Abstract
Vitamin D is a secosteroid that plays an important role in the central nervous system (CNS). Through binding to the vitamin D receptor (VDR), which is found throughout the CNS, 1,25-hydroxyvitamin D3 regulates gene transcription to exert neurotrophic and immunomodulatory effects. As a result of various downstream responses, vitamin D signaling provides neuroprotection, decreasing damage and accelerating recovery from a variety of CNS insults. As a result, a growing body of scientific literature addresses a possible relationship between this steroid, or lack thereof, and a variety of neurodegenerative diseases. In the first sections of this chapter, we will summarize the current understanding of the role vitamin D plays in CNS protection and development. In the latter section, we will examine existing evidence that vitamin D plays a role in neurodegenerative diseases, specifically evaluating if vitamin D availability may modify the risk, prognosis, and treatment outcomes for patients with Parkinson disease, Alzheimer disease, amyotrophic lateral sclerosis, and multiple sclerosis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Neurology
- Neurodevelopment
- Neurodegenerative disease
- Parkinson disease (PD)
- Alzheimer disease (AD)
- Amyotrophic lateral sclerosis (ALS)
- Multiple sclerosis (MS)
Vitamin D is a neurologically active secosteroid essential for the proper development and functioning of the central nervous system (CNS). Despite the expanse of knowledge in the last several decades, many questions still remain about the role vitamin D plays within the CNS. This chapter will review the current understanding of how vitamin D is involved in CNS development and protection as well as the risk, prognosis, and treatment of several neurodegenerative disorders. Throughout this chapter, “vitamin D levels” or “vitamin D status” will refer to the total serum concentration of 25-hydroxyvitamin D, the inactive circulating vitamin D metabolite, while “vitamin D” will refer specifically to 1,25-dihydroxyvitamin D3, the active vitamin D3 metabolite, unless otherwise specified.
The Presence of Vitamin D in the Nervous System
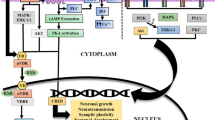
Vitamin D acts similar to other steroid hormones at the cellular level. Following ligand binding, the vitamin D receptor (VDR) forms homodimers or heterodimers with the retinoid X receptor, which bind to vitamin D response elements on target genes to directly regulate gene expression [1]. Notably, while the VDR is found at the plasma membrane in renal and liver cells, it is predominantly a nuclear receptor in the CNS, likely reflecting its role in regulating gene transcription, rather than calcium homeostasis [2]. In the 1980s, the earliest studies implicating a role for vitamin D in the nervous system demonstrated the ability to cross the blood-brain barrier (BBB) and bind to receptors in the CNS [3, 4]. Mapping of VDR expression in the brain using immunohistochemical techniques demonstrated a wide distribution throughout sensory, motor, and endocrine-autonomic regions of the rodent brain, findings which were confirmed in the late 1990s with VDR-targeted antibodies [5, 6].
Later studies revealed a similar pattern in the human CNS, demonstrating the presence of VDR and 1α-hydroxylase, the enzyme that catalyzes the synthesis of 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3, in neurons, glial cells, and pericytes throughout most areas of the human brain [7, 8]. Pericytes, cells that surround CNS vasculature and contribute to the BBB function, also express 25-hydroxylase, the enzyme required to synthesize 25-hydroxyvitamin D3. Pericytes are able to upregulate 25-hydroxylase, 1α-hydroxylase, and VDR expression in response to inflammatory stimuli, not only indicating the presence of a vitamin D paracrine/autocrine pathway within the CNS but also the existence of a local, coordinated mechanism to respond to tissue injury [8].
The Neuroprotective Role of Vitamin D in the Central Nervous System
In vitro and, to a lesser extent, animal studies show vitamin D provides protection against a variety of CNS insults, including ischemia, reperfusion injury, glutamate excitotoxicity, and oxidative damage [9,10,11,12]. While there is evidence vitamin D regulates expression of various BBB efflux transporters, thus decreasing exposure to toxins, most of the neuroprotective effects are attributed to immunomodulation [13]. Vitamin D reduces production of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and TNFα and increases expression of anti-inflammatory signals like IL-10 in vitro, reducing inflammation and decreasing microglial activation [14, 15]. Microglia are the innate immune cells of the CNS, and chronic microglial activation, leading to chronic inflammation, has been implicated in many neurodegenerative disorders [16]. Vitamin D also modulates the adaptive immune response, promoting a more regulatory T cell immunophenotype and reducing B cell immunoreactivity [17, 18].
Vitamin D and Neurodevelopment
In addition to its immunomodulatory actions, vitamin D signaling also triggers pro-differentiation and antiproliferative effects, providing potential protection from CNS malignancies and playing an important role in neurodevelopment [1, 19, 20]. Neural stem cells (NSCs) are pluripotent cells with the ability to differentiate into neurons, astrocytes, and oligodendrocytes and are vital to CNS development and repair [21]. NSCs constitutively express VDR and, in response to vitamin D signaling, upregulate VDR expression, creating a positive feedback loop [22]. Administration of vitamin D not only protects NSCs from neurotoxic insults, but enhances expression of neurotrophic factors, including brain-derived neurotrophic factor (BDNF) , glial-derived neurotrophic factor (GDNF) , ciliary neurotrophic factor (CNTF) , and neurotrophin 3 (NT3) , which promote differentiation of NSCs into neurons and oligodendrocytes [22, 23]. Oligodendrocytes, the cells responsible for myelination in the CNS, are vital to CNS repair and require VDR signaling in order to differentiate from progenitor cells [24].
Vitamin D likely plays an important role in early brain development, as VDR is expressed almost immediately after neural tube closure. Throughout in utero development, VDR continues to be expressed in the CNS, most prominently in neuroepithelium and actively differentiating areas of the brain, spinal cord, and dorsal root ganglia [25]. Research utilizing the developmental vitamin D (DVD)-deficient animal model has helped elucidate some of the important roles vitamin D plays in brain development. DVD-deficient rats are born to mothers who are vitamin D deficient throughout gestation. They are given vitamin D supplementation at birth and typically reach normal serum vitamin D levels within 2–3 weeks [26].
