Abstract
The abiotic and biotic stresses in the environment trigger the synthesis of ethylene, a gaseous plant hormone that leads to many physiological changes in plants that inhibit plant growth and development. Plant growth inhibition that accrues from the stress can be overcome to a large extent by lowering the amount of ethylene that is synthesized in response to stress by the application of ACC deaminase-producing bacteria. The plant growth-promoting bacteria also give other multifacet benefits as they promote plant growth through enhancing nutrient uptake and hormone production and acting as a biocontrol agent. The enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase present in bacterium cleaves ACC, the precursor of ethylene, and thereby lowers the ethylene level and facilitates plant growth. Plants respond similarly to ACC deaminase regardless of whether the enzyme is expressed in the roots of transgenic plants or as part of a root-associated bacterium. The role of growth-promoting bacteria is encouraging in this area. Thus overcoming stress responses in plants through ACC deaminase-producing bacteria has come up as an effective tool, when the agricultural practices worldwide are seeking a sustainable approach to the issues of food security under the changing climatic conditions. The present review discusses the role of ACC deaminase-producing bacteria in ameliorating the various abiotic stress responses in plants induced by ethylene.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
12.1 Introduction
Plant growth in its entire lifetime is challenged by many biotic and abiotic stresses. The abiotic stresses may be extremes of temperature, salt, high light, flooding, drought, presence of toxic metals and organic contaminants, radiation, and wounding, and the biotic stresses may include insect predation and attack by various pathogens like viruses, bacteria, and fungi (Abeles et al. 1992). Most of the adverse effects of stress on plant metabolism occurs in the form of osmotic stress or salt toxicity, ROS production, ethylene production, and nutrient imbalance, which overall affect the plant physiology and inhibit seedling growth, vigor, flowering, and fruit setting (Sairam and Tyagi 2004).
Soil which adheres the plant and provides water and nutrients is rich in microbial diversity. The soil microbial diversity includes the bacteria, actinomycetes, fungi, algae, and protozoa. A fertile soil per gram contains 9 × 107 bacteria, 4 × 106 actinomycetes, 2 × 105 fungi, 3 × 104 algae, 5 × 103 protozoa, and 3 × 101 nematodes (Alexander 1991). The rhizospheric bacterial count is 10–1000 times higher than the count in bulk soil as the root exudates contain carbohydrates (sugars and oligosaccharides), organic acids, vitamins, nucleotides, flavonoids, enzymes, hormones, and volatile compounds that diffuse into the rhizosphere and support microbial growth and activity. Plant growth-promoting rhizobacteria (PGPR) are a group of free-living saprophytic bacteria living in plant rhizosphere that aggressively colonize the root system and promote plant growth and act as biocontrol agents against plant diseases (Kloepper and Beauchamp 1992). Plant growth-promoting bacteria promote plant growth and development through many direct and indirect mechanisms. The indirect mechanisms include their ability to act as biocontrol agent on phytopathogens, and the direct include fixation of nitrogen, enhanced nutrient uptake through iron sequestration and phosphate solubilization, and production of hormones like indole-3-acetic acid (IAA) and cytokinin (Glick et al. 1999). Plant growth-promoting rhizobacteria elicit the so-called induced systemic tolerance (IST) in plants under different abiotic stresses by altering the plant metabolism. Production of IAA is a common growth-promoting trait observed in up to 80% of the soil bacteria and bacterial endophytes (Patten and Glick 1996). Besides the above benefits, PGPR also benefit the plants by lowering plant ethylene levels through the activity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme present in them (Glick et al. 1998). The ethylene level gets elevated during stresses which may be abiotic like temperature, salt, high light, flooding, drought, toxic metals, organic contaminants, and radiation or biotic like attack of viruses, bacteria, and fungi (Abeles et al. 1992). Ethylene was originally regarded as a “stress hormone” due to its accelerated synthesis by plant in response to stress signals (Kende 1993; Johnson and Ecker 1998). Ethylene hormone induces physiological changes in plant growth like overcoming dormancy, differentiation, formation of adventitious roots, abscission of leaf and fruit, induction of flowering and femaleness in dioecious plants, senescence, and fruit ripening (Arshad and Frankenberger 2002; Owino et al. 2006). However, high level of ethylene leads to senescence and abnormal root growth. As ethylene production in plant roots gets accelerated under biotic and abiotic stress factors which have an inhibitory effect on root growth which in turn leads to abnormal plant growth, it becomes vital to regulate the ethylene production in the rhizosphere of plant to achieve normal growth and development. Bacterial strains with ACC deaminase activity are capable of overcoming the ethylene-induced negative responses in plants to a great extent. Bacterial ACC deaminase activity is a widespread character of the rhizospheric bacteria most commonly observed in bacteria residing in stressful conditions (Timmusk et al. 2011). ACC deaminase activity of bacteria endows plants with the capability to withstand the stress better and therefore survive in harsh environmental conditions. Inoculation with PGPR containing ACC deaminase activity has come up as an alternative sustainable approach in improving plant growth and development under stress conditions by reducing stress-induced ethylene production.
12.2 Mechanism of Action
Ethylene is an endogenously produced gaseous plant growth hormone by plants. Plants undergoing any stressed situation show an increased production of ethylene, and for this reason it is also known as a stress hormone. On the onset of stress, an initial small peak of ethylene of low magnitude for a few hours is observed, and then a second much larger peak of high magnitude for 1–3 days is observed (Stearns and Glick 2003; Pierik et al. 2006; Van Loon et al. 2006). The second peak initiates protective response in plants, like transcription of pathogenesis-related genes and acquired resistance (Ciardi et al. 2000; Van Loon and Glick 2004). The second ethylene peak is so large that processes such as senescence, chlorosis, and abscission are initiated, the overall effect of which is generally inhibitory to plant survival. Plant can be released from the inhibitory levels of ethylene by degrading its precursor like S-adenosylmethionine (derived from l-methionine) or ACC, which effectively reduces the ethylene levels. Among these, the enzymes ACC deaminase, S-adenosylmethionine (SAM) hydrolase, and SAM decarboxylase are much being worked on.
