Abstract
Esophageal motility disorders are a broad category of diseases with a variety of symptoms, ranging from dysphagia to chest pain. The pathophysiology is not always fully understood, but may involve alterations in inhibitory or excitatory innervation of the smooth muscle of the distal esophagus and LES. High-resolution manometry is the gold standard for its diagnosis, and the Chicago classification offers an organizational framework for better evaluation and management. Major disorders of motility are generally pathologic, and include achalasia, EGJOO, DES, hypertensive esophagus, and diseases of absent peristalsis, such as scleroderma. Treatments are targeted at the particular diagnosis. In achalasia, endoscopic and surgical options are preferred. For the remaining motility diagnoses, medical management forms the mainstay of treatment, which can be limited by side effect profiles.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Esophageal motility disorders
- Achalasia
- High-resolution manometry
- Heller Myotomy
- Gastroesophageal reflux disease
- Diffuse esophageal spasm
- Ineffective esophageal motility
- Nissen fundoplication
- Partial fundoplication
Introduction
Esophageal motility disorders are a broad range of diseases that can present with a variety of symptoms. A careful clinical assessment can suggest the etiology, but high-resolution esophageal manometry (HREM) is the gold standard for diagnosis. Management depends on the type of motility disorder identified and ranges from medical treatment to more definitive endoscopic and surgical options.
Pathophysiology
Esophageal motility disorders result from dysfunction of one or more components of esophageal peristalsis including esophageal body contraction and lower esophageal sphincter (LES) relaxation. The smooth muscle of the esophageal body and LES are modulated by inhibitory and excitatory innervation, and the specific neurologic defect can dictate the pathologic outcome (Fig. 5.1). Inhibitory innervation , composed of preganglionic neurons in the vagus and postganglionic neurons in the myenteric plexus, results in the release of nitric oxide and vasoactive intestinal peptide, which allows for relaxation of the LES and modulates contractility in the esophageal body. When absent, disorders including achalasia (poor relaxation of the LES) and diffuse esophageal spasm (DES) (poorly modulated contraction in the esophageal body) can result. Conversely, an increase in inhibitory innervation can result in increased transient LES relaxation (TLESR) episodes, accompanied by pathologic reflux.
Excitatory innervation , composed of vagal preganglionic and postganglionic neurons, results in the release of substance P and acetylcholine, establishing basal LES pressure and peristaltic contraction pressure. When absent, disorders including hypotensive LES, which can be associated with reflux, and hypotensive peristalsis, often seen in scleroderma, may result. Conversely, an increase in excitatory innervation may lead to disorders of hypertensive contractility, such as jackhammer esophagus.
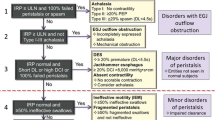
Traditionally, motility disorders have been classified into disorders of hypocontractility (such as achalasia types I and II, and scleroderma) and hypercontractility (such as DES, jackhammer esophagus, and type III “spastic” achalasia). However, with the development of HREM and the Chicago classification to guide interpretation and diagnosis, a new organizational hierarchy for motility disorders was introduced (Fig. 5.2). Major disorders of motility are generally pathologic, and often require advanced treatments such as surgery. These include achalasia, esophagogastric junction outflow obstruction, DES, jackhammer esophagus, and absent contractility. Minor disorders of motility may not always be pathologic or result in clinical symptoms, and can include ineffective esophageal motility (IEM), fragmented peristalsis, and other less well-defined diagnoses. We will be using this framework to guide our discussion of specific motility diagnoses in the following sections.
The Chicago Classification v3.0. Esophageal motility disorders are organized by major disorders of motility (generally pathologic, including disorders of EGJ obstruction with poor LES relaxation), and minor disorders of motility (which can be a normal variant). IRP integrated relaxation pressure, ULN upper limit of normal, PEP pan-esophageal pressurization, DL distal latency, DCI distal contractile integral
Clinical Assessment
The clinical symptoms of patients with esophageal motility disorders depend upon the etiology. Symptoms range from dysphagia to chest pain. Dysphagia to solids and liquids, consisting of a sensation of food or liquid lodged in the esophagus after initiation of a normal swallow, is one of the most common symptoms. Patients often feel that the bolus is hung up at the level of the suprasternal notch, although this may not be reflective of the actual location of the impacted food or liquid bolus. Learned behaviors to aid in swallowing and compensate for symptoms include taking longer to eat a meal, drinking fluids to clear food from the esophagus, eating smaller amounts of food, and performing physical maneuvers such as standing or arching the back to enhance bolus passage. Weight loss is common, and an indication for expedited evaluation. Other possible symptoms include chest pain or discomfort and/or regurgitation of liquid or food bolus.