Alteration in Brain Morphology
Although there is evidence that VDR is at least partly autoregulated by available vitamin D levels in adult models, DVD-deficient rats have normal VDR expression at birth, indicating in utero VDR expression does not depend on vitamin D availability [2, 27]. Genomic studies have demonstrated the binding of VDR by vitamin D results in alterations of many genes, some of which ultimately function to inhibit cell proliferation [1]. Consistent with this data, DVD-deficient rat pups have an increased number of immature and mitotic neural cells at birth in the hippocampus, hypothalamus, and basal ganglia, without a corresponding increase in apoptosis. As a result, these pups have altered brain morphology when compared to healthy pups, including 30% longer cortical hemispheres, 200% larger lateral ventricles (seemingly due to a thin neocortex rather than excess cerebrospinal fluid), and decreased expression of neurotrophic factors in the CNS, including nerve growth factor (NGF), GDNF, and p75NTR [26]. These findings are interesting, given that vitamin D deficiency has been linked to schizophrenia, for which a hallmark radiologic finding is enlarged lateral ventricles [28].
Long-Lasting Effects of Vitamin D Deficiency In Utero
Maternal vitamin D deficiency not only affects early brain development in offspring but can also result in long-lasting changes in the structure and function of the adult rat brain. For example, the enlarged lateral ventricles and reduced NGF expression persist into adulthood for DVD-deficient offspring, even after reintroduction of vitamin D through supplementation [29]. As adults, these animals exhibit abnormal expression of genes involved in cytoskeleton maintenance (MAP2, NF-L) and neurotransmission (GABA-Aα4), as well as abnormal expression of proteins involved in synaptic plasticity and mitochondrial function [29, 30]. There may be a critical period in late pregnancy during which vitamin D deficiency results in abnormal adult phenotypes in rats [31]. While most of the research using an animal model of maternal vitamin D deficiency has been performed with rats, the few studies using mice have observed several opposite morphological brain changes at birth, including decreased length, head size, and lateral ventricle volume [32]. This discrepancy highlights the physiologic differences between animal models and limitations to generalizing results to humans.
Outcomes in Humans
Human studies evaluating the effect of maternal vitamin D deficiency on offspring have typically utilized large prospective cohorts and have focused on whether an association exists between prenatal maternal vitamin D levels and various markers of neurocognitive development in children. One study found no significant independent relationship between third trimester maternal vitamin D level and cognitive or psychological outcomes in children over a 9-year follow-up period [33]. However, another found in multivariate models that maternal vitamin D status in the first half of pregnancy was positively and linearly associated with mental and psychomotor development at 14 months of age [34]. Two additional studies found a positive relationship in multivariate models between second trimester maternal vitamin D level and language development, with nearly twofold higher rates of developmental language difficulties at 5 and 10 years in children whose mothers were vitamin D deficient (<46 nmol/L) [35, 36].
Studies investigating neonatal vitamin D levels from cord blood samples have shown little to no evidence for an association with cognitive development, intelligence, or behavior [37, 38]. Research evaluating neurocognitive outcomes in adolescence and beyond are limited in humans, but thus far one study found no association between third trimester maternal vitamin D level and diagnosis of attention-deficit hyperactivity disorder (ADHD), clinical depression, or standardized exam scores over a 22-year period [39]. While the results of human studies are somewhat inconsistent, they suggest that sufficient maternal vitamin D levels may be important early in pregnancy for proper language development in offspring.
Vitamin D Status and Brain Volume
Unlike in rodents, maternal vitamin D status and offspring brain morphology has not been studied in humans; however, several papers have been published assessing brain volume in adults relative to vitamin D status. A 2014 meta-analysis of nine animal and cross-sectional human studies found serum vitamin D levels were positively associated with brain volume and negatively associated with lateral ventricle size. However, two subsequent cross-sectional studies showed the opposite relationship—in both young and elderly adults, higher serum vitamin D levels were associated with decreased brain volume [40,41,42]. The authors hypothesized a smaller brain volume may be due to the antiproliferative, pro-apoptotic effects of vitamin D or earlier bone maturation leading to skull maturation at a smaller size. Yet, without prospective data to guide our understanding of causality in this relationship, it remains unclear if and how vitamin D levels affect brain size.
Cognition
There is currently a limited understanding of the relationship between vitamin D levels and cognitive function. Animal studies demonstrate deficient vitamin D levels correlate with increased markers of oxidative and nitrosative stress in the brain, consistent with changes seen at the cellular level in the brain with cognitive impairment [43]. Genetic studies of polymorphisms in the VDR gene provide some evidence that alterations in the VDR may influence overall longevity, cognitive performance, and susceptibility to both age-related cognitive decline and depressive symptoms in older adults [44,45,46,47].
Multiple epidemiological studies have been conducted in an attempt to determine whether a relationship exists between vitamin D levels and cognition. However, as with brain volume and most of the neurodegenerative disorders discussed in the following section, it is difficult to infer causality between vitamin D deficiency and cognitive decline given that the majority of studies evaluating these associations are observational, cross-sectional studies. A limited number of prospective studies evaluating cognitive decline among healthy, older adults have been published with conflicting outcomes. One study demonstrated a significantly higher relative risk of cognitive decline over 6 years among vitamin D-deficient elderly adults, while another showed no significant association between the two [48, 49]. A later study with both cross-sectional and prospective components demonstrated a correlation between vitamin D levels and cognitive performance in participants over 65 years of age, with vitamin D levels being predictive of cognitive performance 7–13 years later [45]. Few randomized controlled trials (RCTs) evaluating vitamin D and cognition have been conducted, although a very recent study involving older adults without dementia showed high-dose vitamin D supplementation (4000 IU/day) over 18 weeks improved nonverbal memory [50]. More RCTs are needed to clarify the relationship between cognition and vitamin D, which remains an area of active research.
CNS Disorders
As our understanding of the role vitamin D plays in immune function and inflammation expands, researchers and medical providers are gaining insight into how vitamin D is involved in the development, course, and treatment of specific neurologic diseases. There is strong evidence that links vitamin D deficiency to multiple sclerosis and a growing body of research associating it with Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis as well. Particularly notable is that these four disorders, while varied in many clinical aspects, share common pathophysiological characteristics including increases in oxidative stress, inflammation, mitochondrial dysfunction, and cell death. Throughout the remainder of this chapter, we will summarize the existing literature regarding each of these diseases and the potential role of vitamin D in disease risk, prognosis, and potential treatment options.
Parkinson Disease
Parkinson disease (PD) is a neurodegenerative disease in which specific destruction of dopaminergic neurons in the substantia nigra occurs with accumulation of α-synuclein cytoplasmic inclusions called Lewy bodies, resulting in movement abnormalities such as tremor, bradykinesia, rigidity, and postural instability. Vitamin D has been implicated in the development and proper functioning of dopaminergic neurons, and research is ongoing to elucidate the specific role vitamin D plays in the development of PD [51].
