Abstract
Within the central nervous system (CNS), melanin-concentrating hormone (MCH) participates in a number of functions including sleep-wake behavior. In this respect, MCHergic neurons project widely throughout the central nervous system (CNS) to neural structures involved in the regulation of wakefulness (W). An enhancement of REM sleep time has been described following the microinjection of MCH into the dorsal raphe nucleus (serotonergic neurons), locus coeruleus nucleus (noradrenergic neurons), and basal forebrain [(horizontal limb of the diagonal band of Broca) glutamatergic and cholinergic (W-on) neurons] of rodents. In addition, optogenetic stimulation of MCH terminals in the tuberomammillary nucleus (histaminergic neurons) is followed by an increase in the duration of REM sleep episodes. Moreover, the finding that the neuropeptide negatively modulates the mesolimbic dopaminergic function tends to indicate that the inhibition of nucleus accumbens and ventral tegmental nucleus dopaminergic neurons by MCH could facilitate the occurrence of REM sleep. Thus, the REM sleep-inducing and sleep-facilitating effect of MCH is at least partly related to the deactivation of monoaminergic, glutamatergic, and cholinergic (W-on) neurons.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Melanin-concentrating hormone
- REM sleep
- Wakefulness
- Serotonin
- Norepinephrine
- Dopamine
- Glutamate
- Acetylcholine
1 Neural Structures and Neurotransmitter Systems Involved in the Generation and Maintenance of Wakefulness
The brain regions involved in the promotion of the waking state are found in the brainstem, hypothalamus, and basal forebrain (BFB). The nuclei located in the brainstem include:
-
Neurons containing serotonin [5-HT—dorsal raphe nucleus (DRN), median raphe nucleus (MRN)]
-
Norepinephrine [NE—locus coeruleus]
-
Dopamine [DA—ventral tegmental area (VTA), substantia nigra pars compacta (SNc), ventral periaqueductal gray matter (vPAG)]
-
Acetylcholine [ACh (wakefulness-on)—laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT)]
The hypothalamic component of the arousal system includes cells containing orexin [OX; posterior lateral hypothalamus (LH) around the fornix] and histamine [HA; tuberomammillary nucleus (TMN)]. The cholinergic and glutamatergic neurons of the BFB involved in the regulation of wakefulness (W) are located predominantly in the medial septum, diagonal band of Broca, and substantia innominata (Pace-Schott and Hobson 2002; Jones 2003). The 5-HT-, NE-, HA-, and ACh-containing neurons that participate in the regulation of W give rise to mainly ascending projections to (1) the thalamus (dorsal route) which in turn projects to the cerebral cortex and (2) the BFB (ventral route) where cells in turn project to the cerebral cortex and the hippocampus. The DA-containing cells of the VTA and SNc project to the striatum and the prefrontal cortex, while those corresponding to the vPAG project predominantly to the BFB and midline thalamus. Furthermore, OX-containing neurons carry projections to the entire forebrain and brainstem arousal systems as well as to the thalamus and cortex (Brown et al. 2012). Additionally, most of these neural structures send descending projections to the brainstem and spinal cord regions that modulate muscle tone.
2 Neural Structures and Neurotransmitter Systems Involved in the Generation and Maintenance of NREM Sleep
Neurons of the preoptic area and adjacent BFB constitute the sleep-inducing system (Szymusiak et al. 2007). Sleep active neurons of the preoptic area are mainly located in the ventrolateral preoptic area (VLPO). A majority of these neurons contain γ-aminobutyric acid (GABA) and galanin and project to brainstem and hypothalamic areas involved in the promotion of W including the DRN, LC, LDT/PPT, vPAG, and LH neurons. More recently, melanin-concentrating hormone (MCH) neurons located in the lateral hypothalamus and zona incerta have been proposed to participate also in the regulation of non-rapid-eye movement (NREM) sleep and REM sleep (Torterolo et al. 2011).
