Abstract
The role of extracellular vesicles (EV) in carcinogenesis has become the focus of much research. These microscopic messengers have been found to regulate immune system function, particularly in tumorigenesis, as well as conditioning future metastatic sites for the attachment and growth of tumor tissue. Through an interaction with a range of host tissues, EVs are able to generate a pro-tumor environment that is essential for tumorigenesis. These small nanovesicles are an ideal candidate for a non-invasive indicator of pathogenesis and/or disease progression as they can display individualized nucleic acid, protein, and lipid expression profiles that are often reflective of disease state, and can be easily detected in bodily fluids, even after extended cryo-storage. Furthermore, the ability of EVs to securely transport signaling molecules and localize to distant tissues suggests these particles may greatly improve the delivery of therapeutic treatments, particularly in cancer. In this chapter, we discuss the role of EV in the identification of new diagnostic and prognostic cancer biomarkers, as well as the development of novel EV-based cancer therapies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 EV as Novel Cancer Biomarkers
The need for novel cancer biomarkers is fundamental in improving patient outcomes. This search has resulted in the emergence of EV as new predictive, diagnostic, and prognostic factors in cancer. EV can be obtained from virtually any body fluid or tissue, by safe and minimally invasive or non-invasive methods. Additionally, the intrinsic nature of EV protects the internalized (and external, to a degree) contents from host and environmental degradation, allowing easier EV isolation and storage. As EV are often released in higher concentration from tumor tissue, and the expression profile often mimics/reflects host cell expression profiles, they can be used as a liquid biopsy of the cancer tissue, even tissue that is unreachable via conventional methods [1,2,3,4]. EV may also be used as a future indicator of disease in healthy populations, leading to improved health planning and patient outcomes. This aids in determining the most effective treatment options, resulting in decreased economic burden and fewer unwanted side effects in patients.
Two major challenges exist in the development of EV diagnostics and prognostics in cancer. The first challenge is our limited understanding of the spectrum of signaling options that are available due to the complexity of EV surface expression. The sometimes-low concentrations of certain EVs, as well as the diversity and heterogeneity of EV type and expression profile also hamper development [5,6,7]. This will be improved with biobanking of both healthy and diseased tissue for adequate comparative analyses [7]. This problem is common in emerging diagnostics/prognostics and requires substantial resources and investment to generate a reliable and affordable repository. The second challenge is the development of economical methods of isolating and analyzing EV from samples. Though the liquid biopsy is a safe and effective method, high-sensitivity methods of isolating and characterizing EV are only beginning to be established [7, 8].
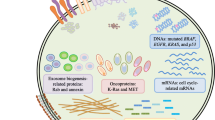
EV contain a varied assortment of factors, that present significant diagnostic and prognostic potential in cancer treatment. For these purposes, EV are most often obtained from patient serum, though plasma and urine are also easily utilized [9, 10]. Factors isolated from EV not only discern healthy from diseased patients but can also be effective in staging disease. Many studies have identified EV nucleic acid, particularly miRNA, as an effective cancer biomarker [11,12,13,14,15,16,17]. These studies identified many indicative miRNA species in a vast array of cancers, often using quantitative PCR and/or sequencing for RNA detection [18, 19]. Undoubtedly many studies utilizing serum miRNA as diagnostic and prognostic disease markers have accidentally harvested exosomal miRNA. In fact, exosomal miRNA may represent a significant fraction of commonly isolated miRNA in some studies. Other nucleic acids that have been identified as demonstrating biomarker potential are mRNA, DNA (containing oncogenic mutations), short non-coding RNA, and circular RNA [20,21,22,23,24,25]. Much like EV miRNA, many studies have utilized mass spectrometry techniques to identify an array of proteins that are highly indicative of disease state [10, 26,27,28]. Protein markers have thus far demonstrated significant potential, with a recent study identifying a marker that displayed unprecedented accuracy in diagnosing and staging disease state in pancreatic cancer patients [29]. Analysis of the lipid composition of EV has shown lipid expression profiles may also be a potential cancer biomarker [30].
