Abstract
American Cutaneous leishmaniasis (ACL) in Brazil is caused by seven Leishmania species. A multitude of sand fly species are implicated in their enzootic and zoonotic transmission. This chapter catalogues the different phlebotomines involved together with their feeding habits, geographical distribution and the impact of climatic and environmental changes. Clades of the same Leishmania species are transmitted by different sand fly genera in different geographical regions. In Amazonia Psychodopygus species are L. (Viannia) ACL vectors. In the Atlantic rainforest zone and the Guyana shield region Nyssomyia species are vectors of ACL caused by L. (Viannia) species. Bichromomyia, Migonemyia and Pintomyia species are also considered to be important ACL vectors. Evidence is accumulating indicating that some parasite may be transmitted by more than one species in an ACL focus.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
The eco-epidemiology of Brazilian American cutaneous leishmaniasis (ACL) is a complex of epidemiological chains involving different parasites, vectors, and reservoirs. The transmission of the seven Leishmania spp. associated with ACL in Brazil involves different phlebotomine species that are closely associated with the parasite’s mammalian reservoirs, which range from Xenathra to rodents to primates, resulting in a variety of transmission cycles in the different geographical regions in the country. However, evidence is also accumulating that indicates that there are geographical clades of the different Leishmania species that may be associated with different vectors. Leishmania species (L. (Leishmania) amazonensis; L. (Viannia) braziliensis; L. (V.) guyanensis; L. (V.) lainsoni; L. (V.) shawi; L. (V.) naiffi; and L. (V.) lindenbergi) are associated with human cutaneous leishmaniasis. However, other Brazilian parasites of the subfamily Leishmaniinae exist, some of which are found in sand flies (L. (V.) utingensis; Endotrypanum spp.), whereas others (L. (Mundinia) enriettii; Porcisia deanei) have so far not been recorded in them (the parasite nomenclature follows that published in Espinosa et al. 2016).
Over the years well-defined and accepted criteria, data from field studies and—in some cases—experimental results have led to some species being considered as ACL vectors. In our opinion, some are primary vectors, and we will discuss these first. However, an increasing amount of data, principally from molecular studies, suggest that other sand-fly species may be participating in the cycles, and we will discuss these towards the end of this chapter.
Nyssomyia intermedia (Lutz & Neiva, 1912)
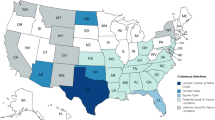
Nyssomyia intermedia was described from specimens collected at Fazenda Ouro Fino in the municipality of Além Paraíba (MG) and also from SP and RJ, where it was found to be abundant in homes. It was one of the first phlebotomine species to be described in the neotropical region (Barretto 1961; Martins et al. 1978; Young and Duncan 1994; Marcondes 1996). The current geographic distribution of Ny. intermedia includes Brazil (PA, PI, MA, PE, PR, SC, RS, MG, MS, GO and TO [Fig. 1]), Argentina and Paraguay (Young and Duncan 1994; Marcondes et al. 1997; Rangel and Lainson 2003, 2009).
Recently published projections under climate-change scenarios predict a global decrease in the climatically suitable areas of Ny. intermedia in Brazil with a slight expansion in specific areas of the Northeast region for the upcoming decades (McIntyre et al. 2017).
One year after the suggestion of the transmission of cutaneous leishmaniasis by sand flies in the Old World (Sergent et al. 1921), Aragão (1922, 1927) reported in the city of Rio de Janeiro (locality Águas Férreas, currently Cosme Velho and Santa Tereza) the importance of Ny. intermedia in the transmission of L. (V.) braziliensis by inoculating a triturated pool of this sand fly in the nose of a dog and thus experimentally reproducing the disease for the first time on the American continent. Subsequently, Costa Lima (1932) recorded the occurrence of Ny. intermedia and Migonemyia migonei in the neighbourhoods of Copacabana and Laranjeiras, near the slopes of Corcovado and Santa Teresa hills, in Rio de Janeiro. In 1952, Forattini and Santos found promastigotes (referred to as “leptomonads”) in Ny. intermedia, like those observed by Aragão (1922).
Forattini (1973) pointed out an irregular seasonal behaviour for this species in observing greater population density during the colder months of the year. However, Rangel et al. (1990) reported the occurrence of the vector throughout the year, and Souza et al. (2003) demonstrated that in Rio de Janeiro, Ny. intermedia has high density during the hottest times of the year.
Epidemiological evidences suggest Ny. intermedia as a vector of L. (V.) braziliensis in endemic areas in the Southeast, considered the main ACL vector in SP and RJ (Forattini 1953, 1973; Forattini et al. 1976; Araújo Filho et al. 1981; Gomes et al. 1986; Rangel et al. 1986, 1990, 1992, 1999; Meneses et al. 2005; Rangel and Lainson 2009). Pita-Pereira et al. (2005) isolated the parasite from Ny. intermedia, captured in a focus of ACL in Jacarepaguá (RJ), and identified it as L. (V.) braziliensis. This reinforced all the ecological and epidemiological evidence regarding this vector. In MG and ES, it shares its vector role with Ny. whitmani (Barretto 1943; Falqueto 1995; Mayrink et al. 1979; Aguiar & Soucasaux 1984; Souza-Rocha et al. 2007).
Ny. intermedia starts its hematophagous activities at dusk and peaks during the first half of the night. It is abundant inside houses; in peri-domiciliary environments in domestic-animal shelters, such as birds, dogs, equines, and rodents; and in the forest to feed on other wild animals (Forattini 1953, 1973, 1976; Araujo Filho et al. 1981; Rangel et al. 1986; Gomes and Galati 1989; Pirmez et al. 1997; Afonso et al. 2005).
Nyssomia neivai (Pinto, 1926)
This species was described from a sample of males collected in the city of São Paulo at the Butantan Institute. It was considered a synonym of Ny. intermedia. Marcondes (1996), however, revalidated Ny. neivai as a species after a study of morphological and morphometric characters of specimens collected in Bolivia and the holotypes of both species. The distinction between Ny. intermedia and Ny. neivai was made possible mainly by morphological differences in the spermathecae (Marcondes 1996; Marcondes and Alexander 2003; Andrade-Filho et al. 2004). The males of the species were not separated by morphology but only by a series of morphometric data (Marcondes and Borges 2000). According to Andrade-Filho and Brazil (2003), the presence of cryptic species, such as Ny. intermedia and Ny. neivai, is a result of allopatry, which in the case of these species must have occurred about 250,000 years ago, thus indicating that the separation of species was recent.
Ny. neivai occurs in colder and drier regions of Brazil compared with Ny. intermedia (Marcondes et al. 1998, Fig. 1). It was suggested as an important vector mainly in the states of the South and Southeast regions. According to McIntyre et al. (2017), the appropriate climatic range of Ny. neivai is currently restricted to the South, Southeast, and Central West regions of Brazil, extending to western Bolivia and Paraguay, like Ny. intermedia. The investigators predict that Ny. neivai will face changes in its climatic range in the future with the Central West region, in particular, becoming less habitable.
The first record of Ny. neivai naturally infected by L. (V.) spp., possibly L. (V.) braziliensis, was described by Marcondes et al. (1999) using PCR techniques of specimens collected in Piçarras (SC). Later, Silva et al. (2008) found a high incidence of Ny. neivai in 37 municipalities of PR where 75.6% of all specimens collected were Ny. neivai, thus suggesting their possible participation in the disease cycle. This hypothesis gained strength with the encounter of specimens of Ny. neivai infected with L. (V.) spp. in this state by multiplex PCR techniques (Oliveira et al. 2011). Since 2002, autochthonous cases of ACL by L. (V.) braziliensis have been recorded in the outskirts of the city of Porto Alegre (RS), in vegetation areas near streams (Pita-Pereira et al. 2009). Entomological studies revealed a large predominance of Ny. neivai in the area of occurrence of the cases (Gonçalves 2003); later, the natural infection by L. (V.) spp. was found in this species by PCR techniques, thus identifying the phlebotomine as a potential vector of L. (V.) braziliensis (Pita-Pereira et al. 2009).
Recent investigations in the Southeast region suggest that the migration of Ny. neivai from forests to residential areas has resulted in the occurrence of ACL cases. However, in a study on the biology of this sand fly in an endemic area in São Paulo, the investigators considered it as having low competence as Leishmania vector (Casanova et al. 2009). Studies on the feeding preferences of Ny. neivai showed that it is an opportunistic sand fly (feeding on domestic animals such as pigs, dogs, rabbits, and chicken) with few specific host preferences (Dias-Sversutti et al. 2007).
Migonemyia migonei (França, 1920)
The literature suggests that Mg. migonei is a sylvatic species found in forests, generally areas of abundant vegetation, occurring less frequently in secondary forests and in capoeiras. However, it is common to observe this phlebotomine inside houses and in domestic animal shelters (Barretto 1943; Forattini 1973; Araújo Filho et al. 1981; Rangel et al. 1986). It is believed that this species has great capacity for adaptation, surviving in degraded areas by man, and approaching impacted environments where it feeds on domestic animals and man, possibly being able to maintain the enzootic cycle of the disease in areas of secondary forest (Ferreira et al. 2001; Queiroz et al. 1994; Rangel and Lainson 2009). It is distributed in Brazil (AP, AC, PA, MA, CE, PB, PE, BA, ES, RJ, SP, PR, SC, RS, MG, MT, Fig. 1), Venezuela, Argentina, Paraguay, Peru, Trinidad, and Tobago (Young and Duncan 1994; Rangel and Lainson 2003).
According to seasonal studies conducted in PE, Mg. migonei can be captured during all months of the year; however, other studies have indicated the absence of the vector during the coldest and driest months (Rangel and Lainson 2009; Guimarães et al. 2012).
Its hematophagous activity begins at dusk and advances into the night. According to Nieves and Pimenta (2002), L. (V.) braziliensis infections were observed in Mg. migonei females that fed on wild rats, opossums, horses, and males. The first citation of the role of Mg. migonei in the ACL-transmission cycle was through the encounter of a species naturally infected by flagellates, probably Leishmania promastigotes, in 1941 by Pessoa and Coutinho in São Paulo. Later studies also showed the coincident increase of ACL cases and Mg. migonei specimens in SP (Camargo-Neves et al. 2002; Rangel and Lainson 2009). In RJ, the species has been implicated as a probable vector in regions, such as Ilha Grande and Jacarepaguá, where the species has a preference for biting dogs and is associated with the maintenance of canine leishmaniasis in addition to its natural infection with L. (V.) braziliensis in Jacarepaguá (Pita-Pereira et al. 2005; Rangel and Lainson 2009; Gouveia et al. 2012; Carvalho et al. 2014). Mg. migonei has also been associated with ACL transmission in MG and ES. In the Northeast region, more precisely in CE, Mg. migonei was found to be naturally infected by L. (V.) braziliensis (Azevedo & Rangel 1991, Queiroz et al. 1994). However, this occurred only as a secondary vector of ACL (Rangel and Lainson 2009). Recently molecular studies using PCR techniques identified Mg. migonei infected by L. (L.) infantum chagasi, the etiological agent of AVL, in PE and CE (Carvalho et al. 2010; Silva et al. 2014a, b; Rodrigues et al. 2016).
Nyssomyia whitmani (Antunes & Coutinho, 1939)
N. whitmani was described by Antunes and Coutinho in 1939 as Flebotomus whitmani in honour of Dr. Whitman of the Rockefeller Foundation, which collaborated with the Brazilian government in the Yellow Fever Campaign. The new species was described based on male and female specimens collected in Ilhéus (BA). Until 1939, it was misidentified as Ny. intermedia. In Brazil, this species was registered throughout the country except for SC and RS (Fig. 1) and beyond in French Guiana, Paraguay, Peru, and Argentina (Young and Duncan 1994).
The participation of Ny. whitmani in the epidemiological chain of ACL is restricted to the Brazilian territory where its first report as a vector was in SP when it was found infected by flagellates, possibly Leishmania (Pessôa & Coutinho 1941). It was considered a sylvatic species, although it could be found inside houses that were located within or near the forest. At dusk, it was found biting man and feeding on dogs and was shown to be present in large numbers in chicken houses. Because the localities Barretto (1943) studied were close to deforested areas, he suggested that the high-density population under these circumstances was simply due to the wide variety of blood sources available to this sand fly.
In 1953, Forattini confirmed the presence of Ny. whitmani in both the forest and nearby domestic animal shelters in SP. Later (1960) he commented on the fact that although initially dependent on primary forest, this sand fly could now be captured during several months of the year inside houses, peri-domestic pigsties, and banana plantations and that there was no doubt that it was now thriving in a domestic environment. In the same publication, Forattini (1953) believed that although there were reports indicating that Ny. whitmani coexists with Ny. intermedia in areas recently occupied by man and may outnumber the latter species as the environmental alterations proceed, more detailed information was needed to confirm this suggestion.
In RJ in general, the Ny. whitmani population has for some time remained at a low level (Rangel et al. 1986, 1990; Oliveira et al. 1995). Souza et al. (2001) registered this sand fly’s presence in the Atlantic Forest and, more importantly, its frequency in residential areas close to the forest. The same investigators recorded both Ny. intermedia and Ny. whitmani biting man in a peri-domestic habitat close to the forest and noted that the former prevailed in the residential area, whereas the latter was the most frequent of the two insects in the forest. They observed a higher density of Ny. whitmani during winter months, whereas Ny. intermedia was most abundant during the hotter months of the year (Souza et al. 2002).
The tendency of Ny. whitmani to occupy residential areas in MG was discussed by Mayrink et al. (1979) and Passos et al. (1991). It was found feeding on man and domestic animals and thought to possibly be breeding in this habitat. Conversely, it was found in very small numbers in the neighbouring forest.
In Northeast Brazil, in BA, CE and PE, its behaviour has been shown to be like that seen in the Southeast region, namely, highly attracted to man and well adapted to the domiciliary habitat (Barreto et al. 1982; Vexenat et al. 1986; Brandão-Filho et al. 2003). In BA, it was suggested that Ny. whitmani might be breeding on cocoa plantations (França et al. 1991).
Regarding seasonality, differences in behaviour have been noted in different regions of Brazil, probably due to differing climatic conditions. In the Southern region, for example, Barretto (1943) noted its presence year-round, whereas in studies in Petrópolis, RJ, it was found in greater numbers during the months of low temperatures in June through August (Souza et al. 2002).
Although the dusk and nocturnal feeding habit of Ny. whitmani follows the usual sand-fly pattern (Barretto 1943), it, too, has been shown to be somewhat variable in different regions of Brazil. In the Northeast, Azevedo and Rangel (1991) showed that it can also be captured during the whole of the dawn period, in chicken houses or feeding on equines, with maximum activity from 1–3 am. Souza et al. (2004) noted that in RJ it could be found feeding on man, in the peri-domestic habitat, between 4 and 6 am, and such behaviour was previously recorded by Teodoro et al. (1993) in PR; studies on host preferences of Ny. whitmani among domestic animals, made in the same state, showed that this insect is an opportunistic feeder resulting in a wide choice of hosts in the peri-domestic environments of human colonization.
In the primary forest, in several different regions of PA, Lainson et al. (1979) noted that Ny. whitmani has very different habits from those discussed previously in other regions. It was found to be essentially sylvatic and was captured principally from large tree trunks and in the forest canopy. It was disinclined to bite man and attempts to demonstrate its invasion of houses located very near the forest were completely unsuccessful. Subsequent studies confirmed these observations and led to the conclusion that any divergence from this behaviour is likely to occur only under special conditions (Ready et al. 1986; Lainson 1988; Shaw et al. 1991).