3 Neural Structures and Neurotransmitter Systems Involved in the Generation and Maintenance of REM Sleep
It has been proposed that the sublaterodorsal tegmental nucleus (SLD) is the brain structure necessary and sufficient to induce and maintain REM sleep in the rat, and the effect would depend upon the activation of glutamatergic neurons located in this neuroanatomical structure (Luppi et al. 2013). Its equivalent in the cat is called the subcoeruleus nucleus or nucleus pontis oralis. In favor of the proposal is the finding that SLD glutamatergic neurons increase their firing rate during REM sleep. Furthermore, microiontophoretic administration of the glutamate agonist kainic acid into the SLD induces a REM sleep-like state (Clément et al. 2011). Luppi et al. (2013) have posed, in addition, that the activation of SLD glutamatergic neurons would depend upon the removal of an inhibitory tone present during W and NREM sleep arising from GABAergic REM-off neurons located in the ventrolateral periaqueductal gray (vlPAG) and the deep mesencephalic nucleus (DPMe) and monoaminergic neurons located in the brainstem and hypothalamus. Accordingly, local microinjection into the SLD of the GABAA receptor antagonists bicuculline or gabazine produced an increase of REM sleep in laboratory animals (Boissard et al. 2002). It has been contended also that inhibition of vlPAG and DPMe REM-off neurons would depend upon the activation of GABAergic REM-on neurons located in the vlPAG and dorsal gigantocellular nucleus (DPGi) and MCH neurons of the lateral hypothalamus (Sapin et al. 2009; Monti et al. 2016). On the other hand, the reciprocal-interaction hypothesis of REM sleep generation originally proposed by McCarley and Hobson (Hobson et al. 1975) identifies interconnected populations of REM-on and REM-off neurons compatible with reciprocal interactions as a physiological basis of sleep cycle oscillation. In the updated version of the reciprocal-interaction model (McCarley 2007), cholinergic neurons of the LDT/PPT are identified as promoting REM sleep and interact with serotonergic and noradrenergic neurons of the DRN and LC that inhibit REM sleep. In addition, McCarley (2007) includes GABAergic mechanisms that deactivate neurotransmitter systems responsible for the inhibition of LDT/PPT cholinergic (REM-on) neurons. This would lead to the activation of pontine reticular formation glutamatergic neurons and the occurrence of REM sleep.
Thus, it can be proposed that there is much agreement with respect to the neural structures and mechanisms of action involved in the regulation of W and NREM sleep. However, discrepancies still exist in relation to the neuroanatomical systems and synaptic processes involved in the facilitation and inhibition of REM sleep.
4 Structure, Mechanism of Action, and Projections of MCH-Containing Neurons
In mammals MCH is a cyclic neuropeptide with 19 amino acids. It is generated by the cleavage of a precursor of 165 amino acids, the prepro-MCH. MCH is confined to a group of neurons in the lateral hypothalamus and incerto-hypothalamic area and acts through its G-protein-coupled receptors named MCHR1 and MCHR2. Rodents present only the MCHR1 (Lembo et al. 1999). The binding of MCH to MCHR1 activates diverse intracellular signaling pathways by coupling to Gi, Gq, and Go proteins, while MCHR2 is known to couple to the Gq protein (Hawes et al. 2000; Sailer et al. 2001). Studies of MCH neurons in vitro have shown a predominantly inhibitory effect of the neuropeptide both at pre- and postsynaptic levels (Gao and van den Pol 2001). Sekiya et al. (1988) determined the distribution of MCH-like immunoreactivity by radio immunoassay in the central nervous system (CNS) of the rat, guinea pig, and man. The highest concentrations of MCH were found in the hypothalamus of all these species. Later on, Bittencourt et al. (1992) characterized the organization of the system using antisera raised against rat MCH. It was shown that medium-sized and multipolar to fusiform MCH-containing cells were localized predominantly in the lateral hypothalamic area and zona incerta of the rat. In addition, monosynaptic fibers stained for MCH were broadly distributed throughout the CNS. In this respect, MCH-immunoreactive (MCH-ir) axons innervate several neuroanatomical structures located in the telencephalon, diencephalon, mesencephalon, and rhombencephalon that are involved in the regulation of the behavioral state. In addition, Bittencourt and Elias (1998) established that the origin of MCH-ir projections in the medial septal nucleus, vertical and horizontal limbs of the diagonal band of Broca, and spinal cord resides mainly in the lateral hypothalamus. Moreover, dense MCH innervation was reported in the cerebral motor cortex and LDT/PPT of the rat, the latter originating mainly from the dorsal half of the lateral hypothalamus (Elias et al. 2008; Hong et al. 2011).
