Abstract
Hsp90 is an essential and abundantly expressed molecular chaperone in any living cell. The multiplicity of Hsp90 cellular functions is driven by its interaction with a broad range of partner proteins and thereby establishing itself as a moonlighting molecule. There are newer insights emerging to ascertain the cellular and physiological roles of Hsp90, such as (and not limited to) chromatin remodeling, gene regulation and developmental pathways. Hsp90 has been recognized as an important therapeutic target and has been linked to an increasing number of diseases, including cancer. Development of Hsp90 therapeutic reagents would be valuable research tools towards the maintenance of the proteome in health and disease. This review revisits the expression, structure-function, and clinical significance of the Hsp90 and its forms and reinforces its impact as a disease target.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heat shock proteins (Hsp) or chaperonins, as they were previously called, are group of evolutionary conserved proteins that show high sequence homology between different species, from bacteria to humans (Morimoto 1993). These molecular chaperones are proteins that interact with and help other client proteins to acquire a functionally active form, and they then dissociate from the client once the final active structure is formed. They are classified based on molecular size, sequence similarities, location within the cell and function. Despite the significant degree of evolutionary conservation, HSP’s are highly immunogenic and it has been postulated that they could activate antigen presenting cells, serving as a danger signal to the immune system (Gallucci and Matzinger 2001).
Of the several Hsp, Hsp90 (with an approximate molecular weight of 90 kilo Daltons) is an abundant, constitutively expressed chaperone constituting around 1–2% of total cellular protein under non-stress conditions (Falsone et al. 2005). In eukaryotes, Hsp90 is found in the cytosol, the nucleus and in organelles such as the endoplasmic reticulum. The nuclear localized Hsp90 represents a small fraction of cytosolic Hsp90 under physiological conditions. Studies have shown that Hsp90 is not only present inside the cell, but also on the cell surface of various cell types and secreted into the extracellular space suggesting distinct extracellular chaperoning activity (Li et al. 2012). Hsp90 has a crucial role in cellular signaling, as it participates in the folding of steroid hormone receptors, protein kinases and other signaling components. However, the well determined function of Hsp90 proteins is to suppress the aggregation of unfolded proteins. The full functional activity of Hsp90 is gained in concert with other co-chaperones, thereby playing an important role in the folding of newly synthesized proteins and stabilization and refolding of denatured proteins after stress. Apart from its co-chaperones, Hsp90 binds to an array of client proteins, where the co-chaperone requirement varies and depends on the actual client (Sreedhar et al. 2004). A current updated list of Hsp90 and its partner proteins is maintained by Dr. Didier Picard (http://www.picard.ch/downloads/downloads.htm). Hsp90 clients were shown to be functionally and structurally diverse thereby making it a central modulator of important processes that range from stress regulation and protein folding to DNA repair, development, the immune response, neuronal signaling and many other processes (Schopf et al. 2017). Very recently, studies by the late Susan Lindquist’ team demonstrated that Hsp90 can modify the consequences of genetic variation in human, something long hypothesized but never proven. The study provided insights into the mechanisms by which Hsp90 buffering can alter the course of human diseases, broadly protective in nature and mitigating the deleterious effects of missense mutations. The study suggested that by buffering these kinds of human genetic variation, Hsp90 enables gene-environment interactions which are capable of shaping disease trajectories (Karras et al. 2017) thus indicating clinical significance.
