Abstract
Bone health is now recognized as an important facet of child health with sufficient evidence to support standardized approaches to diagnosis, monitoring, treatment, and prevention. Current management strategies are based on monitoring at-risk children to identify and then treat earlier rather than later signs of osteoporosis in those with limited potential for spontaneous recovery. Research studies addressing prevention of the first-ever fracture are still needed for children who have both a high likelihood of developing fractures and less potential for recovery.
This chapter focuses on the evidence that shapes the current approach to diagnosis, monitoring, and treatment of osteoporosis in childhood, with emphasis on the key pediatric-specific biological principles that are pivotal to the overall approach and on the main questions with which clinicians struggle during routine care. The scope of this chapter is to review the manifestations of and risk factors for primary and secondary osteoporosis in children, to discuss the definition of pediatric osteoporosis, and to provide specific recommendations for monitoring and prevention. This chapter also reviews when a child is a candidate for osteoporosis therapy, which agents and doses should be prescribed, duration of therapy, how the response to therapy is evaluated, and side effects. With this information, the bone health clinician will be poised to diagnose osteoporosis in children, to identify when children need osteoporosis therapy and the clinical outcomes that gauge efficacy and safety of treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Osteoporosis
- Vertebral fractures
- Bone fragility
- Bone density
- Diagnosis
- Monitoring
- Treatment
- Prevention
- Bisphosphonates
- Denosumab
- Peripheral quantitative computed tomography
- Dual-energy x-ray absorptiometry
- Trans-iliac histomorphometry
- Bone turnover markers
- Vertebral morphometry
-
Osteoporosis in childhood can be associated with serious morbidity including premature loss of ambulation in those with mobility disorders, chronic back pain, and deformity from vertebral fractures and long bone deformity limiting functional mobility; mortality from fat embolism syndrome has also been described following a low-trauma femur fracture.
-
Advanced osteoporosis presentations are no longer acceptable in clinical practice; instead, bone health monitoring should be carried out in those with clinically significant risk factors in order to identify the earliest signs of bone fragility (which are often vertebral fractures).
-
Osteoporosis treatment is divided into four phases: stabilization, maintenance, treatment discontinuation and posttreatment monitoring. Intravenous bisphosphonate therapy remains the mainstay of osteoporosis therapy, with newer anti-resorption and anabolic therapies on the horizon for children with primary and secondary osteoporotic conditions.
-
Osteoporosis therapy should be undertaken by clinicians with sufficient experience in the safe and effective administration of bone-targeted drugs.
1 Introduction
Once considered a disease of the aging, osteoporosis is now recognized as an important facet of clinical care in children with genetic disorders predisposing to bone fragility and in children with serious acute and chronic illnesses. At the same time, approaches to the management of osteoporosis during the pediatric years are complicated by a number of factors, including the impact of variable growth rates and tempos of puberty on size-dependent bone mineral density (BMD) testing, distinguishing pathological fractures from those sustained during the course of normal childhood development, and the fact that informative, well-designed intervention trials are themselves a hurdle due to limitations such as small sample sizes in pediatric compared to adult trials.
While many principles from the adult osteoporosis literature can be adapted to children, the development of the mature skeleton is nevertheless a complex, multi-decade process that gives rise to unique considerations. Some of these differences have been unearthed through long-term natural history studies using standard, widely available evaluative tools, while others have been demonstrated through more recent developments such as peripheral quantitative computed tomography (pQCT) and trans-iliac bone histomorphometry. Knowledge of pediatric-specific principles and their biological basis is essential for forming logical management decisions in the young.
The purpose of this chapter is to review evidence that shapes the current approach to diagnosis, monitoring, and management of osteoporosis in childhood, with particular emphasis on the key biological principles that are pivotal to the overall approach and on the main questions with which clinicians struggle on a daily basis. The scope of this article spans the review of specific disorders and risk factors associated with osteoporosis in childhood, the clinical manifestations of osteoporosis, issues with respect to the definition and the diagnosis, and recommendations for monitoring and prevention of osteoporosis in at-risk children. Finally, this article discusses when a child is a candidate for osteoporosis drug therapy, which agents and doses should be prescribed, the length of therapy, how the response to therapy should be evaluated, and side effects. With this information, the bone health clinician should be poised to identify which children should be targeted for osteoporosis therapy and the clinical outcomes that reflect safety and efficacy.
2 The Etiology and Mechanisms of Childhood Osteoporosis
As highlighted in recent reviews [1,2,3,4,5], childhood osteoporosis is typically divided into primary and secondary etiologies , with osteogenesis imperfecta (OI) representing the prototypical primary osteoporosis of childhood. There is a growing list of secondary osteoporotic conditions of childhood (i.e., osteoporosis caused by underlying diseases and/or their treatment), with most falling into two broad categories: glucocorticoid (GC)-treated diseases and disorders with compromised mobility. A list of the most frequent causes of primary bone fragility disorders (and their related genes, proteins, and phenotypic features) is provided in ◘ Table 24.1. A list of the secondary osteoporotic conditions of childhood is provided in ◘ Table 24.2.
2.1 Primary Osteoporosis
Among the most exciting recent developments in the pediatric bone health field has been discovery of genes implicated in heritable bone fragility disorders. While the phenotypic heterogeneity in congenital bone fragility has been known for years [6], the spectrum of the genetic basis has only recently come to the fore. Most cases of congenital bone fragility are still due to mutations in the coding regions of the type I collagen genes (COL1A1 and COL1A2, classically referred to as OI types I, II, III, and IV based on disease severity); however, over a dozen additional genetic causes have been described with novel pathobiology and often discrete clinical features [7, 8] (◘ Table 24.1). In many cases, heritable bone fragility is suggested by the family history or typical physical findings (blue sclerae, dentinogenesis imperfecta). However, these stigmata are not universal even in the presence of type I collagen mutations [9]. In practical terms, the diagnosis of OI remains a possibility in any child with recurrent fractures once a secondary cause has been ruled out (◘ Fig. 24.1).
Algorithm of the approach to the diagnosis and treatment of children with fractures due to osteoporosis (From: Ward et al. [5]. Reprinted with permission from Springer)
2.2 Secondary Osteoporosis
Advances in pediatric care have led to significant improvements in cure rates for acute disorders such as childhood leukemia [10] and in longevity for chronic disabling conditions such as Duchenne muscular dystrophy (DMD) [11]. With improved outlooks for such children (◘ Table 24.2), there is increasing focus on long-term sequelae and quality of life. Despite advances in chemotherapy and disease-modifying interventions, GC therapy remains the mainstay of treatment for many serious illnesses in the first few years of the illness for disorders, such as leukemia and rheumatic conditions [12, 13], and for decades in boys with DMD [14]. Recently, the use of GC-sparing biological agents has led to improved health outcomes for children with Crohn’s disease [15] and juvenile arthritis [16, 17]; not surprisingly, evidence for a positive effect of these agents on skeletal health has been demonstrated in a number of contemporary studies [16, 18,19,20].
A recent census of our bone health clinic (carried out in a general, tertiary pediatric hospital) showed that out of 89 patients with chronic illnesses and a history of low-trauma fractures treated with osteoporosis therapy, 40% had GC-naïve neuromuscular disorders (cerebral palsy, congenital myopathy), 27% had GC-treated DMD, 24% had other GC-treated disorders (Crohn’s disease, rheumatic disorders myasthenia gravis), and 9% had leukemia or other cancers. This census provides insight into the systemic illness groups likely to present to a pediatric bone health clinic with low-trauma fractures requiring osteoporosis intervention.
3 Clinical Presentations and Predictors of Osteoporotic Fractures
3.1 Vertebral Fractures
A number of studies have highlighted that vertebral fractures (VF) are an important yet underappreciated manifestation of osteoporosis in children. This is particularly true in children with GC-treated disorders given the predilection of GC therapy to adversely impact the trabecular-rich spine [21, 22]. In GC-treated illnesses such as rheumatic disorders, nephrotic syndrome, leukemia, and DMD, the prevalence of VF ranges from 7% to 32% [22,23,24,25] and the 12-month incidence from 6% to 16% [26–28] depending upon the underlying disease. The peak annual incidence in children with GC-treated rheumatic disorders and leukemia occurs at 1 year, at the time during which annual GC exposure is highest for most patients [12, 13]. At the same time, children with chronic diseases who are GC naïve are not exempt from spine fragility, since vertebral collapse has been shown to occur in 25% of children with motor disabilities in the absence of GC therapy [19].
VF often go undetected in children for two main reasons. First, VF can be asymptomatic [23,24,25,26,27,28], despite moderate to severe collapse [12, 23]. Secondly, routine surveillance with intermittent spine x-rays has not historically been signaled an important component of osteoporosis monitoring. However, a recent position statement by the International Society for Clinical Densitometry (ISCD) proposed that monitoring beyond BMD is needed in at-risk children, since the diagnosis of osteoporosis in children with at least one VF no longer requires BMD criteria [30]; furthermore, the position statement acknowledges that BMD Z-scores above −2 standard deviations (SD) do not preclude increased vertebral and non-VF risks.
3.2 Non-vertebral Fractures
Low-trauma non-VF in childhood are observed most frequently at the femur, tibia, forearm, humerus, feet, and ankles [22, 31, 32]. Long bone fractures are the most frequent and disabling of the non-VF in childhood, while hip fractures occur rarely and should prompt consideration of serious underlying diseases such as childhood leukemia [33]. Looser zones, also known as “insufficiency fractures,” may be mistaken for osteoporotic fractures; however, they represent the distinctly different process of osteomalacia, defined histomorphometrically as an increase in osteoid thickness associated with prolongation of the mineralization lag time. Looser zones appear as incomplete cracks in the cortices of the ribs, scapulae, medial shafts of long bones, and pubic rami. In such cases, the patient requires an assessment for a disorder of calcium and/or phosphate metabolism including a hand x-ray (to rule out rickets if the growth plate is still active) and biochemical parameters of bone and mineral ion metabolism (◘ Fig. 24.1).
3.3 The Frequency and Clinical Predictors of Fractures in At-Risk Children
In recent years, there has been an effort to delineate disease-specific risk factors for osteoporosis through natural history studies, by assessing the precise relationship between various illness-related factors and fractures, as well as the relationship between measurable indicators of bone health and fractures (such as BMD and back pain; see ◘ Table 24.3). These studies have provided robust results that fine-tune the clinician’s ability to identify the at-risk child.
3.3.1 Vertebral Fractures
As shown in ◘ Table 24.3, a number of studies have been sufficiently powered to assess clinical predictors of prevalent or incident (new) VF in univariate or multivariable models. Studies which show significant differences in relevant clinical parameters between those with and without VF have also been included in ◘ Table 24.3. Most studies have been retrospective or cross-sectional; relatively few studies have assessed the frequency of incident VF in relation to the evolving clinical trajectory of the child.
From these studies, a number of clinically useful themes have emerged. First, GC exposure is a consistent predictor of both prevalent and incident VF, an observation that is not surprising given clinical experience and the known osteotoxicity of GC therapy. Both cumulative and average daily dose predict VF in a number of different diseases as outlined in ◘ Table 24.3, as well as GC dose intensity (“pulse therapy”) in children with leukemia [12]. Secondly, leukemia studies have shown that prevalent VF around the time of GC initiation are highly predictive of future fractures, a phenomenon known in adults as “the VF cascade” [12, 26]. In fact, even mild (grade 1) VF independently predict future fractures, highlighting the importance of identifying early signs of vertebral collapse [12, 26]. While back pain predicted prevalent VF in two studies of children with GC-treated leukemia and rheumatic disorders [23, 25], pain did not predict new VF [12, 13]. The message arising from these data is that a lack of back pain does not rule out the presence of VF in at-risk children.
The fact that prevalent VF around the time of GC initiation predict future VF draws attention to the clinical importance of understanding the skeletal phenotype early in the child’s disease course. In children with GC-treated rheumatic disorders, other discrete clinical features in the first year were also independent predictors of future VF, including increases in disease activity scores in the first 12 months of GC therapy as well as increases in body mass index and decreases in lumbar spine (LS) BMD Z-scores, both in the first 6 months of GC therapy [13]. In children with solid organ transplantation, older age was another consistent predictor of increased VF risk [34,35,36,37].
