vesicle resorption. The same migrating cell type that reach the same regions in the larval body expresses the CiPO2 gene and produces the protein (Parrinello et al. 2015b). Presumably distinct mesenchyme cell populations may be involved in the expression of CiTNFα and CiPO2. Although in competent larvae the functions of the cytokine and phenoloxidase are not known, these findings disclose that they are shared with the pharynx and tunic inflammatory responses.
Nitric oxide (NO), a pluripotent physiological messenger produced by oxidation of L-arginine catalyzed by the enzyme NO synthase (NOS), is involved in regression of the tail, which is controlled by caspase-dependent apoptosis. Notably, the NO/cGMP signaling pathway, together with the stress-inducible protein HSP90, have been shown to be involved in the metamorphosis of the ascidians B. villosa and Cnemidocarpa finmarkiensis (Bishop et al. 2001).
Finally, in C. intestinalis, NO regulates tail regression and the NO synthase (NOS) gene is always transcribed, reaching the maximum level in late larvae just before tail resorption (Comes et al. 2007). NO regulates metamorphosis in a dose-dependent manner, since any increase or decrease of NO levels result in delay or acceleration of tail resorption. NOS is expressed in the anterior part of the trunk at the early–middle larval stage and in the posterior part of the sensory vesicle at the middle larva stage.
In conclusion, the activation of immune-related genes during the dramatic body reorganization in metamorphosing tadpole larva may be also dependent on endogenous signals that induce transcription necessary for the resorption of some larval tissues. Of course, the origin of the signals is unclear and it remains to be established whether they come from degenerating tissues, or whether other signals could activate the immune-related genes. Therefore, if all of this is true, the larvae of protochordates may be a reliable model to study the damage theory.
Concluding Remarks
Ascidians, like other invertebrates, are a heterogeneous taxon. Many species develop tadpole larvae. All are marine but are widespread in diverse living environments, and they can have distinct lifestyles (colonial or solitary, benthonic, or pelagic). Their populations are geographically isolated, and all of them have proceeded along their own evolutionary routes. Their habitats are typically laden with infectious agents: viruses, bacteria, fungi, protists, and metazoan parasites, associated with a given environment. Moreover, they are filter-feeding organisms that have elaborate obligatory relationships with harmful agents of which they can be tolerant (symbiosis) or can activate defense reactions. There are certainly important immunological commonalities among the ascidian species, but their diversities may be related to their distinct evolutionary lineages as well as possibly depending on diverse selective pressures; thereby, populational/species differences can be found.
Although recombinatory mechanisms for generating adaptive specific recognition are lacking, they have developed a variety of molecular and cellular pathways leading to self-protection that shows the inflammatory basis of their innate immunity. As established for vertebrates, cellular and molecular mechanisms participate in a protective response, eliminating pathogens and repairing damaged tissues. A complex of genes, phylogenetically and functionally linkable to the mammalian inflammatory reactions (Fig. 2), can be upregulated (PRRs , self-receptors, cytokine-like molecules, the lectin complement cascade, and signaling and immune regulatory pathways). Inflammatory cells can be recruited to the injured site, responding to chemokines, disclosing recognition capability for self or nonself, and acting as effector cells. Genomic findings and structural analyses of deduced amino acid sequences highlight structural and/or functional conservation of immune-related domains, modules and motifs, and more or less large variations can lead to molecular divergence in taxa that are phylogenetically distant or close, allowing observation of intrapopulational polymorphism (e.g., CiTNFα). With respect to recognition of nonself, both humoral and cell membrane –associated pattern recognition molecules are capable of binding determinants characteristic of broad groups of pathogens. The germ line PRRs show wide glycan-binding interaction that is further amplified through molecular microheterogeneity and subunit oligomerization. In some cases, their functional role appears to be turned for binding proteins. In addition, the germ line immunoglobulin variable region–containing chitin-binding proteins (VCBPs) with regionalized hyperpolymorphism represent a wide nonself recognition mechanism that in part compensates for the absence of the jawed vertebrate somatic rearrangement.
It is of interest that immune-related genes can have a role in developmental and larval stages, presumably producing multifunctional proteins or responding to damage signals (Zucchetti et al. 2008; Parrinello et al. 2010, 2015a, b).
Another interesting subject concerns the missing-self mechanism that is conserved in mammals . Cytotoxic NK lymphocytes contribute to immune responses and homeostasis through germ line–encoded activating and inhibitory receptors that, in concert, regulate their activities. Receptors recognize self MHC class I that acts as inhibitory, becoming activated when its specific ligands are absent or altered. The B. schlosseri allorecognition system is reminiscent of the missing-self mechanism in which a BsCD94/NKR–like gene homolog of the vertebrate NK receptor has been reported. In addition, a CD94/NKR–like gene has been also identified in C. intestinalis. In both species, this gene is expressed in hemocytes, indicating that, as in mammals, ascidians may be provided with both self and nonself recognition mechanisms. Sensing the self and/or recognizing the nonself lead to immune gene upregulation, and orchestrated responses are performed.
In this respect, the gamete self-incompatibility of the hermaphroditic species displays that another mechanism accompanies the self or nonself recognition; it is intriguing that it is related to the immune system or has evolved independently. In the ascidian fertilization process, gamete allorecognizable receptors from polymorphic loci are evolutionarily selected to interact with alloligands but ignore self-ligands. Some data indicate that immune-related genes may be involved in oocytes and development, i.e., the C. intestinalis Hsp70 and the Botryllus histocompatibility factor. The CiHsp70 and CiLgals are constitutively expressed during oogenesis in the follicle cells of the oocytes, suggesting involvement in self-sterility (Marino et al. 1998; Parrinello et al. 2018).
In invertebrates, the presence of circulating cells with reliable immunocompetencies , as in jawed vertebrate lymphocytes, has not been shown. In ascidians, lymphocyte-like cells appear to be stem cells and may be retained as a primordial form of vertebrate lymphocytes because they can sense various stimuli including cytokine-like molecules, enhance their proliferative activity, and differentiate into effector cells. In spite of the large number of studies, a shared hemocyte differentiation lineage has not been reported, but granulocytes with various features and morula cells, which are activated granulocytes, are pivotal in inflammatory reactions. Inflammatory cells may have originated from LLCs and, in distinct species, may assume several functional and morphological features even when they have the same activity. The CC/MCs that express immune-related products appear to be mature/activated hemocytes. Lymphocyte-like cells could be merely retained pluripotent stem cells; conversely, in allograft rejection of solitary ascidian species and nonfusion allorejection in colonial ascidians, their potential immunocompetence has been indicated, and they represent an intriguing topic in studying protochordate immunoevolution.
The necrotic events that occur during the inflammatory reaction mainly in the tunic could be a source of damage signals that presumable intervene, challenging a complex network of responses. Nonfeeding ascidian larvae and the metamorphosing events could be a suitable model to examine the “damage theory.” The challenges and the expression of immune-related genes occur in the competent larval stage when direct contact with microorganisms has not yet reasonably happened.
In addition, immune-related genes active during the inflammatory response may exert multifunctional roles. In C. intestinalis the genes for CiLgals- and -b are upregulated in oogenesis, and galectins are expressed in accessory cells of the immature oocytes as well as in the ooplasm and nucleus (Parrinello et al. 2018). Furthermore, the CiTNFα-like gene is expressed in swimming larvae, while the transcription of a phenoloxidase gene (CinPO2) is modulated in the development stages and larva.
In conclusion, the extraordinarily sophisticated ascidian inflammatory reaction and gene upregulation may represent the evolutionary pivotal basis of chordate innate immunity, from which the complexity of the vertebrate immune mechanisms originated.