Risk
Patients with PD tend to have higher rates of vitamin D deficiency than age-matched healthy controls and patients with AD, which has also been associated with low vitamin D levels [52,53,54]. However, causality has yet to be determined; while vitamin D deficiency may be a risk factor for PD, having PD may also increase the risk of vitamin D deficiency, with immobility reducing sunlight exposure or leading to malnutrition. Most of the existing data are cross-sectional, and although two recent prospective cohort studies were published on this topic, the results are varied. Data from the Mini-Finland Health Survey, a 29-year cohort with 50 incident PD cases, indicated a relative risk of 0.33 in participants with baseline vitamin D levels ≥50 nmol/L compared to <25 nmol/L [55]. However, evaluation of data from the Atherosclerosis Risk in Communities (ARIC) study, a 17-year cohort with 67 incident PD cases, found no significant relationship between baseline vitamin D level and incident PD [56]. Notably, the former study had nearly double the follow-up duration and significantly lower baseline vitamin D levels across the sample population than the latter.
Genetic studies in many diseases linked to vitamin D, including PD, have investigated specific VDR single nucleotide polymorphisms (SNPs). Polymorphisms are genetic variants that can affect gene expression or protein composition, creating the potential to cause or increase risk of developing a disease. Some of the common VDR polymorphisms studied in neurodegenerative disorders include TaqI, BsmI, and ApaI, which are found in exons 8 and 9 in a region with unknown function, and FokI, which is found in exon 2 and involves a T to C change within a start codon, yielding a longer VDR protein [57]. A meta-analysis in 2014 found no association between BsmI, ApaI, or TaqI VDR polymorphisms and PD risk, but two meta-analyses the following year including the FokI polymorphism found an association between FokI and PD [58,59,60]. Specifically, the FokI C allele, resulting in the longer VDR protein, was associated with an increased risk of PD (OR 1.41, 95% CI 1.14–1.75), which was even greater with the homozygous CC versus TT FokI genotype (OR 2.45, 95% CI 1.52–3.93) [60].
Prognosis
Several studies have evaluated vitamin D as a prognostic indicator of PD severity, with varied results. While one evaluation of Chinese patients with PD observed lower serum vitamin D levels with longer disease duration and greater disease severity, a similar study of Iranian patients showed no association [61, 62]. Gatto et al. found the FokI C allele correlated with faster cognitive decline; on the contrary, Suzuki et al. observed milder disease in patients with the FokI CC genotype [63, 64]. Despite differences in patient population and analyses, the methodology of these studies remains limited by their cross-sectional design and thus an inability to determine the causative direction of any relationship that may exist.
Treatment
Several studies utilizing a preclinical mouse model of PD have suggested vitamin D may have a therapeutic benefit by decreasing inflammation, protecting dopaminergic neurons from glutamate neurotoxicity, and promoting recovery of dopaminergic functioning in injured nigrostriatal neurons [65,66,67]. Studies with rotenone-induced neurotoxicity PD models illustrate the ability of vitamin D to induce autophagy, an intracellular degradation process, which is hypothesized to be abnormal and potentially contribute to pathophysiology in PD [68,69,70]. The only double-blind RCT in humans testing the efficacy of vitamin D as a PD therapy found 1200 IU daily vitamin D3 supplementation slowed PD progression over 12 months, but that the effect was modified by the FokI polymorphism [71]. Patients with the wild-type TT FokI genotype had a strong, consistent response to vitamin D supplementation, while patients with the heterozygous FokI genotype had a moderate response, and patients with the CC FokI genotype had no response, experiencing a similar level of clinical deterioration as the placebo group. No association was seen with the BsmI, Cdx2, ApaI, TaqI, and GC1 polymorphisms [71]. While further research must be done to evaluate the utility of vitamin D as a PD treatment, these results suggest genotype influences the response to vitamin D and highlights the importance of precision medicine in disease prognosis and treatment.
Alzheimer Disease
Alzheimer disease (AD) is a neurodegenerative disease characterized by progressive loss of memory and cognitive function. The pathophysiology is marked by brain accumulation of amyloid-β (Aβ) plaques and hyper-phosphorylated tau protein neurofibrillary tangles, which are neurotoxic, leading to mitochondrial dysfunction, increased oxidative stress, persistent microglial activation, and a chronic inflammatory state [72, 73].
Risk
Patients with AD have been shown to have significantly higher rates of vitamin D deficiency than healthy, age-matched controls [74]. Several large, prospective cohorts have been studied to evaluate the relationship between vitamin D status and Alzheimer disease, with conflicting findings. Data from the Cardiovascular Health Study and the Copenhagen City Heart Study both showed evidence of an association between vitamin D deficiency and increased risk of all-cause dementia and AD [75, 76]. Data from the Framingham Heart Study found an association between lower vitamin D levels and decreased cognitive function and hippocampal volume, but no association with incident all-cause dementia or AD [77]. As with PD, it is impossible to determine whether low vitamin D levels are a cause or effect of AD. In fact, the pathophysiology of AD may directly reduce vitamin D availability and action in the CNS, as the presence of Aβ suppresses VDR expression while simultaneously increasing expression of 24-hydroxylase (CYP24A1), the enzyme required for vitamin D catabolism [78].
Genetic studies evaluating VDR polymorphisms as potential AD risk factors have found a possible association between ApaI and AD, but limited or no evidence for an association with FokI, TaqI, or BsmI [79,80,81]. Where an association is found to exist, the potential risk conferred by the VDR polymorphism seems to be ethnicity-dependent [82]. Surprisingly, genetic studies have demonstrated that the apolipoprotein E (ApoE) ε4 allele, a known genetic risk factor for AD, is associated with higher vitamin D levels in both mice and humans [83]. Further investigation showed the vitamin D deficiency prevalent among patients with AD is predominantly found in patients without the ε4 allele, indicating vitamin D deficiency may be a larger contributing risk factor in these patients [84].
Prognosis
There is some evidence that vitamin D deficiency is associated with accelerated cognitive decline in elderly patients, but these results are from a general population [85]. Little research has been done focusing on vitamin D as a prognostic factor specifically in AD.
Treatment
A major goal in treating AD is decreasing Aβ levels and pro-inflammatory cytokines. Encouraging results have demonstrated that vitamin D supplementation reduces Aβ levels, amyloid plaque formation, and inflammation in animal models of AD [73, 86]. Vitamin D supplementation also results in upregulation of Mdr1a/P-gp, a blood-brain barrier efflux transporter implicated in Aβ secretion, effectively reducing the amount of Aβ in the CNS [13, 87]. In a study utilizing a mouse model of AD, P-gp induction with vitamin D treatment prior to Aβ plaque formation resulted in lower levels of soluble and insoluble Aβ in the CNS, while P-pg induction after plaque formation resulted in decreased soluble Aβ, but had no effect on the insoluble Aβ already incorporated into plaques [88]. These results suggest vitamin D supplementation may play a preventative as well as therapeutic role in limiting Aβ plaques formation and progression of AD.
