Abstract
Gastro-gastric fistula is a complication that might happen after a bariatric procedure like Roux-en-Y gastric bypass. It is defined as an abnormal communication between the excluded gastric pouch and the gastric remnant after gastric bypass. Its incidence has substantially decreased since the technique evolved from a nondivided to a divided gastric pouch. The diagnosis of gastro-gastric fistula could be challenging because of the clinical presentation that varies depending on the etiology, the patient’s objective and subjective response, the time of onset, the medications administered, and the imaging sensitivity. Besides clinical evaluation, imaging studies like upper GI study and CT scan and esophagogastroduodenoscopy are useful tools in achieving a diagnosis. Finally, medical and surgical management are extensively discussed in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Morbid obesity is a worldwide epidemic. It is well established that surgery is the most effective cure for obesity and its comorbidity. Consequently the number of such procedures has exponentially increased, as has surgeons’ familiarity with bariatric procedures and with their related complications commensurately. The increased familiarity with such complications has actually led to their progressive reduction or to changes in techniques in order to avoid complications. In specific instances, procedures that led to high numbers of such complications have been completely abandoned, such as the vertical banded gastroplasty (VBG), or nearly abandoned, such as the laparoscopic adjustable gastric banding (LAGB) . In fact, the VBG resulted in 20–65% of complications, or long-term failure rates, with complications including gastric distention, uncontrollable vomiting, leaks, obstruction, staple line disruption, GERD, and insufficient weight loss needing revisional intervention [1]. The LAGB , on the other hand, experienced a quick peak in popularity due to its relatively technical simplicity and potential reversibility; however, over time, it became evident that up to 50% of patients might require reoperation or removal of the band due to either complications, insufficient weight loss, or weight regain [2].
The described background explains how procedures like laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic sleeve gastrectomy (LSG) gained popularity, both for their more favorable short- and long-term outcomes and their relatively low rates of complications. Obviously, complications remain an inevitable fact of any surgical intervention, and with the exponential increase in number of such procedures, surgeons had to increase their expertise in facing such complications, as well as improving their techniques, in order to avoid them.
The purpose of this chapter is to define and discuss a potential complication that might occur after gastric bypass, specifically the gastro-gastric fistula, and scope here includes its management from diagnosis to treatment.
Definition and Pathogenesis
A gastro-gastric fistula (GGF) is an abnormal communication between the excluded gastric pouch and the gastric remnant after LRYGB. Several hypotheses exist regarding the formation of GGF. The more obvious of the determining factors of a GGF is the incomplete separation of the gastric pouch from the gastric remnant. This factor can be secondary to surgeon’s inexperience, difficult anatomy, or technical problems (staple misfires). Other technical aspects of the procedure can also lead to a higher likelihood of GGF. In fact, Capella et al. in 1999 demonstrated a significantly higher incidence of GGF after nondivided gastric bypasses. In particular, they showed an incidence of 49% of GGF in the subgroup of nondivided or partially divided LRYGBs, compared with an incidence of 2.6% in the divided technique (p < 0.0001). Furthermore, they proposed that interposing a jejunal limb between the pouch and the remnant resulted in additional protection against the formation of a fistula, as the incidence after 492 cases was 0% [3].
Patients with particularly problematic habitus, such as male gender and higher body mass index (BMI), are known to pose challenges of visualization of the gastroesophageal (GE) junction, resulting in higher risk to develop this kind of fistula from incomplete division of the fundus of the stomach. In addition, the accidental presence of adipose tissue within the closed stapler could result in an incomplete resection as well.
Based on the abovementioned reports, the technique for LRYGB evolved from a stapled but nondivided to a stapled and divided pouch. However, a study from Cucchi et al. showed that the incidence of GGF remained substantial (6%) in spite of the divided technique, suggesting additional etiologies for GGF, such as abscess formation after a leak at the gastrojejunal anastomosis [4].