References
Ajjan RA, Watson PF, Weetman AP (1996) Cytokines and thyroid function. Adv Neuroimmunol 6:359–386
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Amparyup P, Sutthangkul J, Charoensapsri W et al (2012) Pattern recognition protein binds to lipopolysaccharide and β-1,3-glucan and activates shrimp prophenoloxidase system. J Biol Chem 287:10060–10069
Anderson RS (1971) Cellular responses to foreign bodies in the tunicate Mogula manhattensis (DeKay). Biol Bull 141:91–98
Arizza V, Parrinello D (2009) Inflammatory hemocytes in Ciona intestinalis innate immune response. Invertebr Surviv J 6:S58–S66
Arizza V, Cammarata M, Tomasino MC et al (1995) Phenoloxidase characterization in vacuolar hemocytes from the solitary ascidians Styela plicata. J Invertebr Pathol 66:297–302
Arizza V, Parrinello D, Cammarata M et al (2011) A lytic mechanism based on soluble phospholypases A2 (sPLA2) and β-galactoside specific lectins is exerted by Ciona intestinalis (ascidian) unilocular refractile hemocytes against K562 cell line and mammalian erythrocytes. Fish Shellfish Immunol 30:1014–1023
Armstrong PB (2010) Role of α2-macroglobulin in the immune responses of invertebrates. Invertebr Surviv J 7:165–180
Asea A, Rehli M, Kabingu E et al (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034
Ashley NT, Zachary M, Weil RJ et al (2012) Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst 43:385–406
Azumi K, De Santis R, De Tomaso AW et al (2003) Genomic analysis of immunity in a urochordate and the emergence of the vertebrate immune system: “waiting for Godot”. Immunogenetics 55:570–581
Azumi K, Sabau SV, Fujie M et al (2007) Gene expression profile during the life cycle of the urochordate Ciona intestinalis. Dev Biol 308:572–582
Anderson P (2010) Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev 10:24–35
Ballarin L (2008) Immunobiology of compound ascidians, with particular reference to Botryllus schlosseri: state of art. Invertebr Surviv J 5:54–74
Ballarin L, Cima F (1998) Phenoloxidase and cytotoxicity in the compound ascidian Botryllus schlosseri. Dev Comp Immunol 22:479–492
Ballarin L, Cima F (2005) Cytochemical properties of Botryllus schlosseri haemocytes: indications for morpho-functional characterisation. Eur J Histochem 49:255–264
Ballarin L, Zaniolo G (2007) Colony specificity in Botrylloides leachi. II. Cellular aspects of the non-fusion reaction. Invertebr Surviv J 4:38–44
Ballarin L, Cima F, Sabbadin A (1994) Phenoloxidase in the colonial ascidian Botryllus schlosseri (Urochordata, Ascidiacea). Anim Biol 3:41–48
Ballarin L, Cima F, Floreani M et al (2002) Oxidative stress induces cytotoxicity during rejection reaction in the compound ascidian Botryllus schlosseri. Comp Biochem Physiol 133C:411–418
Ballarin L, Cammarata M, Franchi N et al (2013) Routes in innate immunity evolution: galectins and rhamnose-binding lectins in ascidians. In: Kim S-K (ed) Marine proteins and peptides: biological activities and applications. John Wiley & Sons, Ltd, Hoboken
Barnum SR (2015) C4a: an anaphylatoxin in name only. J Innate Immun 7:333–339
Bartel Y, Bauer B, Steinle A (2013) Modulation of NK cell function by genetically coupled C-type lectin–like receptor/ligand pairs encoded in the human natural killer gene complex. Front Immunol 4:362. https://doi.org/10.3389/fimmu.2013.00362
Beck G, Habicht GS (1991) Purification and biochemical characterization of an invertebrate interleukin-1. Mol Immunol 28:577–584
Beck G, Vasta R, Marchalonis J, Habicht GS (1989a) Characterization of interleukin-1 activity in tunicates. Comp Biochem Physiol 92B:93–98
Beck G, O'Brien RF, Habicht GS (1989b) Invertebrate cytokines: the phylogenetic emergence of interleukin-1. BioEssays 11:62–67
Beck G, O'Brien RF, Habicht GS, Stillman DL, Cooper EL, Raftos DA (1993) Invertebrate cytokines. III: Invertebrate interleukin-1-like molecules stimulate phagocytosis by tunicate and echinoderm cells. Cell Immunol 146:284–299
Berná L, Alvarez-Valin F (2014) Evolutionary genomics of fast evolving tunicates. Genome Biol Evol 6:1724–1738
Bianchet MA, Ahmed H, Vasta GR, Amzel LM (2008) Structural aspects of lectin–ligand interactions. In: Vasta GR, Ahmed H (eds) Animal lectins: a functional view. CRC Press Taylor & Francis Group, England, pp 17–31
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Bierhaus AJ, Chen B, Liliensiek B et al (2000) LPS and cytokine activated endothelium. Semin Thromb Hemost 26:571–587
Billack B (2006) Macrophage activation: role of Toll-like receptors, nitric oxide, and nuclear factor kappa B. Amer J Pharm Edu 70:102. PMC1637021
Bishop CD, Bates WR, Brandhorst BP (2001) Regulation of metamorphosis in ascidians involves NO/cGMP signaling and HSP90. J Exp Zool 289:374–384
Bobó J, Pál G, Cerkenak L et al (2016) The emerging roles of mannose-binding lectin–associated serine proteases (MASPs) in the lectin pathway of complement and beyond. Immunol Rev 274:98–111
Bock DG, MacIsaac HJ, Cristescu ME (2012) Multilocus genetic analyses differentiate between widespread and spatially restricted cryptic species in a model ascidian. Proc Roy Soc Lond B. https://doi.org/10.1098/rspb.2011.2610
Bodmer JL, Schneider P, Tschopp J (2002) The molecular architecture of the TNF superfamily. Trends Biochem Sci 27:19–26
Bonura A, Vizzini A, Salerno G et al (2009) Isolation and expression of a novel MBL-like collectin cDNA enhanced by LPS injection in the body wall of the ascidian Ciona intestinalis. Mol Immunol 46:2389–2394
Bonura A, Vizzini A, Salerno G, Parrinello D, Parrinello N, Longo V, Montana G, Colombo P (2010) Cloning and expression of a novel component of the CAP superfamily enhanced in the inflammatory response to LPS of the ascidian Ciona intestinalis. Cell Tissue Res 342(3):411–421
Borrego F, Masilamani M, Kabat J et al (2005) The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol Immunol 42:485–488
Botos I, Segal DM, Davies DR (2011) The structural biology of Toll-like receptors. Structure 19:447–495
Boyington JC, Riaz AN, Patamawenu A et al (1999) Structure of CD94 reveals a novel C-type lectin fold: implications for the NK cell–associated CD94/NKG2 receptors. Immunity 10:75–82
Brocker C, Thompson D, Matsumoto A et al (2010) Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics 5:30–55
Brown GD, Gordon S (2001) Immune recognition. A new receptor for beta-glucans. Nature 413:36–37
Brown FD, Tiozzo S, Roux MM, Ishizuka K et al (2009) Early lineage specification of long-lived germline precursors in the colonial ascidian Botryllus schlosseri. Development 136:3485–3494
Buchmann K (2014) Evolution of innate immunity: clues from invertebrates via fish to mammals. Front Immunol 5:459. https://doi.org/10.3389/fimmu.2014.00459
Burighel P, Cloney RA (1997) Urochordata: ascidiacea. In: Harrison FW, Ruppert EE (eds) Microscopic anatomy of invertebrates, vol 15. Wiley-Liss Inc, New York, pp 221–347
Calderwood SK, Murshid A, Gong J (2012) Heat shock proteins: conditional mediators of inflammation in tumor immunity. Front Immunol 3:75. https://doi.org/10.3389/fimmu.2012.00075
Cambi A, Figdor CG (2003) Dual function of C-type lectin–like receptors in the immune system. Curr Opin Cell Biol 15:539–546
Cammarata M, Parrinello N (2009) The ascidian prophenoloxidase activating system. Invert Surv J 6:S67–S76
Cammarata M, Arizza V, Parrinello N et al (1997) Phenoloxidase-dependent cytotoxic mechanism in ascidian (Styela plicata) hemocytes active against erythrocytes and K562 cells. Eur J Cell Biol 74:302–307
Cammarata M, Arizza V, Savona B et al (1999) Prophenoloxidase in the hemocyte of Phallusia mamillata. Anim Biol 8:15–17
Cammarata M, Arizza V, Cianciolo C et al (2008) The prophenoloxidase system is activated during the tunic inflammatory reaction of Ciona intestinalis. Cell Tissue Res 333:481–492
Cammarata M, Parisi M, Benenati G, Vasta G, Parrinello N (2014) A rhamnose-binding lectin from sea bass (Dicentrarchus labrax) plasma agglutinates and opsonizes pathogenic bacteria. Dev Comp Immunol 44:332–340
Cannon JP, Haire RN, Litman GW (2002) Identification of diversified genes that contain immunoglobulin-like variable regions in a protochordate. Nat Immunol 3(12):1200–1207
Cannon JP, Haire RN, Schnitker N, Mueller MG, Litman GW (2004) Individual protochordates have unique immune-type receptor repertoires. Curr Biol 14(12):R465–R466
Caputi L, Andreakis N, Mastrototaro F et al (2007) Cryptic speciation in a model invertebrate chordate. PNAS 104:9364–9369
Cerenius L, Lee BL, Söderhäll K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Cha IS, Segovia del Castillo C, Nho SW et al (2011) Innate immune response in the hemolymph of an ascidian, Halocynthia roretzi, showing soft tunic syndrome, using label-free quantitative proteomics. Dev Comp Immunol 35:809–816
Chaga OY (1980) Ortho-diphenoloxidase system of ascidians. Tsitologia 22:619–625