In humans, there has only been one RCT to date evaluating vitamin D supplementation in AD patients. In that study, the authors did not find an improvement in cognition or level of disability after 8 weeks of 1000 IU daily oral vitamin D2 [89]. Unfortunately, until more large RCTs are conducted, and perhaps with varied doses, it will be unclear whether vitamin D supplementation has a therapeutic benefit for patients with AD.
ALS
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease in which progressive destruction of upper and lower motor neurons occurs over months to years, eventually leading to death. The mechanism of damage is multifactorial, including glutamate excitotoxicity, free radical damage, mitochondrial dysfunction, autoimmune inflammation, and accumulation of intracellular calcium leading to caspase-mediated apoptosis [90, 91].
Risk
There is relatively weak evidence that vitamin D deficiency plays a significant role in ALS risk, and research specifically investigating the association of vitamin D deficiency or VDR polymorphisms with ALS is limited [92]. The earliest study analyzing VDR polymorphisms in ALS patients did so in the context of evaluating susceptibility to lead exposure, the primary ALS risk factor being studied, and no significant association was found [93]. Only one other study, which was published in 2016, addressed VDR polymorphisms as a potential ALS risk factor. The authors of that study found the A allele of the ApaI VDR polymorphism, which encodes a larger protein than the C allele, was significantly more common among ALS patients than healthy controls and may potentially be one genetic risk factor. That study did not find associations between ALS and the BsmI, TaqI, or FokI VDR polymorphisms [91].
Prognosis
Research studies evaluating vitamin D levels and ALS progression have had conflicting results. Camu et al. proposed serum vitamin D levels be used as a prognostic factor in ALS after completing a retrospective analysis showing ALS patients with severe vitamin D deficiency progressed four times more rapidly than patients with normal vitamin D levels and survived a median of 29.5 months compared to 52.8 months [94]. Additionally, vitamin D deficiency in the mouse model of ALS has been noted to worsen motor performance and exacerbate pathophysiology in the spinal cord [95, 96]. However, in contrast to these findings, a prospective study with 125 ALS patients found that higher vitamin D levels were actually associated with worse clinical outcomes, although this association was weak (p = 0.06) [97]. A more recent retrospective analysis concluded that ALS prognosis is not associated with vitamin D levels at all, but rather age at onset and the presence or absence of bulbar features [98]. Thus, whether vitamin D levels have any prognostic value in ALS remains to be determined.
Treatment
The ability of vitamin D to potentiate neurotrophic factors in motor neurons, as well as to rescue motor neurons from Fas-induced cell death in vitro, provided encouraging results that vitamin D may be helpful in the treatment of ALS [94]. Furthermore, studies utilizing the mouse model of ALS indicate vitamin D supplementation at a dose ten times an “adequate” intake improves motor performance, although supplementation at higher doses (50 times the “adequate” intake) fails to provide additional benefit and increases the risk of vitamin D3 toxicity [99, 100]. In a retrospective review of human ALS patients, those given 2000 IU vitamin D3 supplementation by their personal physicians had slightly slower decline at 9 months than patients without supplementation, but this difference was not observed at 3, 6, and 12 months; it is also not clear how these patients may have systematically differed from those patients who were not recommended to take supplements [101]. Despite functional improvements with vitamin D supplementation in both mouse and human studies, no significant effect on disease onset, progression, or survival has been documented [102]. Although it has been determined that supplementation of 2000 IU daily is safe for ALS patients, due to the small number of existing studies and limitations in study design, it remains unknown whether vitamin D supplementation is truly beneficial in slowing the progression of ALS.
Multiple Sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by CNS inflammation and demyelination causing, for most, relapsing and remitting neurologic symptoms which, for a subset of patients, eventually transitions to a progressive deterioration, with a fraction of individuals experiencing only progressive symptoms from disease onset. Of all the neurodegenerative disorders in which vitamin D deficiency has been implicated, the most research and strongest evidence exists for MS.
Risk
Environmental risk factors seem to play a predominant role in the development of MS, demonstrated by a pattern of higher MS prevalence at latitudes farther from the equator, migration studies showing risk depends on area of residence, and twin studies showing only 30% concordance among monozygotic twins [103,104,105,106]. A groundbreaking study in 2006 demonstrated a significant inverse relationship between serum vitamin D levels and MS risk in adult Caucasian patients, with a particularly strong effect for vitamin D status prior to 20 years of age [107]. Vitamin D levels early in life may be particularly important, as maternal vitamin D deficiency in the first trimester as well as low neonatal vitamin D levels increases the risk of developing MS in adulthood [108, 109]. Furthermore, low sun exposure in adolescence has been shown to correlate with earlier age at MS onset, and the migration studies that have been done indicate the risk of MS is greatest with relocation to high-prevalence regions in the first two decades of life [105, 110]. However, a study investigating vitamin D status and evidence of cumulative sunlight exposure showed that while higher vitamin D levels were associated with a decreased risk of developing MS, interestingly, cumulative sunlight exposure had an independent, protective association that was even stronger than vitamin D level [111]. These results do raise the question about whether it is truly vitamin D levels, or UV light exposure (which may have immunomodulatory properties independent of vitamin D), that are truly important in MS [112].
A large body of literature has been published evaluating potential genetic risk factors of MS, and while the HLA locus DRB1*1501 is a known risk factor, case-control investigations into vitamin D enzyme and receptor polymorphisms have been less conclusive [110]. A large meta-analysis summarizing much of the data suggested the ApaI and FokI polymorphisms may be significant risk factors for MS, with the TaqI T allele exerting a protective effect, but also noted individual study results varied by methodology [113]. In a prospective cohort analysis, patients with the homozygous wild-type FokI TT genotype had an 80% reduced MS risk with 400 IU daily vitamin D supplementation (unclear formulation). In an interesting parallel to the response seen in PD patients with the same genotype, vitamin D supplementation in MS patients with the altered CC FokI genotype did not seem to confer any protection against disease development [71, 114].
