Abstract
Plants have been shown to uptake nanoparticles of various composition and size. The uptake is plant specific and can result in negative, positive, or no effect on plants. Some nanoparticles modify the roots and leaves of plants and seed germination and induce genetic alterations. Nanoparticle physicochemical properties, such as size, crystalline structure, and surface charge, influence their translocation and bioaccumulation in plants. Nanoparticles are shown to be transmitted to second-generation plants. While some researchers try to justify the use of nanoparticles for some plant species, the overall negative effects due to accumulation of nanoparticles in soil and plants might overshadow the limited beneficial aspects on some plants. Depending mainly on their size, many nanoparticles can enter the food chain via roots or foliar uptake. Nanoparticles of many materials are already shown to be toxic to humans and animals; therefore nanoparticle uptake by plants may pose serious safety concerns.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1.1 Introduction
Nanoparticles can be defined as very small particles with size in the nanometer range. They can be as small as 1 nm and as large as hundreds of nm.
Due to their small size, nanoparticles can be internalized by plants, animals, and humans. Further, they can enter cells and organelles and affect cellular processes. Nanoparticles with selected compositions had shown some beneficial effects in selected plants, and, as a result, some scientists are promoting their use in agriculture. However, nanoparticles are phytotoxic for many other plants. In addition, nanoparticles are toxic to humans and animals, being associated to a multitude of diseases, ranging from respiratory and cardiovascular to neurological diseases. As a result of their toxicity, it is necessary to environmentally monitor man-made nanoparticles and to pass regulations and laws regarding the use and safe handling of nanoparticles.
What makes nanoparticles different from larger particles of the same material are surface and quantum effects (Buzea and Pacheco 2017). A material in nanoform exhibits different physical, chemical, and mechanical properties than the material in bulk form. Decreasing the size of a nanoparticle, the ratio between the atoms on its surface compared to those in its interior increases, leading to a smooth scaling of its physical and chemical properties. As a result, nanoparticles will have higher surface/volume ratio, increased chemical reactivity, and reduced melting point. Due to the small size of a nanoparticle, its electrons become confined and will have a quantized energy spectrum, resulting in quantum size effects. An example of quantum size effect is the appearance of magnetic moments. For example, there are nanoparticles of materials nonmagnetic in bulk that, when in nanoform, develop magnetic moments. Among these are gold, platinum, and palladium.
Nanoparticles can have various sizes, morphologies, and crystallinities, as illustrated in Fig. 1.1. They can have a short aspect ratio, with spherical or cubic morphologies, or a long aspect ratio, in the form of tubes or long whiskers (Soto et al. 2005; Murr and Soto 2004; Rui et al. 2015; Qiu et al. 2010).
Transmission electron microscopy images of nanoparticles of (a) Ag, (b) Al2O3, (c) Fe2O3, (d) TiO2 rutile, (e) MWCNTs, and (f) chrysotile asbestos. Inserts are showing selected area electron diffraction (SAED) patterns that indicate the degree of crystallinity of nanoparticles. Notice the similarity between the morphology of MWCNTs and asbestos. Images (a–d) are reprinted from Soto K. F. et al. 2005. Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. Journal of Nanoparticle Research, 7, 145–169, with permission from Springer (Soto et al. 2005). Images (e–f) are reproduced from Murr L. E. & Soto K. F. 2004. TEM comparison of chrysotile (asbestos) nanotubes and carbon nanotubes. Journal of Materials Science, 39, 4941–4947. Copyright 2004 Kluwer Academic Publishers. With permission of Springer (Murr and Soto 2004). (g) CeO2 nanoparticles. Reprinted from Environmental Pollution, vol. 198, Rui Y. et al., Transformation of ceria nanoparticles in cucumber plants is influenced by phosphate, pp. 8–14, Copyright (2015), with permission from Elsevier (Rui et al. 2015). (h) Gold nanospheres and (i) gold nanorods; images (h–i) adapted from Biomaterials, Vol 31, issue 30, Qiu Y. et al, Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods, Pages 7606–7619, Copyright (2010), with permission from Elsevier (Qiu et al. 2010)
Nanotoxicology is a branch of toxicology that studies the toxicity of nanoparticles in humans and animals. It encompasses in vitro studies performed on animal and human cell lines, in vivo experiments on animals and humans, epidemiological data related to particle pollution, and occupational exposure studies of workers involved in handling nanoparticles (welding, mining, etc.).
Nanoparticles are being increasingly used in applications, including agriculture. However, many types of nanoparticles are proved to be toxic, despite the fact that the same material in bulk form is harmless. It is impossible to predict the toxicity degree of a nanoparticle type without experimental data. As most of the nanotoxicity studies are published in very specialized journals, the dissemination of information on nanoparticle toxicity is not readily available for the scientists that are starting to use these nanoparticles in applications, including agrichemicals.
The researchers working in their application in agriculture, being unaware of nanoparticle toxicity, are likely to suffer health effects in the coming years due to incorrect handling and inadvertently exposure to nanoparticles. Due to their small size, nanoparticles can easily become airborne and be inhaled and ingested and enter in contact with the skin. Secondly, the use of agricultural nanoparticles poses a risk for the population and ecosystem.
Having remembered asbestos and the severe health effects due to its use in construction, we would like to prevent a similar situation from happening. However, nanoparticles use in agriculture might pose a higher environmental and toxic threat than asbestos. Asbestos use was limited mainly to the construction industry, being confined to buildings, and is now relatively easy to remove. Nanoparticles used in agriculture will not be confined to a specific place; they will enter the atmosphere and become respirable particles, pollute the water, and lead to devastating consequences for humans and other life species.
This chapter will focus on evaluating the beneficial and detrimental effects of nanoparticles on plants together with their toxicity in humans and animals. Weighting the pros and cons will allow the reader to form an idea whether or not nanoparticles should be used in agriculture. We show research regarding nanoparticle uptake and accumulation in plants, together with phytotoxicity studies. We also show selected beneficial effects in some plants. Following are subchapters dedicated to toxic effects of nanoparticles in humans and animals together with comparative toxicity for various compositions. After reading this chapter, the reader should be informed on the pros and cons of using nanoparticles in agriculture and the environmental risks and toxicity that they will pose for life.
1.2 Nanoparticle Physicochemical Properties
Nanoparticle interaction with their environment and uptake and toxicity in plants, humans, and animals depend on their size, aggregation, composition, concentration, shape, porosity, surface area, hydrophobicity, electrical charge, and magnetic properties, as illustrated schematically in Fig. 1.2.
It is important to note that nanoparticles suffer chemical transformation in the soil, within the plants, and within organisms in general. They are able to undergo various transformations, for example, acquiring a protein corona or changing their oxidation state, depending on their environment conditions. These transformations dictate ultimately their uptake, translocation, and toxicity. Even nanoparticles that may be considered stable are still able to change chemically, and their beneficial properties might become detrimental. For example, under hydroponic conditions Ce(IV)O2 in cucumber plants is reduced to Ce(III) (Rui et al. 2015). CeO2 nanoparticles in hydroponic cucumber plants treated with phosphate suffer chemical transformation, being located outside the epidermis, while in phosphate free plants, they were observed only in the intercellular spaces and vacuole of root (Rui et al. 2015).
1.3 Nanoparticles in Agriculture
1.3.1 Pesticides and Fertilizers
The topic of nanoparticle applications in agriculture emerged around the year 2000 (Gogos et al. 2012). Nanoparticles used in agriculture can be solid (such as metal and their oxides) or nonsolids (such as lipid or polymer) (Gogos et al. 2012). They are used for plant crop protection and for soil/water remediation (Fig. 1.3). Nanoparticles in plant protection are used as fungicides, herbicides, and insecticides, as depicted in Fig. 1.4. Nanoparticles can be the active ingredient or an additive that can act for the controlled release of the main ingredient, as dispersing agent, targeted delivery agent, protective agent, or photocatalyst.
Comparative results of nanoparticles used in agriculture. (a) Applications of nanoparticles in agriculture. (b) Types of plant protection products containing nanoparticles, (c) the function of nanoparticles within these products, (d) the role of the additive nanoparticles in plant protection products. Reprinted with permission from Gogos A. et al., Nanomaterials in Plant Protection and Fertilization: Current State, Foreseen Applications, and Research Priorities. Journal of Agricultural and Food Chemistry, vol. 60, pp. 9781–9792. Copyright (2012) American Chemical Society (Gogos et al. 2012)
Figure 1.3 shows a schematic of nanoparticle function used in agriculture together with examples of nanoparticle compositions. Figure 1.4 shows comparative results of nanoparticles used in agriculture. Nanoparticles can act as active constituents and additives: they can serve as delivery devices that targeting specific tissues, nanopesticides (small particles of pesticides), and nanocages filled with pesticides act as controlled release devices. Nanoparticles themselves can have pesticidal properties when in nanoform, such as Ag, Au, TiO2, Cu, and ZnO, several of these having photocatalytic properties. They can be pesticide additives that serve for enhancing the solubility of active ingredients. Some nanoparticles can also be used for soil and water remediation (Aragay et al. 2012). Due to their high surface area, adsorption capacity, and electromagnetic properties, nanoparticles are prospected for the adsorption of organic and inorganic pollutants from soil and water (Gupta and Saleh 2013). Among them are metal-containing particles, CNTs, C60, and zeolites. Magnetic nanoparticles, such as iron oxide (Fe3O4) and zerovalent iron, are unique agents for water treatment (Xu et al. 2012; Deng et al. 2014). Magnetic nanoparticles are used for selected pollutant removal. Heavy metal pollutants are adsorbed by nanoparticles of Fe2O3, Fe3O4, SiO2, and Al2O3 (Bakshi et al. 2015).
Some nanoparticles are found to be beneficial for plant protection and growth of selected plants, as discussed in Sect. 1.6. Unfortunately, the same types of nanoparticles are shown to be toxic to animals, humans, and some plants, such as carbon nanotubes (CNTs), Ag, titanium dioxide (TiO2), silica (SiO2), and alumina (Al2O3), as seen in Sect. 1.7.
The use of nanoparticles in agriculture should be limited by legislation. Very concerning is the increasing number of patents being filed related to nano-agrichemicals. The buildup of nanoparticles in plants, soil, water, and the environment, their trophic transfer, will detrimentally and irreversibly affect the health of humans and animals as well as plants. Many nanoparticles are shown to enter edible plants, and once they are in the food chain, they are likely to cause adverse health effects. It is imperative that regulatory agencies address and control the utilization of nanoparticles in agriculture (Kookana et al. 2014).
The reader interested in finding out more details about nanoparticles used in agriculture as agrichemicals, crop enhancers, crop protection, and soil and water remediation, can research the following reviews: Iavicoli et al. (2017), Khot et al. (2012), Liu and Lal (2015), Servin et al. (2015), Deng et al. (2014), Aragay et al. (2012), Gogos et al. (2012), Kah and Hofmann (2014), Ruttkay-Nedecky et al. (2017), and Wang et al. (2016).
1.3.2 Nanoparticle Soil Interaction and Accumulation
The use of nanoparticles in agriculture results in their accumulation in soil and the environment in general as well as trophic transfer. We must specify that when speaking about soil and nanoparticles, we are referring to man-made nanoparticles. Within the soil there are a multitude of natural nano- and microparticles, some of them having beneficial properties for the soil fertility. For example, clay nanoparticles may prevent leakage of nutrients in the groundwater by forming electrostatic bonds with them (Bernhardt et al. 2010).
Several types of nanoparticles are known for their antibacterial properties; hence their availability in soil is likely to affect soil bacteria, which are essential for their role in various ecosystems (Dinesh et al. 2012). The negative effects on endophytic bacteria symbionts are of special concern (Deng et al. 2014). Nanoparticles in soil will modify their properties in a dynamic manner, affecting their aggregation, dispersibility, dimensions, surface area, charge, and chemistry, which will affect their transport and availability.
Nanoparticle interaction with the soil and their bactericidal properties depends on the soil properties (Bakshi et al. 2015; Layet et al. 2017; Schlich and Hund-Rinke 2015). For example, silver nanoparticle toxicity against ammonia-oxidizing bacteria decreases for soils with higher clay content and larger pH. As a result, the toxicity of nanoparticles on plants may be affected by the soil type (Josko and Oleszczuk 2013).
The existence of nanoparticles with bactericidal properties in soil is likely to affect plants. It was found that the exposure of legumes to some nanoparticles severely lowers nitrogen fixation due to their bactericidal effects. Soybean plants exposed to ceria nanoparticles have a reduced nitrogen fixation correlated with almost absent bacteroids in its nodules (Priester et al. 2012).
1.4 Nanoparticle Uptake in Plants
1.4.1 Nanoparticle Uptake Routes
The interaction of nanoparticle with plants is a relatively new field of study. Nanoparticle uptake is plant specific. While the topic of uptake and transport of nanoparticles within plants is still not entirely understood, there is a consensus that it depends on the type of nanoparticle, their physicochemical properties, plant species, and the plant substrate—soil, hydroponics, or culture medium (Arruda et al. 2015; Aslani et al. 2014; Bakshi et al. 2015; Bernhardt et al. 2010; Chichiricco and Poma 2015; Deng et al. 2014; Dietz and Herth 2011; Ma et al. 2015; Miralles et al. 2012a, b; Navarro et al. 2008; Rico et al. 2011; Yadav et al. 2014; Schwab et al. 2015; Zuverza-Mena et al. 2017; Reddy et al. 2016).
It is known already that some nanoparticles translocate within the plants by forming complexes with membrane transporter proteins or root exudates (Yadav et al. 2014). Nanoparticle properties, such as size, porosity, hydrophobicity, and surface, are modulating the interaction of nanoparticles with plants. A schematic of nanoparticle uptake in plants is shown in Fig. 1.5 (Line et al. 2017).
Schematics of the uptake and translocation of CNTs in plants. Image not at scale. Within the cell blue represents vacuole; green, chloroplasts; purple, nucleus; orange, smooth endoplasmic reticulum; blue, plasmode. 1. The uptake of CNTs by plant roots can occur via osmotic pressures, capillary forces, pores on cell walls, intercellular plasmodesmata, or through direct penetration. 2. Endocytosis allows CNTs to cross both cell wall and cell membrane. 3. CNTs may use the vascular system together with water and nutrients and can translocate to the upper parts of the plants. 4. CNTs may reach the upper part of plants. Their preferential location in leaves is the xylem. 5. Inside the cells CNTs can be found in cytoplasm, cell wall, cell membrane, chloroplast, mitochondria, and plasmodes. Reprinted from Carbon, vol. 123, Line C. et al., Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem—A review, pp. 767–785. Copyright (2017), with permission from Elsevier (Line et al. 2017)
Roots can uptake small nanoparticles through pores (with size around 5–20 nm) within the root epidermal cell walls—called the apoplastic route (Deng et al. 2014). Particles larger than the pore size will be stopped. Small nanoparticles that cross the cell walls may be subjected to osmotic pressure and capillary forces and diffuse through the apoplast and reach the endodermis (Lin et al. 2009; Deng et al. 2014).
Another route of nanoparticle uptake in plants is the symplastic pathway via the inner side of the plasma membrane. The cell wall is a porous matrix of polysaccharide fibers that can be crossed by nanoparticles that bind to protein carriers, via aquaporins, ion channels, and endocytosis, or by piercing the cell membrane and creating new pores (Tripathi et al. 2017; Rico et al. 2011; Wild and Jones 2009). Nanoparticles can migrate to neighboring cells through plasmodesmata (20–50 nm diameter channels) (Deng et al. 2014).
Another way of entry of nanoparticle in plants is via foliar through stomatal pores (Larue et al. 2014a, b; Hong et al. 2014). From leaves nanoparticles can translocate to other parts of the plants, including roots (Hong et al. 2014). Examples of plants that internalize nanoparticles through leaves are rapeseed, wheat, beans, corn, lettuce, and cucumber (Chichiricco and Poma 2015). Nanoparticles ranging from a few nanometers up to several hundred nanometers and with different compositions can be internalized through leaves, such as ceria, titania, iron oxide, zinc oxide, and silver (Chichiricco and Poma 2015).
Within the cells nanoparticles are shown to interact with cell organelles, and depending on their physicochemical properties, many produce oxidative stress, genotoxicity, and metabolic changes (Deng et al. 2014).
1.4.2 Nanoparticle Composition-Dependent Uptake in Plants
In the following, we will focus mostly on the nanoparticle uptake on crops due to their immediate trophic transfer to humans and animals. Many crops exposed to various nanoparticles have been shown to internalize them (Deng et al. 2014). Once inside, they translocate to various plant tissues: stems, leaves, petioles, flowers, and fruits (Deng et al. 2014). While there are some reports on beneficial effects on selected plants, there is an overwhelming evidence of adverse effects of nanoparticles on many crops.
Below are examples of studies showing the uptake of nanoparticles with various compositions in various edible plants:
-
Au—tomato plants (Dan et al. 2015), tobacco (Judy et al. 2011; Sabo-Attwood et al. 2012), Arabidopsis thaliana (Avellan et al. 2017; Taylor et al. 2014), barley (Feichtmeier et al. 2015), rice, radish, pumpkin (Zhu et al. 2012)
-
Ag—Arabidopsis thaliana (Geisler-Lee et al. 2013; Kaveh et al. 2013; Nair and Chung 2014), tomato (Antisari et al. 2015), wheat (Dimkpa et al. 2013b), lettuce (Larue et al. 2014a), mung bean and sorghum (Lee et al. 2012), rice (Mirzajani et al. 2013; Thuesombat et al. 2014), broad bean (Patlolla et al. 2012), corn, cabbage (Pokhrel and Dubey 2013), review (Cox et al. 2016)
-
CeO2—alfalfa, corn (Lopez-Moreno et al. 2010b; Wang et al. 2013b), cucumber (Zhang et al. 2011; Lopez-Moreno et al. 2010b; Rui et al. 2015; Hong et al. 2014), tomato (Antisari et al. 2015; Lopez-Moreno et al. 2010b; Wang et al. 2013b), soybean (Lopez-Moreno et al. 2010a), barley (Rico et al. 2015), lettuce (Gui et al. 2015; Zhang et al. 2015), wheat (Rico et al. 2014)
-
MWCNTs—wheat (Miralles et al. 2012b; Larue et al. 2012b), rapeseed (Larue et al. 2012b), tomato (Khodakovskaya et al. 2013), red spinach (Amaranthus tricolor L), (Begum and Fugetsu 2012), lettuce, rice, cucumber (Begum et al. 2014), onion (Ghosh et al. 2015), alfalfa (Miralles et al. 2012b), corn (Yan et al. 2013), review (Line et al. 2017)
-
TiO2—corn (Asli and Neumann 2009), wheat (Du et al. 2011; Larue et al. 2012a, c), rapeseed (Larue et al. 2012a, c), lettuce (Larue et al. 2014b), Arabidopsis thaliana (Kurepa et al. 2010, Wang et al. 2011b), cucumber (Servin et al. 2012, 2013), tomato (Antisari et al. 2015), onion (Pakrashi et al. 2014; Ghosh et al. 2010), review (Cox et al. 2016; Jacob et al. 2013), tobacco (Ghosh et al. 2010)
-
C60 or C70—Arabidopsis thaliana (Landa et al. 2012; Liu et al. 2010), bitter melon (Kole et al. 2013), rice (Lin et al. 2009), onion (Chen et al. 2010), review (Husen and Siddiqi 2014)
-
Zn and ZnO—Arabidopsis thaliana (Landa et al. 2012), soybean (Lopez-Moreno et al. 2010a), radish, rape, lettuce, corn, cabbage (Pokhrel and Dubey 2013; Lin and Xing 2007), cucumber (Lin and Xing 2007), wheat (Dimkpa et al. 2013a; Du et al. 2011), cress (Josko and Oleszczuk 2013), onion (Kumari et al. 2011), garlic (Shaymurat et al. 2012)
-
Carbon-Fe—pea, sunflower, tomato, wheat (Cifuentes et al. 2010)
-
Fe3O4—pumpkin (Zhu et al. 2008), soybean (Ghafariyan et al. 2013), tomato (Antisari et al. 2015)
-
Al2O3 or Al—onion, cress (Asztemborska et al. 2015), corn (Lin and Xing 2007; Asztemborska et al. 2015), review (Singh et al. 2017b)
-
Co—tomato (Antisari et al. 2015), onion (Ghodake et al. 2011)
-
SnO2—tomato (Antisari et al. 2015)
-
CuO2—radish (Atha et al. 2012), wheat (Dimkpa et al. 2013a), rice (Shaw and Hossain 2013), review (Anjum et al. 2015)
-
CdSe quantum dots—rice (Nair et al. 2011)
-
Rare-earth La2O3, Gd2O3, Yb2O3—rape, radish, wheat, lettuce, cabbage, tomato, cucumber (Ma et al. 2010)
The accumulation of nanoparticles in plants is not yet entirely understood; however several trends are emerging (Deng et al. 2014). Nanoparticle uptake in plants is species specific and depends on the nanoparticle composition and their size. For example, tobacco uptakes Au nanoparticles, while wheat does not (Judy et al. 2012). One must emphasize that future research might show a different picture of nanoparticle uptake, as various researchers uses nanoparticles with different sizes, surface charge and functionalization, crystallinity, etc.
