Abstract
As elsewhere in the gastrointestinal tract, the anatomy, morphology, and physiology of the esophagus and stomach are adapted and designed to subserve their respective functions. Thus, the esophagus transports the food bolus from the posterior pharynx to the stomach and at the same time protects the airway and minimizes gastroesophageal reflux. The stomach then relaxes to accommodate the meal and thereafter ensures the timely and coordinated delivery of the meal to the small intestine in a format that optimizes digestive activity there. These complex and highly integrated functions are based on the intrinsic electrophysiological properties of the esophagogastric musculature and the local regulatory and modulatory role of the enteric nervous system with input from the central nervous system via the autonomic nerves.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Esophagus
- Stomach
- Lower esophageal sphincter
- Acid reflux
- Accommodation
- Gastric emptying
- Myenteric plexus
- Gut smooth muscle
Esophageal Anatomy and Physiology: The Basics
The major functions of the esophagus are threefold:
-
1.
To transport liquids and solids from the pharynx to the stomach in a manner that protects the airway,
-
2.
To prevent the reflux of gastric contents into the esophagus once swallowing has occurred, and
-
3.
To clear the esophagus of any material that may have refluxed into its lumen.
These tasks are achieved, in health, remarkably quickly and efficiently and involve the meticulous and precise coordination of the central nervous system, including a number of cranial nerves, the enteric nervous system within the esophagus, and various muscle layers that comprise the esophageal musculature .
The esophagus is lined, in continuity with the oropharynx, by a squamous epithelium that meets the columnar epithelium of the stomach at the squamo-columnar junction (also referred to as the Z line). The anatomy of the gastroesophageal junction is of considerable clinical importance and in its assessment the following landmarks need to be defined:
-
1.
The diaphragmatic hiatus (also referred to as the diaphragmatic pinch)
-
2.
The gastroesophageal junction—typically defined at endoscopy as the proximal extent of the gastric folds
-
3.
The squamo-columnar junction—defined endoscopically as the meeting of the squamous and columnar epithelia (the Z line)
In health these three locations should be the same or very close together. A separation of the diaphragmatic hiatus from the gastroesophageal junction defines a hiatal hernia and the separation of the gastroesophageal junction from the squamo-columnar junction suggests the presence of Barrett’s esophagus, which must be confirmed by histology [1].
In terms of esophageal disorders that may arise in diabetes, three dominate: esophageal dysmotility , gastroesophageal reflux disease , and candidiasis . It is appropriate, therefore, that this introductory chapter focus on three aspects of esophageal function: motility, protection against acid, and resistance to infection/infestation.
Esophageal Motor Function [2]
The esophagus is a muscular tube composed of three distinct functional regions: the upper esophageal sphincter (UES) , the tubular esophagus (also referred to as the esophageal body), and the lower esophageal sphincter (LES).
The upper esophageal sphincter is a striated muscle structure that consists primarily of the cricopharyngeus, but receives contributions from the inferior pharyngeal constrictor and the adjacent esophagus. It is a slit-like structure that is closed at rest due to the tonic contraction of the UES and the viscoelastic properties of adjacent structures. The UES opens in advance of the arriving bolus and the pharyngeal peristaltic wave. The mechanisms contributing to its opening include traction on the sphincter by contraction of the infrahyoid and suprahyoid muscles, relaxation of the cricopharyngeus, and pressure in the swallowed bolus. One of the main factors contributing to propulsive forces in the posterior pharynx and, thus, to UES opening and the transfer of the bolus to the esophagus is the tongue. Opening of the UES allows transfer of the bolus from the pharynx to the esophagus. Arrival of the pharyngeal contraction closes the UES. During this passage of the bolus from the mouth to the esophagus, the airway and the nasopharynx are sealed off to prevent aspiration and nasal regurgitation, respectively. All of these events have to be accomplished in the right sequence and within a time frame of approximately 1 s; a remarkable achievement (Fig. 4.1).
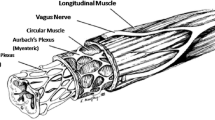
The body of the esophagus is a 20–22 cm muscular tube that consists of an inner circular muscle layer and an outer longitudinal muscle layer. The muscle layers are named according to the axial orientation of their constituent smooth muscle cells . The top 5% of the esophagus is striated muscle, the middle 35–40% is a mixture of striated and smooth muscle with smooth muscle progressively replacing striated muscle, and the lower 50–60% is comprised entirely of smooth muscle. The myenteric plexus is found between the muscular layers. The plexus receives neural input from the CNS and provides the terminal motor innervation to the smooth muscle of the esophagus.