5 Local Brain Delivery of MCH into CNS Structures Involved in the Regulation of the Sleep-Wake Cycle
5.1 Dorsal Raphe Nucleus
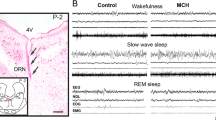
Bittencourt et al. (1992) described for the first time a descending component of MCH fibers that innervated the DRN in the rat. More recently, Yoon and Lee (2013) examined the projections from MCH neurons in the medial and lateral subdivision of the LH to the DRN of rodents. The authors found that MCH axon terminals from both subdivisions of the LH made contact with DRN somata at both rostral and caudal levels. A further study by Urbanavicius et al. (2016) characterized in detail the distribution and density of the MCHergic fibers along the rostro-caudal axis of the rat DRN and their anatomical relationship with 5-HT and GABA-containing neurons. In addition, the authors evaluated the MCH effects on the 5-HT extracellular levels. Accordingly, MCHergic axons reached all the rostro-caudal levels of the DRN, although their density was lower at the most caudal level. Of note, MCH fibers were found to be in apposition with both 5-HT- and GABA-containing cells. The local microinjection of a relatively low dose (30 μM) of the neuropeptide was followed by a significant and long-lasting decrease of stimulated 5-HT levels. The finding of particular dense MCHergic projections to the DRN by Bittencourt et al. (1992) led to the analysis of the effect of microinjections of MCH into this neuroanatomical structure on sleep variables in the rat. To this purpose MCH (50 and 100 ng) and vehicle were microinjected into the DRN of rodents prepared for chronic sleep recordings. Compared with the control vehicle, MCH 100 ng significantly increased REM sleep time during 6-hour polysomnographic recordings (Table 1) (Lagos et al. 2009). The increment of REM sleep time amounted to 70.7% of the control value and was related to a greater number of REM sleep episodes. MCH 100 ng produced, in addition, a small but significant increase in SWS (9.2% increment of the control value). Besides, administration of the neuropeptide significantly reduced the time spent in W and light sleep (33.8% and 26% decrement of the control values, respectively). The analysis of REM sleep values in 2-hour blocks showed that the increase of REM sleep after MCH 100 ng microinjection was maintained up to 6 hours. During a second step, the effect of immunoneutralization of MCH in the DRN on sleep and W was determined in the rat (Lagos et al. 2011). Compared to the control solution, microinjection of anti-MCH antibodies (1/100 solution in 0.2 μl) induced a significant reduction of REM sleep time and the number of REM sleep episodes, while REM sleep latency was increased. Additionally, there was an increase in total W time. Light sleep and SWS remained almost unchanged. These findings strongly support the proposal that MCH released in the DRN facilitates the occurrence of predominantly REM sleep. Because MCH is an inhibitory neuropeptide, it can be hypothesized that MCH would facilitate the generation of REM sleep by inhibiting serotonergic neurons. In support of the suggestion, Devera et al. (2015) described that the microinjection of MCH into lateral ventricle resulted in a significant decrease in the firing rate of 59% of the neurons recorded in the DRN. Moreover, the juxtacellular administration of MCH reduced the discharge in 80% of these cells. Based on the electrophysiological and pharmacological properties of the neurons affected by MCH, including action potential average duration, basal firing rate, and suppression of the discharge following the systemic administration of the selective 5-HT1A receptor agonist 8-OH-DPAT, it was concluded that these neurons were likely serotonergic.
