Abstract
The burden of malignant pleural disease continues to rise, and the discovery of a malignant pleural effusion is a common problem in patients with lung cancer and malignant pleural mesothelioma. Symptomatic pleural involvement can present clinicians with a number of diagnostic and therapeutic challenges. Radiologically apparent pleural involvement requires thorough investigation and its discovery commonly represents metastatic disease across many tumour types. Histopathological pleural molecular subtyping has dramatically increased the availability of novel targeted therapies in advanced thoracic malignancy, and modern pleural intervention strategies can offer patients timely evidence-based fluid management. The impact of modern pleural research has reshaped the way in which patients are managed, shifting from what was historically a more surgical and radiological domain to the more medically focused approach. Developing pleural teams are in an excellent position to influence change in the current economically challenging environment by providing this group of patients with advanced disease, rapid management in an ambulatory setting. The use of validated prognostication tools and image-guided symptom control strategies help us to offer our patients more individualised pleural management. The chapter aims to summarise the available data around the pathophysiological mechanisms of pleural fluid production whilst considering methods of investigation, tumour staging and prognostication through to patient focused, therapeutic intervention strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pleural disease
- Malignant pleural effusion
- Tumour staging
- Lung cancer
- Malignant pleural mesothelioma
- Prognostication
- Pleural aspiration
- Intercostal chest drain
- Thoracoscopy
- Indwelling pleural catheter
Burden of Disease
Cancer is the leading cause of death globally accounting for around 8.8 million deaths annually. The World Health Organisation suggest that with lung cancer accounting more than 1.69 million deaths annually worldwide, it remains one the most frequent causes of cancer-related mortality, and is arguably the most deadly [36]. In men, around 85–90% of the cases of lung cancer are found to be attributable to tobacco smoking, and there remains a year-on-year global increase [36]. The majority of patients with lung cancer will present at stages III and IV with an overall survival of just 9.5–16.8%, respectively [13]. Regardless of gender, lung cancer remains a leading cause of mortality worldwide (WHO 2018 [36]; National Lung Cancer [22]; Torre et al. [31]).
Despite this, one of the greatest advances in modern lung cancer management over the last decade is the concept of personalised medicine, whereby therapeutic intervention is based upon specific histologic and genetic tumour characteristics [32]. In terms of cell types, non-small cell lung cancer (NSCLC) is the commonest type of lung cancer with an overall diagnostic incidence of 85% in all lung cancer cases. The two most common histopathologic large cell subtypes are that of adenocarcinoma and squamous cell carcinoma. Historically, little attention was paid to subtype distinction in those smaller tissues samples, and no therapeutic implications existed within the NSCLC classification. The situation changed dramatically with the discovery of the novel effective inhibitor targets, epidermal growth factor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements in patients with advanced lung adenocarcinoma [32]. Histopathological molecular testing is now a prerequisite in those tumours classified as adenocarcinoma or when an adenocarcinoma cannot be excluded. These advances in the understanding of such specific molecular pathways and genomic subtyping using immunohistochemistry have allowed clinicians offer a much more focused approach with genetically targeted immunological anticancer therapies [36].
Neuroendocrine tumours are found in up to 20% of lung cancer with its histological subtypes including large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC). The tumours are high-grade and largely peripheral, are commonly associated with cigarette smoking and have a much higher incidence in men once again. SCLC is distinguishable from NSCLC due to its rapid doubling time, high growth fraction and the early development of distant metastases [8]. NICE recommend assessment by a thoracic oncologist within one week following a decision to treat SCLC [21]. Although SCLC is known to be highly responsive to chemotherapy and radiotherapy, it is particularly aggressive with the majority of patients seeing a relapse with this broadly resistant disease, often at just a few months from the initial therapy.
In terms of occupationally related deaths, it is estimated that globally there are 2.3 million each year, with asbestos exposure contributing to the largest proportion of deaths [28]. Malignant pleural mesothelioma is a rare but fatal form of thoracic cancer meaning that a thorough occupational history and para-exposure history should be sought in those patients presenting with a suspected pleural malignancies with a causal link to asbestos exposure. High-risk occupations include dock and shipyard workers, electricians, plumbers and launderers [35]. The true global burden of disease from mesothelioma remains unclear; however, Delgermaa et al. [7] suggest crude and age-adjusted mortality rates for all mesothelioma deaths of 6.2 and 4.9 per million population, respectively, with a mean age at death of 70 years. The associated risk factors for a malignant pleural mesothelioma include a male prevalence and occupational exposure, and rarely, in familial cases, it is linked to the mutation of the breast cancer (BAP1) gene [35].
The NICE 2015 clinical guideline recommends a referral for an urgent chest radiograph to exclude mesothelioma in those aged over 40, who present with unexplained symptoms of cough, fatigue, breathlessness, chest pain and anorexia, when they may have never smoked or have evidence of prior asbestos exposure [20]. Pleural malignancy when discovered is typically unilateral, with bilateral involvement accounting for just 3% of cases, and a differentiation between a malignant pleural mesothelioma and metastatic pleural malignancy remains challenging [35]. The more recently published British Thoracic Society Mesothelioma [35] guidelines also advocate a more targeted approach in terms of diagnostic immunohistochemistry from both pleural biopsy and pleural cytology specimens. It is recommended that those who are diagnosed with lung cancer and mesothelioma have a care plan based on a holistic needs assessment at diagnosis and other key stages of care [19].