Chambon J-P, Soule J, Pomies P et al (2002) Tail regression in Ciona intestinalis (prochordate) involves a Caspase dependent apoptosis event associated with ERK activation. Development 129:3105–3114
Chambon JP, Nakayama A, Takamura K et al (2007) ERK-and JNK-signalling regulate gene networks that stimulate metamorphosis and apoptosis in tail tissues of ascidian tadpoles. Development 134:1203–1219
Cima F, Perin A, Burighel P et al (2001) Morphofunctional characterisation of haemocytes of the compound ascidian Botrylloides leachi (Tunicata, Ascidiacea). Acta Zool 82:261–274
Cima F, Basso G, Ballarin L (2003) Apoptosis and phosphatidylserine-mediated recognition during the take-over phase of the colonial life-cycle in the ascidian Botryllus schlosseri. Cell Tissue Res 312:369–376
Cima F, Sabbadin A, Ballarin L (2004) Cellular aspects of allorecognition in the compound ascidian Botryllus schlosseri. Dev Comp Immunol 28:881–889
Cima F, Manni L, Basso G et al (2010) Hovering between death and life: natural apoptosis and phagocytes in the blastogenetic cycle of the colonial ascidian Botryllus schlosseri. Dev Comp Immunol 34:272–285
Cima F, Franchi N, Ballarin L (2016) Origin and function of tunicate hemocytes. In: Malagoli D (ed) The evolution of the immune system. Elsevier, London, pp 29–49
Cloney RA (1982) Ascidian larvae and the events of metamorphosis. Am Zool 22:817–826
Cloney RA, Grimm LM (1970) Transcellular emigration of blood cells during ascidian metamorphosis. Z Zellforsch 107:157–173
Comes S, Locascio A, Silvestre F et al (2007) Regulatory roles of nitric oxide during larval development and metamorphosis in Ciona intestinalis. Dev Biol 306:772–784
Cooper EL (1992) Overview of immunoevolution. Boll Zool 59:119–128
Cooper EL (2009) Putative stem cell origins in solitary tunicates. In: Rinkevich B, Matranga V (eds) Stem cells in marine organisms. Springer, Netherlands, pp 21–32
Cooper EL (2016) Commentary: blurring borders: innate immunity with adaptive features. Front Microbiol 7:358. https://doi.org/10.3389/fmicb.2016.00358
Cooper EL, Parrinello N (2001) Immunodefense in tunicates: cells and molecules. In: Sawada H, Yokosawa H, Lambert CC (eds) The biology of ascidians. Springer, Tokio, pp 383–394
Corey DM, Rosental B, Kowarsky M et al (2016) Developmental cell death programs license cytotoxic cells to eliminate histocompatible partners. Proc Natl Acad Sci U S A 113:6520–6525
Coscia MR, Giacomelli S, Oreste U (2011) Toll-like receptors: an overview from invertebrates to vertebrates. Invert Surv J 8:210–226
Cummings RD, McEver RP (2009) C-type lectins. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. Chapter 31
Cummings RD, Schnaar RL, Esko JD et al. (2017) Principles of glycan recognition. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology. 3rd edn. Cold Spring Harbor Laboratory Press Chapter 29. https://doi.org/10.1101/glycobiology.3e.029
Davidson B, Swalla BJ (2002) A molecular analysis of ascidian metamorphosis reveals activation of an innate immune response. Develop 129:4739–4751
De Barros CM, Andrade LR, Allodi S, Viskov C et al (2007) The hemolymph of the ascidian styela plicata (Chordata–Tunicata) contains heparin inside basophil-like cells and a unique sulfated galactoglucan in the plasma. J Biol Chem 282:1615–1626
De Leo G (1992) Ascidian hemocytes and their involvement in defence reactions. Boll Zool 59:195–213
Deck JD, Hay ED, Revel J-P (1966) Fine structure and origin of the tunic of Perophora viridis. J Morphol 120:267–280
Dehal P, Satou Y, Campbell RK et al (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298:2157–2167
De Leo G, Parrinello N, Parrinello D et al (1997) Encapsulation response of Ciona intestinalis (Ascidiacea) to intratunic erythrocyte injection. J Invertebr Pathol 69:14–23
de Leo G, Parrinello N, Parrinello D, Cassara’ G, di Bella MA (1996) Encapsulation response of Ciona intestinalis (Ascidiacea) to intratunical erythrocyte injection. J Invertebr Pathol 67(3):205–212
Delsuc F, Brinkmann H, Chourrout D et al (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439:965–968
Delsuc F, Tsagkogeorga G, Lartillot N, Philippe H (2008) Additional molecular support for the new chordate phylogeny. Genesis 46:592–594
Di Bella MA, Cassarà G, Russo D et al (1998) Cellular components and tunic architecture of the solitary ascidian Styela canopus (Stolidobranchiata, Styelidae). Tissue Cell 30:352–359
Di Bella MA, Carbone MC, De Leo G (2005) Aspects of cell production in mantle tissue of Ciona intestinalis L. (Tunicata, Ascidiacea). Micron 36:477–481
Di Bella MA, Carbone MC, D’Amato M et al (2009) The identification and localization of two intermediate filament proteins in the tunic of Styela plicata (Tunicata, Styelidae). Tissue Cell 41:381–389
Di Bella MA, Fedders H, De Leo G et al (2011) Localization of antimicrobial peptides in the tunic of Ciona intestinalis (Ascidiacea, Tunicata) and their involvement in local inflammatory-like reactions. Results Immunol 1:70–75
Di Bella MA, Carbone MC, De Leo G (2015) Ultrastructural aspects of naturally occurring wound in the tunic of two ascidians: Ciona intestinalis and Styela plicata (Tunicata). Micron 69:6–14
Di Bella MA, De Leo G (2000) Hemocyte migration during Inflammatory-like reaction of Ciona intestinalis (Tunicata, Ascidiacea). J Invertebr Pathol 76(2):105–111
Di Giammartino DC, Nishida K, Manley JL (2011) Mechanisms and consequences of alternative polyadenylation. Mol Cell 43:853–866
Dinarello CA (2007) Historical review of cytokines. Eur J Immunol 37:S34–S45
Dinasarapu AR, Chandrasekhar A, Fujita T et al (2013) Mannose/mannan-binding lectin. UCSD Molecule 2:8–18. https://doi.org/10.1155/2016/1245049
Dishaw LJ, Giacomelli S, Melillo D, Zucchetti I, Haire RN, Natale L, Russo NA, De Santis R, Litman GW, Pinto MR (2011) A role for variable region-containing chitin-binding proteins (VCBPs) in host gut-bacteria interactions. Proc Natl Acad Sci 108(40):16747–16752
Dishaw LJ, Leigh B, Cannon JP et al (2016) Gut immunity in a protochordate involves a secreted immunoglobulin-type mediator binding host chitin and bacteria. Nat Commun 7:10617
Donaghy L, Hong HK, Park KI et al (2017) Flow cytometric characterization of hemocytes of the solitary ascidian, Halocynthia roretzi. Fish Shellfish Immunol 66:289–299
Dong B, Liu F, Gao H et al (2009) CDNA cloning and gene expression pattern following bacterial challenge of peroxinectin in Chinese shrimp Fenneropenaeus chinensis. Mol Biol Rep 36:2333–2339
Drickamer K, Fadden AJ (2002) Genomic analysis of C-type lectins. Biochem Soc Symp 69:59–72
Drickamer K, Taylor ME (2015) Recent insights into structures and functions of C-type lectins in the immune system. Curr Opin Struct Biol 34:26–34
Du Clos TW (2013) Pentraxins: structure, function, and role in inflammation. ISRN Inflamm 2013:1–22
Du Pasquier L (2004) Innate immunity in early chordates and the appearance of adaptive immunity. C R Biol 327:591–601
Di Meo S, Reed TT, Venditti P et al (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med Cell Longev 2016:1–44
East L, Isacke CM (2002) The mannose receptor family. Biochim Biophys Acta 1572:364–386
Elkon R, Ugalde AP, Agami R (2013) Alternative cleavage and polyadenylation: extent, regulation and function. Nat Rev Genet 14:496–506
Elliot MR, Ravochandran KS (2010) Clearance of apoptotic cells: implications in health and diseases. J Cell Biol 189:1059–1070
Endean R (1961) The test of the ascidian, Phallusia mammillata. Quart J Microsc Sci 102:107–117
Ermak TH (1975a) Cell proliferation in the ascidian Styela clava: an autoradiographic and electron microscopic investigation emphasizing cell renewal in the digestive tract of this and fourteen other species of ascidians. PhD Diss – Univ Cal, San Diego
Ermak TH (1975b) An autoradiographic demonstration of blood cell renewal in Styela clava (Urochordata: Ascidiacea). Experientia 31:837–838
Ermak TH (1976) The hematogenic tissues of tunicates. In: Wright RK, Cooper EL (eds) Phylogeny of thymus and bone marrow-bursa cells. Elsevier, Amsterdam, pp 45–56
Ermak TH (1982) The renewing cell populations of ascidians. Am Zool 22:795–805
Esposito R, D’Aniello S, Squarzoni P et al (2012) New insights into the evolution of metazoan tyrosinase gene family. PLoS One 74:1–10
Ewan R, Huxley-Jones J, Mould AP et al (2005) The integrins of the urochordate Ciona intestinalis provide novel insights into the molecular evolution of the vertebrate integrin family. BMC Evol Biol 5:1–18
Fedders H, Leippe M (2008) A reverse search for antimicrobial peptides in Ciona intestinalis: identification of a gene family expressed in hemocytes and evaluation of activity. Dev Comp Immunol 32:286–298
Fox PL (2015) Discovery and investigation of the GAIT translational control system. RNA 21:615–618