5.2 Locus Coeruleus Nucleus
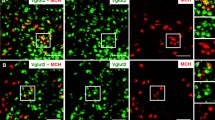
Harthoorn (2007) and Bittencourt (2011) initially reported the existence of MCH terminals within the LC. More recently, Yoon and Lee (2013) examined the distribution to the LC of fibers from MCH neurons located within medial and lateral subdivisions of the lateral hypothalamus. The authors established that MCH projections to the nuclear core of the LC exhibit differential distribution with a predominance of the lateral subdivision over the medial one. Accordingly, the proportions of retrograde-labeled MCH neurons over the total retrograde-labeled cells amounted to 4.4% ± 0.5% at the medial subdivision and 7.4% ± 0.6% at the lateral one. It has been proposed, in addition, that MCH neurons might utilize GABA as a co-transmitter, since their soma contain the enzyme glutamic acid decarboxylase (Gao and van den Pol 2001; Elias et al. 2001; Sapin et al. 2010). In support of the proposal, Del Cid-Pellitero and Jones (2012) found that a small number of MCH varicosities present at the LC in the rat were immunopositive for the vesicular transporter for GABA. Furthermore, the MCH varicosities containing the vesicular transporter for GABA contacted the tyrosine hydroxylase immunostained neurons of the LC.
The effects of MCH microinjection into the right LC on sleep variables during 6-h recording sessions have been characterized in the rat (Table 1). Compared with the control vehicle, MCH 200 ng significantly increased REM sleep from a control value of 27.7 ± 4.1 min (7.7% of the total recording time) to 39.6 ± 3.5 min (11.0% of the total recording time). The increment of REM sleep time was related to a greater number of REM sleep episodes. SWS and REM sleep latency were not significantly modified. Analysis of sleep variables in 2 h blocks showed that MCH 200 ng significantly augmented REM sleep during the first, second, and third 2 h of recording. Additionally, MCH 100 ng induced a significant increase of REM sleep during the first 2 h period after treatment. W, light sleep, and SWS showed slight but inconsistent changes that did not attain significance (Monti et al. 2015).
Thus, it can be proposed that under normal conditions MCH and to a smaller extent GABA released by MCH-containing neurons would inhibit the noradrenergic cells located in the LC and increase REM sleep values.
Interestingly, Bayer et al. (2005) examined the effects of NE and carbachol, a cholinergic agonist, on identified MCH neurons in rat hypothalamic slices. It was found that both receptor agonists hyperpolarized MCH cells by direct postsynaptic actions. The authors concluded that MCH neurons would be inhibited by NE and ACh (W-on cells) during W while disinhibited and active during SWS and REM sleep. Thus, the study by Bayer et al. (2005) further supports the proposal that MCH-induced inhibition of LC noradrenergic neurons favors the occurrence of REM sleep in laboratory animals.
5.3 Lateral Basal Forebrain
The effects of bilateral microinjection of MCH into the horizontal limb of the diagonal band of Broca (HDB) on sleep variables during the light phase of the light-dark cycle have been examined in the rat. The microinjection of MCH was aimed at the HBD because this is where choline-acetyltransferase (ChAT)-immunoreactive cells, glutamic acid decarboxylase (GAD)-immunoreactive cells, and, as judged by the presence of vesicular glutamate transporter 2 (VGLUT2), glutamatergic neurons are present in great numbers (Semba 2000).
