Abstract
Selective dorsal rhizotomy (SDR) is a neurosurgical procedure for the relief of spasticity interfering with motor function in children with spastic cerebral palsy (CP). The goal of the treatment is to improve function as well as reduce pain and discomfort related to severely increased spasticity. SDR is an ablative procedure that results in lifelong effects on function in the central nervous system. One must also be aware that performing SDR does not guarantee that other treatments for spasticity or orthopedic corrective procedures can be avoided. For SDR to be an effective treatment, it must be combined with specific physiotherapy over a long period of time. Today there exists a good body of evidence that SDR is an effective means of treating patients with the CP subtype spastic diplegia, as long as selection criteria are rigorously adhered to. The procedure is also safe with little risk of short or long-term complications. Further studies on long-term effects late in adulthood will show if the treatment effects are stable over time.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Selective dorsal rhizotomy (SDR) is a neurosurgical procedure performed for the relief of spasticity interfering with motor function in children with spastic cerebral palsy (CP). The goal is to improve function and reduce pain and discomfort related to severely increased muscle tone. It is one of the tools for the treatment of muscle hypertonia. The most frequently used neurosurgical treatment is insertion of a pump to infuse intrathecal baclofen (ITB). Deep brain stimulation (DBS) has also been an option during the last 10 years; ITB and DBS are mostly used in cases where a dystonic component is involved.
SDR is the only treatment that reduces spasticity permanently, giving new prerequisites for functional training and contracture prevention. Together with goal-directed physiotherapy, SDR improves motor function development in carefully selected cases (HQO 2017). SDR is included among the rare interventions in CP that have enough scientific evidence to be fully recommended (Novak et al. 2013). Selective dorsal rhizotomy (SDR) is now performed at the lumbosacral level on children diagnosed with bilateral spastic cerebral palsy (BSCP), but the intervention has also been used in adults with BSCP and in unilateral spastic CP. In this chapter, we will only discuss lumbosacral SDR for spasticity management in BSCP, specifically the type of BSCP with more severe involvement of the lower than of the upper limbs (CP spastic diplegia) including all levels of gross motor function defined by GMFCS levels I–V.
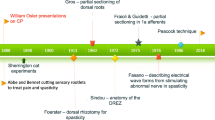
Some History
Dorsal root rhizotomy as a treatment for spasticity is an old procedure. The method was introduced by Foerster (1911) and was based on experimental findings from Sherrington’s work with a spasticity model in cats (Sherrington 1898). In Foerster’s procedure, electric stimulation was used to identify dorsal roots from L2 to S2 that were then subsequently completely cut. He also described in this study that postoperative physiotherapy was mandatory for a good outcome. Gros et al. (1973) introduced some measure of selectivity in the procedure by only cutting 80% of the dorsal roots between L1 and S1. The method was further developed and refined by Fasano et al. (1979). Outcome was improved by introducing intraoperative EMG to determine which rootlets should be cut. However, Fasano’s method still entailed bladder dysfunction since S2 was included in the sectioning. Peacock and Arens (1982) made further improvements on Fasano’s method by changing the surgical approach from Th12–L2 to L2–L5, thereby making it easier to identify sacral nerve roots and subsequently reducing the risk of bladder dysfunction. The selective dorsal rhizotomy procedure as described by Peacock and Arens, including indications and contraindications for the intervention, has now been used in many countries around the world for the last 30 years. During this period, some centers have adopted Park’s modification of the procedure, which entails a more limited laminectomy at the conus level coupled with EMG (Park and Johnston 2006).
In the beginning of the modern SDR era, expectations on outcome were high among many professionals. However, after a decade or two, disappointment with the results increased in many centers. Severe lumbar lordosis and frequent occurrence of spinal problems in the SDR-operated patients were reported even if there hadn’t been control groups (HQO 2017). Spinal problems in other patients with spastic diplegia are also common (Harada et al. 1993). At the same time, as the reports of disappointing results were being published for SDR, new treatments for spasticity were introduced. Intrathecal baclofen administered via implanted pumps (ITB) and intramuscular injections with botulinum toxin became widely used and were seen as attractive alternatives since they were methods that were not based on permanent lesions in the patient’s nervous system. Since the indications for these two therapies and SDR partially overlap, many centers abandoned the SDR procedure.