3.3.2 Non-vertebral Fractures
Predictors of non-VF fractures in children with chronic illnesses are also outlined in ◘ Table 24.3, most of which are cross-sectional or retrospective. Loss of ambulation, anticonvulsant medication, and reductions in BMD at various skeletal sites are among the most consistent predictors of non-VF in this setting. An important observation making use of lateral distal femur BMD, a frequent site of fracture in children with neuromuscular disorders, is that every 1 SD reduction in BMD Z-score at this site was associated with a 15% increase in lower extremity fractures [38].
4 Outcomes and Complications: Morbidity, Mortality, and Recovery
The clinical consequences of osteoporosis arise from the fractures themselves or short- and long-term consequences of bone fragility. Fractures cause pain, and anyone who has sustained a fracture will attest to the clinically significant nature of such pain. Lower extremity fractures invariably compromise mobility in the short-term; however, premature loss of ambulation is seen in disease groups with tenuous ambulation to begin with such as cerebral palsy and DMD. Fractures can also cause deformity of the spine and extremities, both of which can lead to functional impairment . In such cases, surgical intervention may be needed to restore functional abilities. In adults, mortality has long been linked to hip and spine fractures [39]; whether these associations are true in children remains unclear. However, fat embolism syndrome following long bone fractures has been described in pediatric DMD [40, 41], while another study suggested that bisphosphonate therapy for osteoporosis was linked to survival [42].
The pediatric skeleton is a dynamic structure with the distinct capability to not only reclaim BMD lost during transient bone health insults, but to reshape fractured bone (including vertebral bodies) through the process of skeletal modeling. Both indices are important measures of recovery in children, either spontaneously or following osteoporosis therapy (i.e., bisphosphonate treatment). Since vertebral body reshaping appears to be growth-mediated, as it has never been unequivocally reported in adults [43], we postulate that bisphosphonate therapy does not directly bring about reshaping but rather has a permissive effect by enhancing BMD in order to prevent further collapse [44].
The disease that has been best-studied for signs of recovery from skeletal insult in the absence of osteoporosis therapy is acute lymphoblastic leukemia (ALL). This is unsurprising, since ALL represents a transient threat to bone health in the majority of patients undergoing current treatment strategies. Mostoufi-Moab [45] assessed children by tibia pQCT and found that trabecular and cortical BMD Z-scores were low compared to healthy controls within 2 years post-chemotherapy completion, but that significant improvements (on average 0.5 SD) were evident a year later. Cortical dimensions also increased, followed by increases in cortical BMD. Other studies have also shown recovery in bone mass and density in the years following chemotherapy [46, 47]. Lack of BMD restitution is linked to craniospinal radiation, particularly at doses ≥ 24 Gy [47]. However, it should be noted that the lower spine BMD among those with radiation exposure appears to arise in part from hormone deficiency-related short stature. Other recognized risk factors for incomplete BMD recovery in ALL include untreated hypogonadism, vitamin D deficiency, hypophosphatemia, low IGF-binding protein-3, and reduced physical activity [48].
The fact that reshaping can occur during leukemia chemotherapy (i.e., during high-dose GC treatment) is hypothesized to result from the saltatory pattern of GC exposure with current treatment protocols (◘ Fig. 24.2a). Vertebral body reshaping has also been observed in our clinic among children with rheumatic disorders post-GC cessation, though not previously reported (◘ Fig. 24.2b). On the other hand, older children who have insufficient residual growth potential can be left with permanent vertebral deformity after vertebral collapse (◘ Fig. 24.2c). The long-term consequences of permanent deformity remain unstudied; however, reports in adults indicate reduced quality of life due to pain and functional limitation [49, 50]; whether the same is true in later stages of life following permanent vertebral deformity sustained during childhood warrants further study.
a (I) Lateral spine radiographs in a 7.7-year-old girl at diagnosis with pre-B acute lymphoblastic leukemia showing a normal spine radiograph. (II) Vertebral fractures after 1 year of chemotherapy are as follows: grade 3 (severe) wedge fractures at T12 and L1, grade 2 (moderate) biconcave fracture at L2, and grade 3 (severe) biconcave vertebral fractures at L3 and L4. (III–V) These panels show stages in vertebral body reshaping with a “bone within bone” appearance during and after chemotherapy, in the absence of bone-specific (bisphosphonate) therapy. b (I) Lateral spine radiographs showing vertebral fractures in a toddler with systemic-onset juvenile idiopathic arthritis. Grade 2 vertebral fractures at T12 and L1 on GC therapy at 1.4 years of age. (II) At 4.9 years of age, she has almost complete recovery of vertebral height ratios with the typical “bone within bone” appearance, in the absence of bone-specific (bisphosphonate) therapy. c (I) Lateral spine radiographs showing a grade 3 (severe) fracture at L3 in a 15.3-year-old girl with pre-B acute lymphoblastic leukemia 3 months after diagnosis. At diagnosis, she had already attained final adult height. (II, III) Lack of reshaping due to fused epiphyses and absence of endochondral bone formation (From: Ward et al. [5]. Reprinted with permission from Springer)
To understand the vertebral body reshaping phenomenon further, the Canadian STeroid-Induced Osteoporosis in the Pediatric Population (STOPP) Consortium has studied determinants of complete versus incomplete reshaping in bisphosphonate-naïve ALL (quantified by a decrease in a positive spinal deformity index (SDI) [51] by 100% in the 6 years following diagnosis). Preliminary analyses suggest that many children reshape following VF in ALL but those with moderate or severe vertebral collapse and those who are older children appear to reshape less frequently. The next question is whether children with VF and persistent bone health threats in the context of other diseases such as GC-treated DMD can undergo vertebral body reshaping without bisphosphonate therapy. To date, there are no published reports to suggest such they do, a fact that is concordant with our own clinical experience.
5 The Definition of Osteoporosis and Diagnostic Evaluation in At-Risk Children
5.1 Bone Health Monitoring: Goals and Candidates
The ultimate goal of monitoring is to identify high-risk patients for intervention that will prevent the first fracture. However, lack of available data to support such primary prevention has led to monitoring that identifies early rather than late signs of osteoporosis, followed by bone-active treatment in those with limited potential for spontaneous recovery (including limited potential to undergo vertebral body reshaping). This is in line with a secondary prevention approach, which seeks to mitigate the progression of the osteoporosis following identification in its earlier stages.
Two important observations have shifted monitoring away from a BMD-centric to a more functional approach: (1) the use of a BMD Z-score threshold to identify a child who is at risk is problematic due to variability in the Z-scores generated by the different available normative databases [52,53,54] and (2) asymptomatic VF can occur at BMD Z-scores > −2, thereby requiring imaging surveillance for VF detection. Other functional outcomes should also be tracked during monitoring including any history of non-VF, growth, pubertal status, mobility, pain, muscle strength, and the potential for spontaneous recovery (vertebral body reshaping and bone density restitution). BMD remains a vital part of the bone health monitoring approach but as an adjuvant tool to chart the child’s BMD trajectory, thereby signaling a child who is losing ground and thereby at increased risk for fractures or who is showing signs of recovery following a transient bone health threat (potentially obviating the need for osteoporosis treatment).
Patients expected to be GC-treated for ≥3 months should be considered for a baseline spine radiograph (or high-quality dual-energy x-ray absorptiometry (DXA)-based VF assessment (VFA), if available) at the time of GC initiation. GC therapy for ≥3 months is the recommended cutoff since the earliest incident VF reported after GC initiation in children is at 4 months [28]. Children meeting the criteria for baseline spine imaging should also undergo a follow-up radiograph at 12 months, since this is the time point with the highest annual incidence of VF in GC-treated children [12, 28]. Imaging for VF is advised every 1–2 years thereafter for those with ongoing GC exposure. The predictors of VF outlined in ◘ Table 24.3 can facilitate the decision around frequency of VF follow-up assessments beyond 12 months in the various disease groups.
Among children with other risk factors for bone fragility apart from GC exposure (◘ Tables 24.1, 24.2, and 24.3), the same principles apply; that is, the patient should be assessed for both non-VF and VF since GC-naïve children with mobility issues and genetic bone fragility can also develop VF [7, 29]. In youth with impaired mobility due to cerebral palsy and congenital myopathies, a spine radiograph is recommended at the latest by about 6 years of age and then at intervals thereafter until the end of growth or sooner in the presence of back pain. Monitoring is recommended to start by this time since treatment should be initiated before there is insufficient residual growth potential for vertebral body reshaping.
Since BMD is useful as a serial measurement to assist the clinician in understanding the child’s overall bone health trajectory and in making logical decisions about the need for ongoing monitoring, discharge from bone health care, or intervention, it is recommended that a BMD assessment be carried out at least as frequently as spine radiographs according to the above guidelines, with assessments every 6 months in those children at greatest risk [4, 30].
5.2 Axial Skeletal Health: Vertebral Fracture Detection Methods and Imaging Modalities
The most widely used tool for the assessment of VF in both children and adults is the Genant semiquantitative method [55, 56]. According to the Genant method, the definition of a VF is ≥ 20% loss in vertebral height ratio regardless the VF morphology (wedge, bi- or mono-concave, or crush). VF are subjectively graded by trained readers according to the magnitude of the reduction in vertebral body height ratios, without direct measurement. Vertebral height ratios are generated when the anterior vertebral height is compared with the posterior height (for an anterior wedge fracture), middle height to the posterior height (bi- or mono-concave fracture), and posterior height to the posterior height of adjacent vertebral bodies (crush fracture). The Genant scores correspond to the following reductions in height ratios: grade 0 (normal), <20%; grade 1 fracture (mild), ≥20% to 25%; grade 2 fracture (moderate), >25% to 40%; and grade 3 fracture (severe), >40%. Overall, the Genant semiquantitative method is preferred over quantitative (6-point) vertebral morphometry [57], since it is faster, and takes into consideration the expertise of an experienced reader. In addition, it quantifies the severity of VF (an important predictor of the lack of potential for spontaneous vertebral body reshaping following VF in children). Furthermore, the Genant scoring system permits calculation of the SDI, the sum of the Genant grades along the length of the spine [51]. The SDI is a global index of spine morbidity that is useful clinically and can be used as a continuous outcome variable in research studies [58]. The kappa statistics for intra- and interobserver agreement are similar for children compared to adults using the Genant semiquantitative method [55, 59, 60].
A number of recent studies have provided validity for the Genant approach in children. First, Genant-defined VF show a bimodal distribution from T4 to L4 similar to the known distribution in adults [61,62,63,64], with a predilection for the mid-thoracic region (T5 to T8, the site of the natural kyphosis) and the thoracolumbar junction (the site of transition to the natural lordosis) [23, 64]. Secondly, biologically relevant clinical predictors of Genant-defined VF have been identified including back pain, low LS BMD Z-scores, longitudinal declines in LS BMD Z-scores, and GC exposure [13, 23, 26]. One of the most important observations to assert the validity in children is that both mild and moderate-severe Genant-defined VF at leukemia diagnosis are robust clinical predictors of new VF over the next 3 years [12, 26].
To date, the most common imaging tool for VF detection in childhood is lateral thoracolumbar spine radiographs. In view of the high radiation exposure from spine radiographs but nevertheless critical need for VF assessments as part of bone health evaluations, non-radiographic imaging techniques have been developed which use the scoring methods described above. The use of DXA to diagnose VF is called VF assessment (VFA), with images captured on a lateral spine view. VFA is attractive as an assessment tool given its minimal radiation and the fact that fan-beam technology facilitates capture of the entire spine on a single image without divergent beam issues due to parallax. Newer DXA machines have a rotating “c-arm” which obviates the need to reposition the patient from the supine to lateral position. Image quality varies significantly depending on the densitometer [65]. Using a Hologic Discovery A machine, Mayranpaa et al. [66] showed low diagnostic accuracy for VFA compared to lateral spine radiographs and poor visibility in children. Pediatric studies on newer DXA machines are presently underway and preliminary data are promising.
5.3 Axial Skeletal Health: Trans-iliac Bone Biopsies
Iliac crest bone biopsies with tetracycline labeling provide unique diagnostic information about static and dynamic bone properties that cannot be obtained by any other means (i.e., osteoid thickness, bone formation rate, mineralization lag time, and other bone formation and resorption indices) [67]. In practical terms, biopsies are useful in establishing the cause of osteoporosis in special cases such as a child with unexplained bone fragility and negative genetic studies. Idiopathic juvenile osteoporosis has a characteristic histomorphometric appearance – low bone turnover and thin osteoid seams – but clinically may be difficult to distinguish from other forms of osteoporosis such as non-deforming OI without blue sclerae, wormian bones, or a family history [68, 69]. Similarly, patients with OI typically have a histological hallmark (hyperosteocytosis) that is helpful diagnostically in rare cases when studies are falsely negative [68, 69]. At the same time, few clinicians are trained in this technique, and so overall, it is a rarely used tool aside from highly specialized clinics.