This situation led to the suggestion that Ny. whitmani might represent a species complex of two or more taxa (Lainson 1988), and this has resulted in several comparative studies on populations of this sand fly from widely different areas of Brazil. Some investigations suggested the existence of at least three different lineages of this sand fly based on biological characters, geographical variations, and morphometric features together with observations on sequences of mitochondrial DNA (Rangel et al. 1996; Ready et al. 1997, 1998). Rangel et al. (1996) made it clear, however, that they did not discard the possibility that the populations they studied—from PA, CE, and BA—could represent a cline. Ishikawa et al. (1999) studied populations from the North, Northeast, Southeast, and the South regions and indicated the existence of a clade from RO within the lineage of forested areas, which included haplotypes of the Amazon and Atlantic forests and Ilhéus (the type locality of Ny. whitmani). They suggested that their findings did not sustain the hypothesis of a cryptic species complex but rather the occurrence of a recent crossing-over of populations in forested areas.
Margonari et al. (2004) studied populations of Ny. whitmani from the Northeast and the Southeast of Brazil. They confirmed observations on the similar morphometry of these but presented evidence of two biogeographical “clusters.” Later, however, they suggested the existence of a genetic flow between the two lineages.
Regarding the incrimination of Ny. whitmani as an important vector of ACL, the first suggestion of this was made in 1941, when Pessôa and Coutinho found a specimen from SP infected by flagellates, which were considered possibly to be promastigotes of Leishmania: As a result, entomological investigations were intensified in areas of ACL transmission in this region and soon showed that although considered as a sylvatic species, Ny. whitmani was a highly anthropophilic sand fly with a particularly dense population (Barretto 1943; Forattini 1954).
In Southeastern Brazil, data in the literature suggest the participation of this sand fly in the transmission of ACL in a focus of the disease in Caratinga, MG, and in the mountainous area of Afonso Cláudio (Mayrink et al. 1979; Falqueto 1995), and Souza et al. (2002) considered that it might be sharing the role of a vector of L. (V.) braziliensis, together with Ny. intermedia, in rural RJ. Recently, the finding of a specimen of Ny. whitmani infected with a Leishmania of the subgenus Viannia by PCR analysis, in a region very close to Belo Horizonte, MG, has led to the suggestion that this sand fly could be the vector of cutaneous leishmaniasis in that area (Carvalho et al. 2008).
In the South, Ny. whitmani was also considered as a possible vector of ACL in PR, whereas in the northern part of this state a natural infection with L. (V.) braziliensis found in one specimen and the insect’s high population density clearly emphasized this sand fly’s medical importance (Luz et al. 2000; Teodoro et al. 2003).
In all areas of ACL in Northeast Brazil, this same species of sand fly is considered as an important vector of the disease based on the finding of specimens infected by L. (V.) braziliensis in the area of Três Braços, BA (Hoch et al. 1986; Ryan et al. 1990). The sand fly’s predominance in houses and the peri-domestic habitats in general prompted these investigators to suggest the development of a purely domestic cycle of transmission by Ny. whitmani. In Ilhéus, BA, the type locality of this sand fly, its fondness for human blood, and its high population density in the domestic habitat led to the same conclusion (Azevedo and Rangel 1991). In the Serra de Baturité, CE, a parasite of the subgenus Viannia and others positively identified as L. (V.) braziliensis were found in dissected Ny. whitmani (Azevedo et al. 1990; Queiróz et al. 1994) and, once again, these findings—together with population density in the peri-domestic habitat—indicated this fly as the local vector of ACL due to this parasite. Gil et al. (2003) registered Ny. whitmani as the second most prolific sand fly in captures made in the central area of RO, stressed its preferential arboreal habits, and recorded the presence of unidentified trypanosomatid parasites in some specimens.
In the municipalities of Rio Branco, Bujari, and Xapuri, AC, a study of sand-fly fauna and the potential vectors of ACL showed that Ny. whitmani was the most abundant species with its spatial distribution coinciding with proven transmission sites of L. (V.) braziliensis: it was therefore suggested that this sand fly was a probable vector of this parasite in that region (Azevedo 2008).
The state of Tocantins (TO) has suffered environmental impacts resulting in ecological changes due to the construction of hydroelectric plants, agricultural activities, and the establishment of new settlement areas, and the increasingly high incidence of ACL in this region has probably been due to these activities. Ny. whitmani is found in most of the endemic municipalities especially in areas that have been degraded by man (Vilela et al. 2008). In the Central West region, some studies conducted in areas that suffered environmental changes due to human activities have suggested Ny. whitmani as an important vector of L. (V.) braziliensis (Galati et al. 1996; Dorval et al. 2009).
This sand fly is one of the principal ACL vectors in Brazil having been recorded in large numbers of endemic areas (Costa et al. 2007) and in association with a wide vegetation diversity. Environmental and climatic changes most probably account for the spread of ACL in Brazil in recent years (Shaw 2007), and Ny. whitmani adapts readily to new environments, such as degraded areas, in association with domestic animals and man in rural and peri-urban areas (Costa et al. 2007; Shaw 2008). Peterson and Shaw’s (2003) ecological niche modelling of ACL vectors predicted that climate warming would favour the adaptation of Ny. whitmani to new areas as well as its geographical expansion within Brazil.
The very different behaviour of Ny. whitmani in the primary forest in PA, North Brazil, has already been discussed, and until now this sand fly has not been associated with ACL due to L. (V.) braziliensis in this region. The suggestion was made, however, that promastigotes of a member of the subgenus Viannia found in this sand fly in Monte Dourado, PA—an area of ACL due to L. (V.) guyanensis— were probably those of this parasite and that Ny. whitmani was participating in its transmission together with the principal vector, Ny. umbratilis (Lainson et al. 1981b). The parasite was not identified at the time, and in view of later isolations of L. (V.) shawi from Ny. whitmani in another area of primary forest in PA (Lainson et al. 1989), it was suggested that the parasite of Ny. whitmani in Monte Dourado, PA, was also L. (V.) shawi (Rangel et al. 1996, Lainson & Shaw 1998). However, the Monte Dourado Ny. whitmani infections were recently typed (de Souza et al. 2017) and proved to be L. (V.) guyanensis. Therefore, we conclude that in the Brazilian Guiana Shield Ny. whitmani participates in the transmission of L. (V.) guyanensis as previously suggested (Lainson et al. 1981b).
The comment by Lainson (1988), based on years of entomological observations in areas of primary forest in PA by workers in the Instituto Evandro Chagas (i.e. that in Amazonia Ny. whitmani is “seldom observed biting man, and never in large numbers”) conflicts with the frequency of human L. (V.) shawi infections. One explanation could be that other anthropophilic sand flies are involved in the transmission of L. (V.) shawi with Ny. whitmani merely maintaining the enzootic in wild animals. However, Campbell-Lendrum et al. (1999) observed no significant difference in the anthropophily of Ny. whitmani from North Brazil and Ny. whitmani sensu stricto from other areas in Brazil. The Instituto Evandro Chagas team’s observations were made in primary forest biomes, in which it was either absent (de Souza et al. 1996; Ward et al. 1973b) or present in small numbers (de Souza et al. 2016, 2017). Those of Campbell-Lendrum et al. (1999) were performed in a “patch of degraded primary forest” in an area of extensive deforestation. Another study (Donalisio et al. 2012) clearly showed a highly significant difference between the sizes of populations in forested and peri-domiciliary areas of PE. A simple explanation for the apparently conflicting opinions in relation to the anthropophily of Amazonian Ny. whitmani populations is variations in population densities related to environmental conditions. The fact that fewer flies are attracted to men in different areas is perhaps not because of difference in their anthropophily rather but differences in populations sizes.
Bichromomyia flaviscutellata (Mangabeira, 1942)
In their various field trips in Brazil, Mangabeira and collaborators captured approximately 17,000 specimens of sand flies representing 57 different species and with 35 of them being new to science. Among the latter was Mangabeira’s description of Bi. flaviscutellata, which was based only on male insects and collected in the locality of Aurá close to Belém, PA. Later, Sherlock and Carneiro (1962) described the female of the species after the establishment of a laboratory colony of this sand fly from BA. It must be stressed, however, that the taxonomic status of the material from BA has been questioned (Young and Duncan 1994). The specific name of this sand fly was probably chosen in view of the double colouration of the shield where the scutelum is clear and the remainder of the structure a dark brown (Latin flavus = golden; yellow + scutu = shield).
Bi. flaviscutellata has an extensive geographical distribution (Fig. 1) and can be found in very different habitats such as primary forest, secondary or copse-like vegetation, and lowland várzea forest, which during half of the year is subject to various degrees of flooding. Ready et al. (1983) showed that together with the various rodents and marsupials on which it feeds, it rapidly adapts to plantations of introduced trees, such as Pinus and Gmelina, and it is occasionally captured in the peri-domestic habitat of houses located near forest (Lainson et al. 1994). The distribution and population ecology of Bi. flaviscutellata are also influenced by climate, particularly by seasonal precipitation (Shaw and Lainson 1972; Ready et al. 1983). Projections from climate-change scenarios suggest an expansion of climatically suitable areas for Bi. flaviscutellata in the Southeast and South regions of Brazil in the future (Carvalho et al. 2015). Given continuous environmental and climatic changes, there are modifications in the behaviour of some sand-fly vectors of leishmaniasis, and in the Brazilian Cerrado of Central Brazil (extensive, flat areas of low, fire-resistant trees, small palms, and thorny bushes), it is possible to note the spread of Bi. flaviscutellata found in association with domestic-animal shelters and the presence of new cases of anergid diffuse cutaneous leishmaniasis (ADCL) (Vilela et al. 2008, 2011; Shaw 2008; Nunes et al. 2008; Queiroz et al. 2012; Brito et al. 2014).
Bi. flaviscutellata is a low-flying sand fly that is essentially nocturnal in its biting habits and highly attracted to rodents but not greatly attracted to man (Lainson and Shaw 1968; Shaw and Lainson 1968; Shaw et al. 1972; Gomes 1994; Vilela et al. 2006, 2007). This is fortunate because it is the proven vector of Leishmania (L.) amazonensis, which, in addition to being an agent of single-lesion cutaneous leishmaniasis, is also the cause of ADCL in individuals with a faulty immunological system. ADCL is highly disfiguring and cured with difficulty. In Brazil, human cases of ADCL were notified in North, Northeast, Central West, and Southeast regions (Costa et al. 2009). In 2007 the first autochthonous human case of ADCL from RJ was notified in the municipality of Paraty (Azeredo-Coutinho et al. 2007). Despite the records of Bi. flaviscutellata in the neighbouring municipality of Angra dos Reis (Araújo Filho et al. 1981; Carvalho et al. 2013), the vector remains to be detected in Paraty even after 3 years of monthly sand-fly captures in the region (Vieira et al. 2015). Bi. flaviscutellata was not detected in Paraty probably because animal-baited Disney traps were not yet used. This has proven to be the best capture method for zoophilic sand flies such as Bi. flaviscutellata (Shaw & Lainson 1968; Dorval et al. 2007, 2009, 2010).
In 1963, Lainson paid a visit to the Instituto Evandro Chagas in Belém, PA, and during a demonstration of the animal-trapping programme of the Rockefeller Virus Laboratory discussed the unique opportunity this held for the examination of these animals for evidence of Leishmania infections: ACL was a considerable public health problem in the Amazon Region of Brazil. The director of the programme, the late Dr. Otis Causey, was impressed with the similarity of cutaneous lesions he had seen on the tails of wild rodents and those caused by Leishmania (L.) mexicana on the tails of forest rodents in Belize, Central America (Lainson and Strangways-Dixon 1964). He promised to look more closely at the next ones he saw and within a few days presented Lainson with a stained smear of a lesion on the tail of the rodent Oryzomys capito, which was rich in amastigotes. At first it was thought that the parasite was L. (V.) braziliensis (Guimarães & Costa 1966), but after subsequent study of the parasite it was given the name of Leishmania mexicana amazonensis (Lainson & Shaw 1972) and later amended to Leishmania (L.) amazonensis (Lainson & Shaw 1987).
With the knowledge that rodents were important reservoir hosts of the parasite, rodent-baited Disney traps were used to capture sand flies attracted to them. By far the greatest number trapped were Bi. flaviscutellata, and dissection of these revealed 8 of 2706 to be heavily infected with promastigotes, which proved to be those of L. (L.) amazonensis (Lainson & Shaw 1968): During this and continuing studies, a total of 45 heavily infected Bi. flaviscutellata were recorded in 7498 females dissected, and on no occasion was the parasite encountered in other species of sand flies from the same area.
Finally, L. (L.) amazonensis was experimentally transmitted from hamster to hamster by the bite of Bi. flaviscutellata (Ward et al. 1977). This species was replaced in the upper reaches of the Amazon River in the Rondônia and Amazonia states by Bi. olmeca nociva and Bi. reducta. Infections of L. (L.) amazonensis have been found in both, and it seems likely that these two species are its vector in these areas.
Nyssomyia umbratilis (Ward & Fraiha, 1977)
During a study of the epidemiology of cutaneous leishmaniasis in Surinam in 1966, Wijers and Linger recorded flagellate infections in a tree-trunk inhabiting sand fly, which they referred to as Phlebotomus anduzei (syn. Ny. anduzei). It was thought to be the most likely vector of “bosch yaws,” or pian-bois, due to L. (V.) guyanensis, but their attempts to infect hamsters with the flagellates failed, and the parasite remained unidentified.
Lainson et al. (1976) worked in the primary forest of Monte Dourado (Jari), PA, Brazil, north of the Amazon River, where approximately 300 cases of ACL due to L. (V.) guyanensis were recorded, in 1 year, in men working on deforestation. They recorded massive infections with L. (V.) guyanensis in 4 of 55 specimens of a sand fly considered, at the time, to be Ny. anduzei and isolated the parasite after the intradermal inoculation of hamsters. Suspicions were aroused during these studies, however, that the vector was not in fact Ny. anduzei, and subsequent morphological studies showed that it was a closely related and morphologically very similar sand fly that was new to science.
Ward and Fraiha (1977) described the new sand fly as Ny. umbratilis from 10 females collected during the work in Monte Dourado, PA, and an intense study of its behaviour was initiated in the same area (Lainson et al. 1979). It was found that although sand-fly species of the subgenus Psychodopygus predominated at ground level, Ny. umbratilis was extremely abundant in the forest canopy but descended to ground level, presumably to oviposit, by way of the tree-trunks, on which it could be collected in great numbers in the early morning. In studies conducted in RO, in the area of Samuel Ecological Station; however, it was noted that Ny. umbratilis predominated in the canopy (Azevedo et al. 1993). In Monte Dourado, PA, it was noted that Ny. umbratilis flies off the tree-trunks when disturbed by man’s activities and attacks the nearest person. In the same study, L. (V.) guyanensis was isolated from 16 more specimens of Ny. umbratilis and, of 77 sand flies attacking 2 men collecting from the tree trunks, 72 (92.5%) proved to be Ny. umbratilis. Some idea of the efficiency of this sand fly in the transmission of ACL in the Monte Dourado, PA, area may be gained by the fact that the 2 men developed a total of 13 leishmanial lesions due to L. (V.) guyanensis on their arms, probably providing the most conclusive incrimination of a vector of ACL ever obtained.
The explanation of this great number of infected sand flies on tree trunks came with the detection of L. (V.) guyanensis in 27 of 59 specimens of the sloth Choloepus didactylus in the Monte Dourado area, PA (Lainson et al. 1981a, b). This animal spends most of its time in the forest canopy and has thus become the principal mammalian reservoir host of the parasite. Because the animal may remain in the same tree for a considerable time, there is a gradual build-up of infected Ny. umbratilis on a given tree. This sand fly’s similar role as a vector of L. (V.) guyanensis, as well as its common presence in the forest canopy and on large tree-trunks at ground level, has been recorded in some other areas of the Amazon region of Brazil (Arias and Freitas 1977a, 1978) and in French Guiana (Le Pont and Pajot 1980). Infection of an undoubted specimen of Ny. anduzei with a parasite having development consistent with that of members of the subgenus Viannia has been reported in Manaus (AM) (Arias and Freitas 1977b). However, this sand fly can, at most, now be considered only as a possible secondary vector of L. (V.) guyanensis and is probably of low importance with regard to the transmission of ACL to man.