2 HSP90 Nomenclature, Isoforms and Structure
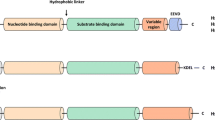
This HSP90 family encodes five members as seen in the Table 12.1 namely HSPC1, HSPC2, HSPC3, HSPC4 and HSPC5. Their chromosomal location all varies and are known by various nick names in literature (Kampinga et al. 2009, Website: HUGO gene nomenclature committee). They can be found in different cell compartment such as cytosol, endoplasmic reticulum and the mitochondria (Csermely et al. 1998). Most studied members of this group are the cytoplasmic isoforms and an ER specific HSPC4. This protein from even the most distantly related eukaryotes has 50% amino acid identity and all have more than 40% identity with the Escherichia coli protein (Bardwell and Craig 1987) and has sequence similarities with other mammalian species as shown in Fig. (12.1a, b). There are two major cytoplasmic forms of Hsp90: Hsp90α [inducible form] and Hsp90β [constitutive form] which possibly arose by gene duplication roughly 500 million years ago (Gupta 1995). In contrast to bacteria, which typically contain only a single Hsp90 gene, budding yeast and humans contain two HSP90 genes that encode cytosolic proteins: Hsc82 and Hsp82 in S. cerevisiae, and Hsp90α and Hsp90β in H. sapiens (Schopf et al. 2017). A minimum of two additional gene duplication events, which took place at a later time, are required to explain the presence of two different forms of HSP90 that are found in these fungi and vertebrate species. In humans, sequence similarities between the alpha and the beta form are around 93.4% using the EBI tool: EMBOSS pairwise alignment algorithm. Hsp90α is a 732-amino acid long protein while Hsp90β has 724 amino acids and 8 amino acids (7–11 and 237–239) present in Hsp90α are missing in Hsp90β as seen in Fig. (12.1c, d). Although both are ubiquitously expressed, Hsp90α is heat-inducible in specific tissues, while Hsp90β typically has a more constitutive pattern of expression (Sreedhar et al. 2004). These regulatory differences translate into varied responses to extracellular signals and stress events, with Hsp90α adopting a cytoprotective role by mediating a rapid response, whereas Hsp90β is associated with long-term cellular adaptation. Owing to its higher levels of expression, Hsp90β is the predominant Hsp90 isoform involved in normal cellular function, including the maintenance of cytoskeletal architecture, cellular transformation, and signal transduction. An important difference is that the α-form readily dimerizes, whereas the β-form does so with much less efficiency. Hsp90 is mainly a constitutive dimer [αα or ββ], however, monomers [α or β], heterodimers [αβ] and higher oligomers of both forms also exist (Sreedhar et al. 2004). Hsp90 contains three conserved domains: a N-terminal ATP-binding domain, a middle domain and a carboxy-terminal domain. All examined forms of Hsp90 bind and hydrolyze ATP (Zuehlke and Johnson 2010). The N-terminal of Hsp90 and the highly charged central region are responsible for the binding of different target proteins. A binding site for ATP/ ADP also can be found in the N-terminal region, while the C-terminal domain contains a dimerization site (Csermely et al. 1998). Some of the biological differences between the Hsp90α and Hsp90β isoforms are likely to be determined by client proteins and by cochaperones associated with Hsp90 chaperone machinery (Rohl et al. 2013; Millson et al. 2007). It is intriguing therefore that the cochaperone, GCUNC45, previously implicated in the Hsp90 chaperoning of the progesterone receptor, was found to bind preferentially to Hsp90β over Hsp90α, resulting in an efficient blockade of progesterone receptor chaperoning in vitro (Chadli et al. 2006, 2008).
Distance relation of Hsp90β across species and Hsp90α versus Hsp90β structure. (a) Hsp90 protein shows high sequence homology between different species, from bacteria to humans as seen in the dendogram which shows the distance relation between the species with respect to human Hsp90β. (b) Percentage sequence similarity with respect to human Hsp90β and other species. The rodents show the highest sequence similarity with respect to human Hsp90β protein. (c) Hsp90α is a 732-amino acid long protein while Hsp90β has 724 amino acids. (d) It can be clearly seen in the stick diagram that 8 amino acids (7–11 and 237–239) present in Hsp90α are missing in Hsp90β
3 Hsp90 Expression Profile
The isoform specificity is not restricted only to the biochemical level but extends to the functional role of Hsp90 in cell differentiation and development. On one hand Hsp90α has been shown to play a regulatory role in muscle cell differentiation of zebra fish while on the other hand it is shown to inhibit cellular differentiation of embryonal carcinoma cells to trophectoderm (Lele et al. 1999; Sreedhar et al. 2004). Hsp90β has been shown to play a major role in trophoblast differentiation, and Hsp90β-deficient homozygous mice with normal expression of Hsp90α failed to differentiate to form placental labyrinths (Voss et al. 2000). Hsp90β overexpression is observed throughout the germ cell lineage from very early stages of development to adult oocytes and spermatocytes (Hilscher et al. 1974) thereby indicating expression in different stages of development, and suggestive of the fact that Hsp90β is required for early embryonic development. Thus, there are several differences between Hsp90 isoforms in cell differentiation and embryonic development in various organisms. Hsp90α expression is lower compared to Hsp90β in most cells. Hsp90α is highly inducible in contrast to Hsp90β whose expression is thought to be constitutive (Hilscher et al. 1974; Gruppi et al. 1991).
