Abstract
Proteins, surfactants and some polymers adsorb spontaneously at the interface between their aqueous solution and a gas, solid, or liquid phase. The surface layer made this way is characterized by different mechanical properties from the bulk solution, which is determined by surface rheology. The surface layer can undergo dilatational deformation and shear. In general, it can be characterized by both viscous and elastic properties. Surface rheology allows understanding the interaction between molecules, change of molecular conformations or molecular aggregations and diffusion rate of molecules towards the surface layer. There are many publications in which attention is drawn to the possibility of a relationship between surface rheology and foam and emulsion stability. This chapter discusses basic measurement techniques used to determine rheological properties of the sur-face layer, examples of test results, and the potential possibility of their use in practice.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
1 Introduction
To ensure stability of emulsion and foam, it is necessary to use additions of surfactants, proteins or polymers. Many of these substances are adsorbed on the interface, and since their surface concentration is much higher than their bulk concentration, rheological properties of the surface layer are diametrically different from the bulk solution.
The first scientific works on rheological properties of the surface layer were published in the 19th century. However, theoretical foundations of surface rheology (surface dilatational rheology) were developed by Boussinesq (1913) at the beginning of the 20th century. Although since then there have been more than 100 violent years, a rapid increase in the number of scientific papers, especially on surface shear rheology, has been observed for about 10 years. This is mainly related to the development of measurement techniques. Devices that allow making rheological measurements, both during shear deformation as well as dilatational deformation, are currently produced by commercial companies. This chapter discusses measurement methods, examples of test results, and the potential use of surface rheology.
2 Definition
Surface layer can be subject to shear and dilatational deformation (Fig. 1). In general, it can be characterized by both viscous and elastic properties.
Deformation of the surface layer with elastic properties that undergoes compression and expansion is characterized by the surface dilatational modulus defined by Gibbs (1878):
whereas during shear the surface shear modulus:
where: σ is the surface tension, A is the area of the interfacial layer, τ s is the surface shear stress (unit: N/m) and γ s is the shear strain of the surface layer.
The two surface viscosities have also been defined: surface dilatational viscosity (introduced first by Boussinesq 1913):
and surface shear viscosity
where \(\dot{\gamma }_{s}\) is the shear rate. Units of η s and η d is (Pa·s·m).
In practice, the surface layer is most often subjected to oscillatory deformation. Measurements of this type allow determining elastic and viscous properties of the surface layer and obtaining information on its microstructure (understanding the interaction between molecules, change of molecular conformations or molecular aggregations). For dilatational deformation, oscillatory changes of the surface layer are induced in accordance with the following equation:
and surface tension changes are measured, which, in general, can be described by the relation:
In Eqs. (5) and (6) A s,0 is the initial or reference interfacial area, A 0 is the amplitude of the area oscillations, ω is the frequency imposed, σ s,0 is the equilibrium reference of interfacial tension, σ 0 is the amplitude of the interfacial tension oscillations, and t is the time. The phase shift θ between the generated change of surface layer A and the interfacial tension allows determining viscous and elastic properties of the surface layer (Fig. 2a). For an ideal elastic and viscus material θ = 0° and θ = 90° respectively.
By using a complex notation it can be introduced a complex dilatational modulus:
Real component E d , also called dilatational storage modulus and imaginary component E η , also called dilatational loss modulus, are described by the following equations:
where the dilatational viscoelastic modulus of ∣E *∣ is calculated from
Modulus E d characterizes the elastic properties of interfacial layer, while modulus E η describes the viscous properties of the interfacial layer during dilatational deformation.
The loss to storage modulus ratio is defined by loss tangent:
Generally, tan θ < 1 indicates a dominance of elastic fluid properties, and tan θ > 1 shows a dominance of viscous fluid characteristics.
In the shear flow, during oscillation measurements, phase shift δ is determined between surface shear stress and deformation (Fig. 2 b).
By analogy to bulk rheology, the complex surface shear modulus has been defined
where: \(G_{s}^{{\prime }}\) is the surface storage modulus and \(G_{s}^{{{\prime \prime }}}\) is the surface loss modulus.