Ready et al. (1986) performed a detailed study of the ecology of Ny. umbratilis in the region of Monte Dourado, PA. It is highly anthropophilic and presumably becomes infected after feeding at night, particularly on the two-toed sloth Choloepus didactylus, but also on other arboreal animals such as the ant-eater Tamandua tetradactyla. In Manaus, AM, precipitin tests on blood in naturally fed Ny. umbratilis showed that 66% of them had fed on sloths (Christensen et al. 1982). In a later evaluation with the same method, Ny. umbratilis females captured in a non-flooded upland forest in Manaus fed predominantly on rodents (34%) followed by dogs (19%), sloths (18%), humans (16%) and chickens (13%) (Nery et al. 2004). In addition to its nocturnal feeding habits, however, this sand fly clearly will feed in the early daylight hours if disturbed from its resting place on tree trunks. It is recorded biting man in the dry season and, particularly, directly after the rainy season.
Areas of high ACL prevalence due to L. (V.) guyanensis may be found in communities located in or very close to primary forest, and this has led to the erroneous impression that Ny. umbratilis is undergoing the process of adapting to a peri-domestic habitat. However, no consistent data exist proving that this is true, and any transmission in this environment is almost certainly due to sand flies that have been attracted to a residential area, from nearby primary forest, by the lights of the houses. Esterre et al. (1986) discussed the acquisition of ACL due to L. (V.) guyanensis in persons living in a small village within forest in French Guyana and came to the same conclusion: When the forest was cleared to about 400–500 m around the village, all peri-domestic transmission ceased. Guerra et al. (2007) discussed this situation in Manaus, AM, and were clearly of the opinion that the eco-epidemiology of ACL there is the same as that recorded in Monte Dourado, PA. In other forested areas on the outskirts of Manaus, however, Ny. umbratilis was considered to be present in equal numbers in both the forest and in the peri-domestic habitat (Barbosa et al. 2008).
Observations exist suggesting that Ny. umbratilis is a vector of L. (V.) guyanensis in the state of Bolivar, Venezuela (Feliciangeli et al. 1985), possibly indicating an expansion of the Brazilian zoonotic cycle.
Rangel et al. (1998) isolated L. (V.) braziliensis from patients with ACL in Peixoto de Azevedo (MT), and Azevedo et al. (2002) noted that one of the most abundant and highly anthropophilic sand fly in the same area was, morphologically, Ny. umbratilis. In addition, they confirmed observations made by workers in the Instituto Evandro Chagas, Belém, PA (Ward et al., 1976) that the population of this sand fly, south of the Amazonas River, behaved very differently from that studied north of the river (Monte Dourado, PA). Although abundant in the forest canopy, it was not found to accumulate on tree trunks at ground level. It was this marked behavioural difference that led Lainson (1988) to suggest that perhaps the populations of Ny. umbratilis north and south of the Amazonas River were not identical and, since that time, the taxonomic status of Ny. umbratilis started to attract special attention.
Azevedo et al. (2002) studied the morphology and the morphometric characters of the head, thorax, and abdomen of populations of the insect from Brazil (in AP, PA, AM, and MT) and Venezuela (state of Bolivar). They found that analysis of the morphological characters could not separate the populations but that the quantitative characters (morphometry) showed that 77% of these separated the Venezuelan population from the Brazilian ones. The analysis did not, however, supply evidence of heterogeneity among the populations from Brazil, but later studies on Ny. umbratilis populations from Brazil and Venezuela suggest the existence of three different populations, which are separated by the geographical barriers of the planalto of RR and the two rivers, Negro and Amazon; One is in Venezuela and the other two in Brazil (north and south of the Amazon River (Azevedo 2008)).
The same investigator recorded 52 different species of sand flies in the municipalities of Rio Branco, Xapuri and Bujari, AC (17 being a new record for that state); Ny. umbratilis was abundant in the forest canopy in close association with the major reservoir of L. (V.) guyanensis, the sloth C. didactylus; and Tojal da Silva et al. (2006) recorded the presence of ACL due to L. (V.) guyanensis in the municipality of Rio Branco. These observations lead Azevedo et al. (2005, 2008) to conclude there is, in fact, a transmission cycle of this parasite south of the Amazonas River involving Ny. umbratilis. More recent sand-fly captures in urban and peri-urban Rio Branco did not detect Ny. umbratilis, but the other ACL vectors—Ny. whitmani, Ny. antunesi, and Bi. flaviscutellata—were present (Araújo-Pereira et al. 2014).
A biological analysis under laboratory conditions compared Ny. umbratilis populations from Manaus and Manacapuru (left and right sides of the Negro River, respectively) and showed differences in their life cycle, fecundity, fertility, adult longevity, and emergence. These differences suggested that some divergence of intrinsic biological features evolved because of their geographical isolation by the Negro River (Justiniano et al. 2004). Further phylogenetic analyses based on mitochondrial DNA detected two distinct lineages in Ny. umbratilis populations of opposite sides of the Amazon and Negro rivers, thus reinforcing the thought that these rivers may be acting as effective barriers, preventing gene flow between them (Scarpassa and Alencar 2012).
In PE, where most ACL cases are caused by L. (V.) braziliensis and transmitted by Ny. whitmani (Brandão-Filho et al. 1999), studies conducted at a forest reserve in Recife detected Ny. umbratilis at very high frequencies (96.5%) and biting rates (≤333.3 flies/person-hour) (Balbino et al. 2001, 2005). Phylogenetic analysis based on wing morphometry and the period clock gene concluded that the Recife population of Ny. umbratilis is significantly closer to the Rio Preto da Eva population (north of the Amazon River, AM) and that both populations are genetically distant from Manacapuru (south of Amazon River, AM) (Souza Freitas et al. 2015, 2016).
Molecular taxonomy studies based on a barcode region of mitochondrial DNA of Ny. umbratilis and Ny. anduzei from different regions of the Amazon clearly separated both species. However, the barcode region did not have enough power to separate the two lineages of Ny. umbratilis from opposite sides of the Amazon River, likely reflecting incipient species that have not yet reached the status of distinct species (Scarpassa and Alencar 2013).
Ny. umbratilis has so far not been associated with the transmission of ACL south of the Amazon River, but its behaviour is markedly different from that of the populations from regions located north of the river. One key behavioural difference is the failure of the southern Ny. umbratilis populations to concentrate at the base of trees. A parasite isolated from Ny. umbratilis captured in Peixoto de Azevedo, MT, proved to be L. (V.) braziliensis and not L. (V.) guyanensis (Azevedo et al. 2002). This raises the question as to its possible participation in the transmission L. (V.) braziliensis. In addition, L. (V.) guyanensis is replaced in the Amazonian forest south of the river by its sister species, L. (V.) shawi, where it is transmitted by Ny. whitmani.
Psychodopygus wellcomei (Fraiha, Shaw & Lainson, 1971)
In 1968, the Meridional Mining Company, undertaking mineral exploration in PA, requested the Instituto Evandro Chagas to investigate an alarming number of men acquiring ACL due to L. (V.) braziliensis whilst working on road construction through primary forest in the Serra dos Carajás. It required only a few days for one particular sand fly to become highly suspected as the vector due to its avid feeding on man.
It proved to be a previously undescribed sand fly, which was named Ps. wellcomei in honour of Sir Henry Wellcome, founder of the Wellcome Trust, London, who was to sponsor the Institute’s leishmaniasis programme for nearly 40 further years.
Ward et al. (1973a) made a study of sand flies captured during a 2-month period (December and January) using human bait, rodent-baited Disney traps, and aspiration from tree trunks, all at ground level, and captures with CDC light traps on platforms built in the trees at 5 and 11 m above the forest floor. A total of 23 different species were caught, and approximately 65% of all the sand flies captured while biting man were Ps. wellcomei. Heavy promastigote infections were encountered in three specimens of this sand fly, and the parasite was isolated in culture and the skin of hamsters; subsequent studies showed it to be L. (V.) braziliensis. Finally, Ryan et al. (1987a) performed experimental transmission of the parasite to hamsters by placing the animals in cages with large numbers of newly caught sand flies. All fed flies were separately maintained in glass vials until they had oviposited, at which time they were dissected to detect promastigotes and the eggs of all infected specimens maintained in order to rear males for positive identification. This was necessary because the females of Ps. wellcomei are morphologically indistinguishable from those of a sympatric species, Ps. complexus, whereas the males have distinctly different morphology.
Ps. wellcomei is an essentially sylvatic and highly anthropophilic species (Ward et al. 1973a; Wilkes et al. 1984). In addition, Ward et al. (1973b) found that 25.5% of all sand flies attracted to rodent-baited traps were of this species: This, and the fact that this sand fly has a vertical flight-range of only 1–2 m above ground level, led to their suggestion that the sylvatic hosts of Ps. wellcomei are terrestrial animals, the most highly suspected being rodents and marsupials (Lainson et al. 1973). The isolation of parasites with the biological characters of L. (V.) braziliensis from the rodents Oryzomys concolor, O. capito, O. nigripes, Akodon arviculoides, Proechimys spp., Rattus, and Rhipidomys leucodactylus—and the opossum Didelphis marsupialis in Brazil—tended to support this view (Lainson and Shaw 1970, 1979; Forattini et al. 1972; Forattini 1973; Lainson et al. 1981b; Rocha et al. 1988). Finally, a more definitive identification of this parasite from the Brazilian rodents Bolomys lasiurus and R. rattus was obtained by multi-locus enzyme electrophoresis (Brandão-Filho et al. 2003).
Regarding its behaviour and seasonality, Ps. wellcomei is most abundant during the rainy season (November–April) and enters into diapause during the dryer months when it is rarely encountered. The same seasonal pattern was observed in more recent studies in areas out of the Amazon Region, in RN, where Ps. wellcomei only occurs in months with greater rainfall and lower temperatures (Pinheiro et al. 2013, 2016a, b). Limiting forest work to the dryer months can therefore greatly reduce the risk of acquiring ACL in areas where this sand fly is found. The great importance of Ps. wellcomei as a vector of L. (V.) braziliensis is due to its tendency to not only feed at night but also during broad daylight, particularly in cloudy weather. The number of infected females captured during the day was, in fact, found to be greater than that obtained during the night suggesting that transmission is actually most frequent during the day (Wilkes et al. 1984).
The presence of Ps. wellcomei has been recorded in other areas out of the Amazon Region such as in forest of the Serra de Baturité, CE (Ready et al. 1983; Azevedo and Rangel 1991). The former investigators suggested that sand flies recorded as Ps. squamiventris by Lucena (1953) in Guaramiranga, CE, were possibly Ps. wellcomei because the females of the two species are morphologically very similar.
In the Serra de Baturité area, CE, Queiroz et al. (1994) detected flagellates in Ps. wellcomei (infection rate 0.05%), but unfortunately the parasites were not identified. Ps. wellcomei was also captured in MA, but it was classified as an accessory species (Pereira Filho et al. 2015) because the local main vector of L. (V.) braziliensis is probably Ny. whitmani (Rebêlo et al. 2010; Azevedo et al. 2011; Campos et al. 2013).
Other areas of Ps. wellcomei recorded outside the Amazon include RN and PE. Despite the recent additional evidence of its high anthropophily in Nísia Floresta, RN (Pinheiro et al. 2016a), the importance of Ps. wellcomei as a vector of ACL in Northeast Brazil still must be confirmed. Although Ps. wellcomei has been found in the Atlantic Rainforest region of PE, again there is so far no association of this species with local ACL in that region (Andrade et al. 2005; Silva and Vasconcelos 2005).
Psychodopygus complexus (Mangabeira, 1941)
This sand fly was described by Mangabeira from a single male, captured in the municipality of Abaetetuba, PA, in 1938, by members of the Commission of Studies of American Visceral Leishmaniasis. Like Ps. wellcomei, the females are highly anthropophilic, although they seem not to share that sand-fly’s daytime biting habits.
The females of Ps. complexus and Ps. wellcomei are morphologically indistinguishable, although the males are easily identified by the structure of the external genitalia. Ready et al. (1991), however, used DNA probes to distinguish the two species and showed that a fragment of DNA highly repetitive for Ps. wellcomei was not detected in either sex of Ps. complexus.
In Serra dos Carajás, PA, the two species share the same forest habitat, which at first created difficulties in pinpointing the principal vector of L. (V.) braziliensis in that area and required the rearing of males from the eggs of infected females to obtain the all-important males. In a transect running from high up on the range of hills down to the lowland forest, Ready et al. (1984) showed that the predominant species at the higher altitude (≥700 m above sea level) was Ps. wellcomei and that this predominance was slowly reversed with decreasing altitude until Ps. complexus predominated, in large numbers, in the forest at the foot of the hills (200 m) and Ps. wellcomei was completely absent at ≤150 m. Because ACL due to L. (V.) braziliensis is commonly found in the latter lowland forest in various regions of PA, this is a clear indication that vectors other than Ps. wellcomei are involved (Shaw et al. 1987). In later studies in Paragominas, where Ps. wellcomei is uncommon, several infected females of the squamiventris group were found and, because all the males captured proved to be Ps. complexus, it was considered sufficient evidence to incriminate this sand fly as the vector of L. (V.) braziliensis in that region (de Souza et al., 1996).
Azevedo et al. (2002) showed that Ps. complexus represented 8.2% of all captured sand flies in an area of ACL transmission in Peixoto de Azevedo, MT, although the participation of this species as a vector in this region has yet to be established.
In a military-training area of the Atlantic Forest in Pernambuco, Andrade et al. (2005) found flagellates characteristic of Leishmania during dissections of Ps. complexus females, but species typing could not be done due to contamination of the cultures. Because Ps. complexus predominated (87%) during periods of military activities that were followed by records of human cases of ACL, the investigators considered Ps. complexus as the principal suspected vector involved in the local transmission of ACL (Andrade et al. 2005).
In the municipality of Guaraí, Tocantins, Ps. complexus was the prevalent sand-fly species in the rural environment associated with human settlements and in captures with Shannon traps, thus confirming its anthropophilic behaviour (Vilela et al. 2013). Additionally, a multiplex PCR analysis of pooled dissected females detected natural infections by L. (V.) braziliensis, which lead the investigators to conclude that although Ny. whitmani is thought of as the most important ACL vector in TO, Ps. complexus may also play an important role in the transmission cycle of ACL in rural settlement areas of Guaraí (Vilela et al. 2013). Recent studies in the same municipality found positive correlations between Ps. complexus abundance and precipitation, which further supports its potential role as a L. (V.) braziliensis vector during the rainy season (Godoy et al. 2017).
Psychodopygus ayrozai (Barretto & Coutinho, 1940)
This species has an extensive geographical distribution in Brazil that encompasses the North, Northeast, Central, Southeast, and South regions (AM, RO, RR, PA, BA, PE, MT, MG, RJ) (Aguiar and Medeiros 2003). However, its level of anthropophily appears to vary in different regions.
Psychodopygus ayrozai is anthropophilic in the more mountainous area in Atlantic Forest of Southeast Brazil (Aguiar and Soucasaux 1984), and its seasonality is associated with the hot and humid months decreasing in frequency during the colder and dryer months of the year. Studies in the Serra dos Órgãos, Rio de Janeiro state, showed that its feeding activity begins at dusk, extending until 12 pm, and that feeding occurred preferentially at ground level (Aguiar and Soucasaux 1984). In the Atlantic Forest of Paraná state, studies indicated it as one of dominant species whose population density fluctuated with temperature and rainfall indices (Marcondes et al. 2001).