Prognosis
Vitamin D status appears to influence disease course after a clinically isolated syndrome (CIS) , a single MS-like episode prior to meeting MS diagnostic criteria, as well as after diagnosis of clinically definite MS (CDMS) . Patients with vitamin D levels ≥50 nmol/L at the time of CIS diagnosis have lower risk of conversion from CIS to CDMS, decreased radiological evidence of disease, and less disability than patients with levels <50 nmol/L [115]. Furthermore, higher vitamin D levels are associated with preserved gray matter volume in CIS patients, which is significant given that gray matter atrophy correlates to greater levels of disability in patients with MS [116]. Lower vitamin D levels are associated with an increased relapse rate in CIS as well as CDMS, which is a consistent finding in both pediatric-onset and adult-onset MS [115,116,117,118,119,120,121]. In fact, for every 10 nmol/L higher serum vitamin D level, patients with MS may experience up to a 12% reduction in risk of relapse [120]. Furthermore, vitamin D has been implicated in progression from relapsing-remitting MS (RRMS) to secondary progressive MS (SPMS) . SPMS patients tend to have lower vitamin D levels, and a retrospective analysis demonstrated that patients with a faster progression to SPMS also had lower vitamin D levels at the time of their MS diagnosis [122].
Prevention and Treatment
Studies evaluating the efficacy of vitamin D as both a preventative and therapeutic agent in the mouse model of MS, experimental autoimmune encephalomyelitis (EAE), have shown promising results. Vitamin D protects neurons from T cell-mediated killing in vitro, and when administered to mice prior to EAE induction reduces disability, CNS inflammation, and axonal damage [123, 124]. In a cuprizone-mediated demyelination mouse model, high-dose vitamin D injected intraperitoneally compared to placebo results in faster clearance of damaged myelin followed by greater numbers of mature oligodendrocytes and higher levels of remyelination [125]. As an alternative to the immunosuppressive therapies currently used to treat MS, some researchers are now focusing on development of a vaccine to prevent development of MS in the first place. One such vaccine, consisting of vitamin D and myelin oligodendrocyte glycoprotein (MOG) injected intraperitoneally, effectively prevents development of EAE in mice, while neither vitamin D nor MOG independently achieves a similar result [126].
In humans, research has not yet focused on MS vaccination, but does suggest vitamin D supplementation is beneficial. RCTs have demonstrated short-term vitamin D3 supplementation up to 10,400 IU daily is safe, tolerable, and effective in increasing serum vitamin D levels in patients with MS [127]. Interestingly, Caucasian women with MS experience a smaller increase in plasma vitamin D levels than healthy controls when given the same oral dose [128]. Additionally, a gender disparity exists in response to vitamin D supplementation; females may experience a stronger anti-inflammatory effect than males do, which has been attributed to potential synergy between vitamin D and estradiol signaling [129]. Nonetheless, administration of high-dose vitamin D (10,400 IU) daily to both male and female MS patients results in significant changes to immunophenotype in vivo, including a reduction in circulating IL-17-producing CD4+ T cells [127].
Administration of vitamin D to improve symptoms and progression of MS is currently an active area of research. Preliminary results from the SOLAR trial presented at the 2016 ECTRIMS (European Committee for Treatment and Research in Multiple Sclerosis) conference indicate high-dose (14,007 IU/day) vitamin D3 supplementation did not affect disease progression, or the proportion of patients free of disease activity,Footnote 1 which was the authors’ primary endpoint, over a 4-year follow-up period. However, vitamin D supplementation did significantly decrease the number of active MS lesions seen on MRI, one of the authors’ secondary endpoints [130]. Other data suggest that high-dose vitamin D supplementation may result in improved mental health and quality of life for patients with MS [131]. Results from additional ongoing trials of vitamin D supplementation and MS are pending [132].
Conclusion
After several decades of research, vitamin D is now recognized as a neurologically active secosteroid with a role in CNS development and function. However, a comprehensive understanding of the far-reaching downstream effects of vitamin D signaling is currently unavailable, and many questions remain regarding the role of vitamin D in human neurologic disease. Research suggests vitamin D likely plays a role in the pathogenesis and progression of MS, while an association between vitamin D and the risk, prognosis, or treatment of AD, PD, and ALS is less apparent. Looking to the future, additional prospective studies and placebo-controlled randomized clinical trials are necessary to determine the relationship between vitamin D and neurologic diseases.
Notes
- 1.
Disease activity was defined as relapse, Expanded Disability Status Scale (EDSS) score progression, new gadolinium-enhancing T1 lesions, or new or enlarging T2 MRI lesions.
Abbreviations
- Aβ:
-
Amyloid-β
- AD:
-
Alzheimer disease
- ALS:
-
Amyotrophic lateral sclerosis
- BBB:
-
Blood-brain barrier
- CDMS:
-
Clinically definite multiple sclerosis
- CIS:
-
Clinically isolated syndrome
- CNS:
-
Central nervous system
- DVD:
-
Developmental vitamin D
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MS:
-
Multiple sclerosis
- NSC:
-
Neural stem cell
- PD:
-
Parkinson disease
- RRMS:
-
Relapsing-remitting multiple sclerosis
- SPMS:
-
Secondary progressive multiple sclerosis
- VDR:
-
Vitamin D receptor
References
Carlberg C, Seuter S. A genomic perspective on vitamin D signaling. Anticancer Res. 2009;29(9):3485–93.
Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. Neuroscience. 2014;268:1–9.
Gascon-Barre M, Huet P-M. Apparent [3H] 1, 25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244(3):E266–71.
Stumpf WE, Sar M, Clark SA, DeLuca HF. Brain target sites for 1,25-dihydroxyvitamin D3. Science. 1982;215(4538):1403–5.
Stumpf WE, O’Brien LP. 1, 25 (OH) 2 vitamin D3 sites of action in the brain. Histochemistry. 1987;87(5):393–406.
Prüfer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1, 25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135–45.
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30.
El-Atifi M, Dreyfus M, Berger F, Wion D. Expression of CYP2R1 and VDR in human brain pericytes: the neurovascular vitamin D autocrine/paracrine model. Neuroreport. 2015;26(5):245–8.
Fu J, Xue R, Gu J, Xiao Y, Zhong H, Pan X, et al. Neuroprotective effect of calcitriol on ischemic/reperfusion injury through the NR3A/CREB pathways in the rat hippocampus. Mol Med Rep. 2013;8(6):1708–14.
Taniura H, Ito M, Sanada N, Kuramoto N, Ohno Y, Nakamichi N, et al. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res. 2006;83(7):1179–89.
Kajta M, Makarewicz D, Ziemińska E, Jantas D, Domin H, Lasoń W, et al. Neuroprotection by co-treatment and post-treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochem Int. 2009;55(5):265–74.
Uberti F, Morsanuto V, Bardelli C, Molinari C. Protective effects of 1alpha,25-Dihydroxyvitamin D3 on cultured neural cells exposed to catalytic iron. Physiol Rep. 2016;4(11). pii: e12769.