The surface storage modulus \(G_{s}^{{\prime }}\) and the surface loss modulus \(G_{s}^{{{\prime \prime }}}\), which are characterized by elastic and viscous properties of the surface layer, are described by equations:
where τ s,0 is the amplitude surface stress and γ s,0 is the amplitude surface strain.
3 Measurement Methods
3.1 Dilation Rheology
Experiments used to measure dynamic surface tension can simultaneously be used for rheological measurements during compression and expansion of the surface layer. The Langmuir trough with an oscillating barrier is a classic device used for this type of research. Changes in surface tension over time are recorded using a Wilhelmy plate. This method can be applied to a frequency of approximately 0.2 Hz (Derkach et al. 2009).
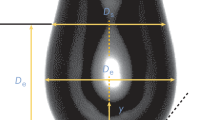
At present, the most commonly used techniques to make surface dilatational rheology measurements are the oscillating bubble method and the oscillating drop method (Fig. 3). In these methods harmonic changes of the surface of a drop or a gas bubble are induced. Surface tension and surface area can be determined based on the image of a drop (bubble) or capillary pressure measurements and volume of a drop (bubble). The surface tension is calculated based on Young-Laplace equation. A certain disadvantage of the oscillating bubble method and the oscillating drop method is the limited frequency range in which they can be carried out. By measuring surface tension changes based on a drop image, the range of attainable frequencies falls from 1·10−3 to 2·10−1 Hz, while using a capillary pressure measurement from approximately 1·10−1 Hz up to approximately 1 Hz (Noskov 2010). At higher frequencies, the shape of the drop begins to significantly deviate from the spherical one.
Thus, the assumption that deformation takes place only in the radial direction is no longer valid, and the results obtained can have a significant error. Classic methods of measuring rheological properties during dilatational deformation also include methods based on the measurements of the characteristics of capillary waves. Their advantage is the possibility to make measurements at high frequencies (up to 1000 Hz), which would not be possible for other methods. Currently, these techniques are rarely used, mainly due to difficulties in interpreting measurement results. Often, negative values of surface dilatational viscosity are obtained which is at variance with the second law of thermodynamics (Noskov 2010).
3.2 Shear Rheology
There are three basic groups of experiments in which rheological measurements are made during shear deformation of the surface layer: indirect methods; direct methods and microrheology. Indirect measurements consist in placing tracer particles on the surface of liquid and observing the trajectory of its movement. In direct methods, torque and angular velocity are measured during the rotation of specially designed elements placed on the liquid surface. Among microrheological methods, the passive ones are the most commonly used in surface layer research as they consist in recording thermal motions of inoculum particles located at the interface. The most well-known indirect devices are a canal surface viscometer and a rotating wall knife-edge surface viscometer, as well as a deep-channel surface viscometer. At present, commercial surface rheometers are produced, which allow for a direct measurement of surface shear stress, which is why indirect methods are rarely used. A description of indirect methods can be found in (Edwards et al. 1991; Krägel and Derkatch 2009), among others.
If direct methods are concerned, different forms of measurement systems are used which are in contact with the surface layer (Fig. 4). The most commonly used are bicone, double wall-ring and Du Noüy ring (Vandebril et al. 2010).
In the past, such systems were suspended on thin torsion wire. Now, they are connected to traditional rotary rheometers equipped with an air-magnetic bearing. When using a rotary rheometer, it is possible to calculate surface tension and deformation rate based on the measured torque and angular frequency. Bicone, double wall-ring and Du Noüy ring can be used to make measurements at the gas-liquid and liquid-liquid interfaces. There are also specialist structures of surface rheometers, an example of which is a device in which the movement of a magnetic needle located on liquid surface between Helmholtz coils (Fig. 5) is recorded (Shahin 1986; Brooks et al. 1999). The movement of the needle is recorded using a digital camera. Magnetic rod rheometers are characterized by very high sensitivity. It is possible to measure surface shear stress in the range from 10−8 to 10−4 N/m. This type of device can be used for measurements at the gas-liquid and liquid-liquid interfaces. With the use of movable barriers during measurement, it is possible to change the surface concentration of surface active agents.