Nanoparticle uptake and toxicity in plants is composition specific. For example, the exposure of tomato plants to nanoparticles with various compositions (CeO2, Fe3O4, SnO2, TiO2, Ag, Co, and Ni) has different effects on root growth, accumulation site, and fruit yield (Antisari et al. 2015). Longer roots are achieved after exposure to iron oxide nanoparticles, while the opposite effect is obtained by using tin oxide. While most metal nanoparticles accumulate in roots, silver and cobalt nanoparticles were found in below- and aboveground plant organs. Tomato fruits had higher amount of silver nanoparticles compared to other compositions (Antisari et al. 2015).
The uptake of nanoparticle by plants is a function of exposure condition, nanoparticle physicochemical properties, and plant species. Similar to the process in humans, the uptake and translocation of nanoparticles within plants can be very swift. The time of translocation from roots to shoots of carbon-coated magnetic nanoparticles in sunflower, tomato, pea, and wheat is less than 24 h (Cifuentes et al. 2010).
1.4.3 Nanoparticle Size-Dependent Plant Uptake
Particle size is one of the most important factors that determine the uptake of nanoparticles in plants. Smaller nanoparticles are internalized by plants, while larger ones are not (Zhu et al. 2008; Wang et al. 2011a). For example, in the case of TiO2 nanoparticles with sizes between 14 and 655 nm, only the smallest ones are able to translocate through the entire wheat plant (Larue et al. 2012a). The ones smaller than 140 nm pass through wheat root epidermis, while those smaller than 36 nm can transfer through root parenchyma and translocate from root to shoot (Larue et al. 2012a). Another example is the uptake of ceria nanoparticles in cucumber (Zhang et al. 2011). Nanoparticles with sizes of 7 and 25 nm are both absorbed by cucumber roots and translocate to leaves; however a larger number of smaller nanoparticles are absorbed compared to larger ones (Zhang et al. 2011).
1.4.4 Nanoparticle Crystalline Structure-Dependent Plant Uptake
Nanoparticles with the same composition but different crystalline structure can suffer a different uptake and translocation in plants. For example, titanium dioxide nanoparticles in anatase and rutile crystalline form are differentially translocated in cucumber plants (Servin et al. 2012). The anatase nanoparticles remained mainly in the roots, while the rutile nanoparticles translocated and accumulated mostly in the aerial tissue of cucumber.
1.4.5 Nanoparticle Charge-Dependent Plant Uptake
Studies show that the uptake of nanoparticles in plants is a function of nanoparticle surface charge or functionalization. Nanoparticles can be neutral; have a positive charge, in which case are called cationic; or have a negative charge—being called anionic. There seems to be a different behavior in the uptake of nanoparticles according to their charge by woody plants compared to herbaceous plants.
Woody Plants
A recent study on the uptake of CdSe/CdZnS quantum dots coated with cationic polyethylenimine (PEI) or poly(ethylene glycol) of anionic poly(acrylic acid) (PAA-EG) in poplar trees shows that both types of nanoparticles are internalized after 2-day exposure (Wang et al. 2014). Cationic quantum dot absorption is tenfolds faster than anionic nanoparticles, most likely due to electrostatic forces between positively charged quantum dots and the negatively charged root cell wall (Wang et al. 2014). Slower absorption of anionic quantum dots might be a result of the repulsive electrostatic forces between the negatively charged root surface and the negatively charged nanoparticles.
Herbaceous Plants
Interestingly, the uptake of cationic and anionic nanoparticles in herbaceous plants differs from the one in woody plants (Koelmel et al. 2013; Zhu et al. 2012).
Rice under hydroponic conditions uptakes and bioaccumulates 2 nm gold nanoparticles. Their distribution is a function of the nanoparticle surface charge (Koelmel et al. 2013). The accumulation in roots follows the order AuNP(+) > AuNP(0) > AuNP(−), where “+,” “0,” and “−” denoted positive, zero, and negative electrical charged nanoparticles, respectively. In contrast, the rice shoots showed a reverse order of nanoparticle charge uptake compared to the roots, having a preferential uptake of anionic nanoparticles.
Similar results were obtained in a study on the uptake of (6−10 nm) gold nanoparticles with different surface charge under hydroponic conditions in rice, radish, pumpkin, and perennial ryegrass (Zhu et al. 2012). Nanoparticle uptake is surface charge and plant specific. Cationic nanoparticles translocate mainly in plant roots, while anionic nanoparticles suffer uptake mainly in plant shoots. A larger number of nanoparticles are found in radish and ryegrass roots than rice and pumpkin roots. Nanoparticles accumulate in rice shoots in larger amounts compared to none in radish and pumpkin shoots (Zhu et al. 2012).
Cerium oxide nanoparticles (4 nm in size) also have a preferential uptake and tissue localization in wheat according to their surface charge (Spielman-Sun et al. 2017). Positively charged CeO2 adhere to wheat roots the strongest, while negatively charged and neutral nanoparticles have higher concentrations in leaves compared to plants exposed to cationic CeO2.
Therefore, the trend for herbaceous plants is to absorb positively charged nanoparticles in roots, while the shoots, stems, and leaves uptake mainly negatively charged nanoparticles.
1.5 Detrimental Effect of Nanoparticles in Plants
1.5.1 Composition and Plant-Specific Phytotoxicity
The interaction between plants and nanoparticles may range from subtle to notable changes in plant morphology, physiology, biochemistry, and genetics (Deng et al. 2014). Plant morphology changes include germination index (germination time and rate), root elongation, shoot and root biomass, root tip morphology, etc. (Deng et al. 2014).
Many studies indicate a detrimental effect of nanoparticles in many plant species, while a minority is trying to promote the use of nanoparticles for selected beneficial effects in a few plants. It is important to note that while some plants will have beneficial effects as a result of exposure to a type of nanoparticle, other plants are negatively affected by the same nanoparticles.
Many types of nanoparticles are phytotoxic, inhibiting plant growth and physiological, biochemical, and genetic traits (Tripathi et al. 2017; Brar et al. 2010; Deng et al. 2014). Table 1.1 shows examples of edible plants adversely affected by nanoparticles with several compositions that are promoted or already being used as agrichemicals (Au, Ag, CNT, C60, CeO2, ZnO, CuO2, Fe3O4). Here “D” refers to detrimental.
Table 1.2 shows examples of plant-specific detrimental effects of nanoparticles as a result of plant exposure to nanoparticles with several compositions. These range from adverse effects in their physiological, biochemical, and genetic traits. Noble metal nanoparticles, such as Au, induce necrosis in tobacco plants (Sabo-Attwood et al. 2012). Exposure to Ag nanoparticles leads to retarded germination in rice and corn (Thuesombat et al. 2014; Pokhrel and Dubey 2013) and reduction in mitotic index and fragmented chromosomes in onion (Kumari et al. 2009). Carbon-based nanoparticles (CNTs, C60) lead to cellular toxicity in rice, spinach, and onion (Shen et al. 2010; Begum and Fugetsu 2012; Chen et al. 2010), reduction in biomass for zucchini (Stampoulis et al. 2009), and delayed flowering together with decreased yield (Lin et al. 2009). Exposure to TiO2 nanoparticle results in damaged chloroplast and reduced photosynthetic rate in spinach (Lei et al. 2008), stress in cucumber (Servin et al. 2013), inhibited leaf growth, and DNA damage in corn (Asli and Neumann 2009; Castiglione et al. 2011). CeO2 nanoparticle adversely affects the nutrition and genetics of soybean, cucumber, rice, and wheat (Lopez-Moreno et al. 2010a; Hong et al. 2014; Rico et al. 2013, 2014; Zhao et al. 2014). ZnO is genotoxic to onion, garlic, and buckwheat (Kumari et al. 2011; Shaymurat et al. 2012; Lee et al. 2013); affects the seed formation in soybean (Yoon et al. 2014); inhibits plant growth in corn, soybean, rice, and cabbage (Lin and Xing 2007; Lopez-Moreno et al. 2010a; Boonyanitipong et al. 2011; Xiang et al. 2015); and affects chlorophyll in green peas (Mukherjee et al. 2014). CuO is genotoxic to radish and buckwheat (Atha et al. 2012; Lee et al. 2013), produces stress in rice (Shaw and Hossain 2013), and severely reduces root length (77%) and biomass (90%) in zucchini (Stampoulis et al. 2009). Nickel nanoparticles induce stress and damage of mitochondria and cells in tomato (Faisal et al. 2013).
A type of nanoparticle can sometimes have both beneficial and detrimental effects on the same plant. For example, barley exposed to CeO2 nanoparticles (500 mg/kg) led to a more than 300% increase in shoot biomass; however it formed no grain (Rico et al. 2015).
In the following subchapters, we will elaborate on the adverse effects of nanoparticles on plant physiological, biochemical, and genetic traits.
1.5.2 Plant Growth Inhibition
Phytotoxicity related to growth inhibition manifests in reduced biomass; decreased germination and leaf growth; reduced root elongation, root biomass, root tip morphology, and shoot growth; delayed flowering; and decreased yield among others (Tripathi et al. 2017). The adverse biochemical traits involve the generation of reactive oxygen species, lipid peroxidation, decreased rate of transpiration, disturbed mitosis, breakdown of cell wall, reduction in chlorophyll content, and reduced photosynthesis (Tripathi et al. 2017). Toxicity at genetic level involves reduction in mitotic index, sticky and fragmented chromosomes, chromosome aberrations, alteration of genes, damaged DNA structure, and decreased cell viability (Tripathi et al. 2017). Examples of toxic effects of nanoparticle on plants are given in Table 1.2.
Some adverse effects of nanoparticles on plant growth are easily assessed by measuring the germination index, the elongation of roots and shoots, root biomass, root tip morphology, total biomass, and flowering (Deng et al. 2014).
For plants exposed to nanoparticles from soil or hydroponically, an important indicator of toxicity is the shoot and root biomass. While studies use different exposure times and doses, the general conclusion is that phytotoxicity is plant and nanoparticle specific. This toxicity can be due to the release and subsequent accumulation of ions in plant tissue and/or nanoparticle uptake and translocation (Deng et al. 2014). Nanoparticles with various compositions have an adverse effect on seedling roots and shoot elongation, mainly due to the adsorption of nanoparticles into the roots. Among phytotoxic materials to roots and shoots are gold, silver, zinc oxide, copper oxide, alumina, and carbon nanotubes (Begum and Fugetsu 2012; Begum et al. 2012; Burklew et al. 2012; Feichtmeier et al. 2015; Deng et al. 2014; Dimkpa et al. 2013b; Ghodake et al. 2011).
Figure 1.6 shows photographs of plants detrimentally affected by exposure to nanoparticles. Figure 1.6a–h illustrates the trend of decreased shoot and root length in a concentration-dependent manner in tomato and cauliflower exposed to CuO nanoparticles (Singh et al. 2017a), wheat exposed to Ag nanoparticles (Dimkpa et al. 2013b), barley seedlings exposed to Au nanoparticles (Feichtmeier et al. 2015), red spinach exposed to MWCNTs (Begum and Fugetsu 2012), rice exposed to MWCNTs (Begum et al. 2012), and rice exposed to CuO nanoparticles (Shaw and Hossain 2013). Figure 1.6i–j shows various aberrant features observed in onion after exposure to titanium dioxide nanoparticles, such as chromosome break and nuclear blebbing (Pakrashi et al. 2014).
Photographs showing detrimental effects following the exposure to different concentrations of various nanoparticles of selected crops. (a) Tomato exposed to CuO nanoparticles, (b) cauliflower exposed to CuO nanoparticles. Images (a–b) are reprinted from Singh A. et al., Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. Journal of Biotechnology, 262, 11–27, Copyright (2017), with permission from Elsevier (Singh et al. 2017a). (c) Wheat exposed to Ag nanoparticles. Reprinted with permission from Dimkpa C. O. et al, Silver Nanoparticles Disrupt Wheat (Triticum aestivum L.) Growth in a Sand Matrix. Environmental Science & Technology, 47, 1082–1090. Copyright (2013) American Chemical Society (Dimkpa et al. 2013b). (d) Barley seedlings exposed to Au nanoparticles. Reprinted from Feichtmeier, N. S. et al, Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles. Environmental Science and Pollution Research, vol. 22, 2015, pp. 8549–8558, with permission from Springer (Feichtmeier et al. 2015). (e–f) Red spinach exposed to MWCNTs. Images (e–f) reprinted from Begum P and Fugetsu B, 2012, Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant, Journal of Hazardous Materials, 243, 212–222, Copyright (2012), with permission from Elsevier (Begum and Fugetsu 2012). (g) Rice exposed to MWCNTs. Image reprinted from Begum P. et al., Applied Surface Science, vol. 262, Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage, pp. 120–124, Copyright (2012), with permission from Elsevier (Begum et al. 2012). (h) Rice exposed to CuO nanoparticles. Reprinted from Shaw A. K. & Hossain Z. 2013. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere, 93, 906–91, Copyright (2013), with permission from Elsevier (Shaw and Hossain 2013). (i, j) Various aberrant features observed in Allium cepa after exposure to titanium dioxide nanoparticles: (i) chromosome break, (j) nuclear blebbing. Images (i–j) reprinted from Pakrashi S. et al., 2014. In Vivo Genotoxicity Assessment of Titanium Dioxide Nanoparticles by Allium cepa Root Tip Assay at High Exposure Concentrations. Plos One, 9, 12 (Pakrashi et al. 2014)
It is important to note that nanophytotoxicity is material and species specific. This can be seen in a study comparing the toxic effects of several rare-earth oxide nanoparticles (CeO2, La2O3, Gd2O3, Yb2O3) on several crops (cabbage, cucumber, lettuce, radish, rape, tomato, wheat) (Ma et al. 2010). For example, only the root elongation of lettuce is affected by CeO2, while all remaining (La2O3, Gd2O3, Yb2O3) nanoparticles lead to a large reduction in root elongations for all studied plants.
Silver nanoparticles are known for their antibacterial, antifungal activity and are consequently used extensively as agrichemicals. As a result, the existence of Ag nanoparticles in soil can have an effect upon soil microbiota (such as nitrogen-fixing bacteria) that will in turn affect the physicochemical characteristics of soil and plants (Anjum et al. 2013). Silver nanoparticles can be internalized and accumulate in edible plants and consequently enter the food chain. Some plants exposed to silver nanoparticles show reduced germination, biomass, transpiration, shoot and root length, and cytotoxicity involving modifications in gene expression, oxidative stress, decreased mitosis, chromosomal abnormalities, and cell death (Anjum et al. 2013; Arruda et al. 2015; Thuesombat et al. 2014; Pokhrel and Dubey 2013; Kumari et al. 2009). Silver nanoparticles have a concentration-dependent growth inhibition effect upon mung bean and sorghum (Lee et al. 2012).
MWCNTs are the type of nanoparticle that shows the entire array of effects on plants, ranging from beneficial to detrimental. They are promoted for their use in aiding germination of some seeds (Khodakovskaya et al. 2011). CNTs are phytotoxic to red spinach, lettuce, and cucumber, showing decreased roots and shoot lengths, while no negative effects were observed for chili and soybeans (Begum et al. 2014). They accumulate in onion plants and are cytotoxic and genotoxic, altering cellular morphology, affecting membrane integrity and mitochondrial function, resulting in DNA damage and chromosome aberration (Ghosh et al. 2015).
1.5.3 Nutrient Depletion in Nanoparticle-Contaminated Plants
The intake of plants and fruits is important for the mineral and nutrients they contain. Plant exposure to nanoparticles results in modified content of nutrients, fruit flavor, antioxidant content, and growth performance (Antisari et al. 2015; Deng et al. 2014; Petersen et al. 2014; Rico et al. 2011; Zhao et al. 2014).
Therefore, the use of nanoparticles as agrichemicals raises some serious concerns.
Cerium oxide nanoparticles affect the amounts of nutrients for important crops, such as rice, corn, soybean, tomato, and cucumber (Antisari et al. 2015; Peralta-Videa et al. 2014; Rico et al. 2013; Zhao et al. 2014, 2015). Rice exposed to ceria nanoparticles yields grains with compromised nutritional value, showing smaller amounts of iron, glutelin, lauric and valeric acids, starch, and some antioxidants (Rico et al. 2013). Cucumber fruits of plants exposed to nanoceria have an altered Mo micronutrient, sugar, and phenolic content in addition to protein fractionation (Zhao et al. 2014). Corn exposed to nanoceria has 38% reduced yield and less calcium translocation from the cob to the kernels compared to control (Zhao et al. 2015).
Zinc oxide nanoparticles have a profound effect on corn plants, accumulating in corncobs, alter its nutrient contents, and reduce photosynthesis and relative chlorophyll content, resulting in a reduced yield by 49% (Zhao et al. 2015).
The fruits of tomato plants exposed to CeO2, Fe3O4, SnO2, TiO2, Ag, Co, and Ni nanoparticles exhibit a depletion of Mg, P, and S (Antisari et al. 2015).
1.5.4 Nanoparticle-Induced Genotoxicity
Due to their small size, nanoparticles are able to enter cells and elicit a genetic response from plants. Nanoparticles with many compositions (CuO, Ag, ZnO, CeO2, TiO2, carbon nanotubes, etc.) induce genotoxicity in various plants (radish, onion, soybean, buckwheat, fava beans, ryegrass, tobacco, etc.) (Atha et al. 2012; Chichiricco and Poma 2015; Ghosh et al. 2015; Kumari et al. 2009, 2011; Lee et al. 2013; Lopez-Moreno et al. 2010a; Pakrashi et al. 2014; Patlolla et al. 2012; Shaymurat et al. 2012; Burklew et al. 2012). The plants with inhibited roots displayed errors in cell division, chromosomal abnormalities, microRNA deregulation, and DNA damage.