On initiating a swallow, the UES relaxes and the primary peristaltic (or stripping) wave which consists of a ring of circular smooth muscle contraction sweeps through the esophagus from the UES to the LES at a rate of 2–4 cm/s. The bolus moves rapidly ahead of this wave and enters the stomach through an open lower esophageal sphincter. As the peristaltic contraction enters the upper esophagus, the longitudinal muscle contracts to shorten the esophagus by 2.0–2.5 cm. How does the esophagus generate this highly programmed sequential response? A number of elements are involved:
-
1.
Central programming in the swallowing center in the CNS seems to be especially relevant to the components involving the pharynx, UES, and related structures,
-
2.
The intrinsic properties of esophageal smooth muscle that lead to a patterned sequential response to stimulation. On stimulation, the esophageal smooth muscle generates a biphasic response:
-
(a)
An immediate (“on”) response while the stimulus is applied which generates a slight contraction in the esophageal body and, most importantly, initiates a prolonged relaxation in the lower sphincter so that the LES remains open throughout the peristaltic sequence.
-
(b)
A later (“off”) response after the stimulus has ceased that leads to the intense peristaltic contraction and whose onset is progressively delayed as one moves down the esophagus, thus generating a peristaltic wave that moves in an orderly and abroad direction through the esophagus.
-
(a)
The Lower Esophageal Sphincter and Protection Against Reflux
The lower esophageal sphincter (LES) is identified anatomically as a muscular thickening at the gastroesophageal junction. It is composed of two muscular components: one is a long bundle of muscle from an inner muscular layer that runs obliquely along the lesser curvature of the stomach, loops around the greater curvature side of the esophagogastric junction, and passes back along the lesser curve; the other is a short bundle of muscle fibers that is oriented transversely around the esophagus at the gastroesophageal junction. The muscles of the LES contract tonically at rest to produce an intraluminal pressure that exceeds intragastric pressure by 10–45 mmHg. The LES relaxes as the swallowed bolus reaches the upper esophagus, and it stays relaxed until the peristaltic contraction arrives at the gastroesophageal junction. The LES then contracts and regains its resting tone. The major neurotransmitter mediating relaxation of the LES is nitric oxide; a loss of this inhibitory innervation is considered central to the development of achalasia.
The LES also relaxes independent of swallows through a mechanism known as transient lower esophageal sphincter relaxation (TLESR) . This is a vago-vagal reflex normally initiated by gastric distension and designed to release air distending the upper stomach—the belch reflex. TLESRs that are a normal phenomenon are characteristically longer in duration than LES relaxations initiated by a swallow [3].
Reflux of gastric contents into the esophagus will inevitably occur in health, primarily through TLESRs [3]. What distinguishes physiological from pathological reflux is simply the duration of acid exposure, which is, in turn, determined by all of the elements that normally act as a barrier to the reflux of gastric contents into the esophagus and protect the esophageal mucosa:
-
1.
The aforementioned lower esophageal sphincter. In pathological reflux (gastroesophageal reflux disease; GERD) the LES may contribute to reflux in one of three ways:
-
(a)
Through chronic hypotension—usually seen in more severe manifestations of GERD where there is free reflux of gastric contents into the esophagus
-
(b)
Through more frequent TLESRs—probably the most common mechanism
-
(c)
By being overwhelmed by very high intragastric pressure
-
(a)
-
2.
The diaphragmatic crura . These muscles that surround the lower esophagus provide a second “extrinsic” sphincter mechanism that augments LES pressure during times of stress such as at the height of inspiration and when intra-abdominal pressure is elevated. When the LES and the crura are no longer aligned, as occurs when a hiatal hernia develops, this relationship is lost and reflux is potentiated, not prevented.
-
3.
Esophageal clearance . When upright, gravity greatly assists clearance of refluxed material. Secondary peristalsis, initiated by reflux or anything else that distends the esophageal lumen also serves to clear refluxed material. Loss of peristalsis or ineffective contractions will prolong contact between acid and the esophageal mucosa—the primary factor determining the development of esophageal injury in GERD.
-
4.
Acid neutralization —provided by salivary bicarbonate and also bicarbonate secreted by the esophageal mucosa. The former is swept through the esophagus by the peristaltic wave and is thought to provide neutralization of any acid which lingers on the mucosal surface after most of the contents have been swept clear.
-
5.