In achalasia , patients may complain of vomit that dribbles out onto their pillow overnight. The main complication of untreated achalasia is the eventual development of megaesophagus, which is irreversible. There is also a small risk of developing squamous cell cancer of the esophagus, although there is insufficient data to support a screening program at present.
In DES , chest pain is often the predominant symptom. This pain can be triggered by emotional stress and radiate to the back, lateral chest and both arms or jaw, which can be confused with cardiac chest pain. In such cases, cardiology evaluation should be prioritized to exclude the presence of cardiovascular disease.
In hypertensive or jackhammer esophagus , patients often complain of chest pain, in addition to or in place of dysphagia. Interestingly, episodes of high-amplitude contractions seen on esophageal manometry do not always correlate with symptoms, and can be a normal variant, so clinical correlation is essential when this pattern is detected on motility testing. This is the only major disorder of motility that may not be pathologic when detected on HREM.
In disorders of absent esophageal contractility , GERD symptoms are most prominent, including heartburn, dysphagia, regurgitation, and chest pain. One such disorder of absent contractility is scleroderma, an autoimmune disease in which patients may also develop concomitant pulmonary interstitial fibrosis from micro-aspiration or from direct scleroderma involvement of lung tissue.
Patients with minor disorders of motility may complain of reflux symptoms or no symptoms at all. Clinical correlation is required when these findings are seen on HREM.
Diagnostic Procedures
Upper endoscopy is generally required in patients with suspected esophageal motility disorders to rule out mechanical causes, including stricture and malignancy. This is especially important in patients presenting with alarm symptoms including dysphagia or weight loss. Additionally, biopsies of the mid- and distal esophagus should be obtained to rule out eosinophilic esophagitis, an inflammatory condition causing dysphagia which occurs when eosinophils infiltrate the esophageal mucosa. Esophageal biopsies may also evaluate for evidence of infectious esophagitis, including HSV and candida, which is in the differential diagnosis of dysphagia. In achalasia, upper endoscopy is important to rule out gastroesophageal junction tumors, which can result in secondary achalasia.
Laboratory testing can be used to support manometric diagnoses. In achalasia, antibodies to the parasite T. cruzi should be considered in patients with risk factors, such as foreign travel, to evaluate for secondary achalasia due to parasitic infection. Additionally, antineuronal antibodies should be checked when paraneoplastic syndromes are suspected as the cause of secondary achalasia, particularly in patients with small cell cancers of the breast or lung. In scleroderma, antibody testing to topoisomerase-1 (Scl 70) can provide additional support for the diagnosis.
Radiologic studies can also be useful in the assessment of motility disorders. Barium swallow may demonstrate classic “bird-beaking” of the lower esophagus in achalasia, or a corkscrew appearance of the esophagus in DES. In advanced achalasia, the esophagus may appear sigmoid with severe dilation and an acute angle in the distal esophagus, or feature evidence of an esophageal diverticulum. There may also be delayed emptying and impaired or absent peristalsis of the esophagus noted as part of the study, which may be a more general signifier of dyskinesia. Chest imaging may be used in achalasia to exclude secondary achalasia due to pulmonary malignancy.
Multichannel intraluminal impedance and pH (MII-pH) testing may be helpful to evaluate for contribution from acid or bolus reflux. Some motility disorders, such as scleroderma, often co-present with reflux symptoms. Other findings, such as IEM, are frequently detected in the presence of GERD, and are often associated with evidence of increased reflux on MII-pH testing.