The mentioned measurement systems cause not only the movement of the surface layer but also of deeper liquid layers. Thus, the total measured drag is the result of the forces induced by the movement of both the surface layer, as well as the subphase which directly adheres thereto. The relation of surface drag to subphase drag is called the Boussinesq number, which is expressed by the equation (Vandebril et al. 2010):
where: η is the bulk viscosity (Pa·s), v is a characteristic velocity (m/s), L 1 and L 2 are the characteristic length scales over which the characteristic velocity decays at the interface and in the subphases, respectively (m), P 1 is the contact perimeter between the surface probe and the interface (m), and A 2 is the contact area between the geometry and the surrounding subphases (m2). The ratio (A 2 ·L 1 )/(P 1 ·L 2 ) has the units of length and defines a characteristic length scale L, which will be dependent on the dimensions of the measurement geometry (Vandebril 2010). In the case of oil–water interface in Eq. (15) η should be substituted by η 1 + η 2 , where η 1 and η 2 are the viscosities of the lower and the upper liquid (Derkach et al. 2009). High values of Bo number (Bo ≫ 1) mean that the measured drag is mainly caused by the surface layer, so surface viscosity is easy to calculate. If Bo values << 1, the measurement system is not suitable for determining surface viscosity of a given liquid. Currently, for the most commonly used methods of measuring the surface shear viscosity (bicone, double wall-ring and magnetic rod rheometer), the lowest values of characteristic length L will be achievable when using the magnetic rod rheometer, however, the solution has its disadvantages. When using one magnetic rod, measurements in a limited range of surface viscosity changes can be made. For this reason, it is necessary to use magnetic rods of different dimensions. With bicone it is possible to make measurements over a wide range of surface viscosities, simultaneously for this value system Bo numbers will be much lower than when using a magnetic rod rheometer at the same viscosity ratio value η S /η. The double wall-ring geometry is an attempt to combine the advantages of both solutions. For this system, the characteristic values of the L dimension are comparable to values obtained for the magnetic rod rheometer, while at the same time it has the advantages of bicone.
4 Examples of Research Results
Most of the research results that have been presented so far cover the rheological properties of surface layers of surfactants, proteins, polymers, and mixtures of proteins and polymers with surfactants (Langevin and Monroy 2010; Lucassen-Reynders et al. 2010). The rheological properties of the surface layer of these systems depend on many factors. The most important are the diffusion rate of surfactant molecules towards the direction of the interfacial surface, and interactions between molecules adsorbed at the interface. The current state of knowledge on this subject matter can be found in (Bos and Vliet 2001; Miller and Liggieri 2009; Krägel and Derkatch 2010; Narsimhan 2016) reviews. This chapter discusses examples of research results.
Surface viscosity measurements of low molecular weight surfactants are most often made under conditions of dilatational deformation. In shear flow of surface viscosity of solutions of these compounds it is usually very low, often unmeasurable. Figure 6 shows the complex dilatational modulus correlation in the function of frequency for aqueous solution of oxyethylated surfactant C14ΕO8 (noninionic surcitrate) obtained by Fainerman and co-workers (Fainerman et al. 2008) using bubble profile analysis tensiometer. The characteristic property of surfactants is the ability to form micelles in solutions. Their presence in a solution influences the dynamics of formation of the surface layer. The data in Fig. 6 shows that the highest values of viscoelatic modulus |E *| are achievable at a concentration of the surfactant close to or slightly below the critical micelle concentration (cmc). Increasing the surface area concentration above the cmc results in a decrease in viscoelatic modulus |E *|. Its value will depend not only on the diffusion rate of molecules towards the surface, but also on the dynamics of micelle formation.
Viscoelasticity module |E *| as a function of frequency f for various C14ΕO8 concentrations below the cmc (◇5 μmol/dm3), at cmc (▢7 μmol/dm3), and above the cmc at 12 (▲), 15 (◆), 20 (△), and 30 μmol/dm3 (■) (Fainerman et al. 2008)
When the interfacial surface is expanded, the adsorption of surfactant molecules from the bulk solution takes place thereon. Thus, their concentration in the subphase adhering to the interfacial surface is lower than in the state of equilibrium, which disintegrates part of the micelles and simultaneously forces them to diffuse from the bulk solution. During compression of the surface layer, the situation is reversed. In this case, monomer desorption, increased concentration of the surfactant in the bulk solution, and formation of new micelles will occur. Thus, dilatational rheology provides information not only on the dynamics of surface layer formation but also on micelles themselves.