Tissue resistance . The esophageal mucosa comprises three layers: the stratum basale/germinativum, stratum intermedium/spinosum, and stratum superficiale/corneum. Protrusions of the lamina propria, known as rete pegs, extend about two-thirds of the way into the mucosa. Several factors contribute to the resistance of the esophageal epithelium to acid and other injurious agents. These include bicarbonate production, the integrity of cell–cell junction and cell membranes, the presence of sodium/hydrogen ion exchange pumps, the ability of the epithelium to undergo restitution, and an adequate blood flow which arrives via the rete pegs and, critically, delivers bicarbonate.
Esophageal Resistance to Infection and Infestation
Long considered to be a sterile organ, high throughput studies now reveal that the esophagus has a distinct and rather diverse microbiome which generally resembles that of the oropharynx which it is, one assumes, derived from [4, 5]. There has been considerable interest in the possible contribution of the esophageal microbiome to GERD, Barrett’s esophagus, and esophageal adenocarcinoma [6]. One must assume that certain disturbances in the esophageal microbiome, such as those that may result from antibiotic exposure, will, in a manner analgous to what occurs elsewhere in the gastrointestinal tract, leave the door open to pathogenic opportunistic infections but this has not been directly studied. Similarly, studies of esophageal immune responses have been largely directed at diseases, such as eosinophilic esophagitis, which are thought to be immunologically mediated [7] and relatively little is known about the impact of systemic immune dysfunction on esophageal susceptibility to infection or infestation though clearly the esophagus is susceptible to fungal and viral infections in such circumstances as has been witnessed in immunosuppressed individuals with human immunodeficiency virus (HIV) infection [8]. In the HIV-infected individual the selective loss of the Th17 functional subset of T helper cells has been shown to set the stage for infection with Candida albicans [9].
Gastric Anatomy and Physiology: The Basics [10,11,12]
In diabetes the most important gastric disorder is gastroparesis ; the focus of our review of gastric anatomy and physiology will, therefore, be on motility.
The main functions of gastric motility are to accommodate and store the ingested meal, grind down or “triturate” solid particles, and then empty all of the constituents of the meal in a carefully controlled and regulated fashion into the duodenum. As already discussed, specialized muscle at the lower esophageal sphincter prevents reflux into esophagus; in an analogous manner the pyloric sphincter regulates transit across the gastroduodenal junction and also prevents orad reflux of duodenal contents. Of considerable relevance to the diabetic patient, the stomach also plays an active role in the generation of satiety and in the regulation of food intake .
To subserve these functions the stomach demonstrates a degree of regional specialization; in functional terms the stomach can be divided into three distinct regions: proximal stomach (incorporating the cardia, fundus, and proximal corpus), antrum , and pylorus . To subserve its role in optimizing the digestion of the food it delivers to the intestine gastric motor function is regulated by feedback from the small intestine.
Gastric Motor Physiology
Contractile activity at any level in the gastrointestinal tract is based on fundamental electrophysiological properties which typically comprise an omnipresent, highly regular, and recurring electrical pattern called the slow wave and intermittent events, spike potentials, which lead to muscle contractions [10]. In the stomach, slow waves occur at a frequency of 3 cycles per minute, the maximum frequency of phasic contractions is, therefore, also 3 cycles per minute. Gastric slow waves originate at the gastric pacemaker along the greater curvature in the proximal corpus and migrate therefrom in both circumferential and longitudinal directions. The electrophysiological properties of the various parts of the stomach can be seen to subserve its motor functions. Thus, though fundic smooth muscle cells are unique in being electrically silent, their resting membrane potential normally lies at or above the mechanical threshold, thereby promoting the maintenance of a sustained tonic contraction. Excitatory or inhibitory neural input to the region will increase or decrease this tone; the magnitude and duration of the response being directly related to the intensity and duration of the neural discharge. The corpus and antrum, in contrast reveal spontaneous and complex action potentials whose duration and characteristics determine the contractile activity of the region. In the stomach, interstitial cells of Cajal are now recognized as the originators of slow wave activity; these cells are electrically coupled to smooth muscle cells via gap junctions.