1.5.5 Nanoparticle Transgenerational Effects in Plants
As shown previously, nanoparticles can accumulate in plants within various tissues, such as leafs, roots, fruits, and seeds. Nanoparticle uptake in seeds has been shown to cause transgenerational effects in some plants (Lin et al. 2009; Wang et al. 2013b). These studies raise the questions if other nanoparticles might cause long-term multigenerational effects in plants.
Nanoparticles can be transmitted to plant progenies through seeds, in the absence of external nanoparticle exposure. For example, C70 can be found in second-generation rice plants (Lin et al. 2009). Seeds harvested from plants exposed to C70 were planted in a media free of C70 nanoparticles. The germinated rice plants (second-generation plants) were found to contain C70 black aggregates near the stem’s vascular system and in leaf tissue.
Some authors found that ceria nanoparticles might have a beneficial effect on some plants. Exposure to ceria nanoparticles had a minor beneficial effect on first-generation seedlings and, however, had a detrimental effect on the growth of second-generation plants (Wang et al. 2013b). Second-generation tomato plants grown from seeds harvested from parent plants exposed to ceria nanoparticles were weaker and had a lower biomass, lower water transpiration, and a higher reactive oxygen species amount (Fig. 1.7).
Effect of nanoparticle used as micronutrients (left) and non-nutrients (right) on crop disease. Reproduced from Journal of Nanoparticle Research, A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield, Servin A. et al, vol. 17, 2015. Copyright (2015) with permission of Springer (Servin et al. 2015)
1.6 Beneficial Effects of Nanoparticles in Plants
Nanoparticles can influence plant phenotype, some plants will be negatively affected, others will show beneficial effects, while others will show no response.
Some nanoparticles are used for their beneficial effects as crop enhancers or/and inhibiting plant pathogens. Several reviews report positive effects of some nanoparticles as crop enhancers in selected plant species, such as enhanced seed germination, crop yield, improved photosynthesis, increased resistance against stress, and suppressed plant disease (Rico et al. 2011; Du et al. 2017; Siddiqi and Husen 2017; Rizwan et al. 2017; Ruttkay-Nedecky et al. 2017; Gardea-Torresdey et al. 2014; Zuverza-Mena et al. 2017; Wang et al. 2016; Khan et al. 2017; Arruda et al. 2015). The composition of nanoparticles that show beneficial effects includes Au, Pd, Cu, Si, CeO2, TiO2, Fe2O3, MWCNTs, and fullerol C60(OH)20 (Arruda et al. 2015; Chichiricco and Poma 2015; Siddiqui and Al-Whaibi 2014; Zheng et al. 2005).
While some authors chose to focus mainly on the positive aspects of nanoparticle in plants, promoting their use as plant growth enhancers (Servin et al. 2015; Khodakovskaya et al. 2012, 2013; Lahiani et al. 2013; Lyu et al. 2017), it is difficult to predict what is the effect of nanoparticle progressive accumulation in soil. Here we must mention hormesis, which describes a beneficial effect for a low-dose agent, while a higher dose is toxic or inhibits growth.
Nanoparticles with selected compositions have been shown to inhibit plant pathogens as a result of their antimicrobial properties (Servin et al. 2015). Examples of pathogen inhibitor nanoparticles are Ag (Lamsal et al. 2011b; Gajbhiye et al. 2009), Cu (Giannousi et al. 2013; Kanhed et al. 2014), ZnO (Wani and Shah 2012; He et al. 2011), MgO (Wani and Shah 2012), and TiO2 (Cui et al. 2009).
MWCNTs
Several authors describe the positive effects of MWCNTs related to increased germination of tomato, barley, soybean, and corn and increased growth of tobacco cells (Khodakovskaya et al. 2009; 2011, 2012, 2013; Lahiani et al. 2013; Alexandru et al. 2012).
MWCNT exposure leads to enhanced germination and growth of tomato seedlings (Khodakovskaya et al. 2009, 2011) and enhanced flowering and fruit yield for tomato plants (Khodakovskaya et al. 2013). Raman spectroscopy detected MWCNT nanoparticles in the fruits of tomato plants (Khodakovskaya et al. 2011), which raises questions about the safety of their consumption. The presence of these nanoparticles in the edible parts of plants together with the increased evidence of their toxicity in humans should not justify their use for increased flowering and fruit yield.
MWCNTs penetrate tomato seeds and increase their germination and growth rates compared to control (Khodakovskaya et al. 2009). The exposure of tomato plants to MWCNTs results in significant changes in total gene expression in leaves and roots (Khodakovskaya et al. 2011). The enhanced germination and growth of tomato plants are due to the upregulation of the stress-related genes, such as those induced by pathogens and water-channel LeAqp2 gene (Khodakovskaya et al. 2011). There are a large number of altered genes with various functions in the leaves of first-generation plants exposed to MWCNTs: 29 genes involved in cellular responses, 39 genes in stress response, 14 genes in transport, 13 genes in signal transduction, and 25 genes in metabolic processes. Similarly, there are a large number of genes in the roots: 19 genes involved in stress responses, 9 genes in cellular processes, 6 genes in transport, and 22 genes related to catabolic, metabolic, and biosynthetic processes.
The biochemical mechanism of MWCNT hormesis in plants is not yet understood. Cell culture experiments indicated that small concentrations (5 μg/ml) of MWCNTs lead to an augmented growth of tobacco cell, while higher concentrations (100–500 μg/ml) showed the opposite effect (inhibited cell growth) (Khodakovskaya et al. 2012).
Some authors studied the effects of CNTs on crops (such as corn, barley, soybean) and observed that they lead to accelerated seed germination also concluding that they have no negative effect on the biological endpoints showing the development of plants grown from the exposed seeds (Lahiani et al. 2013).
One must emphasize that while beneficial effects of nanoparticles were reported for first-generation plants, the nanoparticles accumulated in the seeds might be detrimental to second-generation plants (Wang et al. 2013b).
One must remind that the beneficial effects of carbon nanotubes are plant species specific. While CNTs might be beneficial for tomato plants, they inhibit the growth of red spinach, lettuce, cucumber, and rice in a dose-dependent manner (Begum and Fugetsu 2012; Begum et al. 2012, 2014).
In addition, taking into account their similarities to asbestos, CNTs are believed to have comparable toxicity to humans (Stella 2011).
C60 Fullerenes
The use of C60-based nanoparticles in the growth of bitter melon was reported to increase biomass, water content, and fruit yield (Kole et al. 2013). The authors show that C60 nanoparticles biodistribute to petioles, leaves, flowers, and fruits. Again, the accumulation of nanoparticles in the edible part poses risks for human exposure to this nanoparticle.
Au, Pd, Si, and Cu Nanoparticles
Low concentration of nanoparticles made of Au and Pd and higher concentrations of Si and Cu nanoparticles were found to increase the shoot ratio for lettuce after 15 days of incubation (Shah and Belozerova 2009). Nanoparticles with these compositions are shown to be toxic to humans and animals.
Ag
Silver nanoparticles are used in agriculture for their bactericidal effects. Silver nanoparticles in small concentrations (30 μg/ml) accelerated root growth in rice plants while just doubling the concentration inhibited root growth (Mirzajani et al. 2013). Silver nanoparticles were shown to enter cell wall, damaged cells and produce reactive oxygen species.
TiO2
Titania nanoparticles had some beneficial effects on seeds, especially those with low germination (Zheng et al. 2005; Feizi et al. 2013). Some authors believe that the induction of reactive oxygen species by nanoparticles results in subsequent enhancement of stress resistance and facilitation of seed penetration of water and oxygen (Khot et al. 2012).
Spinach exposed to titania nanoparticles shows increased plant dry weight, chlorophyll, and photosynthetic rate (Zheng et al. 2005).
Another example of hormesis is titanium dioxide in fennel plants. Titania nanoparticles were found to increase fennel seed germination, while larger particles lowered by 50% the shoot biomass compared to the control (Feizi et al. 2013). This fact is important as small nanoparticles can aggregate into larger particles, which can in turn become phytotoxic to the same plant species.
It seems that increased root length is an adaptation process of roots clogged with nanoparticles (Asli and Neumann 2009). Titania nanoparticles are shown to accumulate in roots and block pores in hydroponic maize treated with nanoparticles. As a result, the plants have lower water supply, leaf growth, and transpiration rate (Asli and Neumann 2009). In order to survive, the maize plant adapts by forming a larger root system.
Rare Earth
Rare-earth additives are used in fertilizers for their promotion of larger yields, longer rots, darker green foliage, and better fruit color (Yuan et al. 2001).
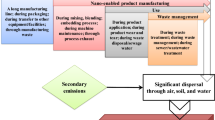
1.7 Nanoparticle Toxicity in Humans and Animals
Unfortunately, many of the nanoparticles that have some agricultural benefits, including those shown to be internalized by crops, have varying degrees of toxicity in humans and laboratory animals. A multitude of reviews discuss the topic of nanoparticle toxicity in humans and animals (Buzea et al. 2007; Ema et al. 2016, 2017; Sohaebuddin et al. 2010; Kendall and Holgate 2012; He et al. 2017; Shah et al. 2015).
While the discipline of nanotoxicology is a fairly new, some older epidemiological studies give a plethora of information on environmental nanoparticle (particulate matter) toxicity on humans. What makes nanoparticles toxic is their size and, as a result, their ability to enter organisms; enter circulatory system; translocate to organs, such as the liver, spleen, kidneys, brain, and heart; enter cells; and go further into organelles (Buzea et al. 2007). They are able to be internalized, depending on their entry and size, within several minutes to several hours following exposure (see Fig. 1.8d) (Nemmar et al. 2002). Once inside cells and organelles, they produce cytotoxicity and genotoxicity. They are associated to inflammation and various diseases, including cancer. In the following we will give examples of such adverse effects of nanoparticles on humans. While our examples are not comprehensive, we expect to be compelling and inform the scientists planning to use nanoparticles in agriculture of the potential adverse effects on humans, including themselves.
High-resolution TEM images showing MWCNTs inside lung cells (a1) and in the bronchoalveolar lavage fluids (a2–a3) of asthmatic children together with MWCNTs from vehicle exhausts (b1) and pollution dust collected near a busy traffic intersection in Paris (b2). N indicates nucleus; blue arrows, lamellar bodies; black arrows, nanospheres; red arrows, MWCNTs. Image (a2) is a magnified view of (a1). Image (b2) is a magnified view of (b1). Reprinted from Kolosnja-Tabi J. et al., Ebiomedicine, vol. 2, Anthropogenic Carbon Nanotubes Found in the Airways of Parisian Children, pp. 1697–1704, Copyright (2015), with permission from Elsevier (Kolosnjaj-Tabi et al. 2015). (c) Pathology of lung with centrilobular emphysema with multiple cavities heavily lined by black carbon deposits. Courtesy of Dr. Edwin P. Ewing, Jr., http://phil.cdc.gov/phil_images/20040517/4/865_lores.jpg. (d) (left) Radioactivity on the bladder and liver compared to lungs versus time of exposure to 99mTc-labeled carbon nanoparticles in humans. (Right) Radioactivity in the body of a human volunteer after 1 h of exposure to 99mTc-labeled carbon nanoparticles. Image reproduced from Nemmar A. et al., Passage of inhaled particles into the blood circulation in humans. Circulation, vol. 105, pp. 411–414. Copyright (2002) American Heart Association, Inc. with permission of Wolters Kluwer Health, Inc. (Nemmar et al. 2002)
1.7.1 Nanoparticle Physicochemical Characteristic-Dependent Toxicity
Nanoparticle toxicity depends on their physical and chemical properties, such as size, aggregation, composition, concentration, shape, porosity, surface area, hydrophobicity, electrical charge, and magnetic properties (Buzea et al. 2007; Podila and Brown 2013; Silva et al. 2014, 2015; Hanley et al. 2009; Chithrani et al. 2006; Naqvi et al. 2010; Schlinkert et al. 2015; Sharma et al. 2014; Li et al. 2015b; Teske and Detweiler 2015). A schematic illustrating this idea is given in Fig. 1.2.
There is no universal law for determining the toxicity of nanoparticles, which cannot be extrapolated from the behavior of the bulk material. Each type of nanoparticles has to be tested in order to assess its toxicity. Nanoparticles of the same material can have different toxicities for different sizes, surface functionalization, or surface charge. Nanoparticles with the same size but made of different materials will also have different toxicities.
Usually nanoparticles with smaller size have higher toxicity than larger ones (Buzea et al. 2007).
Nanoparticles with the same composition but different crystalline form can exhibit different properties and toxicity, such as titanium dioxide in rutile and anatase forms. Rutile titania 200 nm in size induced oxidative DNA damage and other cytotoxic effects in human bronchial epithelial cell, while anatase titania did not (Gurr et al. 2005).
Some nanoparticles are hydrophobic, while others are hydrophilic (Garcia-Ivars et al. 2015). This property can be modulated by coating of nanoparticles of various substances (Podila and Brown 2013). For example, coating with polyethylene glycol (PEG) renders nanoparticles highly hydrophilic (Kettler et al. 2014).
Nanoparticles can have positive, negative, or neutral charge. Nanoparticle surface charge is very important in deciding how a nanoparticle interacts with biological systems (Gatoo et al. 2014; Salatin et al. 2015). Positively charged nanoparticles are attracted to the negatively charged cell membrane and have a higher cellular uptake versus negatively charged or neutral nanoparticles (Kettler et al. 2014). Nanoparticle toxicity depends on whether or not nanoparticles are internalized within cells. For example, cationic gold nanoparticles are toxic, while anionic nanoparticles are nontoxic (Goodman et al. 2004). Studies also show that nanoparticles with large surface charge, either negative or positive, show an increased receptor-mediated endocytic uptake of nanoparticle compared to neutral nanoparticles (Kettler et al. 2014).
1.7.2 Nanoparticle Internalization and Biodistribution
1.7.2.1 Inhalation, Ingestion, and Dermal Exposure
Due to their minute size, nanoparticles can be inhaled and ingested or penetrate through the skin. From the respiratory and gastrointestinal systems, they can rapidly enter blood and lymphatic system (Landsiedel et al. 2012).
Numerous studies indicate that inhaled nanoparticles accumulate in lungs, and some nanoparticles, depending on their size and other physicochemical properties, can reach the alveoli, translocate to organs, and become systemic. They can be found in the circulatory system and lymphatic system and in the brain, heart, thyroid, liver, spleen, colon, bones, and kidney (Anderson et al. 2015; Bakand et al. 2012; Bruinink et al. 2015; Buzea et al. 2007; Davidson et al. 2015; Fischer and Chan 2007; Geiser and Kreyling 2010; Gosens et al. 2014, 2015; Khlebtsov and Dykman 2011, Johnston et al. 2010; Lin et al. 2015; Landsiedel et al. 2012; Nakane 2012; Theodorou et al. 2014).
From lungs, nanoparticles can go further to the circulatory system. Nanoparticles with various compositions have been collected from the blood of patients with various diseases (Gatti and Montanari 2006). In the circulatory system, they interact with plasma and form a protein corona that will determine their toxicity and translocation. Next, nanoparticles move to and accumulate in various organs and tissues: the liver, spleen, pancreas, heart, kidneys, brain, lymph nodes, bone marrow, etc. (Landsiedel et al. 2012; Sonavane et al. 2008). The smaller the nanoparticles, the greater their accumulation in tissues (Sonavane et al. 2008).
Ingested nanoparticles that enter the gastrointestinal tract are partly excreted in feces, and some are absorbed and become systemically available (Hillyer and Albrecht 2001).
The site of accumulation in the body depends on the composition and surface functionalization of the nanoparticles. Metallic nanoparticles usually localize in the liver, spleen, and lymph node (Lin et al. 2015; Johnston et al. 2010).
Nanoparticles are able to cross the placental barrier, reach fetus, and have adverse effects on pregnancy and fetuses, as shown by in vivo and ex vivo studies on animals (Kulvietis et al. 2011; Wick et al. 2010; Semmler-Behnke et al. 2014; Snyder et al. 2015; Melnik et al. 2013; Yamashita et al. 2011).
1.7.2.2 Nanoparticle Persistence and Disease
Nanoparticles can persist in the body for longer than 6 months (Lin et al. 2015). Long-term residence of nanoparticles in the body will produce tissue injuries and inflammation which is the precursor to cancer and other diseases. The residence of metallic nanoparticles within a tissue favors tumorigenesis (Sighinolfi et al. 2016). Indeed, recent studies indicate that nanoparticles accumulate in tissue of patients with various diseases, such as deep-vein thrombosis, pulmonary embolism, colon cancer, prostate cancer, stroke, asthma, emphysema, lung cancer, Crohn’s disease, ulcerative colitis, liver necrosis, renal failure, and Hodgkin’s lymphoma (Gatti 2004; Gatti and Montanari 2006; Gatti and Rivasi 2002; Roncati et al. 2015a, b; Ballestri et al. 2001; Iannitti et al. 2010).
Figure 1.8a–c shows nanoparticles persistent in the lungs that are likely to be the cause of the disease observed in the respective subjects. Figure 1.8a shows images of MWCNTs inside lung cells and in the bronchoalveolar lavage fluids of asthmatic children living in Paris (Kolosnjaj-Tabi et al. 2015). The carbon nanotubes found inside the lungs of asthmatic children are very similar in morphology and shape to those from Fig. 1.8b, which shows MWCNTs from vehicle exhausts and from pollution dust collected near a busy traffic intersection in Paris. Figure 1.8c shows black carbon deposits inside the lung of a patient diagnosed with emphysema, which are likely to be the cause of his emphysema. Figure 1.8d illustrates very fast internalization of inhaled 99mTc-labeled carbon nanoparticles in a human volunteer, nanoparticles being detected within 5–60 min of exposure (Nemmar et al. 2002).
In some of the following paragraphs, we will discuss in more detail various diseases associated to nanoparticle exposure.
1.7.2.3 Nanoparticle Size-Dependent Accumulation
Experiments on mice with orally ingested gold nanoparticles having size between 4 and 28 nm show translocation to the blood, brain, lung, heart, kidney, spleen, liver, small intestine, and stomach, while nanoparticles with larger size (58 nm) were not detected in most studied tissue (Hillyer and Albrecht 2001).
Inhaled nanoparticles smaller than 50–100 nm can travel to and accumulate in the brain, through olfactory nerves and blood-brain barrier (Buzea et al. 2007; Lin et al. 2015; Sonavane et al. 2008).
The maximum size of nanoparticles that can enter and be cleared from the body is between 200 and 250 nm (Bruinink et al. 2015). If nanoparticles existent in tissues aggregate in complexes with larger size, their clearance becomes less likely. Consequently, long-term exposure to small amounts of nanoparticles can lead to adverse health effects due to their accumulation without clearance.
Experiments on human volunteers show fast internalization and long-term persistence of gold nanoparticles in humans (Miller et al. 2017). Within 15 min following inhalation, gold nanoparticles are detected in the blood of human volunteers. They can persist 3 months following inhalation exposure. Smaller nanoparticles (5 nm diameter) are more persistent than the larger ones (30 nm).
1.7.2.4 Nanoparticle Corona
When in contact with organic matter, nanoparticles will interact dynamically with biomolecules via electrostatic and van der Waals forces (Kumar et al. 2014). This interaction will result in acquiring a corona formed of biomolecules, which will determine their subsequent interaction with cells and tissue/organ accumulation (Grillo et al. 2015; Khlebtsov and Dykman 2011). This corona will dictate the degree of nanoparticle toxicity in addition to the intrinsic properties of the nanoparticle (Foroozandeh and Aziz 2015). There are cases when nanoparticle corona might be more important than the intrinsic physical properties of a nanoparticle in deciding its toxicity (Walkey et al. 2014). In general, nanoparticle physicochemical properties determine to some extent the composition of its corona together with the composition of the biological environment (Kreyling et al. 2014). It is believed that the existence of a corona, with its overall negative charge, will diminish nanoparticle toxicity due to a reduced interaction with the negatively charged cell wall (Docter et al. 2015). On the other side, nanoparticles with a positive charge (without a corona), being electrostatically attracted to the negatively charged cell membrane, will have an increased cellular uptake.