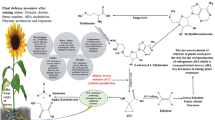
Normally the root exudates are estimated to contain 5–30% of the photosynthetically fixed carbon. The plant root exudates contain tryptophan which is taken by the rhizospheric bacteria and used as a precursor in the synthesis of indole-3-acetic acid (IAA), some of which is taken up by the plant. This bacterial IAA together with endogenously synthesized plant IAA stimulates cell proliferation and cell elongation. It also induces the transcription of ACC synthase which leads to the formation of 1-aminocyclopropane-1-carboxylic acid (ACC) which is the intermediate precursor of ethylene in higher plants (Yang and Hoffman 1984). Small amount of this ACC is exuded from the seeds or roots (Penrose et al. 2001; Grichko and Glick 2001a) which may be taken up by the rhizospheric bacteria. The enzyme present in rhizobacteria ACC deaminase hydrolyzes the ethylene precursor ACC into ammonia and α-ketobutyrate (Glick et al. 1994, 1998; Mayak et al. 1999; Shaharoona et al. 2006). This activity of the rhizobacteria decreases the amount of ACC, and in turn the ethylene level, in the spermosphere and rhizoplane and thereby eliminates its potential inhibitory effects on plants (Glick et al. 1998). Thus the rhizobacterium acts as a sink for the plant-synthesized ACC (Fig. 12.1). Plants inoculated with PGPR possessing ACC deaminase activity are relatively more tolerant to environmental stress (Naveed et al. 2014). When plants get exposed to stress conditions, the gaseous hormone ethylene endogenously regulates plant homeostasis, resulting in reduced root and shoot growth.
The increased ethylene levels in plant cause a feedback inhibition of the IAA signal transduction pathway and thereby limit the ACC synthase transcription (Burg and Burg 1966; Glick et al. 2007) by IAA. Bacteria capable of synthesizing IAA and possessing ACC deaminase activity are more beneficial in plant growth promotion as they do not allow the elevated levels of ethylene being formed in the plants. This prevents feedback inhibition of IAA signal transduction by ethylene, and the plant growth continues in response to the auxin.
12.3 Biochemistry of ACC Deaminase
ACC deaminase belongs to the tryptophan synthase beta superfamily of pyridoxal phosphate-binding proteins (Glick et al. 2007). ACC deaminase is a multimeric enzyme that is cytoplasmically localized. ACC deaminase subunit mass is approximately 35–42 kD, while its native size is estimated to be approximately 100–112 kD (Sheehy et al. 1991; Jacobson et al. 1994; Hontzeas et al. 2004a). The affinity of this enzyme for the substrate is not particularly high (Km = 1.5–6.0 mM). The coenzyme pyridoxal phosphate is a cofactor of ACC deaminase (Honma 1985), and even ACC synthase, the enzyme that catalyzes the formation of ACC, requires pyridoxal phosphate for its enzyme activity. ACC deaminase enzyme is present in bacteria at a very low amount, and at the same time, ACC oxidase has a much higher affinity for ACC compared to ACC deaminase (Glick et al. 1998). The ethylene levels in bacteria depend upon the ratio of the two, i.e., ACC oxidase and ACC deaminase (Glick et al. 1998). However, ACC deaminase synthesis is induced by ACC, at levels as low as 100 nM (Jacobson et al. 1994), with full induction requiring up to 10 h. The amino acids l-Ala, dl-Ala, and dl-Val can also induce enzyme activity to a small extent, and g-aminoisobutyric acid can induce activity to almost the same level as ACC (Honma 1983). Maximal enzyme activity typically occurs at 30 °C and pH 8.5. The affinity for the substrate ACC and the competitive inhibitors l-Ala and l-Ser is also highest at pH 8.5 (Hontzeas et al. 2006). acdS genes that have been traced in some stramenopiles, bacteria, and various fungi (Ascomycota and Basidiomycota) are believed to have a common ancestor (Nascimento et al. 2014). The genes are commonly transmitted vertically in various microorganisms, and occasional horizontal gene transfer is also observed including inter-kingdom transfer events. ACC deaminase genes (including both the structural gene acdS and the regulatory gene acdR) have been found in many different rhizobacteria (rhizospheric, endophytic, and rhizobia), including Azospirillum spp., Rhizobium spp., Agrobacterium spp., Achromobacter spp., Burkholderia spp., Ralstonia spp., Pseudomonas spp., and Enterobacter spp. (Blaha et al. 2006). More importantly, even if some strains of a particular genus and species have an acdS gene, not all strains do.