Other centers that had experienced good results, probably because of strict adherence to indications and contraindications for the operation as described by Peacock and Staudt (1991), continued to perform SDR. A comparative meta-analysis from three randomized controlled trials (RCTs), comparing SDR with intense physiotherapy (PT) against controls receiving only the same PT intervention as the operated patients, showed clinically and statistically significant motor function improvement up to 2 years after inclusion in the study (MacLaughlin et al. 2002).
Scientific reporting on long-term outcome has increased steadily, but skepticism toward SDR still remains among many professionals working with CP patients. Despite this skepticism, the amount of operations has increased during recent years, probably because of increased awareness among parents and patients that the procedure exists (the Internet) and that the operation has become regarded as an evidence-based intervention (Novak et al. 2013; HQO 2017).
Parallel to increased knowledge on outcome from long-term follow-up of surgery over the past three decades, a greater understanding of natural gross motor development in children with CP has developed. This has been possible through the Ontario motor growth study (OMG study) and the creation of the OMG curves (Rosenbaum et al. 2002). The OMG curves are based on long-term follow-up using valid and reliable classifications and measurements of motor function, the Gross Motor Function Classification System (GMFCS) (Palisano et al. 1997), and the Gross Motor Function Measure (Russel et al. 2002). Both of these were developed at CanChild. The OMG study sample included all CP subtypes and excluded children with ITB or SDR treatment.
The OMG motor growth curves show that the average gross motor development, as measured with GMFM-66, levels off much earlier than previously recognized and occurs earliest in the most severe CP (GMFCS level V) at 2–3 years of age and latest in milder CP (GMFCS I) just before 5 years of age. After plateauing of the gross motor development, deterioration was seen in the GMFCS III–V groups between about 7 years and 13–15 years of age (Hanna et al. 2009). Increasing body size with age and muscle weakness (a part of the CP syndrome), malnutrition, contracture development, and pain were shown to be major causes of this deterioration (Bartlett et al. 2010). Continuing decrease of motor function, pain, and fatigue are described in several cross-sectional studies which include adults with comparatively good motor function during childhood, GMFCS levels I–II (Opheim et al. 2009).
Treatment
Patient Selection
As stressed in this chapter, correct patient selection is essential for achieving good results with SDR. The potentially beneficial effect of spasticity must be evaluated when assessing the patient. Spasticity may be needed for antigravity control as it can compensate for muscle weakness and even high levels of spasticity may not interfere with function or well-being. Since SDR is an irreversible intervention that has lifelong consequences and can lead to deterioration of motor function if selection criteria are not adhered to, all aspects of the patient’s function must be evaluated prior to surgery.
A review of the literature shows that selection of patients for SDR varies in different studies (Grunt et al. 2013). Some centers focus on ambulant young children (GMFCS I–III, 2–7 years), while other centers have wider ranges regarding age and included all GMFCS levels. The contraindications for SDR also vary between centers. The selection criteria and procedures are not consistently described, classified, or quantified (Grunt et al. 2013). The decision procedures and criteria used in the SDR programs in Lund and Uppsala, Sweden, are described here.
Decision Procedure
A child where spasticity is judged to be a major underlying problem for motor function should be referred to a clinic with a broad experience of the different treatments for motor problems. A thorough medical history and physical examination should be performed by both a physiotherapist and a pediatric neurologist. Repeated examinations are often necessary. The child’s motor function is assessed in many situations and activities and with different instruments including the GMFM. The GMFCS level should be determined. The level of spasticity is quantified according to the methods of Ashworth and Bohannon or Tardieu (Bohannon and Smith 1987; Gracies et al. 2000). Tendon reflexes and presence of clonus are noted. Goniometry of joints in the legs should be performed.
The underlying diagnosis of CP should be verified. In most of the cases, the diagnosis is supported by neuroimaging.
The presence of the parents at the examination is very important. It is of great advantage if the child’s personal physiotherapist can participate in the evaluation. When SDR or ITB are considered as treatment options, the child should meet the entire multidisciplinary team (pediatric neurologist, physiotherapist, neurosurgeon, and orthopedic surgeon) for further evaluation.