Earlier studies from my group (Pires and Khole 2009a; Pires et al. 2011a) using isoform specific antibodies showed that Hsp90β is the predominant form in the ovary, while the testes express Hsp90α form. This data was supported by a Western blot as shown in Fig. 12.2, which clearly demonstrates the predominance of the beta isoform of Hsp90 in ovarian protein extracts (lane 1). No immunoreactivity to the 90 kDa locus was seen when the blot was probed with commercially available Hsp90α polyclonal antibody (lane 2), thereby suggesting Hsp90β to be the predominant isoform in ovary. Mouse testes extract was used as a positive control for Hsp90α (lane 3). Data from mass spectrometry analysis of the immunoreactive dominant EP90 protein demonstrated dominance of Hsp90β peptides where 15 of the 18 LC/MS generated peptides and 9 of the 12 MS/MS generated peptides, other 2 peptides in each MS run belong to Hsp90α (Pires and Khole 2009a). Immunohistochemical (IHC) analysis using immunoreactive patient serum containing Hsp90 AOAs against a panel of multiple rodent tissue sections as seen in Fig. (12.3) shows strong immunoreactivity not only to oocyte ooplasm (panel O) but also to spermatogonial and spermatocytes of testes (panel N) and cilia of principal cells in epididymis (panel M). Since the mass spectrometry data revealed major beta peptides and minor alpha peptides, antibody cross reactivity to the alpha form in the male reproductive system is of a high probability keeping in mind the polyclonal nature of human serum antibodies.
Hsp90α and Hsp90β gamete specific expression. Hsp90β is the predominant isoform present in the ovary. Western blot using commercially available antibodies demonstrates the predominance of the beta isoform of Hsp90 in ovarian protein extracts (lane 1). No immunoreactivity to the 90 kDa locus was seen when the strip was probed with Hsp90α commercially available polyclonal antibody (lane 2), thereby suggesting Hsp90β to be the predominant isoform in ovary. Mouse testes extract was used as a positive control for Hsp90α (lane 3). A ‘no primary’ control showed no immunoreactivity to any of the ovarian proteins (lane 4). GAPDH served as an equal loading control
Multiple tissue immunohistochemistry with Hsp90 specific immunoreactive patient serum antibodies. IHC using a Hsp90β AOA positive patient serum against a panel of multiple rodent tissue sections shows immunoreactivity oocyte ooplasm (panel O), to spermatocytes of testes (panel N) and cilia of principal cells in epididymis (panel M) indicating that the protein could be a reproductive specific protein
4 Clinical Significance of Hsp90
4.1 Hsp90 Expression in Diseases
Tumor cells seem to be in a ‘stressed’ state, which puts additional pressure on controlling proteostasis. Hsp90 plays an important part in the survival of cancer cells, also because of their extensive dependence on Hsp90-assisted signaling pathways (Schopf et al. 2017). In breast cancer, Hsp90 up-regulation has been found as being linked to the expression of the estrogen receptor and Her-2/Erb-B2 associated with bad prognosis and decreased survival (Pick et al. 2007). A study with 52 epithelial ovarian carcinomas revealed an association of the Hsp90 expression with higher stages, but not with prognosis indicating that it might be a reliable indicator of aggressiveness (Elpek et al. 2003).
Studies on the predictive role of Hsp90 in prostate cancer are shown to be inconsistent. On the one hand, abnormal up-regulated levels of Hsp90 have been observed in human prostatic carcinoma cells with a stage-dependent and malignancy-dependent expression (Cardillo and Ippoliti 2006; Cornford et al. 2000). On the other hand, no significant association between Hsp90 expression and conventional diagnostic factors could be detected in 193 patients with clinically organ-confined prostate cancer who underwent radical prostatectomy without any neoadjuvant therapies (Miyake et al. 2010).