The gold standard for diagnosis of esophageal motility disorders is HREM. This diagnostic tool measures esophageal peristalsis, baseline LES pressure and relaxation, and bolus transit. Esophageal manometry involves placement of a thin flexible catheter with sequential pressure sensors transnasally into the stomach, traversing the esophagus and LES in the process. Patients are asked to take ten swallows with 5 mL of water in the supine position, and in some centers, an additional ten swallows with thick gel is also performed. Recently, as mentioned above, the Chicago classification was proposed to provide an organizational scheme for the diagnosis of motility disorders, dividing them into major and minor disorders based on manometric parameters [1]. This classification system allows for greater standardization in the diagnosis and classification of motility disorders. The Chicago parameters include the distal contractile integral (DCI) , distal latency (DL) , contractile deceleration point (CDP) , and integrated relaxation pressure (IRP) (Table 5.1, Figs. 5.2 and 5.3). The DCI is the product of the mean amplitude of contraction in the distal esophagus (mmHg), duration of contraction (seconds), and the length of the distal esophageal segment (cm)—essentially a measure of distal esophageal contractile force. It is considered failed if less than 100 mmHg·s·cm, weak if less than 450 mmHg·s·cm, and hypercontractile if greater than 8000 mmHg·s·cm. DL is measured from the time of upper esophageal sphincter relaxation to an inflection in the peristaltic axis, or CDP, and is considered premature if less than 4.5 s. DL is a measure of esophageal spasm. The IRP is measured as the mean EGJ pressure during the 4 s of maximal relaxation in the first 10 s after upper esophageal sphincter relaxation, relative to gastric baseline, and is considered elevated if greater than 15 mmHg, though this cutoff is dependent on the model of HREM machine. IRP is a measure of LES relaxation.
The first two diagnoses in the Chicago classification hierarchy are ones in which the median IRP is elevated, reflecting poor LES relaxation.
HREM is a sensitive method to diagnose achalasia [2]. Manometric parameters that meet criteria for achalasia include median IRP >15 mmHg across all swallows, with 100% failed peristalsis or esophageal spasm. HREM allows for achalasia to be classified into three subtypes based on the pattern of contractility in the esophagus, with implications for treatment success. Type I “classic” achalasia is defined by failed contractions without esophageal pressurization. Type II achalasia is characterized by aperistalsis with pan-esophageal pressurization. Type III “spastic” achalasia is defined by high amplitude spastic or premature contractions [1]. Type II achalasia is the most common subtype and is most responsive to treatment, whereas type III achalasia is the least common subtype and is also the least treatment responsive.
Esophagogastric junction outflow obstruction (EGJOO) is characterized manometrically by impaired LES relaxation with median IRP >15 mmHg but normal or weak peristalsis. In some cases, this may reflect incompletely expressed achalasia, and should be monitored closely. It is also important to exclude mechanical obstruction.
The next three diagnoses feature normal IRP, accompanied by other abnormal parameters.
DES is characterized manometrically by a normal IRP, but with simultaneous, non-peristaltic contractions featuring DL less than 4.5 s, in at least 20% of swallows. The non-peristaltic contractions are due to loss of inhibitory nerve function in the esophagus, similar to achalasia, and the contractions themselves can have either increased or decreased amplitudes. One distinguishing feature from Type III achalasia is that with DES, the lower esophageal sphincter is spared and relaxes normally.
Hypertensive esophagus, or “jackhammer” esophagus , is characterized manometrically by a normal IRP and normal DL, but with a DCI greater than 8000 in at least 20% of swallows.
Absent contractility is defined by normal IRP with evidence of aperistalsis. It encompasses diagnoses such as scleroderma, which is not a distinct diagnosis in the Chicago classification. Hypotensive LES may also be seen in scleroderma.
The preceding diagnoses are considered major disorders of peristalsis, which are generally pathologic.
Minor disorders of peristalsis include IEM defined by greater than 50% failed or weak swallows, and fragmented peristalsis defined by greater than 50% contractions with peristaltic breaks of at least 5 cm. These findings are not always clinically significant, so clinical correlation is recommended before pursuing treatment.
Treatment Options
Achalasia
Treatment ranges from pharmacologic therapy, to endoscopic and surgical interventions, which are more invasive but also more effective. The goal of therapy for all approaches is to decrease the resting LES pressure, allowing for passage of solids and liquids into the stomach. Overall, treatment response is highest for patients with Type II achalasia.
Pharmacologic Options
Medical therapy has limited efficacy in the treatment of achalasia. In patients who cannot tolerate any endoscopic or surgical intervention, nitrates and calcium channel blockers may be used to decrease LES pressure to enhance bolus clearance. However, these medications tend to be short-acting, with maximum pharmacologic duration of 120 min, and are often limited by side effects including dizziness and headaches [3]. Thus, medical therapy should be avoided as long as endoscopic or surgical options remain viable.