1.7.2.5 Nanoparticle Uptake by Cells
Depending on their physicochemical properties, nanoparticles can be internalized by cells and locate within various organelles, cytoplasm, mitochondria, nucleus, lysosomes, endoplasmic reticulum, etc. (Singh et al. 2010; Huk et al. 2015; Sathuluri et al. 2011). The cellular uptake of nanoparticles is also cell specific and depends on the experimental conditions (Kettler et al. 2014). Cell internalization of nanoparticles usually results in cytotoxicity. Inside the cells, nanoparticles have been observed to affect cellular processes; produce reactive oxygen species, DNA damage, and epigenetic changes; and even cause cell death (Gatoo et al. 2014; Karlsson et al. 2008; Stoccoro et al. 2013). Nanoparticles can be genotoxic simply by their direct interaction with the genetic material or due to generating reacting oxygen species (Tortiglione 2014).
1.7.3 Nanoparticle Association to Respiratory Diseases
Epidemiological studies indicate that exposure to particulate pollution is associated with different respiratory diseases, such as chronic obstructive pulmonary disease (COPD), respiratory infections, lung cancer, and asthma (Mannucci et al. 2015; Rückerl et al. 2011). For each 10 μg/m3 increase in particulate pollution, there is a 2.5% increase in hospital admissions of patients with COPD (Mannucci et al. 2015). Children exposed to air pollution have more respiratory tract infections and asthma episodes. Figure 1.8 shows nanoparticles accumulated in the lung of subjects with asthma and emphysema.
Nanoparticles with composition of iron, manganese, and chromium were found in the lungs of welders suffering from various respiratory diseases (Andujar et al. 2014). Welders that are exposed to welding fumes for a long time have higher incidence of high blood pressure (Li et al. 2015a; Xu et al. 2017).
1.7.4 Nanoparticle Association to Cardiovascular Diseases
In vivo, in vitro, and epidemiological studies show that exposure to nanoparticles of various compositions is associated to cardiovascular diseases (Franklin et al. 2015; Yu et al. 2016; Savi et al. 2014; Cosselman et al. 2015; Rückerl et al. 2011). Epidemiological studies show a relationship between particulate pollution and a gamut of cardiovascular diseases. Among them are blood clot formation, pulmonary embolism, increased blood pressure, atherosclerosis, arrhythmia, ischemic heart disease, myocardial infarction, and heart failure; stroke and stroke mortality correlate to particulate pollution in a dose-dependent manner (Mannucci et al. 2015; Cosselman et al. 2015; Shah et al. 2015; Yu et al. 2016).
The composition of nanoparticles that are associated to adverse cardiovascular effects includes, but is not limited to, titanium dioxide, silver, silicon, silica, carbon black, carbon nanotubes, zinc oxide, etc. (Many of these nanoparticles are promoted for their use in agriculture due to some benefits on selected plants.)
Nanoparticles of metal oxide produce clotting irrespective of their charge (Steuer et al. 2014). Studies show that environmental nanoparticles are associated with an increased risk of thrombotic complications that lead to an increased and worse prognosis of cardiovascular events (Ilinskaya and Dobrovolskaia 2013).
Nanoparticles are found in biopsies and various human specimens of diseased tissue or collected from the blood of patients with various diseases (Gatti 2004; Gatti and Montanari 2006; Gatti and Rivasi 2002; Bitounis et al. 2016; Rinaldo et al. 2015; Ballestri et al. 2001). Nanoparticles tend to accumulate at the site of vascular lesions (Miller et al. 2017).
Figure 1.9a shows microscopy images of nanoparticles found in blood clots collected from diseased patients by using a vena cava filter (Gatti and Montanari 2006). With the help of environmental scanning electron microscopy (ESEM) and energy-dispersive spectroscopy (EDS), nanoparticles and their composition are identified in thrombi and fibrotic tissue taken from patients at risk of developing deep-vein thrombosis or to prevent pulmonary embolisms in potentially relapsing patients (Gatti et al. 2005; Gatti and Montanari 2006). The composition of micro- and nanoparticles collected from the tissue of the patients had various compositions, such as Fe, Cr, Cu, W, and Al. It is likely that the particles within the thrombi are the sole cause for thrombosis (Gatti et al. 2005).
Scanning electron microscope (SEM) images of nanoparticles observed in the blood, colon, liver, and kidney biopsies of patients with various diseases together with their EDS spectra indicating their compositions. (A) Nanoparticles in thrombotic tissues collected from diseased patients by using a vena cava filter; (a1) SEM image shows thrombus containing nanoparticle with the composition of Ag, S, and O; (a2) SEM image of red blood cells with a cluster of nanoparticles having the composition: Ti, O, and. Images (a1) and (a2) are reprinted from Gatti A. M. & Montanari S., Journal of Biomedical Materials Research Part B-Applied Biomaterials 77B (2006) 307, with permission from John Wiley & Sons, Inc. (Gatti and Montanari 2006). (B) SEM images showing nanoparticles with different compositions in the colon of patients with various diseases. Upper side shows the electron microscopy image and bottom the EDS spectrum indicating the composition of the nanoparticles; (b1) nanoparticles of calcium and silicon in the colon of a patient with adenocarcinoma; (b2) nanoparticles of stainless steel (Fe, Cr, Ni) in the colon of a patient with adenocarcinoma; (b3) nanoparticles of Ag in the colon of a patient with colon cancer. Images (b1), (b2), and (b3) are reprinted from Biomaterials, vol. 25, Gatti A. M., Biocompatibility of micro- and nano-particles in the colon. Part II., pp. 385–392, Copyright (2004), with permission from Elsevier (Gatti 2004). (C) SEM images of the liver and kidneys from diseased patients together with inset showing the EDS spectrum indicating the composition of nanoparticles; (c1) liver section with giant-cell granuloma showing nanoparticles with composition of Si, Na, Al, Mg, Ca, O, and C; (c2) kidney granuloma with ceramic nanoparticles; (c3) kidney granuloma with an alumina particle. Images (c1), (c2), and (c3) are reprinted from Biomaterials, vol. 23, Gatti A. M. & Rivasi F., Biocompatibility of micro- and nanoparticles. Part I: in liver and kidney, pp. 2381–2387, Copyright (2002), with permission from Elsevier (Gatti and Rivasi 2002)
Nanoparticles from automobile exhaust pollution lead to adverse cardiovascular effects which can occur as fast as within a few hours in the case of acute exposure, or within a few years for chronic exposure (Donaldson et al. 2013). Living near highway is associated with increased risk of high blood pressure (Chung et al. 2015).
Each 10 μg/m3 increased in particulate pollution is associated with a 21% increase in fatal and nonfatal coronary artery disease according to an epidemiological study in more than 65,000 postmenopausal US women (Mannucci et al. 2015). An increase in the levels of particulate matter of 10 μg/m3 results in a 35–85% increase in nonfatal and fatal strokes for population suffering a long-term exposure to particulate pollution (Mannucci et al. 2015).
Particulate pollution is also associated to higher mortality due to cardiovascular and respiratory events (Cohen et al. 2017; Chen et al. 2012; Brook 2008). The segment of population exposed to particulate pollution has a lower life expectancy, the life span being reduced by several months to several years (Brook 2008).
High concentration of particulate pollution is associated with increased hospital admission, stroke, acute myocardial infarction, and mortality (Shah et al. 2015). Even small increases in particulate pollution were associated with a large (19%) increase for developing cerebrovascular disease (ischemic and hemorrhagic) (Shah et al. 2015).
1.7.5 Nanoparticles in the Central Nervous System
Increasing evidence show that nanoparticles can reach and accumulate in the brain and are associated to neurotoxicity (Cupaioli et al. 2014; Buzea et al. 2007; Song et al. 2015; Hillyer and Albrecht 2001; Wang et al. 2017; Heusinkveld et al. 2016; Maher et al. 2016). In vitro, in vivo, and epidemiological studies relate nanoparticle exposure to neuro-inflammation and neurodegenerative disease, nanoparticles being involved in oxidative stress, inflammation, and impaired activity of cellular organelles. It is believed that the accumulation of nanoparticles in the brain may accelerate the appearance of neurodegenerative diseases (Wang et al. 2017).
Animal Experiments
In vivo experiments show that titania nanoparticle-exposed rodents suffer impaired ability of recognition, spatial memory, and learning (Song et al. 2015).
Occupational Exposure
Manganese nanoparticles generated during welding and mining operations are associated with increased risk of neurological diseases in miners and welders (Buzea et al. 2007). For example, some welders develop Parkinson’s disease in their mid-1940s, while the general population is affected by this disease at around 60 years of age.
Environmental Nanoparticle Pollution
Environmental nanoparticles are linked to neurodegenerative disease, such as Alzheimer’s disease, Parkinson’s disease, and dementia (Calderon-Garciduenas et al. 2016a, b; Gonzalez-Maciel et al. 2017; Chin-Chan et al. 2015).
A recent study showed that pollution nanoparticles are able to translocate to the brain of adults and children living in a polluted environment, can enter cells and organelles, and produce neurotoxicity and cellular damage (Gonzalez-Maciel et al. 2017). Spherical incomplete combustion nanoparticles (29 nm size) have been observed in the neurons, endothelium, nasal, and olfactory epithelium of Mexico City residents that suffered accidental deaths. They were found at sites with abnormal mitochondria, vascular damage in the prefrontal white matter, and other disease pathologies. Samples from control residents living in less polluted environment had intact mitochondria usually with no nanoparticles, compared to those from Mexico City residents that had numerous abnormal mitochondria containing nanoparticles (Gonzalez-Maciel et al. 2017).
Children and adults residing in polluted environments show neuropathological traits of neurodegenerative disease, such as amyloid-beta diffuse plaques and tau hyper-phosphorylation with pre-tangle disease (Calderon-Garciduenas et al. 2016a). Nanoparticles are present inside cells and organelles with abnormal pathologies of adults and children exposed to high concentrations of pollutant nanoparticles (residents of Mexico City) (Calderon-Garciduenas et al. 2016b; Gonzalez-Maciel et al. 2017).
A population-based cohort study of all Ontario adults between 2001 and 2012 found that living closer than 300 m from heavy traffic was associated with a higher incidence of dementia as opposed to those living further away than 300 m (Chen et al. 2017). In addition, dementia involved predominantly urban residents versus rural residents, with urban environments being known to have a higher concentration of particulate matter pollutants than rural ones.
Proof of nanoparticle internalization by human brain tissue is shown in Figs. 1.10a–b). Figure 1.10a shows nanoparticles of iron oxide, silica, and titania inside human cerebral endothelial cells (HCEC) (Kenzaoui et al. 2012).
Nanoparticles inside human brain cells. (A) Uptake of nanoparticles with various compositions by human cerebral endothelial cells (HCEC); (a1) 8 nm iron oxide, (a2) 25 nm silica, and (a3) 21 nm titanium dioxide nanoparticles. Reproduced from reference (Kenzaoui et al. 2012), courtesy of Portland Press. (B) Nanoparticles in the prefrontal white matter of children living in Mexico City and exposed to high concentrations of nanoparticulate pollution. (b1) Nanoparticles in a red blood cell (RBC), an endothelial cell mitochondria (M), and the basement membrane (arrowheads); (b2) one 30 nm diameter nanoparticle inside a mitochondria without cristae in a poorly preserved unmyelinated (short arrow); (b3) a nanoparticle (short arrow) inside a degenerating myelinated axon with remnants of myelin (arrowheads). Reprinted from Environmental Research, vol. 146, Calderon-Garciduenas L. et al., Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease, pp. 404–417, Copyright (2016), with permission from Elsevier (Calderon-Garciduenas et al. 2016b). (c) Titanium dioxide nanoparticles in the rat ventricular myocardium after tracheal instillation. Nanoparticles are located in longitudinally oriented cardiomyocytes and in the wall of a vascular structure. Reprinted from (Savi et al. 2014) courtesy of Particle and Fibre Toxicology
Figure 1.10b shows nanoparticles in the prefrontal white matter of children living in Mexico City that were exposed to high concentrations of particulate pollution. Nanoparticles were internalized inside (b1) a red blood cell (RBC), (b2) mitochondria, (b3) and a degenerating myelinated axon (Calderon-Garciduenas et al. 2016b).
1.7.6 Nanoparticle Toxicity Following Maternal Exposure
Nanoparticles with different compositions and sizes can be transmitted from mother to offspring through the placental barrier or through milk (Muoth et al. 2016; Ema et al. 2016, 2017; Kulvietis et al. 2011; Melnik et al. 2013; Semmler-Behnke et al. 2014; Snyder et al. 2015; Wick et al. 2010; Yamashita et al. 2011). Rats exposed to titanium dioxide nanoparticle during gestation result in negative cardiovascular effects in progenies that last into adulthood (Hathaway et al. 2017). The potential mechanism of impaired functionality of the heart is believed to be related to mitochondrial dysfunction.
1.7.7 Nanoparticles in the Liver, Kidneys, and Other Organs
Systemically available nanoparticles larger than 6 nm locate mainly in the liver and spleen, and other reticular connective tissues (Lu and Gu 2017). Spleen localization is likely for nanoparticles with a diameter of 200–500 nm, comparable to the inter-endothelial slit. Liver fenestration size of ~100 nm allows accumulation of nanoparticles with smaller and comparable sizes, while the urinary system will eliminate those smaller than ~6 nm (Lu and Gu 2017; Landsiedel et al. 2012).
The long-term retention of nanoparticles in organs can be cytotoxic (Wang et al. 2013a). For example, 20 nm gold nanoparticles accumulate in the liver of rats and change the expression of gene expression involved in detoxification, lipid metabolism, and the cell cycle (Kermanizadeh et al. 2014). Silver nanoparticles with sizes of 20 nm can be found in the cytoplasm and nuclei of liver cells and upregulate pro-inflammatory genes (Kermanizadeh et al. 2014). Nanoparticles of copper and zinc oxide produce renal damage in mice (Wang et al. 2013a).
Nanoparticles of different materials have been found in the liver and kidneys of humans with different diseases (Gatti and Rivasi 2002). Some examples are illustrated in Fig. 1.9b–c where one can see nanoparticles in diseased tissue of the liver and kidneys (Gatti and Rivasi 2002).
Nanoparticles can be found in heart tissue as well. Titanium dioxide nanoparticles are internalized by heart cells following tracheal instillation of nanoparticles in rats (Savi et al. 2014). Figure 1.10c shows nanoparticles in the rat ventricular myocardium located in longitudinally oriented cardiomyocytes and in the wall of a vascular structure.
Nanoparticle exposure is also associated to diabetes, exacerbation of allergic diseases, and immune system disruption (Mannucci et al. 2015; Ilinskaya and Dobrovolskaia 2014).
A recent meta-analysis study found a positive association between particulate pollution and type 2 diabetes mellitus (He et al. 2017). In individuals suffering long-term exposure to particulate matter, for each 10 μg/m3 increase in particulate concentration PM2.5, the risk of type 2 diabetes mellitus increases by 25% (He et al. 2017).
Nanoparticles with different compositions (Co, ZnO, TiO2) are toxic to immune cells or immune system (Bregoli et al. 2009; Hanley et al. 2009; Andersson-Willman et al. 2012; Moon et al. 2011). When the immune system is deregulated, it can trigger the onset of cancer and autoimmune diseases (Ilinskaya and Dobrovolskaia 2014).
1.7.8 Toxicity of Nanoparticles with Various Compositions
In the following we will present information on the toxicity of nanoparticles with different compositions in humans and animals. Many of these nanoparticles are suggested to be beneficial agrichemicals.
1.7.8.1 Toxicity of Gold Nanoparticles
One might think that, just because bulk gold is chemically inert and biocompatible, gold nanoparticles should react very little and be biocompatible. Indeed, some gold nanoparticles can be chemically inert and have little or no toxicity depending on their size and surface functionalization. However, there is experimental evidence that indicates gold nanoparticles are toxic, the toxicity degree depending on their size and functionalization.
Gold Nanoparticle Magnetism
The toxicity of gold nanoparticles might be related to the fact that when sufficiently small, gold in nanoform develops magnetic moments. Several materials at the nanoscale develop magnetic moments, while their bulk counterparts do not show magnetism (Pacheco and Buzea 2018). Gold in bulk form is diamagnetic—an external magnetic field will have a very weak effect on it; after the removal of the field, the material will not show a magnetic moment. Decreasing the size of gold particles below 3 nm, they become ferromagnetic (Hori et al. 1999, 2004; Yamamoto et al. 2004; Maitra et al. 2011; Greget et al. 2012; Nealon et al. 2012). A similar phenomenon happens to platinum (Sakamoto et al. 2011) and palladium (Hori et al. 1999), these materials becoming magnetic in nanoform. Hence, gold nanoparticles smaller than 3 nm become magnetic, and their aggregation and chemical reactivity will differ from that of larger nanoparticles.
The magnetic behavior of nanoparticles can be modified by coating with various ligands (Krishna et al. 2014; Crespo et al. 2013). Gold nanoparticles of selected sizes can be made diamagnetic, paramagnetic, or ferromagnetic, just by changing surface ligand types (Krishna et al. 2014). In addition, gold surface functionalization can modify their electrical charge rendering them positively or negatively charged (Gerber et al. 2013; Cheng et al. 2013). The electromagnetic behavior of nanoparticles will influence their translocation and accumulation and hence their toxicity.
Accumulation
Experiments demonstrated that intravenously administered gold nanoparticles accumulate in the liver, spleen, kidney, lung, brain, heart, thymus, skin, and testis (Johnston et al. 2010). For example, 8.5% of radiolabeled (1.4 nm size) gold nanoparticles are found in secondary targets, including the blood and liver of rats after 24 h of exposure (Semmler-Behnke et al. 2008). Gold nanoparticles administered via intravenous injection in pregnant rodents were detected in fetal tissue as soon as 2 h after exposure (Lin et al. 2015).
Mice injected intraperitoneally with gold nanoparticles with sizes 8–37 nm showed fatigue, loss of appetite, fur color changes, weight loss, camel-like back, and crooked spine; most of the mice died within 21 days (Chen et al. 2009). Gold nanoparticles with small size translocate from maternal blood into fetus as showed by experiments on rats (Semmler-Behnke et al. 2014).
Cytotoxicity
The cytotoxicity to gold nanoparticles is cell specific and depends on their surface coating (Cheng et al. 2013; Schlinkert et al. 2015). Gold nanoparticles were found to be internalized by cells and, as a function of their surface functionalization, may locate in endosomes/lysosomes, mitochondria (Cheng et al. 2013), vacuoles (Khlebtsov and Dykman 2011), and nuclei (Ojea-Jimenez et al. 2012). In vivo cytotoxicity studies showed the presence of gold nanoparticles inside liver Kupffer cells and spleen macrophages accompanied by acute inflammation and apoptosis in the liver (Cho et al. 2009). Analysis of liver and spleen samples of rats exposed to gold nanoparticle via injection indicate gene expression changes in genes pertaining to detoxification, lipid metabolism, the cell cycle, defense response, and circadian rhythm (Balasubramanian et al. 2010).