For children regarded suitable for SDR, some special aspects of the evaluation are very important. Muscle strength in the trunk and legs should be evaluated in various activities. Selective motor control is also estimated as well as trunk stability and balance in the sitting position. If the muscle strength is suspected to be low, the child can be put on a training program for a few months and then reevaluated. The ability of the child and family to cooperate in intensive physiotherapy, for mobilization and future training, is of utmost importance to be evaluated as well as the possibility to organize such training in the long term.
In Lund and Uppsala, the criteria proposed by Peacock and Staudt (1991) are used in the selection process. Presence of ataxia and dystonia is regarded as a contraindication as well as severe contractures and previous orthopedic operations (except for adductor tenotomy). Hip dislocation and spinal abnormalities are in most cases contraindications for SDR in ambulant children. Cognitive disability is not seen as a contraindication as long as the child shows a drive to improve and willingness to train motor activities. Three through seven years of age are considered as optimal timing for surgery, at least in ambulant children (GMFCS I–III). Children with any GMFCS level could be considered as candidates if the spasticity is judged to be the main problem and if there is no major contraindication (such as dystonia or a severe spinal abnormality). In Uppsala, ITB treatment is usually chosen for children with GMFCS III–IV and severe spasticity, while in Lund, SDR is also recommended for these children provided that there are no dystonic traits.
To set realistic individual functional goals for the procedure are very important. The goals are of course related to the GMFCS level of the child. It is important to discuss the goals in detail with the parents and also with the local physiotherapist. SDR should also always be combined with long-lasting physiotherapy in order to reach the best possible result.
It is very important to have a detailed documentation of the child’s motor function using the methods described above. The findings of the preoperative evaluation are also important for the neurosurgeon and neurophysiologist as a basis for decisions during the operation.
Preoperative X-ray of the spine is often valuable. If there is any doubt regarding the CP diagnose, neuroimaging is sometimes relevant and has in many cases been performed earlier. If earlier imaging exists, a reevaluation of previous imaging is recommended to look for changes in the thalamus, basal ganglia, or cerebellum which are contraindications for the operation. Findings of white matter injury of immaturity on imaging strengthen the clinical diagnosis of spastic diplegia. Since the procedure is on the lumbosacral level, continence functions should be evaluated, and a bladder scan of the urinary bladder should be performed. Standard preoperative blood tests should be taken.
General Selection Criteria for SDR
-
A confirmed diagnosis of cerebral palsy. There must not be any signs of progressive brain disorder and no neurometabolic disorder. CP due to postneonatally acquired brain injury is excluded.
-
Confirmed subtype spastic diplegia, as classified in the Swedish classification of CP subtypes (Westbom et al. 2007), with more involvement of the legs than of the arms.
-
Pure spasticity. CP subtypes are classified according to “dominant neurological symptom” meaning that also other neurological signs may coexist with the dominating spasticity in spastic CP. Ataxic or other than subtle dyskinetic signs are regarded as contraindications for SDR.
-
Absence of dystonic traits or dystonia is an important selection criterium. Dystonia may be mistaken for spasticity. Spasticity is also often present in the dystonic CP subtype, although dystonia is the dominating neurological sign. Foot clonus and positive Babinski are often present in dystonic CP, often times with more intense clonus, toe-spread, and spontaneous Babinski sign than you see in pure spastic CP. Young children with dystonia (tonus-changing CP) are often relaxed during sleep. Another clinical sign of dystonia is that the child may stiffen especially when held standing in an upright position. Children with CP after severe asphyxia at term usually have hyperkinesia and/or dystonia, and some even have severe spasticity. SDR is contraindicated in these patients. Children with CP following term asphyxia have neonatal hypoxic ischemic encephalopathy often evident in the thalamus and basal ganglia on brain MRI.
-
Spasticity interfering with functional development – operationalized as:
-
1.
Spasticity involving both proximal and distal muscle groups in the lower parts of the body with difficulties to preserve joint range of motion (PROM), despite full conservative treatment (physiotherapy, orthoses, plasters)
-
2.
Poor sitting ability with pelvis tilt due to spasticity, with too small sitting base and rounded spine (thoracolumbar kyphotic position)
-
3.