Pathophysiology and Pleural Burden
The pleura is a delicate membrane of mesothelial cells covering the lung and inner surface of the chest cavity, creating a pleural space or cavity that usually contains around 0.1–0.2 mL/Kg of fluid bilaterally. The pleural cavity is enclosed between the parietal mesothelium which is located on the inner surface of the thorax, the diaphragm and the mediastinal tissues and the visceral mesothelium present on the lung surfaces, and both membranes are joined at the level of the hilae. The pleural space and the physiological composition of the serous pleural fluid allows an almost frictionless apposition of the mesothelia throughout respiration, thus limiting any damage to opposing sliding surfaces (Negrini in Astoul [23]). The pleural fluid provides the essential lubrication which enables a synchronous lung and chest wall movement of which has been thought to facilitate adequate ventilation. Interestingly however, human studies do not appear to show any long lasting ventilatory effects following surgical removal in pleurectomy or following a chemical pleurodesis of this complex structure, thus raising the question of its physiological relevance [14].
In health, fluid enters the pleural space through the capillary network following which it is efficiently removed via the lymphatics of the parietal pleura. The normal volume of pleural fluid in an adult is around 17mls/day for a 70 kg person with a total pleural drainage of up to 1–2 L/day. The pleural lymphatics system has a large absorption capacity with a rate of reabsorption at 20 times the rate of production [17]. A pleural effusion quite simply describes an excess volume of fluid within the pleural cavity between the parietal and visceral pleura. Excessive fluid accumulation occurs when there are pathophysiological processes involving inflammation and impaired lymphatic drainage causing an imbalance between fluid production and fluid absorption [2]. An accumulation of pleural fluid can give rise to a restriction in forced vital capacity resulting in a ventilation defect; however, this commonly depends upon the amount of fluid, the rate of development and the underlying aetiology of disease.
It is well known that a number of both pulmonary and systemic causes can give rise to the pathological accumulation of pleural fluid. The diagnostic differentiation of pleural transudates and exudates following thoracentesis remains the single most important step in determining the aetiology of a pleural effusion [14]. A pleural fluid exudate is determined by a pleural protein of >30 g/L, and subsequent evaluation of the pleural pH, glucose, lactate dehydrogenase (LDH) red cell counts and gram staining and cytological analysis are required to further identify the underlying cause.
These are complex pathophysiological processes, and there can be an overlap within identification, for example, 25% of heart failure-related effusions may be exudative, especially when a patient is taking diuretics whereby a small number of transudative effusions may be proven malignant [14]. Light’s criteria can help further determine a transudate from an exudate. The three determinants that can be used are (a) pleural protein/serum protein ratio of >0.5 (b) pleural LDH/serum LDH ratio >0.6 or (c) pleural LDH >two thirds the upper limit of the laboratory reference range of serum LDH [12].
A radiologically apparent pleural effusion can be found in up to 0.3% of the population per annum [16]. A confirmed exudative pleural effusion following thoracentesis suggests disease from within the parietal pleura, and this can be due to a variety of inflammatory conditions with pneumonia being the most common. Patients with a symptomatic pleural effusion often present through an emergency route, commonly requiring both urgent diagnostic and therapeutic intervention of common symptoms that may be suspicious for malignancy. Unilateral pleural effusions or persistent bilateral effusions will almost always require additional evaluation to exclude an underlying malignancy in those fit enough for further investigation. The discovery of a malignant pleural effusion represents advanced metastatic disease and is seen in around 7–23% of patients with lung cancer, significantly affecting tumour staging and overall prognosis [16].
The precise pathophysiological mechanisms of pleural fluid formation and absorption will often depend upon the underlying aetiology [27]; however, radiologically apparent pleural effusions, pleural thickening or nodularity is concerning for malignancy, and such discovery will almost always require thorough investigation. A malignant pleural effusion is simply defined as an excess accumulation of exudative pleural fluid with the discovery of malignant cells [26]. Modern guidance and diagnostic pathways can help guide timing and urgency within the investigation of a pleural effusion, with the primary aim of establishing an underlying definitive diagnosis and excluding malignant disease [2] (Fig. 15.1).
Suggested algorithm for the early investigation of suspected pleural effusion [2]. (Permission granted by BMJ Publishing Group)
It is well described that lung cancer commonly affects the pleura in a number of ways, and whilst most confirmed pleural effusions in this setting are proven to be malignant, a nodular malignant extension to the pleural may not always produce a radiographically apparent effusion on chest imaging [16]. The goal of any initial evaluation in a suspected pleural malignancy is to obtain sufficient clinical and radiological information in order to inform suitable diagnostic tissue biopsy sampling, tumour staging and targeted treatment [30]. Pleural cytology has a mean sensitivity of 60%; however, yield depends upon the underlying tumour, sample preparation and pathologist experience [26]. Tumour cells tend to metastasise through the ipsilateral visceral pleura via the pulmonary vessels, and secondary dissemination of the parietal pleura occurs by seeding along adhesions in the pleural fluid [26].