Endoscopic Botulinum Toxin Injection
Botulinum toxin A (Botox) inhibits acetylcholine release. When injected into the LES, it lowers LES pressure. Approximately 25 units of Botox total are injected into the four quadrants of the LES. Initial response rates are high at 80%, but the effect seems to be transient, with many patients requiring repeat injections with diminished efficacy over time [4,5,6]. This may be due to antibody formation to the toxin as well as fibrosis of the LES from repeated treatments. In spite of this, Botox does benefit from minimal side effects and ease of delivery, and is therefore most often reserved for non-surgical candidates with achalasia, such as elderly patients.
Pneumatic Dilation
Pneumatic dilation uses air filled balloons under high pressure to mechanically disrupt the smooth muscle of the distal esophagus and LES. The dilators typically range in size from 30 to 40 mm. Pneumatic dilation is performed using fluoroscopic guidance with the balloon crossing the LES. Usually two or more dilations are required, resulting in high remission rates at 1–5 years after treatment. Four percent of dilations cause perforations requiring surgical repair, which is more common in patients requiring serial dilations compared to single dilation [7]. Perforation, though rare, is more frequent with balloons greater then 30 mm, and with the initial dilation [8]. Relapse is more likely to occur in males, subjects with extreme esophageal dilatation, younger age (<40), and poor bolus emptying on timed barium swallow [7, 9, 10]. Because pneumatic dilation is less invasive than myotomy, it is often the preferred approach in subjects with surgical risk factors, such as older age and medical comorbidities.
Heller Myotomy
First performed in 1913, the Heller myotomy is now performed laparoscopically and usually with an extended myotomy into the cardia of the stomach. The extended myotomy allows for further reduction of LES pressures to a goal of <10 mmHg. However, this comes at the risk of significant reflux. To help mitigate this risk, a partial fundoplication (either anterior Dor with 180° wrap, or posterior Toupet with 270° wrap) is also performed [11, 12]. Symptomatic improvement occurs in 90% of patients post-operatively, though efficacy does decrease with time [4]. The most common complication is GERD requiring PPI treatment in upwards of 40% of patients, even when a partial wrap is performed. However, a complete wrap or Nissen fundoplication is usually avoided, since it can become difficult to distinguish post-operative dysphagia from a tight wrap versus an incomplete myotomy. In certain cases of Type III achalasia, the myotomy can be extended proximally in the esophagus to address severe esophageal spasticity.
Laparoscopic Heller myotomy is superior to a single pneumatic dilation but this difference dissipates with graded pneumatic dilations guided by clinical symptoms. A meta-analysis comparing graded pneumatic dilation to laparoscopic myotomy determined myotomy was more effective than pneumatic dilation, but there were no differences in reflux rates and LES pressure [13]. The largest trial included in the meta-analysis found no significant difference in success rate for pneumatic dilation (90%) versus Heller myotomy (93%) at 1 year [14]. Five years after treatment, there remained no significant difference in success rates between the myotomy and pneumatic dilation groups [15].
Endoscopic Myotomy
Per-oral endoscopic myotomy (POEM ) is a newer alterative to surgical myotomy. It is a form of natural orifice transluminal endoscopic surgery (NOTES) . This is an incisionless surgery performed with a flexible endoscope, with submucosal tunneling made distal to the mucosal incision. Contraindications include severe esophagitis, coagulation disorders, prior therapy with possible submucosal fibrosis such as radiation, endoscopic mucosal resection, and portal hypertension. The technique involves four steps: (1) mucosal incision with entry into the submucosa, (2) formation of a submucosal tunnel, (3) myotomy, and (4) closure of the mucosal incision. In POEM , the circular muscle of the LES is disrupted and the longitudinal muscle layer is left intact. The technique involves insertion of a flexible endoscope into the esophagus and use of a very small electrosurgical knife through the instrument channel of the endoscopy. A small mucosal incision is made in the mid esophagus so that the endoscope can then enter into the 1–2 mm submucosal space between the mucosa and muscularis propria. This space is expanded with saline injections to provide space for insertion of the 10-mm endoscope, which is subsequently advanced to create a tunnel into the gastric cardia. A myotomy is then performed within the tunnel. The length of the myotomy depends upon the underlying disorder and is typically longer in spastic esophageal disorders compared to achalasia subtype I or II. Finally the original mucosal incision is closed with sutures or endoscopic clips [16]. Typically patients are admitted to the hospital overnight for observation and given prophylactic antibiotics and antiemetics. An esophagram is obtained the day after the procedure to rule out an esophageal leak and the diet is advanced to a soft diet for the next 2 weeks. POEM is a safe procedure with low rates of adverse events which include pneumoperitoneum, pneumothorax, bleeding, mucosal perforation and gastroesophageal reflux (which is of concern since a partial fundoplication cannot be performed at the time of POEM unlike during laparoscopic Heller myotomy). POEM has demonstrated a high rate of clinical success (82–100%) and is comparable to laparoscopic Heller myotomy in safety and efficacy based on a recent meta-analysis [17]. POEM is also effective and safe in patients who have refractory or recurrent symptoms despite prior surgical or endoscopic treatment [18].