Periodic functional improvement and less muscle pain with repeated injections of botulinum toxin, but with too little or too short effect, or with problems to carry out regular repeated treatments
-
1.
-
Enough muscle control and strength under the spasticity to reach the individually formulated goals. Previous lengthening operations of Achilles tendons or hamstring muscles are contraindications for SDR if the goals include standing transfers, standing without support, or walking.
-
Available physiotherapy and orthotic services and willingness to perform pre- and postoperative functional training activities and to use orthoses when needed
-
Any gross motor function level – GMFCS I–V
Individual Selection Criteria for SDR
Every child’s individual goals, as expressed by themselves or for young children by the parents, are important parts of the selection criteria. The goals should be established by the family and the child’s personal PT together with the SDR team. The goals should be based on the child’s actual function and the child’s and family’s priorities.
General goals in GMFCS I–II are improved balance, endurance, and flexibility in standing, walking, running, and jumping; in GMFCS III stability and variability in sitting, to attain and maintain standing, walking, and enabling self-propelled wheeled transfers; and for GMFCS IV–V independent sitting, supported standing, enabling wheelchair transfers, and to ease daily burden for caregivers. Reduction of pain and discomfort from spasticity or inactivity may be a goal for some children in all GMFCS levels. To “preserve joint ROM” is usually not a goal formulated by the child but is important in order to reach many of the mentioned functional goals.
Timing of SDR Surgery
In practice, it is good to operate young children as soon as the diagnosis is clear and the individual child’s development has been followed for some time. Effective contracture prevention becomes easier after SDR and is important at a young age in severe spasticity to avoid nonreversible contractures and tendon-lengthening operations which will reduce muscle power permanently. SDR in early preschool years coincides with ongoing gross motor development and is a time when the child may have more time and interest for the intense physiotherapy needed during the first postoperative year than they have once they have started school. In older children, 6–8 years of age, the initial postoperative period seems to be more demanding before they have regained their usual function and independence.
Depending on individual goals, SDR in pure spastic CP may be beneficial also in older age, even in adults, but this and other new indications have to be scientifically evaluated.
Surgical Procedure
Under general anesthesia, the patient is placed in the prone position. Muscle relaxants should not be used after induction of anesthesia. Appropriate bolstering is placed under the chest and pelvis. EMG needle electrodes are inserted into muscles bilaterally including all spinal root levels that are potential candidates for sectioning (see description neurophysiology strategy).
Access to the spinal canal follows standard neurosurgical procedures for midline approach to the intradural space. Today, SDR is commonly performed through single-level laminectomy (Park and Johnston 2006)/laminoplasty (Funk and Haberl 2016) at the L1 level or multilevel laminoplasty at L1–L5. Peacock and Arens’ (1982) original approach was a multilevel laminectomy L2–S1 but has been replaced by single-level laminectomy/laminoplasty or multilevel laminoplasty instead of laminectomy to reduce the risk of developing spinal deformity (Turi and Kalen 2000; Johnson et al. 2004; Spiegel et al. 2004). The single-level approach is considered to be more difficult because of the limited exposure to the nerve roots that it gives at the conus level. With the multilevel opening, there are clear anatomical landmarks since the nerves can be followed to the level where they exit the spinal canal.
Next the dura mater is opened in the midline.
Once the dura is open, nerve stimulation is used to identify the motor and sensory nerve roots for L2–S2 and L1 if sectioning is being considered at this level (i.e., in patients with hip flexor spasticity). This is done with Peacock probes. Silicone nerve retractors, cut silicone strips, or cut surgical cottonoids can be used to keep the sensory and motor nerves separated once they have been identified. The sensory roots are then subdivided into rootlets using sharp dissection.
At this point, a systematic stimulation of the afferent rootlets with intraoperative EMG is performed for each level. Pathological rootlet response is graded according to the degree of spread beyond the nerve’s normal myotome. This stimulation response is evaluated using both neurophysiological measures and manual evaluation of relevant muscle response by a physiotherapist. The sensory rootlets with abnormal spread are sectioned but should not exceed 70% of the total amount of rootlets. Different centers describe sectioning percentages that vary between 40 and 80% (Grunt et al. 2013). At the L4 and S2 levels, special considerations should be made concerning muscle strength in the quadriceps muscles and in sphincter responses, respectively.