In a study on Hsp90α-deficient male mice (Grad et al. 2010), testicular atrophy and infertility was observed which was mediated likely due to apoptosis of spermatocytes, indicating that the Hsp90α isoform is required for spermatogenesis. A conditional deletion of Hsp90α showed that the chaperone is essential for germ cell development in mature adult testes (Kajiwara et al. 2012). Hsp90 was shown to be localized in the neck, midpiece, and tail regions of human sperm, and its expression increased during capacitation thus suggesting roles in intracellular calcium homeostasis, protein tyrosine phosphorylation regulation, and progesterone-stimulated sperm function (Li et al. 2014). Hsp90 (pro-apoptotic in nature) was shown to play an important role in apoptosis in mitochondrially-mediated aging and male infertility (Purandhar et al. 2014). This study clearly indicated a lucid expression and functional role of the Hsp90 during spermatogenesis and/or the process of aging.
4.2 Hsp90 in Ovarian Autoimmunity
With a continuous interest in ovarian biology (Pires et al. 2013), female reproductive tract diseases (Pires et al. 2015) and the role Hsp90 plays in reproductive immunology (Pires 2010; Pires 2017), our laboratory focused on identifying targets that played a role in female reproduction and infertility. Efforts were spent on establishing a diagnostic test to detect serum anti-ovarian antibodies (AOA) which could have a pathological role in this disease thereby leading to premature ovarian failure (POF) or insufficiencies (POI) and we were keen in identifying the proteins under target (Pires et al. 2006). The specificity of existing immunoassays detecting these AOAs were questioned. We reported earlier on the presence of naturally occurring anti-albumin antibodies as the likely factor for non-specificity (Pires et al. 2006). Having developed a novel blocking recipe, we show substantial elimination of this non-specificity. Subsequently using patient sera we reported multiple targets at the protein and histological levels. Our study demonstrated that 15 of 50 (30%) patients with POF/POI and 13 of 50 (26%) in vitro fertilization- embryo transfer (IVF-ET) patients showed the presence of these AOAs (Pires et al. 2011b). Western blotting showed a large number of patients making AOAs to a 90-kDa protein, followed by 97-kDa and 120-kDa proteins (Pires et al. 2007; Pires and Khole 2009a, b). The immunodominant 90-kDa protein was found to be conserved across species, was serine–threonine phosphorylated, and was expressed from the primordial stage to the Graafian-stage ooplasm of the oocytes during follicular development (Pires and Khole 2009a).
Using high-throughput proteomic technologies like liquid chromatography/mass spectrometry (LC-MS), matrix-assisted laser desorption/ionization time-of-flight/time-of-flight (MALDI-TOF/TOF), and tandem mass spectrometry analysis revealed the identity of this protein to be Hsp90β (Pires and Khole 2009a). Using an immunoreactive Hsp90β patient sera to immunostain isolated mouse germinal vesicle breakdown oocyte (GVBD eggs) and growing blastocyst, expression of this protein was seen in the cytosol of the oocytes (ooplasm) as well as cells of the inner cell mass/trophectoderm as seen in Fig. (12.4a, b) respectively. Commercially available recombinant protein immunoreacted with the sera from patients with AOAs against the 90-kd antigen. In parallel, using monoclonal antibody to human Hsp90, we found that it reacts with the eluted protein from a crude ovarian extract (Pires and Khole 2009a). With an aim to identify potential immunoreactive Hsp90 epitopes using patient sera containing AOA to Hsp90, experimentally and statistically peptide EP6 (amino acids 380–389) seems to be the major antigenic epitope followed by EP1 (amino acids 1–12) and EP8 (amino acids 488–498). Predicted 3D structures of these peptides demonstrated that they exist in the loop conformation which is the most mobile part of the protein. Also, analysis of the sequences of Hsp90 beta across several species reveals that EP6 peptide forms a part of a well conserved motif. The polyclonal antibody generated to the immunodominant epitope-EP6 confirmed similar biochemical and cellular immunoreactivity as seen with the patients’ sera having anti-Hsp90 autoantibodies (Pires et al. 2011a). The study proposed options to generate new tools for the detection of disease-inducing epitopes and a possible therapeutic intervention. Since our initial evidence suggested the involvement of Hsp90β in human ovarian autoimmunity, identifying the protein was a significant causative factor in early ovarian failure. These observations were supported by results from a mouse model in which fertilization and embryo development were highly disrupted in animals immunized with in vitro generated Hsp90 antibodies. The study showed that there was a significant drop in the fertility index due to an increase in pre- and post-implantation loss, associated with an increased incidence of degenerated eggs and embryos. The ovaries showed an increase in the number of empty and degenerated follicles and extensive granulosa cell deaths, which was reflected by the decrease in the levels of Nobox and Gja1 gene expression. (Choudhary and Khole 2013).