Bilateral microinjection of MCH 100 ng into the HDB significantly reduced W during the 6-hour recording sessions from a control value of 81.7 ± 10.8 min (22.6% of the total recording time) to 64.7 ± 9.3 min (17.9% of the total recording time) (Table 1). REM sleep time showed a slight increase that did not attain significance. Notwithstanding this, MCH 100 ng significantly decreased REM sleep latency and augmented the number of REM episodes during the first 2-h period after treatment (Table 1). Analysis of sleep variables in 2 h blocks showed that MCH 50 and 100 ng significantly reduced W during the first 2 h of recording. In contrast, only the 100 ng dose of the neuropeptide significantly increased REM sleep during the first 2 h after treatment. Light sleep and SWS showed slight but inconsistent changes that did not attain significance (Lagos et al. 2012). MCHergic projections from the lateral hypothalamus and zona incerta have been described to the nuclei of the HDB (Bittencourt et al. 1992; Hervieu et al. 2000). In addition, the MCHR1 receptor is densely expressed in the basal forebrain (Saito et al. 2001). Cholinergic, glutamatergic, and GABAergic neurons of the basal forebrain have been shown to be involved in the promotion of W (Jones 2005; Lee et al. 2005). The former two neurotransmitter systems influence directly cortical and hippocampal activities, whereas GABAergic neurons target inhibitory interneurons and through disinhibition activate pyramidal cells (Freund and Gulyas 1991; Deurveilheur and Semba 2011). In addition, BFB GABAergic and non-GABAergic, mainly cholinergic, neurons have been proposed to contribute to the modulation of REM sleep via descending projections acting on REM sleep-regulatory neurons located in the brainstem (Rodrigo-Angulo et al. 2008; Deurveilheur and Semba 2011; Semba 2011). The former would induce a direct inhibition of REM sleep generating and maintaining neurons, while the latter would exert an indirect inhibition through the activation of local GABAergic cells. It can be proposed that MCH microinjected into the HDB would inhibit cholinergic, glutamatergic, and GABAergic neurons that express MCHR1R. Inhibition of the acetylcholine- and glutamate-containing cell groups involved in the occurrence of W would be followed by its reduction. Moreover, the deactivation of REM sleep-off GABAergic and non-GABAergic cells that project to the brainstem would lead to the disinhibition of neuroanatomical structures involved in the induction and maintenance of REM sleep. As a result, values corresponding to this behavioral state would be increased. Thus, it can be proposed that under normal conditions, the MCH-induced deactivation of HDB neurons would contribute to the regulation of W and REM sleep.
5.4 Dopaminergic Mesocorticolimbic System
The DAergic neuronal system relevant to sleep and W is located in the upper mesencephalon. Dopamine neurons project to several brain areas via a number of tracts. One group of DA neurons arises in the SNc and projects via the nigrostriatal tract to the dorsal striatum (caudate-putamen). A second group of DA neurons arises in the VTA and projects via the mesolimbic and the mesocortical tract to limbic areas [septal area, olfactory tubercles, nucleus accumbens (ACb), amygdaloid complex, hippocampus, and piriform cortex] and the cerebral cortex [medial prefrontal, cingulate, and entorhinal areas], respectively (Monti and Jantos 2009). Additionally, DA neurons have been characterized in the vPAG of the rat (Lu et al. 2006). Dopamine D1 and D2 receptors are present within these structures. Behavioral arousal is impaired in DA D1 receptor knockout mice. On the other hand, systemic administration of a selective DA D1 agonist induces an increase of W and a reduction of SWS and REM sleep in laboratory animals. Mice with genetically induced lesions that target the D2 receptor show reduced levels of spontaneous locomotor activity. Similar results have been observed following systemic, i.c.v., or intra-accumbens injection of a selective DA D2 autoreceptor agonist. Systemic administration of DA D2 agonists induces biphasic effects such that low doses reduce W and increase SWS and REM sleep, whereas large doses induce the opposite effects (Monti and Monti 2007).