5.4 Axial and Appendicular Skeletal Health: Dual-Energy X-Ray Absorptiometry
DXA is the most commonly used and widely available technique to measure bone mass and density in children, since it is highly reproducible and inexpensive and confers low radiation exposure. LS and total body less head are the preferred measuring sites [70]; recently, lateral distal femur BMD Z-scores have also been useful in children with neuromuscular disorders who prefer to position on their side [38, 71] (◘ Table 24.3). BMD raw values are converted to age- and sex-specific SD scores (Z-scores) and require additional interpretation in view of body size, ethnicity, and pubertal staging or skeletal maturity (the latter, by bone age) [72]. Since BMD can be underestimated in children with familial short stature, and children with chronic illnesses may be transiently or permanently short due to the effects of the disease/treatment on linear growth and puberty, adjustment for bone size using a technique such as derivation of bone mineral apparent density (BMAD, in g/cm3) [73] or height Z-score-corrected BMD Z-scores [74] is required to avoid underestimation of BMD parameters. BMAD has the advantage that it has been tested for its ability to accurately predict VF [75], whereas height Z-score-corrected BMD Z-scores have not. Lateral distal femur BMD Z-scores predicted non-VF in children with neuromuscular disorders [38] and furthermore, this assessment method is taken at a clinically relevant site, since children with neuromuscular disorders often fracture at this location. Despite challenges in BMD interpretation due to variable growth rates and timing and tempos of puberty, numerous studies (◘ Table 24.3) confirm an inverse relationship between BMD and fracture rates, and serial measurements provide additional information about the child’s overall bone health trajectory that can inform whether there is a need for ongoing bone health monitoring.
5.5 Appendicular Skeletal Health: Peripheral Quantitative Computed Tomography
pQCT at the radius and tibia provides information that cannot be obtained by DXA about musculoskeletal geometry as well as “true” (volumetric) cortical and trabecular BMD. For example, in children with cerebral palsy, it has been shown that smaller bone and cortical cross-sectional area are the main structural defect, rather than lower cortical BMD [76]; pQCT studies have also shown that cortical thickness and not density is the main parameter impacted by growth hormone deficiency and treatment [77]. pQCT is particularly useful when DXA studies are precluded due to spine deformity, hip and knee contractures, or metallic hardware. The newest technique, high-resolution pQCT, has the spatial resolution to measure trabecular geometry and microarchitecture. At the moment, pQCT and high-resolution pQCT are research tools in most centers.
5.6 Bone Turnover Markers
Bone turnover markers (BTM) are often measured in children undergoing a bone health assessment or while on osteoporosis therapy. Recently, two markers have been recommended by the International Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine [78]: serum procollagen type I N-terminal propeptide (PINP, a marker of bone formation) and serum collagen type I cross-linked C-telopeptide (CTx, a marker of bone resorption), both of which have been studied in healthy children in order to generate reference data [79,80,81,82]. These analytes were chosen because of their specificity to bone and relationship to relevant outcomes in adult clinical studies as well as their stability, wide availability, and ease of analysis and procurement.
BTM are influenced by several factors that lead to high intra- and interindividual variability, including age/pubertal stage, gender, time of day, food intake, physical activity, recent fractures, serum 25-hydroxyvitamin D status, assay methods, and sample transport and storage conditions. One of the main factors that have limited their use in children, particularly for those with chronic illness and growth delay, is that BTM are largely a reflection of linear growth and not bone turnover per se. In children, the only available method to determine the bone turnover status with certainty is to directly measure bone formation and resorption on trabecular surfaces via trans-iliac bone biopsy; however, this tool is not in widespread clinical use.
In children, BTM provide some insight into general diagnostic categories; for example, urinary NTx levels are high pre-bisphosphonate treatment in children over 3 years of age with OI [83] and correlate with an increased trabecular bone formation rate on trans-iliac biopsies [84]. Low BTM and trabecular bone formation are frequently observed in chronic illness osteoporosis both before [44, 85] and after years [44] of GC therapy. LRP5 mutations causing juvenile osteoporosis are also characterized by low BTM and trabecular bone formation [86, 87]. On the other hand, brisk increases in BTM can signal recovery from growth failure and bone mass deficits as observed in children undergoing effective treatment for Crohn’s disease [16]. A low alkaline phosphatase can separate patients with OI from those with hypophosphatasia – an important distinction since bisphosphonates are contraindicated in hypophosphatasia and furthermore, a life-saving medical therapy is now available to treat the severe infantile form [88].
To date, there are no studies in childhood which have assessed fracture risk reduction or frequency of adverse effects according to thresholds of bone turnover marker reduction with bisphosphonate therapy. At the present time, BTM during pediatric osteoporosis therapy serve to document that the drug is exerting the anticipated biological effect and provide a measure of compliance.
5.7 The Definition and Diagnosis of Osteoporosis in Children
The definition and diagnosis of osteoporosis in children has been fraught with challenges and controversy over the years, following the widespread availability of BMD by DXA that led to zealous testing in myriad pediatric populations. In recent years, there has been a move away from a BMD-centric diagnostic focus to a more functional approach. While the clinical relevance of BMD testing has been clearly affirmed by numerous studies showing consistent, inverse relationships between BMD Z-scores and low-trauma fractures in children (◘ Table 24.3), the proportion of children assigned a BMD Z-score ≤ −2.0 varies considerably depending on the BMD normative database that is used to generate the Z-scores [52,53,54]. Specifically, the Canadian STOPP Consortium reported the magnitude of the disparity in LS BMD Z-scores generated by normative databases from both Hologic and Lunar machines in children with ALL at diagnosis [54], showing a difference of as much as 2.0 SD depending upon which database was used to generate the Z-scores. Secondly, the Consortium reported that 48% of children with VF at the time of leukemia diagnosis had BMD Z-scores > −2.0.
These disparate results in BMD Z-scores depending on the reference data that is used plus the fact that VF can occur above the −2.0 threshold suggested that the use of a LS BMD Z-score cutoff as part of the definition of osteoporosis in children with VF was not valid [54]. This view has been underscored by the ISCD in an updated (2013) position statement [30] which notes a BMD Z-score threshold of ≤ −2.0 is no longer required to diagnose osteoporosis in a child with a VF; in fact, there are no longer BMD Z-score requirements at all in the setting of a low-trauma VF. In the 2013 ISCD recommendation, the use of a BMD Z-score threshold (−2.0 or worse) has been retained to denote osteoporosis in children with long bone fractures, provided such children also have a clinically significant fracture history defined as ≥ 2 long bone fractures by age 10 and ≥ 3 long bone fractures by age 18 [30]. At the same time, the 2013 ISCD position statement notes that a BMD (or bone mineral content) Z-score > −2.0 does not preclude an increased fracture risk of long bone fractures. This caveat is affirmed by Henderson et al.’s report that up to about 15% of children with neuromuscular disorders and lower limb fractures had lateral distal femur BMD Z-scores > −2.0 [38]; similar observations have been made in adolescents with anorexia nervosa [89].
Despite the disparity in LS BMD Z-score generated by different normative databases, Ma et al. [54] showed in children with ALL at diagnosis that the relationships between LS BMD Z-scores and VF are consistent regardless of the reference databases that are used to generate the Z-scores. This is not surprising, since the available reference databases are all highly correlated with one another (with r value ranges from 0.85 to 0.99) [54]. These findings suggest that while the use of a LS BMD Z-score threshold is not valid for the diagnosis of osteoporosis in children with VF, and that this is likely also true in relation to other BMD sites in children with extremity fractures [38], the use of LS BMD Z-scores as a continuous variable risk factor for VF in clinical research studies nevertheless remains valid.
Where does this leave the clinician in the pivotal decision to label a child with osteoporosis? On balance, current evidence puts the weight of the diagnosis on the fracture history. Among children with risk factors for osteoporosis, a low-trauma fracture is usually apparent (falling from a wheelchair, sustaining a fracture during a seizure); in such cases, a size-corrected BMD Z-score > −2.0 should not deter the clinician from the osteoporosis diagnostic label.
On the other hand, in the case of an otherwise healthy child with recurrent fractures but absence of risk factors, stigmata of OI, or a genetically confirmed family history of osteoporosis, it is incumbent upon the clinician to find evidence of additional features to support the diagnosis of osteoporosis (◘ Fig. 24.1). A VF without a history of trauma is highly suggestive of an underlying bone fragility condition, and the lower the BMD, the more likely an osteoporotic phenotype (although a normal BMD does not categorically rule out osteoporosis as discussed). Genetic testing is indicated in such children, since even children with type I collagen mutations can lack typical stigmata. Overall, about 7% of patients with a mutation in the type I collagen genes will be without either blue sclerae or dentinogenesis imperfecta [Frank Rauch, personal communication].
Since over a dozen genes have now been implicated in OI or “OI-like” bone fragility (◘ Table 24.1), questions have been raised about the best way to describe the various forms of mild, moderate, and severe genetic forms of osteoporosis. While some reports retain the original OI subtype nomenclature [90] (i.e., types I to XVI, expanding on the initial classification proposed by Sillence [91]), recently it has been proposed that congenital bone fragility should be described according to the implicated gene and that the term OI should be reserved for genetic forms which involve type I collagen pathobiology [92]. This approaches simplifies the diagnosis of genetic bone fragility for the clinician, clustering diagnoses into broad categories based on known genetic underpinnings (see ◘ Table 24.1 for phenotypic characteristics associated with each). ◘ Figure 24.1 provides an overview of the approach to the diagnosis of osteoporosis in children. It should be remembered that a young child with unexplained fractures, lack of evidence for a secondary cause of osteoporosis, and normal genetic studies may be the victim of non-accidental trauma.
6 Treatment
6.1 General Measures for Optimization of Bone Health
First-line measures to optimize bone health fall into three main categories: nutrition (calcium, vitamin D, protein, potassium, magnesium, copper, iron, fluoride, zinc, and vitamins A, C, and K), physical activity, and treatment of the underlying condition and associated comorbidities; these have been recently reviewed extensively elsewhere [1, 2, 5, 93,94,95,96,97,98,99] and will not be reiterated here with the exception of vitamin D status given its tendency to be entrenched in controversy, making it difficult for the clinician to see the forest for the trees.
The recommended intake of vitamin D is a minimum of 600 IU/day [100], although higher doses are often required to meet target levels, particularly in those with malabsorption, obesity, and darker skin [100]. Adequate total body vitamin D stores have been defined at a serum 25-hydroxyvitamin D level ≥ 50 nmol/L (20 ng/mL) [100, 101] or ≥ 75 nmol/L (30 ng/mL) [102], mostly based on adult studies. In children, the optimal serum 25OHD threshold remains under debate. A meta-analysis showed a lack of significant effect of vitamin D supplementation and 25OHD levels ≥ 50 nmol/L on BMD in healthy youth [103], a bone histomorphometric study in children with OI failed to show an association between serum 25OHD levels and bone mineralization or bone mass [104], and calcium plus vitamin D supplementation had no effect on spine BMD in children with inflammatory bowel disease [105] and leukemia [106]. Overall, the optimal serum 25OHD threshold associated with health benefits across the life cycle remains controversial as discussed in a large contemporary “umbrella” assessment of published systematic reviews and meta-analyses [107]. From a practical perspective, a minimum 25-hydroxyvitamin D level of 50 nmol/L (20 ng/mL) is recommended in youth through diet and/or supplementation, with measurement of 25-hydroxyvitamin D in high risk populations ideally at the end of winter in order to determine compliance with and efficacy of prescribed doses at the time of the nadir.