Hsp90β protein expression in mouse oocytes and developing embryos. (a) Indirect immunofluorescence using an immunoreactive patient sera to Hsp90β protein shows strong ooplasm reactivity (green stain for Hsp90β, red is counterstain for DNA) to the cytoplasm of the germinal vesicle breakdown oocyte. (b) As the embryo develops and transforms to the blastocyst stage, it was seen that, apart from a strong signal (green stain for Hsp90β) in the inner cell mass, slight immunostaining also was seen in the cells of the trophectoderm. Neither the control sera nor the secondary alone control showed immunoreactivity to any of the stages in embryogenesis or to the cumulus cells (data not shown here)
In view of this, we proposed that as a result of a prolonged or repeated asymptomatic chronic infection early in the life of these infertile women they could have anti-Hsp90 antibodies in circulation. In the course of their reproductive life, these antibodies could then target the ovarian antigens (exposed to the immune system due to reasons such as accidents or trauma, also immune system has memory) leading to early ovarian failure (Pires 2017). This may be relevant to human reproduction, since many couples with fertility problems have had a previously undetected genital tract infection (Witkin et al. 1994a). In general, HSP are among the first proteins produced during embryogenesis (Bensaude and Morange 1983). Constitutive form of Hsp90 is known to be expressed at high levels during preimplantation mouse embryo development (Loones et al. 1997). Therefore presence of anti Hsp90 antibodies in women aiming for pregnancy is likely to have detrimental consequences. Women who have antibodies to Hsp90 in their circulation, the possible interactions between Hsp90 and the cytoskeletal proteins (such as actin and tubulin) may be disturbed and destroyed. Thus, there could likely be a collapse of the ovarian cytoarchitecture as the main role of Hsp90 as a chaperone is to maintain this cytoskeletal framework (Pires 2017).
4.3 Hsp90 and Immunotherapy
Immunotherapies involving patients own T-cells have shown immense potential as new treatment strategies. Although inhibition of Hsp90 has received attention for therapeutic purposes in solid tumors and hematologic malignancies, Hsp90 inhibition has shown limited responses as single agents in cancer patients (Pacey et al. 2012; Solit et al. 2008). Although success rates vary, there is a need for improvement with the combinatorial approach. Hwu’ group at MD Anderson recently discussed enhancement of cancer immunotherapy utilizing Hsp90 inhibition through an upregulation of interferon response genes (Mbofung et al. 2017). The highlights of this study include: [a] Hsp90 inhibition shows enhanced T-cell killing of tumors, [b] This mechanism is mediated via IFN-induced protein with tetratricopeptide repeats [IFIT genes], [c] Thereby potentiating immune checkpoint blockade therapy, [d] Combining with anti-CTLA4 enhances CD8 T-cell function. All in all, evidence that Hsp90 inhibition can potentiate T-cell-mediated anti-tumor immune responses and supports exploration of the combination of immunotherapy and Hsp90 inhibitors in the clinic was established.
An interesting paper was presented at the Society for immunotherapy of cancer (SITC; Fecek et al. 2015) where the group reported treatment of melanoma cell lines in vitro or in vivo with Hsp90 inhibitor, lead to the rapid proteasome-dependent degradation of Hsp90 client proteins, with the resulting peptides used to load MHC class I complexes on the tumor cell surface, thereby conditionally-enhancing specific CD8+ T cell recognition. These results suggested that a polyepitope vaccine based on BRAFi-resistance associated Hsp90 client proteins could define a novel immunotherapeutic strategy for the co-treatment of patients with advanced-stage melanomas. Thus, there is a growing wealth of evidence indicating the focal role of Hsp90 in tumorigenesis.