12.4 Role of ACC Deaminase Bacteria in Ameliorating Various Stress Responses in Plants
A common invariable observation in plants exposed to stress is an increased ethylene level, which leads to damage. Ethylene production upregulates in response to the presence of metals, organics, salt, temperature extremes, drought, ultraviolet light, damage by insects, nematode, and phytopathogens (Abeles et al. 1992). The second ethylene peak observed in plants exposed to stress is more detrimental to plant growth as this initiates processes such as senescence, chlorosis, and leaf abscission. Any treatment whether it is chemical or biological that lowers the magnitude of the second peak of ethylene reduces the damage caused to the plant as a stress consequence. Microorganisms exhibit wide range (>100-fold) in ACC deaminase activity, and it has been observed that organisms that express high ACC deaminase are beneficial as they are nonspecific toward their host (Glick 2005). This group encompasses most of the rhizospheric and phyllospheric microbes along with the endophytes. Such microbes reduce the ethylene by acting as a sink for ACC produced as a consequence of stress. Addition of an ACC deaminase producing PGPR and its negative mutant strain in canola roots (Hontzeas et al. 2004b) showed down regulation of genes involved in ethylene induced plant stress responses and up-regulation of genes involved in plant growth. The results supported that plant growth-promoting bacteria expressing ACC deaminase are able to overcome the stress response in plants. Different ACC deaminase-producing bacteria have been demonstrated for their efficacy in protecting plants against yield loss induced by various abiotic stresses as listed in Table 12.1.
12.4.1 Salt Stress
Salinity, which was a natural feature of ecosystems in arid and semiarid regions, has now become a major constrain due to the anthropogenic activities, primarily due to irrigation of agricultural fields (Abrol et al. 1988). Of the total global cultivable area, 20% is under salinity stress, and this is continuously increasing as a direct consequence of irrigation (Flowers 2004). Around 800 million hectares of land is estimated to be affected by salinity throughout the world (FAO 2008). Salinity stress creates an oxidative burst in cells resulting in an increased accumulation of reactive oxygen species (ROS) which affects the plasma membrane, cell metabolism, and homeostasis. Salt stress imbalances the ethylene production and causes its overproduction which accelerates leaf and petal abscission and organ senescence, leading to premature death (Cheng et al. 2007; Mayak et al. 2004a, b; Zahir et al. 2009).
Reducing the ethylene level, one can alleviate some of the effects of stresses on plants (Glick 2004). Plant losses approximately 40% of photosynthates, through root exudates (Lynch and Whipps 1991), and it has been estimated that during stress much of the released carbon is in the form of ACC, which is a precursor of ethylene, and is exuded from plant roots (Bayliss et al. 1997). Thus, PGPR, with ACC deaminase activity, can be used to convert ACC to ammonia and α-ketobutyrate that are used up by the plant as a nitrogen source simultaneously reducing the negative effects of salinity stress (Cheng et al. 2007; Mayak et al. 2004a, b; Zahir et al. 2009). However, the efficiency of PGPR depends on environmental factors such as the climate, weather conditions, soil characteristics, and interaction with other indigenous microbial flora in the soil (Giongo et al. 2008; Sinha and Raghuwanshi 2015). Salt-tolerant ACC deaminase-producing bacteria can survive well in a saline environment, and their beneficial properties help plants to overcome stress effects (Mayak et al. 2004a, b). Halotolerant bacteria are a group of microorganisms able to grow in media containing a wide range of NaCl up to 1–33% or in the absence of NaCl (Larsen 1986). A significant decrease in the level of ethylene was observed in tomato plants exposed to high salt concentration on inoculation with Achromobacter piechaudii ARV8, an isolate obtained from the rhizosphere of Lycium shawii plant wildly growing in the Arava region of Israel (Mayak et al. 2004b). The inoculated tomato seedlings showed an increased fresh and dry weight, but this however did not reduce the content of sodium in the plant. Plants inoculated with Achromobacter piechaudii ARV8 had four times higher biomass compared to controls, as there was a significant reduction of the ethylene level (Mayak et al. 2004b). Similar effect of the bacterial strain, i.e., lowering the ethylene level, was observed in peppers and tomatoes growing under drought stress (Mayak et al. 2004a). Studies done on maize plant growing in saline–sodic soil when treated with fertilizer along with ACC deaminase-producing Pseudomonas strains showed 198% augmented plant dry weight (Zafar-ul-Hye et al. 2014). Studies done on wild-type bacterial endophytes showed protection against salt stress in plants by limiting the buildup of salt and thereby improving plant survival. Inoculating ACC deaminase bacteria do not alter the sodium level in plants, but the uptake of phosphorous and potassium gets slightly increased, which supports plant growth under salt stress. Similar reports by Saravanakumar and Samiyappan (2007) revealed Pseudomonas fluorescens strain TDK1 possessing ACC deaminase activity not only enhanced the resistance toward salinity in groundnut plants but also increased yield. Compared to mutant-inoculated or non-inoculated plants, the plants inoculated with ACC deaminase-producing strain show augmented level of chlorophyll content. High chlorophyll content has been linked with stress tolerance in many plants (Vurukonda et al. 2016). Encouraging results were also obtained by the tripartite interaction of Arthrobacter protophormiae, Rhizobium leguminosarum, and Glomus mosseae which increased plant weight by 53%, reduced proline content and lipid peroxidation, and increased pigment content under 200 mM salt condition (Barnawal et al. 2013). Inoculating plants with wild-type ACC deaminase-producing strain tend to prevent salt buildup in plant tissues; however, few contradictory results have also been observed where more salt was deposited per gram of dry biomass in the plants inoculated with the ACC deaminase-producing strains. Study done to evaluate the growth of canola in the presence of wild-type ACC deaminase-containing plant growth-promoting rhizospheric bacterium P. putida UW4 showed the accumulation of much higher concentrations of sodium in the shoots compared to the plants treated with ACC deaminase mutants (Cheng et al. 2007).