Many experimental results suggest that in small enough concentrations, gold nanoparticles with sizes down to 3–5 nm are not toxic; however, smaller nanoparticles may be cytotoxic due to their irreversible binding to biomolecules such as DNA (Khlebtsov and Dykman 2011). This fact could be explained by the newly acquired magnetic behavior of very small gold nanoparticles (Pacheco and Buzea 2018). Some in vitro genotoxicity studies indicate that gold nanoparticles may induce DNA strand break and chromosomal damage via oxidative stress in some mammalian cells (Li et al. 2011; Hadrup et al. 2015).
Charge-Dependent Cytotoxicity
Cytotoxicity studies of gold with different surface properties on Cos-1 cells (kidney fibroblasts from the African monkey) indicate that cationic nanoparticles are several times more toxic than anionic ones (Khlebtsov and Dykman 2011). Study on other four cell lines (HeLa, Sk-Mel-28, L929, J774A1) shows that 1.4 nm nanoparticles cause cell necrosis 12 h after exposure, while 1.2 nm gold nanoparticles caused apoptosis (Khlebtsov and Dykman 2011).
1.7.8.2 Toxicity of Silver Nanoparticles
Silver nanoparticles are promoted as agrichemicals due to their broad-spectrum antimicrobial properties (Cox et al. 2016; Anjum et al. 2013; Jo et al. 2009; Lamsal et al. 2011a). However, silver in nanoform is found to be toxic to humans and animals.
Due to its antibacterial properties, silver is used in wound dressing (Marx and Barillo 2014). Sometimes overexposure of humans to silver from drugs or wound dressing leads to a condition called argyria, where the skin has a blue-gray discoloration, being accompanied by liver toxicity (Hadrup and Lam 2014; Christensen et al. 2010).
Accumulation
Experiments on mice show that following silver nanoparticle inhalation, ingestion, and injection, they accumulate in the blood, stomach, liver, spleen, kidney, lung, brain, heart, and testis (Johnston et al. 2010; Gaillet and Rouanet 2015). Inhaled silver nanoparticles have a long residence time in the lung, about a third being still found in the lungs 2 months after exposure, as indicated by in vivo studies in rats (Anderson et al. 2015). Studies on animals showed that radioactive isotope-labeled silver nanoparticles with size of 35 nm can be passed through the placenta to fetuses and can be passed from mother to infants from breast milk (Melnik et al. 2013).
Toxicity
Animal studies show that silver is toxic to animals resulting in immune system effects, enlarged heart, weight loss, pulmonary toxicity, changes in liver enzymes and blood biochemistry, and liver damage (Ahlberg et al. 2014; Gaillet and Rouanet 2015; Hadrup and Lam 2014; Kim et al. 2008). Silver nanoparticles accumulated in immune system organs are accompanied by damage in the liver, kidneys, thymus, and spleen, and possible genotoxicity due to chromosomal breakage (Wen et al. 2017). Experiments also indicated that silver nanoparticles might have a toxic effect on myocardial electrophysiology and induce lethal brady-arrhythmias in mice (Lin et al. 2017). They produce cardiac dysfunction and genotoxicity in chicken, heart malformation in fish, and thrombus formation in rats (Yu et al. 2016). A low concentration of silver nanoparticles with size of 45 nm produces vasoconstriction in isolated rat aortic rings, while a high concentration stimulates vasodilation (Rosas-Hernandez et al. 2009).
Cytotoxicity
Silver nanoparticles enter cells and may localize in endosomes/lysosomes, mitochondria, and nuclei (Cheng et al. 2013). Once inside the cells and organelles, they interfere with their functions. Smaller Ag nanoparticles seem to be more toxic than larger nanoparticles (Avalos et al. 2014; Carlson et al. 2008). Experimental evidence indicate that human and animal exposure to silver nanoparticles is associated to oxidative stress, apoptosis, genotoxic effects, chromosome aberration, and DNA breaks (Ahlberg et al. 2014; Cheng et al. 2013; Hackenberg et al. 2011; Kim and Ryu 2013; Liu et al. 2015).
1.7.8.3 Toxicity of Titanium Dioxide Nanoparticles
Titanium dioxide nanoparticles are found to have some beneficial effect on selected plants (Zheng et al. 2005; Feizi et al. 2013). They are already widely used as additives in food and other consumer products; however there are studies that indicate they might be toxic (Song et al. 2015; Coccini et al. 2015; Buzea et al. 2007).
Accumulation
Animal experiments in vivo demonstrate that 3 nm titania nanoparticles enter blood, cross the blood-brain barrier, and accumulate in the brain (Li et al. 2010; Moon et al. 2011). Fluorescently labeled titanium dioxide nanoparticles with size of 35 nm intravenously injected into pregnant mice crossed and distributed into the placenta, fetal liver, and brain (Yamashita et al. 2011).
Toxicity
Animal models show that regardless of their way of internalization (instillation, inhalation, injection, or ingestion), titanium dioxide nanoparticles are toxic (Tortiglione 2014). Toxicity includes immune system effects, reduced sperm production, neurobehavior alteration, abnormal fetal brain development, smaller fetuses, fetal deformities, and mortality. Titanium dioxide nanoparticles promote arrhythmia in rats as a result of a direct interaction with cardiac tissue (Savi et al. 2014). Just one single dose of nanoparticles results in higher cardiac conduction velocity and tissue excitability, increasing the probability of arrhythmias. Titania nanoparticles accumulate in heart tissue of rodents and are associated with cardiac damage and dysfunction, myocarditis, vascular dysfunction, arrhythmia, and inflammatory responses (Yu et al. 2016).
Cytotoxicity
Titanium dioxide nanoparticles are cytotoxic and/or genotoxic in various human cell lines (Karlsson et al. 2008; Coccini et al. 2015; Gurr et al. 2005; Yu et al. 2016).
1.7.8.4 Toxicity of Zinc Oxide Nanoparticles
Zinc oxide nanoparticles are known for their antibacterial properties (Saliani et al. 2015; Seil and Webster 2012). Despite being widely used in cosmetics, recent studies show that ZnO are toxic (Roy et al. 2015; Vandebriel and De Jong 2012). They lead to DNA and mitochondrial damage, decreased cell viability, chromosome aberration, and oxidative lesions and upregulate genes controlling apoptosis (Roy et al. 2015; Karlsson et al. 2008). Inhalation of Zn nanoparticles in rats results in their translocation to the liver and was found to produce liver and lung tissue damage (Vandebriel and De Jong 2012). Zn nanoparticles were also found to produce cardiac inflammation, DNA damage, and apoptosis in mice and rats (Yu et al. 2016).
1.7.8.5 Toxicity of Copper Oxide Nanoparticles
Experiments on human and animal cell lines demonstrate that copper oxide nanoparticles are cytotoxic, neurotoxic, and genotoxic and generate oxidative stress in many cell types (Karlsson et al. 2008; Magaye et al. 2012). Copper nanoparticles degrade DNA via generation of oxygen species. In vivo experiments on mice indicate that copper oxide nanoparticles translocate to the liver, kidneys, and spleen and produce inflammation in these organs. Copper smelter workers exposed to copper dusts or fumes have an increased risk of cancer (Magaye et al. 2012).
1.7.8.6 Toxicity of Cerium Oxide Nanoparticles
Cerium oxide nanoparticles are special in the sense that can have either a beneficial or a toxic effect on mammalian cells depending on cerium oxidation state and oxygen vacancies. The preparation method seems to have an essential role in dictating their toxicity (Gagnon and Fromm 2015; Kumar et al. 2014). Synthesis at lower temperature usually results in reduced toxicity ceria, with a higher number of oxygen vacancies and hence a more beneficial antioxidant effect (Gagnon and Fromm 2015). In addition to synthesis, the storage conditions, aging, and oxidation state influence ceria nanoparticle properties (Kumar et al. 2014). Cerium oxide nanoparticles can be cytotoxic, most likely as a result of reactive oxygen species, and lead to apoptosis in different cell lines (Gagnon and Fromm 2015; Mittal and Pandey 2014). Some CeO2 nanoparticles with size smaller than 20 nm can have beneficial properties: protection of cells against irradiation, inhibition of oxidative stress, and prevention of inflammation (Gagnon and Fromm 2015; Niu et al. 2011).
1.7.8.7 Iron Oxide, Cobalt, and Nickel Nanoparticle Toxicity
Nanoparticles made of Fe, Ni, and Co are ferromagnetic. Magnetic nanoparticles are chemically unstable over long periods of time, oxidizing easily in air (Rao et al. 2015). Magnetism makes nanoparticles more prone to aggregate. As learned from their applications for in vivo imaging, there is always a concern of their aggregation in organs that may lead to inflammation and immunological responses (Markides et al. 2012). Intravenously administered ultrasmall superparamagnetic iron oxide nanoparticles (USPION) promote the formation of blood clots, cardiac oxidative stress, and DNA damage in mice (Nemmar et al. 2016). Injected magnetite nanoparticles with size of 10 nm were found to accumulate in the liver and spleen of rats (Ruiz et al. 2016).
Cobalt exhibits extreme toxicity in large amounts, and cobalt long-term exposure is associated to health effects related to the thyroid gland, lungs, skin, and immune system (Simonsen et al. 2012). Cobalt and nickel compounds are carcinogenic; their inhalation and dermal exposure lead to skin allergies, lung fibrosis, and cancer (Magaye et al. 2012). Nickel nanoparticles elicit severe cytotoxicity producing oxidative stress, genotoxicity, and cell death (Magaye et al. 2012).
1.7.8.8 Toxicity of Silicon and Silica Nanoparticles
Silicon and silicon dioxide (silica) nanoparticles have been found to produce cardiovascular disorders: cerebrovascular toxicity, coagulation disorders, and endothelium dysfunction in rodents (Yu et al. 2016). They are cytotoxic and genotoxic to a range of human cell types, such as human umbilical vein endothelial cells, epithelial cells, microvascular endothelial cells, platelets, and aortic vessel cells (Yu et al. 2016).
1.7.8.9 Toxicity of Carbon-Based Nanoparticles
Carbon-based nanoparticles include carbon black, fullerenes C60 and C70, single-walled carbon nanotubes, SWCNTs, and multiple-walled carbon nanotubes MWCNTs. Sometimes SWCNTs and MWCNTs are referred to as simply “carbon nanotubes,” CNTs.
Carbon black particles are a by-product of automobile exhaust and were found to be toxic to humans and animals. Carbon black nanoparticles were detected in the urine of 289 children at an average of 98.2 × 105 particles/ml (Saenen et al. 2017). Children living close to a major road (<160 m) had a higher amount of urinary particles (Saenen et al. 2017). Carbon black and CNTs were found to produce cardiac and endothelial dysfunction, vasorelaxation, thrombus formation, placenta vessel damage, and thrombus formation in rodents (Yu et al. 2016). They produce cytotoxicity and genotoxicity in a wide range of cell types, such as human umbilical vein endothelial cells, blood cells, smooth muscle cells, human dermal microvascular endothelial cell, and human aortic endothelial cells, to name a few (Yu et al. 2016).
A review of CNT toxicity and exposure concludes that CNTs lead to oxidative stress and inflammation, resulting to fibrosis and granulomas with possible genotoxic and carcinogenic effects (Aschberger et al. 2010). Figure 1.11a shows the persistence of MWCNT in mice lungs. These nanoparticles can be found in lungs and diaphragm of mice 17 months following inhalation exposure and promote lung adenocarcinoma (Sargent et al. 2014). Due to their morphological similarities, MWCNTs are suspected to be as toxic as asbestos (Stella 2011). A comparison between CNTs and asbestos can be seen in Fig. 1.1e–f (Murr and Soto 2004).
Examples of adverse health effects in mice exposed to MWCNTs. (A) Light and enhanced dark-field imaging of MWCNT in lungs and diaphragm of mice exposed with MWCNTs 17 months following inhalation exposure. (a1) MWCNTs (black fibers) can be observed in the alveolar interstitium; (a2) enhanced dark-field imaging shows MWCNT (upper two arrows) in the diaphragm. MWCNTs are bright white; nuclei, brown-to-orange; muscle cells, green; and red blood cells, yellow. The lower arrow indicated the parietal pleural border; (a3) image showing a focal adenomatous hyperplasia in a mouse following the inhalation of MWCNT. Images (a1–a3) are reproduced from Sargent L. M. et al. 2014, Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Particle and Fibre Toxicology, 11, 18 (Sargent et al. 2014). (B) Fetal external and skeletal malformations in mice administered with intratracheal instilled MWCNTs at 4 mg/kg on GD 9. Malformations include (b1) reduction deformity of the forelimbs, (b2) short or absent tail, and (b3) fusion of the vertebrae and ribs. (Courtesy of Dr. T. Fujitani, Tokyo Metropolitan Institute of Public Health, Japan, Fujitani et al. (2012). Reproduced from Reproductive and developmental toxicity of carbon-based nanomaterials: A literature review, Ema M. et al., Nanotoxicology vol. 10 (2016) 391–412, reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com) (Ema et al. 2016)
MWCNTs are shown to be teratogenic in mice, resulting in deformity of limbs and fusion of vertebrae and ribs (Fujitani et al. 2012; Ema et al. 2016). Figure 1.11b shows fetal external and skeletal malformations in mice administered with MWCNTs. The mice show deformity of the forelimbs, short or absent tail, and fusion of the vertebrae and ribs (Ema et al. 2016).
Consequently, the use of CNTs in agriculture is not justified taking into account their adverse effects on humans and animals, as well as environment overload.
1.7.9 Comparative Toxicity of Nanoparticles with Various Compositions
Comparative toxicity studies indicate that some nanoparticles are more toxic than others. For example, metallic nanoparticles are inherently toxic due to their composition, such as metal dust and welding fumes (cobalt, nickel, cadmium, zinc, manganese) (Buzea et al. 2007). Even though some materials are not considered toxic in bulk, in nanoparticle form they cause cancer in animals, as in the case of titanium dioxide (Borm et al. 2004). The toxicity of nanoparticles is also cell specific, as can be seen below.
Table 1.3 illustrates comparative toxicity of nanoparticles with various compositions on murine macrophage cells (Soto et al. 2005). The scale for the comparison of nanoparticles toxicities is based on value “1” for asbestos, a known carcinogen. Nanoparticles with values smaller than “1” are less toxic than asbestos. At a concentration of 5 μg/ml, silver nanoparticles and SWCNTs are more toxic than asbestos. They are followed in decreasing toxicity order by MWCNTs and iron oxide, carbon black, zirconia and alumina, and titanium dioxide. At a concentration of 10 μg/ml, the materials show the following toxicity in decreasing order: SWCNTs, MWCNTs and silver, carbon black and zirconia, alumina, and titanium dioxide. It is interesting to note that the aggregation strongly modulates the cytotoxicity of silver.
In Fig. 1.12 is illustrated the comparative cytotoxicity of CuO, TiO2, ZnO, CuZnFe2O4, Fe2O3, Fe3O4, C, and CNT nanoparticles in human lung epithelial cell line A549 (Karlsson et al. 2008). This study reveals that CuO nanoparticles show the highest level of toxicity, followed by ZnO, carbon nanotubes, CuZnFe2O4, and Fe2O3. Titania and Fe3O4 did not decrease cell viability; however they produced oxidative lesions.
(a) Cytotoxicity of various nanoparticles in cultures of human A549 cells (human adenocarcinomic alveolar basal epithelial cells) after exposure to 20 and 40 μg/cm2 of nanoparticles. (b) Oxidative DNA lesions. (c) Transmission electron microscopy images of nanoparticles used in the experiment: CuO, TiO2, ZnO, CuZnFe2O4, Fe2O3, Fe3O4, C, and CNTs. Reprinted with permission from Karlsson H. L. et al. 2008. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol, 21, 1726–32. Copyright (2008) American Chemical Society 45 (Karlsson et al. 2008)
The discrepancy between results on comparative cytotoxicity from different studies is likely due to the fact that the nanoparticles used in various experiments have different sizes, aggregations, and synthesis methods, which will result in different reactivity and interaction with cells and subcellular structures (Staszek et al. 2015). In addition, cytotoxicity is cell dependent, as we will see in the following.
Table 1.4 shows cellular responses of two types of cells (ht bronchial epithelial cells and RAW macrophages) subjected to TiO2, SiO2, and MWCNTs, having different sizes (Sohaebuddin et al. 2010). The response to nanoparticles is cell specific. For example, titania nanoparticles are more cytotoxic to macrophages than the ht bronchial epithelial cells, showing a higher apoptosis rate. In general, macrophages are more prone to cytotoxicity than fibroblasts and epithelial cells as a response to nanoparticle exposure.
Other cytotoxicity studies on pulmonary cell lines show that Cu and Zn nanoparticles have the highest toxicity, followed by TiO2, Al2O3, CeO2, Ag, Ni, and ZrO2 (Lanone et al. 2009).
1.8 Beneficial or Detrimental Effect of Nanoparticles?
Some nanoparticles are promoted as agrichemical for selected beneficial effects on some plants. While they show benefits for some plants, they are phytotoxic to others. If used in agriculture, nanoparticles will become ubiquitous in the soil, atmosphere, and water and will be available for uptake in other plant species for which they are phytotoxic. Last but not least, most nanoparticles are shown to be associated to many diseases in humans and animals. The environmental overload with agricultural nanoparticles together with the consumption of plants containing nanoparticles will pose a serious health risk to humans and animals. For example, silver, which is increasingly used in agriculture, can be more cytotoxic than asbestos. Another example is carbon in the form of nanotubes. Carbon nanotubes have morphologies very similar to asbestos and show similar toxicities in animal cells.
Here below we give the most important arguments explaining why nanoparticles should not be used in agriculture:
-
Most studies of beneficial effects of nanoparticles were carried out on first-generation plants. The benefits shown in first-generation plants might not be present in second-generation plants, as in the case of ceria nanoparticles on tomato plants.
-
The use of agrichemical nanoparticles as plant growth regulators will result in their environmental accumulation in soil and water. Due to their small size, it is very likely they will become airborne.
-
Nanoparticle benefits are found to be plant species specific. Some nanoparticles show benefits for one plant, while they are phytotoxic for another plant type. Agrichemical nanoparticles cannot be confined to a specific location, becoming airborne and bioavailable for other plants that might suffer phytotoxic effects as a result of exposure.
-
The accumulation of nanoparticles in soil will make a higher concentration of nanoparticles available to plants for uptake. In many cases a higher concentration of nanoparticles has been shown to have detrimental effects compared to the lower concentrations that might show beneficial effects.
-
Edible plants have been shown to uptake and accumulate nanoparticles. The possible toxic effect on humans from nanoparticles within edible plants poses serious concerns.
-
The use of nanoparticles can adversely affect soil microbiota, creating an imbalance in the bacterial diversity.
-
Last but not least, most nanoparticles are shown to have toxic effects on humans and animals, leading to a plethora of diseases, ranging from respiratory, cardiovascular, and neurodegenerative diseases to various cancers. Agricultural nanoparticles can become available to humans via ingestion of edible plants that contain nanoparticles, via environmental exposure with nano-agrichemicals from atmosphere, soil, and water.
We should ask ourselves: are the beneficial effects of nanoparticles in some plants enough to warrant their use in agriculture with possible catastrophic consequences for humans, animals, and other plants?
Figure 1.13 shows a schematic of adverse effects of nanoparticles in humans, animals, and plants. There is overwhelming evidence demonstrating that nanoparticles with different composition are associated with health effects in humans and animals, such as arteriosclerosis, high blood pressure, blood clots, stroke, arrhythmia, heart disease, heart attack, respiratory diseases, neurodegenerative diseases, reproductive system diseases, and various cancers. Nanoparticle toxicity in plants relate to growth inhibition, physiological and biochemical traits, and toxicity at genetic level.