EGJ Outflow Obstruction
There are no specific treatments for EGJOO since the etiology of this entity is not well understood. It may be a variant of or represent early achalasia. Alternatively, it may be caused by abnormal anatomy at the cardia including a hiatal hernia. The same treatment options available for achalasia may be applied for EGJOO. However, as many as one-third of patients diagnosed with EGJOO experience spontaneous symptom resolution without specific intervention. Medical therapies such as calcium channel blockers and nitrates may be used, but only 50% of patients experience a response [19]. More invasive treatment options including Botox injection, pneumatic dilation, or surgery are all highly efficacious with favorable outcomes, but given the unclear natural history of this diagnosis, are reserved for severe cases. Recently, the use of acotiamide hydrochloride, a prokinetic drug approved for functional dyspepsia, may offer some treatment benefit in EGJOO . Acotiamide acts as an acetylcholinesterase inhibitor with prokinetic activity and improved gastric emptying, and 83.3% of EGJOO patients reported at least some symptomatic improvement as well as normalization of the IRP following its use [20,21,22].
Diffuse Esophageal Spasm
Treatment of DES can be difficult and therapy is mainly focused on symptom control, primarily because of the current lack of understanding of the underlying etiology of DES and dearth of controlled therapeutic trials. Proton pump inhibitors and histamine receptor antagonists may be used to address any contribution from acid reflux, which has the potential to induce or be a consequence of esophageal spasm. Smooth muscle relaxants including nitrates and calcium channel blockers, anticholinergics, and phosphodiesterase-5 inhibitors are used to decrease LES pressure and esophageal contraction amplitude. While nitrates have not been tested in a controlled fashion in patients with DES or other spastic disorders, they have been demonstrated manometrically to prolong the DL and decrease distal contraction amplitude [23]. Phosphodiesterase-5 inhibitors such as sildenafil block the breakdown of nitric oxide and thereby prolong smooth muscle relaxation [24]. Many of these therapies are limited by side effects such as headache and dizziness. Low dose antidepressants (tricyclic antidepressants, serotonin receptor inhibitors, trazodone) are effective in improving chest pain caused by DES, though data does not demonstrate any effect on motility. This suggests that visceral hypersensitivity could be a major driver of symptoms [25]. Studies have demonstrated that more invasive techniques such as empiric bougie dilation or Botox injection of the LES alone do not significantly improve symptoms, though data is more promising when considering Botox injection of the esophageal body [26, 27]. Pneumatic dilation, while effective in the treatment of achalasia, has not been proven in DES. Limited data demonstrates some improvement with an extended Heller myotomy, but this invasive approach is reserved for refractory patients [28]. A recent systematic review and meta-analysis demonstrated POEM as an effective and safe treatment modality for spastic esophageal disorders including type III achalasia, diffuse esophageal spasm and jackhammer esophagus [29].
Overall, treatment of DES should be approached in a stepwise fashion. First, the patient should be placed on antireflux medication. If this therapy is ineffective, a smooth muscle relaxant such as a calcium channel blocker or a treatment for visceral hypersensitivity such as tricyclic antidepressant can be tried. If symptoms persist, more invasive treatments such as Botox injection can be considered. Finally, surgery is reserved for patients who fail all other treatment modalities.
Hypercontractile (Jackhammer) Esophagus
Similar to DES, therapy of hypercontractile esophagus is aimed at symptom control, as the underlying pathophysiology remains poorly understood. Treatment is dictated by the predominant clinical complaint. Smooth muscle relaxants including nitrates and calcium channel blockers, as well as anticholinergic medications, may be applied to treat symptoms of dysphagia. For subjects with noncardiac chest pain, tricyclic antidepressants may help address the clinical contribution from visceral hypersensitivity. In severe cases, Botox injection in the esophageal body has resulted in clinical improvement in dysphagia symptoms and may be an option for select patients [27].