Pea crop has been reported to suffer approximately 50% yield loss at 100 mM NaCl (Subbarao and Johansen 1994). Ethylene formed in response to salt stress inhibits the development of rhizobial infection threads in Pisum sativum cv. Sparkle (Lee and LaRue 1992). Plants undergoing symbiotic association with microbes, like Rhizobium, and mycorrhizal fungi also show a slight increase in ethylene levels during the establishment period. As the nitrogen fixation is a high energy-demanding process, there are fair chances of ethylene production by the plant, which may lead to nodule senescence (Murset et al. 2012). During this period the ACC deaminase-producing bacteria residing in the rhizosphere help in establishment of symbiosis by locally lowering the ethylene levels.
Thus effects of soil salinity on crop productivity can be alleviated by bacterial inoculations having ACC deaminase activity. Microbe-assisted plant stress management has emerged as an important strategy, and their role in improving growth and productivity has been well established (Venkateswarlu et al. 2008; Yang et al. 2009).
12.4.2 Waterlogging Stress
Soil flooding or waterlogging causes major changes in the normal functioning of plant roots (Jackson and Drew 1984) as the gas diffusion rates get reduced in flooded soil (Jackson 1985), and at the same time, respiration by microorganisms and plant roots leads to a rapid buildup anaerobic conditions in the soil. Anaerobic conditions of the soil lead to toxicity primarily due to Fe2+, Mn+ and sulfide and due to accumulated gases like carbon dioxide, methane, ethane, and ammonia (Ernst 1990). This also affects some of the vital processes like ion uptake in root (Jackson and Drew 1984). These stressful conditions trigger the synthesis of enzyme ACC synthase as well as other stress proteins in the plant which elevate the level of ACC in its roots (Li et al. 2012). This newly synthesized ACC cannot be converted to ethylene in the roots, as ethylene synthesis requires oxygen, so the ACC is transported to the shoots where under aerobic environment the ACC gets converted into ethylene (Bradford and Yang 1980; Else and Jackson 1988) causing epinasty (wilting), leaf chlorosis, necrosis, and stunted growth. Accumulation of ethylene in plants may also lead to adoptive response like shoot elongation (Voesenek and Blom 1989) and formation of aerenchyma (Armstrong et al. 1994), and a few studies also report the formation of adventitious roots (Drew 1992). Waterlogging induces several physiological alterations like reduced photosynthetic rate, stomatal closure, plant growth inhibition, and low yield. A sustainable solution to this problem comes from the ACC deaminase-producing plant growth-promoting bacteria (Barnawal et al. 2012; Grichko and Glick 2001a; Li et al. 2013) which mitigate the stress by lowering the ethylene level in plants and making them more capable to withstand flood (Saleem et al. 2007). Studies have shown that flooded plants inhabiting ACC deaminase-producing microbes are able to overcome the flood response partially. Even plants are genetically engineered to express this enzyme in root-specific manner resulting in less accumulation of ethylene in the roots and thereby minimizing the adverse effects of flooding (Grichko and Glick 2001a, b).
12.4.3 Metal and Organic Pollutants
Industrial revolution has accelerated the toxic metal accumulation rate in the biosphere and has come up as a serious current environmental problem. Metal in soil beyond a limit becomes toxic to plant growth as they interfere with normal growth and development. Soils dumped with heavy metals also cause a severe stress induction in plants that leads to the synthesis of stress ethylene up to an inhibitory level. Plants interact with these heavy metals present in the environment through phytostabilization, phytoextraction, and phytovolatilization (Pilon-Smits 2005). The easy and preferred way to get rid of the metals is phytoextraction. Cleaning heavy metal pollutants through plants, i.e., phytoremediation (Salt et al. 1995), is an eco-friendly and cost-effective approach compared to the traditional soil remediation approaches of metal removal through chemical and physical extraction. However, the limitation lies that not all plants are capable to naturally tolerate and accumulate heavy metals. Many plants effective in phytoremediation are small sized and slow in growth, which limit their practical use (Khan et al. 2000). An effective plant to remediate the soil must be tolerant to one or more pollutants, highly competitive, and fast growing and produce a high biomass. Healthy and robust plants are preferred as they have better ability to phytoremediate metal contaminants. The commonly used plants in heavy metal accumulation belong to the Brassicaceae family (Kumar et al. 1995). The literature is well documented with the role of metal-resistant ACC deaminase-producing bacteria in improving plant growth by decreasing the stress effects due to ethylene. The first report on role of ACC deaminase-containing bacterium in phytoremediation of nickel-contaminated soil indicated that toxicity of nickel toward canola plants was reduced in the presence of the bacteria (Burd et al. 1998). Bacteria increased the uptake of Cd in Brassica napus (Sheng and Xia 2006) and Ni in Alyssum murale (Abou-Shanab et al. 2006). Rhizobial microfloras are known to affect heavy metals mobility and availability to the plant through release of chelating agents, acidification, and redox changes (Abou-Shanab et al. 2003; Smith and Read 1997). The root-associated ACC deaminase-producing bacteria not only reduce the ethylene levels but also provide multifaceted benefits to the plant (Glick 1995; Glick et al. 1999). Bacteria produce indole-3-acetic acid, siderophores, and solubilize phosphate, which stimulate plant growth (Glick 1995; Chabot et al. 1996a, b; Rajkumar et al. 2006). Heavy metal-contaminated soil often become iron depleted, and this effect of heavy metals can be overcome by inoculating ACC deaminase and siderophore-producing bacteria (Burd et al. 1998, 2000; Reed and Glick 2005). Thus, besides reducing the ethylene level in plants, microbes also enhance the mobility and availability of minerals to the plants (Abou-Shanab et al. 2003; Idris et al. 2004) which improve plant growth.