2 EV Biomarker Technology in Cancer
The future of EV as diagnostic and prognostic markers in cancer relies on the development of systems that rapidly capture and identify markers of disease. Common methods for isolating EV for biomarker analyses include standard isolation techniques based on filtration combined with ultracentrifugation, and immunoaffinity capture methods [6, 7]. Though effective, the cost of these technologies is currently prohibitive for large scale implementation [31]. Thus, new technologies are being developed to utilize the vast content of EV for therapeutic purposes. Recent developments in the modification of existing technologies used in liquid biopsy analysis have already provided new diagnostic methods [8, 31]. These include several effective immunoaffinity capture methods, including the ExoChip, ExoScreen and ExoSearch technologies, that allow rapid identification of specific EV markers associated with oncogenesis [32,33,34]. Fortunately, EV factors can be identified using a range of methods including PCR, mass spectrometry, nuclear magnetic resonance, and immunofluorescence [26, 35,36,37,38,39,40,41]. Two diagnostic EV technologies are currently available that identify RNA signatures in the urine of prostate cancer patients and the serum of lung cancer patients (www.exosomedx.com) [42, 43]. These markers help diagnose disease and determine treatment options. Although only two methods are currently available, many clinical trials utilizing EV-based technologies in cancer diagnostics are under investigation.
3 Novel Role of EV in Cancer Therapy
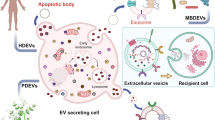
The burgeoning area of EV function in cellular communication derives from their ability to protect and transport a range of cargoes to a wide array of tissues [3, 19, 44,45,46,47,48]. This ability is being utilized in the development of novel therapies in the treatment of many diseases, particularly cancer [8, 49,50,51]. Most EV-based therapies utilized natively-derived (obtained from patients) or semi-synthetic/bioengineered EV (mimetics) that deliver compounds which either activate/enhance antitumoral immune responses (cancer vaccines) or deliver antiproliferative agents directly to the tumor tissue (therapy delivery) [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Apart from the aforementioned vaccination and therapy delivery, the removal of EV or inhibition of EV production to reduce cancer growth and/or pre-metastatic niche formation is also evaluated [70,71,72,73,74]. This has been investigated via the reduction of Rab27a protein expression, as well as the removal of circulating EV via filtration or immunoaffinity capture [6, 31, 75,76,77,78].
EV make excellent delivery vehicles due to their bioavailability and lack of unwanted immunogenicity. When compared with the delivery of soluble factors alone, EV-internalized or associated factors often display increased efficacy with minimal off-target/side effects [56, 79,80,81]. The complexity and hence similarity of exosomal surface expression to host cells both increases the effectiveness of EV as delivery systems, as opposed to synthetic vehicles, and reduces unwanted immune responses due to their syngeneic nature [8, 80, 82]. This can result in increased uptake of exosomal contents by host cells compared to synthetic particles, such as liposomes [49, 50]. This is advantageous in the delivery of certain compounds, such as chemotherapeutics, where tumor uptake is enhanced (increased tumor cytotoxicity) while unwanted drug deposition is reduced (reduced side effects). This complexity also permits the encapsulation of multiple compounds that could target several cell types or targets.
However, there are also disadvantages to using biological EVs as therapeutic vehicles [8, 51]. Sometimes generalized increased uptake is not required, but more limited and specific uptake in certain sites or tissues. Although synthetic EV can have unwanted toxicity and immunogenicity, enhanced immunogenicity may be required to maximize antitumor effects. These issues require a modified delivery system that does not necessarily prevent uptake of the nanovesicle, but prevents content release unless the desired inter/intracellular conditions are met. With current technology, synthetic particles have been advantageous in this respect, as the regulation of surface expression is far easier, and the particle structure can be easily modified to prevent release at unwanted sites, such as low or neutral pH [83,84,85,86]. Thus, the two main advantages of synthetic and semi-synthetic EV delivery systems are that the manufacturing process limits unwanted variability/heterogeneity (an issue when utilizing current biological systems for EV generation), and that synthetic EV can be generated on large scale, suitable for drug delivery or vaccination. Future therapies will most likely rely on a combination of these methods, as well as the generation of EV mimetics, a type of EV of biological origin, generated via non-biological mechanisms [67, 68, 87, 88].