In order to understand the pathophysiology of a fistula, we need to review some basic concepts of general surgery that could explain its development. A fistula could originate from a chronic evolution of an abscess, as the inflammatory capsule of the abscess can erode into adjacent tissues and finally drains itself into a space other than the original, whether being the peritoneal cavity, another hollow organ, or outside the skin. This abnormal connection can be classified as blind, complete, or complex, according to its extension, complexity of tract through the organs, or whether it extends to the skin. Usually the chronic process moves toward loose tissues or follows the direction of existing forces, like gravity or peristaltic movements. Consequently, it has been hypothesized that a leak through a surgical suture, like the gastrojejunal anastomosis or the remnant stump, could evolve into an abscess secondary to the presence of acidic fluid, the inflammatory response, and the superinfection by common bacteria. If the fluid collection reaches and erodes through the gastric serosa, it can evacuate inside the gastric remnant, resulting in a fistula. The clinical manifestation depends on the degree of infection and varies from completely asymptomatic to frank sepsis. This scenario is described by Cucchi et al. who reported signs and symptoms of early localized sepsis with postoperative fever and tachycardia in all of their six patients with a GGF, in spite of negative routine contrast studies [4].
However, the clinical presentation is not always abrupt and can develop over weeks with moderate symptoms. This can be explained by an internal decompression via the gastric remnant of an abscess that has caused a breakdown in staple lines without irritating the peritoneal serosa [5].
Based on this principle of GGF formation, all patient-related risk factors for poor blood supply and tissue healing at the staple lines could increase the incidence of leaks at the anastomosis despite a thorough technique. These commonly known risk factors, such as diabetes, smoking status, steroid use, and hepatic disease with hypoalbuminemia, often coexist in the bariatric population and could have a considerable impact on outcomes.
More rarely, technical aspects related to the surgical staplers can also play a role in the formation of GGF. These includes staple misfires, wrong choice of staple height with failure of staples to penetrate the gastric tissue, which can potentially result in a leak and consequently to a fistula.
Among other hypothesized etiologies of GGF, there is also the formation of a marginal ulceration. The incidence of marginal ulcers after LRYGB has been reported, ranging between 0.6% and 16% [6], and the coexistence between a marginal ulcer and a GGF has been described in up to 52% [7]. This close association sparked some controversy regarding whether a marginal ulcer is a risk factor for a fistula formation or vice versa. A break in gastric mucosa’s continuity due to prolonged exposition to an acidic environment could predispose to a fistula formation, but conversely the presence of a fistula surely exposes the gastric pouch to low pH fluids originating from the gastric remnant.
Other proposed risk factors in the development of a fistula are gastric tissue migration, foreign body erosion, and anastomotic ischemia.
The ability of the gastric tissue to migrate and attach to other surfaces is well described; thus it is possible that gastric mucosa cells could spill out of the anastomosis and reach the gastric remnant through serosal attachments leading to a fistula formation.
Foreign bodies like preanastomotic rings and buttress material are potentially capable of eroding into the gastric wall and facilitating a pathological communication between the pouch and the remnant.
Finally, a crucial role is played by excessive tension in performing the anastomosis, as an under-tension suture is susceptible to ischemia and consequently to rupture and leak.
The discussed etiologic factors for GGF formation are summarized in Table 7.1.
Diagnosis
Clinical
The diagnosis of GGF could be challenging because of the clinical presentation that varies depending on the etiology, the patient’s objective and subjective response, the time of onset, the medications administered, and the imaging sensitivity.
In the series from Cucchi et al. the typical symptoms of a leak and abscess formation are reported in six out of six patients. In fact, in the early postoperative period, fever, tachycardia, and abdominal pain were present and supported the pathogenic hypothesis of a leak [4].
On the contrary, Carrodeguas et al. reported non-specific symptoms including nausea, vomiting, and epigastric pain, presenting after a variable time from 3 to 384 days (average 80 days) in 80% of patients [8]. The same author also showed that in 26.7% of the patients, a gastrojejunal anastomosis leak was previously diagnosed and treated, and consequently the diagnosis of GGF was achieved earlier (average 25 days) [8].
Yao et al. reported pain in 42% of the patients, nausea/vomiting in 21%, and the presence of an ulcer in 21% of the patients later diagnosed with GGF [9].
Nevertheless, for a considerable number of patients, the principal sign leading to the diagnosis of GGF was insufficient weight loss or weight regain. This percentage varies from 26.7% to 64%.
In the latter situation, since no additional symptoms were present, the diagnosis was achieved later (range from 17.1 to 19.8 months) [8, 9].
In another series the major complaint was epigastric pain (78%) followed by weight regain (44%) and gastrointestinal bleeding (11%) [10].
As demonstrated by O’Brien et al., another possible presentation of GGF could be the relapse of diabetes after its initial remission. It is well described, in fact, that the bypass of the duodenum and an accelerated transit of nutrients through the distal intestine enhance the release of peptide YY and GLP-1, improving insulin release and decreasing insulin resistance early in the postoperative course. Therefore, in the absence of other previously described symptoms, a relapse of diabetes, often concurrent with weight regain, reveals a restored transit of nutrients through the physiological route [11].