For children with chronic illnesses, adequate treatment of the underlying illness is the mainstay of osteoporosis prevention and treatment. The situation is complicated by the fact that some of the standard therapies are osteotoxic , including GC, high-dose methotrexate in the cancer setting [108], calcineurin inhibitors [109], hepatic microsomal enzyme-inducing anti-epileptics increasing catabolism of 25-hydroxyvitamin D, and long-term use of anticoagulants [110] and medroxyprogesterone [111]. Wherever possible, these agents should be used sparingly in children with risk factors for osteoporosis, a principle that is not always practical given, for example, the need for GC therapy to treat systemic inflammatory diseases and leukemia and to slow the progression of the myopathy in DMD.
Identification of endocrine comorbidities is also appropriate, including treatment of delayed puberty, growth hormone deficiency, hyperthyroidism, and diabetes. Growth hormone therapy increases areal BMD even after final adult height attainment and should be continued through adulthood in those with low size-adjusted BMD or fractures [112]. A word of caution in the use of growth hormone to treat GC-induced growth failure in DMD – in addition to a paucity of data to support the safety and efficacy of this approach, one of the current hypotheses is that short stature may be beneficial to muscle strength in DMD since stresses on the sarcolemma are higher with increases in the size of the muscle fiber [113]. A comprehensive review of the management of specific chronic conditions such as anorexia nervosa is beyond the scope of this chapter and is discussed elsewhere (► Chap. 12).
6.2 Drug Therapy: Candidates for Medical Intervention and Timing of Treatment Initiation
When to initiate medical treatment is a frequently posed question by clinicians. To date, intervention studies in children have largely been limited to case series and small observational or case-control studies, given the relative paucity of patients with various diseases at any one medical center and the challenges in securing funding for large, multicenter drug trials in the young. The absence of treatment trials targeting prevention of first-ever fractures in children has led to a conservative approach overall, with therapy typically reserved for children with overt bone fragility. Among those with chronic illness and osteoporosis, there is an additional consideration – not every child with symptomatic osteoporotic fractures and chronic illness requires osteoporosis therapy given the potential for spontaneous (medication unassisted) recovery if risk factors are transient, including reshaping of previously fractured vertebral bodies. The potential for spontaneous recovery in children with transient risk factors demands controlled trials in this setting.
Where primary prevention with drug therapy prior to the first fracture is concerned, at the present time, there is insufficient data to recommend osteoporosis therapy other than the general measures discussed previously. In the future, primary prevention drug trials should target priority disease groups including the progressive neuromuscular disorders like GC-treated DMD. Here, there is an urgent need for well-designed trials on sufficient numbers of patients to effectively assess functional outcomes including fractures, pain, and mobility when treatment is started before the first fracture.
Since there are insufficient data to recommend drug therapy for the primary prevention of osteoporotic fractures in children with any condition at the present time, careful monitoring in at-risk children to identify those with early signs of bone fragility, particularly in those with limited potential for spontaneous recovery, is indicated. Such an approach follows the principles of secondary prevention – to mitigate osteoporosis progression and foster recovery in those with earlier (rather than later) signs of osteoporosis. Given the knowledge that has emerged about the clinical populations at risk for osteoporosis and the disease-specific predictors of fractures, it is no longer appropriate for children to present to medical attention with, for example, back pain due to advanced vertebral collapse necessitating “rescue therapy.” Rather, pediatric programs should be established to effectively monitor at-risk children in order to identify earlier stages of vertebral collapse, followed by an assessment of the child’s potential for medication-unassisted recovery versus need for osteoporosis treatment. A monitoring program also provides the clinician with an opportunity to identify and treat vitamin D, mineral, and hormonal deficiencies, to encourage a healthy weight, to promote physical activity within the limits of the child’s underlying condition, and to encourage compliance with treatment of the underlying condition [16, 114].
Bisphosphonate therapy is typically reserved for children with a history of low-trauma fractures but also limited potential for spontaneous (i.e., medication-unassisted) recovery due to permanent or persistent osteoporosis risk factors (◘ Fig. 24.1). Low-trauma long bone fractures and symptomatic VF (or asymptomatic VF that are moderate or severe) are the most frequent indications for treatment. Extremity fractures at sites other than long bones (such as hands and feet, fingers and toes) do not usually warrant treatment. Studies are currently underway to evaluate the safety and efficacy of treating mild (Genant grade 1) asymptomatic or minimally symptomatic VF in pediatric osteoporosis; for now it is recommended that such fractures be closely monitored for symptomatology and/or progressive vertebral height loss that would prompt treatment.
After determining the child’s vertebral and long bone fractures status, the clinician assesses the potential for medication-unassisted recovery in view of the osteoporosis severity (including degree of vertebral collapse), residual growth potential, and whether risk factors are persistent or resolving. In the face of resolving risk factors at a young age (such as withdrawal of GC therapy in a pre-pubertal child), a conservative approach can often be taken that involves monitoring to document the child’s anticipated recovery. In contrast, children who are peri-pubertal or older as well as younger children with ongoing risk factors or heritable forms of osteoporosis will have less potential for spontaneous reshaping of vertebral bodies and reclamation of BMD – such children are optimal candidates for osteoporosis therapy. Of course, symptomatic osteoporosis (such as pain from VF limiting the child’s quality of life) is itself an indication for treatment; in such cases, osteoporosis therapy is recommended to relieve pain and allow the child to regain quality of life regardless of the child’s potential for spontaneous recovery in the future.
Following these steps facilitates the decision to start treatment in a child with a clear diagnosis of primary or secondary osteoporosis. As shown in ◘ Fig. 24.1, a frequent conundrum is whether to start treatment without a specific underlying diagnosis – a scenario referred to as “low-trauma, recurrent (usually extremity) fractures in otherwise healthy children.” In such cases, the clinician needs to make every effort to unearth a known cause, including the now expanded etiologies of heritable bone fragility outlined in ◘ Table 24.1 or chronic illnesses with insidious onset (such as Crohn’s or rheumatic diseases) outlined in ◘ Table 24.2. A low-trauma VF in this setting is highly suggestive of a bone fragility condition. When genetic and chronic illness evaluations are negative, a trans-iliac bone biopsy can also provide important clues although it is less readily available. When no specific diagnosis is forthcoming despite a comprehensive evaluation, the criteria to label a child with osteoporosis provided in the most recent ISCD position statement support the decision to initiate osteoporosis treatment: ≥ 2 long bone fractures by age 10 or ≥ 3 or more long bone fractures by age 18 and a size-corrected BMD or bone mineral content Z-score of −2 [30]. Low-trauma VF may also prompt treatment in these cases.
6.3 Bisphosphonate Treatment of Primary and Secondary Osteoporosis in Childhood
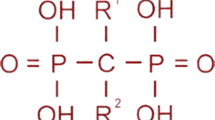
Bisphosphonates , synthetic analogues of pyrophosphate , are the most extensively published agents to treat osteoporosis in childhood [115, 116], despite the fact that they remain off-label in most countries. The vast majority of publications describing the effect of bisphosphonate therapy in children are observational, pre-post studies; there are relatively few controlled studies of bisphosphonate therapy in children, and even fewer studies have been sufficiently powered to assess fracture outcomes. The paucity of fracture outcome data in controlled trials reflects a number of considerations when studying children: the relatively small numbers of patients available for study, the historically adult focus of industry-sponsored trials, and the logistical and philosophical challenges enrolling younger patients. The latter issue includes pressure from families and health-care providers alike to treat individual pediatric patients despite insufficient evidence, instead of enrolling children in controlled trials that address uniquely pediatric safety and efficacy issues. Nevertheless, the few controlled studies available in addition to a number of key observational studies provide important and useful information about pediatric patients’ responses to bisphosphonate therapy.
6.4 Oral Versus Intravenous Bisphosphonate Therapy
The use of oral versus intravenous (IV) bisphosphonate therapy for pediatric osteoporosis has long been debated [117]. Overall, IV pamidronate is the mostly extensively reported agent in children following the inaugural, observational study in the late 1990s which showed improved pain, mobility, and reshaping of vertebral bodies following pamidronate therapy in children with moderate to severe OI [118]. Children were treated with cyclical, IV pamidronate at a dose of 9 mg/kg/year divided every 2–4 months up to 5 years’ duration [118]. In recent years, IV zoledronic acid has been introduced given the advantage that it can be given over a shorter period of time and less frequently [44, 119]; zoledronic acid is 100 times more potent than pamidronate [120]. Both agents are nitrogen-containing bisphosphonates that inhibit farnesyl diphosphate synthase and thereby protein prenylation, a process crucial for osteoclast survival. A randomized study comparing the two agents in OI showed that zoledronic acid had similar effects on LS BMD Z-scores and fracture rates over 12 months [119]. Of the oral agents, alendronate and risedronate have been the most extensively studied, with one report confirming that the oral bioavailability of alendronate in children is <1%, similar to adults [121].
◘ Figure 24.3 shows the mean difference in LS areal BMD Z-score change in published, controlled trials of bisphosphonate therapy for the treatment of childhood osteoporosis, with comparison of results in the treatment versus placebo/untreated control groups. As shown in ◘ Fig. 24.3, increases in spine BMD Z-scores were a consistent finding in all of the available controlled studies using oral alendronate or risedronate in children with OI; one report showed no effect of oral alendronate in a study of girls with anorexia nervosa [122]. In addition, a controlled study by Gatti et al. in pediatric OI (◘ Table 24.4) showed a significant effect of IV neridronate on the percent change in spine and hip BMD compared to controls after 1 year. Overall, it appears that IV and oral bisphosphonates consistently increase BMD parameters in children, as confirmed in recent Cochrane reviews on the use of bisphosphonates in pediatric secondary osteoporosis [116] and OI [115].
Mean difference in the lumbar spine area . BMD Z-score in published, controlled trials of bisphosphonate therapy for the treatment of children with osteoporosis, with comparison of results for the treatment versus placebo/untreated control groups. Studies were included with the following criteria: (1) at least ten patients per group, (2) prospective design with a placebo or untreated control arms, and (3) available data on either the pre- and posttreatment change in LS BMD Z-score with standard error OR the percent change in LS BMD Z-score. *Details about the magnitude of the mean change in LS BMD Z-score were not reported; however, the effect size with 95% CI was provided. &Seikaly 2005 was a placebo-controlled crossover study design with the results from the first year of the study presented. $Bishop 2013 reported least-squares mean difference. Abbreviations: ALN alendronate, AN anorexia nervosa, CF cystic fibrosis, GC glucocorticoids, IV intravenous, yrs. years, NER neridronate, OI osteogenesis imperfecta, OLP olpadronate, PO oral, Pts patients, RIS risedronate (From: Ward et al. [5]. Reprinted with permission from Springer)
On the other hand, the effects of IV versus oral bisphosphonates on fracture outcomes are less homogeneous, an observation that is evident in ◘ Fig. 24.4 (describing the relative risk of fractures in controlled bisphosphonate trials from data on the number of patients with fractures in the two groups) and ◘ Fig. 24.5 (showing the incidence rate of fractures in controlled trials from data on the number of fracture events in each group). Of the nine studies which permitted calculation of the relative risk of non-VF, only one by Bishop et al. [123] using risedronate in pediatric OI showed a decrease in non-VF risk. The other studies in ◘ Fig. 24.4 [122,123,124,125,126,127,128,129] found no significant differences compared to placebo or untreated controls in the relative risks of non-VF after oral alendronate, oral olpadronate, and IV neridronate. At the same time, ◘ Fig. 24.4 highlights that the direction of effects for non-VF risks in the nonsignificant studies was favorable for treatment in all but one study [122]. ◘ Figure 24.5 shows the incidence rate ratio of fractures using the number of fracture events in the two groups (a more powerful calculation since there are typically more fracture events than patients with at least one fracture). Two studies with nonsignificant results for the relative risk of non-VF had positive results when the incidence rate ratio was calculated [127, 128]. Most of the nonsignificant estimates in ◘ Figs. 24.4 and 24.5 had extremely wide confidence intervals but directions of effect in favor of treatment, suggesting that sample sizes were likely inadequate to show differences in fracture rates between the two groups.