Absent Peristalsis/Scleroderma Esophagus
The current treatment of absent esophageal peristalsis includes aggressive reflux management, with use of proton pump inhibitors at maximum dose. Unfortunately, prokinetic medications have not demonstrated clinical utility in this patient population. Scleroderma esophagus is a connective tissue disorder that affects the smooth muscle of the esophagus, resulting in aperistalsis and decreased LES pressure. Antireflux surgery has been discouraged because of the risk of significant post-operative dysphagia with decreased or absent peristalsis of the esophageal body, although some studies have proposed partial fundoplication to manage severe GERD symptoms in select patients with absent peristalsis [30]. Esophageal strictures may develop from significant uncontrolled reflux and often require dilation.
Ineffective Esophageal Motility
Treatment options are limited for IEM. Because most cases are associated with GERD, treatment is aimed at antireflux control. Buspirone is a serotonin receptor agonist which may enhance LES resting pressure in IEM, and presents a possible treatment for IEM regardless of reflux association, in patients with clinical symptoms of dysphagia [31].
Fragmented Peristalsis
Similar to IEM, treatment options are limited in fragmented peristalsis because the clinical implications of this diagnosis remain unclear. Treatment tends to be focused on management of concomitant GERD.
Summary
Esophageal motility disorders are a broad category of diseases with a variety of symptoms, including dysphagia and chest pain. The pathophysiology is not always fully understood, but may involve alterations in inhibitory or excitatory innervation of the smooth muscle of the distal esophagus and LES. The gold standard in diagnosis is HREM, and the Chicago classification offers an organizational framework for better evaluation and management. Major disorders of motility are generally pathologic, and include achalasia, EGJOO, DES, hypertensive esophagus, and absent peristalsis, such as scleroderma. Minor disorders including IEM may be associated with GERD or have no clinical correlation. Treatments are targeted at the particular diagnosis. In achalasia, endoscopic and surgical options are preferred. For the remaining motility diagnoses, medical management forms the mainstay of treatment, which can be limited by side effect profiles.
References
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–74. https://doi.org/10.1111/nmo.12477.
Richter JE. High-resolution manometry in diagnosis and treatment of achalasia: help or hype. Curr Gastroenterol Rep. 2015;17(1):420. https://doi.org/10.1007/s11894-014-0420-2.
Hoogerwerf WA, Pasricha PJ. Pharmacologic therapy in treating achalasia. Gastrointest Endosc Clin N Am. 2001;11(2):311–24, vii. http://www.ncbi.nlm.nih.gov/pubmed/11319064
Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249(1):45–57. https://doi.org/10.1097/SLA.0b013e31818e43ab.
Fishman VM, Parkman HP, Schiano TD, et al. Symptomatic improvement in achalasia after botulinum toxin injection of the lower esophageal sphincter. Am J Gastroenterol. 1996;91(9):1724–30. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=8792688
Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46(5):597–600. https://doi.org/10.1136/gut.46.5.597.
Katzka DA, Castell DO. Review article: an analysis of the efficacy, perforation rates and methods used in pneumatic dilation for achalasia. Aliment Pharmacol Ther. 2011;34(8):832–9. https://doi.org/10.1111/j.1365-2036.2011.04816.x.
Richter JE, Vela MF, Richter JE, et al. Update on the management of achalasia: balloons, surgery and drugs. Clin Gastroenterol Hepatol. 2006;4(3):580–7. https://doi.org/10.1586/17474124.2.3.435.
Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103(6):1732–8. https://doi.org/10.1053/j.gastro.2008.07.022.Achalasia.
Chan KC, Wong SKH, Lee DWH, et al. Short-term and long-term results of endoscopic balloon dilation for achalasia: 12 years’ experience. Endoscopy. 2004;36:690–4. https://doi.org/10.1055/s-2004-825659.
Richards WO, Torquati A, Holzman MD, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240(3):405–15. https://doi.org/10.1097/01.sla.0000202000.01069.4d.
Oelschlager BK, Chang L, Pellegrini CA. Improved outcome after extended gastric myotomy for achalasia. Arch Surg. 2003;138(5):490–495-497. https://doi.org/10.1001/archsurg.138.5.490.