Although there is no direct evidence regarding the interaction between MCHergic and DAergic neurons in the control of the behavioral state, indirect evidence strongly suggests an interplay between both systems in the control of W and sleep. In this respect, MCH-containing fibers and MCHR1 expression are abundant in the ACb and VTA (Bittencourt et al. 1992; Hervieu et al. 2000; Saito et al. 2001). Pissios et al. (2008) evaluated the striatal-dependent functions in MCH knockout (MCH−/−) mice and distinguished a number of functional changes in the ACb shell, including increased electrically evoked DA release and augmented reuptake of the neurotransmitter in the presynaptic terminals. The latter was consistent with the increased expression of the DA transporter in the ACb. Moreover, MCH−/− mice showed a significantly greater increase in locomotor activity following repeated d-amphetamine injection compared to wild-type animals, which led the authors to propose that chronic absence of MCH enhanced the sensitizing effects of d-amphetamine in mice. The selective DA D1 receptor agonist SKF81297 induced also a significantly greater enhancement of locomotor activity as compared to the MCH+/+ animals. Furthermore, Smith et al. (2005) determined whether basal and evoked tissue levels of DA within the ACb and locomotor activity were altered in MCHR1−/− as compared to controls. No significant differences in basal or evoked tissue levels of DA were observed in MCHR1−/− mice compared with wild-type animals. Concerning the mice behavior, it was observed that MCHR1−/− animals became hyperactive when exposed to a novel environment. In addition, they were supersensitive to the locomotor stimulating effect of d-amphetamine and the selective DA D1 receptor agonist SKF38393. Thus, presently available evidence tends to indicate that deletion of MCH or MCHR1 results in an upregulation of mesolimbic DA receptors, indicating that the neuropeptide may negatively modulate mesolimbic DA function. This outcome could tentatively result in a reduction of W and increase of NREM sleep and REM sleep following the microinjection of MCH into the ACb.
Related to our topic, Alberto et al. (2011) and Conductier et al. (2011) investigated the effect of DA on MCH neurons using whole-cell patch-clamp recordings in hypothalamic mouse brain slices. It was observed that DA hyperpolarizes MCH cells through activation of α2 NE receptors and the opening of G-protein-activated inward rectifier K+ (GIRK) channels. The finding by van den Pol et al. (2004) that α2 NE receptors are present on MCH neurons further supports the proposal that DA indirectly regulates MCH neurons. Thus, it can be proposed that DA induces a reduction in MCH neuron excitability and decreases MCH release. Interestingly, Yang et al. (2014) described that DA induces a hyperpolarization of the membrane potentials in the rat SLD glutamatergic neurons and a decrease of their firing, which is mediated also by α2-noradrenergic receptors.
5.5 Histaminergic Tuberomammillary Nucleus
Skofitsch et al. (1985) characterized the distribution of MCH-like immunoreactivity in the rat brain and concluded that only a moderate number of axons reach the TMN. In contrast, Bittencourt and Elias (1998) found that the TMN, mainly the medial mammillary nucleus and the supramammillary nucleus, receive a large number of MCH axons in the rat. On the other hand, a very low number of MCH fibers occurred in the lateral mammillary nucleus.
Jego et al. (2013) tested the hypothesis that MCH neurons induce an inhibitory effect on W-promoting HA-containing neurons in the TMN of mice. In this respect, it was found that in vitro activation of MCH neuron terminals induces inhibitory postsynaptic currents in histaminergic neurons of the TMN. Moreover, in vivo stimulation of TMN MCH terminals by means of optogenetic tools augmented the duration of REM sleep episodes in mice prepared for sleep recordings (Table 1). It can be concluded that MCH-induced inhibition of W-promoting histaminergic neurons of the TMN facilitates REM sleep occurrence in mice.
6 Conclusions
Presently available evidence tends to indicate that MCH inhibition of neurotransmitter systems involved in the occurrence of W, including the serotonergic, noradrenergic, glutamatergic, cholinergic (W-on cells), and histaminergic ones, is followed by an increase of REM sleep.