5 Conclusions
Hsp90 is one of the oldest proteins found in all organisms ranging from the simplest prokaryotic bacteria to the highly complexed eukaryotic human beings. The amino acid compositions have not changed dramatically as HSP of highly divergent species are similar to each other and thus is one of the classical evolutionary proteins studied till date. It’s solely ascertained functions viz., molecular chaperoning and stress-elicited responses still holds good but studies have described newer functional predictions and thus Hsp90 rightly fits under “moonlighting” category. Of interest to many is to be able to differentiate Hsp90 in its “housekeeping” role in comparison to its “pathobiochemical role”. Exploiting its structure-function towards human health and disease is of immense importance and attempts are being made by the Hsp90 pioneers towards this effect.
Abbreviations
- AOA:
-
Anti-ovarian antibodies
- GVBD:
-
Eggs, germinal vesicle breakdown oocyte
- Hsp:
-
Heat shock proteins
- IHC:
-
Immunohistochemistry
- IVF-ET:
-
In vitro fertilization- embryo transfer
- LC-MS:
-
Liquid chromatography/mass spectrometry
- MALDI-TOF/TOF:
-
Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight
- POF:
-
Premature ovarian failure
- POI:
-
Primary ovarian insufficiency
References
Bardwell, J. C. A., & Craig, E. A. (1987). Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 84, 5177–5181.
Bensaude, O., & Morange, M. (1983). Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. The EMBO Journal, 2, 173–177.
Cardillo, M. R., & Ippoliti, F. (2006). IL-6, IL-10 and HSP-90 expression in tissue microarrays from human prostate cancer assessed by computer-assisted image analysis. Anticancer Research, 26, 3409–3416.
Chadli, A., Graham, J. D., Abel, M. G., et al. (2006). GCUNC45 is a novel regulator for the progesterone receptor/Hsp90 chaperoning pathway. Molecular and Cellular Biology, 26(5), 1722–1730.
Chadli, A., Felts, S. J., & Toft, D. O. (2008). GCUNC45 is the first Hsp90 co-chaperone to show α/β isoform specificity. Journal of Biological Chemistry, 283(15), 9509–9512.
Choudhury, A., & Khole, V. V. (2013). Hsp90 antibodies: a detrimental factor responsible for ovarian dysfunction. American Journal of Reproductive Immunology, 70(5), 372–385.
Cornford, P. A., et al. (2000). Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Research, 60, 7099–7105.
Csermely, P., Schnaider, T., Soti, C., Prohaszka, Z., & Nardai, G. (1998). The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacology & Therapeutics, 79, 129–168.
Elpek, G. O., Karaveli, S., Simsek, T., Keles, N., & Aksoy, N. H. (2003). Expression of heat-shock proteins Hsp27, Hsp70 and Hsp90 in malignant epithelial tumour of the ovaries. APMIS, 111, 523–530.
Falsone, F. S., Bernd, G., Florian, T., Anna-Maria, P., Andreas, J., & Kung, l. (2005). A proteomic snapshot of the human heat shock protein 90 interactome. FEBS Letters, 579, 6350–6354.
Fecek, R. J., Simeng, W., & Walter, J. S. (2015). Immunotherapeutic targeting of Hsp90 client proteins in BRAF-inhibitor resistant melanoma. Journal for ImmunoTherapy of Cancer, 3(Suppl2), 432.
Gallucci, S., & Matzinger, P. (2001). Danger signals: SOS to the immune system. Current Opinion in Immunology, 13, 114–119.
Grad, I., Cederroth, C. R., Walicki, J., et al. (2010). The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One, 5(12), e15770.
Gruppi, C. M., Zakeri, Z. F., & Wolgemuth, D. J. (1991). Stage and lineage-regulated expression of two hsp90 transcripts during mouse germ cell differentiation and embryogenesis. Molecular Reproduction and Development, 28(3), 209–217.
Gupta, R. S. (1995). Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Molecular Biology and Evolution, 12, 1063–1073.
Hilscher, B., Hilscher, W., Bulthoff-Ohnolz, B., Kramer, U., Birke, A., Pelzer, H., & Gauss, G. (1974). Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell and Tissue Research, 154, 443–470.
Kajiwara, C., Kondo, S., Uda, S., et al. (2012). Spermatogenesis arrest caused by conditional deletion of Hsp90α in adult mice. Biology Open, 1(10), 977–982.
Kampinga, H. H., Hageman, J., Vos, M. J., Kubota, H., Tanguay, R. M., Bruford, E. A., et al. (2009). Guidelines for the nomenclature of the human heat shock proteins. Cell Stress & Chaperones, 14(1), 105–111.