Up to 70% of exudative pleural effusions will have malignancy confirmed with histological analysis [38]. Lung cancer, breast cancer and lymphoma are the commonest causes of malignant pleural effusion. It has been estimated that as many as 100,000 patients per year who have been diagnosed with lung cancer will go on to develop a pleural effusion with associated poor quality of life, affecting morbidity and mortality [16]. Malignant pleural mesothelioma is the most common type of primary pleural malignancy associated with a malignant pleural effusion [26]. The incidence of all-cause pleural malignancy continues to rise despite tumour type due to an ageing population with greater comorbidity [14]. There remains a steady increase in the number of confirmed lung cancer and malignant pleural mesothelioma cases, with presentation at stage IV remaining our biggest challenge [22].
Tumour Staging
The majority of malignant pleural effusion is caused by metastatic disease, and most commonly associated with lung cancer in men [26]. Modern lung cancer strategies continue to improve, and accurate tumour staging remains an essential element of such lung cancer management and prognostication. The globally validated TNM system considers anatomical spread of cancer by factors of tumour size and invasion, extent of lymphatic spread and presence of metastatic disease, and it informs multidisciplinary strategies for both clinical and surgical staging investigations and appropriate treatment strategies [37].
The International Association for the Study of Lung Cancer (IASLC) staging and prognostic factors committee examined data from 94,708 cases of lung cancer from around 16 countries around the globe. After exclusions, 70,967 cases of NSCLC and 6189 cases of SCLC were analysed to inform the eighth edition of the TNM classification for lung cancer. Updated descriptors and categories led to the migration of certain TNM subsets based upon survival analysis [9]. In terms of malignant pleural mesothelioma, data from 1987 patients across 29 centres was analysed. This comprised of 509 cases with only clinical staging information, 836 cases with only pathological staging information and 642 cases with both clinical and pathological information available [35] (Fig. 15.2).
Imaging
It is essential to confirm the aetiology of a pleural effusion in order to provide the most clinically appropriate and timely treatment. The role of imaging is firmly established in the workup of a suspected malignant pleural effusion [26]. It is known that malignant infiltration of the pleura is common across a number of different tumour types; however, pleural burden in the setting of lung cancer remains the highest incidence and often indicates an overall poor prognosis. Confirming malignant pleural disease in any setting can be challenging for practitioners and requires appropriate clinical examination, targeted chest radiographic techniques and timely referral. Pleural disease burden represents a significant challenge to both patients and healthcare resources in such a demanding economic environment.
Various imaging modalities are available to help guide diagnosis and optimise ongoing management strategies in pleural burden. Whilst there are several available imaging techniques, a posterior-anterior chest radiograph (CXR) remains the primary imaging method in an initial survey, often providing early indicators within initial tumour staging [33]. Pleural effusions may be radiologically apparent as blunting of the costophrenic recess on a chest radiograph with as little as 200mls of fluid and pleural deposits or thickening may be seen. Up to 15% of patients with lung cancer will have a demonstrable pleural effusion on chest radiograph at diagnosis [16]. Further diagnostic evaluation of the pleura will require the application of other imaging modalities such as thoracic ultrasound, computed tomographic (CT) scans of the thorax and abdomen, magnetic resonance imaging (MRI) and F-18-fluorodeoxyglucose positron emission tomography (FDG-PET) in order to detect thickened pleura or the malignant invasion of underlying structures.
Contrast-enhanced CT scanning is the current gold standard imaging modality for the pleura when seeking a diagnosis in a newly discovered pleural effusion; it may not only reveal a primary tumour, pleural thickening or nodularity but also may identify potential biopsy targets [2]. However, CT is not perfect, and data suggests that this modality will not demonstrate definitive evidence of malignancy in up to one in three patients with a pleural malignancy. Therefore, careful follow-up and assessment of suitability for further invasive diagnostic investigation depending upon performance status may be indicated if malignant radiological characteristics are not identifiable [26].
As in all areas of medicine, improving safety within invasive thoracic investigations is essential. The National Patient Safety Agency [11] undertook a review of 12 deaths and 15 cases of serious harm following pleural intervention. Common themes were identified around level of experience, supervision, site of intervention, anatomical anomalies and inadequate imaging. The report suggested that trainees should consider certain variables for pleural intervention including timing, training and familiarity of equipment, and it strongly advised the use of thoracic ultrasound for pleural intervention. The NPSA reports recommendation was mirrored by the British Thoracic Society pleural guidelines, whose evidence concur that ultrasound guidance will both increase the likelihood of success and reduce the risk of organ puncture in pleural intervention [10].