Psychodopygus ayrozai has been implicated as a vector of L. (V.) naiffi in the Amazon Region, especially in PA (Lainson and Shaw 1998; Rangel and Lainson 2009). In fact, human L. (V.) naiffi cases are infrequent, probably because Ps. ayrozai does not reveal itself as anthropophilic sand-fly species in this region (Lainson and Shaw 1998; Rangel and Lainson 2009). Specimens of this phlebotomine have also been found in L. (V.) naiffi in AP and RO (de Souza et al. 2017; Arias et al. 1985).
In recent studies carried out in TO, in the Cerrado biome, Ps. ayrozai, which was first recorded in TO, was found with natural infection by L. (V.) braziliensis. These infections occurred in settlements in rural areas in the municipality of Guaraí, an endemic area for ACL with a local transmission profile related to environmental impacts by different purposes. However, the species was not among the most frequent in the study, and the investigators suggest that it may not play a secondary role in local epidemiology (Vilela et al. 2013).
Pintomyia fischeri (Pinto, 1926)
This species was described based on specimens from SP with its occurrence in secondary forested areas from many municipalities (Barretto 1943). Because it could be found close to domestic-animal shelters, it was suggested that it was adapting to a domiciliary environment (Barretto 1943). Currently, the species has its distribution mainly in the states of the South and Southeast regions of Brazil (SC, RS, PR, SP, RJ, MG, ES, MS, MT, and GO) (Aguiar and Medeiros 2003).
Discussion of the epidemiological importance of Pi. fischeri began when it was recorded in peri-domestic habitats of São Paulo state where ACL occurred (Forattini 1953). In addition, in MG and SC it was found in endemic ACL areas (Alexander et al. 2002; Marcondes et al. 2005). A study conducted from 1986 to 1995 again found this sand fly in the domiciliary habitat in areas with ACL of SP cases (Camargo-Neves et al. 2002).
Even though it had not found naturally infected with a Leishmania sp., there were strong grounds for considering it to be a potential vector. It is highly anthropophilic, and its spatial distribution coincides with reports of human ACL cases in deforested areas, Lainson (1983) suggested that this sand fly could be maintaining transmission of L. (V.) braziliensis among wild animals in forest fragments.
Recently its importance as a vector of L. (V.) braziliensis was reinforced with records of natural infections in females captured in endemic ACL areas of ES (Rocha et al. 2010). In another study (Pita-Pereira et al. 2011), in the periphery of Porto Alegre (RS), where human cases of L. (V.) braziliensis have occurred, L. (Viannia) sp. was found infected. This result led the investigators to suggest that it was participating as an ACL vector in the region. In the metropolitan area of Greater Sao Paulo, cases of ACL are sporadic and are associated with fragments of the Atlantic rain forest. In the latter, both within the forest and outside in peri-domiciliary ecotopes Pi. fischeri was the dominant species (Moschin et al. 2013). It is interesting to note that neither Ny. intermedia nor Ny. neivai were found in this habitat but that Mg. migonei was present in smaller numbers.
All the previously cited literature reinforces the importance of Pi. fischeri in the eco-epidemiology of ACL in Southeast and Southern Brazil, particularly in forested habitats.
Lutzomyia gomezi (Nitzulescu 1931)
This species was described from female sand flies captured in San Cristobal, Tachira state, Venezuela. The male of this species was described from Panama by Rozeboom in 1940 as Phlebotomus suis, which was synonymized by Fairchild and Hertig (1948).
In Brazil, this sand fly has been recorded mainly in Northern regions, but it has also been recorded in the Northeast and Central regions (AC, AP, AM, RO, RR, PA, MA, GO, MT, and BA) (Young and Duncan 1994; Aguiar and Medeiros 2003).
Although in northern Brazil, specimens of Lu. gomezi were found infected with promastigotes, suggested as being a Leishmania sp., belonging to the subgenus L. (Viannia), this was not confirmed (Rangel and Lainson 2009).
Historically, this phlebotomine has been associated with L. (V.) panamensis transmission in some South American countries without any evidence of transmission of Leishmania spp. in Brazil. However, recently, a natural infection of L. (V.) shawi was found in Lu. gomezi captured in Amazonian forest of PA (de Souza et al. 2016). The investigators suggest that this phlebotomine may participate in ACL eco-epidemiology, especially because of its arboreal habits, which is where the mammalian reservoirs of L. (V.) shawi occur.
Other vectors
We consider that the species discussed previously (Bichromomyia flaviscutellata, Lutzomyia gomezi, Ny. intermedia, Ny. whitmani, Ny. neivai, Ny. umbratilis, Migonemyia migonei, Pintomyia fischeri, Psychodopygus wellcomei, Ps. complexus, and Ps. ayrozai) are primary ACL vectors. However, others exist based on either epidemiological or parasitological evidence, or both, that may be playing roles in ACL transmission.
Trichophoromyia ubiquitalis, the only known vector of L. (V.) lainsoni (Silveira et al. 1991) but an L. (L.) amazonensis infection, was recorded by molecular methods in flies from Lábrea (AM) (Silva et al. 2014b). This sand fly is found in the Brazilian Amazonian forests and is anthropophilic being taken off man in larger numbers where the population is higher. Shannon-trap catches are considered to reflect anthropophily because a man catches the flies as they alight on the traps’ surface. In forests, south of the Amazon River, PA, it ranked 16th of 68 species of the females in Shannon-trap catches, but it ranked 3rd in abundance when light-trap catches were included in the calculations (de Souza et al. 2016). In the Brazilian Guiana Shield forest of AP in Shannon-trap catches, it ranked sixth and also sixth when light-trap catches were included in the calculation (de Souza et al. 2017). These figures reflect moderate levels of anthropophily. It is interesting to note that in both places the proportion of males to females in Shannon-trap catches was almost equal (e.g. 21 of 23 in the PA catches and 31 of 36 in those from AP), thus suggesting no great differences in population sizes despite considerable ecological differences reflected by the dominant anthropophilic species in PA being Ps. complexus/wellcomei and in AP Ny umbratilis. In CDC catches in Lábrea (AM), it was the second most common species (Silva et al. 2014b). Its constant presence in relatively high numbers is consistent with the number of L. (V.) lainsoni ACL cases in forested regions and it being considered this parasite’s principal vector.
Evidence exists that Ps. davisi participates in the transmission of L. (V.) braziliensis (Grimaldi et al. 1991), but there is stronger evidence for that of L. (V.) naiffi (Gil et al. 2003; de Souza et al. 2016). This sand fly has an extensive distribution throughout Amazonia and in pockets of the Atlantic rainforest. Infections of L. (V.) naiffi have been found in Ps. davisi in RO (Gil et al. 2003) and PA (de Souza et al. 2016). It was the dominant species in RO and ranked fifth in the PA study, but consecrated anthropophilic species—such as the complexus/wellcomei group and Ny. umbratilis—were present in large numbers. However, in the RO study area, L. (V.) braziliensis was the most common parasite, and no cases of human cases of L. (V.) naiffi were detected (Shaw et al. 2007). A possible explanation is that L.(V.) naiffi infections in man are mild and thus go unnoticed.
Another highly anthropophilic species is Ps. squamiventris, which occurs in the AM, AP, RR, and regions of PA north of the Amazon River (Ready et al. 1982). Its level of anthropophily is reflected by the fact that 4 times the number of females were captured in Shannon traps than in ground-level CDC traps (de Souza et al. 2017). Infections of L. (V.) naiffi have been found in specimens captured in AM, AP, and PA (Grimaldi et al. 1991; de Souza et al. 2017; Naiff et al. 1991). It has also been found infected with L. (V.) braziliensis, and a natural infection was transmitted experimentally to a hamster (Ryan et al. 1987b). Given its avidity for man, as well as the fact that it has been found infected with two Leishmania (Vianna) must be considered a highly probable ACL vector.
Records exist in the literature of infections in wild-caught Brazilian phlebotomines that were not identified to the species level. The first was that of Pessôa and Pestana (1940), who found flagellates in Mg. migonei and suggested that they were probably L. (V.) braziliensis. Such findings should not be ignored nor forgotten because they are strong circumstantial evidence for the possible role of a species in ACL transmission that needs confirmation. In some cases, subsequent identifications indicate what they most probably were. An example of this are studies (Ryan et al. 1987a) performed more than 30 years ago in the Serra das Carajás. Flagellates were found in 114 of 11,586 phlebotomines, and many identified as “Leishmania braziliensis subspecies” were found in Lu. gomezi, Ny. richardwardi, Ny. shawi, Ny. whitmani, Th. ubiquitalis, Ps. hirsutus, and Ps. “wellcomei.” Worthy of mention is 11 infections occurring in Th. ubiquitalis. They were almost certainly L. (V.) lainsoni because this species was later identified in this same sand-fly species (Silveira et al. 1991), and this adds weight to the importance of this species as the primary vector of this parasite. Ps. hirsutus had also been found infected with Leishmania (Vianna) in Rio de Janeiro (Rangel et al. 1985), but we do not know what the parasites were. L. (V.) shawi was described in arboreal mammals captured in the Carajás, and it seems quite likely that the infections in the three Nyssomyia species belong to this species, but this needs confirmation. In addition to the above-mentioned infections, others have been recorded by different investigators in Lu. renei, Ny. umbratilis, Pintomyia pessoai, Psathyromyia aragaoi, Pa. dendrophyla, Psychodopygus amazonensis, Ps. claustrei, Ps. davisi, and Ps. paraensis. The Leishmania species were not identified; however, based on epidemiological and molecular data they probably belonged to an ACL Leishmania species.
Table 1 lists infections of L. (L.) amazonensis in species of Nyssomyia and Trichophoromyia captured in forests and in Lutzomyia, Martinsmyia, and Nyssomyia species captured in peri-domestic habitats. L. (V.) braziliensis has been documented in Evandromyia, Martinsmyia, Micropygomyia, and Psychodopygus species obtained from sylvatic habitats as well as a smaller number near human dwellings. Similarly, infections of L. (V.) guyanensis have been recorded in species of Martinsmyia and Micropygomyia from forests. The question is this: What do these infections mean in relation to ACL transmission? They may or may not be participating in enzootic or zoonotic ACL cycles, but future studies are needed to answer these questions. The finding of infections using molecular methods in pools of flies must be viewed with caution. It does not mean that the species in question should immediately be considered as a vector. Was blood present? Where were the parasites located? How many were there? Were metacyclic forms present? These are just a few questions, some of which are only answered by viewing the dissected insect’s gut. A technique used extensively in the past that can lead to the parasite’s isolation. For many years, epidemiological data favored the one parasite/vector hypothesis. However, depending on the Leishmania species, recent parasitological results now suggest a more complex situation where one species may be the dominant vector with other species being involved in enzootic and zoonotic ACL transmission.
Impacts of Environmental and Climatic Changes
Global human population is facing the impacts of centuries of constant changes in natural environments. Climate change is happening now and impacts in the dynamics of infectious diseases are not only expected but can already be noticed (IPCC 2014; Woodward et al. 2014). Vector-borne diseases are particularly susceptible to environmental and climatic changes because their occurrence depends on the ecological balance between different species in complex transmission cycles (Walsh et al. 1993; Patz et al. 2000; McMichael 2004). Leishmaniases are among the vector-borne diseases most affected by this ecological chaos driven by human actions (Shaw 2008), and one of the expected impacts is the expansion of its geographical distribution (Ashford 2000; Dujardin 2006; WHO 2010).
Sand flies are affected by climate, especially by precipitation, humidity, and temperature. These variables influence their distribution, metabolism, and interactions with Leishmania (Ready 2008; WHO 2010; Hlavacova et al. 2013). One of the expected impacts of climate change in the eco-epidemiology of leishmaniasis is the expansion of the geographical distribution of its vectors (Peterson and Shaw 2003; González et al. 2010; Moo-Llanes et al. 2013; Carvalho et al. 2015; McIntyre et al. 2017). Given the wide latitudinal range of Brazil, regional climates play a major role in delimiting the distribution of species. Most projections under climate-change scenarios agree that disease vectors should find climatic conditions favourable to their geographic expansions towards higher latitudes in the upcoming decades (Carvalho et al. 2017).
In Brazil, the concept of leishmaniases as a sylvatic zoonosis is restricted to the Amazon Forest, Atlantic Forest fragments, and parts of Cerrado. A new transmission profile has emerged driven mostly by human-made environmental changes. In past decades, human migration of different origins and purposes resulted in major deforestation and unplanned settlements. These changes favour the dispersion of sylvatic animals (some Leishmania reservoir hosts) and sand flies (especially those species with eclectic feeding habits) to peri-domestic areas where new transmission cycles may establish close to human dwellings (Rangel 1995; Rangel and Lainson 2009; Costa et al. 2007).
Brazil is currently facing an increasing geographical expansion of ACL, which can probably be explained by the growing environmental changes, which in turn affect vector behaviour (Rangel et al. 2014). Some ACL-vector species have been showing evidences of adaptation to man-modified environments by establishing in peri-domestic areas, even in outskirts of large cities (Brasil 2007; Rangel and Lainson 2009). In this case, two sand-fly species are particularly good examples in different eco-epidemiological situations: Ny. whitmani and Bi. flaviscutellata. However, there are records of other species (see Table 1) that have been found in or near human dwellings that may be playing secondary or even primary roles in ACL- transmission cycles.
Because of its extensive geographical distribution and its association with two ACL parasites (L. (V.) braziliensis and L. (V.) shawi), Ny. whitmani is currently considered the most important ACL vector in Brazil, especially in impacted areas. This sand-fly species was found in several localities associated with the exploitation of natural environments and deforestation caused by the construction of roads, hydroelectric power plants, human settlements, wood extraction, agricultural activities, military training, and ecotourism. These epidemiological patterns occur throughout Brazil and together are considered to be responsible for the geographical expansion of ACL in the country.
Peterson and Shaw (2003) published the first projections of future potential distributions of Brazilian leishmaniasis vectors under climate-change scenarios. The investigators concluded that the ACL vectors Ny. whitmani, Ny. intermedia, and Mg. migonei should expand their distributions by the middle of the twenty-first century in different directions, most notably southwards, with Ny. whitmani showing the most dramatic range changes (Peterson and Shaw 2003). More recent projections of the potential distribution of Ny. whitmani reinforce the trends described by Peterson and Shaw (2003) and indicate a greater area of expansion of climate suitability in the North region (Costa et al., 2018, Fig. 2). Although climate-change scenarios show that the Amazon Region will become progressively drier (Joetzjer et al. 2013), the updated results state that Ny. whitmani will remain present in the region and should expand its area of climate suitability in the future (Costa et al. 2018).
The presence of Bi. flaviscutellata in peri-domestic areas, especially in the Cerrado biome, confirms the process of ruralisation of an L. (L.) amazonensis transmission cycle that was previously considered to be strictly sylvatic. Future projections under climate-change scenarios indicate that Bi. flaviscutellata might also expand its distribution beyond its current range limits in the Amazon and the Cerrado southwards into the Southeast and South regions (Carvalho et al. 2015, Fig. 3). Human cases of ADCL in Southeast Brazil are currently rare (Costa et al. 2009; Azeredo-Coutinho et al. 2007), although the disease seems to be gradually expanding its occurrence southwards. If the vector reaches these climatically suitable areas and its dispersion is followed by competent hosts and parasites, these can become ADCL-risk areas, especially because these are the most populated areas within the species’ range. The possibility of this enzootic cycle to be maintained in secondary forests and even become peri-domestic was previously discussed (Lainson et al. 1994). This could be happening, in part, because of the adaptation process of the vector to man-modified environments. At first, it would be logical to think that a strictly sylvatic cycle would disappear with the deforestation of primary forests (Campbell-Lendrum et al. 2001), but the L. (L.) amazonensis cycle shows evidences of occurrence in secondary forests and peri-domestic areas, where the vector could be dispersing to domestic-animal shelters (Rangel and Lainson 2009).