Schematics of adverse effects of nanoparticles in humans, animals, and plants. Nanoparticles with different compositions shown in the upper part were found to localize in humans at the site of various diseases. Several health effects in humans and animals are associated to exposure to nanoparticles. Nanoparticle toxicity in plants relate to growth inhibition, physiological and biochemical traits, and toxicity at genetic level
It is likely that many authors working in the agricultural field are not aware of the current knowledge on nanoparticle toxicity to humans and animals and are conducting experiments expected to improve plant yield and promote their growth. We hope that this chapter has shed some light on the most stringent problems related to nanoparticles use in agriculture and will help scientists decide whether or not to pursue research in different avenues of nano-agrichemicals.
References
Ahlberg S, Antonopulos A, Diendorf J et al (2014) PVP-coated, negatively charged silver nanoparticles: a multi-center study of their physicochemical characteristics, cell culture and in vivo experiments. Beilstein J Nanotechnol 5:1944–1965 (references therein). https://doi.org/10.3762/bjnano.5.205
Alexandru SB, Mariya VK, Biris AS et al (2012) Method of using carbon nanotubes to affect seed germination and plant growth. US Patent
Anderson DS, Patchin ES, Silva RM et al (2015) Influence of particle size on persistence and clearance of aerosolized silver nanoparticles in the rat lung. Toxicol Sci 144:366–381. https://doi.org/10.1093/toxsci/kfv005
Andersson-Willman B, Gehrmann U, Cansu Z et al (2012) Effects of subtoxic concentrations of TiO2 and ZnO nanoparticles on human lymphocytes, dendritic cells and exosome production. Toxicol Appl Pharmacol 264:94–103. https://doi.org/10.1016/j.taap.2012.07.021
Andujar P, Simon-Deckers A, Galateau-Salle F et al (2014) Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part Fibre Toxicol 11:13. https://doi.org/10.1186/1743-8977-11-23
Anjum NA, Gill SS, Duarte AC et al (2013) Silver nanoparticles in soil-plant systems. J Nanopart Res 15:1896–1897 (Unsp 1896)
Anjum NA, Adam V, Kizek R et al (2015) Nanoscale copper in the soil-plant system – toxicity and underlying potential mechanisms. Environ Res 138:306–325. https://doi.org/10.1016/j.envres.2015.02.019
Antisari LV, Carbone S, Gatti A et al (2015) Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag, Co, Ni) engineered nanoparticles. Environ Sci Pollut Res 22:1841–1853. https://doi.org/10.1007/s11356-014-3509-0
Aragay G, Pino F, Merkoci A (2012) Nanomaterials for sensing and destroying pesticides. Chem Rev 112:5317–5338. https://doi.org/10.1021/cr300020c
Arruda SCC, Silva ALD, Galazzi RM et al (2015) Nanoparticles applied to plant science: a review. Talanta 131:693–705. https://doi.org/10.1016/j.talanta.2014.08.050
Aschberger K, Johnston HJ, Stone V et al (2010) Review of carbon nanotubes toxicity and exposure-appraisal of human health risk assessment based on open literature. Crit Rev Toxicol 40:759–790. https://doi.org/10.3109/10408444.2010.506638
Aslani F, Bagheri S, Julkapli NM et al (2014) Effects of engineered nanomaterials on plants growth: an overview. Sci World J. https://doi.org/10.1155/2014/641759
Asli S, Neumann PM (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584. https://doi.org/10.1111/j.1365-3040.2009.01952.x
Asztemborska M, Steborowski R, Kowalska J et al (2015) Accumulation of aluminium by plants exposed to nano- and microsized particles of Al2O3. Int J Environ Res 9:109–116
Atha DH, Wang HH, Petersen EJ et al (2012) Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 46:1819–1827. https://doi.org/10.1021/es202660k
Avalos A, Haza AI, Mateo D et al (2014) Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J Appl Toxicol 34:413–423. https://doi.org/10.1002/jat.2957
Avellan A, Schwab F, Masion A et al (2017) Nanoparticle uptake in plants: gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging. Environ Sci Technol 51:8682–8691. https://doi.org/10.1021/acs.est.7b01133
Bakand S, Hayes A, Dechsakulthorn F (2012) Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal Toxicol 24:125–135. https://doi.org/10.3109/08958378.2010.642021
Bakshi S, He ZLL, Harris WG (2015) Natural nanoparticles: Implications for environment and human health. Crit Rev Environ Sci Technol 45:861–904. https://doi.org/10.1080/10643389.2014.921975
Balasubramanian SK, Jittiwat J, Manikandan J et al (2010) Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 31:2034–2042. https://doi.org/10.1016/j.biomaterials.2009.11.079
Ballestri M, Baraldi A, Gatti AM et al (2001) Liver and kidney foreign bodies granulomatosis in a patient with malocclusion, bruxism, and worn dental prostheses. Gastroenterology 121:1234–1238. https://doi.org/10.1053/gast.2001.29333
Begum P, Fugetsu B (2012) Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant. J Hazard Mater 243:212–222. https://doi.org/10.1016/j.jhazmat.2012.10.025
Begum P, Ikhtiari R, Fugetsu B et al (2012) Phytotoxicity of multi-walled carbon nanotubes assessed by selected plant species in the seedling stage. Appl Surf Sci 262:120–124. https://doi.org/10.1016/j.apsusc.2012.03.028
Begum P, Ikhtiari R, Fugetsu B (2014) Potential impact of multi-walled carbon nanotubes exposure to the seedling stage of selected plant species. Nanomaterials 4:203–221. https://doi.org/10.3390/nano4020203
Bernhardt ES, Colman BP, Hochella MF et al (2010) An ecological perspective on nanomaterial impacts in the environment. J Environ Qual 39:1954–1965. https://doi.org/10.2134/jeq2009.0479
Bitounis D, Pourchez J, Forest V et al (2016) Detection and analysis of nanoparticles in patients: a critical review of the status quo of clinical nanotoxicology. Biomaterials 76:302–312. https://doi.org/10.1016/j.biomaterials.2015.10.061
Boonyanitipong P, Kositsup B, Kumar P et al (2011) Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int J Biosci Biochem Bioinform 1(4):282–285
Borm PJA, Schins RPF, Albrecht C (2004) Inhaled particles and lung cancer, part B: paradigms and risk assessment. Int J Cancer 110:3–14. https://doi.org/10.1002/ijc.20064
Brar SK, Verma M, Tyagi RD et al (2010) Engineered nanoparticles in wastewater and wastewater sludge – evidence and impacts. Waste Manage 30:504–520. https://doi.org/10.1016/j.wasman.2009.10.012
Bregoli L, Chiarini F, Gambarelli A et al (2009) Toxicity of antimony trioxide nanoparticles on human hematopoietic progenitor cells and comparison to cell lines. Toxicology 262:121–129. https://doi.org/10.1016/j.tox.2009.05.017
Brook RD (2008) Cardiovascular effects of air pollution. Clin Sci 115:175–187. https://doi.org/10.1042/cs20070444
Bruinink A, Wang J, Wick P (2015) Effect of particle agglomeration in nanotoxicology. Arch Toxicol 89:659–675. https://doi.org/10.1007/s00204-015-1460-6
Burklew CE, Ashlock J, Winfrey WB et al (2012) Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PLoS One 7. https://doi.org/10.1371/journal.pone.0034783
Buzea C, Pacheco I (2017) Nanomaterials and their classification. In: Shukla AK (ed) EMR/ESR/EPR spectroscopy for characterization of nanomaterials, Advanced structured materials, vol 62. Springer, New York, pp 3–45
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2:MR17–MR71. https://doi.org/10.1116/1.2815690
Calderon-Garciduenas L, Avila-Ramirez J, Calderon-Garciduenas A et al (2016a) Cerebrospinal fluid biomarkers in highly exposed PM2.5 urbanites: the risk of Alzheimer’s and Parkinson’s diseases in Young Mexico City residents. J Alzheimers Dis 54:597–613. https://doi.org/10.3233/jad-160472
Calderon-Garciduenas L, Reynoso-Robles R, Vargas-Martinez J et al (2016b) Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ Res 146:404–417. https://doi.org/10.1016/j.envres.2015.12.031
Carlson C, Hussain SM, Schrand AM et al (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112:13608–13619. https://doi.org/10.1021/jp712087m
Castiglione MR, Giorgetti L, Geri C et al (2011) The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J Nanopart Res 13:2443–2449. https://doi.org/10.1007/s11051-010-0135-8
Chen YS, Hung YC, Liau I et al (2009) Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett 4:858–864. https://doi.org/10.1007/s11671-009-9334-6
Chen R, Ratnikova TA, Stone MB et al (2010) Differential uptake of carbon nanoparticles by plant and mammalian cells. Small 6:612–617. https://doi.org/10.1002/smll.200901911
Chen RJ, Kan HD, Chen BH et al (2012) Association of particulate air pollution with daily mortality. Am J Epidemiol 175:1173–1181. https://doi.org/10.1093/aje/kwr425
Chen H, Kwong JC, Copes R et al (2017) Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 389:718–726. https://doi.org/10.1016/s0140-6736(16)32399-6
Cheng LC, Jiang X, Wang J et al (2013) Nano-bio effects: interaction of nanomaterials with cells. Nanoscale 5:3547–3569. https://doi.org/10.1039/c3nr34276j
Chichiricco G, Poma A (2015) Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 5:851–873. https://doi.org/10.3390/nano5020851
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:124. https://doi.org/10.3389/fncel.2015.00124
Chithrani BD, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668. https://doi.org/10.1021/nl052396o
Cho WS, Cho MJ, Jeong J et al (2009) Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol 236:16–24. https://doi.org/10.1016/j.taap.2008.12.023
Christensen FM, Johnston HJ, Stone V et al (2010) Nano-silver – feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology 4:284–295. https://doi.org/10.3109/17435391003690549
Chung M, Wang DD, Rizzo AM et al (2015) Association of PNC, BC, and PM2.5 measured at a central monitoring site with blood pressure in a predominantly near highway population. Int J Environ Res Public Health 12:2765–2780. https://doi.org/10.3390/ijerph120302765
Cifuentes Z, Custardoy L, de la Fuente JM et al (2010) Absorption and translocation to the aerial part of magnetic carbon-coated nanoparticles through the root of different crop plants. J Nanobiotechnol 8. https://doi.org/10.1186/1477-3155-8-26
Coccini T, Grandi S, Lonati D et al (2015) Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology 48:77–89. https://doi.org/10.1016/j.neuro.2015.03.006
Cohen AJ, Brauer M, Burnett R et al (2017) Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389:1907–1918. https://doi.org/10.1016/s0140-6736(17)30505-6
Cosselman KE, Navas-Acien A, Kaufman JD (2015) Environmental factors in cardiovascular disease. Nat Rev Cardiol 12:627–642. https://doi.org/10.1038/nrcardio.2015.152
Cox A, Venkatachalam P, Sahi S et al (2016) Silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol Biochem 107:147–163. https://doi.org/10.1016/j.plaphy.2016.05.022
Crespo P, de la Presa P, Marin P et al (2013) Magnetism in nanoparticles: tuning properties with coatings. J Phys-Condes Matter 25. https://doi.org/10.1088/0953-8984/25/48/484006
Cui HX, Zhang P, Gu W et al (2009) Application of anatase TiO(2) sol derived from peroxotitannic acid in crop diseases control and growth regulation. Nanotech Conference & Expo 2009, vol 2, Technical Proceedings. CRC Press-Taylor & Francis Group, Boca Raton
Cupaioli FA, Zucca FA, Boraschi D et al (2014) Engineered nanoparticles. How brain friendly is this new guest? Prog Neurobiol 119:20–38. https://doi.org/10.1016/j.pneurobio.2014.05.002
Dan YB, Zhang WL, Xue RM et al (2015) Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma-mass spectrometry analysis. Environ Sci Technol 49:3007–3014. https://doi.org/10.1021/es506179e
Davidson RA, Anderson DS, Van Winkle LS et al (2015) Evolution of silver nanoparticles in the rat lung investigated by X-ray absorption spectroscopy. J Phys Chem A 119:281–289. https://doi.org/10.1021/jp510103m
Deng YQ, White JC, Xing BS (2014) Interactions between engineered nanomaterials and agricultural crops: implications for food safety. J Zhejiang Univ Sci A 15:552–572. https://doi.org/10.1631/jzus.A1400165
Dietz KJ, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16:582–589. https://doi.org/10.1016/j.tplants.2011.08.003
Dimkpa CO, Latta DE, McLean JE et al (2013a) Fate of CuO and ZnO nano- and microparticles in the plant environment. Environ Sci Technol 47:4734–4742. https://doi.org/10.1021/es304736y
Dimkpa CO, McLean JE, Martineau N et al (2013b) Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol 47:1082–1090. https://doi.org/10.1021/es302973y
Dinesh R, Anandaraj M, Srinivasan V et al (2012) Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 173:19–27. https://doi.org/10.1016/j.geoderma.2011.12.018
Docter D, Westmeier D, Markiewicz M et al (2015) The nanoparticle biomolecule corona: lessons learned – challenge accepted? Chem Soc Rev 44:6094–6121. https://doi.org/10.1039/c5cs00217f
Donaldson K, Duffin R, Langrish JP et al (2013) Nanoparticles and the cardiovascular system: a critical review. Nanomedicine 8:403–423. https://doi.org/10.2217/nnm.13.16
Du WC, Sun YY, Ji R et al (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828. https://doi.org/10.1039/c0em00611d
Du W, Tan W, Peralta-Videa JR et al (2017) Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol Biochem 110:210–225. https://doi.org/10.1016/j.plaphy.2016.04.024
Ema M, Hougaard KS, Kishimoto A et al (2016) Reproductive and developmental toxicity of carbon-based nanomaterials: a literature review. Nanotoxicology 10:391–412. https://doi.org/10.3109/17435390.2015.1073811
Ema M, Okuda H, Gamo M et al (2017) A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod Toxicol 67:149–164. https://doi.org/10.1016/j.reprotox.2017.01.005
Faisal M, Saquib Q, Alatar AA et al (2013) Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater 250:318–332. https://doi.org/10.1016/j.jhazmat.2013.01.063
Feichtmeier NS, Walther P, Leopold K (2015) Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles. Environ Sci Pollut Res 22:8549–8558. https://doi.org/10.1007/s11356-014-4015-0
Feizi H, Kamali M, Jafari L et al (2013) Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 91:506–511. https://doi.org/10.1016/j.chemosphere.2012.12.012
Fischer HC, Chan WCW (2007) Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol 18:565–571. https://doi.org/10.1016/j.copbio.2007.11.008
Foroozandeh P, Aziz AA (2015) Merging worlds of nanomaterials and biological environment: factors governing protein corona formation on nanoparticles and its biological consequences. Nanoscale Res Lett 10:221. https://doi.org/10.1186/s11671-015-0922-3
Franklin BA, Brook R, Pope CA (2015) Air pollution and cardiovascular disease. Curr Probl Cardiol 40:207–238. https://doi.org/10.1016/j.cpcardiol.2015.01.003
Fujitani T, Ohyama K, Hirose A et al (2012) Teratogenicity of multi-wall carbon nanotube (MWCNT) in ICR mice. J Toxicol Sci 37:81–89
Gagnon J, Fromm KM (2015) Toxicity and protective effects of cerium oxide nanoparticles (Nanoceria) depending on their preparation method, particle size, cell type, and exposure route. Eur J Inorg Chem:4510–4517. https://doi.org/10.1002/ejic.201500643
Gaillet S, Rouanet JM (2015) Silver nanoparticles: their potential toxic effects after oral exposure and underlying mechanisms—a review. Food Chem Toxicol 77:58–63. https://doi.org/10.1016/j.fct.2014.12.019
Gajbhiye M, Kesharwani J, Ingle A et al (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed-Nanotechnol Biol Med 5:382–386. https://doi.org/10.1016/j.nano.2009.06.005
Garcia-Ivars J, Iborra-Clar MI, Alcaina-Miranda MI et al (2015) Comparison between hydrophilic and hydrophobic metal nanoparticles on the phase separation phenomena during formation of asymmetric polyethersulphone membranes. J Membr Sci 493:709–722. https://doi.org/10.1016/j.memsci.2015.07.009
Gardea-Torresdey JL, Rico CM, White JC (2014) Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ Sci Technol 48:2526–2540. https://doi.org/10.1021/es4050665
Gatoo MA, Naseem S, Arfat MY et al (2014) Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int 2014:498420. https://doi.org/10.1155/2014/498420
Gatti AM (2004) Biocompatibility of micro- and nano-particles in the colon. Part II. Biomaterials 25:385–392. https://doi.org/10.1016/s0142-9612(03)00537-4
Gatti AM, Montanari S (2006) Retrieval analysis of clinical explanted vena cava filters. J Biomed Mater Res Part B 77B:307–314. https://doi.org/10.1002/jbm.b.30361
Gatti AM, Rivasi F (2002) Biocompatibility of micro- and nanoparticles. Part I: in liver and kidney. Biomaterials 23:2381–2387. https://doi.org/10.1016/s0142-9612(01)00374-x
Gatti AM, Montanari S, Gambarelli A et al (2005) In-vivo short- and long-term evaluation of the interaction material-blood. J Mater Sci Mater Med 16:1213–1219. https://doi.org/10.1007/s10856-005-4731-6
Geiser M, Kreyling WG (2010) Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol 7:2. https://doi.org/10.1186/1743-8977-7-2
Geisler-Lee J, Wang Q, Yao Y et al (2013) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7:323–337. https://doi.org/10.3109/17435390.2012.658094
Gerber A, Bundschuh M, Klingelhofer D et al (2013) Gold nanoparticles: recent aspects for human toxicology. J Occup Med Toxicol 8:32. https://doi.org/10.1186/1745-6673-8-32
Ghafariyan MH, Malakouti MJ, Dadpour MR et al (2013) Effects of magnetite nanoparticles on soybean chlorophyll. Environ Sci Technol 47:10645–10652. https://doi.org/10.1021/es402249b
Ghodake G, Seo YD, Lee DS (2011) Hazardous phytotoxic nature of cobalt and zinc oxide nanoparticles assessed using Allium cepa. J Hazard Mater 186:952–955. https://doi.org/10.1016/j.jhazmat.2010.11.018
Ghosh M, Bandyopadhyay M, Mukherjee A (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels plant and human lymphocytes. Chemosphere 81:1253–1262. https://doi.org/10.1016/j.chemosphere.2010.09.022
Ghosh M, Bhadra S, Adegoke A et al (2015) MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat Res 774:49–58. https://doi.org/10.1016/j.mrfmmm.2015.03.004
Giannousi K, Avramidis I, Dendrinou-Samara C (2013) Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv 3:21743–21752. https://doi.org/10.1039/c3ra42118j
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60:9781–9792. https://doi.org/10.1021/jf302154y
Gonzalez-Maciel A, Reynoso-Robles R, Torres-Jardon R et al (2017) Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: culprit hidden in plain sight in Alzheimer’s disease development. J Alzheimers Dis 59:189–208. https://doi.org/10.3233/jad-170012
Goodman CM, McCusker CD, Yilmaz T et al (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15:897–900. https://doi.org/10.1021/bc049951i
Gosens I, Mathijssen LE, Bokkers BG et al (2014) Comparative hazard identification of nano- and micro-sized cerium oxide particles based on 28-day inhalation studies in rats. Nanotoxicology 8:643–653. https://doi.org/10.3109/17435390.2013.815814
Gosens I, Kermanizadeh A, Jacobsen NR et al (2015) Comparative hazard identification by a single dose lung exposure of zinc oxide and silver nanomaterials in mice. PLoS One 10:e0126934. https://doi.org/10.1371/journal.pone.0126934