Treatment of plants with ACC deaminase-producing plant growth-promoting bacteria not only relieves the plant with the growth inhibition effects of ethylene but also allows the plant to grow normal and restore the nutrient cycling (Huang et al. 2004, 2005; Reed and Glick 2005; Greenberg et al. 2006). Therefore, bacterial strains utilized in plant growth promotion under metal stress should be screened for their abilities to resist the targeted toxic metal, synthesize IAA to promote root growth (Patten and Glick 2002), secrete siderophore (Burd et al. 2000) that helps plants to acquire iron from the metal-contaminated soil, and possess ACC deaminase activity which can prevent the building up of inhibitory levels of ethylene in the plants (Glick et al. 1998).
Organic pollutants in the soil, if present above a permissible limit, inhibit plant growth and drag the plant toward senescence by accelerated ethylene production (Abeles et al. 1992). Phytoremediation has come up as a technology to clean soil contaminated with organic oil spills, polycyclic aromatic hydrocarbons (PAHs), and polycyclic biphenyls (PCB), and is being practiced at commercial scale. PGPR possessing ACC deaminase activity has multifold benefits in phytoremediation of organic-, metal-, and salt-contaminated soils. Reduction in stress ethylene partially alleviates the damage caused by the target contaminant (Mayak et al. 2004a, b). Therefore the growth of plants exposed to organic contaminants in the soil should be facilitated by the presence of ACC deaminase-containing plant growth-promoting bacteria. In fact, this strategy of bacterially assisted phytoremediation appears to be particularly effective for removal and/or degradation of organic contaminants from impacted soils. Helianthus annuus L. seedlings inoculated with Achromobacter xylosoxidans (SF2) and Bacillus pumilus (SF3 and SF4) bacterial strains increased the production of auxins, salicylic acid, abscisic acid, jasmonic acid, as well as the plant dry matter. High salicylic acid concentration in stressed seedlings played key role in abiotic stress tolerance (Castillo et al. 2013). Plant growth-promoting bacteria improve plant competitiveness and responses in a stressed ecosystem (Egamberdiyeva and Hoflich 2004).
12.4.4 Drought
Drought stress can adversely affect plant growth and yield and is one of the most fatal reasons for economic losses in agriculture and forestry. Its affects plant water relations at the cellular and whole plant levels, altering the plant physiology and leading to specific and nonspecific phenotype (Pereyra et al. 2009; Arzanesh et al. 2011). In order to combat drought, plants adopt altered gaseous exchange and water relation strategies (Sinha and Raghuwanshi 2016). Bacteria adhering to plant roots containing ACC deaminase enzyme hydrolyze ACC and use it as the source of carbon and nitrogen (Glick 2014), and the process continues until a dynamic equilibrium between the roots and rhizosphere bacteria is maintained and the modulated root system starts normal functioning under low water condition. It has been well documented that inoculation of plants with certain PGPR at seedling stage improves biomass production through their effects on root system, which enhance plant growth and yield (Prasad et al. 2017).
Different ACC deaminase-producing bacteria have been demonstrated for their efficacy in protecting plants against yield loss induced by drought stress (Table 12.1). Studies done by Mayak et al. (2004a, b) on ACC deaminase PGPR Achromobacter piechaudii ARV8 showed that during water stress although the bacterium did not influence the water content of plants, it improved the recovery of plants when watered. Exposure of Bacillus subtilis-inoculated plants to 8 weeks drought stress led to significant increase in shoot and root biomass by 26.6 and 63.8%, and the photosynthesis and stomatal conductance too got enhanced by 55.2% and 214.9%, respectively (Bourque et al. 2016). Azospirillum brasilense sp. 245 uninoculated seeds of Triticum aestivum when sown under drought conditions had a yield loss of 26.5% and got reduced to 14.1% on inoculation with Azospirillum brasilense sp. 245. Grain Mg and K diminished in nonirrigated, non-inoculated plots. Grains harvested from Azospirillum-inoculated plants had significantly higher Mg, K, and Ca than non-inoculated plants (Creus et al. 2004). Cucumber plants treated with a consortium of three plant growth-promoting rhizobacterial strains (Bacillus cereus AR156, Bacillus subtilis SM21, and Serratia sp. XY21) induced tolerance to drought stress. The treatment decreased the leaf monodehydroascorbate (MDA) content and increased the leaf proline content and the root recovery intension by 3.45-fold and 50%, respectively. It also maintained the leaf chlorophyll content in cucumber plants under drought stress (Wang et al. 2012). Azospirillum lipoferum strain B3 having phosphate-solubilizing and ACC deaminase activities, when inoculated in wheat under drought, produced the highest amounts of N and auxin and increased wheat yield up to 109% (Arzanesh et al. 2011). Many studies have proven the positive effects of ACC deaminase bacterial activity on plant biomass, leaf area, and transpiration ratio of plants under drought (Saleem et al. 2007). Inoculation with ACC deaminase bacterial activity has restored nodulation in pea plants under drought which is comparable with the well-irrigated plants (Arshad et al. 2008).
12.5 Conclusion and Future Prospects
It is well proven that bacteria with ACC deaminase activity are potent in improving plant growth and productivity under varied abiotic stress. Knowing the potentially serious environmental health damage caused by the excessive use of chemicals and pesticides in the agricultural sector, we need a major paradigm shift in agricultural practices. As the cost of engineering and developing transgenic plants that are able to defend well the variety of pathogens and other abiotic stresses, it is rather economical to isolate and screen an efficient plant growth-promoting bacterium able to combat the adverse conditions. The major challenge in the large-scale application of these bacteria is their survival under varied geographical and harsh environmental conditions, but a potential solution to the problem can be the exploitation of a potent endophytic plant growth-promoting bacteria. Unrevealing the fundamental mechanism of action of these bacteria will facilitate the wider application of this technology and overcome the bottleneck.