Relative risk of vertebral and non-vertebral fractures in published, controlled trials of intravenous or oral bisphosphonate therapy for the treatment of children with osteoporosis, with comparison of the number of children with fractures in the treatment versus placebo/untreated groups. Studies were included in the figure if they met the following criteria: (1) at least ten patients per group, (2) prospective design with a placebo or untreated control arm, and (3) available data on the number of patients with fractures in each group. Abbreviations: ALN alendronate, AN anorexia nervosa, CF cystic fibrosis, GC glucocorticoid-treated, IV intravenous, yrs. years, NER neridronate, OI osteogenesis imperfecta, OLP olpadronate, PO oral, Pts patients, RIS risedronate (From: Ward et al. [5]. Reprinted with permission from Springer)
The incidence rate ratio in published, controlled trials of intravenous or oral bisphosphonate therapy for the treatment of children with osteoporosis with comparison of the number of fracture events in the treatment versus placebo/untreated control groups. Studies were included with the following criteria: (1) at least ten patients per group, (2) prospective design with a placebo or untreated control arm, and (3) data available on the number of fractures in each intervention group. &Seikaly 2005 was a placebo controlled crossover study design with the results from the first year of the study presented. Abbreviations: ALN alendronate, CF cystic fibrosis, GC glucocorticoid-treated, IV intravenous, yrs. years, NER neridronate, OI osteogenesis imperfecta, OLP olpadronate, PO oral, Pts patients, RIS risedronate (From: Ward et al. [5]. Reprinted with permission from Springer)
So how do we adjudicate whether oral or IV bisphosphonate therapy is more efficacious in the presence of such little controlled data and inadequate sample sizes to determine the effects on fractures? The answer appears to lie in the VF and vertebral body reshaping data. Based on observational studies, it is expected that fractured vertebral bodies will undergo reshaping with bisphosphonate therapy [44, 58, 130, 131], thereby providing a key index of benefit. The controlled trials to date which quantified vertebral body height clearly showed increases in those receiving IV bisphosphonate therapy [127, 132, 133], whereas none of the controlled oral bisphosphonate studies in which it was measured showed a positive effect on vertebral height [124, 128, 134]. Furthermore, in a large randomized trial of daily oral alendronate for moderate and severe pediatric OI [129], there was no effect of alendronate on the cortical width of trans-iliac specimens. In contrast, this is a key structural index derived from a precise measurement which has shown a positive response in OI to IV bisphosphonate therapy [84]. Another compelling observation that supports IV over oral therapy is from a controlled OI trial [124], where risedronate did not lead to an increase in the trabecular volumetric BMD at the distal radius compared to placebo; on the other hand, IV therapy caused significant increases in BMD at this site [135]. Overall, these data support the use of IV instead of oral bisphosphonate therapy first-line. At the same time, ◘ Figs. 24.4 and 24.5 underscore the need for controlled trials of osteoporosis therapies, especially in the secondary osteoporoses where there are only three controlled trials published to date and none sufficiently powered to address any fracture outcomes.
6.5 Monitoring the Efficacy of Bisphosphonate Treatment
Gauging the efficacy of bisphosphonate therapy rests on a number of clinical parameters, most of which are focused on the functional musculoskeletal health of the child. One of the main goals of therapy is remittance of back and bone pain which typically occurs within 2–6 weeks following IV bisphosphate therapy [44, 118]. In a child with VF, follow-up spine radiographs should be carried out in order to evaluate a number of efficacy parameters as outlined in ◘ Fig. 24.1.
In addition, the history of new non-VF should be recorded, along with details about the site of fracture, degree of trauma associated with the injury, need for surgical management , impact to quality of life, and duration of healing. Improvements in energy level [118], mobility, and muscle strength [136] are also monitored. BMD parameters are tracked as a measure of efficacy following initiation of bisphosphonate therapy; however, there are no studies which have addressed which BMD increment or cutoff is associated with a clinically acceptable decrease in fracture rates posttreatment initiation. In the absence of such data, it is advised that the areal BMD Z-score should stabilize (if previously on the decline) or increase beyond the precision of the measurement and furthermore, the areal BMD Z-score will approximate the patient’s height Z-score. Another approach is to aim for a BMD Z-score > −2 SD [58].
6.6 Bisphosphonate Dose Adjustments, Duration of Treatment, and the Effect of Treatment Discontinuation
The most frequently prescribed IV bisphosphonate regimen is cyclical IV pamidronate (maximum dose 9 mg/kg/year for children ≥3 years, 3 mg/kg divided equally over 3 days given every 4 months) [6, 83, 116, 118, 137]. Due to high bone turnover in younger children, pamidronate is dosed more frequently (2.25 mg/kg divided equally over 3 days, every 3 months for children 2–3 years of age, and 1.5 mg/kg divided equally over 3 days, every 2 months to children <2 years of age). Zoledronic acid is increasingly used in clinical care due to its ease of less frequent dosing intervals and shorter infusion time compared to pamidronate (maximum dose 0.1 mg/kg/year given as two equal doses (0.05 mg/kg) every 6 months in children ≥ 2 years and 0.025 mg/kg every 3 months in children <2 years) [119, 138, 139]. Some investigators have favored a lower annual starting dose (such as a single-day pamidronate infusion 1 mg/kg every 3 months, 4 mg/kg/year) [140, 141]. Apart from these regimens, other IV doses and intervals have also been reported (◘ Table 24.4) though none has gone head to head in controlled, comparative trials, the exception being pamidronate versus zoledronic acid which showed similar effects on BMD and fracture rates in OI [119]. With such little controlled comparative data, it is impossible to state which IV agents and regimens achieve the best results for mitigating fractures and pain and improving overall function. Regardless, bisphosphonate therapy should only be administered by clinicians with the appropriate expertise and infrastructure to support peri-infusion care, and the maximum, published annual doses should not be exceeded so as to avoid iatrogenic osteopetrosis arising from toxic doses [142].
The approach to dose adjustments and the duration of bisphosphonate therapy are also questions frequently posed by pediatricians. A number of key observations unique to children have influenced practice in this regard. The first observation has led to continuing bisphosphonate therapy until final height attainment in those with permanent or persistent risk factors, as follows. Among children with open epiphysis and ongoing endochondral bone formation, following treatment discontinuation the newly formed bone adjacent to the growth plate will be “treatment naïve” and thereby low density, creating a stress riser between high (previously treated) and low (untreated) density bone [135]. Not surprisingly, metaphyseal fractures have occurred post-bisphosphonate discontinuation in children with OI (i.e., persistent risk factors for low bone density) at the interface between the treated and untreated bone [143]. In fact, metaphyseal fractures have even occurred during intermittent IV bisphosphonate therapy at the interface between the dense metaphyseal lines created at the time of therapy and the (2 mm) adjacent treatment-naïve bone [144]. This latter report raises the question whether IV bisphosphonates should be administered with as short an infusion interval as possible, a line of thinking that is challenged by the demands on the patient from frequent infusions.
Further support for continuation of therapy to final height in those with persistent or permanent risk factors arises from a study by Rauch et al. [143]. These investigators showed using pQCT that there were significant declines in trabecular BMC Z-scores at the distal radius following pamidronate discontinuation in children with OI who were still growing. On the other hand, discontinuation after epiphyseal fusion was associated with more stable BMD Z-scores 2 years later. Balancing these observations with the lingering concern about over-suppression with longer-term therapy, the current recommended approach is to treat patients initially with a higher-dose regimen until the patient is clinically stable (◘ Fig. 24.1). Usually this equates to a minimum of 2 years, the time point at which the maximum benefit from bisphosphonate therapy has been observed in children with OI [84]. Once the patient is clinically stable, a lower (half-dose or less) [58, 145] maintenance protocol is given until the patient attains final adult height, at which time treatment can be discontinued if the patient is stable [58]. The goal of the maintenance phase of therapy in children with permanent or persistent risk factors is to preserve the gains realized during high-dose therapy while avoiding overtreatment [58, 145]. To this end, the dose of IV bisphosphonate therapy in the maintenance phase may require further downward titration to avoid unnecessarily high BMD Z-scores – this can be achieved by decreasing the dose or by increasing the interval between infusions. Palomo et al. [58] recently reported that long-term (at least 6 years) bisphosphonate therapy with downward dose titration in pediatric OI led to higher BMD Z-scores compared to historical controls and to vertebral body reshaping, although it was notable that non-VF rates were still high and most patients developed scoliosis. An outstanding question about the duration of therapy in those who stop around the time of adult height attainment but have persistent risk factors for fractures (e.g., OI, ongoing GC exposure) is whether they will require reintroduction of bisphosphonate therapy in the adult years and, if so, at what time point.
In children with resolution of risk factors during growth (i.e., cessation of GC therapy, resolution of inflammation, recuperation of mobility), discontinuation of therapy can be considered once the child has been fracture-free (VF and non-VF) for at least 6–12 months, previously fractured vertebral bodies have stabilized or undergone reshaping, and BMD Z-scores are appropriate for height. Reintroduction of therapy may be required during growth if the prior risk factors for osteoporosis recur and patients once again meet the criteria for treatment initiation.
6.7 Bisphosphonate Therapy Side Effects and Contraindications
6.7.1 Short-Term
The most frequent side effects of bisphosphonate therapy , reported with both oral and IV treatment [118, 121, 127], are collectively referred to as “the acute phase reaction” and include fever, malaise, back and bone pain, nausea, and vomiting. These symptoms usually begin 24–72 h following the initial dose, remit over a few days, typically do not occur with subsequent infusions or oral doses, and are effectively managed with anti-inflammatory and antiemetic medications. Asymptomatic hypocalcemia is frequent even with repeat infusions (though most marked with the first), reaching a nadir usually 1–3 days post-infusion [83]. The frequency of first-dose hypocalcemia appears to be mitigated by reducing the initial dose [130], a practice that is now in widespread use. Interestingly, a lower dose with the first infusion does not appear to mitigate the frequency of acute phase side effects [130]. Symptoms have been reported in up to 30% of children with first-infusion hypocalcemia [44, 130]. This has led to the widespread practice of prescribing calcium supplementation at published doses [100] for 5–10 days following the first bisphosphonate infusion, as well as ensuring vitamin D adequacy pre- and posttreatment. Children at risk for either hypocalcemia or its consequences (i.e., children with hypoparathyroidism or seizure disorders) may require even more aggressive hypocalcemia prevention such as an active form of vitamin D. Untreated hypocalcemia, hypophosphatemia, vitamin D deficiency, and rickets/osteomalacia are contraindications to bisphosphonate therapy. In these cases, the underlying vitamin D and/or mineral ion deficiency must be adequately treated before bisphosphonate therapy is administered (i.e., 25-hydroxyvitamin D level ≥ 50 nmol/L (20 ng/mL) and calcium intake sufficient for age).
The more serious acute side effects associated with bisphosphonate therapy in adults (such as uveitis, thrombocytopenia, and mucosal ulcerations with oral agents) are rare in children. Furthermore, a recent review of bisphosphonates in adults concluded that there is no link between bisphosphonates and atrial fibrillation, while the association between oral agents and esophageal cancer remains inconclusive [146]. In any patient with poor renal function (estimated glomerular filtration rate < 35 ml/min), bisphosphonates are contraindicated. Recently, the US Food and Drug Administration updated the label for zoledronic acid, stating it is also contraindicated in patients with acute renal impairment and that patients should be screened for renal insufficiency prior to initiating treatment. To this end, it should be noted that serum creatinine may not be a reliable marker of renal function in those with myopathies such as DMD, raising the need for other measures such as cystatin C to ensure adequate renal function prior to each zoledronic acid infusion. In our center, we also verify normal renal function prior to all pamidronate infusions.
6.7.2 Long-Term
Concern about the effects of bisphosphonates on linear growth have ultimately been quelled by studies which confirm expected growth rates in children with bisphosphonate-treated OI [137] and osteoporosis [147]; there are even reports of improved growth with long-term bisphosphonate therapy [58], likely attributable to a positive effect on vertebral height. On the other hand, chronic bone turnover suppression has two rare but serious sequelae in adults: osteonecrosis of the jaw (ONJ) and atypical subtrochanteric or metaphyseal “fatigue” fractures (AFF). Both are proposed to arise from accumulated microdamage due to suppressed osteoclast activity. ONJ is defined as exposed bone in the maxillofacial area that does not heal within 8 weeks following identification by a health-care provider, in the absence of radiation therapy [148]. In children, there are no reports of ONJ despite three studies which examined over 350 bisphosphonate-treated children with OI following dental procedures [149,150,151]. Despite the lack of reported ONJ in children to date, one position statement has nevertheless recommended to safeguard the bisphosphonate-treated child’s oral health by referral to a dentist prior to bisphosphonate initiation, completion of necessary invasive dental procedures prior to treatment initiation, regular dental evaluations by a dentist during treatment, and good daily oral hygiene [152].