Fasting Motor Activity: The Migrating Motor Complex
Along the length of the gut, patterns of motor activity during fasting and after food differ fundamentally [12]. In the fasted state, motor activity is highly organized into a distinct and cyclically recurring sequence of events known as the migrating motor complex (MMC) which consists of three distinct phases of motor activity that occur in sequence and migrate slowly along the length of the small intestine. Each sequence begins with a period of motor quiescence (phase I), is followed by a period of apparently random and irregular contractions (phase II), and culminates in a burst of uninterrupted phasic contractions (Phase III or the activity front). Individual cycles last between one and 2 h, originate in the proximal small intestine, and migrate aborally; the velocity of propagation slowing as the activity front progresses distally. Cyclical motor activity has also been identified in the stomach, lower esophageal sphincter, gallbladder, and sphincter of Oddi. In the stomach, patterns of MMC activity tend to commence and end simultaneously at all sites rather than propagate, as occurs in the small bowel. As phase III develops in the proximal duodenum, several associated motor events occur in the stomach. Basal tone in the lower esophageal sphincter is increased and exhibits superimposed phasic contractions, thereby preventing reflux of gastric contents during this time of intense gastric contractile activity. Tone increases in the proximal stomach, and superimposed phasic waves can be identified. At the same time, 1 cycle per minute high-amplitude waves develop in the body of the stomach. True rhythmic activity occurs only in the distal antrum where contractions at 3–5 cycles per minute may be seen at the end of Phase III. As Phase III approaches and develops, antro-pyloro-duodenal coordination increases and high-amplitude contractions propagate through the antrum across the pylorus into the proximal duodenum where they are associated with brief clusters of phasic contractions.
The Motor Response to a Meal
On initiation of a swallow sequence, the gastric fundus undergoes vagally mediated receptive relaxation [13]. As the meal enters the stomach, tone and phasic contractions in the proximal stomach are inhibited leading to accommodation. Accommodation results in a dramatic, two- to threefold increase in gastric volume leading to the retention of food in the stomach until it may be distributed to the antrum and is triggered by distension-induced stimulation of mechanoreceptors and mediated by a vago-vagal reflex which relays in the nucleus of the tractus solitarius and is effected through projections to the dorsal motor nucleus of the vagus.
Food ingestion also results in the abolition of the cyclical pattern of the MMC and its replacement by a band of random contractions called the fed pattern which may last from 2.5 to 8 h, at which time the fasted pattern resumes, assuming that no more food has been ingested. In the stomach, the duration of the fed pattern is related to the caloric content and the nature of the meal.
An important component of the normal response to a meal and of the gastric emptying process is the ability of the antro-pyloric region to discriminate solids by size and to restrict emptying of solid particles greater than 1 mm in diameter. The antro-pyloric mill grinds down or triturates larger particles which are then emptied with the liquid phase, thus promoting optimal digestion. While trituration proceeds, solid emptying does not occur, thus giving rise to the lag phase; the duration of which is directly related to the size and consistency of the solid component of the meal. After a typical solid/liquid meal, the lag phase lasts approximately 60 min. The solid component is first retained in the proximal stomach; as liquids empty, the solid component moves to the antrum during the lag phase where it is triturated until solid particles reach a size of not more than 1 mm when they can be emptied, suspended in the liquid phase, through the pylorus.
Gastric emptying is regulated, largely through feedback from sensors in the duodenum, by the size, caloric content, osmolality, pH, and temperature of the meal [13]. The larger the meal, the higher its caloric content, the lower its pH, and the more its osmolality deviates from that of plasma, the slower it is emptied. The larger the size of the solid particles, the longer it will take for trituration to be accomplished. Furthermore, fat is emptied more slowly than protein, which, in turn, is slower than carbohydrate. The regulation of gastric emptying is so finely tuned and coordinated that it ensures the delivery of nutrients to the intestine at a rate of 2–3 kcal/min; an extraordinary accomplishment.
The rate of gastric emptying and, especially, that of solids is slower in females, and while this relative delay is assumed to be hormonal in origin, not all studies have documented a relationship between gastric emptying rate, the phase of the menstrual cycle, and cyclical variations in estradiol and progesterone.
Satiety, Food Intake, and the Stomach
Several factors contribute to the role of the stomach in the maintenance of intestinal homeostasis . These include the several feedback mechanisms already described above whereby nutrients and fat, in particular, delay gastric emptying, inhibit antral motility, and relax the fundus, thereby retarding the delivery of the ingested meal to the small intestine [13]. Enterogastric reflexes originating at more distal sites may play a similar role. One of the best described is the so-called ileal brake whereby the instillation of nutrients into the ileum delays gastric emptying. This mechanism could also be envisioned as fulfilling a homeostatic role in situations where absorption and digestion have not been optimal and nutrients may be lost. Various neuropeptides, such as cholecystokinin (CCK), leptin, and ghrelin which are involved in satiety and the regulation of food intake, also exert motility effects on the stomach.