4 Generation and Modulation of EV for Cancer Therapy
As of 2016, there were no commercial EV-based therapies available for the treatment of cancer. Although synthetic nanovesicle delivery systems have been established in the treatment of array of diseases, the potential of EV to deliver therapeutic compounds is beginning to be elicited [8, 51]. The generation of EV to be used in cancer treatment relies, fundamentally, on two methods; the isolation of EV from the patient, tissue, or cell culture, followed by modification (drug, protein, nucleic acid, lipid) and reintroduction to the patient as treatment; or the large-scale isolation/fabrication of EV from cell culture, bioreactor or animal body fluid, again, followed by modification and introduction to the patient. Ex vivo modification of EV is often required to regulate antigen presentation or surface expression in order to modulate immunostimulatory potential and enhance selective uptake and delivery of EV contents [80, 85, 89, 90]. These contents can be internalized utilizing a range of methods. The cells used to generate the EV can be treated with factors that regulate EV expression of protein and nucleic acid, and to produce exosomes that contain said factor [60, 85]. EV themselves can also be treated to incorporate specific contents. Simple incubation can facilitate uptake of certain compounds, while more complex methods, such as electroporation or enzymatic poration can also be used [49, 51, 62].
Though EV can be isolated from nearly all cell types and bodily fluids, exosome production for cancer therapy is limited. This includes primarily dendritic cells, cancer cells, and stem cells, each having distinct advantages and disadvantages. The first study to demonstrate the effectiveness of EVs as a mechanism for delivery showed that EV could deliver siRNA while effectively crossing the blood blain barrier [19, 57]. Though not a cancer treatment, the use of the host’s EV for therapy propogated widespread interest in this method. In this study, dendritic cells were harvested and modified before reintroduction into the host, but these are not the only cell types that can be used in the production of therapeutic EV [19]. Regardless of the method utilized, substantial data indicates the necessity for diligent selection of the cell type to be used due to unwanted side-effects. These effects are intrinsic due to the heterogeneity in surface expression of EV.
Besides the significant changes in yield between and within these methods, the most important consideration is the surface expressed factors that dictate uptake, as complex EV expression profiles can obscure other functions [91]. The use of EVs as therapy requires the utmost stringency in the selection, isolation, and preservation to ensure patient safety. Exosomes derived from cancer cells tend to express higher levels (sometimes only) of MHC class I and a diverse array of growth factors, while EV from dendritic cells tends to express higher levels of MHC class II and lower amounts of growth mediators [8, 81, 92,93,94,95,96,97]. EV from mesenchymal stem cells (MSC) have been shown to be anti-inflammatory but can both enhance and inhibit tumor growth in different contexts [98]. Depending on whether the chosen method is to engage the immune system or directly kill tumor tissue, certain complications are inherent to EV-producing cell types and may have both positive and negative effects for the development of novel treatments. For example; aiming to generate an immune response that engages and destroys tumor tissue may have indirect proliferative effects on tumor tissue, while directly targeting tissue with EV cytotoxic drugs may compromise anti-tumor immune responses. Thus, modification of surface expressed factors is often required to elicit effectiveness, by improving immunogenicity or cytotoxicity.
Of the cell types discussed, MSC have shown the most potential, due to their low immunogenicity and ability to generate substantial quantities of EV [99, 100]. They are also relatively easy to obtain from patients allowing for personalized treatment. Recently, the use of bioreactors to culture adipose-derived MSC was shown to increase EV yield approximately 100-fold compared to conventional culturing methods [101]. Other methods for the large-scale purification of EV include harvesting from bovine milk, or the generation of EV mimetics, generated via serial extrusion [67, 68, 87, 88, 102]. This process generates nanovesicles of identical biological composition to EV, opening their potential for use in therapy.
5 Therapeutic Contents of EV in Cancer Therapy
Therapeutic contents of EV utilized in the treatment of cancer consist primarily of RNA or chemotherapeutics. Several studies have investigated the delivery of compounds via modified EV derived primarily from MSC. MSC-derived EV containing miRNA and anti-miRNA could increase sensitivity or re-sensitize tumor tissue to chemotherapeutics, and inhibit tumor growth [53, 64,65,66] . The efficacy of these methods can be improved my modifying the expression profile of the EV, resulting in greater uptake by target cells. The use of therapeutic siRNA is also being investigated, where preliminary studies have shown significant increases in mRNA depletion, leading to substantial decreases in cancer cell proliferation and viability [54, 56, 103]. EV, particularly from MSC, have also been used to enhance the effect of chemotherapeutics [58, 59, 61]. MSC treated with chemotherapeutics release large quantities of drug-containing EV. These EV can be more effectively used to deliver compounds to target cells [60]. Off-target effects can be further minimized by delivering modified EV that contain enzymes which activate prodrugs in tumor tissue [54]. Prodrug accumulation in other tissues is insignificant as the negligible levels of EV uptake by non-cancerous cells minimize drug activation. Currently, only two trials have investigated EV as method for drug delivery in cancer treatment, both utilizing plant-derived EV to either enhance the delivery of chemotherapeutics to tumor tissue (NCT01294072) or minimize side-effects of standard therapy (NCT01668849).