Franchi N, Ballarin L (2014) Preliminary characterization of complement in a colonial tunicate: C3, Bf and inhibition of C3 opsonic activity by compstatin. Dev Comp Immunol 46(2):430–438
Franchi N, Ballarin L (2016) Cytotoxic cells of compound ascidians. In: Ballarin L, Cammarata M (eds) Lessons in immunity: from single-cell organisms to mammals. Elsevier, London, pp 193–203
Franchi N, Ballarin L (2017) Morula cells as key hemocytes of the lectin pathway of complement activation in the colonial tunicate Botryllus schlosseri. Fish Shellfish Immunol 63:157–164
Fugmann SD (2010) The origins of the RAG genes—from transposition to V(D)J recombination. Semin Immunol 22:10–16
Fugmann SD, Messier C, Novack LA et al (2006) An ancient evolutionary origin of the Rag1/2 gene locus. Proc Natl Acad Sci U S A 103:3728–3733
Fujikawa T, Munakata T, S-i K et al (2010) Stress response in the ascidian Ciona intestinalis: transcriptional profiling of genes for the heat shock protein 70 chaperone system under heat stress and endoplasmic reticulum stress. Cell Stress Chaperones 15:193–204
Fujita H, Sawano F (1979) Fine structural localization of endogenous peroxidase in the endostyle of ascidians, Ciona intestinalis. A part of phylogenetic studies of the thyroid gland. Arch Histol Jpn 42:319–326
Fujita T, Endo Y, Nonaka M (2004a) Primitive complement system—recognition and activation. Mol Immunol 41:103–111
Fujita T, Matsushita M, Endo Y (2004b) The lectin–complement pathway—its role in innate immunity and evolution. Immunol Rev 198:346–353
Fuke MT (1980) “Contact reaction” between xenogeneic or allogeneic celomic cells of solitary ascidians. Biol Bull 158:304–315
Futosi K, Fodor S, Mócsai A (2013) Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:638–650
Gallucci S, Matzinger P (2001) Danger signals: SOS to the immune system. Curr Opin Immunol 13:114–119
Gasparini F, Franchi N, Spolaore B et al (2008) Novel rhamnose-binding lectins from the colonial ascidian Botryllus schlosseri. Dev Comp Immunol 32:1177–1191
Giacomelli S, Melillo D, Lambris JD et al (2012) Immune competence of the Ciona intestinalis pharynx: complement system-mediate activity. Fish Shellfish Immunol 33:946–952
Gibbs GM, Roelants K, O’Bryan MK (2008) The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—roles in reproduction, cancer, and immune defense. Endocr Rev 29:865–897
Gijtenbeel TBH, Inghuis GR (2009) Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9:465–479
Goetz FW, Planas JV, MacKenzie S (2004) Tumor necrosis factors. Dev Comp Immunol 28:487–497
Green PL, Nair SV, Raftos DA (2003) Secretion of a collectin-like protein in tunicates enhanced during inflammatory responses. Dev Comp Immunol 27:3–9
Gu C, Wu L, Li X (2013) IL-17 family: cytokines, receptors and signaling. Cytokine 64:477–485
Guilliams M, Ginhoux F, Jakubzick C et al (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14:571–578
Gupta G, Surolia A (2007) Collectins: sentinels of innate immunity. BioEssays 29:452–464
Hansen JD, Vojtech LN, Laing KJ (2011) Sensing disease and danger: a survey of vertebrate PRRs and their origins. Dev Comp Immunol 35:886–897
Hata S, Azumi K, Yokosawa H (1998) Ascidian phenoloxidase: its release from hemocytes, isolation, characterization and physiological roles. Comp Biochem Physiol 119:769–776
Hibino T, Loza-Coll M, Messier C et al (2006) The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol 300:349–365
Hirose E (2009) Ascidian tunic cells: morphology and functional diversity of free cells outside the epidermis. Invertebr Biol 128:83–96
Hirose E, Ishii T, Saito Y, Taneda Y (1994) Phagocytic activity of tunic cells in the colonial ascidian Aplidium yamazii (Polyclinidae, Aplousobranchia). Zool Sci 11:203–208
Hirsiger S, Simmen H-P, Werner CML et al (2012) Danger signals activating the immune response after trauma. Mediators Inflamm 315941:10. https://doi.org/10.1155/2012/315941
Houzelstein D, Goncalves IR, Fadden AJ et al (2004) Phylogenetic analysis of the vertebrate galectin family. Mol Biol Evol 21:1177–1187
Hoving JC, Wilson GJ, Brown GD (2014) Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol 16:185–194
Hsu PI, Liu CH, Tseng DY et al (2006) Molecular cloning and characterisation of peroxinectin, a cell adhesion molecule, from the giant freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol 21:1–10
Huang S, Yuan S, Guo L et al (2008) Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Res 18:1112–1126
Hughes TK, Smith EM, Chin R et al (1990) Interaction of immunoactive monokines (interleukin 1 and tumor necrosis factor) in the bivalve mollusc Mytilus edulis. Proc Natl Acad Sci U S A 87:4426–4429
Huminiecki L, Goldovsky L, Freilich S et al (2009) Emergence, development and diversification of the TGF-b signalling pathway within the animal kingdom. BMC Evol Biol 3:9–28
Idriss TH, Naismith JH (2000) TNF alpha and the TNF receptor superfamily: structure function relationship(s). Microsc Res Tech 1:184–195
Immesberger A, Burmester T (2004) Putative phenoloxidases in the tunicate Ciona intestinalis and the origin of the arthropod hemocyanin superfamily. J Comp Physiol B 174:169–180
Inoue J, Ishida T, Tsukamoto N et al (2000) Tumor necrosis factor receptor–associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res 254:142–144
Ip EWK, Takahashi K, Ezekowitz AR et al (2009) Mannose-binding lectin and innate immunity. Immunol Rev 230:9–21
Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol 38:128–150
Jackson AD, Smith VJ, Peddie CM (1993) In vitro phenoloxidase activity in the blood of Ciona intestinalis and other ascidians. Dev Comp Immunol 17:97–108
Janeway CA Jr, Travers P, Walport M et al (2001) Immunobiology: the immune system in health and disease. Receptors of the innate immune system, 5th edn. Garland Science, New York. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27110/
Jensen LE, Whitehead AS (2001) IRAK1b, a novel alternative splice variant of interleukin-1 receptor associated kinase (IRAK), mediates interleukin-1 signaling and has prolonged stability. J Biol Chem 276:29037–29044
Ji X, Azumi K, Sasaki M, Nonaka M (1997) Ancient origin of the complement lectin pathway revealed by molecular cloning of mannan binding protein-associated serine protease from a urochordate, the Japanese ascidian, Halocynthia roretzi. Proc Natl Acad Sci 94(12):6340–6345
Jiang Y, Doolittle RF (2003) The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci U S A 100:7527–7532
Jimbo M, Usui R, Sakai R et al (2007) Purification, cloning and characterization of egg lectins from the teleost Tribolodon brandti. Comp Biochem Physiol 147B:164–171
Johansson MW, Lind MI, Holmblad T et al (1995) Peroxinectin, a novel cell adhesion protein from crayfish blood. Biochem Biophys Res Commun 216:1079–1087
Jouault T, Abed-El Behi ME, Martínez-Esparza M et al (2006) Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol 177:4679–4687
Johansson MW (1999) Cell adhesion molecules in invertebrate immunity. Dev Comp Immunol 23:303–315
Johansson MW, Söderhäll K (1989) A cell adhesion factor from crayfish haemocytes has degranulating activity towards crayfish granular cells. Insect Biochem 19(2):183–190
Kamesh N, Aradhyam GK, Manoj N (2008) The repertoire of G protein-coupled receptors in the sea squirt Ciona intestinalis. BMC Evol Biol 8:129 doi:10.1186/1471-2148-8-129://www.biomedcentral.com/1471-2148/8/129
Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38:209–223
Karpova AY, Ronco LV, Howley PM (2001) Functional characterization of interferon regulatory factor 3a (IRF3a), an alternative splice isoform of IRF3. Mol Cell Biol 21:4169–4176
Kawamura K, Sunanaga T (2010) Hemoblasts in colonial tunicates: are they stem cells or tissue-restricted progenitor cells? Develop Growth Differ 52:69–76
Kawashima A, Yamazaki K, Hara T et al (2013) Demonstration of innate immune responses in the thyroid gland: potential to sense danger and a possible trigger for autoimmune reactions. Thyroid 23:477–487
Kelley J, Walter L, Trowsdale J (2015) Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet 1(2):e27. https://doi.org/10.1371/journal.pgen.0010027
Kelly KL, Cooper EL, Raftos DA (1992) In vitro allogeneic cytotoxicity in the solitary urochordate Styela clava. J Exp Zool 262:202–208
Kenjo A, Takahashi M, Matsushita M et al (2001) Cloning and characterization of novel ficolins from the solitary ascidian Halocynthia roretzi. J Biol Chem 276:19959–19965
Kerrigan AM, Brown GD (2009) C-type lectins and phagocytosis. Immunobiology 214:562–575
Khalturin K, Becker M, Rinkevich B, Bosch TCG (2003) Urochordates and the origin of natural killer cells: identification of a CD94/NKR-P1-related receptor in blood cells of Botryllus. PNAS 100:622–627
Kirkitadze M, Barlow P (2001) Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev 180:146–161