References
Alberto CO, Trask RB, Hirasawa M (2011) Dopamine acts as a partial agonist for α2A adrenoceptor in melanin-concentrating hormone neurons. J Neurosci 31:10671–10676
Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M et al (2005) Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience 130:807–811
Bittencourt JC (2011) Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endocrinol 172:185–197
Bittencourt JC, Elias CF (1998) Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neural tracers. Brain Res 805:1–19
Bittencourt JC, Press F, Arias C, Peto C, Vaughan J, Nahon JL et al (1992) The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319:218–245
Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH (2002) The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci 16:1959–1973
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
Clément O, Sapin E, Bérod A, Fort P, Luppi PH (2011) Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep 34:419–423
Conductier G, Nahon JL, Guyon A (2011) Dopamine depresses melanin-concentrating hormone neuronal activity through multiple effects on α2-noradrenergic, D1 and D2-like dopaminergic receptors. Neuroscience 178:89–100
Del Cid-Pellitero E, Jones BE (2012) Immunohistochemical evidence for synaptic release of GABA from MCH-containing varicosities in the locus coeruleus. Neuroscience 223:269–276
Deurveilheur S, Semba L (2011) Basal forebrain regulation of cortical activity and sleep-wake states: roles of cholinergic and non-cholinergic neurons. Sleep Biol Rhythms 9(Suppl. 1):65–70
Devera A, Pascovich C, Lagos P, Falconi A, Sampogna S, Chase MH et al (2015) Melanin-concentrating hormone (MCH) modulates the activity of dorsal raphe nucleus. Brain Res 1598:114–128
Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB et al (2001) Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol 432:1–19
Elias CF, Sita LV, Zambon BK, Oliveira ER, Vasconcelos LAP, Bittencourt JC (2008) Melanin-concentrating hormone projections to areas involved in somatomotor responses. J Chem Neuroanat 35:188–201
Freund TF, Gulyas AI (1991) GABAergic interneurons containing calbindin D28K or somatostatin are major targets of GABAergic basal forebrain afferents in the rat neocortex. J Comp Neurol 314:187–199
Gao XB, van den Pol AN (2001) Melanin-concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol 55:237–252
Harthoorn LF (2007) Projection-dependent differentiation of melanin-concentrating hormone-containing neurons. Cell Mol Neurobiol 27:49–55
Hawes BE, Kil E, Green B, O’Neill K, Fried S, Graziano MP (2000) The melanin-concentrating hormone receptor couples to multiple G proteins to activate diverse intracellular signaling pathways. Endocrinology 141:4524–4532
Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S et al (2000) The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, sic-1, in the central nervous system of the rat. Eur J Neurosci 12:1194–1216
Hobson JA, McCarley RW, Wyzinski PW (1975) Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189:55–58
Hong EY, Yoon YS, Lee HS (2011) Differential distribution of melanin-concentrating hormone (MCH) and hypocretin (Hcrt)-immunoreactive neurons projecting to the mesopontine cholinergic complex in the rat. Brain Res 1424:20–31
Jego S, Glasgow SD, Gutierrez-Herrera C, Ekstrand M, Reed SJ, Boyce R et al (2013) Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci 16:1637–1644
Jones BE (2003) Arousal systems. Front Bioci 8:438–451
Jones BE (2005) From waking to sleeping: neuronal and chemical substrates. TIPS 26:578–586
Lagos P, Torterolo P, Jantos H, Chase MH, Monti JM (2009) Effects on sleep of melanin-concentrating hormone (MCH) microinjections into the dorsal raphe nucleus. Brain Res 1265:103–110
Lagos P, Torterolo P, Jantos H, Monti JM (2011) Immunoneutralization of melanin-concentrating hormone (MCH) in the dorsal raphe nucleus: effects on sleep and wakefulness. Brain Res 1369:112–111
Lagos P, Monti JM, Jantos H, Torterolo P (2012) Microinjections of the melanin-concentrating hormone into the lateral basal forebrain increases REM sleep and reduces wakefulness in the rat. Life Sci 90:895–899
Lee M, Hassani O, Alonso A, Jones BE (2005) Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci 25:4365–4369
Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C et al (1999) The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol 1:267–271
Lu J, Jhou TC, Saper CB (2006) Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 26:191–202