EV can also be utilized to deliver cargo that activates or enhances anti-tumor immune responses, producing a retroactive cancer vaccine [80, 95, 97, 104,105,106]. EV from tumor cells, and particularly dendritic cells, can contain be induced/modified to express/contain increased levels of MHC complexes for antigen presentation, as well as immunostimulatory components, such as heat shock proteins, interferon, and granulocyte macrophage colony stimulating factor [8, 81, 92,93,94,95,96,97]. These EV serve to enhance cytotoxic T-cell and Natural Killer cell responses against tumor tissue. Thus far, trials have investigated EV as an anti-cancer vaccine in lung (NCT01159288) and colorectal cancer, as well as malignant glioma (NCT01550523, NCT02507583). Studies investigating malignant glioma utilized a novel method for EV delivery. Rather than systemic delivery of EV, modified glioma cells captured within diffusion chambers were surgically inserted in the patient. As the glioma cells undergo apoptosis due to prior ex vivo modification, they release a range of vesicles, in particular EV, that serve to stimulate glioma-specific anti-tumor immune responses [107]. Although showing great promise, EV-based therapies for cancer have yet to make it to market.
6 Summary
EV are intriguing and present a new paradigm in our understanding of the dynamics of cancer pathology and treatment. Though the function of exocytosis in oncogenesis is not fully understood, many studies have demonstrated the capabilities of EV in many aspects of cancer diagnostics and treatment. Though EV-based cancer treatments are still in clinical trials, EV-based biomarkers have recently become available for cancer diagnosis. With an increased understanding of the complex signaling potential of EV, combined with rapid and sensitive analysis methods, these nano-sized particles will undoubtedly provide a range of new options in cancer treatment.
References
Haber DA, Velculescu VE (2014) Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 4(6):650–661
Webb S (2016) The cancer bloodhounds. Nat Biotechnol 34(11):1090–1094
Henderson MC, Azorsa DO (2012) The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol 2:1–9
Brock G, Castellanos-Rizaldos E, Hu L, Coticchia C, Skog J (2015) Liquid biopsy for cancer screening, patient stratification and monitoring. Transl Cancer Res 4(3):280–290
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci 113(8):E968–E977
Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3(Unit 3):22
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2. https://doi.org/10.3402/jev.v2i0.20360
Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M (2014) A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 1846(1):75–87
Zeringer E, Barta T, Li M, Vlassov AV (2015) Strategies for isolation of exosomes. Cold Spring Harb Protoc 2015(4):pdb.top074476
Simpson RJ, Lim JW, Moritz RL, Mathivanan S (2009) Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 6(3):267–283
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838
Cappello F, Logozzi M, Campanella C, Bavisotto CC, Marcilla A, Properzi F, Fais S (2017) Exosome levels in human body fluids: a tumor marker by themselves? Eur J Pharm Sci 96:93–98
Gusachenko ON, Zenkova MA, Vlassov VV (2013) Nucleic acids in exosomes: disease markers and intercellular communication molecules. Biochemistry (Mosc) 78(1):1–7
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci 105(30):10513–10518
Raponi M, Dossey L, Jatkoe T, Wu X, Chen G, Fan H, Beer DG (2009) MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res 69(14):5776–5783
Wu M, Jolicoeur N, Li Z, Zhang L, Fortin Y, L’Abbe D, Yu Z, Shen SH (2008) Genetic variations of microRNAs in human cancer and their effects on the expression of miRNAs. Carcinogenesis 29(9):1710–1716
Zheng D, Haddadin S, Wang Y, Gu L-Q, Perry MC, Freter CE, Wang MX (2011) Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol 4(6):575–586
Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101(10):2087–2092
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659
Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D (2014) Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24(6):766–769
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10(12):1470–1476
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J (2011) Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2:180
Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R (2014) Identification of double stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 289(7):3869–3875
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S (2015) Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 25(8):981–984
Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, Brown D, Russo LM (2010) Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78(2):191–199
Duijvesz D, Burnum-Johnson KE, Gritsenko MA, Hoogland AM, Vredenbregt-van den Berg MS, Willemsen R, Luider T, Pasa-Tolic L, Jenster G (2013) Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS One 8(12):e82589
Duijvesz D, Luider T, Bangma CH, Jenster G (2011) Exosomes as biomarker treasure chests for prostate cancer. Eur Urol 59(5):823–831
Jakobsen KR, Paulsen BS, Baek R, Varming K, Sorensen BS, Jorgensen MM (2015) Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 4:26659
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523(7559):177–182
Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K (2013) Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 1831(7):1302–1309
Ko J, Carpenter E, Issadore D (2016) Detection and isolation of circulating exosomes and microvesicles for cancer monitoring and diagnostics using micro-/nano-based devices. Analyst 141(2):450–460