Studies of the response to a meal indicate an interaction between gastric and oro-sensory factors; for example, while the direct infusion of the nutrient into the small intestine will induce neither fullness nor satiety, direct infusion of the nutrient into the stomach will provoke appetite suppression . However, the greatest suppression of appetite occurs when the nutrient is taken orally, suggesting roles for central, oral, and gastric factors in the induction of satiety [14].
By virtue of its release by nutrients, its known interaction with gastric sensory receptors , its effects in the central nervous system, and its proposed effects on a variety of gastric motor phenomena, CCK has become a prime candidate as the crucial link between food intake, gastric motor function, and the conscious response (fullness, satiety) to a meal. Leptin has been isolated from the stomach and seems to be involved in an early, CCK-mediated response to food intake. Ghrelin is a key regulator of food intake and seems to be responsible for meal initiation [15]. It also has effects on gastric motility and emptying .
Abbreviations
- CCK:
-
Cholecystokinin
- CNS:
-
Central nervous system
- GERD:
-
Gastroesophageal reflux disease
- HIV:
-
Human immunodeficiency virus
- LES:
-
Lower esophageal sphincter
- MMC:
-
Migrating motor complex
- TLESR:
-
Transient lower esophageal sphincter relaxation
- UES:
-
Upper esophageal sphincter
References
Alvarez Herrero L, Curvers WL, van Vilsteren FG, Wolfsen H, Ragunath K, Wong Kee Song LM, et al. Validation of the Prague C&M classification of Barrett's esophagus in clinical practice. Endoscopy. 2013;45:876–82.
Miller L, Clavé P, Farré R, Lecea B, Ruggieri MR, Ouyang A, et al. Physiology of the upper segment, body, and lower segment of the esophagus. Ann N Y Acad Sci. 2013;1300:261–77.
Hershcovici T, Mashimo H, Fass R. The lower esophageal sphincter. Neurogastroenterol Motil. 2011;23:819–30.
Di Pilato V, Freschi G, Ringressi MN, Pallecchi L, Rossolini GM, Bechi P. The esophagealmicrobiota in health and disease. Ann N Y Acad Sci. 2016;1381:21–33.
Hunt RH, Yaghoobi M. The esophageal and gastric microbiome in health and disease. Gastroenterol Clin N Am. 2017;46:121–41.
Snider EJ, Freedberg DE, Abrams JA. Potential role of the microbiome in Barrett’s esophagus and esophageal adenocarcinoma. Dig Dis Sci. 2016;61:2217–25.
Weinbrand-Goichberg J, Segal I, Ovadia A, Levine A, Dalal I. Eosinophilic esophagitis: an immune-mediated esophageal disease. Immunol Res. 2013;56:249–60.
Wilcox CM, Karowe MW. Esophageal infections: etiology, diagnosis, and management. Gastroenterologist. 1994;2:188–206.
Cassone A, Cauda R. Candida and candidiasis in HIV-infected patients: where commensalism, opportunistic behavior and frank pathogenicity lose their borders. AIDS. 2012;26:1457–72.
Greenwood-Van Meerveld B, Johnson AC, Grundy D. Gastrointestinal physiology and function. Handb Exp Pharmacol. 2017;239:1–16.
Quigley EMM. Gastrointestinal functions. In: Reference module in neuroscience and biobehavioral psychology. New York: Elsevier Science; 2017. p. 1–8.
Quigley EMM. Gastric motor and sensory function and motor disorders of the stomach. In: Feldman F, Friedman LS, Sleisenger MH, editors. Gastrointestinal and liver disease. Pathophysiology/diagnosis/management. 7th ed. Philadelphia: WB Saunders; 2002. p. 691–714.
Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33:880–94.
Holtmann G, Talley NJ. The stomach-brain axis. Best Pract Res Clin Gastroenterol. 2014;28:967–79.
Howick K, Griffin BT, Cryan JF, Schellekens H. From belly to brain: targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci. 2017;18:pii:E273.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Oglat, A., Quigley, E.M.M. (2018). Esophageal and Gastric Function. In: Duvnjak, M., Smirčić-Duvnjak, L. (eds) Gastrointestinal Complications of Diabetes . Clinical Gastroenterology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-75856-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-75856-5_4
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-75855-8
Online ISBN: 978-3-319-75856-5
eBook Packages: MedicineMedicine (R0)