Greget R, Nealon GL, Vileno B et al (2012) Magnetic properties of gold nanoparticles: a room-temperature quantum effect. Chemphyschem 13:3092–3097. https://doi.org/10.1002/cphc.201200394
Grillo R, Rosa AH, Fraceto LF (2015) Engineered nanoparticles and organic matter: a review of the state-of-the-art. Chemosphere 119:608–619. https://doi.org/10.1016/j.chemosphere.2014.07.049
Gui X, Zhang ZY, Liu ST et al (2015) Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PLoS One 10:e0134261. https://doi.org/10.1371/journal.pone.0134261
Gupta VK, Saleh TA (2013) Sorption of pollutants by porous carbon, carbon nanotubes and fullerene – an overview. Environ Sci Pollut Res 20:2828–2843. https://doi.org/10.1007/s11356-013-1524-1
Gurr JR, Wang ASS, Chen CH et al (2005) Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213:66–73. https://doi.org/10.1016/j.tox.2005.05.007
Hackenberg S, Scherzed A, Kessler M et al (2011) Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett 201:27–33. https://doi.org/10.1016/j.toxlet.2010.12.001
Hadrup N, Lam HR (2014) Oral toxicity of silver ions, silver nanoparticles and colloidal silver – a review. Regul Toxicol Pharmacol 68:1–7. https://doi.org/10.1016/j.yrtph.2013.11.002
Hadrup N, Sharma AK, Poulsen M et al (2015) Toxicological risk assessment of elemental gold following oral exposure to sheets and nanoparticles – a review. Regul Toxicol Pharmacol 72:216–221. https://doi.org/10.1016/j.yrtph.2015.04.017
Hanley C, Thurber A, Hanna C et al (2009) The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res Lett 4:1409–1420. https://doi.org/10.1007/s11671-009-9413-8
Hathaway QA, Nichols CE, Shepherd DL et al (2017) Maternal-engineered nanomaterial exposure disrupts progeny cardiac function and bioenergetics. Am J Physiol-Heart Circ Physiol 312:H446–H458. https://doi.org/10.1152/ajpheart.00634.2016
He LL, Liu Y, Mustapha A et al (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166:207–215. https://doi.org/10.1016/j.micres.2010.03.003
He D, Wu SW, Zhao HP et al (2017) Association between particulate matter 2.5 and diabetes mellitus: a meta-analysis of cohort studies. J Diabetes Investig 8:687–696. https://doi.org/10.1111/jdi.12631
Heusinkveld HJ, Wahle T, Campbell A et al (2016) Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56:94–106. https://doi.org/10.1016/j.neuro.2016.07.007
Hillyer JF, Albrecht RM (2001) Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci 90:1927–1936. https://doi.org/10.1002/jps.1143.abs
Hong J, Peralta-Videa JR, Rico C et al (2014) Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ Sci Technol 48:4376–4385. https://doi.org/10.1021/es404931g
Hori H, Teranishi T, Nakae Y et al (1999) Anomalous magnetic polarization effect of Pd and Au nano-particles. Phys Lett A 263:406–410. https://doi.org/10.1016/s0375-9601(99)00742-2
Hori H, Yamamoto Y, Iwamoto T et al (2004) Diameter dependence of ferromagnetic spin moment in Au nanocrystals. Phys Rev B 69:174411. https://doi.org/10.1103/PhysRevB.69.174411
Huk A, Izak-Nau E, el Yamani N et al (2015) Impact of nanosilver on various DNA lesions and HPRT gene mutations – effects of charge and surface coating. Part Fibre Toxicol 12:20. https://doi.org/10.1186/s12989-015-0100-x
Husen A, Siddiqi KS (2014) Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol 12:16. https://doi.org/10.1186/1477-3155-12-16
Iannitti T, Capone S, Gatti A et al (2010) Intracellular heavy metal nanoparticle storage: progressive accumulation within lymph nodes with transformation from chronic inflammation to malignancy. Int J Nanomedicine 5:955–960. https://doi.org/10.2147/ijn.s14363
Iavicoli I, Leso V, Beezhold DH et al (2017) Nanotechnology in agriculture: opportunities, toxicological implications, and occupational risks. Toxicol Appl Pharmacol 329:96–111. https://doi.org/10.1016/j.taap.2017.05.025
Ilinskaya AN, Dobrovolskaia MA (2013) Nanoparticles and the blood coagulation system. Part II: safety concerns. Nanomedicine 8:969–981. https://doi.org/10.2217/nnm.13.49
Ilinskaya AN, Dobrovolskaia MA (2014) Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol 171:3988–4000. https://doi.org/10.1111/bph.12722
Jacob DL, Borchardt JD, Navaratnam L et al (2013) Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int J Phytoremediation 15:142–153. https://doi.org/10.1080/15226514.2012.683209
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043. https://doi.org/10.1094/pdis-93-10-1037
Johnston HJ, Hutchison G, Christensen FM et al (2010) A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol 40:328–346. https://doi.org/10.3109/10408440903453074
Josko I, Oleszczuk P (2013) Influence of soil type and environmental conditions on ZnO, TiO2 and Ni nanoparticles phytotoxicity. Chemosphere 92:91–99. https://doi.org/10.1016/j.chemosphere.2013.02.048
Judy JD, Unrine JM, Bertsch PM (2011) Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ Sci Technol 45:776–781. https://doi.org/10.1021/es103031a
Judy JD, Unrine JM, Rao W et al (2012) Bioavailability of gold nanomaterials to plants: importance of particle size and surface coating. Environ Sci Technol 46:8467–8474. https://doi.org/10.1021/e03019397
Kah M, Hofmann T (2014) Nanopesticide research: current trends and future priorities. Environ Int 63:224–235. https://doi.org/10.1016/j.envint.2013.11.015
Kanhed P, Birla S, Gaikwad S et al (2014) In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater Lett 115:13–17. https://doi.org/10.1016/j.matlet.2013.10.011
Karlsson HL, Cronholm P, Gustafsson J et al (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 21:1726–1732. https://doi.org/10.1021/tx800064j
Kaveh R, Li YS, Ranjbar S et al (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47:10637–10644. https://doi.org/10.1021/es402209w
Kendall M, Holgate S (2012) Health impact and toxicological effects of nanomaterials in the lung. Respirology 17:743–758. https://doi.org/10.1111/j.1440-1843.2012.02171.x
Kenzaoui BH, Bernasconi CC, Guney-Ayra S et al (2012) Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J 441:813–821. https://doi.org/10.1042/bj20111252
Kermanizadeh A, Gaiser BK, Johnston H et al (2014) Toxicological effect of engineered nanomaterials on the liver. Br J Pharmacol 171:3980–3987. https://doi.org/10.1111/bph.12421
Kettler K, Veltman K, van de Meent D et al (2014) Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ Toxicol Chem 33:481–492. https://doi.org/10.1002/etc.2470
Khan MN, Mobin M, Abbas ZK et al (2017) Role of nanomaterials in plants under challenging environments. Plant Physiol Biochem 110:194–209. https://doi.org/10.1016/j.plaphy.2016.05.038
Khlebtsov N, Dykman L (2011) Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 40:1647–1671. https://doi.org/10.1039/c0cs00018c
Khodakovskaya M, Dervishi E, Mahmood M et al (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth (retracted article. See vol. 6, pg. 7541, 2012). ACS Nano 3:3221–3227. https://doi.org/10.1021/nn900887m
Khodakovskaya MV, de Silva K, Nedosekin DA et al (2011) Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc Natl Acad Sci USA 108:1028–1033. https://doi.org/10.1073/pnas.1008856108
Khodakovskaya MV, de Silva K, Biris AS et al (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6:2128–2135. https://doi.org/10.1021/nn204643g
Khodakovskaya MV, Kim BS, Kim JN et al (2013) Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 9:115–123. https://doi.org/10.1002/smll.201201225
Khot LR, Sankaran S, Maja JM et al (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70. https://doi.org/10.1016/j.cropro.2012.01.007
Kim S, Ryu DY (2013) Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J Appl Toxicol 33:78–89. https://doi.org/10.1002/jat.2792
Kim YS, Kim JS, Cho HS et al (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol 20:575–583. https://doi.org/10.1080/08958370701874663
Koelmel J, Leland T, Wang HH et al (2013) Investigation of gold nanoparticles uptake and their tissue level distribution in rice plants by laser ablation-inductively coupled-mass spectrometry. Environ Pollut 174:222–228. https://doi.org/10.1016/j.envpol.2012.11.026
Kole C, Kole P, Randunu KM et al (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 13:37. https://doi.org/10.1186/1472-6750-13-37
Kolosnjaj-Tabi J, Just J, Hartman KB et al (2015) Anthropogenic carbon nanotubes found in the airways of Parisian children. EBioMedicine 2:1697–1704. https://doi.org/10.1016/j.ebiom.2015.10.012
Kookana RS, Boxall ABA, Reeves PT et al (2014) Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J Agric Food Chem 62:4227–4240. https://doi.org/10.1021/jf500232f
Kreyling WG, Fertsch-Gapp S, Schaffler M et al (2014) In vitro and in vivo interactions of selected nanoparticles with rodent serum proteins and their consequences in biokinetics. Beilstein J Nanotechnol 5:1699–1711. https://doi.org/10.3762/bjnano.5.180
Krishna KS, Tarakeshwar P, Mujica V et al (2014) Chemically induced magnetism in atomically precise gold clusters. Small 10:907–911. https://doi.org/10.1002/smll.201302393
Kulvietis V, Zalgeviciene V, Didziapetriene J et al (2011) Transport of nanoparticles through the placental barrier. Tohoku J Exp Med 225:225–234. https://doi.org/10.1620/tjem.225.225
Kumar A, Das S, Munusamy P et al (2014) Behavior of nanoceria in biologically-relevant environments. Environ-Sci Nano 1:516–532. https://doi.org/10.1039/c4en00052h
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246. https://doi.org/10.1016/j.scitotenv.2009.06.024
Kumari M, Khan SS, Pakrashi S et al (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater 190:613–621. https://doi.org/10.1016/j.jhazmat.2011.03.095
Kurepa J, Paunesku T, Vogt S et al (2010) Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett 10:2296–2302. https://doi.org/10.1021/nl903518f
Lahiani MH, Dervishi E, Chen JH et al (2013) Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl Mater Interfaces 5:7965–7973. https://doi.org/10.1021/am402052x
Lamsal K, Kim S-W, Jung JH et al (2011a) Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology 39:26–32. https://doi.org/10.4489/myco.2011.39.1.026
Lamsal K, Kim SW, Jung JH et al (2011b) Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 39:194–199. https://doi.org/10.5941/myco.2011.39.3.194
Landa P, Vankova R, Andrlova J et al (2012) Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J Hazard Mater 241:55–62. https://doi.org/10.1016/j.jhazmat.2012.08.059
Landsiedel R, Fabian E, Ma-Hock L et al (2012) Toxico-/biokinetics of nanomaterials. Arch Toxicol 86:1021–1060. https://doi.org/10.1007/s00204-012-0858-7
Lanone S, Rogerieux F, Geys J et al (2009) Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol 6:12. https://doi.org/10.1186/1743-8977-6-14
Larue C, Laurette J, Herlin-Boime N et al (2012a) Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ 431:197–208. https://doi.org/10.1016/j.scitotenv.2012.04.073
Larue C, Pinault M, Czarny B et al (2012b) Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J Hazard Mater 227:155–163. https://doi.org/10.1016/j.jhazmat.2012.05.033
Larue C, Veronesi G, Flank AM et al (2012c) Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J Toxicol Environ Health Part A 75:722–734. https://doi.org/10.1080/15287394.2012.689800
Larue C, Castillo-Michel H, Sobanska S et al (2014a) Foliar exposure of the crop Lactuca sativa to silver nanoparticles: evidence for internalization and changes in Ag speciation. J Hazard Mater 264:98–106. https://doi.org/10.1016/j.jhazmat.2013.10.053
Larue C, Castillo-Michel H, Sobanska S et al (2014b) Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J Hazard Mater 273:17–26. https://doi.org/10.1016/j.jhazmat.2014.03.014
Layet C, Auffan M, Santaella C et al (2017) Evidence that soil properties and organic coating drive the phytoavailability of cerium oxide nanoparticles. Environ Sci Technol 51:9756–9764. https://doi.org/10.1021/acs.est.7b02397
Lee WM, Kwak JI, An YJ (2012) Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity. Chemosphere 86:491–499. https://doi.org/10.1016/j.chemosphere.2011.10.013
Lee S, Chung H, Kim S et al (2013) The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut 224(11). https://doi.org/10.1007/s11270-013-1668-0
Lei Z, Su MY, Xiao W et al (2008) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res 121:69–79. https://doi.org/10.1007/s12011-007-8028-0
Li YF, Li JG, Yin JL et al (2010) Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. J Nanosci Nanotechnol 10:8544–8549. https://doi.org/10.1166/jnn.2010.2690
Li JJ, Lo SL, Ng CT et al (2011) Genomic instability of gold nanoparticle treated human lung fibroblast cells. Biomaterials 32:5515–5523. https://doi.org/10.1016/j.biomaterials.2011.04.023
Li HQ, Hedmer M, Karedal M et al (2015a) A cross-sectional study of the cardiovascular effects of welding fumes. PLoS One 10:15. https://doi.org/10.1371/journal.pone.0131648
Li X, Liu W, Sun L et al (2015b) Effects of physicochemical properties of nanomaterials on their toxicity. J Biomed Mater Res A 103:2499–2507. https://doi.org/10.1002/jbm.a.35384
Lin DH, Xing BS (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250. https://doi.org/10.1016/j.envpol.2007.01.016
Lin SJ, Reppert J, Hu Q et al (2009) Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5:1128–1132. https://doi.org/10.1002/smll.200801556
Lin ZM, Monteiro-Riviere NA, Riviere JE (2015) Pharmacokinetics of metallic nanoparticles. Wiley Interdiscip Rev-Nanomed Nanobiotechnol 7:189–217. https://doi.org/10.1002/wnan.1304
Lin CX, Yang SY, Gu JL et al (2017) The acute toxic effects of silver nanoparticles on myocardial transmembrane potential, I-Na and I-K1 channels and heart rhythm in mice. Nanotoxicology 11:827–837. https://doi.org/10.1080/17435390.2017.1367047
Line C, Camille LA, Flahaut E (2017) Carbon nanotubes: impacts and behaviour in the terrestrial ecosystem – a review. Carbon 123:767–785. https://doi.org/10.1016/j.carbon.2017.07.089
Liu RQ, Lal R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 514:131–139. https://doi.org/10.1016/j.scitotenv.2015.01.104
Liu QL, Zhao YY, Wan YL et al (2010) Study of the inhibitory effect of water-soluble fullerenes on plant growth at the cellular level. ACS Nano 4:5743–5748. https://doi.org/10.1021/nn101430g
Liu F, Mahmood M, Xu Y et al (2015) Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci 9:115. https://doi.org/10.3389/fnins.2015.00115
Lopez-Moreno ML, de la Rosa G, Hernandez-Viezcas JA et al (2010a) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 Nanoparticles on soybean (Glycine max) plants. Environ Sci Technol 44:7315–7320. https://doi.org/10.1021/es903891g
Lopez-Moreno ML, de la Rosa G, Hernandez-Viezcas JA et al (2010b) X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem 58:3689–3693. https://doi.org/10.1021/jf904472e
Lu Y, Gu Z (2017) Kidney physiology: a size bandpass filter. Nat Nanotechnol 12:1023–1025. https://doi.org/10.1038/nnano.2017.200
Lyu SH, Wei XY, Chen JJ et al (2017) Titanium as a beneficial element for crop production. Front Plant Sci 8:19. https://doi.org/10.3389/fpls.2017.00597
Ma YH, Kuang LL, He X et al (2010) Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78:273–279. https://doi.org/10.1016/j.chemosphere.2009.10.050
Ma CX, White JC, Dhankher OP et al (2015) Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol 49:7109–7122. https://doi.org/10.1021/acs.est.5b00685
Magaye R, Zhao J, Bowman L et al (2012) Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med 4:551–561. https://doi.org/10.3892/etm.2012.656
Maher BA, Ahmed IAM, Karloukovski V et al (2016) Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci USA 113:10797–10801. https://doi.org/10.1073/pnas.1605941113
Maitra U, Das B, Kumar N et al (2011) Ferromagnetism exhibited by nanoparticles of noble metals. Chemphyschem 12:2322–2327. https://doi.org/10.1002/cphc.201100121
Mannucci PM, Harari S, Martinelli I et al (2015) Effects on health of air pollution: a narrative review. Intern Emerg Med 10:657–662. https://doi.org/10.1007/s11739-015-1276-7
Markides H, Rotherham M, El Haj AJ (2012) Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J Nanomater 614094. https://doi.org/10.1155/2012/614094
Marx DE, Barillo DJ (2014) Silver in medicine: the basic science. Burns 40(Suppl 1):S9–S18. https://doi.org/10.1016/j.burns.2014.09.010
Melnik EA, Buzulukov YP, Demin VF et al (2013) Transfer of silver nanoparticles through the placenta and breast milk during in vivo experiments on rats. Acta Naturae 5:107–115
Miller MR, Raftis JB, Langrish JP et al (2017) Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11:4542–4552. https://doi.org/10.1021/acsnano.6b08551
Miralles P, Church TL, Harris AT (2012a) Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ Sci Technol 46:9224–9239. https://doi.org/10.1021/es202995d
Miralles P, Johnson E, Church TL et al (2012b) Multiwalled carbon nanotubes in alfalfa and wheat: toxicology and uptake. J R Soc Interface 9:3514–3527. https://doi.org/10.1098/rsif.2012.0535
Mirzajani F, Askari H, Hamzelou S et al (2013) Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol Environ Safe 88:48–54. https://doi.org/10.1016/j.ecoenv.2012.10.018
Mittal S, Pandey AK (2014) Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. Biomed Res Int 891934. https://doi.org/10.1155/2014/891934
Moon EY, Yi GH, Kang JS et al (2011) An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J Immunotoxicol 8:56–67. https://doi.org/10.3109/1547691x.2010.543995
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S et al (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138. https://doi.org/10.1039/c3mt00064h
Muoth C, Aengenheister L, Kucki M et al (2016) Nanoparticle transport across the placental barrier: pushing the field forward! Nanomedicine 11:941–957. https://doi.org/10.2217/nnm-2015-0012
Murr LE, Soto KF (2004) TEM comparison of chrysotile (asbestos) nanotubes and carbon nanotubes. J Mater Sci 39:4941–4947. https://doi.org/10.1023/b:jmsc.0000035342.99587.96
Nair PMG, Chung IM (2014) Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ Sci Pollut Res 21:8858–8869. https://doi.org/10.1007/s11356-014-2822-y
Nair R, Poulose AC, Nagaoka Y et al (2011) Uptake of FITC labeled silica nanoparticles and quantum dots by rice seedlings: effects on seed germination and their potential as biolabels for plants. J Fluoresc 21:2057–2068. https://doi.org/10.1007/s10895-011-0904-5
Nakane H (2012) Translocation of particles deposited in the respiratory system: a systematic review and statistical analysis. Environ Health Prev Med 17:263–274. https://doi.org/10.1007/s12199-011-0252-8
Naqvi S, Samim M, Abdin M et al (2010) Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine 5:983–989. https://doi.org/10.2147/IJN.S13244
Navarro E, Baun A, Behra R et al (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386. https://doi.org/10.1007/s10646-008-0214-0
Nealon GL, Donnio B, Greget R et al (2012) Magnetism in gold nanoparticles. Nanoscale 4:5244–5258. https://doi.org/10.1039/c2nr30640a