Climatic suitability of Bi. flaviscutellata under a “business as usual” climate-change scenario (average for years 2041–2060). Dark red represent areas that will only become suitable in the future; light red areas are currently suitable and will remain suitable in the future; grey areas are currently suitable but will become unsuitable in the future. RCP representative concentration pathway
The closely related species Ny. intermedia and Ny. neivai were treated as Ny. intermedia sensu lato by Peterson and Shaw (2003), who concluded that its distribution might expand southwards. A recent study reviewed the projections for both species separately, demonstrating that it is only Ny. neivai that should expand southwards, whereas Ny. intermedia might show some discrete expansions in the Northeast region (McIntyre et al. 2017, Figs. 4 and 5).
Climatic suitability of Ny. intermedia under a “business as usual” climate-change scenario (average for years 2041–2060). Dark red represent areas that will only become suitable in the future; light red areas are currently suitable and will remain suitable in the future; grey areas are currently suitable but will become unsuitable in the future. RCP representative concentration pathway
Climatic suitability of Ny. neivai under a “business as usual” climate-change scenario (average for years 2041–2060). Dark red represent areas that will only become suitable in the future; light red areas are currently suitable and will remain suitable in the future; grey areas are currently suitable but will become unsuitable in the future. RCP: representative concentration pathway
Climate change poses new challenges to the control of leishmaniasis. In addition to the long-term effects on the geographic distribution of vectors, interannual fluctuations of climate phenomena, such as the El Niño, might impact the seasonality of the sand flies and leishmaniasis (Franke et al. 2002; Chaves and Pascual 2006; Cardenas et al. 2006, 2008). Further studies are needed about the effects of climate in sand-fly densities including long-term monitoring of natural populations and climate variability. Such studies should also include spatial and temporal variations in leishmaniasis. Results from climate-based models must be validated with robust external data before they can effectively be applied in programs of the surveillance and control of leishmaniasis.
Considering the great challenge that is controlling ACL, a disease with complex epidemiology directly associated with environmental changes, studies that aim to characterize and monitor its spatial and temporal trends can support the epidemiological and entomological surveillance actions of health departments. These studies can help to identify receptive areas for new ACL outbreaks and population groups at higher risk of infection so that control actions can be better planned and more effective.
References
Afonso MMS, Gomes AC, Meneses CRV, Rangel EF (2005) Studies on the feeding habits of Lutzomyia (N.) intermedia (Diptera: Psychodidae), vector of cutaneous leishmaniasis in Brazil. Cad Saude Publica 21:1816–1820
Aguiar GM, Medeiros WM (2003) Distribuição regional e habitats das espécies de flebotomíneos do Brasil. In: Elizabeth Rangel & Ralph Lainson (org.). Flebotomíneos do Brasil. Editora Fiocruz, Rio de Janeiro. pp 291–310
Aguiar GM, Soucasaux T (1984) Ecological aspects of Phlebotomus of Parque Nacional da Serra dos Órgãos, Rio de Janeiro. I. Monthly frequency in human baits (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz 79:197–209
Alexander B, Oliveira EB, Haigh E, Almeida LL (2002) Transmission of Leishmania in coffee plantations of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 97:627–630
Andrade MS, Valença HF, da Silva AL, Almeida EL, de Brito ME, Brandão-Filho SP (2005) Sand fly fauna in a military training area endemic for American tegumentary leishmaniasis in the Atlantic Rain Forest region of Pernambuco, Brazil. Cad Saude Publica 21:1761–1767
Andrade-Filho JD, Galati EA, Falcão AL (2004) Biology of the first generation of a laboratory colony of Nyssomyia intermedia (Lutz & Neiva, 1912) and Nyssomyia neivai (Pinto, 1926) (Diptera: Psychididae). Mem Inst Oswaldo Cruz 99:597–601
Andrade Filho JD, Brazil RP (2003) Relationships of new word phlebotomine sand flies (Diptera: Psychodidae) based on fossil evidence. Mem Inst Oswaldo Cruz 98(Suppl. 1):145–149
Aragão HB (1922) Leishmaniose tegumentar e a sua transmissão pelo Phlebotomus. Mem Inst Oswaldo Cruz 82:143
Aragão HR (1927) Leishmaniose Tegumentar e sua Transmissão pelos Flebótomos. Mem Inst Oswaldo Cruz 20(2):177–185
Araújo Filho NA, Sherlock IA, Coura JR (1981) Leishmaniose Tegumentar Americana na Ilha Grande, Rio de Janeiro. V. Observações sobre a biologia dos transmissores em condições naturais. Rev Soc Bras Med Trop 14(4–6):171–183
Araujo-Pereira T, Fuzari AA, Andrade Filho JD, Pita-Pereira D, Britto C, Brazil RP (2014) Sand fly fauna (Diptera: Psychodidae: Phlebotominae) in an area of leishmaniasis transmission in the municipality of Rio Branco, State of Acre, Brazil. Parasit Vect 7(1):360
Arias JR, Freitas RA (1977a) On the vectors of cutaneous leishmaniasis in the Central Amazon of Brazil. I. Preliminary findings. Acta Amazon 7:293–294
Arias JR, Freitas RA (1977b) Flebótomos da Amazônia Central. 1. Resultados obtidos das capturas feitas com iscas humanas e eqüina (Diptera: Psychodidae). Acta Amazon 7:507–527
Arias JR, Freitas RA (1978) Sobre os vetores de leishmaniose cutânea na Amazônia Central do Brasil. 2. Incidência de flagelados em flebótomos selváticos. Acta Amazon 8:387–396
Arias JR, Miles MA, Naiff RD, Povoa MM, de Freitas RA, Biancardi CB, Castellon EG (1985) Flagellate infections of Brazilian sand flies (Diptera: Psychodidae): isolation in vitro and biochemical identification of Endotrypanum and Leishmania. Am J Trop Med Hyg 34:1098–1108
Ashford RW (2000) The leishmaniasis as emerging and reemerging zoonoses. Int J Parasitol 30:1269–1281
Azeredo-Coutinho RBG, Conceição-Silva F, Schubach A, Cupolillo E, Quintella LP, Madeira MF, Pacheco RS, Valete-Rosalino CM, Mendonça SCF (2007) First report of diffuse cutaneous leishmaniasis and Leishmania amazonensis infection in Rio de Janeiro State, Brazil. Trans Roy Soc Trop Med Hyg 101:735–737
Azevedo ACR (2008) Contribuição ao conhecimento da fauna de flebotomíneos do Estado do Acre com ênfase em Lutzomyia (Nyssomyia) umbratilis Ward & Fraiha, 1977, importante transmissor de leishmaniose tegumentar americana na Amazônia: biologia, morfologia, morfometria e discussão da identidade taxonômica, PnD Thesis, Instituto Oswaldo Cruz, Rio de Janeiro, 144 pp
Azevedo ACR, Bessa-Luz S, Vilela ML, Rangel EF (1993) Studies on the sand fly fauna of Samuel Ecological Station, Porto Velho municipality, Rondônia State, Brazil. Mem Inst Oswaldo Cruz 88:509–512
Azevedo ACR, Costa SM, Pinto MC, Souza JL, Cruz HC, Vidal J, Rangel EF (2008) Studies on the sand fly fauna (Diptera: Psychodidae: Phlebotominae) from transmission areas of American cutaneous leishmaniasis in State of Acre, Brazil. Mem Inst Oswaldo Cruz 103:760–767
Azevedo ACR, Lainson R, Afonso MMS, Rangel EF (2005) Estudio sobre Lutzomyia (Nyssomyia) umbratilis Ward & Frailha, 1977 (Psychodidae: Phlebotominae), vector de la leishmaniasis tegumentar Americana, en el Amazonas. Salud Ciencia 14:30–33
Azevedo ACR, Rangel EF (1991) A study of sand fly species (Diptera: Psychodidae: Phlebotominae) in a focus of cutaneous leishmaniasis in the Municipality of Baturité, Ceará, Brazil. Mem Inst Oswaldo Cruz 85:251
Azevedo ACR, Rangel EF, Costa EM, David J, Vasconcelos AW, Lopes UG (1990) Natural infection of Lutzomyia (Nysomyia) whitmani (Antunes & Coutinho, 1939) by Leishmania of the braziliensis complex in Baturité, Ceará State, Northeast Brazil. Mem Inst Oswaldo Cruz 85:251
Azevedo ACR, Souza NA, Meneses CRV, Costa WA, Costa SM, Lima JB, Rangel EF (2002) Ecology of sand flies (Diptera: Psychodidae: Phlebotominae) in the North of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz 97(4):459–464
Azevedo PCB, Lopes GN, Fonteles RS, Vasconcelos GC, Moraes JLP, Rebêlo JMM (2011) The effect of fragmentation on phlebotomine communities (Diptera: Psychodidae) in areas of ombrophilous forest in São Luís, state of Maranhão, Brazil. Neotrop Entomol 40(2):271–277
Balbino VQ, Coutinho-Abreu IV, Sonoda IV, Marques da Silva W, Marcondes CB (2005) Phlebotomine sandflies (Diptera: Psychodidae) of the Atlantic forest in Recife, Pernambuco state, Brazil: the species coming to human bait, and their seasonal and monthly variations over a 2-year period. Ann Trop Med Parasit 99(7):683–693
Balbino VQ, Marcondes CB, Alexander B, Luna LK, Lucena MM, Mendes A, Andrade PP (2001) First report of Lutzomyia (Nyssomyia) umbratilis Ward & Frahia, 1977 outside of Amazonian Region, in Recife, State of Pernambuco, Brazil (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz 96(3):315–317
Barbosa MG, Fé NF, Marcião AH, Silva AP, Monteiro WM, Guerra JA (2008) Sand fly fauna (Diptera: Psychodidae) in a focus of American cutaneous leishmaniasis on the urban periphery of Manaus, state of Amazonas. Rev Soc Bras Med Trop 41:485–491
Barreto AC, Vexenat JA, Cuba-Cuba CA, Marsden PD (1982) Fauna flebotomínica de uma região endêmica de leishmaniose cutâneo-mucosa no Estado da Bahia. IX Reunião Anual sobre Pesquisa Básica em Doenças de Chagas, 147 pp
Barretto MP (1943) Observações sobre a biologia em condições naturais dos flebótomos do estado de São Paulo (Ditera: Psychodidae). Tese de Livre-Docência, Faculdade de Medicina da USP, São Paulo, p 162
Barretto MP (1961) Subfamilias e gêneros neotropicais da família Psychodidae Big, 1854 (Diptera). Pap Av Dep Zool S Paulo 14:211–225
Brandão-Filho SP, Brito ME, Carvalho FG, Ishikawa EA, Cupolillo E, Floeter-Winter L, Shaw JJ (2003) Wild and synanothropic hosts of Leishmania (Viannia) braziliensis in the endemic cutaneous leishmaniasis locality of Amaraji, Pernambuco state, Brazil. Trans R Soc Trop Med Hyg 97:291–296
Brandão-Filho SP, Campbell-Lendrum D, Brito ME, CR SJJD (1999) Epidemiological surveys confirm an increasing burden of cutaneous leishmaniasis in North–East Brazil. Trans Roy Soc Trop Med Hyg 93:488–494
Brasil (2007) Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de Vigilância da Leishmaniose Tegumentar Americana 2a. ed. Editora do Ministério da Saúde, Brasília
Brito VND, Almeida ADBPF, Nakazato L, Duarte R, Souza CDO, Sousa VRF (2014) Phlebotomine fauna, natural infection rate and feeding habits of Lutzomyia cruzi in Jaciara, state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz 109(7):899–904
Camargo-Neves VLF, Gomes AC, Antunes JLF (2002) Correlação da presença de espécies de flebotomíneos (Diptera: Psychodidae) com registros de casos da leishmaniose tegumentar americana no Estado de São Paulo, Brasil. Rev Soc Bras Med Trop 35(4):1–11
Campbell-Lendrum D, Dujardin JP, Martinez E, Feliciangeli MD, Perez JE, Silans LNMP, Desjeux P (2001) Domestic and peridomestic transmission of American cutaneous leishmaniasis: changing epidemiological patterns present new control opportunities. Mem Inst Oswaldo Cruz 96(2):159–162
Campbell-Lendrum DH, Brandão-Filho SP, Ready PD, Davies CR (1999) Host and/or site loyalty of Lutzomyia whitmani (Diptera: Psychodidae) in Brazil. Med Vet Entomol 13:209–211
Campos AM, Matavelli R, Santos CD, Moraes LS, Rebêlo JMM (2013) Ecology of phlebotomines (Diptera: Psychodidae) in a transitional area between the Amazon and the Cerrado in the state of Maranhão, Brazil. J Med Entomol 50(1):52–58
Cardenas R, Sandoval CM, Rodríguez-Morales AJ, Franco-Paredes C (2006) Impact of climate variability in the occurrence of leishmaniasis in northeastern Colombia. Am J Trop Med Hyg 75(2):273–277
Cardenas R, Sandoval CM, Rodríguez-Morales AJ, Vivas P (2008) Zoonoses and climate variability. The example of leishmaniasis in southern departments of Colombia. Ann New York Acad Sci 1149:326–330
Carvalho BM, Dias CMG, Rangel EF (2014) Phlebotomine sand flies (Diptera, Psychodidae) from Rio de Janeiro State, Brazil: species distribution and potential vectors of leishmaniases. Rev Bras Entomol 58(1):77–87
Carvalho BM, Rangel EF, Ready PD, Vale MM (2015) Ecological niche modelling predicts southward expansion of Lutzomyia (Nyssomyia) flaviscutellata (Diptera: Psychodidae: Phlebotominae), vector of Leishmania (Leishmania) amazonensis in South America, under climate change. PLoS One 10(11):e0143282
Carvalho BM, Rangel EF, Vale MM (2017) Evaluation of the impacts of climate change on disease vectors through ecological niche modelling. Bull Ent Res 107:419–430
Carvalho BM, Maximo M, Costa WA, de Santana ALF, da Costa SM, da Costa Rego TAN, Pita-Pereira D, Rangel EF (2013) Leishmaniasis transmission in an ecotourism area: potential vectors in Ilha Grande, Rio de Janeiro State, Brazil. Parasit Vect 6:325
Carvalho RM, Valença HF, Silva FJ, Pita-Pereira D, Pereira TA, Britto C, Brazil RP, Brandão Filho SP (2010) Natural Leishmania infantum in Migonemyia migonei (França, 1920) (Diptera:Psychodidae: Phlebotominae) the putative vector of visceral leishmaniasis in Pernambuco. State, Brazil. Acta Trop 116:108–110
Carvalho GM, Andrade Filho JD, Falcão AL, Rocha Lima AC, Contijo CM (2008) Naturally infected Lutzomyia sand flies in a Leishmania-endemic area of Brazil. Vector Borne Zoonotic Dis 8:407–414