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S (2014) Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14(11):1891–1900
Zhao Z, Yang Y, Zeng Y, He M (2016) A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16(3):489–496
Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, Furuta K, Nakajima T, Hayashi H, Sugisaki H, Higashimoto H, Kato T, Takeshita F, Ochiya T (2014) Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun 5:3591
Noerholm M, Balaj L, Limperg T, Salehi A, Zhu LD, Hochberg FH, Breakefield XO, Carter BS, Skog J (2012) RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer 12:22
Shao H, Chung J, Lee K, Balaj L, Min C, Carter BS, Hochberg FH, Breakefield XO, Lee H, Weissleder R (2015) Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun 6:6999
Goda T, Masuno K, Nishida J, Kosaka N, Ochiya T, Matsumoto A, Miyahara Y (2012) A label-free electrical detection of exosomal microRNAs using microelectrode array. Chem Commun 48(98):11942–11944
Tavoosidana G, Ronquist G, Darmanis S, Yan J, Carlsson L, Wu D, Conze T, Ek P, Semjonow A, Eltze E, Larsson A, Landegren UD, Kamali-Moghaddam M (2011) Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci 108(21):8809–8814
Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE (2009) The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112(1):55–59
Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H (2012) Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 18(12):1835–1840
Jeong S, Park J, Pathania D, Castro CM, Weissleder R, Lee H (2016) Integrated magneto-electrochemical sensor for exosome analysis. ACS Nano 10(2):1802–1809
Enderle D, Spiel A, Coticchia CM, Berghoff E, Mueller R, Schlumpberger M, Sprenger-Haussels M, Shaffer JM, Lader E, Skog J, Noerholm M (2015) Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One 10(8):e0136133
Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O’Neill V, Cochran JS, Brown GA (2015) A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis 18(4):370–375
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820(7):940–948
el-Andaloussi S, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12(5):347–357
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579
Kharaziha P, Ceder S, Li Q, Panaretakis T (2012) Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta 1826(1):103–111
Tickner JA, Urquhart AJ, Stephenson S-A, Richard DJ, O’Byrne KJ (2014) Functions and Therapeutic Roles of Exosomes in Cancer. Front Oncol 4:127
Kotmakci M, Bozok Cetintas V (2015) Extracellular vesicles as natural nanosized delivery systems for small-molecule drugs and genetic material: steps towards the future nanomedicines. J Pharm Pharm Sci 18(3):396–413
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ (2015) Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 199:145–155
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6(4):287–296
Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L, Baranov V, Gianni D, Tannous BA, Sena-Esteves M, Breakefield XO, Skog J (2012) Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther 20(5):960–971
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21(1):185–191
Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O (2013) Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21(1):101–108
Lee YS, Kim SH, Cho JA, Kim CW (2011) Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp Mol Med 43(5):281–290
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32(6):2003–2014
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35(7):2383–2390
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12(3):655–664
Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigano L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A (2014) Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release 192:262–270
Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M (2015) Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release 220(Pt B):727–737
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44
Katakowski M, Zheng X, Jiang F, Rogers T, Szalad A, Chopp M (2010) MiR-146b-5p suppresses EGFR expression and reduces in vitro migration and invasion of glioma. Cancer Invest 28(10):1024–1030
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M (2013) Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335(1):201–204
Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P (2013) Delivery of functional anti-mir-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids 2:e126
Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G (2013) Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 22(5):758–771
Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM (2012) Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine 7:1525–1541
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS (2013) Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7(9):7698–7710
Gobbo J, Marcion G, Cordonnier M, Dias AM, Pernet N, Hammann A, Richaud S, Mjahed H, Isambert N, Clausse V, Rebe C, Bertaut A, Goussot V, Lirussi F, Ghiringhelli F, de Thonel A, Fumoleau P, Seigneuric R, Garrido C (2016) Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst 108(3)
Alderton GK (2012) Metastasis: exosomes drive premetastatic niche formation. Nat Rev Cancer 12(7):447. https://doi.org/10.1038/nrc3304
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293
Sceneay J, Smyth MJ, Moller A (2013) The pre-metastatic niche: finding common ground. Cancer Metastasis Rev 32(3-4):449–464. https://doi.org/10.1007/s10555-013-9420-1