References
Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in plant biology, 2nd edn. Academic, New York
Abou-Shanab RA, Angle JS, Delorme TA, Chaney RL, van Berkum P, Moawad H, Ghanem K, Ghozlan HA (2003) Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytol 158:219–224
Abou-Shanab RA, Angle JS, Chaney RL (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol Biochem 38:2882–2889
Abrol IP, Yadav JSP, Massoud FI (1988) Salt affected soils and their management. Food and Agriculture Organization (FAO), UN, Soils Bulletin, Rome, p 39
Ait Bakra E, Nowak J, Clément C (2006) Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth promoting rhizobacterium Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol 72:7246–7252
Alexander M (1991) Introduction to soil microbiology, 2nd edn. Krieger, Malabar
Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167
Armstrong W, Bundle R, Jackson MB (1994) Mechanisms of flood tolerance in plants. Ada Bot Neerl 43:307–358
Arshad M, Shaharoona B, Mahmood T (2008) Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.) Pedosphere 18:611–620
Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA, Miransari M (2011) Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J Microbiol Biotechnol 27:197–205
Babu AG, Shea PJ, Sudhakar D, Jung I-B, Oh BT (2015) Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J Environ Manage 151:160–166
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kaira A (2012) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol Biochem 58:227–235
Barnawal D, Maji D, Bharti N, Chanotiya CS, Kalra A (2013) ACC deaminase-containing Bacillus subtilis reduces stress ethylene-induced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J Plant Growth Regul 32:809–822
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol 171:884–894
Barnawal D, Bharti N, Tripathi A, Panday SS, Chanotiya CS, Kalra A (2016) ACC-deaminase-producing endophyte Brachybacterium paraconglomeratum strain SMR20 ameliorates Chlorophytum salinity stress via altering phytohormone generation. J Plant Growth Regul 35:553–564
Bayliss C, Bent E, Culham DE, MacLellan S, Clarke AJ, Brown GL, Wood JM (1997) Bacterial genetic loci implicated in the Pseudomonas putida GR12-2R3-canola mutualism: identification of an exudate-inducible sugar transporter. Can J Microbiol 43:809–818
Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1- aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47:242–252
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S (2005) Cadmium-tolerant plant growth-promoting bacteria associated with roots of Indian mustard (Brassica juncea L Czern.) Soil Biol Biochem 37:241–250
Blaha D, Prigent-Combaret C, Mirza MS, Moënne-Loccoz Y (2006) Phylogeny of the 1-aminocyclopropane-1-carboxylic acid deaminase-encoding gene acdS in phytobeneficial and pathogenic Proteobacteria and relation with strain biogeography. FEMS Microbiol Ecol 56:455–470
Bourque FG, Bertrand A, Claessens A, Aliferis KA, Jabaji S (2016) Alleviation of drought stress and metabolic changes in Timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front Plant Sci 7:584
Bradford KJ, Yang SF (1980) Stress-induced ethylene production in the ethylene-requiring tomato mutant diageotropica. Plant Physiol 65:327–330
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46:237–245
Burg SP, Burg EA (1966) The interaction between auxin and ethylene and its role in plant growth. Am J Bot 55:262–269
Castillo P, Escalante M, Gallardo M, Alemano S, Abdala G (2013) Effects of bacterial single inoculation and co-inoculation on growth and phytohormone production of sunflower seedlings under water stress. Acta Physiol Plant 35:2299–2309
Chabot R, Antoun H, Cescas MP (1996a) Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar phaseoli. Plant Soil 184:311–321
Chabot R, Antoun H, Kloepper JW, Beauchamp CJ (1996b) Root colonization of maize and lettuce by bioluminescent phosphate solubilizing Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol 62:2767–2772
Cheng Z, Park E, Glick BR (2007) 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can J Microbiol 53:912–918
Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123:81–92
Creus CM, Sueldo RJ, Barassi CA (2004) Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can J Bot 82:273–281
Di Gregorio S, Barbafieri M, Lampis S, Sanangelantoni AM, Tassi E, Vallini G (2006) Combined application of Triton X-100 and Sinorhizobium sp. Pb002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere 63:293–299
Drew MC (1992) Soil aeration and plant root metabolism. Soil Sci 154:259–268
Egamberdieva D, Botir H, Abeer H, Abd-Allah EF (2014) Characterization of salt tolerant Enterobacter hormaechei strain associated with tomato root grown in arid saline soil. J Pure Appl Microbiol 8:4231–4239
Egamberdiyeva D, Hoflich G (2004) Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea on a semi-arid region of Uzbekistan. J Arid Environ 56:293–301
Else MA, Jackson MB (1988) Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Aust J Plant Physiol 25:453–458
Ernst WHO (1990) Ecophysiology of plants in waterlogged and flooded environments. Aquat Bot 38:73–90
Etesami H, Mirsyed Hosseini H, Alikhani HA, Mohammadi L (2014) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul:1–17
FAO (2008) FAO land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush
Farwell AJ, Vesely S, Nero V, Rodriguez H, McCormack K, Shah S (2007) Tolerance of transgenic canola plants (Brassica napus) amended with plant growth-promoting bacteria to flooding stress at a metal contaminated field site. Environ Pollut 147:540–545
Flowers T (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Frankenberger WT (2002) Environmental chemistry of arsenic. Marcel Dekker, New York, p 410
German MA, Burdman S, Okon Y, Kigel J (2000) Effects of Azospirillum brasilense on root morphology of common bean (Phaseolus vulgaris L.) under different water regimes. Biol Fertil Soils 32:259–264