AFF are also rare in adults, and while there is no direct causal link between bisphosphonates and AFF, the number of case series and cohort analyses suggesting an association is increasing, as summarized in a recent report [146]. These fractures are located in the subtrochanteric region or femoral shaft, arise from minimal or no trauma, and are characterized by transverse or short oblique fracture lines without comminution and a medial spike when the fracture is complete [153]. They are often bilateral (in up to two-thirds of cases) and may be associated with prodromal thigh pain. In the pediatric setting, Hegazy et al. [154] reported unusual femur stress fractures in children with OI and intramedullary rods on long-term bisphosphonate therapy (6–11 years); two patients had a “drug holiday” of 18–24 months prior to the femoral fractures. Of 72 children on IV pamidronate therapy, 18 had femur fractures and of these, 6/72 met the adult criteria for AFF (8%). All children had intramedullary rodding, none of the fractures were displaced, and all were treated successfully with protected weight-bearing and a hiatus from bisphosphonate therapy. While the duration of bisphosphonate therapy in those with AFF was reported in this study [154], there was no record of the frequency of such fractures in bisphosphonate-naïve children, nor the approach to pamidronate dosing (starting dose, maximum dose, dose titration and total cumulative pamidronate dose). As such, it is difficult to know whether these results are generalizable to other centers; nevertheless, the observation is call for concern and underscores the need for clinicians to report similar observations. Whether downward dose titration with long-term therapy such as currently practiced can obviate AFF remains unknown. Similarly, the benefits and risks of drug holidays in children with permanent or persistent bone health threats needing long-term therapy remain unexplored. Although rare, AFF have led adult care providers to consider drug holidays in those with a low risk of first-ever fractures and in those with a moderate risk who are clinically well after 3–5 years of therapy [146]. High-risk adult patients – those with a history of bone fragility or a T score ≤ −2 SD – are not considered candidates for drug holidays [146].
Delayed osteotomy but not fracture healing has been shown in children with bisphosphonate-treated OI and intramedullary rods; higher mobility scores was the only positive predictor of delayed healing that was identified [155]. This observation has led to withholding bisphosphonate therapy in the week leading up to surgery, and withholding therapy following intramedullary rodding until adequate fracture healing has been documented on x-ray, usually about 4 months. Surgical management has also switched to the use of an osteotome instead of a power saw. With these changes to medical and surgical management, a recent study has reported a significant reduction in the frequency of delayed osteotomy healing [156].
Since the skeleton acts as an endogenous reservoir of bisphosphonates that theoretically can be mobilized in subsequent years, concern has been raised about the safety of preconceptual use. Despite this theoretical concern, there have been no human reports to date of a significant adverse effect of bisphosphonates when administered either preconception or during pregnancy. This appears to stem from the fact that the amount of bisphosphonate mobilized from the skeleton in subsequent years is clinically insignificant. For example, data from Papapoulos [157] shows that 4–10 years after daily oral pamidronate administration to children with osteoporosis, a maximum of 0.13 mg/kg/year is excreted in the urine (less than 0.02% of the annual dose). The fact that the amount released from the skeleton is clinically insignificant is supported by numerous human reports. Reviews of women or girls who have received bisphosphonates preconception or during pregnancy reported an absence of skeletal abnormalities or congenital malformations in the infants, apart from marginal decreases in gestational age, weight, and transient, asymptomatic hypocalcemia [158,159,160,161]. While these data are reassuring, clinicians should ensure that menstruating females have negative pregnancy tests prior to each infusion and/or they are using a medically approved form of contraception if sexually active.
7 Novel Therapies
A number of important signaling pathways that modulate bone mass have led to novel drug developments in recent years. RANKL is an essential mediator of osteoclast formation, function, and survival [162], and both preclinical and clinical data suggest that inhibition of RANKL is a viable strategy for the treatment of osteoporosis [163]. Denosumab is a human, monoclonal antibody administered subcutaneously that targets RANKL to prevent the activation of RANK, thus inhibiting bone resorption and increasing bone strength at both trabecular and cortical sites without directly interacting with bone surfaces [164]. A large study on close to 8000 women with postmenopausal osteoporosis (the FREEDOM trial) showed that denosumab 60 mg every 6 months reduced vertebral, non-vertebral, and hip fracture risk without an increased risk of side effects compared to placebo [165]. Given its convenient route of administration, favorable safety profile and proven efficacy in adults, denosumab now merits exploration in children. To date, its use on compassionate grounds has been reported in a few children with osteoporosis due to OI (type VI, a subtype which is not as responsive to IV bisphosphonate therapy as other OI forms) [166] and in children with giant cell tumors [167], aneurysmal bone cysts [168], and fibrous dysplasia [169]. Importantly, there is no evidence to date of an adverse effect of denosumab on human growth plate activity [170].
Sclerostin, the product of the SOST gene, binds to LRP5/6 receptors and is a powerful inhibitor of the canonical Wnt signaling pathway that results in decreased bone formation. In a mouse model of moderate-severe OI, anti-sclerostin antibody resulted in improved bone mass and reduced long bone fragility [73]; emerging studies in humans show similar promise [171, 172]. Not surprisingly, sclerostin levels are elevated in patients with bone loss due to immobilization disorders, a clinical setting that may benefit from sclerostin suppression. Another novel agent, odanacatib, is a potent and selective inhibitor of cathepsin K (Cat K), which suppresses CatK-mediated bone resorption, but it does not suppress bone formation to the same extent as bisphosphonate therapy [173]. This oral agent is interesting in the chronic illness osteoporosis and GC-induced bone fragility settings where bone turnover is typically low [44, 85]; however, clinical trials in adults have shown an increased risk of atrial fibrillation and stroke and have subsequently been halted [174]. Finally, excessive transforming growth factor-β (TGF-β) signaling has been implicated in the pathogenesis of both CRTAP recessive and type I collagen dominant OI; anti-TGF-antibody rescues the phenotype in both forms of the disease, garnering interest in other high bone turnover osteoporotic states.
Case Studies
The following two cases highlight disparate clinical trajectories depending on whether osteoporosis is diagnosed and treated in its earlier versus later stages. In the first case, a 14-year-old boy presented to a bone health clinic with back pain following a history of GC therapy for 8 years due to severe asthma. A spine x-ray (◘ Fig. 24.6a) confirmed multiple grade 3 (severe) vertebral fractures. Intravenous bisphosphonate therapy was initiated which resulted in significant back pain relief; however, the patient was close to final adult height attainment at the time of presentation; therefore, there was insufficient time to completely reshape vertebral bodies resulting in permanent vertebral deformity. At the same time, the signs of early vertebral collapse were evident on chest x-rays carried out years before to assess his respiratory status. This case highlights the lack of routine spine health surveillance in a child with a chronic, GC-treated illness that resulted in life-long spine deformity. As discussed previously in this chapter, adults with permanent vertebral deformity have been shown to suffer from chronic back pain and functional limitation, raising the importance of early identification and treatment of spine collapse in those with risk factors through periodic spine radiographs.
a (I) 10-year-old boy on high-dose GC therapy for asthma since the age of 4 years. Early signs of vertebral collapse on a radiograph taken to assess his respiratory status at 10 years of age. No treatment or bone-specific monitoring was initiated. (II to IV) No significant deterioration in vertebral collapse despite ongoing GC therapy. (V) Presented with back pain at age 14 years due to multiple severe vertebral fractures. After 1 year of intravenous bisphosphonate therapy, back pain was improved but permanent vertebral deformity was presented due to final adult height attainment in the interim. b (I): 10-year-old boy with GC-treated DMD and back pain. X-ray shows early signs of vertebral fractures at T9 and 10 (Genant Grade 1 (mild) VF). (II) Lateral spine radiograph following 7 years of treatment with intravenous bisphosphonate therapy. Back pain has gone and vertebral bodies are dense and have undergone reshaping
In contrast, an 8-year-old boy with DMD was referred to the pediatric bone health clinic for a bone health evaluation shortly after starting deflazacort 0.9 mg/kg/day. At 10 years of age, he showed early signs of vertebral collapse (◘ Fig. 24.6b, Genant grade 1 VF at T9 and T10) and had minimal back pain elicited on direct questioning. He was started immediately on intravenous zoledronic acid therapy as per the dosing guidelines in ◘ Fig. 24.1; 7 years later, despite continued high-dose GC therapy, his vertebral fractures have undergone reshaping, and there were no new vertebral fractures nor back pain. This case highlights the success of early monitoring and intervention, which in this case allowed the patient to remain on GC therapy without any further signs of osteotoxicity.
8 Summary and Future Directions
There have been significant advances in the pediatric osteoporosis field over the past decade following identification of numerous heritable bone fragility genes, including those associated with phenotypes that lack the classic features of OI. In children with chronic illness, we now better understand the frequency of fractures and time points at which they are most likely to occur, as well as clinical predictors of fractures and potential for spontaneous recovery. This knowledge has facilitated the development of logical osteoporosis monitoring strategies in children with chronic illnesses and improved our understanding about best candidates for osteoporosis therapy. Advances in our understanding of the pathobiology of osteoporosis have led to recently discovered novel drug therapies which hold promise for children with both high and low bone turnover states; their efficacies and safety now merit testing in well-designed trials. As pediatric researchers go forward, there is a need for greater consensus on the methods and clinical outcomes for reporting treatment trials so that data across studies can be better aggregated in order to draw overall conclusions. Importantly, children are not small adults and this is particularly true in the study of skeletal disorders, where bone growth and modeling distinguish the pediatric skeleton from that of the more staid adult situation. A classic example is that vertebral bodies can undergo medication assisted and unassisted reshaping following fractures; at the same time, declines in BMD can be profound when treatment is stopped while the child is still growing. As well, pediatricians, funding agencies, and policy makers need to consider the challenges to clinical care that are created by lack of controlled clinical trials that address these issues [175]. Overall, optimal bone strength across the life span rests on outcomes which take place during the time when the skeleton is under construction – the growing years. This reminds us that it is incumbent upon the pediatric bone health communities to champion the diagnosis, treatment, and study of osteoporosis in childhood.
Review Questions
-
1.
What are the two main risk factors for osteoporosis in children with chronic illnesses?
-
2.
What is the definition of osteoporosis in children?
-
3.
What is the preferred first-line therapy to treat osteoporosis – oral or IV bisphosphonate drugs?
-
4.
Name at least one absolute contraindication to zoledronic acid therapy .
-
5.
What are the clinical goals of treatment efficacy in those prescribed bisphosphonate therapy ? Name at least five.
-
6.
What are the most common side effects of intravenous bisphosphonate therapy? How are these monitored and treated?
Answers
-
1.
Glucocorticoid therapy and impaired mobility.
-
2.
The definition of osteoporosis in children is based on the presence of a clinically significant fracture history: either a low-trauma vertebral fracture at any age or a low-trauma long bone fractures (≥2 or more long bone fractures by age 10 and ≥ 3 long bone fractures by age 19). In the presence of long bone fractures but absent vertebral fractures, a size-corrected BMD or BMC Z-score ≤ 2.0 is recommended; however, a BMD or BMC Z-score > 2.0 does not preclude an increases fracture risk [30].
-
3.
IV bisphosphonates.
-
4.
Glomerular filtration rate < 35 ml/min or acute renal impairment.
-
5.
Absence of new VF, absence of new long bone fractures, absence of deterioration of existing VF, reshaping of VF (restoration of vertebral dimensions), improved mobility, resolution of back pain due to VF, improved quality of life.
-
6.
First infusion side effects (fever, malaise, bone pain, nausea, vomiting – monitored clinically and treated with anti-inflammatory medications, antipyretics, and anti-nausea agents), hypocalcemia (prevented by ensuring calcium and vitamin D sufficiency pretreatment (documented with serum levels)), treated with calcium and vitamin D supplementation and occasionally 1,25-dihydroxyvitamin D3 therapy.
References
Bachrach LK. Diagnosis and treatment of pediatric osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2014;21:454–60.
Makitie O. Causes, mechanisms and management of paediatric osteoporosis. Nat Rev Rheumatol. 2013;9:465–75.
Saraff V, Schneider J, Colleselli V, Ruepp M, Rauchenzauner M, Neururer S, Geiger R, Hogler W. Sex-, age-, and height-specific reference curves for the 6-min walk test in healthy children and adolescents. Eur J Pediatr. 2015;174(6):837–40.