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, Filipazzi P, Rivoltini L, Tagliabue E, Pupa SM (2012) Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 227(2):658–667
Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L (2012) Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One 7(12):e50999
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C (2012) Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 72(19):4920–4930
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319(5867):1244–1247
Peinado H, cacute MsaAck, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, a-Santos GGi, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Bromberg J, Lyden D, Chapman PB, Kang Y (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18(6):883–891
Marleau AM, Chen CS, Joyce JA, Tullis RH (2012) Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 10:134
Mignot G, Roux S, Thery C, Ségura E, Zitvogel L (2006) Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med 10(2):376–388
Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq J-B, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 3(1):1–13
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G (2008) Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 16(4):782–790
Stremersch S, Vandenbroucke RE, Van Wonterghem E, Hendrix A, De Smedt SC, Raemdonck K (2016) Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release 232:51–61
Karanth H, Murthy RS (2007) pH-sensitive liposomes—principle and application in cancer therapy. J Pharm Pharmacol 59(4):469–483
Mortensen JH, Jeppesen M, Pilgaard L, Agger R, Duroux M, Zachar V, Moos T (2013) Targeted antiepidermal growth factor receptor (cetuximab) immunoliposomes enhance cellular uptake in vitro and exhibit increased accumulation in an intracranial model of glioblastoma multiforme. J Drug Deliv 2013:209205
Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, Villanueva J, Khine S, Le Pecq JB (2005) Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis 35(2):158–168
Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D’Ilio S, Lugini L, Violante N, Azzarito T, Majorani C, Brambilla D, Fais S (2014) Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One 9(2):e88193
Yang XZ, Dou S, Sun TM, Mao CQ, Wang HX, Wang J (2011) Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J Control Release 156(2):203–211
Moore C, Kosgodage U, Lange S, Inal JM (2017) The emerging role of exosome and microvesicle- (EMV-) based cancer therapeutics and immunotherapy. 141(3):428–436. https://doi.org/10.1002/ijc.30672
Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Brinkmann K, Dirmeier U, Laus R, Delcayre A (2011) Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res 71(15):5235–5244
Clayton A, Mason MD (2009) Exosomes in tumour immunity. Curr Oncol 16(3):46–49
Rana S, Yue S, Stadel D, Zoller M (2012) Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 44(9):1574–1584
Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N (2010) Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res 70(4):1281–1285
Chaput N, Taieb J, Andre F, Zitvogel L (2005) The potential of exosomes in immunotherapy. Expert Opin Biol Ther 5(6):737–747
Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadiere B, Amigorena S, Thery C (2008) Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 68(4):1228–1235
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4(5):594–600
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9(8):581–593
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton M, Delcayre A, Hsu D-H, Le Pecq J-B, Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3(1):9
Phinney DG, Pittenger MF (2017) Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35(4):851–858
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65(3):336–341
Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo ABH, Padmanabhan J, Lee CN, de Kleijn DPV, Lim SK (2011) Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med 9:47–47
Schirmaier C, Jossen V, Kaiser SC, Jüngerkes F, Brill S, Safavi-Nab A, Siehoff A, van den Bos C, Eibl D, Eibl R (2014) Scale-up of adipose tissue-derived mesenchymal stem cell production in stirred single-use bioreactors under low-serum conditions. Eng Life Sci 14(3):292–303
Munagala R, Aqil F, Jeyabalan J, Gupta RC (2016) Bovine milk-derived exosomes for drug delivery. Cancer Lett 371(1):48–61
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV (2013) Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 11(1):88
Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N (2016) Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5(4):e1071008
Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L (2004) Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol 172(4):2137–2146
Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y, Cao X (2007) Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol 133(6):389–399
Harshyne LA, Hooper KM, Andrews EG, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC (2015) Glioblastoma exosomes and IGF-1R/AS-ODN are immunogenic stimuli in a translational research immunotherapy paradigm. Cancer Immunol Immunother 64(3):299–309
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Tickner, J.A., Richard, D.J., O’Byrne, K.J. (2018). EV, Microvesicles/MicroRNAs and Stem Cells in Cancer. In: Mettinger, K., Rameshwar, P., Kumar, V. (eds) Exosomes, Stem Cells and MicroRNA. Advances in Experimental Medicine and Biology, vol 1056. Springer, Cham. https://doi.org/10.1007/978-3-319-74470-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-74470-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74469-8
Online ISBN: 978-3-319-74470-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)