Casanova C, Natal D, Santos FA (2009) Survival, population size and gonotrophic cycle duration of Nyssomyia neivai (Diptera: Psychodidae) at an endemic area of American cutaneous leishmaniasis in Southeastern Brazil. J Med Entomol 46:42–50
Chaves LF, Pascual M (2006) Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med 3(8):e295
Christensen HA, Arias JR, de Vasques AM, de Freitas R (1982) Hosts of sand fly vectors of Leishmania braziliensis guyanensis in the central Amazon of Brazil. Am J Trop Med Hyg 31:239–242
Costa JML, Costa AAUML, Elkhoury NA, Bezerril ACR, Barral A, Saldanha ACR (2009) Leishmaniose cutânea difusa (LCD) no Brasil após 60 anos de sua primeira descrição. Gazeta Médica da Bahia 79(Supl.3):16–24
Costa SM, Cechinel M, Bandeira V, Zannuncio JC, Lainson R, Rangel EF (2007) Lutzomyia (Nyssomyia) whitmani s.l. (Antunes & Coutinho, 1939) (Diptera: Psychodidae: Phlebotominae) and the epidemiology of American cutaneous leishmaniasis in Brazil. Mem Inst Oswaldo Cruz 102:149–153
Costa Lima AM (1932) Sobre os Phlebotomus Americanos (Diptera, Psychodidae). Mem Inst Oswaldo Cruz 26(1):15–69
da Costa SM, Luís Passos Cordeiro J, Rangel EF (2018) Environmental suitability for Lutzomyia (Nyssomyia) whitmani (Diptera: Psychodidae: Phlebotominae) and the occurrence of American cutaneous leishmaniasis in Brazil. Parasites & Vectors 11(1)
Dias-Sversutti A, Scodro RB, Reinhold-Castro KR, Neitzke HC, Teodoro U (2007) Preliminary study on feeding preference of Nyssomyia neivai (Pinto) and Nyssomyia whitmani (Antunes & Coutinho) (Diptera: Psychodidae) in a rural area of the state of Paraná, South Brazil. Neotrop Entomol 36:953–959
de Souza AAA, Santos TV, Jennings YLL, Ishikawa EAY, Barata IR, Silva MGS, Lima JAN, Shaw J, Lainson R, Silveira FT (2016) Natural Leishmania (Viannia) spp. infections in phlebotomine sand flies (Diptera: Psychodidae) from the Brazilian Amazon region reveal new putative transmission cycles of American cutaneous leishmaniasis. Parasite 23:22
de Souza A, Ishikawa E, Braga R, Silveira F, Lainson R, Shaw J (1996) Psychodopygus complexus, a new vector of Leishmania braziliensis to humans in Pará state, Brazil. Trans R Soc Trop Med Hyg 90:112–113
de Souza AAA, da Rocha BI, das Gracas Soares Silva M, JAN L, YLL J, EAY I, Prevot G, Ginouves M, Silveira FT, Shaw J, Dos Santos TV (2017) Natural Leishmania (Viannia) infections of phlebotomines (Diptera: Psychodidae) indicate classical and alternative transmission cycles of American cutaneous leishmaniasis in the Guiana Shield, Brazil. Parasite 24:13
Donalisio MR, Peterson AT, Costa PL, da Silva FJ, Valença HF, Shaw JJ, Brandão Filho SP (2012) Microspatial distributional patterns of vectors of cutaneous leishmaniasis in Pernambuco, northeastern Brazil. J Trop Med 2012:642910
Dorval MEC, Alves TP, Oliveira AGD, Brazil RP, Galati EAB, Cunha RVD (2007) Modification of Disney trap for capture of sand flies (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz 102(7):877–878
Dorval MEC, Alves TP, Cristaldo G, Rocha HCD, Alves MA, Oshiro ET, Oliveira AG, Brazil RP, Galati EAB, Cunha RVD (2010) Sand fly captures with Disney traps in area of occurrence of Leishmania (Leishmania) amazonensis in the State of Mato Grosso do Sul, mid-western Brazil. Rev Soc Bras Med Trop 43(5):491–495
Dorval ME, Cristaldo G, Rocha HC, Alves TP, Alves MA, Oshiro ET, Brazil RP, Galati EA, Cunha RV (2009) Phlebotomine fauna (Diptera: Psychodidade) of an endemic area in the state of Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz 104:695–702
Dujardin JC (2006) Risk factors in the spread of leishmaniases: towards integrated monitoring? Trends Parasitol 22(1):4–6
Espinosa OA, Serrano MG, Camargo EP, Teixeira MM, Shaw JJ (2016) An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology, ePub, 1–13. https://doi.org/10.1017/S0031182016002092
Esterre P, Chippaux JP, Lefait JF, Dedet JP (1986) Evaluation of a cutaneous leishmaniasis control program in a forest village of French Guyana. Bull World Health Organ 64:559–565
Fairchild GB, Hertig M (1948) An improved method for mounting small insects. Science 108(2792):20
Falqueto A (1995) Especificidade alimentar de flebotomíneos em duas áreas endêmicas de leishmaniose tegumentar no estado do Espírito Santo, PhD Thesis, Fiocruz, Rio de Janeiro, 84 pp
Feliciangeli MD, Ramirez Perez J, Ramirez A (1985) First Venezuelan record of Lutzomyia umbratilis Ward & Fraiha, 1977 (Diptera: Psychodidae), a proven vector of Leishmania braziliensis guyanensis. Trans R Soc Trop Med Hyg 79:878
Ferreira AL, Sessa PA, Varejão JB, Falqueto A (2001) Distribution of sand flies (Diptera: Psychodidae) at different altitudes in an endemic region of American cutaneous leishmaniasis in the state of Espirito Santo, Brazil. Mem Inst Oswaldo Cruz 96:1061–1067
Forattini OP, Santos MR (1952) Nota sobre infecção natural de Phlebotomus intermedius Lutz & Neiva, 1912, por formas leptomonas, em um foco ativo de leishmaniose tegumentar americana. Arq Hig S Paulo 17:171–174
Forattini OP (1953) Nota sobre criadouros naturais de flebótomos em dependências peri-domiciliares no estado de São Paulo. Arq Fac Hig Saude Publica Univ Sao Paulo 7:157–168
Forattini OP (1954) Nota sobre a biologia de Phlebotomus (Diptera: Psychodidae) em região da Bacia do Rio Paraná (Brasil). Arq Fac Hig Saude Publica Univ Sao Paulo 8:15–136
Forattini OP (1973) Entomologia Médica, vol 4. Editora Edgard Blücher & Editora da Universidade de São Paulo, São Paulo, 658 pp
Forattini OP, Pattoli DBG, Rabello EX, Ferreira OA (1972) Infecção natural de flebotomíneos em foco enzoótico de leishmaniose tegumentar no estado de São Paulo, Brasil. Rev Saude Publica 6:431–433
Forattini OP, Rabello EX, Serra OP, Cotrim MD, Galati EAB, Barata JMS (1976) Observações sobre a transmissão da leishmaniose tegumentar no estado de São Paulo, Brasil. Rev Saude Publica 10:31–43
França F, Lago EL, Tada S, Costa JM, Vale K, Oliveira J, Costa MA, Osaki M, Cheever L, Netto EM, Barretto AC, Johnson WD, Marsden PD (1991) An outbreak of human Leishmania (Viannia) braziliensis infection. Mem Inst Oswaldo Cruz 86:169–174
Franke CR, Ziller M, Staubach C, Latif M (2002) Impact of the El Niño/Southern Oscillation on visceral leishmaniasis, Brazil. Emerg Infect Dis 8(9):914–917
Galati EAB, Nunes VLB, Dorval MEC, Oshiro ET, Cristaldo G, Espínola MA, Rocha HC, Garcia WB (1996) Study of the phlebotomines (Diptera: Psychodidade) in area of cutaneous leishmaniasis in the Mato Grosso state, Brazil. Rev Saude Publica 30:115–128
Gil LH, Basano AS, Souza AA, Silva MG, Barata I, Ishikawa EA, Camargo LM, Shaw JJ (2003) Recent observations on the sand fly (Diptera: Psychodidae) fauna of the state of Rondônia, western Amazonia, Brazil: the importance of Psychodopygus davisi as a vector of zoonotic cutaneous leishmaniasis. Mem Inst Oswaldo Cruz 98:751–755
Godoy RE, de Santana ALF, Graser C, Rangel EF, Vilela ML (2017) Aspects on the ecology of phlebotomine sand flies (Diptera: Psychodidae) from Guaraí, state of Tocantins, Brazil, endemic area for American cutaneous leishmaniasis. J Med Entomol 54(1):229–235
Gomes AC (1994) Sand fly vectorial ecology in the state of São Paulo. Mem Inst Oswaldo Cruz 89:457–460
Gomes AC, Rabello EX, Santos JFL, Galati EAB (1986) Ecological aspects of American cutaneous leishmaniasis. 4. Observation on the endophilic behavior of the sand fly and the vectorial role of Psychodopygus intermedius in the Ribeira Valley region of the S. Paulo state, Brazil. Rev Saude Publica 20:280–287
Gomes AC, Galati EA (1989) Aspectos ecológicos da leishmaniose tegumentar americana. 7. Capacidade vetorial flebotominea em ambiente florestal primário do sistema da Serra do Mar, região do Vale do Ribeira, estado de São Paulo, Brasil. Rev Saude Publica 23:136–142
Gonçalves BRD (2003) Identificação da Fauna de Flebotomíneos em Função de Casos Autóctones de LTA. Boletim Epidemiológico. Secretaria Municipal de Saúde de Porto Alegre 21(5):5
González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, Sarkar S (2010) Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis 4(1):e585
Gouveia C, Oliveira RM, Zwetsch A, Motta-Silva D, Carvalho BM, Santana AF, Rangel EF (2012) Integrated tools for American cutaneous leishmaniasis surveillance and control: intervention in an endemic area in Rio de Janeiro, RJ, Brazil. Interdis Perspec Infect Dis 2012:1–9
Grimaldi G Jr, Momen H, Naiff RD, McMahon-Pratt D, Barrett TV (1991) Characterization and classification of leishmanial parasites from humans, wild mammals, and sand flies in the Amazon region of Brazil. Am J Trop Med Hyg 44:645–661
Guerra JA, Barbosa MG, Loureiro AC, Coelho CP, Rosa GG, Coelho LI (2007) American tegumentary leishmaniasis in children: epidemiological aspects of cases treated in Manaus, Amazonas, Brazil. Cad Saude Publica 23:2215–2223
Guimarães FN, Costa O (1966) Novas observações sobre a Leishmania isolada de “Oryzomys goeldi” na Amazônia (4a nota). Hospital RJ 69:161–168
Guimarães VCFV, Costa PL, Silva FJ, Silva KT, Silva KG, Araújo AI, Rodrigues EHF, Brandão-Filho SP (2012) Phlebotomine sand flies (Diptera: Psychodidae) in São Vicente Férrer, a sympatric area to cutaneous and visceral leishmaniasis in Pernambuco, Brazil. Rev Soc Bras Med Trop 45:66–70
Hlavacova J, Votypka J, Volf P (2013) The effect of temperature on Leishmania (Kinetoplastida: Trypanosomatidae) development in sand flies. J Med Entomol 50(5):955–958
Hoch A, Ryan L, Vexenet JA, Rosa AC, Barretto AC (1986) Isolation of Leishmania braziliensis braziliensis and other trypanosomatids from phlebotomines in mucocutaneous leishmaniasis endemic area, Bahia, Brazil. Mem Inst Oswaldo Cruz 81(Suppl):BI 44
IPCC. Core Writing Team, Pachauri RK, Meyer LA (eds) (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva
Ishikawa EAY, Ready PD, de Souza AA, Day JC, Rangel EF, Davies CR, Shaw JJ (1999) A mitochondrial DNA phylogeny indicates close relationship between populations of Lutzomyia whitmani (Diptera: Psychodidae: Phlebotominae) from the rain-forest region of Amazônia and Northeast Brazil. Mem Inst Oswaldo Cruz 94:339–345
Joetzjer E, Douville H, Delire C, Ciais P (2013) Present-day and future Amazonian precipitation in global climate models: CMIP5 versus CMIP3. Clim Dyn 41(11–12):2921–2936
Justiniano SCB, Chagas AC, Pessoa FAC, Queiroz RG (2004) Comparative biology of two populations of Lutzomyia umbratilis (Diptera: Psychodidae) of central Amazonia, Brazil, under laboratory conditions. Braz J Biol 64:227–235
Lainson R (1983) The American leishmaniases: some observation on their ecology and epidemiology. Trans R Soc Trop Med Hyg 77:569–596
Lainson R (1988) Ecological interactions in the transmission of the leishmaniases. Philos Trans R Soc Lond Series B 321:389–404
Lainson R, Braga RR, Souza AA, Povoa MM, Ishihawa EAY, Silveira FT (1989) Leishmania (Viannia) shawi sp.n, a parasite of monkeys, sloths and procyonids in Amazonian Brazil. Ann Parasit Hum Comp 64:200–207
Lainson R, Shaw JJ (1968) Leishmaniasis in Brazil I. Observations on enzootic rodent leishmaniasis – incrimination of Lutzomyia flaviscutellata (Mangabeira) as the vector in the lower Amazonian Basin. Trans R Soc Trop Med Hyg 62:385–395
Lainson R, Shaw JJ (1970) Leishmaniasis in Brazil. V: studies on the epidemiology of cutaneous leishmaniasis in Mato Grosso state and observations on two distinct strains of Leishmania isolates from man and forest animals. Trans R Soc Trop Med Hyg 64:654–667
Lainson R, Shaw JJ (1972) Leishmaniasis of the new world: taxonomic problems. Brit Med Bull 28:44–48
Lainson R, Shaw JJ (1979) The role of animals in the epidemiology of South American leishmaniasis. In: Lumsden WHR, Evans DA (eds) Biology of the Kinetoplastida, vol 2. Academic Press, London, pp 1–116
Lainson R, Shaw JJ (1987) Evolution, classification and geographic distribution. In: Peters W, Killick-Kendrick R (eds) The leishmaniases in biology and medicine, vol 1. Academic Press, London, pp 1–20
Lainson R, Shaw JJ (1998) New World leishmaniasis. The neotropical Leishmania species. In: Cox FEG, Kreier JP, Wakelin D (eds), Topley & Wilson’s microbiology & microbiol infections, 9th edn. vol 5. Parasitology, Arnold, London, pp 242–266
Lainson R, Shaw JJ, Póvoa M (1981a) The importance of edentates (sloths and anteaters) as primary reservoirs of Leishmania brazi-liensis guyanensis, causative agent of “pian-bois” in North Brazil. Trans R Soc Trop Med Hyg 75:611–612
Lainson R, Shaw JJ, Póvoa M (1981b) Leishmaniasis in Brazil: XVI. Isolation and identification of Leishmania species from sand flies, wild mammals and man in north Pará state, with particular reference to L. braziliensis guyanensis causative agent of “pian-bois”. Trans R Soc Trop Med Hyg 75:530–536
Lainson R, Shaw JJ, Silveira FT, de Souza AA, Braga RR, Ishikawa EA (1994) The dermal leishmaniases of Brazil, with special reference to the eco-epidemiology of the disease in Amazônia. Mem Inst Oswaldo Cruz 89:435–443
Lainson R, Shaw JJ, Ward RD, Fraiha H (1973) Leishmaniasis in Brazil. IX. Considerations on the Leishmania braziliensis complex. Importance of sand flies of the genus Psychodopygus (Mangabeira) in the transmission of Leishmania braziliensis braziliensis in North Brazil. Trans R Soc Trop Med Hyg 67:184–196