Klebanoff JS (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625
Klyosov AA (2008) Galectins and their functions in plain language. In: Klyosov AA, Witczak ZJ, Platt D (eds) Galectins. Wiley & Sons, Hoboken, pp 9–32
Kobayashi M, Johansson MW, Söderhäll K (1990) The 76 kDa cell adhesion factor from crayfish haemocytes promotes encapsulation in vitro. Cell Tissue Res 260:113–118
Koh TJ, DiPietro LA (2011) Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 13:e23. https://doi.org/10.1017/S1462399411001943
Kondos SC, Hatfaludi T, Voskoboinik I et al (2010) The structure and function of mammalian membrane-attack complex/perforin-like proteins. Tissue Antigens 76:341–351
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289
Konrad MW (2016) Blood circulation in the ascidian tunicate Corella inflata (Corellidae). Wang L (ed) PeerJ 4:2771. https://doi.org/10.7717/peerj.2771
Kvell K, Cooper E, Engelmann P, Bovari J, Nemeth P (2007) Blurring borders: innate immunity with adaptive features. Clin Dev Immunol 2007:83671. https://doi.org/10.1155/2007/83671
Laird DJ, De Tomaso AW, Weissman IL (2005) Stem cells are units of natural selection in a colonial ascidian. Cell 123:1351–1360
Lauzon RJ, Ishizuka KJ, Weissman IL (1992) A cyclical, developmentally-regulated death phenomenon in a colonial urochordate. Dev Dyn 1941:71–83
Lauzon RJ, Brown C, Kerr L, Tiozzo S (2013) Phagocyte dynamics in a highly regenerative urochordate: insights into development and host defense Devel. Biol 374:357–373
Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743
Levasseur A, Pontarotti P (2011) The role of duplications in the evolution of genomes highlights the need for evolutionary-based approaches in comparative genomics. Biol Direct 6:11. https://doi.org/10.1186/2F1745-6150-6-11
Li MO, Wan YY, Sanjabi S et al (2006) Transforming growth factor-b regulation of immune responses. Annu Rev Immunol 24:99–146
Li K, Fazekasova H, Wang N et al (2011) Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol 48:1121–1127
Linger RM, Keating AK, Earp HS, Graham DK (2008) TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 100:35–83
Liu Y, Wang Y, Yamakuchi M et al (2001) Upregulation of Toll-like receptor 2 gene expression in macrophage response to peptidoglycan and high concentration of lipopolysaccharide is involved in NF-κB activation. Infect Immun 69:2788–2796
Liu CH, Cheng W, Chen JC (2005) The peroxinectin of white shrimp Litopenaeus vannamei is synthesised in the semi-granular and granular cells, and its transcription is up-regulated with Vibrio alginolyticus infection. Fish Shellfish Immunol 18:431–444
Liu CH, Yeh SP, Hsu PY, Cheng W (2007) Peroxinectin gene transcription of the giant freshwater prawn Macrobrachium rosenbergii under intrinsic, immunostimulant, and chemotherapeutant influences. Fish Shellfish Immunol 22:408–417
Liu FT, Hsu DK, Yang RY et al (2008) Galectins in regulation of inflammation and immunity. In: Klyosov AA, Witczak ZJ, Platt D (eds) Galectins. Wiley & Sons, Hoboken, pp 97–114
Liu FT, Yang RY, Hsu DK (2012) Galectins in acute and chronic inflammation. Ann N Y Acad Sci 1253:80–91
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501
López-Botet M, Carretero M, Pérez-Villar J et al (1997) The CD94/NKG2 C-type lectin receptor complex: involvement in NK cell-mediated recognition of HLA class I molecules. Immunol Rev 16:175–185
Lubbers R, van Essen MF, van Kooten C et al (2017) Production of complement components by cells of the immune system. Clin Exp Immunol 188:183–194
Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK (2006) Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med 203:2785–2791
MacKenzie S, Planas JV, Goetz FW (2003) LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev Comp Immunol 27:393–400
Mak TW, Saunders ME (2006) Innate immunity. In: Mak TW, Saunders ME (eds) The immune response. Basic and clinical principles. Elsevier Academic Press, Burligton MA USA, pp 69–92
Mantovani A, Biswas SK, Galdiero MR et al (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229:176–185
Marino R, Pinto MR, Cotelli F, Lamia CL, De Santis R (1998) The hsp70 protein is involved in the acquisition of gamete self-sterility in the ascidian Ciona intestinalis. Development 125:899–907
Marino R, Kimura Y, DeSantis R et al (2002) Complement in urochordates: cloning and characterization of two C3-like genes in the ascidian Ciona intestinalis. Immunogenetics 53:1055–1064
Marshall ASJ, Gordon S (2004) C-type lectins on the macrophage cell surface—recent findings. Eur J Immunol 34:18–24
Martchenko M, Levitin A, Hogues H, Nantel A, Whiteway M (2007) Transcriptional rewiring of fungal galactose-metabolism circuitry. Curr Biol: CB 17(12). https://doi.org/10.1016/j.cub.2007.05.017
Massagué J, Gomis RR (2006) The logic of TGF-b signaling. FEBS Lett 580:2811–2820
Matsumoto J, Nakamoto C, Fujiwara S et al (2001) A novel C-type lectin regulating cell growth, cell adhesion and cell differentiation of the multipotent epithelium in budding tunicates. Development 128:3339–3347
Matsushita M, Fujita T (2001) Ficolins and the lectin complement pathway. Immunol Rev 180:78–85
Matsushita M, Endo Y, Fujita T (1998) MASP1 (MBL-associated serine protease 1). Immunobiology 199:340–347
Matsushita M, Endo Y, Fujita T (2000) Cutting edge: complement-activating complex of ficolin and mannose-binding lectin–associated serine protease. J Immunol 164:2281–2284
Matzinger P (1994) Tolerance, danger, and the extended family. Annu Rev Immunol 12:991–1045
Matzinger P (2002) The danger model: a renewed sense of self. Science 296:301–305
McKitrick TR, De Tomaso AW (2010) Molecular mechanisms of allorecognition in a basal chordate. Semin Immunol 22(1). https://doi.org/10.1016/j.smim.2009.12.001.
Meager A, Wadhwa M (2013) An overview of cytokine regulation of inflammation and immunity. In: eLS. John Wiley & Sons Ltd, Chichester
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Melillo D, Sfyroera G, De Santis R et al (2006) First identification of a chemotactic receptor in an invertebrate species: structural and functional characterization of Ciona intestinalis C3a receptor. J Immunol 177:4132–4140
Menin A, Ballarin L (2010) Immunomodulatory molecules in the compound ascidian Botryllus schlosseri: evidence from conditioned media. Dev Comp Immunol 34:272–285
Menin A, Del Favero M, Cima F et al (2005) Release of phagocytosis-stimulating factor(s) by morula cells in a colonial ascidian. Mar Biol 148:225–230
Merle NS, Church SE, Fremeaux-Bacchi V et al (2015a) Complement system part I—molecular mechanisms of activation and regulation. Front Immunol 6:262. https://doi.org/10.3389/fimmu.2015.00262
Merle NS, Noe R, Halbwachs-Mecarelli L et al (2015b) Complement system part II: role in immunity. Front Immunol 6:257. https://doi.org/10.3389/fimmu.2015.00257
Metchnikoff E (1887) Sur la lutte des cellules de l'organisme contre l’invasion des microbes. Ann Inst Pasteur 1:321–345
Michel ML, Mendes-da-Cruz D, Keller AC et al (2008) Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A 105:19845–19850
Miyazawa S, Nonaka M (2004) Characterization of novel ascidian beta integrins as primitive complement receptor subunits. Immunogenetics 55:836–844
Miyazawa S, Azumi K, Nonaka M (2001) Cloning and characterization of integrin a subunits from the solitary ascidian, Halocynthia roretzi. J Immunol 166:1710–1715
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273
Moodley Y, Rigby P, Bundell C et al (2003) Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol 162:771–779
Moreno E, Yan M, Basler K (2002) Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol 12:1263–1268
Nair SV, Pearce S, Green PL et al (2000) A collectin-like protein from tunicates. Comp Biochem Physiol 125B:279–289
Nair SV, Ramsden A, Raftos DA (2005) Ancient origins: complement in invertebrates. Invertebr Surviv J 2:114–123
Nappi AJ, Ottaviani E (2000) Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays 22:469–480
Nappi AJ, Vass E (1993) Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res 6:117–126
Nesargikar PN, Spiller B, Chavez R (2012) The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol 2:103–111
Nesmelova IV, Dings RPM, Mayo KH (2008) Understanding galectin structure. Function relationships to design effective antagonists. In: Klyosov AA, Witczak ZJ, Platt D (eds) Galectins. Wiley & Sons, Hoboken, pp 33–70
Nonaka M (2014) Evolution of the complement system. In: Anderluh G, Gilbert R (eds) MACPF/CDC proteins—agents of defence, attack and invasion. Subcellular biochemistry, vol 80. Springer, Dordrecht, pp 31–43. https://doi.org/10.1007/978-94-017-8881-6
Nonaka M, Azumi K (1999) Opsonic complement system of the solitary ascidian Halocynthia roretzi. Dev Comp Immunol 23:421–427
Nonaka M, Kimura A (2006) Genomic view of the evolution of the complement system. Immunogenetics 58:701–713