Durk MR, Fan J, Sun H, Yang Y, Pang H, Pang KS, et al. Vitamin D receptor activation induces P-glycoprotein and increases brain efflux of quinidine:an Intracerebral microdialysis study in conscious rats. Pharm Res. 2015;32(3):1128–40.
Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol. 2016;292:126–36.
Dulla YAT, Kurauchi Y, Hisatsune A, Seki T, Shudo K, Katsuki H. Regulatory mechanisms of vitamin D3 on production of nitric oxide and pro-inflammatory cytokines in microglial BV-2 cells. Neurochem Res. 2016;41(11):2848–58.
Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. https://doi.org/10.1002/jnr.20093.
Mann EH, Chambers ES, Chen Y-H, Richards DF, Hawrylowicz CM. 1α,25-dihydroxyvitamin D3 acts via transforming growth factor-β to up-regulate expression of immunosuppressive CD73 on human CD4+ Foxp3- T cells. Immunology. 2015;146(3):423–31.
Haas J, Schwarz A, Korporal-Kuhnke M, Faller S, Jarius S, Wildemann B. Hypovitaminosis D upscales B-cell immunoreactivity in multiple sclerosis. J Neuroimmunol. 2016;294:18–26.
Cui X, McGrath JJ, Burne THJ, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci. 2007;25(4):227–32.
Anic GM, Thompson RC, Burton Nabors L, Olson JJ, Browning JE, Madden MH, et al. An exploratory analysis of common genetic variants in the vitamin D pathway including genome-wide associated variants in relation to glioma risk and outcome. Cancer Causes Control. 2012;23(9):1443–9.
Magnus T, Rao MS. Neural stem cells in inflammatory CNS diseases: mechanisms and therapy. J Cell Mol Med. 2005;9(2):303–19.
Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang G-X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol. 2015;98(2):240–5.
Jang W, Park H-H, Lee K-Y, Lee YJ, Kim H-T, Koh S-H. 1,25-dyhydroxyvitamin D3 attenuates l-DOPA-induced neurotoxicity in neural stem cells. Mol Neurobiol. 2015;51(2):558–70.
de la Fuente AG, Errea O, van Wijngaarden P, Gonzalez GA, Kerninon C, Jarjour AA, et al. Vitamin D receptor–retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol. 2015;211(5):975–85.
Veenstra TD, Prufer K, Koenigsberger C, Brimijoin SW, Grande JP, Kumar R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res. 1998;804(2):193–205.
Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118(3):641–53.
Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheum Dis Clin North Am. 2012;38(1):13–27.
Chiang M, Natarajan R, Fan X. Vitamin D in schizophrenia: a clinical review. Evid Based Ment Health. 2016;19(1):6–9.
Feron F, Burne THJ, Brown J, Smith E, McGrath JJ, Mackay-Sim A, et al. Developmental vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65(2):141–8.
Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769–80.
O’Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne THJ. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32(3):227–34.
Hawes JE, Tesic D, Whitehouse AJ, Zosky GR, Smith JT, Wyrwoll CS. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav Brain Res. 2015;286:192–200.
Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77.
Morales E, Guxens M, Llop S, Rodriguez-Bernal CL, Tardon A, Riano I, et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130(4):e913–20.
Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, et al. Gestational vitamin 25(OH)D status as a risk factor for receptive language development: a 24-month, longitudinal, observational study. Nutrients. 2015;7(12):9918–30.
Whitehouse AJO, Holt BJ, Serralha M, Holt PG, Kusel MMH, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129(3):485–93.
Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol. 2014;28(5):434–44.
Gould JF, Anderson AJ, Yelland LN, Smithers LG, Skeaff CM, Zhou SJ, et al. Association of cord blood vitamin D with early childhood growth and neurodevelopment. J Paediatr Child Health. 2016;53(1):75–83.
Strom M, Halldorsson TI, Hansen S, Granstrom C, Maslova E, Petersen SB, et al. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab. 2014;64(3–4):254–61.
Annweiler C, Annweiler T, Montero-Odasso M, Bartha R, Beauchet O. Vitamin D and brain volumetric changes: systematic review and meta-analysis. Maturitas. 2014;78(1):30–9.
Plozer E, Altbacker A, Darnai G, Perlaki G, Orsi G, Nagy SA, et al. Intracranial volume inversely correlates with serum 25(OH)D level in healthy young women. Nutr Neurosci. 2015;18(1):37–40.
Annweiler C, Bartha R, Goncalves S, Karras SN, Millet P, Feron F, et al. Vitamin D-related changes in intracranial volume in older adults: a quantitative neuroimaging study. Maturitas. 2015;80(3):312–7.
Keeney JTR, Förster S, Sultana R, Brewer LD, Latimer CS, Cai J, et al. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med. 2013;65:324–34.
Varzaneh FN, Sharifi F, Hossein-Nezhad A, Mirarefin M, Maghbooli Z, Ghaderpanahi M, et al. Association of vitamin D receptor with longevity and healthy aging. Acta Med Iran. 2013;51(4):236.
Jorde R, Mathiesen EB, Rogne S, Wilsgaard T, Kjærgaard M, Grimnes G, et al. Vitamin D and cognitive function: the Tromsø study. J Neurol Sci. 2015;355(1–2):155–61.
Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RGJ, van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466–73.
Beydoun MA, Ding EL, Beydoun HA, Tanaka T, Ferrucci L, Zonderman AB. Vitamin D receptor and megalin gene polymorphisms and their associations with longitudinal cognitive change in US adults. Am J Clin Nutr. 2012;95(1):163–78.
Llewellyn DJ. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135.
Slinin Y, Paudel ML, Taylor BC, Fink HA, Ishani A, Canales MT, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74(1):33–41.
Pettersen JA. Does high dose vitamin D supplementation enhance cognition?: a randomized trial in healthy adults. Exp Gerontol. 2017;90:90–7.
Cui X, Pelekanos M, Liu P-Y, Burne THJ, McGrath JJ, Eyles DW. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. 2013;236:77–87.
Evatt ML, DeLong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65(10):1348–52. https://doi.org/10.1001/archneur.65.10.1348. [cited 2016 Dec 22].
Ding H, Dhima K, Lockhart KC, Locascio JJ, Hoesing AN, Duong K, et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease Harvard Biomarker Study. Neurology. 2013;81(17):1531–7.
Rimmelzwaan LM, van Schoor NM, Lips P, Berendse HW, Eekhoff EMW. Systematic review of the relationship between vitamin D and Parkinson’s disease. J Parkinsons Dis. 2016;6(1):29–37.
Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67(7):808–11.
Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley THJ, Chen H. Serum 25-hydroxyvitamin D concentrations in mid-adulthood and Parkinson’s disease risk. J Mov Disord Soc. 2016;31(7):972–8.
Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371(1–2):1–12.
Zhang Z-T, He Y-C, Ma X-J, Li D-Y, Lu G-C. Association between vitamin D receptor gene polymorphisms and susceptibility to Parkinson’s disease: a meta-analysis. Neurosci Lett. 2014;578:122–7.
Niu M-Y, Wang L, Xie A-M. ApaI, BsmI, FokI, and TaqI polymorphisms in the vitamin D receptor gene and Parkinson’s disease. Chin Med J (Engl). 2015;128(13):1809.
Li C, Qi H, Wei S, Wang L, Fan X, Duan S, et al. Vitamin D receptor gene polymorphisms and the risk of Parkinson’s disease. Neurol Sci. 2015;36(2):247–55.
Liu Y, Zhang B. Serum 25-hydroxyvitamin D predicts severity in Parkinson’s disease patients. Neurol Sci. 2014;35(1):67–71.
Chitsaz A, Maracy M, Basiri K, Izadi Boroujeni M, Tanhaei AP, Rahimi M, et al. 25-Hydroxyvitamin D and severity of Parkinson’s disease. Int J Endocrinol. 2013;2013:1–4.
Gatto NM, Paul KC, Sinsheimer JS, Bronstein JM, Bordelon Y, Rausch R, et al. Vitamin D receptor gene polymorphisms and cognitive decline in Parkinson’s disease. J Neurol Sci. 2016;370:100–6.
Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Kawasaki K, Noya M, et al. 25-hydroxyvitamin D, vitamin D receptor gene polymorphisms, and severity of Parkinson’s disease. Mov Disord. 2012;27(2):264–71.
Cass WA, Peters LE, Fletcher AM, Yurek DM. Calcitriol promotes augmented dopamine release in the lesioned striatum of 6-hydroxydopamine treated rats. Neurochem Res. 2014;39(8):1467–76.
Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R, et al. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol. 2016;12(2):327–39. https://doi.org/10.1007/s11481-016-9720-7. [cited 2016 Dec 22].
Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40(6):761–71.
Xiong N, Xiong J, Jia M, Liu L, Zhang X, Chen Z, et al. The role of autophagy in Parkinson’s disease: rotenone-based modeling. Behav Brain Funct. 2013;9(1):13.
Li H, Jang W, Kim HJ, Jo KD, Lee MK, Song SH, et al. Biochemical protective effect of 1,25-dihydroxyvitamin D3 through autophagy induction in the MPTP mouse model of Parkinson’s disease. Neuroreport. 2015;26(12):669–74.
Jang W, Kim HJ, Li H, Jo KD, Lee MK, Song SH, et al. 1,25-Dyhydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem Biophys Res Commun. 2014;451(1):142–7.
Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, et al. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97(5):1004–13.
Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–44.
Raha S, Lee HJ, Yumnam S, Hong GE, Venkatarame Gowda Saralamma V, Ha YL, et al. Vitamin D2 suppresses amyloid-β 25–35 induced microglial activation in BV2 cells by blocking the NF-κB inflammatory signaling pathway. Life Sci. 2016;161:37–44.
Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone. 1998;23(6):555–7.
Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–8.
Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014;10(3):296–302.
Karakis I, Pase MP, Beiser A, Booth SL, Jacques PF, Rogers G, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham Heart Study. Shea T, editor. J Alzheimers Dis. 2016;51(2):451–61.
Dursun E, Gezen-Ak D, Yilmazer S. Beta amyloid suppresses the expression of the vitamin D receptor gene and induces the expression of the vitamin D catabolic enzyme gene in hippocampal neurons. Dement Geriatr Cogn Disord. 2013;36(1–2):76–86.
Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci Lett. 2011;504(2):79–82.
Gezen-Ak D, Dursun E, Ertan T, Hanagasi H, Gurvit H, Emre M, et al. Association between vitamin D receptor gene polymorphism and Alzheimer’s disease. Tohoku J Exp Med. 2007;212(3):275–82.
Luedecking-Zimmer E, DeKosky ST, Nebes R, Kamboh MI. Association of the 3’ UTR transcription factor LBP-1c/CP2/LSF polymorphism with late-onset Alzheimer’s disease. Am J Med Genet. 2003;117B(1):114–7.
Laczmanski L, Jakubik M, Bednarek-Tupikowska G, Rymaszewska J, Sloka N, Lwow F. Vitamin D receptor gene polymorphisms in Alzheimer’s disease patients. Exp Gerontol. 2015;69:142–7.
Huebbe P, Nebel A, Siegert S, Moehring J, Boesch-Saadatmandi C, Most E, et al. APOE 4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB J. 2011;25(9):3262–70.
Dursun E, Alaylıoğlu M, Bilgiç B, Hanağası H, Lohmann E, Atasoy IL, et al. Vitamin D deficiency might pose a greater risk for ApoEɛ4 non-carrier Alzheimer’s disease patients. Neurol Sci. 2016;37(10):1633–43.
Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, Reed BR, et al. Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol. 2015;72(11):1295.
Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, et al. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis. 2011;25(2):295–307.
Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, et al. beta-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76(4):1121–8.
Durk MR, Han K, Chow ECY, Ahrens R, Henderson JT, Fraser PE, et al. 1,25-Dihydroxyvitamin D3 reduces cerebral amyloid-accumulation and improves cognition in mouse models of Alzheimer’s disease. J Neurosci. 2014;34(21):7091–101.
Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. J Alzheimers Dis. 2011;26(3):477–84.
Gordon PH. Amyotrophic lateral sclerosis. CNS Drugs. 2011;25(1):1–15.
Torok N, Torok R, Klivenyi P, Engelhardt J, Vecsei L. Investigation of vitamin D receptor polymorphisms in amyotrophic lateral sclerosis. Acta Neurol Scand. 2016;133(4):302–8.
Wang M-D, Little J, Gomes J, Cashman NR, Krewski D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 2017;61:101–30. http://www.sciencedirect.com/science/article/pii/S0161813X16301164.
Kamel F, Umbach DM, Lehman TA, Park LP, Munsat TL, Shefner JM, et al. Amyotrophic lateral sclerosis, lead, and genetic susceptibility: polymorphisms in the δ-aminolevulinic acid dehydratase and vitamin D receptor genes. Environ Health Perspect. 2003;111(10):1335–9.
Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, Pageot N, et al. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35(5):1198–205.
Solomon JA, Gianforcaro A, Hamadeh MJ. Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis. Klein R, editor. PLoS One. 2011;6(12):e29354.