Following the sectioning of the sensory rootlets, hemostasis is controlled, and the dura is closed in a watertight manner with running sutures. Different means of administering analgesia are used in different centers and include intradural, epidural, or intravenous strategies. If intra- or epidural catheters are used, it is at this stage of the operation that they are put in place. The laminoplasty is performed with sutures or wires. The paravertebral muscles, fascia, and skin are sutured in a conventional manner for spine surgery.
Patient mobilization and physiotherapy can start 2–3 days postsurgery according to local protocols.
Neurophysiologic Intraoperative Monitoring
The key procedure in SDR is repetitive (50 Hz) electrical stimulation of the afferent rootlets for 1 s, which reliably (Mittal et al. 2001) identifies those rootlets that upon stimulation generate pathological reflex activity from those that produce normal muscle reflex activity and consequently should be spared.
Irrespective of the surgical approach to expose the cauda equina, it is imperative to identify the different sensory roots (commonly L2–S2 bilaterally) before longitudinally subdividing the dorsal roots into several natural rootlets. Even if anatomical landmarks are helpful in identifying lumbosacral nerve roots (particularly after L2–S1 laminotomy exposing the entire cauda), it is important to realize that muscle innervation is variable (Phillips and Park 1989) and that sectioning dorsal rootlets based on anatomical textbook knowledge solely is unacceptable in this context. With the aid of intraoperative electrical stimulation and electromyography (EMG), it is possible to identify the different lumbosacral roots and their target myotomes. In case of uncertainty, it is also possible to distinguish posterior from the anterior (motor) roots. Typically, a tenfold higher stimulus intensity is required for a single electrical pulse to elicit a reflex response from a sensory root compared to obtaining a direct motor response from anterior root stimulation. However, the main purpose of this single pulse stimulation technique is usually made with the intent to determine the stimulus threshold for the actual subsequent “spasticity” test (i.e., the 1 s train stimulation of the surgically separated rootlets). Long-lasting muscle relaxants are avoided, and anesthetic regimens that abolish spinal reflex activity should not be used. An indication of suppressed spinal reflex activity related to inappropriate anesthesia level or agent is when test stimulation of anterior roots elicits a motor response, while concurrent stimulation of sensory roots does not.
Ideally, the intraoperative classification into normal or pathological (spastic) reflex motor responses from electrical stimulation should be performed in collaboration with a neurophysiologist and a physiotherapist. Whereas the physiotherapist, positioned close to the patient, is able to observe the strength and pattern of stimulus-induced muscle contractions, the neurophysiologist is provided with records of the corresponding EMG reflex pattern from multiple lumbosacral root myotomes. To avoid common pitfalls, it is important that EMG interpretation is performed by an experienced examiner. For instance, “crosstalk” between different recording sites, i.e., a phenomenon where activity in one muscle is detected in a nearby non-contraction muscle, may easily be misinterpreted as widespread pathological reflex activity. In similar situations, where reports from the physiotherapist and the neurophysiologist contradict each other, stimulation should be repeated and results reassessed. To determine the extent of spasticity, the EMG response from stimulation is graded according to criteria that commonly focus on the spread of reflex activity beyond the spinal segment stimulated (Fasano et al. 1979). This is based on a grading scale from 0 to 4 where the most severe grade 4 represents reflex activity that not only engages multiple ipsilateral spinal levels (grade 3) but also produces contralateral muscle activity. Typically, rootlets producing such abnormal responses from electrical stimulation are sectioned. The physiotherapist, familiar with the patient’s clinical pattern of spasticity, should also be influential in deciding the extent of rootlet sectioning at a given level (Mittal et al. 2001).
The use of intraoperative neurophysiology may also prevent permanent bladder and bowel dysfunction from SDR. For instance, at sacral root levels, reflex activity from the anal sphincter indicates that the stimulated posterior root carry sensory information from the pudendal area. In this situation, it is possible to use another probe for recording sensory nerve action potentials from a single sacral rootlet and examine whether stimulation of pudendal afferents in the genital and/or anal region elicits a response in the recordings (Mittal et al. 2001; Huang et al. 1997) that would in turn preclude sectioning of that rootlet.