Following this concept, also a loss of food-intake restriction could be a hint of the presence of a GGF from a mechanical standpoint. Indeed, a communication between the gastric pouch and the gastric remnant enlarge the volume capacity available for food intake leading to weight regain.
As previously described, a marginal ulceration is often concurrent, and it will clinically result in epigastric pain.
Finally, a significant number of patients can be asymptomatic, and the true incidence of GGF could be underestimated, as suggested by case reports in which patients were incidentally diagnosed in spite of achieving a satisfying and sustained weight loss [12].
Imaging Studies
As discussed, the clinical presentation can be extremely variable, and further imaging investigations are often attained to confirm the clinical suspicion. Routine postoperative upper GI series (UGI) are often performed in many centers, but the sensitivity of this exam is hard to define given the large variability of the condition to examine.
It is however the first choice to investigate a symptomatic patient with a history of LRYGB.
Corcelles et al. described a sensitivity of 70% in detecting a GGF in patients readmitted for suspicious symptoms; however, the results were confirmed by an endoscopy [13].
Lee et al. discussed the impact of a selective use of upper GI studies after LRYGB and showed that morbidity and mortality were not adversely affected when a radiologic analysis is ordered after clinical suspicion driven by symptoms and surgeon’s experience. They also advocated a routine sample of drain amylase as an adjunct tool to detect the presence of a leak with a sensitivity of 100% [14].
In a review from Quartararo et al. comprising 15,022 postoperative routine upper GIs after LRYGB , a sensitivity of 78% for leakage was found [15].
However, as a GGF may have other etiology than a leak, we cannot assume the sensitivity of an upper GI study being equal in detecting a leak and a GGF.
Another important consideration is timing, since the period of development of a GGF can vary depending on the mechanism of formation. This can partially explain the immediate positive postoperative finding occurring after an incompletely divided remnant stomach, compared to a late onset following a chronic ulceration. A different sensitivity is also found depending on the type of contrast used for the upper GI study. In fact, in the immediate postoperative period, a water-soluble oral contrast (Gastrografin® ) is usually used as a potential leak will result in barium-related peritonitis. However, barium sulfate presents a slightly higher sensitivity for small leaks as compared to Gastrografin® , justifying its use in a later clinical scenario in which a GGF is most likely to present.
Ribeiro-Parenti et al. described a 100% rate of confirmation of diagnosis by UGI studies in their nine-case series [10].
Carrodeguas et al. finally showed that up to 80% of GGF may be diagnosed with an UGI study and endoscopy together, suggesting that a single study will not ensure a thorough evaluation [8] (Fig. 7.1). It is paramount to perform the UGI series in various patient positions, including supine. In fact, some of these fistulae are posterior and a standard upright UGI might miss the GGF.
Furthermore, as already discussed, GGF may be asymptomatic in patients with good weight loss results, thus discouraging the physician pursuing UGI. Consequently, the actual incidence of GGF might be underestimated and the radiological sensitivity biased.
Nevertheless, as advised by Huang et al. a barium UGI study is useful when performed in conjunction to an upper endoscopy, in the evaluation of patients with symptoms after LRYGB. They also stated that UGI provides important anatomical information that may be helpful to endoscopists not familiar with this patient population [16].
It is advisable then to associate this first level study with another, either being an endoscopy or a computed tomography (CT).
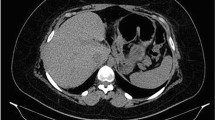
The literature is sparse regarding the use of a CT scan for the diagnosis of GGF. In general, a CT scan is used as a confirmatory tool with a high rate of success after a suspected diagnosis. The key elements of a CT scan in the diagnosis of GGF are either the presence of oral contrast into the excluded stomach or the direct demonstration of the fistulous tract, which can also be useful in defining the anatomy for an eventual surgical treatment (Fig. 7.2).
What appears to be mandatory is an esophagogastroduodenoscopy (EGD) evaluation, which is usually performed whenever a bariatric patient presents with upper GI symptoms. EGD allows for direct visualization of the defect, defines the position and the extent of the defect, and establishes the potential for successful endoscopic treatment, as discussed later (Fig. 7.3).