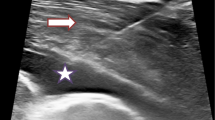
Much data exists suggesting thoracic ultrasound is a highly specific and sensitive tool used within imaging of the pleura. Thoracic ultrasound provides pleural teams with an instantly available imaging modality of increased sensitivity when quantifying and detecting pleural fluid in comparison to that of a plain chest radiograph alone. The discovery of pleural or diaphragmatic thickening and nodularity on thoracic ultrasound is highly specific for malignancy and can inform timely investigation into suspected malignancy [26]. The use of thoracic ultrasound is now commonplace in modern pleural management, and its diagnostic role in pleural burden extends beyond the identification of safe aspiration of fluid [10]. Ultrasound is not only more sensitive in detecting pleural fluid to help guide pleural drainage techniques and pleural-based masses, pleural thickening or nodularity is also easily visualised to improve reliability within the acquisition of targeted pleural biopsies [16]. Thoracic ultrasound guidance is well known to improve the rate of successful pleural intervention and reduce the risk of complications, with a sensitivity and specificity of 76.6% and 60.3% when compared to clinical judgement versus ultrasound, respectively [10].
Thoracic imaging modalities in pleural malignancy may confirm the presence of a non-expandable or trapped lung, suggesting an inability of the lung to expand normally within the pleural space [3]. Such radiological diagnoses of pleural burden combined with the subsequent discovery of malignant cells within the parietal pleura and pleural fluid reflect disseminated disease and poor performance status. Overwhelming symptomatic dyspnoea is described as the commonest symptom in this setting, reflecting a reduced compliance of the chest wall, depression of the ipsilateral diaphragm, mediastinal shift leading to a reduction in lung volume and impaired ventilation [27] (Fig. 15.3).
(a) Chest radiograph showing complete whiteout of the left hemithorax. The central location of the trachea concurs with the presence of underlying lung collapse and pleural effusion. (b) Thoracic ultrasound showing an echogenic pleural effusion, diaphragmatic inversion and nodularity. Transverse (c) and coronal (d) computed tomography images confirming complete left lung collapse and an associated pleural effusion. (Produced with permission from Wilczynska and Davies [34])
Patients with such extensive pleural burden often present in a debilitated state with a myriad of disabling symptoms including cough, chest discomfort, poor appetite, weakness and lethargy. Abnormal thoracic imaging can require a number of diagnostic and therapeutic strategies using invasive techniques such as thoracentesis, intercostal chest drain insertion, thoracoscopy and targeted pleural biopsy depending upon performance status and overall prognosis [18].
Prognostication
Many factors are used to predict overall survival in malignant pleural disease burden with the decision to offer pleural intervention depending upon the patients wishes, presence of symptoms and mean survival rate. Prognostication in malignant pleural disease depends upon a number of variables, and an individualised approach to predicting survival should be taken. Appropriate patient selection is vital, and it is essential that any pleural procedures are carried out in the patients’ best interest and not just because of technical possibility [3]. Treatment options are often determined by symptoms, performance status, tumour type and its response to systemic therapy and the degree of expansile lung [27].
It is known that a malignant pleural effusion represents advanced metastatic disease, and data suggests a median survival of 3–12 months, dependent upon a number of individual factors, with the shortest recognised survival time commonly observed in those malignant effusions secondary to lung cancer [4, 15, 27]. Research suggests that a massive pleural effusion is associated with a worse prognosis, independent of age or histological tumour type, and survival time is worse in all stages of lung cancer [38].
A large number of mainly retrospective studies have examined factors determining prognosis. Analysis of demographics, pathological tumour type, symptoms, performance status and markers of inflammation have been examined in an attempt to determine accurate prognosis when guiding management [35]. The retrospective data consistently demonstrated that performance status was an independent predictor of survival in malignant pleural disease allowing appropriate selection for palliative management; however, there was a need for wider research [38]. Chest wall pain and weight loss have also been examined as prognostic variables in malignant pleural mesothelioma, and both were independently associated with poorer overall survival and the recent mesothelioma guidelines recommending the use of prognostic scores at diagnosis [35].
Clive et al. [4] provided the largest series of prospective prognostication data to inform their LENT predictive tool. Data were obtained from 221 patients from the UK, the Netherlands and Australian cohorts, and survival analysis was examined. The data found a wide range of median survival, with a 74-day median survival in the lung cancer group when compared with 339 days in the malignant pleural mesothelioma group. A clinical risk score was created to help predict survival and guide management in those with a malignant pleural effusion. The LENT score examined four key variables inclusive of pleural fluid lactate dehydrogenase (LDH), eastern cooperative oncology group (ECOG) performance status, serum neutrophil to lymphocyte ratio (NLR), and histological tumour type. The combined LENT prognostication score risk stratified patients into low-, medium- and high-risk groups with a median (IQR) survival of 319, 130 and 44 days, respectively. Again, those with lung cancer commonly fell into the highest-risk category, and systemic inflammation was an important risk factor. A higher LENT score was found to be associated with a worse overall prognosis, and combined scoring was found to be statistically superior to that of performance status alone [4].