Bianchi ML, Leonard MB, Bechtold S, Hogler W, et al. Bone health in children and adolescents with chronic diseases that may affect the skeleton: the 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17:281–94.
Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int. 2016;27:2147–79.
Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–85.
Ben Amor IM, Roughley P, Glorieux FH, Rauch F. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in COL1A1. J Bone Miner Res. 2013;28:2001–7.
Marini JC, Reich A, Smith SM. Osteogenesis imperfecta due to mutations in non-collagenous genes: lessons in the biology of bone formation. Curr Opin Pediatr. 2014;26:500–7.
Rauch F, Lalic L, Roughley P, Glorieux FH. Genotype-phenotype correlations in nonlethal osteogenesis imperfecta caused by mutations in the helical domain of collagen type I. Eur J Hum Genet. 2010;18:642–7.
Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50:185–96.
Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93.
Cummings EA, Ma J, Fernandez CV, Halton J, et al. Incident vertebral fractures in children with leukemia during the four years following diagnosis. J Clin Endocrinol Metab. 2015;100:3408–17.
LeBlanc CM, Ma J, Taljaard M, Roth J, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res. 2015;30:1667–75.
McMillan HJ, Campbell C, Mah JK. Duchenne muscular dystrophy: Canadian paediatric neuromuscular physicians survey. Can J Neurol Sci Le J Can des Sciences Neurologiques. 2015;37:195–205.
Hyams JS. Biologics in pediatric Crohn’s disease: is it time to move to an earlier therapeutic approach? Expert Rev Clin Immunol. 2014;10:1423–6.
Thayu M, Leonard MB, Hyams JS, Crandall WV, et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: results of the REACH study. Clin Gastroenterol Hepatol. 2008;6:1378–84.
Kessler EA, Becker ML. Therapeutic advancements in juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2014;28:293–313.
Billiau AD, Loop M, Le PQ, Berthet F, Philippet P, Kasran A, Wouters CH. Etanercept improves linear growth and bone mass acquisition in MTX-resistant polyarticular-course juvenile idiopathic arthritis. Rheumatology (Oxford). 2010;49:1550–8.
Simonini G, Giani T, Stagi S, de Martino M, Falcini F. Bone status over 1 yr of etanercept treatment in juvenile idiopathic arthritis. Rheumatology (Oxford). 2005;44:777–80.
Griffin LM, Thayu M, Baldassano RN, DeBoer MD, Zemel BS, Denburg MR, Denson LA, Shults J, Herskovitz R, Long J, Leonard MB. Improvements in bone density and structure during anti-TNF-alpha therapy in pediatric Crohn’s disease. J Clin Endocrinol Metab. 2015;100:2630–9.
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28.
King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, Mendell JR, Kissel JT. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–13.
Halton J, Gaboury I, Grant R, Alos N, et al., Canadian STOPP Consortium. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24:1326–1334.
Feber J, Gaboury I, Ni A, Alos N, et al. Skeletal findings in children recently initiating glucocorticoids for the treatment of nephrotic syndrome. Osteoporos Int. 2012;23:751–60.
Huber AM, Gaboury I, Cabral DA, Lang B, et al. Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arthritis Care Res (Hoboken). 2010;62:516–26.
Alos N, Grant RM, Ramsay T, Halton J, et al. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:2760–7.
Phan V, Blydt-Hansen T, Feber J, Alos N, et al. Skeletal findings in the first 12 months following initiation of glucocorticoid therapy for pediatric nephrotic syndrome. Osteoporos Int. 2014;25:627–37.
Rodd C, Lang B, Ramsay T, Alos N, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res. 2012;64:122–31.
Kilpinen-Loisa P, Paasio T, Soiva M, Ritanen UM, Lautala P, Palmu P, Pihko H, Makitie O. Low bone mass in patients with motor disability: prevalence and risk factors in 59 Finnish children. Dev Med Child Neurol. 2010;52:276–82.
Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, Makitie O, Munns CF, Shaw N. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 pediatric official positions. J Clin Densitom. 2014;17:275–80.
Hogler W, Wehl G, van Staa T, Meister B, Klein-Franke A, Kropshofer G. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: comparison of fracture risk with the general practice research database. Pediatr Blood Cancer. 2007;48:21–7.
van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–8.
Baty JM, Vogt EC. Bone changes of leukemia in children. Am J Roentgenol. 1935;34:310–3.
Helenius I, Remes V, Salminen S, Valta H, et al. Incidence and predictors of fractures in children after solid organ transplantation: a 5-year prospective, population-based study. J Bone Miner Res. 2006;21:380–7.
Valta H, Jalanko H, Holmberg C, Helenius I, Makitie O. Impaired bone health in adolescents after liver transplantation. Am J Transplant. 2008;8:150–7.
Valta H, Makitie O, Ronnholm K, Jalanko H. Bone health in children and adolescents after renal transplantation. J Bone Miner Res. 2009;24:1699–708.
Vautour LM, Melton LJ 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int. 2004;15:160–7.
Henderson RC, Berglund LM, May R, Zemel BS, et al. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J Bone Miner Res. 2010;25:520–6.
Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(Suppl 1):58–63.
McAdam LC, Rastogi A, Macleod K, Douglas Biggar W. Fat embolism syndrome following minor trauma in Duchenne muscular dystrophy. Neuromuscul Disord. 2012;22:1035–9.
Medeiros MO, Behrend C, King W, Sanders J, Kissel J, Ciafaloni E. Fat embolism syndrome in patients with Duchenne muscular dystrophy. Neurol. 2013;80:1350–2.
Gordon KE, Dooley JM, Sheppard KM, MacSween J, Esser MJ. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics. 2011;127:e353–8.
Nelson DA, Kleerekoper M, Peterson EL. Reversal of vertebral deformities in osteoporosis: measurement error or “rebound”? J Bone Miner Res. 1994;9:977–82.
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, Ward LM. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int. 2012;23:2703–11.
Mostoufi-Moab S, Brodsky J, Isaacoff EJ, Tsampalieros A, Ginsberg JP, Zemel B, Shults J, Leonard MB. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97:3584–92.
Marinovic D, Dorgeret S, Lescoeur B, Alberti C, Noel M, Czernichow P, Sebag G, Vilmer E, Leger J. Improvement in bone mineral density and body composition in survivors of childhood acute lymphoblastic leukemia: a 1-year prospective study. Pediatrics. 2005;116:e102–8.
Gurney JG, Kaste SC, Liu W, Srivastava DK, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2014;61:1270–6.
Makitie O, Heikkinen R, Toiviainen-Salo S, Henriksson M, Puukko-Viertomies LR, Jahnukainen K. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: a cohort study. Eur J Endocrinol. 2013;168:281–8.
Burger H, Van Daele PL, Grashuis K, Hofman A, Grobbee DE, Schutte HE, Birkenhager JC, Pols HA. Vertebral deformities and functional impairment in men and women. J Bone Miner Res. 1997;12:152–7.
Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793–800.
Kerkeni S, Kolta S, Fechtenbaum J, Roux C. Spinal deformity index (SDI) is a good predictor of incident vertebral fractures. Osteoporos Int. 2009;20:1547–52.
Kocks J, Ward K, Mughal Z, Moncayo R, Adams J, Hogler W. Z-score comparability of bone mineral density reference databases for children. J Clin Endocrinol Metab. 2010;95:4652–9.
Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI. Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. J Pediatr. 2010;135:182–8.
Ma J, Siminoski K, Alos N, Halton J, et al. The choice of normative pediatric reference database changes spine bone mineral density Z-scores but not the relationship between bone mineral density and prevalent vertebral fractures. J Clin Endocrinol Metab. 2015;100:1018–27.
Genant HK, CY W, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48.
Grigoryan M, Guermazi A, Roemer FW, Delmas PD, Genant HK. Recognizing and reporting osteoporotic vertebral fractures. Eur Spine J. 2003;12(Suppl 2):S104–12.
Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ 3rd. Classification of vertebral fractures. J Bone Miner Res. 1991;6:207–15.
Palomo T, Fassier F, Ouellet J, Sato A, Montpetit K, Glorieux FH, Rauch F. Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: skeletal findings during follow up throughout the growing years. J Bone Miner Res. 2015;30(12):2150–7.
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:984–96.
Siminoski K, Lentle B, Matzinger MA, Shenouda N, Ward LM. Observer agreement in pediatric semiquantitative vertebral fracture diagnosis. Pediatr Radiol. 2014;44:457–66.
Adachi JD, Olszynski WP, Hanley DA, Hodsman AB, et al. Management of corticosteroid-induced osteoporosis. Semin Arthritis Rheum. 2000;29:228–51.
Vallarta-Ast N, Krueger D, Wrase C, Agrawal S, Binkley N. An evaluation of densitometric vertebral fracture assessment in men. Osteoporos Int. 2007;18:1405–10.
Spiegel LR, Schneider R, Lang BA, Birdi N, Silverman ED, Laxer RM, Stephens D, Feldman BM. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402–9.
Siminoski K, Lee KC, Jen H, Warshawski R, et al. Anatomical distribution of vertebral fractures: comparison of pediatric and adult spines. Osteoporos Int. 2012;23:1999–2008.
Buehring B, Krueger D, Checovich M, Gemar D, Vallarta-Ast N, Genant HK, Binkley N. Vertebral fracture assessment: impact of instrument and reader. Osteoporos Int. 2010;21:487–94.
Mayranpaa MK, Helenius I, Valta H, Mayranpaa MI, Toiviainen-Salo S, Makitie O. Bone densitometry in the diagnosis of vertebral fractures in children: accuracy of vertebral fracture assessment. Bone. 2007;41:353–9.
Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26:103–9.
Bacchetta J, Wesseling-Perry K, Gilsanz V, Gales B, Pereira RC, Salusky IB. Idiopathic juvenile osteoporosis: a cross-sectional single-centre experience with bone histomorphometry and quantitative computed tomography. Pediatr Rheumatol Online J. 2013;11:6.
Rauch F. Bone biopsy: indications and methods. Endocr Dev. 2009;16:49–57.
Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17:225–42.
Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Harcke HT, Henderson RC. Revised pediatric reference data for the lateral distal femur measured by Hologic discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom. 2009;12:207–18.
Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford: Stanford University Press; 1959.
Kroger H, Kotaniemi A, Vainio P, Alhava E. Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner. 1992;17:75–85.
Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–9.
Crabtree NJ, Hogler W, Cooper MS, Shaw NJ. Diagnostic evaluation of bone densitometric size adjustment techniques in children with and without low trauma fractures. Osteoporos Int. 2013;24:2015–24.
Binkley T, Johnson J, Vogel L, Kecskemethy H, Henderson R, Specker B. Bone measurements by peripheral quantitative computed tomography (pQCT) in children with cerebral palsy. J Pediatr. 2005;147:791–6.
Hogler W, Shaw N. Childhood growth hormone deficiency, bone density, structures and fractures: scrutinizing the evidence. Clin Endocrinol (Oxf). 2010;72:281–9.
Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, Kanis JA. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011;49:1271–4.
Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkensammer G, Griesmacher A, Finkenstedt G, Hogler W. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92:443–9.
Bayer M. Reference values of osteocalcin and procollagen type I N-propeptide plasma levels in a healthy Central European population aged 0-18 years. Osteoporos Int. 2014;25:729–36.
Morovat A, Catchpole A, Meurisse A, Carlisi A, Bekaert AC, Rousselle O, Paddon M, James T, Cavalier E. IDS iSYS automated intact procollagen-1-N-terminus pro-peptide assay: method evaluation and reference intervals in adults and children. Clin Chem Lab Med. 2013;51:2009–18.
Huang Y, Eapen E, Steele S, Grey V. Establishment of reference intervals for bone markers in children and adolescents. Clin Biochem. 2011;44:771–8.
Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH. Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab. 2003;88:986–92.
Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110:1293–9.
Ward LM, Rauch F, Matzinger MA, Benchimol EI, Boland M, Mack DR. Iliac bone histomorphometry in children with newly diagnosed inflammatory bowel disease. Osteoporos Int. 2010;21:331–7.
Hartikka H, Makitie O, Mannikko M, Doria AS, Daneman A, Cole WG, Ala-Kokko L, Sochett EB. Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res. 2005;20:783–9.