Shear flow measurements have been used in research on the surface properties of protein solutions and their mixtures with surfactants and polymers, as well as nanoparticles. High values of surface viscosity characterize aqueous solutions β-lactoglobulin (BLG), hence the solutions of this protein are often used as model liquids. Oscillatory shear measurements allow following the process of forming the structure of the surface layer of protein solutions. Figure 7 shows the dependence of the surface storage modulus \(G_{s}^{{\prime }}\) and the surface loss modulus \(G_{s}^{{{\prime \prime }}}\) on time for aqueous BLG solutions obtained in shear flow (pH = 6.3, tests were made using a bicon system connected to the Physica MCR 501 rheometer). For protein concentration of 750 ppm, values of the surface storage modulus \(G_{s}^{{\prime }}\) clearly increase as a function of time, reaching a maximum, and then they slightly decrease. The initial growth of \(G_{s}^{{\prime }}\) modulus growth demonstrates that the surface layer formation process is controlled by BLG diffusion from the bulk solution. Depending on the type of protein, it can last from a few to several dozen hours (Bos and Vliet 2001 ). Increased BLG concentration to 1250 ppm results in module values \(G_{s}^{{\prime }}\) and \(G_{s}^{{{\prime \prime }}}\) being very high at the beginning, and then decreasing and reaching a constant value. Occurrence of the maximum value of modulus \(G_{s}^{{\prime }}\) for a solution of 750 ppm and a drop in its value for concentration at 1250 ppm is the result of unfolding BLG molecules adsorbed on the interfacial surface.
Dependence of \(G_{s}^{{\prime }}\) and \(G_{s}^{{{\prime \prime }}}\) modules on time function t for BLG solutions (Broniarz-Press et al. 2015)
Oscillatory measurements in the shear flow of surface layer, as in the case of bulk fluids, can be made in linear and nonlinear viscoelastic regimens. The linear viscoelasticity range is determined on the basis of the dependence of the surface storage modulus \(G_{s}^{{\prime }}\) and the surface loss modulus \(G_{s}^{{{\prime \prime }}}\) in the function of strain (stress) amplitude (stress or strain sweep experiments) (Fig. 8a). Based on this graph, it is also possible to determine surface yield stress τ s,y . As in the case of bulk rheology, the analysis of nonlinear measurements is made based on Lissajous curves. In the range of linear viscoelasticity, measurements of modules \(G_{s}^{{\prime }}\) and \(G_{s}^{{{\prime \prime }}}\) as a function of frequency sweep experiments are made as well (Fig. 8b). The results of this experiment allow obtaining some information on the microstructure of the surface layer. For example, an approximate parallel course of dependence and as a function of angular frequency for BLG solutions provides funding for the formation of gel-like films.
Examples of: a strain sweep experiments, b frequency sweep experiments for BLG solution (Broniarz-Press et al. 2015)
5 Possible Practical Use
Rheological research on surface layer are made for diluted solutions of such substances as proteins, surfactants, polymers and mixtures thereof. Solutions of this type are found in food and cosmetic products in which surface-active substances are used as emulsifiers or foaming agents because of their amphiphilic character.
Examples of food products with complex rheological properties of the surface layer are milk, instant coffee and beer (Piazza et al. 2008; Maldonado-Valderrama and Patino 2010; Drusch et al 2012; Dan et al. 2013; Broniarz-Press et al. 2014). Multiphase systems are also common in the chemical industry. In literature on the subject, attention has been paid to the possibility of a relationship between surface rheology and flotation, microflotation and microfiltration (Dukhin et al. 2009; He 2016). In many food products and industrial processes, foams play an important role. Some foams are impermanent materials, which is why their rheological properties are difficult to be determined by methods used in classic rheology. Hence the idea to use surface rheology to analyse factors affecting the stability and mechanical properties of foam. Unfortunately, according to analysis made by Erni and co-workers (Erni et al. 2009), at present it cannot be clearly stated that there is a relationship between surface rheology of the solutions and foam stability. Of course, along with the increase in protein concentration (for example, ovalbumin) the surface shear viscosity and foam lifetime increase as well. Simultaneously, low molecular weight surfactants also stabilize foams and emulsions, although the surface shear viscosity and surface dilatational viscosity of their solutions are very low. Lexis and Willenbacher (Lexis and Willenbacher 2014) compared the results of research on the surface rheology of solutions (surface shear and dilatational elastic moduli) and bulk rheology of foam (apparent yield stress and storage modulus) obtained for casein solutions, whey protein isolate and two surfactants: nonionic Triton X and anionic sodium dodecyl sulphate.