Although some small hidden fistulae could be missed if a thorough inspection is not performed, an experienced endoscopist may take advantage of probing all the mucosal folds with a sphincterotome or add fluoroscopy to indirectly visualize the presence of air or radiologic contrast in the excluded stomach [5]. Additionally, an upper endoscopy can usually detect a concomitant marginal ulcer, which is often not visible with an upper GI series, and subsequently guides the medical and surgical treatment.
The sensitivity of upper GI series and endoscopy together in diagnosis of a GGF is reported by Carrodeguas et al. to be 80%, whereas Corcelles et al. reported a 72.2% for endoscopy alone [8, 13].
In conclusion, the diagnosis of a GGF after LRYGB might be challenging, due to the extreme variability of the symptoms, the heterogeneity of etiologies, the time of presentation, and the relatively low sensitivity of diagnostic tools. Therefore, the diagnosis of GGF should be driven by a solid clinical judgment.
Management
The management of GGF has been described in the several few case series published in the literature, due to its relatively low incidence. However, some algorithms have been proposed, and different interventional approaches have been described.
The management of GGF can be observational, medical, surgical, or endoscopic.
Whenever the GGF is completely asymptomatic and incidentally found, observation is considered to be the best option.
Medical
Medical treatment is basically dependent on symptoms and on endoscopic findings. The clinical presentation should dictate the generic therapy in order to stabilize the patient and make him/her suitable for a possible surgery.
As already discussed, the presenting symptoms are unspecific and can vary from sepsis to mild abdominal pain. Medical therapy should be instituted based on main symptoms, comprising pain control, antiemetic, antibiotics if there is a suspected infection, and intravenous (IV) resuscitation or nutrition whenever an indication is present.
The endoscopic findings are then crucial for the decision to institute gastro-protective therapy. Given the close association between a GGF and a marginal ulcer, the latter must be investigated by endoscopy in order to reinforce the gastro-protective therapy. As proposed by Carrodeguas et al., if a GGF fistula is found, medical therapy with full-dose protein pump inhibitors (PPI) should be initiated, in order to reduce the acid production from G cells of the remnant stomach that could lead to development of a marginal ulcer through the fistula. If an ulcer is diagnosed simultaneously, sucralfate should be administered in order to create a protective layer to the pouch and the small bowel mucosa. Patients should be then reevaluated after 4–6 weeks to be reassessed and eventually elected to surgery (Fig. 7.4) [8].
Gumbs et al. reported a case of complete resolution of a GGF associated with a marginal ulcer after 6 weeks of medical therapy alone [17].
It is also advisable to test patients who tested positive for a marginal ulcer for the presence of Helicobacter pylori (H. pylori), in order to initiate proper antibiotic and gastro-protective therapy. After 3–4 weeks, patients should be reevaluated with endoscopy to assess for resolution of the ulcer and to discuss further treatment, whether observational or surgical.
Although in some cases medical therapy could lead to a resolution of small fistulae and to remission of symptoms with an acceptable weight loss, chronic PPI treatment is associated with important adverse effects, like vitamin B12 deficiency, which might be exacerbated by a malabsorptive mechanism following LRYGB [18] (Table 7.2).
Surgical
There is no standardized surgical procedure to treat a GGF, since the clinical scenario may vary depending on the time of diagnosis, the etiology, the association to marginal ulcer or a foreign body, and the clinical conditions of the patient. Also, factors such as the technique initially utilized (open or laparoscopic) or the experience of the surgeon may play a role in the timing and type of intervention. The decision to perform this kind of revisional operation laparoscopically or not is based on the individual surgeon’s skills and experience. The case series published report a preference of the laparoscopic approach, except for patients who underwent the primary intervention in an open fashion. Also, Filho et al. stated that a laparoscopic approach is easier when performed in the acute postoperative course, but still reasonable in chronic GGF, even when a disrupted anatomy is due to the expected inflammation [19].
Ribeiro-Parenti et al. accomplished 87.5% of the revisions (n = 9) laparoscopically and reported no deaths. They also reported one postoperative leak (12.5%) that required reoperation.
The authors also proposed a simple classification of GGF based on the location and consequent involvement of the gastrojejunal anastomosis that might be helpful in guiding the surgical approach. They classified a type 1 fistula if it is found >2 cm above the anastomosis and type 2 if <2 cm from the anastomosis. The 2 cm cutoff was chosen because it is a reasonable distance that allows firing of the stapler vertically to the pouch and transection of the fistulous tract without involving or narrowing the anastomosis. In their technique, the remnant was also resected laterally to the fistula. The type 2 fistula was instead approached with a complete resection of the previous anastomosis and a subsequent gastrojejunal anastomosis [10].