Validated prognostic scores are easy to calculate and can often help inform clinicians when considering suitability for pleural intervention. Pleural drainage may not offer an overall survival benefit; however, any subsequent interventions should be aimed at relieving disabling respiratory symptoms and improving quality of life. Therapeutic pleural drainage can rapidly improve performance status thus having a positive effect upon quality of life in patients with advanced malignancy. It is well reported that symptomatic malignant pleural effusion represents an advanced metastatic disease, and given the continued rise in new cancer diagnoses, the pleural burden for the patient often remains high [4]. In terms of prognostication, when expected survival is short, a less-invasive and palliative-focused end of life approach is preferred [38].
Pleural Management Strategies
Modern oncological treatments are more advanced, and accurate prognostication at presentation may help individualise treatment strategies [4]. Pleural disease is recognised as an important subspecialty within respiratory medicine, and more recently there has been a paradigm shift from the traditional surgical approach to a more medically and patient focused perspective. Historically, patients with malignant pleural disease were often managed conservatively, whereas more recently patients presenting with a suspected pleural malignancy are now “genotyped, phenotyped and treated on an ambulatory basis” [26].
Specialist pleural teams are in a position to offer patients who have traditionally needed extended admissions, more timely pleural intervention in an ambulatory setting. Not only does this approach demonstrate improved patient safety, it also has positive effects upon waiting times, admission duration and overall bed day costs. Hospital pleural teams are truly multidisciplinary usually with a lead respiratory consultant with an interest in pleural disease management at its core. A clinical nurse specialist is essential for holistic needs assessments and advance care planning, research and, more recently, many have developed practical skills within thoracic ultrasound and autonomous pleural intervention. A successful pleural service depends upon the support of a wide array of specialist services across oncology, radiology, pathology, nursing, clerical and surgical teams. Pleural teams are in an excellent position to streamline care and enhance a patient-focused pathway, and such specialist review not only informs early diagnoses and prognosis but also provides rapid therapeutic intervention whilst strengthening practical training across specialities [1]. Enhancing pleural disease pathways however remains dependent upon the availability of pleural facilities available within individual organisations [3].
Guidelines suggest that a malignant pleural effusion is best managed through complete pleural drainage and instillation of a sclerosant to promote pleurodesis to prevent reaccumulation or by the insertion of a more permanent device to enable repeated community drainage [27]. The Clive et al. [5] Cochrane meta-analysis examined 62 studies involving 3248 patients to try to determine the optimal management for adults with a malignant pleural effusion in terms of pleurodesis success [5]. They examined administration of a pleurodesis agent using a chest tube or thoracoscopy and indwelling pleural catheters. The outcome suggested that talc poudrage following medical thoracoscopy appeared to be the most effective method of preventing fluid reaccumulation; however, patient-centred outcomes including side effects, quality of life and patient satisfaction were inconsistently reported calling for wider research in this area [5] (Fig. 15.4).
Management algorithm for malignant pleural effusion [27]. (Produced with permission from BMJ Publishing Group Ltd. & British Thoracic Society)
Patient presentation and the subsequent urgency of intervention for a pleural effusion will always depend upon the magnitude of pleural burden, the rate of fluid accumulation and the patients underlying respiratory reserve [2]. The majority of patients with malignant pleural disease, especially in those with a massive pleural effusion, will be symptomatic, and modern guidance advocates timely and definitive management strategies over repeated thoracentesis [24]. Patients should always be offered an initial therapeutic procedure to assess both symptomatic improvement and rate of fluid reaccumulation before considering patient-focused definitive management [26]. If the patient does not gain relief from pleural drainage, then further invasive management is rarely indicated, and a more supportive, palliative care-based approach should be taken. Any informed treatment decisions should always be patient-centred and recommendations should be based upon performance status, burden of symptoms and expected survival times using evidence-based prognostication [3, 4, 27, 35].
Observation
All patients presenting with a unilateral pleural effusion should have timely and appropriate investigations to exclude malignancy, and pleural investigations for bilateral pleural effusion may be considered if there are atypical features or a failure to respond to initial therapy [10]. Standard blood tests can be helpful to assess for the presence of co-existing infection or blood loss, and they also help evaluate cardiac, renal and hepatic function in order to inform differential diagnoses [2]. Observation is rarely indicated in the setting of confirmed malignancy as most patients with a radiologically apparent pleural effusion will have presented with significant symptoms of breathlessness and cough, with a number experiencing disabling chest pain due to disseminated pleural malignancy. Howevere, there are a small proportion of patients in whom pleural disease is found incidentally upon routine chest imaging, and they may describe minimal symptoms. In those with a confirmed malignant pleural effusion, observation may be recommended if a patient is asymptomatic and the tumour type is known; however, most patients should be offered early follow-up and be made aware of the available treatment options for definitive pleural intervention as most will become symptomatic over time [27].