In general, more flexible foam was formed from protein solutions then surfactants. At the same time, the surface layer of protein solutions was characterized by greater elasticity than the surface layer of low molecular weight surfactants. It was found that in the case of protein solutions, the increase in surface storage modulus was not always accompanied by an increase in bulk storage modulus of foam. As in the case of foams, the relationship between surface rheology and stability and bulk rheology of emulsions is not fully explained (Georgieva et al. 2009; Maldonado-Valderrama and Patino 2010).
Another area of potential practical use of surface rheology is medical diagnostics. The advantage of this type research is the possibility to record changes in properties of surface layers with very little change in the concentration of general active substance. Kazakov and co-workers (Kazakov et al. 2009) discussed the published results of measurements of dynamic surface tensions and surface rheological parameters of various human liquids, such as blood serum, urine, breathing air condensates, cerebrospinal liquor and others. Research on samples of human liquids taken from healthy and ill patients showed a relationship between surface rheology and kidney stones, rheumatism and pulmonary diseases. Only dilational rheology is used in this research. This is mainly due to the need to use a relatively large amount of liquid in the case of surface shear measurements, which is most often impossible in the case of human liquids. Scheuble and co-workers (Scheuble et al. 2014) used surface shear rheology to monitor the properties of medium-chain triglyceride oil/water layer characteristics under various gastric digestion steps. The authors showed that increased emulsion stability in the gastric environment might lead to homogeneously distributed lipid droplets in the stomach, which could be faster transported to the duodenum and sensed by the body leading to a faster satiety signal.
In the work by Çelebioğlu and co-workers (Çelebioğlu et al. 2017) it was also suggested that the rheological properties of the surface layer of emulsion can influence their perception. However, there has been no evidence of such a relationship so far.
6 Conclusion
Surface rheology allows collecting information on the kinetics of formation and structure of the surface layer being formed at the liquid-liquid and gas-liquid inter-face. The results of this research are very interesting for cognitive reasons, but they have relatively little practical significance. It seems that the biggest challenge is to establish a relation between dynamic interfacial properties and multiphase system properties. This will allow using surface rheology in the description of industrial processes.
References
Bos MA, Vliet T (2001) Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Interfac 91:437–471
Boussinesq MJ (1913) Sur l’existence d’une viscosité seperficielle, dans la mince couche de transition séparant un liquide d’un autre fluide contigu. Ann Chim Phys 29(8):349–357
Broniarz-Press L, Różański J, Różańska S et al (2014) Rheological properties of liquid surface layer in selected commercial beers. Food Sci Tech Qual 95:43–52
Broniarz-Press L, Różański J, Kmiecik J (2015) Reologia warstw powierzchniowych – zastosowanie, metody pomiaru, przykładowe wyniki. Inż Ap Chem 54(6):306–307
Brooks CF, Fuller GG, Frank CW et al (1999) An interfacial stress rheometer to study rheological transitions in monolayers at the water–air interface. Langmuir 15(7):2450–2458
Çelebioğlu HY, Kmiecik-Palczewska J, Lee S et al (2017) Interfacial shear rheology of β-lactoglobulin—Bovine submaxillarymucin layers adsorbed at air/water interface. Int J Biol Macromol 102:857–867
Dan A, Gochev G, Krägel J et al (2013) Interfacial rheology of mixed layers of food proteins and surfactants. Curr Opin Colloid In 18:302–310
Derkach SR, Krägel J, Miller R (2009) Methods of measuring rheological properties of interfacial layers (experimental methods of 2D rheology). Colloid J 71(1):5–22