Yaghoobi M, Mayrand S, Martel M, Roshan-Afshar I, Bijarchi R, Barkun A. Laparoscopic Heller’s myotomy versus pneumatic dilation in the treatment of idiopathic achalasia: a meta-analysis of randomized, controlled trials. Gastrointest Endosc. 2013;78(3):468–75. https://doi.org/10.1016/j.gie.2013.03.1335.
JCY W. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. J Neurogastroenterol Motil. 2011;17(3):324–6. https://doi.org/10.5056/jnm.2011.17.3.324.
Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut. 2016;65:732–9. https://doi.org/10.1136/gutjnl-2015-310602.
Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42(4):265–71. https://doi.org/10.1055/s-0029-1244080.
Marano L, Pallabazzer G, Solito B, et al. Surgery or peroral esophageal myotomy for achalasia. Medicine (Baltimore). 2016;95(10):e3001. https://doi.org/10.1097/MD.0000000000003001.
Stavropoulos SN, Modayil RJ, Friedel D, Savides T. The international per oral endoscopic myotomy survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc. 2013;27(9):3322–38. https://doi.org/10.1007/s00464-013-2913-8.
Pérez-Fernández MT, Santander C, Marinero A, Burgos-Santamaría D, Chavarría-Herbozo C. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil. 2016;28(1):116–26. https://doi.org/10.1111/nmo.12708.
Ishimura N, Mori M, Mikami H, et al. Effects of acotiamide on esophageal motor function and gastroesophageal reflux in healthy volunteers. BMC Gastroenterol. 2015;15(1):1–8. https://doi.org/10.1186/s12876-015-0346-7.
Kusunoki H, Haruma K, Manabe N, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24(6):e250–1. https://doi.org/10.1111/j.1365-2982.2012.01897.x.
Muta K, Ihara E, Fukaura K, Tsuchida O, Ochiai T, Nakamura K. Effects of acotiamide on the esophageal motility function in patients with esophageal motility disorders: a pilot study. Digestion. 2016;94:9–16.
Konturek JW, Gillessen A, Domschke W. Diffuse esophageal spasm: a malfunction that involves nitric oxide? Scand J Gastroenterol. 1995;30(11):1041–5. https://doi.org/10.3109/00365529509101604.
Eherer AJ, Schwetz I, Hammer HF, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut. 2002;50(6):758–64. https://doi.org/10.1136/gut.50.6.758.
Hershcovici T, Achem SR, Jha LK, Fass R. Systematic review: the treatment of noncardiac chest pain. Aliment Pharmacol Ther. 2012;35(1):5–14. https://doi.org/10.1111/j.1365-2036.2011.04904.x.
Bashashati M, Andrews C, Ghosh S, Storr M. Botulinum toxin in the treatment of diffuse esophageal spasm. Dis Esophagus. 2010;23(7):554–60. https://doi.org/10.1111/j.1442-2050.2010.01065.x.
Vanuytsel T, Bisschops R, Farre R, et al. Botulinum toxin reduces dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol. 2013;11(9):1115–21.e2. https://doi.org/10.1016/j.cgh.2013.03.021.
Leconte M, Douard R, Gaudric M, Dumontier I, Chaussade S, Dousset B. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg. 2007;94(9):1113–8. https://doi.org/10.1002/bjs.5761.
Khan MA, Kumbhari V, Ngamruengphong S, et al. Is POEM the answer for management of spastic esophageal disorders? A systematic review and meta-analysis. Dig Dis Sci. 2017;62(1):35–44. https://doi.org/10.1007/s10620-016-4373-1.
Lund RJ, Wetcher GH, Raiser F, et al. Laparoscopic Toupet fundoplication for gastroesophageal reflux disease with poor esophageal body motility. J Gastrointest Surg. 1997;1(4):301–8.
Scheerens C, Tack J, Rommel N. Buspirone, a new drug for the management of patients with ineffective esophageal motility? United European Gastroenterol J. 2015;3(3):261–5. https://doi.org/10.1177/2050640615585688.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Goldin, A., Lo, WK. (2018). Approach to Esophageal Motility Disorders. In: Oleynikov, D., Fisichella, P. (eds) A Mastery Approach to Complex Esophageal Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-75795-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-75795-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75794-0
Online ISBN: 978-3-319-75795-7
eBook Packages: MedicineMedicine (R0)