Luppi PH, Clément O, Fort P (2013) Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Curr Opin Neurobiol 23:1–7
McCarley RW (2007) Neurobiology of REM and NREM sleep. Sleep Med 8:302–330
Monti JM, Jantos H (2009) The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Progr Brain Res 172:625–646
Monti JM, Monti D (2007) The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev 11:113–133
Monti JM, Lagos P, Jantos H, Torterolo P (2015) Increased REM sleep after intra-locus coeruleus nucleus microinjection of melanin-concentrating hormone (MCH) in the rat. Prog Neuro-Psychopharmacol Biol Psychiatry 56:185–188
Monti JM, Torterolo P, Jantos H, Lagos P (2016) Microinjection of the melanin-concentrating hormone into the sublaterodorsal tegmental nucleus inhibits REM sleep in the rat. Neurosci Lett 630:66–69
Pace-Schott EF, Hobson JA (2002) Basic mechanisms of sleep: new evidence on the neuroanatomy and neuromodulation of the NREM-REM cycle. In: Charney D, Nemeroff C (eds) Neuropsychopharmacology—the fifth generation of progress. Lippincott, Williams and Wilkins, Philadelphia, pp 1859–1877
Pissios P, Frank L, Kennedy AR, Porter DR, Marino FE, Liu FF et al (2008) Dysregulation of the mesolimbic dopamine system and reward in MCH−/− mice. Biol Psychiatry 64:184–191
Rodrigo-Angulo M, Heredero S, Rodríguez-Veiga E, Reinoso-Suárez F (2008) GABAergic and non-GABAergic thalamic, hypothalamic and basal forebrain projections to the oral pontine reticular nucleus: their implication in REM sleep regulation. Brain Res 1210:116–125
Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS et al (2001) Identification and characterization of a second melanin-concentrating hormone receptor MCH-2R. Proc Natl Acad Sci U S A 98:7564–7569
Saito Y, Cheng M, Leslie FM, Civelli O (2001) Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol 435:26–40
Sapin E, Lapray D, Bérod A, Goutagny R, Léger L, Ravassard P et al (2009) Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One 4:1–12
Sapin E, Bérod A, Léger L, Herman PA, Luppi PH, Peyron C (2010) A very large number of GABAergic neurons are activated in the tuberal hypothalamus during paradoxical (REM) sleep hypersomnia. PLoS One 5(7):e11766. https://doi.org/10.1371/journal.pone.0011766
Sekiya K, Ghatei MA, Lacoumenta S, Burnet PW, Zamir N, Burrin JM et al (1988) The distribution of melanin-concentrating hormone-like immunoreactivity in the central nervous system of rat, guinea-pig and man. Neuroscience 25:925–930
Semba K (2000) Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res 115:117–141
Semba K (2011) Preoptic and basal forebrain modulation of REM sleep. In: Mallick BN, Pandi-Perumal SR, McCarley R, Morrison AR (eds) REM sleep: regulation and function. Cambridge University Press, Cambridge, pp 99–109
Skofitsch G, Jacobowitz DM, Zamir N (1985) Immunohistochemical localization of melanin-concentrating hormone-like peptide in the rat brain. Brain Res Bull 15:653–649
Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R et al (2005) Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci 25:914–922
Szymusiak R, Gvilia I, McGinty D (2007) Hypothalamic control of sleep. Sleep Med 8:291–301
Torterolo P, Lagos P, Monti JM (2011) Melanin-concentrating hormone: a new sleep factor? Front Neurol 2:2–12
Urbanavicius J, Lagos P, Torterolo P, Abin-Carriquiry JA (2016) Melanin-concentrating hormone projections to the dorsal raphe nucleus: an immunofluorescence and in vivo microdialysis study. J Chem Neuroanat 72:16–24
van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK (2004) Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron 42:635–652
Yang N, Zhang KY, Wang FF, Hu ZA, Zhang J (2014) Dopamine inhibits neurons from the rat dorsal subcoeruleus nucleus through the activation of α2-adrenergic receptors. Neurosci Lett 559:61–66
Yoon YS, Lee HS (2013) Projections from melanin-concentrating hormone (MCH) neurons to the dorsal raphe or the nuclear core of the locus coeruleus in the rat. Brain Res 1490:72–82
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Monti, J.M., Pandi-Perumal, S.R., Torterolo, P. (2018). The Effects of Melanin-Concentrating Hormone on Neurotransmitter Systems Involved in the Generation and Maintenance of Wakefulness. In: Pandi-Perumal, S., Torterolo, P., Monti, J. (eds) Melanin-Concentrating Hormone and Sleep . Springer, Cham. https://doi.org/10.1007/978-3-319-75765-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-75765-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75764-3
Online ISBN: 978-3-319-75765-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)