Nonaka M, Satake H (2010) Urochordate immunity. In: Söderhall K (ed) Invertebrate immunity. Landes Bioscience and Springer Science, Boston, pp 302–310
Nonaka M, Yoshizaki F (2004) Primitive complement system of invertebrates. Immunol Rev 198:203–215
Nonaka M, Azumi K, Ji X et al (1999) Opsonic complement component C3 in the solitary ascidian Halocynthia roretzi. J Immunol 162:387–391
Norling LV, Perretti M, Cooper D (2009) Endogenous galectins and the control of the host inflammatory response. J Endocrinol 201:169–184
Nydam ML, Harrison RG (2007) Genealogical relationships within and among shallow-water Ciona species (Ascidiacea). Mar Biol 151:1839–1847
Nydam ML, Harrison RG (2011) Introgression despite substantial divergence in a broadcast spawning marine invertebrate. Evolution 65:429–442
Nydam ML, Hoang TA, Shanley KM, De Tomaso AW (2013) Molecular evolution of a polymorphic HSP40-like protein encoded in the histocompatibility locus of an invertebrate chordate. Dev Comp Immunol 41(2):128–136
Ogasawara M, Di Lauro R, Satoh N (1999) Ascidian homologs of mammalian thyroid peroxidase genes are expressed in the thyroid-equivalent region of the endostyle. J Exp Zool 285:158–169
Ogawa T, Watanabe M, Naganuma T, Muramoto K (2011) Diversified carbohydrate-binding lectins from marine resources. J Amino Acids 2011:838914, 20. https://doi.org/10.4061/2011/838914
Oren M, Douek J, Fishelson Z et al (2007) Identification of immune relevant genes in histoincompatible rejecting colonies of the tunicate Botryllus schlosseri. Dev Comp Immunol 31:889–902
Oren M, Escande M-l, Paz G et al (2008) Urochordate histoincompatible interactions activate vertebrate-like coagulation system components. PLoS One:3. https://doi.org/10.1371/journal.pone.0003123
Oren M, Paz G, Douek J et al (2013) Marine invertebrates cross phyla comparisons reveal highly conserved immune machinery. Immunobiology 218:484–495
Ottaviani E, Franchini A, Cassanelli S et al (1995) Cytokines and invertebrate immune responses. Biol Cell 85:87–91
Ottaviani E, Franchini A, Kletsas D et al (1996) Presence and role of cytokines and growth factors in invertebrates. Ital J Zool 63:317–323
Pancer Z, Gershon H, Rinkevich B (1995) Cloning of a urochordate cDNA featuring mammalian short consensus repeats (SCR) of complement-control protein superfamily. Comp Biochem Physiol 111B:625–632
Pancer Z, Diehl-Seifert B, Rinkevich B et al (1997) A novel tunicate (Botryllus schlosseri) putative C-type lectin features an immunoglobulin domain. DNA Cell Biol 16:801–806
Pandolfi F, Altamura S, Frosali S, Conti P (2016) Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther 38:1017–1028
Pappu R, Ramirez-Carrozzi V, Ota N et al (2010) The IL-17 family cytokines in immunity and disease. J Clin Immunol 30:185–195
Parker JS, Mizuguchi K, Gay NJ (2001) A family of proteins related to Spätzle, the Toll receptor ligand, are encoded in the Drosophila genome. Proteins 45:71–80
Parrinello N (1981) The reaction of Ciona intestinalis L. to subcuticular erythrocyte and protein injection. Dev Comp Immunol 5:105–110
Parrinello N (1995) Humoral and cellular lectins of ascidians. J Mar Biotechnol 3:29–34
Parrinello N (1996) Cytotoxic activity of tunicates hemocytes. In: Cellular, biochemical and molecular aspects of invertebrate immunology. Müller WEG, Rinkevich B (eds). Progress in molecular and subcellular biology, Springer, Berlin, pp 190–217
Parrinello N, Patricolo E (1984) Inflammatory-like reaction in the tunic of Ciona intestinalis (Tunicata). II. Capsule components. Biol Bull 167:238–250
Parrinello N, Patricolo E, Canicatti C (1984) Inflammatory-like reaction in the tunic of Ciona intestinalis (Tunicata). I. Encapsulation and tissue injury. Biol Bull 167:229–237
Parrinello N, De Leo G, Di Bella MA (1990) Fine structural observations of granulocytes involved in the tunic inflammatory-like reaction of Ciona intestinalis (Tunicata). J Invertebr Pathol 56:181–189
Parrinello N, Cammarata M, Lipari L et al (1995) Sphingomyelin inhibition of Ciona intestinalis hemocytes assayed against sheep erythrocytes. Dev Comp Immunol 19:31–41
Parrinello N, Cammarata M, Vazzana M et al (2001) Immunological activity of ascidian hemocytes. In: Sawada H, Yokosawa H, Lambert CC (eds) The biology of ascidians. Springer, Tokyo, pp 395–401
Parrinello N, Arizza V, Chinnici C et al (2003) Phenoloxidases in ascidian hemocytes: characterization of the pro-phenoloxidase activating system. Comp Biochem Physiol B Biochem Mol Biol 135B:583–591
Parrinello N, Arizza V, Cammarata M et al (2007) Inducible lectins with galectin properties and human IL1alpha epitopes opsonize yeast during the inflammatory response of the ascidian Ciona intestinalis. Cell Tissue Res 329:379–390
Parrinello N, Vizzini A, Arizza V et al (2008) Enhanced expression of a cloned and sequenced Ciona intestinalis TNF alpha like (CiTNF alpha) gene during the LPS-induced inflammatory response. Cell Tissue Res 334:305–317
Parrinello N, Vizzini A, Salerno G et al (2010) Inflamed adult pharynx tissues and swimming larva of Ciona intestinalis share CiTNFα-producing cells. Cell Tissue Res 341:299–311
Parrinello D, Sanfratello MA, Vizzini A et al (2015a) Ciona intestinalis galectin (CiLgals-a and CiLgals-b) genes are differentially expressed in endostyle zones and challenged by LPS. Fish Shellfish Immunol 42:171–176
Parrinello D, Sanfratello MA, Vizzini A, Cammarata M (2015b) The expression of an immune-related phenoloxidase gene is modulated in Ciona intestinalis ovary, test cells, embryos and larva. J Exp Zool B Mol Dev Evol 324B:141–151
Parrinello N, Cammarata M, Parrinello D et al (2016) Inflammatory response of the ascidian Ciona intestinalis. In: Ballarin L, Cammarata M (eds) Lessons in immunity: from single-cell organisms to mammals. Elsevier, London, pp 177–192
Parrinello D, Sanfratello MA, Vizzini A et al (2017) The Ciona intestinalis immune-related galectin genes (CiLgals-a andCiLgals-b) are expressed by the gastric epithelium. Fish Shellfish Immunol 62:24–30
Parrinello D, Sanfratello MA, Parisi MG et al (2018) In the ovary of Ciona intestinalis (type A), immune-related galectin and phenoloxidase genes are differentially expressed by the follicle accessory cells. Fish Shellfish Immunol 72:452–458
Pérez-Portela R, Bishop JDD, Davis AR et al (2009) Phylogeny of the families Pyuridae and Styelidae (Stolidobranchiata, Ascidiacea) inferred from mitochondrial and nuclear DNA sequences. Mol Phylogenet Evol 50:560–570
Petersen JK (2007) Ascidian suspension feeding. J Exp Mar Biol Ecol 342:127–137
Pineda MC, Turon X, López-Legentil S (2012) Stress levels over time in the introduced ascidian Styela plicata: the effects of temperature and salinity variations on hsp70 gene expression. Cell Stress Chaperones 17:435–444
Pinto MR, Chinnici CM, Kimura Y et al (2003) CiC3-1 mediated chemotaxis in the deuterostome invertebrate Ciona intestinalis (Urochordata). J Immunol 171:5521–5528
Poget SF, Legge GB, Proctor MR et al (1999) The structure of a tunicate C-type lectin from Polyandrocarpa misakiensis complexed with D-galactose. J Mol Biol 290:867–879
Pradeu T, Cooper EL (2012) The danger theory: 20 years later. Front Immunol Hypoth Theory 3:287, 1 https://doi.org/10.3389/fimmu.2012.00287
Prasobh R, Manoj N, Kelso J (2009) The repertoire of heterotrimeric G proteins and RGS proteins in Ciona intestinalis. PLoS One 4(10):e7349
Peddie CM, Smith VJ (1993) In vitro spontaneous cytotoxic activity against mammalian target cells by the hemocytes of the solitary ascidian,Ciona intestinalis. J Exp Zool 267(6):616–623
Peddie CM, Smith VJ (1995) Lymphocyte-like’cells in ascidians: precursors for vertebrate lymphocytes? Fish Shellfish Immunol 5:613–629
Quesenberry MS, Ahmed H, Elola MT et al (2003) Diverse lectin repertoires in tunicates mediate broad recognition and effector innate immune responses. Integr Comp Biol 43:323–330
Rabinovich G (2002) Role of galectins in inflammatory and immunomodulatory processes. Biochim Biophys Acta Gen Subj 1572(2–3):274–284
Rabinovich GA, Croci DO (2012) Regulatory circuits mediated by lectin–glycan interactions in autoimmunity and cancer. Immunity 36:322–335
Rabinovich GA, Gruppi A (2005) Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of the disease. Parasite Immunol 27(4):103–114
Raftos D (1996a) Interactions of tunicate immunomodulatory proteins with mammalians cells. Immunol Cell Biol 74:26–31
Raftos DA (1996b) Adoptive transfer of alloimmune memory in the solitary tunicate, Styela plicata. J Exp Zool 274:310
Raftos DA, Cooper EL (1991) Proliferation of lymphocyte-like cells from the solitary tunicate, Styela clava, in response to allogeneic stimuli. J Exp Zool 260:391–400
Raftos DA, Tait NN, Briscoe DA (1987a) Allograft rejection and alloimmune memory in the solitary urochordate, Styela plicata. Dev Comp Immunol 11:343–351