Karras, G. I., Song, Y., Sahni, N., Máté, F., Jenny, X., Marc, V., Alan, D., Luke, W., & Lindquist, S. (2017). Hsp90 shapes the consequences of human genetic variation. Cell, 168(5), 856–866.
Lele, Z., Hartson, S. D., Martin, C. C., Whitesell, L., Matts, R. L., & Krone, P. H. (1999). Developmental Biology, 210, 56–70.
Li, J., Soroka, J., & Buchner, J. (2012). The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1823(3), 624–635.
Li, K., Xue, Y., Chen, A., Jiang, Y., Xie, H., Shi, Q., et al. (2014). Heat shock protein 90 has roles in intracellular calcium homeostasis, protein tyrosine phosphorylation regulation, and progesterone-responsive sperm function in human sperm. PLoS One, 9(12), e115841.
Loones, M. T., Rallu, M., Mezger, V., & Morange, M. (1997). HSP gene expression and HSF2 in mouse development. Cellular and Molecular Life Sciences, 53, 179–190.
Mbofung, R. M., McKenzie, J. A., Malu, S., et al. (2017). Hsp90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nature Communications, 8, 451.
Millson, S. H., Truman, A. W., Racz, A., et al. (2007). Expressed as the sole Hsp90 of yeast, the α and β isoforms of human Hsp90 differ with regard to their capacities for activation of certain client proteins, whereas only Hsp90β generates sensitivity to the Hsp90 inhibitor radicicol. The FEBS Journal, 274(7), 4453–4463.
Miyake, H., Muramaki, M., Kurahashi, T., Takenaka, A., & Fujisawa, M. (2010). Expression of potential molecular markers in prostate cancer: Correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urologic Oncology, 28, 145–151.
Morimoto, R. I. (1993). Cells in stress: Transcriptional activation of heat shock genes. Science, 259(5100), 1409–1410.
Pacey, S., et al. (2012). A phase II trial of 17-allylamino, 17-demethoxygeldanamycin [17-AAG, tanespimycin] in patients with metastatic melanoma. Investigational New Drugs, 30, 341–349.
Pick, E., et al. (2007). High Hsp90 expression is associated with decreased survival in breast cancer. Cancer Research, 67, 2932–2937.
Pires, E. S. (2010). Multiplicity of molecular and cellular targets in human ovarian autoimmunity an update. Journal of Assisted Reproduction and Genetics, 27, 519–524.
Pires, E. S. (2017). The Unmysterious roles of Hsp90: Ovarian pathology and autoantibodies. In D. MacPhee (Ed.), The Role of Heat Shock Proteins in Reproductive System Development and Function, Advances in Anatomy, Embryology and Cell Biology (Vol. 222, pp. 29–44). Springer.
Pires, E. S., & Khole, V. V. (2009a). A ‘block’ in the road to fertility: Autoantibodies to an immunodominant heat shock protein 90-beta in human ovarian autoimmunity. Fertility and Sterility, 92, 1395–1409.
Pires, E. S., & Khole, V. V. (2009b). Anti- ovarian antibodies: Specificity, prevalence, multipleantigenicity and significance in human ovarian autoimmunity. In Current Paradigm of Reprod Immunol (pp. 159–190). Trivandrum: Research signpost Trivandrum. ISBN: 978-81-308-0373-9.
Pires, E. S., Parte, P. P., Meherji, P. K., Khan, S. A., & Khole, V. V. (2006). Naturally occurring anti-albumin antibodies are responsible for false positivity in diagnosis of autoimmune premature ovarian failure. The Journal of Histochemistry and Cytochemistry, 54(4), 397–405.
Pires, E. S., Meherji, P. K., Vaidya, R. R., Parikh, F. R., Ghosalkar, M. N., & Khole, V. V. (2007). Specific and sensitive immunoassays detect multiple anti-ovarian antibodies in women with infertility. The Journal of Histochemistry and Cytochemistry, 55(12), 1181–1190.
Pires, E. S., Choudhury, A. K., Idicula-Thomas, S., & Khole, V. V. (2011a). Anti-Hsp90 autoantibodies in sera of infertile women identify a dominant, conserved epitope EP6 (380–389) of Hsp90 beta protein. Reproductive Biology and Endocrinology, 9, 16.