Giongo A, Ambrosini A, Vargas LK, Freire JRJ, Bodanese-Zanettini MH, Passaglia LMP (2008) Evaluation of genetic diversity of Bradyrhizobia strains nodulating soybean [Glycine max (L.) Merrill] isolated from South Brazilian fields. Appl Soil Ecol 38:261–269
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv Appl Microbiol 56:291–312
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251:1–7
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Glick BR, Jacobson CB, Schwarze MMK Pasternak JJ (1994) 1-aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacteria Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can J Microbiol 40:911–915
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol 190:63–68
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth-promoting bacteria. Imperial College Press, London
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth ACC deaminase-containing soil bacteria. Eur J Plant Pathol 119:329–339
Greenberg BM, Huang XD, Gurska Y, Gerhardt KE, Wang W, Lampi MA, Zhang C, Khalid A, Isherwood D, Chang P, Wang H, Dixon DG, Glick BR (2006) Successful field tests of a multi-process phytoremediation system for decontamination of persistent petroleum and organic contaminants. In: Proceedings of the 29th Arctic and Marine oil spill program technical seminar, pp 389–400
Grichko VP, Glick BR (2001a) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39:11–17
Grichko VP, Glick BR (2001b) Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlled by the 35S, rolD or PRB-1b promoter. Plant Physiol Biochem 39:19–25
Honma M (1983) Enzymatic determination of 1-aminocyclopropane-1-carboxylate deaminase. Agric Biol Chem 47:617–618
Honma M (1985) Chemically reactive sulfhydryl groups of 1-aminocyclopropane-1-carboxylate deaminase. Agric Biol Chem 49:567–571
Hontzeas N, Zoidakis J, Glick BR, Abu-Omar MM (2004a) Expression and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the rhizobacterium Pseudomonas putida UW4: a key enzyme in bacterial plant growth promotion. Biochim Biophys Acta 1703:11–19
Hontzeas N, Saleh SS, Glick BR (2004b) Changes in gene expression in canola roots induced by ACC deaminase-containing plant growth-promoting bacteria. Mol Plant Microbe Interact 17:865–871
Hontzeas N, Hontzeas CE, Glick BR (2006) Reaction mechanisms of the bacterial enzyme 1-aminocyclopropane-1-carboxylate deaminase. Biotechnol Adv 24:420–426
Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) Responses of plants to creosote during phytoremediation and their significance for remediation processes. Environ Pollut 130:453–463
Huang XD, El-Alawai Y, Gurska J, Glick BR, Greenberg BM (2005) A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem J 81:139–147
Idris EES, Bochow H, Ross H, Borriss R (2004) Use of Bacillus subtilis as biocontrol agent. Phytohormone like action of culture filtrates prepared from plant growth-promoting Bacillus amyloliquefaciens FZB24, FZB42, FZB45 and Bacillus subtilis FZB37. J Plant Dis Prot 111:583–597
Jackson MB (1985) Ethylene and responses of plants to soil waterlogging and submergence. Annu Rev Plant Physiol 36:145–174
Jackson MB, Drew MC (1984) Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT (ed) Flooding and plant growth. Academic, New York, pp 47–128
Jacobson CB, Pasternak JJ, Glick BR (1994) Partial purification and characterization of ACC deaminase from the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol 40:1019–1025
Jiang CY, Sheng XF, Qian M, Wang QY (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal polluted soil. Chemosphere 72:157–164
Jiang F, Chen L, Belimov AA, Shaposhnikov AI, Gong F, Meng X, Hartung W, Jeschke DW, Davies WJ, Dodd IC (2012) Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum. J Exp Bot 63:6421–6430
Johnson PR, Ecker JR (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32:227–254
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44:283–307
Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41:197–207
Kloepper JW, Beauchamp CJ (1992) A review of issues related to measuring colonization of plant roots by bacteria. Can J Microbiol 38:1219–1232
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction-the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Kumar KV, Singh N, Behl HM, Srivastava S (2008) Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 72:678–683
Larsen H (1986) Halophilic and halotolerant microorganisms an overview and historical perspective. FEMS Microbiol Rev 39:3–7
Lee KH, LaRue TA (1992) Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol 100:1759–1763
Li K, Pang CH, Ding F, Sui N, Feng ZT, Wang BS (2012) Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S Afr J Bot 78:235–245
Li J, McConkey BJ, Cheng Z, Guo S, Glick BR (2013) Identification of plant growth-promoting rhizobacteria-responsive proteins in cucumber roots under hypoxic stress using a proteomic approach. J Proteomics 84:119–131
Lim JH, Kim SD (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol J 29:201–208
Lynch JM, Whipps JM (1991) Substrate flow in the rhizosphere. In: Keister DL, Cregan B (eds) The rhizosphere and plant growth. Kluwer, Dordrecht, pp 15–24
Mayak S, Tirosh T, Glick BR (1999) Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul 18:49–53
Mayak S, Tirosh T, Glick BR (2004a) Plant growth-promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci 166:525–530
Mayak S, Tirosh T, Glick BR (2004b) Plant growth-promoting bacteria that confer resistance in tomato to salt stress. Plant Physiol Biochem 42:565–572
Murset V, Hennecke H, Pessi G (2012) Disparate role of rhizobial ACC deaminase in root-nodule symbioses. Symbiosis 57:43–50
Nascimento FX, Rossi MJ, Soares CRFS, McConkey BJ, Glick BR (2014) New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS One 9:99168
Naseem H, Bano A (2014) Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance in maize. J Plant Interact 9:689–701
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39
Nie L, Shah S, Rashid A, Burd GI, Dixon DG, Glick BR (2002) Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth-promoting bacterium Enterobacter cloacae CAL2. Plant Physiol Biochem 40:355–361