Raftos DA, Tait NN, Briscoe DA (1987b) Cellular basis of allograft rejection in the solitary urochordate, Styela plicata. Dev Comp Immunol 11:713–725
Raftos DA, Briscoe DA, Tait NN (1988) The mode of recognition of allogeneic tissue in the solitary urochordate Styela plicata. Transplantation 45:1123–1126
Raftos DA, Stillman DL, Cooper EL (1991a) Interleukin-2 and phytohemagglutinin stimulate proliferation of tunicate cells. Immunol Cell Biol 69:225–234
Raftos DA, Cooper EL, Habicht GS et al (1991b) Invertebrate citokines: tunicate cell proliferation stimulated by an interleukin 1–like molecule. Proc Natl Acad Sci 88:9518–9522
Raftos D, Green P, Mahajan D et al (2001) Collagenous lectins in tunicates and the proteolytic activation of complement. Adv Exp Med Biol 484:229–236
Raftos DA, Nair SV, Robbins J et al (2002) A complement component C3–like protein from the tunicate, Styela plicata. Dev Comp Immunol 26:307–312
Raftos DA, Robbins J, Newton RA et al (2003) A complement component C3a–like stimulates chemotaxis by hemocytes from an invertebrate chordate—the tunicate, Pyura stolonifera. Comp Biochem Physiol 134A:377–386
Raftos DA, Fabbro M, Nair SV (2004) Exocytosis of a complement component C3–like protein by tunicate hemocytes. Dev Comp Immunol 28:181–190
Rast JP, Smith LC, Loza-Coll M, Hibino T, Litman GW (2006) Genomic insights into the immune system of the sea urchin. Science 314:952–956
Reddy AL, Bryan B, Hidelmann WH (1975) Integumentary allograft versus autograft reactions in Ciona intestinalis: a protochordate species of solitary tunicate. Immunogenetics 1:584–590
Reynold JM, Dong C (2013) Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol 34:511–519
Rinkevich B (2002) The colonial urochordate Botryllus schlosseri: from stem cells and natural tissue transplantation to issues in evolutionary ecology. Bioessays 24:730–740
Rinkevich B (2005) Rejection pattern in botryllid ascidian immunity: the first tier of allorecognition. Can J Zool 83:101–121
Rinkevich B, Rabinowitz C (1993) In vitro culture of blood cells from the colonial protochordate Botryllus schlosseri. In Vitro Cell Dev Biol Anim 29:79–85
Rinkevich B, Weissman IL (1987) A long-term study on fused subclones in the ascidian Botryllus schlosseri: the resorption phenomenon (Protochordata: Tunicata). J Zool (Lond) 213:717–733
Rinkevich B, Weissman IL (1992) Allogeneic resorption in colonial protochordates—consequences of nonself recognition. Dev Comp Immunol 16:275–286
Rinkevich B, Tartakover S, Gershon H (1998) Contribution of morula cells to allogeneic responses in the colonial urochordate Botryllus schlosseri. Mar Biol 131:227–236
Rinkevich Y, Douek J, Haber O, Rinkevich B, Reshef R (2007) Urochordate whole body regeneration inaugurates a diverse innate immune signaling profile. Dev Biol 312(1):131–146
Rinkevich B, Douek J, Rabinowitz C, Paz G (2012) The candidate FuHC gene in B. schlosseri (Urochordata) and ascidians’ historecognition—an oxymoron? Dev Comp Immunol 36:718–772
Roberts S, Gueguen Y, De Lorgeril J et al (2008) Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev Comp Immunol 32:1099–1104
Robinson JM (2008) Reactive oxygen species in phagocytic leukocytes. Histochem Cell Biol 130:281–297
Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA (2004) The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 64:1–12
Rybakin V, Clemen CS (2005) Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. BioEssays 27:625–632
Sano H, Hsu DK, Apgar JR et al (2003) Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest 112:389–397
Sasaki N, Ogasawara M, Sekiguchi T et al (2009) Toll-like receptors of the ascidian Ciona intestinalis. J Biol Chem 284:27336–27343
Satake H, Sasaki N (2010) Comparative overview of Toll-like receptors in lower animals. Zool Sci 27:154–161
Satake H, Sekiguchi T (2012) Toll-like receptors of deuterostome invertebrates. Front Immunol 3:34. https://doi.org/10.3389/fimmu.2012
Satake M, Kawazoe Y, Kasuya A (2003) Hemocytes of Ciona intestinalis express multiple genes involved in innate immune host defense. Biochem Biophys Res Commun 302:207–218
Sato S, Nieminen J (2004) Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj J 19:583–591
Sato A, Satoh N, Bishop JDD (2012) Field identification of ‘types’ A and B of the ascidian Ciona intestinalis in a region of sympatry. Mar Biol 159:1611–1619
Satoh N, Satau Y, Davidson B, Levine M (2003) Ciona intestinalis: an emerging model for whole-genome analyses. Trends Genet 19:376–381
Schmitz F, Mages J, Heit A et al (2004) Transcriptional activation induced in macrophages by Toll-like receptor (TLR) ligands: from expression profiling to a model of TLR signalling. Eur J Immunol 34:2863–2873
Scofield VL, Nagashima LS (1983) Morphology and genetics of rejection reactions between oozooids from the tunicate Botryllus schlosseri. Biol Bull 165:733–744
Sekine H, Kenjo A, Azumi K et al (2001) An ancient lectin-dependent complement system in an ascidian: novel lectin isolated from the plasma of the solitary ascidian, Halocynthia roretzi. J Immunol 167:4504–4510
Shaw LM, Olsen BR (1991) FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem Sci 16:191–194
Shi Y, Massagué J (2003) Mechanisms of TGF-b signaling from cell membrane to the nucleus. Cell 113:685–700
Shida K, Terajima D, Uchino R et al (2003) Hemocytes of Ciona intestinalis express multiple genes involved in innate immune host defense. Biochem Biophys Res Commun 302:207–218
Shirae M, Saito Y (2000) A comparison of hemocytes and their phenoloxidase activity among botryllid ascidians. Zool Sci 17:881–891
Shirae M, Hirose E, Saito Y (1999) Behavior of hemocytes in the allorejection reaction in two compound ascidians, Botryllus scalaris and Symplegma reptans. Biol Bull 197:188–197
Sidney LE, Branch MJ, Dunphy SE et al (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells (Dayton, Ohio) 32:1380–1389
Silerova M, Prochazkova P, Joskova R et al (2006) Comparative study of the CCFlike pattern recognition protein in different lumbricid species. Dev Comp Immunol 30:765–771
Silva MT, Correia-Neves M (2012) Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol 3:174. https://doi.org/10.3389/fimmu.2012.00174
Sim RB, Laich A (2000) Serine proteases of the complement system. Biochem Soc Trans 28:545–550
Skjoedt MO, Palarasah Y, Rasmussen K et al (2010) Two mannose-binding lectin homologues and an MBL-associated serine protease are expressed in the gut epithelia of the urochordate species Ciona intestinalis. Dev Comp Immunol 34:59–68
Smith VJ, Söderhäll K (1991) A comparison of phenoloxidase activity in the blood of marine invertebrates. Dev Comp Immunol 15:251–261
Smith LC, Azumi K, Nonaka M (1999) Complement systems in invertebrates. The ancient alternative and lectin pathways. Immunopharmacology 42:107–120
Söderhäll K, Cerenius L (1998) Role of prophenoxidase-activating system in invertebrate immunity. Curr Opin Immunol 10:23–28
Springer SA, Gagneux P (2013) Glycan evolution in response to collaboration, conflict, and constraint. J Biol Chem 288:6904–6911
Sritunyalucksana K, Wongsuebsantati K, Johansson MW et al (2001) Peroxinectin, a cell adhesive protein associated with the proPO system from the black tiger shrimp, Penaeus monodon. Dev Comp Immunol 25:353–363
Suzuki MM, Nishikawa T, Bird A (2005) Genomic approaches reveal unexpected genetic divergence within Ciona intestinalis. J Mol Evol 61:627–635
Swalla BJ, Smith AB (2008) Deciphering deuterostome phylogeny: molecular, morphological and paleontological perspectives. Philos Trans R Soc 363B:1557–1568
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14
Taketa DA, De Tomaso AW (2015) Botryllus schlosseri Allorecognition: Tackling the enigma. Dev Comp Immunol 48:254–265
Tarallo R, Sordino P (2004) Time course of programmed cell death in Ciona intestinalis in relation to mitotic activity and MAPK signaling. Dev Dyn 230:251–262
Tecchio C, Micheletti A, Cassatella MA (2014) Neutrophil-derived cytokines: facts beyond expression. Front Immunol | Molecular Innate Immunity 5:508. https://doi.org/10.3389/fimmu.2014.00508
Terada T, Watanabe Y, Tateno H, Naganuma T, Ogawa T, Muramoto K, Kamiya H (2007) Structural characterization of a rhamnose binding glycoprotein (lectin) from Spanish mackerel (Scomberomorous niphonius) eggs. Biochim Biophys Acta 1770:617–629
Terajima D, Yamada S, Uchino R et al (2003) Identification and sequence of seventy-nine new transcripts expressed in hemocytes of Ciona intestinalis, three of which may be involved in characteristic cell–cell communication. DNA Res 10:203–212
Thornqvist PO, Johansson MW, Söderhäll K (1994) Opsonic activity of cell adhesion protein and b-1,3-glucan-binding proteins from two crustaceans. Dev Comp Immunol 18:3–12