In summary, intraoperative neurophysiology is an integral part of SDR and requires expert knowledge in EMG stimulation and recording techniques.
Postoperative Rehabilitation Program
After the operation, there is marked hypotonus and weakness in the lower extremities. The child stays in the hospital for about 10 days and the mobilization follows a tight schedule. In our centers, the child is transferred to the pediatric habilitation unit for a further 2 weeks of intensified physiotherapy.
Initially the physiotherapy is focused on mobilization such as sitting, rolling, and different functional activities. Later on standing is introduced. The training should be incorporated in common daily activities and play. Hydrotherapy can be introduced after the scar has healed.
After the first intensive postoperative weeks, the responsibility for the rehabilitation is transferred to the local habilitation unit. In Lund, this includes repeated assessments and discussions with the SDR team PTs regarding goals, treatment content, and orthoses (Nordmark et al. 2008; Josenby et al. 2014). Recommended frequency of physiotherapy is 1 h twice a week for the first 6 months and thereafter 1 h once a week, but this may vary. Parents and preschool teachers are advised how to include training and contracture prophylaxis (standing shells and other orthoses) into daily and leisure time activities. Improvement in motor function and daily functional performance can be seen many years after SDR (Josenby et al. 2012, 2014).
Expected Outcome
Differences in patient selection between studies as well as the extent and intensity of the postoperative rehabilitation program and other surgical interventions affect the outcome after SDR. Most studies show a positive effect of SDR in carefully selected children, but there are no RCTs with follow-up beyond 1–2 years postoperatively. The results in different studies must also be looked upon in the context of the knowledge of the normal motor development for children with CP, since it has been shown that the capacity to acquire new motor skills levels off at low age (Rosenbaum et al. 2002). The notion in the literature of better results of SDR (as measured by GMFM) in younger than in older children is probably due to this difference in “natural development,” as also the notion that SDR results are better (as measured with GMFM) in GMFCS I–II than in the more severe GMFCS levels.
In a meta-analysis of three RCTs, it was shown that children with spastic diplegia at follow-up about 1 year after SDR had gained more in motor function after SDR in combination with intense physiotherapy than after intense physiotherapy alone (MacLaughlin et al. 2002). Outcome in the short term (1–2 years) after SDR has also been reported in systematic reviews with positive results regarding reduced spasticity, increased passive range of motion in joints in the legs, improved gait, and improvement in gross motor function (Grunt et al. 2011; Novak et al. 2013; Ont Health Technol Assess Ser 2017).
Long-term follow-up reports (>5 years postop) give somewhat conflicting results. There are also few studies with a high scientific level. Most studies however show positive results after SDR. Gross motor function, functional independence, and caregiver assistance have been shown to improve, although most of the improvement takes place during the first years postoperatively. Spasticity is reported to be permanently reduced, but in the systematic reviews, no evidence was found regarding effects in the ICF activity and participation domains. In scientific studies regarding long-term treatment satisfaction from the patients and their relatives, the information is scarce. However, in many studies, parents and children report improvements and satisfaction at follow-up. Future studies with long follow-up periods are needed to further clarify the long-term effects of SDR (Grunt et al. 2011; Novak et al. 2013; HQO 2017).
Complications
SDR has shown to be a safe procedure. Postoperative problems during the first weeks are not uncommon and include dysesthesia/hypoesthesia in the legs, urinary incontinence or retention, urinary tract infections, and constipation. Transient postoperative hypotonia in the lower extremities is also common.
There are few reports regarding manifest lasting continence problems related to SDR. A significant proportion of CP children have urinary incontinence as a part of the clinical picture. Lasting sensory abnormalities after SDR are uncommon (HQO 2017).
An area of concern has been the reports of spinal bony abnormalities and back pain years after SDR. Scoliosis and lumbar lordosis are reported with rather high frequencies. Kyphosis, spondylolysis, and spondylolisthesis are not uncommon but reported to be less common (Turi and Kalen 2000; Johnson et al. 2004; Golan et al. 2007; Langerak et al. 2008). The frequency of spinal abnormalities increases with age in all children with CP and marked spasticity. It is therefore unclear if SDR increases this risk significantly. The proportion of patients that have undergone SDR and later need spinal surgery seems to be low (HQO 2017).