Thoracentesis
Thoracentesis or pleural aspiartion is defined as a minimally invasive, sterile procedure, whereby a needle or catheter is inserted through the subcutaneous tissues of the thorax, over the superior surface of the rib, avoiding the intercostal neurovascular bundle, through the parietal pleura and into the pleural cavity in order to obtain a pleural fluid sample. This is usually the first-line enquiry when investigating an unexplained unilateral pleural effusion or persistent bilateral pleural effusion. In those patients presenting with larger pleural effusions in the setting of a known malignancy, both a diagnostic and therapeutic approach is also required, not only to confirm if the histological cell type is related to a known malignancy or if a synchronous primary exists. The risks of thoracentesis include bleeding, infection, pneumothorax and visceral injury, but these risks are reduced with an experienced operator or an appropriately supervised trainee. With re-expansion pulmonary oedema after the removal of larger volumes of fluid, however, the risk is low [26]. The safety of pleural intervention will be further enhanced through the use of point-of-care thoracic ultrasound, leading to a greater chance of success within both diagnostic aspiration and therapeutic drainage of larger volumes [2]. The primary aims of thoracentesis in this setting are to both secure a pathological tissue diagnosis and alleviate any disabling symptoms that a pleural effusion may cause.
The sensitivity of a cytological yield following thoracentesis in a suspected malignant pleural effusion often depends upon the underlying malignancy but in diagnostic terms, pleural sampling may give rise to an overall initial diagnostic sensitivity in up to 60% of cases [27]. Histopathological analysis of pleural fluid in lung adenocarcinoma may have a 78% yield, whereas confirmation of mesothelioma and squamous cell carcinoma represents 27% and 25%, respectively [25]. Conversely, there are also a small number of patients with lung cancer in whom microscopic pleural analysis actually excludes a metastatic pleural malignancy, in which case it is recommended that the pleural effusion is excluded as an M1a staging descriptor [9]. The diagnosis of a malignant pleural mesothelioma from cytological pleural fluid testing is known to be highly variable, ranging from 16% to 73%, with immunohistochemistry from a pleural biopsy shown to give a more consistent yield in the mesothelial subtypes of epithelioid, sarcomatoid and biphasic mesothelioma. Although lung cancer is reported to be associated with short median survival times, non-epithelioid histology in the setting of malignant pleural mesothelioma is also associated with a significantly shorter overall survival [35].
Histological confirmation in all-cause malignancy within the pleura or pleural fluid represents advanced disease and presents practitioners with complex management challenges around early symptom control, prognostication and choice, in terms of suitability for definitive pleural management. This patient group have a high burden of disease with a worse prognosis and minimal life expectancy with a deteriorating performance status. Repeated therapeutic thoracentesis is only usually recommended for those with chemotherapy-sensitive tumours such as SCLC and lymphoma to enable early treatment or in those obviously at the end of life [26]. A more definitive approach is usually preferred in all tumour types to enhance longer-term symptomatic relief and reduce the risk of pleural adhesions that may complicate thoracoscopic poudrage or indwelling pleural catheter insertion at a later date.
Intercostal Chest Drain
Small-bore Seldinger tube drains are traditionally used in the drainage of malignant pleural effusions allowing subsequent insertion of sterile-graded talc as a sclerosant to aid pleurodesis. Chest drains for pleural effusions are inserted using point-of-care thoracic ultrasound, and larger effusions should be drained in a controlled manner in order to reduce the risk of re-expansion pulmonary oedema. The insertion of talc slurry into a chest drain is thought to cause an acute inflammatory response through the local activation of the coagulation cascade and fibrin deposition [27]. Successful pleurodesis is defined as fusion of the parietal to visceral pleural with resulting obliteration of the pleural space [26]. The most important requirement that informs the potential effectiveness of pleurodesis is a radiologically confirmed apposition of the parietal and visceral pleura. Incomplete expansion may be caused by pleural thickening in a non-expandable or trapped lung, and proximal large airway obstruction from tumour or persistent air leaks are known to be associated with pleurodesis failure [27]. Intercostal chest drains play an important part in the management of pleural effusions; however, they are usually associated with prolonged hospital stays of between 4 and 7 days for talc pleurodesis [26] and a greater risk of complications including unintentional displacement, persistent air leaks, and interpleural infection. Although more modern pleural intervention has largely replaced the standard use of Seldinger drains, they still play a part in those decompensated patients presenting an emergency with a massive pleural effusion requiring urgent pleural intervention.
Thoracoscopy
Thoracoscopy either under sedation or general anaesthesia is the investigation of choice for the diagnosis of a suspected malignant exudative pleural effusion in those with inconclusive pleural cytology [10]. It is also useful for complete pleural drainage and talc poudrage in those with a better performance status and a confirmed malignant pleural effusion, and it is associated with a more successful chance of pleurodesis in around 80–90% of patients [10, 27]. The ultrasound-guided procedure involves the introduction of an induced pneumothorax followed by complete drainage of pleural fluid, acquisition of fluoroscopic pleural biopsies and, finally, directly visualised talc poudrage in the majority of patients. An overnight admission is usually required in order to reinflate the induced pneumothorax; however, larger centres have demonstrated shorter stays with the use of portable suction devices.