Pires, E. S., Parikh, F. R., Mande, P. V., Uttamchandani, S. A., Savkar, S., & Khole, V. V. (2011b). Can anti-ovarian antibody testing be useful in an IVF-ET clinic? Journal of Assisted Reproduction and Genetics, 28(1), 55–64.
Pires, E. S., Hlavin, C., Macnamara, E., Ishola-Gbenla, K., Doerwaldt, C., Chamberlain, C., Klotz, K., Herr, A. K., Khole, A., Chertihin, O., Curnow, E., Feldman, S. H., Mandal, A., Shetty, J., Flickinger, C., & Herr, J. C. (2013). SAS1B protein [Ovastacin] shows temporal and spatial restriction to oocytes in several eutherian orders and initiates translation at the primary to secondary follicle transition. Developmental Dynamics, 242, 1405–1426.
Pires, E. S., D’Souza, R., Needham, M., Herr, A., Jazaeri, A., Li, H., Stoler, M., Anderson-Knapp, K., Thomas, T., Mandal, A., Gougeon, A., Flickinger, C., Bruns, D., Pollok, B., & Herr, J. C. (2015). Membrane associated cancer-oocyte neoantigen SAS1B/ovastacin is a candidate immunotherapeutic target for uterine tumors. Oncotarget, 6(30), 30194–30211.
Purandhar, K., Jena, P. K., Prajapati, B., Rajput, P., & Seshadri, S. (2014). Understanding the role of heat shock protein Isoforms in male fertility, Aging and Apoptosis. The World Journal of Men’s Health, 32(3), 123–132.
Röhl, A., Rohrberg, J., & Buchner, J. (2013). The chaperone Hsp90: Changing partners for demanding clients. Trends in Biochemical Sciences, 38(5), 253–262.
Schopf, H. F., Maximilian, M. B., & Johannes, B. (2017). The Hsp90 chaperone machinery. Nature Reviews Molecular Cell Biology, 18, 345–360.
Solit, D. B., et al. (2008). Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clinical Cancer Research, 14, 8302–8307.
Sreedhar, S. A., Kalmar, E., Csermely, P., & Shen, Y. F. (2004). Hsp90 isoforms: functions, expression and clinical importance. FEBS Letters, 562, 11–15.
Website: http://www.picard.ch/downloads/downloads.htm
Website: https://www.genenames.org/cgi-bin/genefamilies/set/586
Voss, A. K., Thomas, T., & Gruss, P. (2000). Development, 127, 1–11.
Witkin, S. S., Sultan, K. M., Neal, G. S., et al. (1994). Unsuspected Chlamydia trachomatis infections in the female genital tract and in vitro fertilization outcome. American Journal of Obstetrics and Gynecology, 171, 1208–1214.
Zuehlke, A., & Johnson, J. L. (2010). Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers, 93(3), 211–217.
Acknowledgements
The author would also like to place on record his gratitude to his Ph.D. mentor, the late Dr. Vrinda V Khole from the National Institute for Research in Reproductive Health (ICMR), Mumbai, India who mentored him during the course of his graduate degree. The author thanks the journal Fertility & Sterility for permitting reproduction of one figure from his previously published work [Fig. 12.4. Panel B2 and B5 of Pires ES and Khole VV. 2009: A ‘block’ in the road to fertility: autoantibodies to an immunodominant heat shock protein 90-beta in human ovarian autoimmunity. Fertility & Sterility 92:1395–1409]. Thank you to the journal of Reproductive Biology and Endocrinology for allowing him to reproduce one figure from his earlier work [Fig. 12.1a of Pires ES, Choudhury AK, Idicula-Thomas S, and Khole VV. Anti-Hsp90 autoantibodies in sera of infertile women identify a dominant, conserved epitope EP6 (380–389) of Hsp90 beta protein. Reprod Biol Endocrinol. 2011; 9: 16].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Pires, E.S. (2018). Heat Shock Protein 90: Truly Moonlighting!. In: Asea, A., Kaur, P. (eds) Regulation of Heat Shock Protein Responses. Heat Shock Proteins, vol 13. Springer, Cham. https://doi.org/10.1007/978-3-319-74715-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-74715-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74714-9
Online ISBN: 978-3-319-74715-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)