Lainson R, Shaw JJ, Ward RD, Ready PD, Naiff RD (1979) Leishmaniasis in Brazil: XIII. Isolation of Leishmania from armadillos (Dasypus novemcinctus) and observation on the epidemiology of cutaneous leishmaniasis in north Pará state. Trans R Soc Trop Med Hyg 73:239–242
Lainson R, Strangways-Dixon J (1964) The epidemiology of dermal leishmaniasis in British Honduras. Parte III. Reservoir-host of Leishmania mexicana among forest rodents. Trans R Soc Trop Med Hyg 58:136–153
Lainson R, Ward RD, Shaw JJ (1976) Cutaneous leishmaniasis in North Brazil: Lutzomyia anduzei as a major vector. Trans R Soc Trop Med Hyg 70:171–172
Le Pont F, Pajot FX (1980) La leishmaniose en Guyane Française. I. Etude de l’ecologie et du taux d’infection naturelle d’un vecteur Lutzomyia (Nyssomyia) umbratilis Ward et Fraiha, 1977 en saison sèche. Considerations epidémiologiques. Cah ORSTOM Ser Ent Med Parasitol 18:359–383
Lucena DT (1953) Flebótomos do Nordeste. Morfologia de algumas espécies e sua distribuição. Papéis Avulsos de Zoologia 11:89–107
Luz E, Membrive N, Castro EA, Dereure J, Pratlong F, Dedet JA, Pandey A, Thomaz-Soccol V (2000) Lutzomyia whitmani (Diptera: Psychodidae) as vector of Leishmania (V.) braziliensis in Paraná state, Southern Brazil. Ann Trop Med Parasitol 94:623–631
Marcondes CB (1996) A redescription of Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912) and resurrection of L. neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz 91:457–462
Marcondes CB, Day JC, Ready PD (1997) Introgression between Lutzomyia intermedia and both Lu. neivai and Lu. whitmani and their roles as vectors of Leishmania braziliensis. Trans R Soc Trop Med Hyg 91:725–726
Marcondes CB, Alexander B (2003) Correlation of male genital filaments and female spermathecal ducts in new world sand flies of the Lutzomyia intermedia species complex (Diptera: Psychodidae, Phlebotominae). Mem Inst Oswaldo Cruz 98:611–613
Marcondes CB, Borges PSS (2000) Distinction of males of Lutzomyia intermedia (Lutz & Neiva, 1912) complex by ratios between dimensions and by an artificial neural network (Diptera, Psychodidae, Phlebotominae). Mem Inst Oswaldo Cruz 95:685–688
Marcondes CB, Conceição MB, Portes MG, Simão BP (2005) Phlebotomine sand flies in a focus of dermal leishmaniasis in the eastern region of the Brazilian state of Santa Catarina: preliminary results (Diptera: Psychodidae). Rev Soc Bras Med Trop 38:353–355
Marcondes CB, Lozovei AL, Falqueto A, Brazil RP, Galati EAB, Aguiar GM, Souza NA (1999) Influence of altitude, latitude and season of collection (Bergmann’s rule) on the dimension of Lutzomyia intermedia (Lutz & Neiva, 1912) (Diptera, Psychodidae, Phlebotominae). Mem Inst Oswaldo Cruz 94:693–700
Marcondes CB, Lozovei L, Vilela JH (1998) Distribuição geográfica de flebotomíneos do complexo Lutzomyia intermedia (Lutz & Neiva, 1912) (Diptera, Psychodidae) geographic distribution of phlebotomine sandflies of the Lutzomyia intermedia (Lutz & Neiva, 1912) complex (Diptera, Psychodidae). Rev Soc Bras Med Trop 31(1):51–58
Marcondes CB, Santos-Neto LG, Lozovei AL (2001) Ecology of phlebotomine sand flies (Diptera: Psychodidae) in Brazilian Atlantic Forest. Rev Soc Bras Med Trop 34:255–260
Margonari CS, Dias-Forte CL, Dias ES (2004) Genetic variability in geographical populations of Lutzomyia whitmani elucidated by RAPD-PCR. J Med Entomol 41:187–192
Martins AV, Williams P, Falcão A (1978) American sand flies. Academia Brasileira de Ciências, Rio de Janeiro
Mayrink W, Williams P, Coelho MV, Dias M, Martins AV, Magalhães PA, Costa CA, Falcão AR, Melo MN, Falcão AL (1979) Epidemiology of dermal leishmaniasis in the Rio Doce Valley, state of Minas Gerais, Brazil. Ann Trop Med Parasitol 73:123–137
McIntyre S, Rangel EF, Ready PR, Carvalho BM (2017) Species-specific ecological niche modelling predicts different range contractions for Lutzomyia intermedia and a related vector of Leishmania braziliensis following climate change in South America. Parasit Vect 10:157
McMichael AJ (2004) Environmental and social influences on emerging infectious diseases: past, present and future. Phil Trans Roy Soc London B 359:1049–1058
Meneses CR, Cupolillo E, Monteiro F, Rangel EF (2005) Micro-geographical variation among male populations of the sand fly, Lutzomyia (Nyssomyia) intermedia, from an endemic area of American cutaneous leishmaniasis in the state of Rio de Janeiro, Brazil. Med Vet Entomol 19:38–47
Moo-Llanes D, Ibarra-Cerdeña CN, Rebollar-Téllez EA, Ibáñez-Bernal S, González C, Ramsey JM (2013) Current and future niche of North and Central American sand flies (Diptera: Psychodidae) in climate change scenarios. PLoS Negl Trop Dis 7(9):e2421
Moschin JC, Ovallos FG, Sei IA, Galati EA (2013) Ecological aspects of phlebotomine fauna (Diptera, Psychodidae) of Serra da Cantareira, Greater São Paulo Metropolitan region, state of São Paulo, Brazil. Rev Bras Epi 16(1):190–201
Naiff RD, Freitas RA, Naiff MF, Arias JR, Barrett TV, Momen H, Grimaldi Junior G (1991) Epidemiological and nosological aspects of Leishmania naiffi Lainson & Shaw, 1989. Mem Inst Oswaldo Cruz 86:317–321
Nery LCR, Lorosa ES, Franco AMR (2004) Feeding preference of the sand flies Lutzomyia umbratilis and L. spathotrichia (Diptera: Psychodidae, Phlebotominae) in an urban forest patch in the city of Manaus, Amazonas, Brazil. Mem Inst Oswaldo Cruz 99(6):571–574
Nieves E, Pimenta PF (2002) Influence of vertebrate blood meals on the development Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae). Am J Trop Med Hyg 67(6):640–647
Nunes VLB, Galati EAB, Cardozo C, Rocca MEG, Andrade AROD, Santos MFDC, Aquino RB, Rosa DD (2008) Estudo de flebotomíneos (Diptera, Psychodidae) em área urbana do município de Bonito, Mato Grosso do Sul, Brasil. Rev Bras Entomol 52(3):446–451
Oliveira DM, Reinhold-Castro KR, Bernal MVZ, Legriffon CMO, Lonardoni MVC, Teodoro U, Silveira TGV (2011) Natural infection of Nyssomyia neivai by Leishmania (Viannia) spp in the state of Paraná, southern Brazil, detected by multiplex polymerase chain reaction. Vector Borne Zoonotic Dis 11:137–143
Oliveira MR, Macedo VO, de Carvalho EM, Barral A, Marotti JG, Bittencourt A, de Abreu MV, Orge M de La G, Lessa Hde A, Marsden PD (1995) An evolutionary study of mucosal leishmaniasis (a 7 to 17-year follow-up) due to Leishmania (Viannia) braziliensis in Três Braços, Bahia. Rev Soc Bras Med Trop 28:325–332
Passos VNA, Silva RE, Falcão AL (1991) Fauna flebotomínica de municípios da região metropolitana de Belo Horizonte. Rev Soc Bras Med Trop 24(Suppl. 11):107
Patz JA, Thaddeus KG, Geller N, Vittor AY (2000) Effects of environmental change on emerging parasitic diseases. Int J Parasitol 30:1395–1405
Pereira Filho AA, Bandeira MDCA, Fonteles RS, Moraes JLP, Lopes CRG, Melo MN, Rebêlo JMM (2015) An ecological study of sand flies (Diptera: Psychodidae) in the vicinity of Lençóis Maranhenses National Park, Maranhão, Brazil. Parasit Vect 8(1):442
Pessôa SB, Pestana BR (1940) Infecção natural de “Phlebotomus migonei” por formas em leptomonas provavelmente da “Leishmania brasiliensis”. Acta Méd Rio de Janeiro 5:106–111
Pessôa SB, Coutinho JO (1941) Infecção natural e experimental dos flebótomos pela Leishmania braziliensis no estado de São Paulo. Hospital 20:25–35
Peterson AT, Shaw J (2003) Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions and climate change effects. Int J Parasitol 33:919–931
Pinheiro MPG, da Silva JHT, Inacio CLS, de Melo MDFF (2016a) Anthropophily of Lutzomyia wellcomei (Diptera: Psychodidae) in an Atlantic Forest Conservation Unit in Northeast Brazil. J Med Entomol 53(6):1444–1448
Pinheiro MPG, Silva JHT, Cavalcanti KB, de Azevedo PRM (2013) Ecological interactions among phlebotomines (Diptera: Psychodidae) in an agroforestry environment of northeast Brazil. J Vect Ecol 38(2):307–316
Pinheiro MPG, de Medeiros Silva MM, Júnior JBS, da Silva JHT, de Lima AM, De Melo MDFF (2016b) Sand flies (Diptera, Psychodidae, Phlebotominae), vectors of Leishmania protozoa, at an Atlantic Forest Conservation Unit in the municipality of Nísia Floresta, Rio Grande do Norte state, Brazil. Parasit Vect 9(1):83
Pirmez C, Oliveira-Neto MP, Franco A, Meneses C, Rangel E, Mayrink A, Gonçalves AJ, Fernandes O, Grimaldi G (1997) Edentates as a possible reservoir of L. (V.) braziliensis in an endemic area of Rio de Janeiro. Mem Inst Oswaldo Cruz 92(Suppl. I):119
Pita-Pereira D, Alves CR, Souza MB, Brazil RP, Bertho AL, Figueiredo AB, Britto CC (2005) Identification of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridisation assay. Trans R Soc Trop Med Hyg 99:905–913
Pita-Pereira D, Souza GD, Araújo Pereira T, Zwetsch A, Britto C, Rangel EF (2011) Lutzomyia (Pintomyia) fischeri (Diptera: Psychodidae: Phlebotominae), a probable vector of American cutaneous leishmaniasis: detection of natural infection by Leishmania (Viannia) DNA in specimens from the municipality of Porto Alegre (RS), Brazil, using multiplex PCR assay. Acta Trop 120(3):273–275
Pita-Pereira D, Souza GD, Zwetsch A, Alves CR, Britto C, Rangel EF (2009) First report of Lutzomyia (Nyssomyia) neivai (Diptera: Psychodidae: Phlebotominae) naturally infected by Leishmania (Viannia) braziliensis in a periurban area of South Brazil using a multiplex polymerase chain reaction assay. Am J Trop Med Hyg 80:593–595
Queiroz MFM, Varjão JR, Moraes SCD, Salcedo GE (2012) Analysis of sandflies (Diptera: Psychodidae) in Barra do Garças, State of Mato Grosso, Brazil, and the influence of environmental variables on the vector density of Lutzomyia longipalpis (Lutz & Neiva, 1912). Rev Soc Bras Med Trop 45(3):313–317
Queiroz RG, Vasconcelos IA, Vasconcelos AW, Pessoa FA, Souza RN, David JR (1994) Cutaneous leishmaniasis in Ceará state in Northeasten Brazil: incrimination of Lutzomyia whitmani (Diptera: Psychodidae) as a vector of Leishmania braziliensis in Baturité municipality. Ann J Trop Med Hyg 50:693–698
Rangel EF (1995) Tropical Diseases, Society and the Environment. SAREC Documentation/TDR, pp 103–110
Rangel EF, Costa SM, Carvalho BM (2014) Environmental changes and the geographic spreading of American cutaneous leishmaniasis in Brazil. In: Claborn D (ed) Leishmaniasis–trends in epidemiology, diagnosis and treatment. InTech, Rijeka
Rangel EF, Lainson R (2003) Transmissores de Leishmaniose Tegumentar Americana. In: Elizabeth Rangel & Ralph Lainson (org.). Flebotomíneos do Brasil. Editora Fiocruz, Rio de Janeiro, pp 291–310
Rangel EF, Lainson R (2009) Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz 104(7):937–954
Rangel EF, Ryan L, Lainson R, Shaw JJ (1985) Observations on the sandfly (Diptera: Psychodidae) fauna of Além Paraiba, State of Minas Gerais, Brazil, and the isolation of a parasite of the Leishmania braziliensis complex from Psychodopygus hirsuta hirsuta. Mem Inst Oswaldo Cruz 80:373–374
Rangel EF, Azevedo ACR, Andrade CA, Souza NA, Wermelinger ED (1990) Studies on sand fly fauna (Diptera: Psychodidae) in a foci of cutaneous leishmaniasis in Mesquita, Rio de Janeiro state, Brazil. Mem Inst Oswaldo Cruz 85:39–45
Rangel EF, Azevedo ACR, Orosko S, Lima JB, Souza NA, Pereira T, Meneses CRV, Costa WA, Cupollilo E, Brahim L (1998) Lutzomyia (Nyssomyia) umbratilis (Ward & Fraiha, 1977) and the ecology of American cutaneous leishmaniasis in Mato Grosso state. I Bienal de Pesquisa da Fundação Oswaldo Cruz, Fiocruz, Rio de Janeiro, 122 pp
Rangel EF, Meneses CRV, Cupolillo E, Azevedo ACR, Costa WA, Costa SM (1999) Aspectos da ecologia de Lutzomyia intermedia (Lutz & Neiva, 1912) e a fauna flebotomínica (Diptera: Psychodidae) em área de transmissão da Leishmania (V.) braziliensis no Rio de Janeiro. Rev Soc Bras Med Trop 32(Suppl. 1):115
Rangel EF, Souza NA, Wermelinger ED, Barbosa AF, Andrade CA (1986) Biology of Lutzomyia intermedia Lutz & Neiva, 1912 and Lutzomyia longipalpis Lutz & Neiva, 1912 (Diptera: Psychodidae) under experimental conditions. I. Feeding aspects of larvae and adults. Mem Inst Oswaldo Cruz 81:431–438
Rangel EF, Lainson R, Souza AA, Ready P, Azevedo ACR (1996) Variation between geographical populations of Lutzomyia (Nyssomyia) whitmani (Antunes & Coutinho, 1939) sensu lato (Diptera: Psychodidae: Phlebotominae) in Brazil. Mem Inst Oswaldo Cruz 91:43–50
Rangel EF, Barbosa AF, Andrade CA, Souza NA, Wermelinger ED (1992) Development of Leishmania (Viannia) braziliensis Viannia, 1991 in Lutzomyia intermedia (Lutz & Neiva, 1912) (Diptera: Psychodidae) under experimental conditions. Mem Inst Oswaldo Cruz 87:235–238
Ready P (2008) Leishmaniasis emergence and climate change. Rev Sci Tech L’Off Int Epiz 27(2):399–412
Ready PD, Fraiha H, Lane RP, Arias JR, Pajot FX (1982) On distinguishing the female of Psychodopygus wellcomei, a vector of mucocutaneous leishmaniasis, from other squamiventris series females. I. Characterization of Ps. squamiventris squamiventris and Ps. s. maripaensis stat. nov. (Diptera: psychodidae). Ann Trop Med Parasitol 76:201–214
Ready PD, Lainson R, Shaw JJ (1983) Leishmaniasis in Brazil: XX. Prevalence of “enzootic rodent leishmaniasis” (Leishmania mexicana amazonensis) and apparent absence of pian-bois (Le. braziliensis guyanensis), in plantations of introduced tree species and in other non-climax forests in eastern Amazonia. Trans R Soc Trop Med Hyg 77:775–785
Ready PD, Lainson R, Shaw JJ (1984) Habitat and seasonality of Psychodopygus wellcomei help incriminate it as a vector of Leishmania in Amazônia and Northeast Brazil. Trans R Soc Trop Hyg 78:543–544