Regarding hip problems, the situation was radiologically stable for most children after SDR on follow-up (HQO 2017).
Conclusions
Lumbosacral SDR is an effective method for permanently reducing spasticity and is not associated with major negative side effects. SDR as a treatment for spastic diplegia in young patients provides lasting functional benefits over a period of at least 10 years postoperatively if the procedure is combined with physiotherapy. A prerequisite for good results is adherence to strict selection criteria and that there is a systematic follow-up of children. A multiprofessional approach is necessary. SDR is a safe procedure since severe peri- and postoperative problems are rare and long-term complications are uncommon.
References
Bartlett DJ, Hanna SE, Avery L, Stevenson RD, Galuppi B (2010) Correlates of decline in gross motor capacity in adolescents with cerebral palsy in Gross Motor Function Classification System levels III to V: an exploratory study. Dev Med Child Neurol 52:e155–e160
Bohannon RW, Smith MB (1987) Interrater reliability of modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Fasano VA, Barolat-Romana G, Zeme S, Sguazzi A (1979) Electrophysiological assessment of spinal circuits in spasticity by direct dorsal root stimulation. Neurosurgery 4:146–151
Foerster O (1911) Resection of the posterior spinal nerve.-roots in the treatment of gastric crisis and spastic paralysis. Proc R Soc Med 4(surg sect):226–246
Funk JF, Haberl H (2016) Monosegmental laminoplasty for selective dorsal rhizotomy- operative technique and influence on the development of scoliosis in ambulatory children with cerebral palsy. Childs Nerv Syst 32:819–825. https://doi.org/10.1007/s00381-016-3016-3
Golan JD, Hall JA, O’Gorman G, Poulin C, Benaroch TE, Cantin MA, Farmer JP (2007) Spinal deformity following selective dorsal rhizotomy. J Neurosurg 106(6 suppl):441–449
Gracies JM, Marosszeky JE, Renton R, Sandanam J, Gandevia SC, Burke D (2000) Short-term effects of dynamic lycra splints on upper limbs in hemiplegic patients. Arch Phys Med Rehabil 81:1547–1777
Gros C, Frerebeau PH, Kuhner A, Perez-Dominguez E (1973) Technical modification in the Foerster operation. Selective posterior lumbar root section. The results of 18 years of practice. 5th International Congress of Neurosurgery, Tokyo 1973
Grunt S, Becher JG, Vermeulen RJ (2011) Long-term outcome and adverse effects of selective dorsal rhizotomy in children with cerebral palsy: a systematic review. Dev Med Child Neurol 53:490–498
Grunt S, Fieggen AG, Vermeulen RJ, Becher JG, Langerak NG (2013) Selection criteria for selective dorsal rhizotomy in children with spastic cerebral palsy: a systematic review of the literature. Dev Med Child Neurol 56:302–312. https://doi.org/10.1111/dmcn.12277
Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, Russel DJ (2009) Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol 51:295–302
Harada T, Ebara S, Anwar MM, Kajiura I, Oshita S, Hiroshima K, et al (1993) The lumbar spine in spastic diplegia. A radiographic study. J Bone Joint Surg Br 75(4):534–537
Health Quality Ontario (HQO) (2017) Lumbosacral dorsal rhizotomy for spastic cerebral palsy: a health technology assessment. Ont Health Technol Assess Ser Jul 17(10):1–186. Available from: http://www.hqontario.ca/evidence-to-improve-care/journal-ontario-health-technology-assessment-series
Huang JC, Deletis V, Vodusek DB, Abbott R (1997) Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery 41(2):411–415
Johnson MB, Goldstein L, Thomas SS, Piatt J, Arona M, Sussman M (2004) Spinal deformity after selective dorsal rhizotomy in ambulatory patients with cerebral palsy. J Pediatr Orthop 24:529–536
Josenby AL, Wagner P, Jarnlo GB, Westbom L, Nordmark E (2012) Motor function after selective dorsal rhizotomy: a 10-year practice-based follow-up study. Dev Med Child Neurol 54(5):429–435. https://doi.org/10.1111/j.1469-8749.2012.04258.x. Epub 2012