Owino WO, Manabe Y, Mathooko FM, Kubo Y, Inaba A (2006) Regulatory mechanisms of ethylene biosynthesis in response to various stimuli during maturation and ripening in fig fruit (Ficus carica L.) Plant Physiol Biochem 44:335–342
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Patten CL, Glick BR (2002) The role of bacterial indole acetic acid in the development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47:77–80
Pereyra MA, Ballesteros FM, Creus CM, Sueldo RJ, Barassi CA (2009) Seedlings growth promotion by Azospirillum brasilense under normal and drought conditions remains unaltered in Tebuconazole-treated wheat seeds. Eur J Soil Biol 45:20–27
Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11:176–183
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Płociniczak T, Sinkkonen A, Romantschuk M, Piotrowska-Seget Z (2013) Characterization of Enterobacter intermedius MH8b and its use for the enhancement of heavy metals uptake by Sinapis alba L. Appl Soil Ecol 63:1–7
Prasad JK, Gupta SK, Raghuwanshi R (2017) Screening multifunctional plant growth promoting rhizobacteria strains for enhancing seed germination in wheat (Triticum aestivum L.). Int J Agric Res 12:64–72
Rajkumar M, Nagendran R, Kui JL, Wang HL, Sung ZK (2006) Influence of plant growth promoting bacteria and Cr (VI) on the growth of Indian mustard. Chemosphere 62:741–748
Reed MLE, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51:1061–1069
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Salt DE, Blaylock M, Kumar PBAN, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a nove1 strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102(5):1283–1292
Sarma RK, Saikia R (2014) Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant and Soil 377:111–126
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.) Lett Appl Microbiol 42:155–159
Sheehy RE, Honma M, Yamada M, Sasaki T, Martineau B, Hiatt WR (1991) Isolation, sequence, and expression in Escherichia coli of the Pseudomonas sp. strain ACP gene encoding 1-aminocyclopropane-1-carboxylate deaminase. J Bacteriol 173:5260–5265
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64:1036–1042
Singh RP, Jha PN (2016) A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front Plant Sci 7:1890
Sinha S, Raghuwanshi R (2015) Influence of water availability on microbial populations of rhizospheric soils of E. Prostrata (L.) L and A. paniculata Burm Wall Nees plant under different biofertilizer treatment. In: Khan MMAA, John KG, Dilfuza E, Abid M, Maheshwari RK, Naqvi TS (eds) New dimensions in microbiology. Lenin Media, Delhi, pp 43–52
Sinha S, Raghuwanshi R (2016) Synergistic effect of arbuscular mycorrhizal helper bacteria on physiological mechanism to tolerate drought in Eclipta prostrate (L.). Pure. Appl Microbiol 10:1117–1129
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, San Diego. ISBN: 0-12-652840-3
Stearns J, Glick BR (2003) Transgenic plants with altered ethylene biosynthesis or perception. Biotechnol Adv 21:193–210
Subbarao GV, Johansen C (1994) Strategies and scope for improving salinity tolerance in crop plants. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 1069–1087
Timmusk S, Wagner EGH (1999) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant Microbe Interact 12:951–959
Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E (2011) Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS One 6:17968
Trung NT, Hieu HV, Thuan NH (2016) Screening of strong 1-aminocyclopropane-1-carboxylate deaminase producing bacteria for improving the salinity tolerance of cowpea. Appl Micro Open Access 2:1000111
Van Loon LC, Glick BR (2004) Increased plant fitness by rhizobacteria. In: Sandermann H (ed) Molecular ecotoxicology of plants. Springer, Berlin, pp 177–205
Van Loon LC, Geraats BPJ, Linthorst HJM (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11:184–191
Venkateswarlu B, Desai S, Prasad YG (2008) Agriculturally important microorganisms for stressed ecosystems: challenges in technology development and application. In: Khachatourians GG, Arora DK, Rajendran TP, Srivastava AK (eds) Agriculturally important microorganisms. Academic World, Bhopal, pp 225–246
Voesenek LACJ, Blom CWPM (1989) Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ 12:433–439
Vurukonda SSKP, Vardharajula S, Shrivastava M, Skz A (2016) Multifunctional Pseudomonas putida strain FBKV2 from arid rhizosphere soil and its growth promotional effects on maize under drought stress. Rhizosphere 1:4–13
Wang C, Yang W, Wang C, Gu C, Niu D, Liu H, Wang Y, Guo J (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One 7:52565
Yan J, Smith MD, Glick BR, Liang Y (2014) Effects of ACC deaminase containing rhizobacteria on plant growth and expression of Toc GTPases in tomato (Solanum lycopersicum) under salt stress. Botany 92:775–781
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yang J, Kloepper J, Ryu CN (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Zafar-ul-Hye M, Farooq HM, Zahir ZA (2014) Combined application of ACC-deaminase biotechnology and fertilizers improves maize productivity subjected to drought stress in salt affected soils. Int J Agric Biol 16:591–596
Zahir ZA, Munir A, Asghar HN, Shaharoona B, Arshad M (2008) Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J Microbiol Biotechnol 18:958–963
Zahir AZ, Ghani U, Naveed M, Nadeem SM, Asghar HN (2009) Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch Microbiol 191:415–424
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Raghuwanshi, R., Prasad, J.K. (2018). Perspectives of Rhizobacteria with ACC Deaminase Activity in Plant Growth Under Abiotic Stress. In: Giri, B., Prasad, R., Varma, A. (eds) Root Biology. Soil Biology, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-319-75910-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-75910-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75909-8
Online ISBN: 978-3-319-75910-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)