The more invasive, surgical video-assisted thoracoscopy that is performed under general anaesthesia and requires single lung ventilation. It remains appropriate in a subset of patients with benefits demonstrated in those with smaller pleural effusions containing more septations and adhesions [29]. The overall advantage, however, is that the surgeons are in a better position to proceed with other thoracic surgical options at the time of procedure if deemed appropriate [10]. The alternative approach of local anaesthetic thoracoscopy performed by respiratory physicians under sedation, when compared to surgical video-assisted thoracoscopy, is an increasingly available alternative offering a similar diagnostic sensitivity of 92.6% in comparison to 95%, respectively [2, 10]. Thoracoscopy is a safe and well-tolerated procedure in carefully selected patients, it has a low perioperative mortality rate of <0.5% [27], and major complications such as empyema, haemorrhage and pneumonia are rare [10].
Indwelling Pleural Catheter
Traditionally, patients presenting with a suspected pleural effusion would be admitted to a secondary care facility for a battery of diagnostic investigations and extended therapeutic intervention; however, changes in attitudes and technology now facilitate an ambulatory care model in this setting [2].
Fenestrated indwelling silicone pleural catheters are now commonplace in the developed world, and although traditionally only recommended for those with an underlying non-expandable or trapped lung, or failed pleurodesis, there has been a shift towards their first-line use as an alternative to pleurodesis following the TIME-2 trial [3, 6]. Patients may wish to minimise the time spent in hospital by choosing an indwelling pleural catheter over attempted pleurodesis given median predicted survival times of just 44 days (Clive et al. [4]). Indwelling pleural catheters were reported to improve breathlessness when compared to talc slurry pleurodesis, despite lower pleurodesis success rates (Clive et al. [5]; Fig. 15.5).
Tunnelled catheters are inserted as a day case using point-of-care ultrasound guidance, allowing community drainage with the cost-effective devices [16]. Complications following insertion are rare but may include pain, pneumothorax and infection due to tunnelling, with many centres advising prophylactic antimicrobials immediately following insertion. Smaller-scale studies suggest that tunnelled catheters are safe in chemotherapy [2]. Patients or family members can be trained using drainage bottles that are attached to the one-way valve, and community nurses and palliative care teams are in a valuable position to offer ongoing support.
The most commonly reported symptom when established tunnelled catheters is pain during drainage. However, this can be easily managed by administrating pre drainange opiate analgesia and ensuring controlled pleural drainage. Spontaneous pleurodesis will occur in between 50% and 70% of cases, and this can be measured by radiological confirmation and the absence of pleural fluid following which the catheter may be removed (Mishra et al. [16]). Indwelling pleural catheters are highly suitable for use in patients with symptomatic malignant pleural disease, lung entrapment, poorer performance statuses and higher LENT prognostic scores.
Conclusion
In summary, classifying the underlying aetiology of pleural burden is vital to inform tumour staging, prognostication and therapeutic intervention strategies. Despite novel advances within histopathologic subtyping and therapeutics, many patients develop with advanced and incurable lung cancer requiring a palliative approach. Patients are more commonly presenting with stage III and IV disease at an older age and with multiple pre-existing comorbidities [13]. The majority of patients with malignant pleural disease will be symptomatic, and modern guidance advocates timely and definitive pleural strategies. Patients with disseminated pleural disease in suspected or confirmed thoracic malignancies will often have disabling symptoms requiring early specialist therapeutic intervention. Despite whichever pleural management approach is chosen, a supportive holistic assessment is always recommended to explore the patient’s wishes, and support them with their ability to cope both physically and psychologically with intervention and the timing of recovery [3, 15, 19].
References
Bhatnagar R, Maskell N. Developing a ‘pleural team’ to run a reactive pleural service. Clin Med. 2013;13(5):452–6.
Bhatnagar R, Maskell N. The modern diagnosis and management of pleural effusions. Br Med J. 2015;351:h4520:1–8.
Bhatnagar R, Cocoran JP, Maldonado F, Feller-Kopam D, Janssen J, Astoul P, Rahman NM. Advanced medical interventions in pleural disease. Eur Respir Rev. 2016;25(140):199–213.
Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69(12):1098–104.
Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016;5:CD010529.
Davies HE, Mishra EK, Brennen CK, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnoea in patients with malignant pleural effusion: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnoea in patients with malignant pleural effusion. The TIME2 randomised control trial. J Am Med Assoc. 2012;307(22):2383–9.
Delgermaa V, Takahashi K, Park E, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89(10):701–76.
Glisson BS, Byers LA. Pathobiology and staging of small cell carcinoma of the lung. 2017. https://www.uptodate.com/contents/pathobiology-and-staging-of-small-cell-carcinoma-of-the-lung. Accessed 12 Feb 2018.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhard WEE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol. 2015;11(1):39–51.
Hooper C, Lee YGC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society guideline. Thorax. 2010;65:ii4–17.
Lamont T, Ml S-P, Scarpello J, Durand M, Hooper C, Maskell N, et al. Insertion of chest drains: summary of a safety report from the National Patient Safety Agency. Br Med J. 2009;339:b4923.