Fahiminiya S, Majewski J, Roughley P, Roschger P, Klaushofer K, Rauch F. Whole-exome sequencing reveals a heterozygous LRP5 mutation in a 6-year-old boy with vertebral compression fractures and low trabecular bone density. Bone. 2013;57:41–6.
Whyte MP, Greenberg CR, Salman NJ, Bober MB, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. NEJM. 2012;366:904–13.
Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, Mendes N, Snelgrove D, Meenaghan E, Misra M, Klibanski A. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47:458–66.
Marini JC, Blissett AR. New genes in bone development: what’s new in osteogenesis imperfecta. J Clin Endocrinol Metab. 2013;98:3095–103.
Sillence DO, Rimoin DL. Classification of osteogenesis imperfect. Lancet. 1978;1:1041–2.
Palomo T, Al-Jallad H, Moffatt P, Glorieux FH, Lentle B, Roschger P, Klaushofer K, Rauch F. Skeletal characteristics associated with homozygous and heterozygous WNT1 mutations. Bone. 2014;67:63–70.
Mitchell PJ, Cooper C, Dawson-Hughes B, Gordon CM, Rizzoli R. Life-course approach to nutrition. Osteoporos Int. 2005;26:2723–42.
Specker B, Thiex NW, Sudhagoni RG. Does exercise influence Pediatric bone? A systematic review. Clin Orthop Relat Res. 2015;473:3658–72.
Golden NH, Abrams SA. Optimizing bone health in children and adolescents. Pediatrics. 2014;134:e1229–43.
Abrams SA, Coss-Bu JA, Tiosano D, Vitamin D. Effects on childhood health and disease. Nat Rev Endocrinol. 2013;9:162–70.
Julian-Almarcegui C, Gomez-Cabello A, Huybrechts I, Gonzalez-Aguero A, Kaufman JM, Casajus JA, Vicente-Rodriguez G. Combined effects of interaction between physical activity and nutrition on bone health in children and adolescents: a systematic review. Nutr Rev. 2015;73:127–39.
Handel MN, Heitmann BL, Abrahamsen B. Nutrient and food intakes in early life and risk of childhood fractures: a systematic review and meta-analysis. Am J Clin Nutr. 2015;102:1182–95.
Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, McKay HA. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29:2161–81.
Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011.
Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Vitamin D. Deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.
Edouard T, Glorieux FH, Rauch F. Predictors and correlates of vitamin D status in children and adolescents with osteogenesis imperfecta. J Clin Endocrinol Metab. 2011;96:31930–8.
Benchimol EI, Ward LM, Gallagher JC, Rauch F, Barrowman N, Warren J, Beedle S, Mack DR. Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:538–45.
Kaste SC, Qi A, Smith K, Surprise H, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2014;61:885–93.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Mandel K, Atkinson S, Barr RD, Pencharz P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol. 2004;22:1215–21.
Kalayci AG, Kansu A, Girgin N, Kucuk O, Aras G. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics. 2001;108:E89.
Avgeri M, Papadopoulou A, Platokouki H, Douros K, Rammos S, Nicolaidou P, Aronis S. Assessment of bone mineral density and markers of bone turnover in children under long-term oral anticoagulant therapy. J Pediatr Hematol Oncol. 2008;30:592–7.
Modesto W, Bahamondes MV, Bahamondes L. Prevalence of low bone mass and osteoporosis in long-term users of the injectable contraceptive depot medroxyprogesterone acetate. J Womens Health (Larchmt). 2015;24:636–40.
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–609.
Bodor M, McDonald CM. Why short stature is beneficial in Duchenne muscular dystrophy. Muscle Nerve. 2013;48:336–42.
Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–30.
Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2014;(7):CD005088.
Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, Rauch F, Tugwell P, Moher D. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev. 2007;(4):CD005324.
Ward LM, Rauch F. Oral bisphosphonates for paediatric osteogenesis imperfecta? Lancet. 2013;282:1388–9.
Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. NEJM. 1998;339:947–52.
Barros ER, Saraiva GL, de Oliveira TP, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2012;25:485–91.
Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR. Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Miner Res. 2010;25:2251–5.
Ward LM, Denker AE, Porras A, Shugarts S, Kline W, Travers R, Mao C, Rauch F, Maes A, Larson P, Deutsch P, Glorieux FH. Single-dose pharmacokinetics and tolerability of alendronate 35- and 70-milligram tablets in children and adolescents with osteogenesis imperfecta type I. J Clin Endocrinol Metab. 2005;90:4051–6.
Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, Hertz S, Shenker IR. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:3179–85.
Bishop N, Adami S, Ahmed SF, Anton J, et al. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1424–32.
Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24:1282–9.
Bianchi ML, Colombo C, Assael BM, Dubini A, et al. Treatment of low bone density in young people with cystic fibrosis: a multicentre, prospective, open-label observational study of calcium and calcifediol followed by a randomised placebo-controlled trial of alendronate. Lancet Respir Med. 2013;1:377–85.
Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatol (Oxford). 2005;44:813–8.
Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tato L, Viapiana O, Adami S. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res. 2005;20:758–63.
Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, Verbout A, Schweitzer D, Uiterwaal C. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet. 2004;363:1427–31.
Ward LM, Rauch F, Whyte MP, D’Astous J, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96:355–64.
Munns CF, Rauch F, Travers R, Glorieux FH. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical and histomorphometric outcome. J Bone Miner Res. 2005;20:1235–43.
Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone. 2006;39:901–6.
Antoniazzi F, Zamboni G, Lauriola S, Donadi L, Adami S, Tato L. Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. J Pediatr. 2006;149:174–9.
Astrom E, Jorulf H, Soderhall S. Intravenous pamidronate treatment of infants with severe osteogenesis imperfecta. Arch Dis Child. 2007;92:332–8.
Ward LM, Glorieux FH, Rauch F, Verbruggen N, Heyden N, Lombardi AA. Randomized, placebo-controlled trial of oral alendronate in children and adolescents with osteogenesis imperfecta. Bone. 2005;36:0–18.
Rauch F, Munns C, Land C, Glorieux FH. Pamidronate in children and adolescents with osteogenesis imperfecta: effect of treatment discontinuation. J Clin Endocrinol Metab. 2006;91:1268–74.
Land C, Rauch F, Montpetit K, Ruck-Gibis J, Glorieux FH. Effect of intravenous pamidronate therapy on functional abilities and level of ambulation in children with osteogenesis imperfecta. J Pediatr. 2006;148:456–60.
Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics. 2003;111:1030–6.
Vuorimies I, Toiviainen-Salo S, Hero M, Makitie O. Zoledronic acid treatment in children with osteogenesis imperfecta. Horm Res Paediatr. 2011;75:346–53.
Ooi HL, Briody J, Biggin A, Cowell CT, Munns CF. Intravenous zoledronic acid given every 6 months in childhood osteoporosis. Horm Res Paediatr. 2013;80:179–84.
Gandrud LM, Cheung JC, Daniels MW, Bachrach LK. Low-dose intravenous pamidronate reduces fractures in childhood osteoporosis. J Pediatr Endocrinol Metab. 2003;16:887–92.
Steelman J, Zeitler P. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J Pediatr. 2003;142:417–23.
Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. NEJM. 2003;349:457–63.
Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone. 2007;40:821–7.
Biggin A, Briody JN, Ormshaw E, Wong KK, Bennetts BH, Munns CF. Fracture during intravenous bisphosphonate treatment in a child with osteogenesis imperfecta: an argument for a more frequent, low-dose treatment regimen. Horm Res Paediatr. 2014;81:204–10.
Biggin A, Zheng L, Briody JN, Coorey CP, Munns CF. The long-term effects of switching from active intravenous bisphosphonate treatment to low-dose maintenance therapy in children with osteogenesis imperfecta. Horm Res Paediatr. 2015;83:183–9.
Brown JP, Morin S, Leslie W, Papaioannou A, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60:324–33.
Unal E, Abaci A, Bober E, Buyukgebiz A. Efficacy and safety of oral alendronate treatment in children and adolescents with osteoporosis. J Pediatr Endocrinol Metab. 2006;19:523–80.
Khosla S, Burr D, Cauley J, Dempster DW, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91.
Malmgren B, Astrom E, Soderhall S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med. 2008;37:196–200.
Chahine C, Cheung MS, Head TW, Schwartz S, Glorieux FH, Rauch F. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J Pediatr. 2008;153:719–20.
Brown JJ, Ramalingam L, Zacharin MR. Bisphosphonate-associated osteonecrosis of the jaw: does it occur in children? Clin Endocrinol. 2008;68:863–7.
Bhatt RN, Hibbert SA, Munns CF. The use of bisphosphonates in children: review of the literature and guidelines for dental management. Aust Dent J. 2014;59:9–19.
Shane E, Burr D, Abrahamsen B, Adler RA, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23.
Hegazy A, Kenawey M, Sochett E, Tile L, Cheung AM, Howard AW. Unusual femur stress fractures in children with osteogenesis imperfecta and intramedullary rods on long-term intravenous pamidronate therapy. J Pediatr Orthop. 2016;36(7):757–61.
Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004;19:1779–86.
Anam EA, Rauch F, Glorieux FH, Fassier F, Hamdy R. Osteotomy healing in children with osteogenesis imperfecta receiving bisphosphonate treatment. J Bone Miner Res. 2015;30:1362–8.
Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. NEJM. 2007;356:1075–6.
Djokanovic N, Klieger-Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy? J Obstet Gynaecol Can. 2008;30:1146–8.
Green SB, Pappas AL. Effects of maternal bisphosphonate use on fetal and neonatal outcomes. Am J Health Syst Pharm. 2014;71:2029–36.
Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, Moretti M, Koren G, Ito S. Pregnancy outcome following in utero exposure to bisphosphonates. Bone. 2009;44:428–30.
Munns CF, Rauch F, Ward L, Glorieux FH. Maternal and fetal outcome after long-term pamidronate treatment before conception: a report of two cases. J Bone Miner Res. 2004;19:1742–5.
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–30.
Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24:182–95.
Hofbauer LC, Zeitz U, Schoppet M, Skalicky M, Schuler C, Stolina M, Kostenuik PJ, Erben RG. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009;60:1427–37.
Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: a review of preclinical and clinical data. Clinical Ther. 2012;34:521–36.
Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2012;12:183–8.
Karras NA, Polgreen LE, Ogilvie C, Manivel JC, Skubitz KM, Lipsitz E. Denosumab treatment of metastatic giant-cell tumor of bone in a 10-year-old girl. J Clin Oncol. 2013;31:e200–2.
Lange T, Stehling C, Frohlich B, Klingenhofer M, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22:1417–22.
Boyce AM, Chong WH, Yao J, Gafni RI, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27:1462–70.
Wang HD, Boyce AM, Tsai JY, Gafni RI, Farley FA, Kasa-Vubu JZ, Molinolo AA, Collins MT. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. 2014;99:891–7.
Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, Jang G. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014;54:168–78.
Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26.
Feurer E, Chapurlat R. Emerging drugs for osteoporosis. Expert Opin Emerg Drugs. 2014;19:385–95.
Mullard A. Merck & Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. 2016;15:669.
Weintraub JA, Breland CE. Challenges, benefits, and factors to enhance recruitment and inclusion of children in pediatric dental research. Int J Paediatr Dent. 2015;25:310–6.
Makitie O, Doria AS, Henriques F, Cole WG, Compeyrot S, Silverman E, Laxer R, Daneman A, Sochett EB. Radiographic vertebral morphology: a diagnostic tool in pediatric osteoporosis. J Pediatr. 2005;146:395–401.
Acknowledgments
This work was supported by the following programs and organizations: 1. LMW: the Canadian Institutions for Health Research Operating Grants Program, the Canadian Institutes for Health Research New Investigator Program, the Canadian Child Health Clinician Scientist Program, the Children’s Hospital of Eastern Ontario (CHEO) Research Institute, the University of Ottawa Research Chair Program, and the CHEO Departments of Pediatrics and Surgery; JM: The CHEO Research Institute.
Conflict of Interest
LMW has been a consultant to Novartis, Amgen, and Alexion Pharmaceuticals and has participated in clinical trials sponsored by Novartis and Amgen. JM has no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ward, L.M., Ma, J. (2018). Osteoporosis: Diagnosis and Management. In: Radovick, S., Misra, M. (eds) Pediatric Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-73782-9_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-73782-9_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73781-2
Online ISBN: 978-3-319-73782-9
eBook Packages: MedicineMedicine (R0)