Josenby AL, Wagner P, Jarnlo GB, Westbom L, Nordmark E (2014) Functional performance in self-care and mobility after selective dorsal rhizotomy: a 10-year practice-based follow-up study. Dev Med Child Neurol 57(3):286–293. https://doi.org/10.1111/dmcn.12610. Epub 2014
Langerak NG, Lamberts RP, Fieggen AG, Peter JC, van der Merwe L, Peacock WJ, Vaughan CL (2008) A prospective gait analysis study in patients with diplegic cerebral palsy 20 years after selective dorsal rhizotomy. J Neurosurg Pediatr 1(3):180–186. https://doi.org/10.3171/PED/2008/1/3/180
MacLaughlin J, Bjornson K, Temkin N, Steinbok P, Wright V, Reiner A, Roberts T, Drake J, O’Donnell M, Rosenbaum P, Barber J, Ferrel A (2002) Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol 44(1):17–25
Mittal S, Farmer JP, Poulin C, Silver K (2001) Reliability of intraoperative electrophysiological monitoring in selective posterior rhizotomy. J Neurosurg 95:67–75
Nordmark E, Josenby AL, Lagergren J, Andersson G, Strömblad LG, Westbom L (2008) Long-term outcome five years after selective dorsal rhizotomy. BMC Pediatr 8(54):1–15
Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, Stumbles E, Wilson S-A, Goldsmith S (2013) A systematic review of interventions for cerebral palsy: state of the evidence. Dev Med Child Neurol 5:885–910. https://doi.org/10.1111/dmcn.12246
Opheim A, Jahnsen R, Olsson E, Stanghelle JK (2009) Walking function, pain and fatigue in adults with cerebral palsy: a 7-year follow-up study. Dev Med Child Neurol 51:381–388. https://doi.org/10.1111/j.1469-8749.2008.03250.x
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214–223
Park TS, Johnston JM (2006) Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Neurosurg Focus 21(2):E7
Peacock WJ, Arens LJ (1982) Selective posterior rhizotomy for the relief of spasticity in cerebral palsy. S Afr Med J 62(4):119–124
Peacock WJ, Staudt LA (1991) Functional outcomes following selective dorsal rhizotomy in children with cerebral palsy. J Neurosurg 74:380–385
Phillips LH, Park TS (1989) Electrophysiological studies of selective posterior rhizotomy patients. In: Park TS, Phillips LH, Peacock WJ (eds) Management of Spasticity in cerebral palsy and spinal cord injury. Neurosurgery state of the art reviews, vol 4. Hanley and Belfus, Philadelphia, pp 459–469
Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, Wood E, Bartlett DJ, Galuppi BE (2002) Prognosis for gross motor function in cerebral palsy. Creation of motor development curves. JAMA 288:1357–1368
Russel DJ, Rosenbaum PL, Avery LM, Lane M (2002) Gross motor function measure (GMFM-66 and GMFM-88) user’s manual. Mac Keith Press, London
Sherrington CS (1898) Decerebrate rigidity and reflex coordination of movements. J Physiol Lond 22:319–337
Spiegel DA, Loder RT, Alley KA, Rowley S, Gutknecht S, Smith-Wright DL, Dunn ME (2004) Spinal deformity following selective dorsal rhizotomy. J Pedriatr Orthop 24(1):30–36
Turi M, Kalen V (2000) The risk of spinal deformity after selective dorsal rhizotomy. J Pediatr Orthop 20:104–107
Westbom L, Hägglund G, Nordmark E (2007) Cerebral palsy in a total population of 4-11 year olds in southern Sweden. Prevalence and distribution according to different CP classification systems. BMC Pediatr 7:41
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Nilsson, P., Wesslén, N., Axelsson, H., Ahlsten, G., Westbom, L. (2020). Dorsal Rhizotomy for Spasticity Management in Cerebral Palsy. In: Miller, F., Bachrach, S., Lennon, N., O'Neil, M.E. (eds) Cerebral Palsy. Springer, Cham. https://doi.org/10.1007/978-3-319-74558-9_46
Download citation
DOI: https://doi.org/10.1007/978-3-319-74558-9_46
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74557-2
Online ISBN: 978-3-319-74558-9
eBook Packages: MedicineReference Module Medicine