Light RW, Macgregor MI, Luchsinger PC, Wilmot C, Ball JR. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(44):507–13.
Lim RBL. End of life care in patients with advanced lung cancer. Ther Adv Respir Dis. 2016;10(5):455–67.
Maldonado F, Lentz RJ, Light RW. Diagnostic approach to pleural diseases: new tricks for an old trade. F1000Res. 2017;1135:1–6.
Maskell N. Treatment options for malignant pleural effusion: patient preference does matter. J Am Med Assoc. 2012;307(22):2432–3.
Mishra A, Davies HE, Lee YCG. Malignant pleural disease in primary lung cancer. Eur Respir Mon. 2009;44:318–35.
Mordant P, Arame A, Legras A, Le Pimpec A, Barthes F, Riquet M. Pleural lymphatics and effusions. Rev Pneumol Clin. 2013;69(3):175–80.
Mountain CF, Hermes KE. The role of imaging in lung cancer. Cancer Imaging. 2008;1:163–70.
National Institute for Clinical Excellence. Quality standard for lung cancer in adults. 2012. www.nice.org.uk/guidance/qs17. Accessed 4 Feb 2018.
National Institute for Clinical Excellence. Suspected cancer; recognition and referral. 2015. https://www.nice.org.uk/guidance/ng12/resources/suspected-cancer-recognition-and-referral-pdf-1837268071621. Accessed 12 Feb 2018.
National Institute for Clinical Excellence CG 121. Lung cancer: diagnosis and management. 2011. https://www.nice.org.uk/guidance/cg121/resources/lung-cancer-diagnosis-and-management-pdf-35109444863941. Accessed 12 Feb 2018.
National Lung Cancer Audit annual report 2017. Royal College of Physicians. Healthcare Quality Improvement Partnership (HQIP). 2018. Accessed 4 Feb 2018.
Negrini D. Physiology and pathophysiology of the pleural space. In: Astoul P, Tassi G, Tschopp JM, editors. Thoracoscopy for pulmonologists. Berlin, Heidelberg: Springer; 2014.
Ost DE, Niu J, Zhao H, Grosu HB, Giordano SH. Quality gaps and comparative effectiveness of management for recurrent malignant pleural effusions. Chest. 2018;153(2):438–52.
Porcel JM, Esquerda A, Vives M, Bielsa S. Aetiology of pleural effusions: analysis of more than 3000 consecutives thoracentesis. Archivos de Bronconeumología. 2014;50(5):161–5.
Psallidas I, Kalomenidis I, Porcel JM, Robinson BW, Stathopoulos T. Malignant pleural effusion: from bench to bedside. Eur Respir Rev. 2016;25:189–98.
Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ on behalf of the BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline. Thorax. 2010;65(Suppl 2):ii32–40.
Rushton L. The global burden of occupational disease. Curr Environ Health Rep. 2017;4:340–8.
Schiech L. Malignant pleural effusions. 2015. http://www.theoncologynurse.com/ton-issue-archive/2015-issues/march-vol-8-no-2/16361-malignant-pleural-effusions. Accessed 12 Feb 2018.
Thomas KW, Gould MK. Overview of the initial evaluation, diagnosis and staging of patients with lung cancer. 2018. https://www.uptodate.com/contents/overview-of-the-initial-evaluation-diagnosis-and-staging-of-patients-with-suspected-lung-cancer. Accessed 12 Feb 2018.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65(2):87.
Travis WD, Brambilla MD, Nicolson AG, Yasushi Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organisation classification of lung tumours. Impact of genetic, clinical and radiographic advances since the 2004 classification. J Thorac Radiol. 2015;10(9):1243–60.
Walker S, Bibby A, Maskell N. Current best practice in the evaluation and management of malignant pleural effusions. Therapeutic Advances in Respiratory Disease. 2017:105–14. https://doi.org/10.1177/1753465816671697.
Wilczynska MM, Davies HE. Management of a refractory malignant pleural effusion in a patient with small cell lung cancer: a case report. Int J Respir Pulm Med. 2015;2:2–3.
Woolhouse I, Bishop L, Darlison L, De Foneska D, Edey A, Edwards J, et al. British Thoracic Society guideline for the investigation and management of malignant pleural mesothelioma. Thorax. 2018;73(i):1–130.
World Health Organisation. Key cancer facts. 2018. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 12 Feb 2018.
Yim APC, Ng CSH. Lung cancer staging. In: Schwab M, editor. Encyclopedia of cancer. Berlin, Heidelberg: Springer; 2011.
Zamboni MM, Teixeria da Silva C, Baretta R, Cunha ET, Cardosa GP. Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med. 2015;15:29.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Parsonage, M. (2019). Management of Pleural Burden in Metastatic Lung Cancer and Malignant Pleural Mesothelioma. In: Charnay-Sonnek, F., Murphy, A. (eds) Principle of Nursing in Oncology . Principles of Specialty Nursing. Springer, Cham. https://doi.org/10.1007/978-3-319-76457-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-76457-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76456-6
Online ISBN: 978-3-319-76457-3
eBook Packages: MedicineMedicine (R0)