Trapani MR, Sanfratello MA, Mangano V et al (2015) Phenoloxidases of different sizes are modulated by LPS inoculation into Ciona intestinalis tunic and pharynx. Inv Surv J 12:75–81
Trepels T, Zeiher AM, Fichtlscherer S (2006) The endothelium and inflammation. Endothelium 13:423–429
Tu Q, Cameron RA, Worley KC, Gibbs RA, Davidson EH (2012) Gene structure in the seaurchin Strongylocentrotus purpuratus based on transcriptome analysis. Genome Res 22:2079–2087
Turner MW (2003) The role of mannose-binding lectin in health and disease. Mol Immunol 40:423–429
Vabulas RM, Hmad-Nejad P, Ghose S et al (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107–15112
Valanne S, Wang J-H, Rämet M (2011) The Drosophila Toll signaling pathway. J Immunol 186:649–656
Van Lookeren Campagne M, Weismann C, Brown EJ (2007) Macrophage complement receptors and pathogen clearance. Lit Rev Cell Microbiol 9:2095–2102
Vanlangenakker N, Vanden Berghe T, Vandenabeele P (2012) Many stimuli pull the necrotic trigger, an overview. Cell Death Differ 19:75–86
Varki A, Acids SRS (2009) In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor Chapter 14
Vasta GR (2012) Galectins as pattern recognition receptors: structure, function, and evolution, current topics in innate immunity II. Lambris JD, Hajishengallis G (eds) Adv Exp Med Biol 946:21–36
Vasta GR, Hunt JC, Marchalonis JJ et al (1986) Galactosyl-binding lectins from the tunicate Didemnum candidum. Purification and physicochemical characterization. J Biol Chem 261:9174–9181
Vasta GR, Quesenberry MS, Ahmed H et al (1999) C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol 23:401–420
Vasta GR, Ahmed H, Odom EW (2004) Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr Opin Struct Biol 14:617–630
Vasta GR, Ahmed H, Nita-Lazar M et al (2012) Galectins as self/non-self recognition receptors in innate and adaptive immunity: an unresolved paradox. Front Immunol 3:199. https://doi.org/10.3389/fimmu.2012.00199
Vestweber D, Blanks JE (1999) Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79:181–213
Vizzini A, Arizza V, Cervello M et al (2001) Identification of type I and IX collagens in the ascidian Ciona intestinalis. In: Sawada H, Yokosawa H, Lambert CC (eds) The biology of ascidians. Springer, Tokyo, pp 402–407
Vizzini A, Arizza V, Cervello M et al (2002) Cloning and expression of a type IX–like collagen in tissues of the ascidian Ciona intestinalis. Biochim Biophys Acta 1577:38–44
Vizzini A, Pergolizzi M, Vazzana M et al (2008) FACIT collagen (1alpha-chain) is expressed by hemocytes and epidermis during the inflammatory response of the ascidian Ciona intestinalis. Dev Comp Immunol 32:682–692
Vizzini A, Parrinello D, Sanfratello MA et al (2012) Inducible galectins are expressed in the inflamed pharynx of the ascidian Ciona intestinalis. Fish Shellfish Immunol 32:101–109
Vizzini A, Parrinello D, Sanfratello MA et al (2013a) Ciona intestinalis peroxinectin is a novel component of the peroxidase–cyclooxygenase gene superfamily upregulated by LPS. Dev Comp Immunol 41:59–67
Vizzini A, Bonura A, Parrinello D et al (2013b) LPS challenge regulates gene expression and tissue localization of a Ciona intestinalis gene through an alternative polyadenylation mechanism. PLoS One 8:63235
Vizzini A, Parrinello D, Sanfratello MA et al (2015a) Upregulated transcription of phenoloxidase genes in the pharynx and endostyle of Ciona intestinalis in response to LPS. J Invertebr Pathol 126:6–11
Vizzini A, Di Falco F, Parrinello D et al (2015b) Ciona intestinalis interleukin 17-like genes expression is upregulated by LPS challenge. Dev Comp Immunol 48:129–137
Vizzini A, Di Falco F, Parrinello D et al (2016a) Transforming growth factor b (CiTGF-b) gene expression is induced in the inflammatory reaction of Ciona intestinalis. Dev Comp Immunol 55:102–110
Vizzini A, Bonura A, Longo V et al (2016b) LPS injection reprograms the expression and the 3' UTR of a CAP gene by alternative polyadenylation and the formation of a GAIT element in Ciona intestinalis. Mol Immunol 77:174–183
Vizzini A, Parisi MG, Cardinale L et al (2017) Evolution of Ciona intestinalis tumor necrosis factor alpha (CiTNFα): polymorphism, tissues expression, and 3D modeling. Dev Comp Immunol 67:107–116
Voogdt CGP, van Putten JPM (2016) The evolution of the Toll-like receptor system. In: Malagoli D (ed) The evolution of the immune system. Conservation and diversification. Acad Press, London, pp 311–330
Voskoboynik A, Rinkevich B, Weiss A et al (2004) Macrophage involvement for successful degeneration of apoptotic organs in the colonial urochordate Botryllus schlosseri. J Exp Biol 207:2409–2416
Voskoboynik A, Soen Y, Rinkevich Y et al (2008) Identification of the endostyle as a stem cell niche in a colonial chordate. Cell Stem Cell 3:456–464
Voskoboynik A, Neff NF, Sahoo D et al (2013a) The genome sequence of the colonial chordate, Botryllus schlosseri. elife 2:00569
Voskoboynik A, Newman AM, Corey DM et al (2013b) Identification of a colonial chordate histocompatibility gene. Science 341(6144). https://doi.org/10.1126/science.1238036
Vyas K, Chaudhuri S, Leaman DW et al (2009) Genome-wide polysome profiling reveals an inflammation-responsive post-transcriptional operon in gamma interferon-activated monocytes. Mol Cell Biol 29:458–470
Wada H, Matsumoto N, Maenaka K et al (2004) The inhibitory NK cell receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind the top of HLA-E through mostly shared but partly distinct sets of HLA-E residues. Eur J Immunol 34:81–90
Wada S, Hamada M, Satoh N (2006) A genomewide analysis of genes for the heat shock protein 70 chaperone system in the ascidian Ciona intestinalis. Cell Stress Chaperones 11:23–33
Wallis R (2007) Interactions between mannose-binding lectin and MASPs during complement activation by the lectin pathway. Immunobiology 212:289–299
Wang J, Slungaard A (2006) Role of eosinophil peroxidase in host defense and disease pathology. Arch Biochem Biophys 15:256–260
Wang KS, Frank DA, Ritz J (2000) Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood 95:3183–3190
Ward-Kavanagh L, Lin WW, Šedý JS et al (2016) The TNF receptor superfamily in costimulating and coinhibitory responses. Immunity 44:1005–1019
Weaver CT, Hatton RD, Mangan PR et al (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852
Weissman I (2000) Stem cells: units of development, review units of regeneration, and units in evolution. Cell 100:157–168
Wright RK, Cooper EL (1983) Inflammatory reactions of the protochordata. Am Zool 23:205–211
Wu S-Z, Huang X-D, Li Q, He M-X (2013) Interleukin-17 in pearl oyster (Pinctada fucata): molecular cloning and functional characterization. Fish Shellfish Immunol 34(5):1050–1056
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44:450–462
Yousef GM, Diamandis EP (2003) An overview of the kallikrein gene families in humans and other species: emerging candidate tumour markers. Clin Biochem 36:443–452
Yu Y, Yuan S, Yi Y, Huang H et al (2007) Molecular and biochemical characterization of galectin from amphioxus: primitive galectin of chordates participated in the infection processes. Glycobiology 17:774–783
Zanoni I, Ostuni R, Marek LR et al (2011) CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147:868–880
Zederbauer M, Furtmuller PG, Bellei M et al (2007a) Distruption of the aspartate to heme ester linkage in human myeloperoxidase: impact on ligand binding, redox chemistry and interconversion of redox intermediates. J Biol Chem 282:17041–17052
Zederbauer M, Furtmuller PG, Ganster B et al (2007b) Manipulating the vinyl–sulfonium bond in human myeloperoxidase: impact on compound I formation and reduction by halides and thiocyanate. Biochem Biophys Res Commun 356:450–456
Zelensky AN, Gready JE (2005) The C-type lectin–like domain superfamily. FEBS J 272:6179–6217
Zhang X, Luan W, Jin S et al (2008) A novel tumor necrosis factor ligand superfamily member (CsTL) from Ciona savignyi: molecular identification and expression analysis. Dev Comp Immunol 32:1362–1373
Zhang X, Angkasekwinai P, Dong C et al (2011) Structure and function of interleukin-17 family cytokines. Protein Cell 2:26–40
Zucchetti I, Marino R, Pinto MR et al (2008) CiCD94-1, an ascidian multipurpose C-type lectin–like receptor expressed in Ciona intestinalis hemocytes and larval neural structures. Differentiation 76:267–283
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Parrinello, N., Cammarata, M., Parrinello, D. (2018). The Inflammatory Response of Urochordata: The Basic Process of the Ascidians’ Innate Immunity. In: Cooper, E. (eds) Advances in Comparative Immunology. Springer, Cham. https://doi.org/10.1007/978-3-319-76768-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-76768-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76767-3
Online ISBN: 978-3-319-76768-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)