Drusch S, Hamann S, Berger A et al (2012) Surface accumulation of milk proteins and milk protein hydrolysates at the air–water interface on a time-scale relevant for spray-drying. Food Res Int 47:140–145
Dukhin SS, Kovalchuk VI, Aksenenko EV et al (2009) Influence of surface rheology on particle bubble interaction in flotation. In: Miller R, Liggieri L (ed) Interfacial Rheology. Leiden, Boston, pp 567–613
Edwards DA, Brenner H, Wasan DT (1991) Interfacial transport processes and rheology. Butterworth-Heinemann, Oxford
Erni P, Windhab EJ, Fischer P (2009) Interfacial rheology in food science and technology. In: Miller R, Liggieri L (eds) Interfacial rheology. Leiden, Boston, pp 614–653
Fainerman VB, Petkov JT, Miller R (2008) Surface dilational viscoelasticity of C14EO8 micellar solution studied by bubble profile analysis tensiometry. Langmuir 24(13):6447–6452
Georgieva D, Schmitt V, Leal-Calderon F et al (2009) On the possible role of surface elasticity in emulsion stability. Langmuir 25(10):5565–5573
Gibbs JW (1878) On the equilibrium of heterogeneous substances. Am J Sci 16(96):441–458
He Z, Miller DJ, Kasemset S, Wang L et al (2016) Fouling propensity of a poly(vinylidene fluoride) microfiltration membrane to several model oil/water emulsions. J Membrane Sci 514:659–670
Kazakov VN, Knyazevich VM, Sinyachenko OV et al (2009) Interfacial rheology of biological liquids: application in medical diagnostics and treatment monitoring. In: Miller R, Liggieri L (eds) Interfacial rheology. Leiden, Boston, pp 519–566
Krägel J, Derkatch SR (2009) Interfacial shear rheology—an overview of measuring techniques and their applications. In: Miller R, Liggieri L (eds) Interfacial rheology. Leiden, Boston, pp 373–420
Krägel J, Derkatch SR (2010) Interfacial shear rheology. Curr Opin Colloid Interface Sci 15:246–255
Langevin D, Monroy F (2010) Interfacial rheology of polyelectrolytes and polymer monolayers at the air–water interface. Curr Opin Colloid Interface Sci 15(4):283–293
Lexis M, Willenbacher N (2014) Yield stress and elasticity of aqueous foams from protein and surfactant solutions—The role of continuous phase viscosity and interfacial properties. Colloids Surf A 459:177–185
Lucassen-Reynders EH, Benjamins J, Fainerman VB (2010) Dilational rheology of protein films adsorbed at fluid interfaces. Curr Opin Colloid Interface Sci 15(4):264–270
Maldonado-Valderrama J, Patino JMR (2010) Interfacial rheology of protein–surfactant mixtures. Curr Opin Colloid Interface Sci 15(4):271–282
Miller R, Liggieri L (eds) (2009) Interfacial rheology. Leiden, Boston
Narsimhan G (2016) Characterization of interfacial rheology of protein-stabilized air-liquid interfaces. Food Eng Re 8:367–392
Noskov BA (2010) Dilational surface rheology of polymer and polymer/surfactant solutions. Curr Opin Colloid Interface Sci 15(4):229–236
Piazza L, Gigli J, Bulbarello A (2008) Interfacial rheology study of espresso coffee foam structure and properties. J Food Eng 84:420–429
Scheuble N, Geue T, Windhab EJ et al (2014) Tailored interfacial rheology for gastric stable adsorption layers. Biomacromol 15:3139–3145
Shahin GT (1986) The stress deformation interfacial rheometer, Ph.D. Thesis, University of Pennsylvania, Philadelphia
Vandebril S, Franck A, Fuller GG et al (2010) A double wall-ring geometry for interfacial shear rheometry. Rheol Acta 49(2):131–144
Acknowledgements
This work was supported by PUT research grant No. 03/32/DSPB/0702.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Różański, J., Kmiecik-Palczewska, J. (2018). Measuring Techniques and Potential Applications of Interface Rheology. In: Ochowiak, M., Woziwodzki, S., Doligalski, M., Mitkowski, P. (eds) Practical Aspects of Chemical Engineering. Lecture Notes on Multidisciplinary Industrial Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-73978-6_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-73978-6_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73977-9
Online ISBN: 978-3-319-73978-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)