Salimath et al. converted to open surgery 2 patients out of 22 that primarily underwent laparoscopic GGF repair with remnant gastrectomy.
Another option is to proceed with a remnant gastrectomy. The laparoscopic remnant gastrectomy technique is described as follows.
With the patient placed in a standard supine position and a seven-trocar approach (Fig. 7.5), the first step is to define the anatomy by lysing the adhesions between the liver, gastric pouch, gastric remnant, and alimentary limb. Then the short gastric vessels are dissected to mobilize the gastric remnant at the level of the GE junction. Intraoperative endoscopy is used to better identify the fistula, and a 32 Fr gastric lavage tube is placed to identify the GE junction and the anastomosis. A window is created between the gastric pouch and the gastric remnant to allow positioning of a linear stapler and transecting transversally the remnant at the level of the antrum (Fig. 7.6). If the pouch is sufficiently enlarged, it could be directly trimmed by means of a linear stapler in order to complete the remnant gastrectomy (Fig. 7.7). If this maneuver is too risky for the anastomosis, the gastric remnant is excised, leaving a margin of remnant tissue attached to the GGF side, and secondarily oversewn (Fig. 7.8). Finally, the gastric remnant is extracted, endoscopic and methylene blue tests are performed, and drains are left closed to both pouch and antrum. Outcomes were comparable to those shown after bariatric revision interventions, but neither mortalities nor recurrence of the fistula was reported [20].
In the series reported from Corcelles et al. 19.5% out of 36 patients required a conversion to open surgery, and 80.5% underwent revision of the gastrojejunal anastomosis, leading to a significant increase of overall postoperative complications compared to those who received a remnant gastrectomy (19.5%, p = 0.01) [13].
Endoscopic
Recently, the endoscopic approach for bariatric surgery complications has gained increasing interest, probably due to a sum of factors like increasing endoscopic expertise, technological development, and increasing number of bariatric surgeries and related complications. Nonetheless, there are still some concerns regarding this approach because of its relatively poor long-term outcomes, despite promising low complication rates having been described.
Fernandez-Esparrach et al. described a case series of 95 patients diagnosed with post-LRYGB GGF. They highlighted a 95% initial success rate in complete closure of the fistula, but also a 65% rate of reopening after a mean interval of 177 days. Some patients were endoscopically treated again, and after a median time of 217 days, 81% of patients still presented with a GGF. They advocated that the size of the fistula may play an important role in foreseeing endoscopic failure, as they found a higher rate of success in fistulae <10 mm, namely, 32%, at the end of the follow-up period. On the other hand, they encountered an acceptable 2.1% rate of complications that did not require surgical intervention [21].
Bhardwaj et al. reported a small case series of eight patients with a success rate of 50% after 8–46 months of follow-up with no complications [22].
Flicker et al. reported a group of 22 patients who underwent at least one endoscopic attempt of fistula closure before going to surgery and compared it with 13 patients who directly underwent revisional surgery for GGF. They showed no significant differences in minor and major complications after endoscopy or surgery, but endoscopy revealed a non-encouraging 9.1% of minor and 31.8% of major complications. All the patients treated endoscopically underwent surgery anyway, with no underlying fistula closure by endoscopic treatment alone [23].
Finally Niland et al. published their results with 14 patients treated with an over the scope clip technique and showed a 50% initial success rate and a 33% success rate after 6 months, again with no complications reported [24].
Based on these results, it appears that endoscopic treatment should be considered in carefully selected patients, with minor symptoms and with small defects (<10 mm) that are likely to close and in addition to medical therapy. Surgical treatment, although undermined by a considerable risk of complication, has shown to be more definitive and therefore remains the preferred approach in the treatment of success rate. The technologic drive will surely make more effective instruments available for either surgical or endoscopic approach.
References
Switzer NJ, Karmali S, Gill RS, Sherman V. Revisional bariatric surgery. Surg Clin North Am. 2016;96(4):827–42.
Elnahas A, Graybiel K, Farrokhyar F, Gmora S, Anvari M, Hong D. Revisional surgery after failed laparoscopic adjustable gastric banding: a systematic review. Surg Endosc. 2013;27(3):740–5.
Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9(1):22–7. discussion 8
Cucchi SG, Pories WJ, MacDonald KG, Morgan EJ. Gastrogastric fistulas. A complication of divided gastric bypass surgery. Ann Surg. 1995;221(4):387–91.
Pauli EM, Beshir H, Mathew A. Gastrogastric fistulae following gastric bypass surgery-clinical recognition and treatment. Curr Gastroenterol Rep. 2014;16(9):405.
Coblijn UK, Lagarde SM, de Castro SMM, Kuiken SD, van Wagensveld BA. Symptomatic marginal ulcer disease after Roux-en-Y gastric bypass: incidence, risk factors and management. Obes Surg. 2015;25(5):805–11.
Cho M, Kaidar-Person O, Szomstein S, Rosenthal RJ. Laparoscopic remnant gastrectomy: a novel approach to gastrogastric fistula after Roux-en-Y gastric bypass for morbid obesity. J Am Coll Surg. 2007;204(4):617–24.
Carrodeguas L, Szomstein S, Soto F, et al. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1(5):467–74.
Yao DC, Stellato TA, Schuster MM, Graf KN, Hallowell PT. Gastrogastric fistula following Roux-en-Y bypass is attributed to both surgical technique and experience. Am J Surg. 2010;199(3):382–5. discussion 5–6
Ribeiro-Parenti L, De Courville G, Daikha A, Arapis K, Chosidow D, Marmuse JP. Classification, surgical management and outcomes of patients with gastrogastric fistula after Roux-En-Y gastric bypass. Surg Obes Relat Dis. 2016;
O'Brien CS, Wang G, McGinty J, et al. Effects of gastrogastric fistula repair on weight loss and gut hormone levels. Obes Surg. 2013;23(8):1294–301.
Gustavsson S, Sundbom M. Excellent weight result after Roux-en-Y gastric bypass in spite of gastro-gastric fistula. Obes Surg. 2003;13(3):457–9.
Corcelles R, Jamal MH, Daigle CR, Rogula T, Brethauer SA, Schauer PR. Surgical management of gastrogastric fistula. Surg Obes Relat Dis. 2015;11(6):1227–32.
Lee SD, Khouzam MN, Kellum JM, et al. Selective, versus routine, upper gastrointestinal series leads to equal morbidity and reduced hospital stay in laparoscopic gastric bypass patients. Surg Obes Relat Dis. 2007;3(4):413–6.
Quartararo G, Facchiano E, Scaringi S, Liscia G, Lucchese M. Upper gastrointestinal series after Roux-en-Y gastric bypass for morbid obesity: effectiveness in leakage detection. A systematic review of the literature. Obes Surg. 2014;24(7):1096–101.
Huang CS, Forse RA, Jacobson BC, Farraye FA. Endoscopic findings and their clinical correlations in patients with symptoms after gastric bypass surgery. Gastrointest Endosc. 2003;58(6):859–66.
Gumbs AA, Duffy AJ, Bell RL. Management of gastrogastric fistula after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(2):117–21.
Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931–50.
Filho AJ, Kondo W, Nassif LS, Garcia MJ, Tirapelle Rde A, Dotti CM. Gastrogastric fistula: a possible complication of Roux-en-Y gastric bypass. JSLS. 2006;10(3):326–31.
Salimath J, Rosenthal RJ, Szomstein S. Laparoscopic remnant gastrectomy as a novel approach for treatment of gastrogastric fistula. Surg Endosc. 2009;23(11):2591–5.
Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis. 2010;6(3):282–8.
Bhardwaj A, Cooney RN, Wehrman A, Rogers AM, Mathew A. Endoscopic repair of small symptomatic gastrogastric fistulas after gastric bypass surgery: a single center experience. Obes Surg. 2010;20(8):1090–5.
Flicker MS, Lautz DB, Thompson CC. Endoscopic management of gastrogastric fistulae does not increase complications at bariatric revision surgery. J Gastrointest Surg. 2011;15(10):1736–42.
Niland B, Brock A. Over-the-scope clip for endoscopic closure of gastrogastric fistulae. Surg Obes Relat Dis. 2017;13(1):15–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Giambartolomei, G., Menzo, E.L., Szomstein, S., Rosenthal, R. (2018). Gastro-Gastric Fistula Following Gastric Bypass. In: Camacho, D., Zundel, N. (eds) Complications in Bariatric Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-75841-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-75841-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75840-4
Online ISBN: 978-3-319-75841-1
eBook Packages: MedicineMedicine (R0)