Ready PD, Lainson R, Shaw JJ, Souza AA (1991) DNA probes for distinguishing Psychodopygus wellcomei from Psychodopygus complexux (Diptera: Psychodidae). Mem Inst Oswaldo Cruz 86:41–49
Ready PD, Lainson R, Shaw JJ, Ward D (1986) The ecology of Lutzomyia umbratilis (Ward & Fraiha, 1977) (Diptera: Psychodiade), the major vector to man of Leishmania braziliensis guyanensis in north-eastern Amazonian Brazil. Bull Entomol Res 76:21–40
Ready PD, Day JC, Souza AA, Rangel EF, Davies CR (1997) Mitochondrial DNA characterization of populations of Lutzomyia whitmani (Diptera: Psychodiade) incriminated in the peridomestic and silvatic transmission of Leishmania species in Brazil. Bull Ent Res 87:187–195
Ready PD, Souza AA, Rebêlo JMM, Day JC, Silveira FT, Campbell-Lendum D, Davies CR, Costa JML (1998) Phylogenetic species and domesticity of Lutzomyia whitmani at the south-east boundary of Amazonian Brazil. Bull Entomol Res 87:187–195
Rebêlo JMM, Júnior A, Nascimento A, Silva O, Moraes JLP (2010) Occurrence of sand flies (Diptera, Psychodidae) in leishmaniasis foci in an ecotourism area around the Lençóis Maranhenses National Park, Brazil. Cad Saude Publ 26(1):195–198
Rocha LS, Falqueto A, dos Santos CB, Ferreira AL, da Graça GC, Grimaldi G, Cupolillo E (2010) Survey of natural infection by Leishmania in sand fly species collected in southeastern Brazil. Trans R Soc Trop Med Hyg 104(7):461–466
Rocha NMM, Melo MN, Babá EH, Dias M, Michalick MSM, Da Costa CA, Williams P, Mayrink W (1988) Leishmania braziliensis braziliensis isolated from Akodon arviculoides captured in Ca-ratinga, Minas Gerais, Brazil. Trans R Soc Trop Med Hyg 82:68
Rodrigues ACM, Melo LM, Magalhães RD, de Moraes NB, Júnior ADS, Bevilagua CML (2016) Molecular identification of Lutzomyia migonei (Diptera: Psychodidae) as a potential vector for Leishmania infantum (Kinetoplastida: Trypanosomatidae). Veterinary Parasitogy 220:28–32
Ryan L, Lainson R, Shaw JJ (1987a) Leishmaniasis in Brazil. XXIV. Natural flagellate infections of sandflies (Diptera: Psychodidae) in Pará state, with particular reference to the role of Psychodopygus wellcomei as the vector of Leishmania braziliensis braziliensis in the Serra dos Carajás. Trans R Soc Trop Med Hyg 81:353–359
Ryan L, Lainson R, Shaw JJ, Braga RR, Ishikawa EA (1987b) Leishmaniasis in Brazil. XXV. Sandfly vectors of Leishmania in Pará State, Brazil. Med Vet Entomol 1:383–395
Ryan L, Vexenet A, Marsden PD, Lainson R (1990) The importance of rapid diagnoses of new cases of cutaneous leishmaniasis in pinpointing the sand fly vector. Trans R Soc Trop Med Hyg 84:786
Scarpassa VM, Alencar RB (2012) Lutzomyia umbratilis, the main vector of Leishmania guyanensis, represents a novel species complex? PLoS One 7(5):e37341
Scarpassa VM, Alencar RB (2013) Molecular taxonomy of the two Leishmania vectors Lutzomyia umbratilis and Lutzomyia anduzei (Diptera: Psychodidae) from the Brazilian Amazon. Parasit Vect 6(1):258
Sergent ED, Sergent ET, Parrot L, Donatieu A, Béguet M (1921) Transmission du clou de Biskra par le phlébotome (Phlebotomus papatasi Scop.) C R Acad Sci 173:1030–1032
Shaw J (2007) The leishmaniases – survival and expansion in a changing world. A mini- review. Mem Inst Oswaldo Cruz 102:541–547
Shaw J (2008) How climatic and environmental variations affect the eco-epidemiology of the leishmaniases and their control. III Workshop de Genética e Biologia Molecular de Insetos Vetores de Doenças Tropicais, 13 pp
Shaw JJ, Ishikawa EAY, Lainson R, Braga RR, Silveira FT (1991) Cutaneous leishmaniasis of man due to Leishmania (Viannia) shawi Lainson, de Souza, Póvoa, Ishikawa & Silveira in Pará state, Brazil. Ann Parasitol Hum Comp 66:243–246
Shaw JJ, Lainson R (1968) Leishmaniasis in Brazil: II. Observations on enzootic rodent leishmaniasis in the lower Amazon region – the feeding habits of the vector Lutzomyia flaviscutellata in reference to man, rodents and other animals. Trans R Soc Trop Med Hyg 62:396–405
Shaw JJ, Lainson R (1972) Leishmaniasis in Brazil: VI. Observations on the seasonal variations of Lutzomyia flaviscutellata in different types of forest and its relationship to enzootic rodent leishmaniasis (Leishmania mexicana amazonensis). Trans Roy Soc Trop Med Hyg 66(5):709–717
Shaw JJ, Lainson R, Ryan L, Braga RR, Me-Mahon-Pratt D, David JR (1987) Leishmaniasis in Brazil. XXII. The identification of Leishmania braziliensis braziliensis in wild-caught neotropical sand flies using monoclonal antibodies. Trans R Soc Trop Med Hyg 81:69–72
Shaw JJ, Lainson R, Ward RD (1972) Leishmaniasis in Brazil. VII. Further observations on the feeding habitats of Lutzomyia flaviscutellata (Mangabeira) with particular reference to its biting habits at different heights. Trans R Soc Trop Med Hyg 66:718–723
Shaw JJ, de Faria DL, Basano SA, Corbett CE, Rodrigues CJ, Ishikawa EA, Camargo LM (2007) The aetiological agents of American cutaneous leishmaniasis in the municipality of Monte Negro, Rondônia state, western Amazonia, Brazil. AnnTrop Med Parasitol 101:681–688
Sherlock I, Carneiro M (1962) Algumas fêmeas de Phlebotomus do Brasil (Diptera: Psychodidae). Mem Inst Oswaldo Cruz 60:423–435
Silva AM, de Camargo NJ, dos Santos DR, Massafera R, Ferreira AC, Postai C, Cristóvão EC, Konolsaisen JF, Bisetto A Jr, Perinazo R, Teodoro U, Galati EA (2008) Diversity, distribution and abundance of sand flies (Diptera: Psychodidae) in Paraná state, Southern Brazil. Neotrop Entomol 37:209–225
Silva DF, Vasconcelos SD (2005) Phlebotomine sand flies in fragments of rain forest in Recife, Pernambuco state. Rev Soc Bras Med Trop 38:264–266
Silva DT, Starke-Buzetti WA, Alves-Martin MF, Paixão MS, Tenório MS, Lopes MLM (2014a) Comparative evaluation of several methods for Canine Visceral Leishmaniasis diagnosis. Rev Bras Parasitol Vet 23(2):179–186
Silva TR, Assis MD, Freire MP, Rego FD, Gontijo CM, Shimabukuro PH (2014b) Molecular detection of Leishmania in sand flies (Diptera: Psychodidae: Phlebotominae) collected in the Caititu Indigenous Reserve of the Municipality of Lábrea, State of Amazonas, Brazil. J Med Entomol 51:1276–1282
Silveira FT, Souza AA, Lainson R, Shaw JJ, Braga RR, Ishikawa EE (1991) Cutaneous leishmaniasis in the Amazon region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará State, Brazil. Mem Inst Oswaldo Cruz 86:127–130
Souza CM, Pessanha JE, Barata RA, Monteiro EM, Costa DC, Dias ES (2004) Study on phlebotomine sand fly (Diptera: Psychodidae) fauna in Belo Horizonte, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 99:795–803
Souza Freitas MT, Ríos-Velasquez CM, Costa CRL, Figueirêdo CAS, Aragão NC, da Silva LG, Batista MVA, Balbino TCL, Pessoa FAC, Queiroz BV (2015) Phenotypic and genotypic variations among three allopatric populations of Lutzomyia umbratilis, main vector of Leishmania guyanensis. Parasit Vect 8(1):448
Souza Freitas MT, Ríos-Velasquez CM, da Silva LG, Costa CRL, Marcelino A, Leal-Balbino TC, Balbino VQ, Pessoa FAC (2016) Analysis of the genetic structure of allopatric populations of Lutzomyia umbratilis using the period clock gene. Acta Trop 154:149–154
Souza MB, Cardoso PG, Sanavria A, Marzochi MCA, de Carvalho RW, Ribeiro PC, Ponte CS, Meira AM, Meródio JC (2003) Fauna flebotomínica do município de Bom Jardim, Região Serrana do estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 12:150–153
Souza NA, Andrade-Coelho CA, Vilela ML, Peixoto AA, Rangel EF (2002) Seasonality of Lutzomyia intermedia and Lutzomyia whitmani (Diptera: Psychodidae: Phlebotominae), occurring sympatrically in area of cutaneous leishmaniasis in the state of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 97:759–765
Souza NA, Vilela ML, Andrade-Coelho CA, Rangel EF (2001) The Phlebotominae sand fly (Diptera: Psychodidae) fauna of two Atlantic Rain Forest Reserves in the state of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 96:319–324
Souza-Rocha L, Falqueto A, dos Santos CB, Grimaldi G Jr, Cupolillo E (2007) Genetic structure of Lutzomyia (Nyssomyia) intermedia population from two ecologic regions in Brazil where transmission of Leishmania (Viannia) braziliensis reflects distinct eco-epidemiologic features. Am J Trop Med Hyg 76:559–565
Teodoro U, Salvia Filho VL, de Lima EM, Spinosa RP, Barbosa OC, Ferreira MEMC, Verzignassi TG (1993) Flebotomíneos em áreas de transmissão de leishmaniose na região norte do estado do Paraná - Brasil: variação sazonal e atividade noturna. Rev Saude Publica 27:190–194
Teodoro U, Silveira TG, dos Santos DR, dos Santos ES, dos Santos AR, de Oliveira O, Kuhl JB, Alberton D (2003) Influence of rearrangement and cleaning of the peridomiciliary area and building disinsectization on sand fly population density in the municipality of Doutor Camargo, Paraná state, Brazil. Cad Saude Publica 19:1801–1813
Tojal da Silva AC, Cupolillo E, Volpini AC, Almeida R, Romero GA (2006) Species diversity causing human cutaneous leishmaniasis in Rio Branco, State of Acre, Brazil. Tropical Med Int Health 11:1388–1398
Vexenat JA, Barretto AC, Rosa AC (1986) Infecção experimental de Lutzomyia whitmani em cães infectados com Leishmania braziliensis braziliensis. Inst Oswaldo Cruz 81:125–126
Vieira VR, Azevedo ACR, Alves JRC, Guimarães AE, Aguiar GM (2015) Ecological aspects of phlebotomine sand flies (Diptera, Psychodidae, Phlebotominae) in areas of American Cutaneous Leishmaniasis, in the Municipality of Paraty, Rio de Janeiro, Brazil. I-index of abundance by location and type of capture. J Med Entomol 52(5):886–895
Vilela ML, Azevedo ACR, Costa SM, Costa WA, Silva DM, Grajauskas AM, Moreira-de-Carvalho B, Paes LRDNB, Kozlowsky D, Rangel EF (2008) Sand fly survey in the influence área of Peixe Angical Hydroelectric Plant, state of Tocantins, Brazil. In: 6th International Symposium on Phlebotomine Sandflies, Lima, 95 pp
Vilela ML, Azevedo ACR, Afonso MMS, Silva DM, Rangel EF (2006) Estudos dos vetores das leishmanioses na área de influência do Aproveitamento Hidroelétrico Peixe Angical, estado de Tocantins. XLII Congresso da Sociedade Brasileira de Medicina Tropical. Rev Soc Bras Med Trop 39(Suppl. I):76
Vilela ML, Azevedo ACR, Motta-Silva D, Costa WA, Rangel EF (2007) Estudos dos vetores das leishmanioses em áreas de influência do Aproveitamento Hidroelétrico Peixe Angical, estado de Tocantins. XLIII Congresso da Sociedade Brasileira de Medicina Tropical. Rev Soc Bras Med Trop 40(Suppl. I):127
Vilela ML, Azevedo CG, Carvalho BM, Rangel EF (2011) Phlebotomine fauna (Diptera: Psychodidae) and putative vectors of leishmaniases in impacted area by hydroelectric plant, state of Tocantins, Brazil. PLoS One 6(12):e27721
Vilela ML, Pita-Pereira D, Azevedo AC, Godoy RE, Britto C, Rangel EF (2013) The phlebotomine fauna (Diptera: Psychodidae) of Guaraí, state of Tocantins, with an emphasis on the putative vectors of American cutaneous leishmaniasis in rural settlement and periurban areas. Mem Inst Oswaldo Cruz 108(5):578–585
Walsh JF, Molyneux DH, Birley MH (1993) Deforestation: effects on vector-borne disease. Parasitology 106(Suppl):55–75
Ward RD, Fraiha H (1977) Lutzomyia umbratilis, a new species of sand fly from Brazil (Diptera: Psychodidae). J Med Entomol 14:313–317
Ward RD, Lainson R, Shaw JJ (1973a) Further evidence of the role of Lutzomyia flaviscutellata (Mangabeira) as the vector of Leishmania mexicana amazonensis in Brazil. Trans R Soc Trop Med Hyg 67:608–609
Ward RD, Lainson R, Shaw JJ (1977) Experimental transmissions of Leishmania mexicana amazonensis Lainson & Shaw, between hamsters by the bite of Lutzomyia flaviscutellata (Mangabeira). Trans R Soc Trop Med Hyg 71:265–266
Ward RD, Shaw JJ, Lainson R, Fraiha H (1973b) Leishmaniasis in Brazil. VIII. Observations on the phlebotomine fauna of an area highly endemic for cutaneous leishmaniasis in the Serra dos Carajás, Pará state. Trans R Soc Trop Med Hyg 67:174–183
WHO (2010) World Health Organization. Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010 (WHO Technical Report Series, n° 949). WHO Press, Genebra
Wijers DJB, Linger R (1966) Man-biting sand flies in Surinam (Dutch Guiana): Phlebotomus anduzei as a possible vector of Leishmania braziliensis. Ann Trop Med Parasitol 60:501–508
Wilkes TJ, Ready PD, Lainson R, Killick-Kendrick R (1984) Biting periodicities of nulliparous and parous females of Psychodopygus wellcomei. Trans R Soc Trop Med Hyg 78:846–847
Woodward A, Smith KR, Campbell-Lendrum D, Chadee DD, Honda Y, Liu Q, Olwoch J, Revich B, Sauerborn R, Chafe Z, Confalonieri U, Haines A (2014) Climate change and health: on the latest IPCC report. Lancet 383:1185–1189
Young DC, Duncan NA (1994) Guide to the identification and geographic distribution of Lutzomyia sand flies in México, the West Indies, Central and South America (Diptera: Psychodidae). Mem Ann Entomol Institut 54:1–881
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Rangel, E.F., Lainson, R., Carvalho, B.M., Costa, S.M., Shaw, J.J. (2018). Sand Fly Vectors of American Cutaneous Leishmaniasis in Brazil. In: Rangel, E., Shaw, J. (eds) Brazilian Sand Flies . Springer, Cham. https://doi.org/10.1007/978-3-319-75544-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-75544-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75543-4
Online ISBN: 978-3-319-75544-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)