Moghimi E, Solomon JA, Gianforcaro A, Hamadeh MJ. Dietary vitamin D3 restriction exacerbates disease pathophysiology in the spinal cord of the G93A mouse model of amyotrophic lateral sclerosis. Sensi SL, editor. PLoS One. 2015;10(5):e0126355.
Blasco H, Madji Hounoum B, Dufour-Rainfray D, Patin F, Maillot F, Beltran S, et al. Vitamin D is not a protective factor in ALS. CNS Neurosci Ther. 2015;21(8):651–6.
Yang J, Park J-S, Oh K-W, Oh S, Park H-M, Kim SH. Vitamin D levels are not predictors of survival in a clinic population of patients with ALS. J Neurol Sci. 2016;367:83–8.
Gianforcaro A, Hamadeh MJ. Dietary vitamin D3 supplementation at 10× the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study: dietary vitamin D3 supplementation in transgenic mouse model of ALS. CNS Neurosci Ther. 2012;18(7):547–57.
Gianforcaro A, Solomon JA, Hamadeh MJ. Vitamin D3 at 50x AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. Borchelt DR, editor. PLoS One. 2013;8(2):e30243.
Karam C, Barrett MJ, Imperato T, MacGowan DJL, Scelsa S. Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J Clin Neurosci. 2013;20(11):1550–3.
Gianforcaro A, Hamadeh MJ. Vitamin D as a potential therapy in amyotrophic lateral sclerosis. CNS Neurosci Ther. 2014;20(2):101–11.
Simpson S, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1132–41.
Tao C, Simpson S, van der Mei I, Blizzard L, Havrdova E, Horakova D, et al. Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(12):1343–9. https://doi.org/10.1136/jnnp-2016-314013.
Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47(4–5):425–48.
Ebers GC, Bulman DE, Sadovnick AD, Paty DW, Warren S, Hader W, et al. A population-based study of multiple sclerosis in twins. N Engl J Med. 1986;315(26):1638–42.
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–8.
Nielsen NM, Munger KL, Koch-Henriksen N, Hougaard DM, Magyari M, Jørgensen KT, et al. Neonatal vitamin D status and risk of multiple sclerosis: a population-based case-control study. Neurology. 2016;88(1):44–51. https://doi.org/10.1212/WNL.0000000000003454.
Munger KL, Aivo J, Hongell K, Soilu-Hanninen M, Surcel H-M, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol. 2016;73(5):515–9.
Laursen JH, Søndergaard HB, Sørensen PS, Sellebjerg F, Oturai AB. Association between age at onset of multiple sclerosis and vitamin D level–related factors. Neurology. 2016;86(1):88–93.
Lucas RM, Ponsonby A-L, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76(6):540–8.
Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11(9):584–96.
Tizaoui K, Kaabachi W, Hamzaoui A, Hamzaoui K. Association between vitamin D receptor polymorphisms and multiple sclerosis: systematic review and meta-analysis of case–control studies. Cell Mol Immunol. 2015;12(2):243–52.
Simon KC, Munger KL, Yang X, Ascherio A. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16(2):133–8.
Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014;71(3):306.
Mowry EM, Pelletier D, Gao Z, Howell MD, Zamvil SS, Waubant E. Vitamin D in clinically isolated syndrome: evidence for possible neuroprotection. Eur J Neurol. 2016;23(2):327–32.
Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric-onset MS. Ann Neurol. 2010;67(5):618–24.
Brola W, Sobolewski P, Szczuchniak W, Góral A, Fudala M, Przybylski W, et al. Association of seasonal serum 25-hydroxyvitamin D levels with disability and relapses in relapsing-remitting multiple sclerosis. Eur J Clin Nutr. 2016;70(9):995–9. http://www.nature.com/ejcn/journal/vaop/ncurrent/full/ejcn201651a.html. [cited 2016 Dec 22].
Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79(3):261–6.
Simpson SJ, Taylor B, Blizzard L, Ponsonby A-L, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68(2):193–203.
Muris A-H, Smolders J, Rolf L, Klinkenberg LJJ, van der Linden N, Meex S, et al. Vitamin D status does not affect disability progression of patients with multiple sclerosis over three year follow-up. Ramagopalan SV, editor. PLoS One. 2016;11(6):e0156122.
Muris A-H, Rolf L, Broen K, Hupperts R, Damoiseaux J, Smolders J. A low vitamin D status at diagnosis is associated with an early conversion to secondary progressive multiple sclerosis. J Steroid Biochem Mol Biol. 2016;164:254–7.
Nashold FE, Miller DJ, Hayes CE. 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;103(2):171–9.
Sloka S, Zhornitsky S, Silva C, Metz LM, Yong VW. 1,25-Dihydroxyvitamin D3 protects against immune-mediated killing of neurons in culture and in experimental autoimmune encephalomyelitis. Nataf S, editor. PLoS One. 2015;10(12):e0144084.
Nystad AE, Wergeland S, Aksnes L, Myhr K-M, Bø L, Torkildsen Ø. Effect of high-dose 1.25 dihydroxyvitamin D3 on remyelination in the cuprizone model. APMIS. 2014;122(12):1178–86.
Mimura LAN, Chiuso-Minicucci F, Fraga-Silva TFC, Zorzella-Pezavento SFG, Franca TGD, Ishikawa LLW, et al. Association of myelin peptide with vitamin D prevents autoimmune encephalomyelitis development. Neuroscience. 2016;317:130–40.
Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, et al. Safety and immunologic effects of high-vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86(4):382–90.
Bhargava P, Steele SU, Waubant E, Revirajan NR, Marcus J, Dembele M, et al. Multiple sclerosis patients have a diminished serologic response to vitamin D supplementation compared to healthy controls. Mult Scler J. 2016;22(6):753–60.
Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain. 2009;132(5):1146–60.
Smolders J, Hupperts R, Vieth R, Holmøy T, Marhardt K, Schluep M, et al. High dose cholecalciferol (vitamin D3) oil as add-on therapy in subjects with relapsing-remitting multiple sclerosis receiving subcutaneous interferon β-1a. London: ECTRIMS Online Library; 2016.
Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. High dose vitamin D intake and quality of life in relapsing-remitting multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neurol Res. 2016;38(10):888–92.
Bhargava P, Cassard S, Steele SU, Azevedo C, Pelletier D, Sugar EA, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials. 2014;39(2):288–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Roman, S., Mowry, E.M. (2018). Vitamin D and the Central Nervous System: Development, Protection, and Disease. In: Liao, E. (eds) Extraskeletal Effects of Vitamin D. Contemporary Endocrinology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-73742-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-73742-3_12
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-73741-6
Online ISBN: 978-3-319-73742-3
eBook Packages: MedicineMedicine (R0)