Nemmar A, Hoet PHM, Vanquickenborne B et al (2002) Passage of inhaled particles into the blood circulation in humans. Circulation 105:411–414. https://doi.org/10.1161/hc0402.104118
Nemmar A, Beegam S, Yuvaraju P et al (2016) Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part Fibre Toxicol 13:22. doi:https://doi.org/10.1186/s12989-016-0132-x
Niu JL, Wang KK, Kolattukudy PE (2011) Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-kappa B activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharmacol Exp Ther 338:53–61. https://doi.org/10.1124/jpet.111.179978
Ojea-Jimenez I, Garcia-Fernandez L, Lorenzo J et al (2012) Facile preparation of cationic gold nanoparticle-bioconjugates for cell penetration and nuclear targeting. ACS Nano 6:7692–7702. https://doi.org/10.1021/nn3012042
Pacheco I, Buzea C (2018) Metal nanoparticles and their toxicity. In: Thota S, Crans DC (eds) Metal nanoparticles. Synthesis and applications in pharmaceutical sciences. Wiley, Weinheim
Pakrashi S, Jain N, Dalai S et al (2014) In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS One 9:12. https://doi.org/10.1371/journal.pone.0087789
Patlolla AK, Berry A, May L et al (2012) Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. Int J Environ Res Public Health 9:1649–1662. https://doi.org/10.3390/ijerph9051649
Peralta-Videa JR, Hernandez-Viezcas JA, Zhao LJ et al (2014) Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem 80:128–135. https://doi.org/10.1016/j.plaphy.2014.03.028
Petersen EJ, Henry TB, Zhao J et al (2014) Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environ Sci Technol 48:4226–4246. https://doi.org/10.1021/es4052999
Podila R, Brown JM (2013) Toxicity of engineered nanomaterials: a physicochemical perspective. J Biochem Mol Toxicol 27:50–55 (references therein). https://doi.org/10.1002/jbt.21442
Pokhrel LR, Dubey B (2013) Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ 452:321–332. https://doi.org/10.1016/j.scitotenv.2013.02.059
Priester JH, Ge Y, Mielke RE et al (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci USA 109:E2451–E2456. https://doi.org/10.1073/pnas.1205431109
Qiu Y, Liu Y, Wang LM et al (2010) Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 31:7606–7619. https://doi.org/10.1016/j.biomaterials.2010.06.051
Rao JP, Gruenberg P, Geckeler KE (2015) Magnetic zero-valent metal polymer nanoparticles: current trends, scope, and perspectives. Prog Polym Sci 40:138–147. https://doi.org/10.1016/j.progpolymsci.2014.07.002
Reddy PVL, Hernandez-Viezcas JA, Peralta-Videa JR et al (2016) Lessons learned: are engineered nanomaterials toxic to terrestrial plants? Sci Total Environ 568:470–479. https://doi.org/10.1016/j.scitotenv.2016.06.042
Rico CM, Majumdar S, Duarte-Gardea M et al (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59:3485–3498. https://doi.org/10.1021/jf104517j
Rico CM, Morales MI, Barrios AC et al (2013) Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J Agric Food Chem 61:11278–11285. https://doi.org/10.1021/jf404046v
Rico CM, Lee SC, Rubenecia R et al (2014) Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J Agric Food Chem 62:9669–9675. https://doi.org/10.1021/jf503526r
Rico CM, Barrios AC, Tan WJ et al (2015) Physiological and biochemical response of soil-grown barley (Hordeum vulgare L.) to cerium oxide nanoparticles. Environ Sci Pollut Res 22:10551–10558. https://doi.org/10.1007/s11356-015-4243-y
Rinaldo M, Andujar P, Lacourt A et al (2015) Perspectives in biological monitoring of inhaled nanosized particles. Ann Occup Hyg 59:669–680. https://doi.org/10.1093/annhyg/mev015
Rizwan M, Ali S, Qayyum MF et al (2017) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16. https://doi.org/10.1016/j.jhazmat.2016.05.061
Roncati L, Gatti AM, Capitani F et al (2015a) Heavy metal bioaccumulation in an atypical primitive neuroectodermal tumor of the abdominal wall. Ultrastruct Pathol 39:286–292. https://doi.org/10.3109/01913123.2015.1013655
Roncati L, Gatti AM, Pusiol T et al (2015b) ESEM detection of foreign metallic particles inside ameloblastomatous cells. Ultrastruct Pathol 39:329–335. https://doi.org/10.3109/01913123.2015.1042608
Rosas-Hernandez H, Jimenez-Badillo S, Martinez-Cuevas PP et al (2009) Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol Lett 191:305–313. https://doi.org/10.1016/j.toxlet.2009.09.014
Roy R, Das M, Dwivedi PD (2015) Toxicological mode of action of ZnO nanoparticles: impact on immune cells. Mol Immunol 63:184–192. https://doi.org/10.1016/j.molimm.2014.08.001
Rückerl R, Schneider A, Breitner S et al (2011) Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol 23:555–592. https://doi.org/10.3109/08958378.2011.593587
Rui Y, Zhang P, Zhang Y et al (2015) Transformation of ceria nanoparticles in cucumber plants is influenced by phosphate. Environ Pollut 198:8–14. https://doi.org/10.1016/j.envpol.2014.12.017
Ruiz A, Mancebo A, Beola L et al (2016) Dose-response bioconversion and toxicity analysis of magnetite nanoparticles. IEEE Magn Lett 7:1502205. https://doi.org/10.1109/lmag.2016.2535414
Ruttkay-Nedecky B, Krystofova O, Nejdl L et al (2017) Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnol 15:19. https://doi.org/10.1186/s12951-017-0268-3
Sabo-Attwood T, Unrine JM, Stone JW et al (2012) Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 6:353–360. https://doi.org/10.3109/17435390.2011.579631
Saenen ND, Bove H, Steuwe C et al (2017) Children’s urinary environmental carbon load a novel marker reflecting residential ambient air pollution exposure? Am J Respir Crit Care Med 196:873–881. https://doi.org/10.1164/rccm.201704-0797OC
Sakamoto Y, Oba Y, Maki H et al (2011) Ferromagnetism of Pt nanoparticles induced by surface chemisorption. Phys Rev B 83:104420. https://doi.org/10.1103/PhysRevB.83.104420
Salatin S, Dizaj SM, Khosroushahi AY (2015) Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int 39:881–890. https://doi.org/10.1002/cbin.10459
Saliani M, Jalal R, Kafshdare Goharshadi E (2015) Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J Microbiol 8:e17115. https://doi.org/10.5812/jjm.17115
Sargent LM, Porter DW, Staska LM et al (2014) Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol 11:18. https://doi.org/10.1186/1743-8977-11-3
Sathuluri RR, Yoshikawa H, Shimizu E et al (2011) Gold nanoparticle-based surface-enhanced Raman scattering for noninvasive molecular probing of embryonic stem cell differentiation. PLoS One 6:13. https://doi.org/10.1371/journal.pone.0022802
Savi M, Rossi S, Bocchi L et al (2014) Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Part Fibre Toxicol 11:63. https://doi.org/10.1186/s12989-014-0063-3
Schlich K, Hund-Rinke K (2015) Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environ Pollut 196:321–330. https://doi.org/10.1016/j.envpol.2014.10.021
Schlinkert P, Casals E, Boyles M et al (2015) The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J Nanobiotechnology 13(1). https://doi.org/10.1186/s12951-014-0062-4
Schwab F, Zhai G, Kern M et al (2015) Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants – critical review. Nanotoxicology 1–22. https://doi.org/10.3109/17435390.2015.1048326
Seil JT, Webster TJ (2012) Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine 7:2767–2781. https://doi.org/10.2147/IJN.S24805
Semmler-Behnke M, Kreyling WG, Lipka J et al (2008) Biodistribution of 1.4-and 18-nm gold particles in rats. Small 4:2108–2111. https://doi.org/10.1002/smll.200800922
Semmler-Behnke M, Lipka J, Wenk A et al (2014) Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Part Fibre Toxicol 11:33. https://doi.org/10.1186/s12989-014-0033-9
Servin AD, Castillo-Michel H, Hernandez-Viezcas JA et al (2012) Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ Sci Technol 46:7637–7643. https://doi.org/10.1021/es300955b
Servin AD, Morales MI, Castillo-Michel H et al (2013) Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ Sci Technol 47:11592–11598. https://doi.org/10.1021/es403368j
Servin A, Elmer W, Mukherjee A et al (2015) A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J Nanopart Res 17:92. https://doi.org/10.1007/s11051-015-2907-7
Shah V, Belozerova I (2009) Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut 197:143–148. https://doi.org/10.1007/s11270-008-9797-6
Shah ASV, Lee KK, McAllister DA et al (2015) Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 350:10. https://doi.org/10.1136/bmj.h1295
Sharma VK, Siskova KM, Zboril R et al (2014) Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv Colloid Interface Sci 204:15–34. https://doi.org/10.1016/j.cis.2013.12.002
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93:906–915. https://doi.org/10.1016/j.chemosphere.2013.05.044
Shaymurat T, Gu JX, Xu CS et al (2012) Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): A morphological study. Nanotoxicology 6:241–248. https://doi.org/10.3109/17435390.2011.570462
Shen CX, Zhang QF, Li JA et al (2010) Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. Am J Bot 97:1602–1609. https://doi.org/10.3732/ajb.1000073
Siddiqi KS, Husen A (2017) Plant response to engineered metal oxide nanoparticles. Nanoscale Res Lett 12. https://doi.org/10.1186/s11671-017-1861-y
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J Biol Sci 21:13–17. https://doi.org/10.1016/j.sjbs.2013.04.005
Sighinolfi GL, Artoni E, Gatti AM et al (2016) Carcinogenic potential of metal nanoparticles in BALB/3T3 cell transformation assay. Environ Toxicol 31:509–519. https://doi.org/10.1002/tox.22063
Silva T, Pokhrel LR, Dubey B et al (2014) Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ 468–469:968–976. https://doi.org/10.1016/j.scitotenv.2013.09.006
Silva RM, Anderson DS, Franzi LM et al (2015) Pulmonary effects of silver nanoparticle size, coating, and dose over time upon intratracheal instillation. Toxicol Sci 144:151–162. https://doi.org/10.1093/toxsci/kfu265
Simonsen LO, Harbak H, Bennekou P (2012) Cobalt metabolism and toxicology—a brief update. Sci Total Environ 432:210–215. https://doi.org/10.1016/j.scitotenv.2012.06.009
Singh S, Kumar A, Karakoti A et al (2010) Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst 6:1813–1820. https://doi.org/10.1039/c0mb00014k
Singh A, Singh NB, Hussain I et al (2017a) Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J Biotechnol 262:11–27. https://doi.org/10.1016/j.jbiotec.2017.09.016
Singh S, Tripathi DK, Singh S et al (2017b) Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot 137:177–193. https://doi.org/10.1016/j.envexpbot.2017.01.005
Snyder RW, Fennell TR, Wingard CJ et al (2015) Distribution and biomarker of carbon-14 labeled fullerene C-60 ( C-14(U) C-60) in pregnant and lactating rats and their offspring after maternal intravenous exposure. J Appl Toxicol 35:1438–1451. https://doi.org/10.1002/jat.3177
Sohaebuddin SK, Thevenot PT, Baker D et al (2010) Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol 7:17. https://doi.org/10.1186/1743-8977-7-22
Sonavane G, Tomoda K, Makino K (2008) Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloid Surf B-Biointerfaces 66:274–280. https://doi.org/10.1016/j.colsurfb.2008.07.004
Song B, Liu J, Feng X et al (2015) A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res Lett 10:1042. https://doi.org/10.1186/s11671-015-1042-9
Soto KF, Carrasco A, Powell TG et al (2005) Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. J Nanopart Res 7:145–169. https://doi.org/10.1007/s11051-005-3473-1
Spielman-Sun E, Lombi E, Donner E et al (2017) Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum). Environ Sci Technol 51:7361–7368. https://doi.org/10.1021/acs.est.7b00813
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479. https://doi.org/10.1021/es901695c
Staszek M, Siegel J, Rimpelova S et al (2015) Cytotoxicity of noble metal nanoparticles sputtered into glycerol. Mater Lett 158:351–354. https://doi.org/10.1016/j.matlet.2015.06.021
Stella GM (2011) Carbon nanotubes and pleural damage: perspectives of nanosafety in the light of asbestos experience. Biointerphases 6:P1–P17. https://doi.org/10.1116/1.3582324
Steuer H, Krastev R, Lembert N (2014) Metallic oxide nanoparticles stimulate blood coagulation independent of their surface charge. J Biomed Mater Res Part B 102:897–902. https://doi.org/10.1002/jbm.b.33051
Stoccoro A, Karlsson HL, Coppede F et al (2013) Epigenetic effects of nano-sized materials. Toxicology 313:3–14. https://doi.org/10.1016/j.tox.2012.12.002
Taylor AF, Rylott EL, Anderson CWN et al (2014) Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS One 9:e93793. https://doi.org/10.1371/journal.pone.0093793
Teske SS, Detweiler CS (2015) The biomechanisms of metal and metal-oxide nanoparticles’ interactions with cells. Int J Environ Res Public Health 12:1112–1134. https://doi.org/10.3390/ijerph120201112
Theodorou IG, Ryan MP, Tetley TD et al (2014) Inhalation of silver nanomaterials—seeing the risks. Int J Mol Sci 15:23936–23974. https://doi.org/10.3390/ijms151223936
Thuesombat P, Hannongbua S, Akasit S et al (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Safe 104:302–309. https://doi.org/10.1016/j.ecoenv.2014.03.022
Tortiglione C (2014) The heritable effects of nanotoxicity. Nanomedicine 9:2829–2841. https://doi.org/10.2217/nnm.14.152
Tripathi DK, Shweta SS et al (2017) An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem 110:2–12. https://doi.org/10.1016/j.plaphy.2016.07.030
Vandebriel RJ, De Jong WH (2012) A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl 5:61–71. https://doi.org/10.2147/NSA.S23932
Walkey CD, Olsen JB, Song FY et al (2014) Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8:2439–2455. https://doi.org/10.1021/nn406018q
Wang HH, Kou XM, Pei ZG et al (2011a) Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 5:30–42. https://doi.org/10.3109/17435390.2010.489206
Wang SH, Kurepa J, Smalle JA (2011b) Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ 34:811–820. https://doi.org/10.1111/j.1365-3040.2011.02284.x
Wang B, He X, Zhang ZY et al (2013a) Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res 46:761–769. https://doi.org/10.1021/ar2003336
Wang Q, Ebbs SD, Chen YS et al (2013b) Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 5:753–759. https://doi.org/10.1039/c3mt00033h
Wang J, Yang Y, Zhu HG et al (2014) Uptake, translocation, and transformation of quantum dots with cationic versus anionic coatings by populus deltoides x nigra cuttings. Environ Sci Technol 48:6754–6762. https://doi.org/10.1021/es501425r
Wang P, Lombi E, Zhao FJ et al (2016) Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 21:699–712. https://doi.org/10.1016/j.tplants.2016.04.005
Wang Y, Xiong LL, Tang M (2017) Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol 37:644–667. https://doi.org/10.1002/jat.3451
Wani AH, Shah MA (2012) A unique and profound effect of MgO and ZnO nanoparticles on some plant pathogenic fungi. J App Pharm Sci 2:40–44
Wen H, Dan M, Yang Y et al (2017) Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS One 12:e0185554. https://doi.org/10.1371/journal.pone.0185554
Wick P, Malek A, Manser P et al (2010) Barrier capacity of human placenta for nanosized materials. Environ Health Perspect 118:432–436. https://doi.org/10.1289/ehp.0901200
Wild E, Jones KC (2009) Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ Sci Technol 43:5290–5294. https://doi.org/10.1021/es900065h
Xiang L, Zhao HM, Li YW et al (2015) Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Environ Sci Pollut Res 22:10452–10462. https://doi.org/10.1007/s11356-015-4172-9
Xu PA, Zeng GM, Huang DL et al (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10. https://doi.org/10.1016/j.scitotenv.2012.02.023
Xu YY, Li HQ, Hedmer M et al (2017) Occupational exposure to particles and mitochondrial DNA – relevance for blood pressure. Environ Health 16:10. https://doi.org/10.1186/s12940-017-0234-4
Yadav T, Mungray AA, Mungray AK (2014) Fabricated nanoparticles: current status and potential phytotoxic threats. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol 230. Springer, Cham
Yamamoto Y, Miura T, Suzuki M et al (2004) Direct observation of ferromagnetic spin polarization in gold nanoparticles. Phys Rev Lett 93:116801
Yamashita K, Yoshioka Y, Higashisaka K et al (2011) Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol 6:321–328. https://doi.org/10.1038/nnano.2011.41
Yan SH, Zhao L, Li H et al (2013) Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J Hazard Mater 246:110–118. https://doi.org/10.1016/j.jhazmat.2012.12.013
Yoon SJ, Kwak JI, Lee WM et al (2014) Zinc oxide nanoparticles delay soybean development: a standard soil microcosm study. Ecotoxicol Environ Safe 100:131–137. https://doi.org/10.1016/j.ecoenv.2013.10.014
Yu XH, Hong FS, Zhang YQ (2016) Bio-effect of nanoparticles in the cardiovascular system. J Biomed Mater Res Part A 104:2881–2897. https://doi.org/10.1002/jbm.a.35804
Yuan DG, Shan XQ, Huai Q et al (2001) Uptake and distribution of rare earth elements in rice seeds cultured in fertilizer solution of rare earth elements. Chemosphere 43:327–337. https://doi.org/10.1016/s0045-6535(00)00142-9
Zhang ZY, He X, Zhang HF et al (2011) Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 3:816–822. https://doi.org/10.1039/c1mt00049g
Zhang P, Ma YH, Zhang ZY et al (2015) Species-specific toxicity of ceria nanoparticles to Lactuca plants. Nanotoxicology 9:1–8. https://doi.org/10.3109/17435390.2013.855829
Zhao LJ, Peralta-Videa JR, Rico CM et al (2014) CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J Agric Food Chem 62:2752–2759. https://doi.org/10.1021/jf405476u
Zhao LJ, Sun YP, Hernandez-Viezcas JA et al (2015) Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ mu-XRF mapping of nutrients in kernels. Environ Sci Technol 49:2921–2928. https://doi.org/10.1021/es5060226
Zheng L, Hong FS, Lu SP et al (2005) Effect of nano-TiO2 on strength of naturally and growth aged seeds of spinach. Biol Trace Elem Res 104:83–91. https://doi.org/10.1385/bter:104:1:083
Zhu H, Han J, Xiao JQ et al (2008) Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717. https://doi.org/10.1039/b805998e
Zhu ZJ, Wang HH, Yan B et al (2012) Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ Sci Technol 46:12391–12398. https://doi.org/10.1021/es301977w
Zuverza-Mena N, Martínez-Fernández D, Du W et al (2017) Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses – a review. Plant Physiol Biochem 110:236–264. https://doi.org/10.1016/j.plaphy.2016.05.037
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Pacheco, I., Buzea, C. (2018). Nanoparticle Uptake by Plants: Beneficial or Detrimental?. In: Faisal, M., Saquib, Q., Alatar, A., Al-Khedhairy, A. (eds) Phytotoxicity of Nanoparticles. Springer, Cham. https://doi.org/10.1007/978-3-319-76708-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-76708-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76707-9
Online ISBN: 978-3-319-76708-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)