Abstract
Despite being considered one of the cornerstones of treatment, many aspects of intravenous fluid administration to patients with sepsis remain controversial. While recent data have provided considerable insights, there remains uncertainty as to the type, rate and volume of fluid that should be administered. In addition, the appropriate balance between fluids and vasopressors to achieve adequate end-organ perfusion at various stages of the septic insult is open to debate. Nonetheless, there is increasing evidence that the volume, nature and timing of fluid given can have a significant influence upon patient outcome. Finally, the conventional paradigms regarding fluid administration and fluid bolus therapy are being increasingly challenged by newer evidence.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Despite being considered one of the cornerstones of treatment, many aspects of intravenous fluid administration to patients with sepsis remain controversial. While recent data have provided considerable insights, there remains uncertainty as to the type, rate and volume of fluid that should be administered. In addition, the appropriate balance between fluids and vasopressors to achieve adequate end-organ perfusion at various stages of the septic insult is open to debate. Nonetheless, there is increasing evidence that the volume, nature and timing of fluid given can have a significant influence upon patient outcome. Finally, the conventional paradigms regarding fluid administration and fluid bolus therapy are being increasingly challenged by newer evidence.
1 Definitions
Fluid resuscitation can be defined as intravenous fluid administered because the clinician judges that there is inadequate end-organ perfusion. This is distinct from the ongoing maintenance fluids administered, intravenously or enterally, to meet ongoing patient needs. Sometimes the line between the two may blur.
2 Causes of Organ Dysfunction in the Septic Patient
Understanding of several key aspects of organ dysfunction of sepsis has increased markedly over the last 20 years, reflected in the updated International Consensus Definitions for Sepsis and Septic Shock published in 2016 [1].
In patients challenged by severe infection, the endogenous release of cytokines (e.g., tumour necrosis factor, interleukins, etc.), eicosanoids and other mediators activates an inflammatory cascade, and organ dysfunction may be due to both circulatory and cellular abnormalities that follow. In particular, several mechanisms of lethal cell injury have now been described (necroptosis [2], apoptosis [3], ferroptosis [4]) that are independent of perfusion and much more closely related to immunological and metabolic events. These observations make it uncertain whether organ injury in sepsis is an immune injury-dependent phenomenon or a tissue hypoxia-induced event or both.
The rationale underlying a ‘fluid-liberal’ approach is that organ dysfunction is mainly hypoperfusion-related and therefore reversible with fluid. A positive fluid balance when applied beyond the first 24 h has, however, been associated with worse outcomes [5, 6].
Here, we briefly revisit some of the major causes of circulatory disturbance and organ dysfunction in sepsis.
2.1 Circulatory Disturbances in Sepsis
Absolute volume depletion may be present due to poor intake or excess losses (e.g., from diarrhoea, vomiting). Ongoing fluid loss through capillary leakiness may exacerbate volume depletion beyond this initial stage [7].
Vasodilatation, mediated by increased production of nitric oxide by inflammatory mediators, contributes to vascular smooth muscle relaxation, producing the so-called vasoplegia of sepsis, presenting in its most extreme form as septic shock. The Third International Consensus Definition for Sepsis and Septic Shock (Sepsis-3) defined shock as a vasopressor requirement to achieve a mean arterial pressure of at least 65 mmHg, accompanied by a serum lactate level > 2 mmol/L, despite adequate fluid resuscitation [1]. It should be recognized that ‘adequate’ fluid resuscitation is challenging to define.
Septic myocardial depression, a cytokine-mediated phenomenon of decreased right and left ventricular contractility, impaired response to filling, and reversibility with resolution of sepsis [8], is common in patients with sepsis and septic shock [9]. In 1 series of 67 mechanically ventilated septic patients free from known previous cardiac disease, it was reported in 60% of patients within the first 3 days of admission [10]. In its most extreme form, septic myocardial depression can lead to profound coexisting cardiogenic shock.
2.2 Organ Dysfunction in Sepsis
Any of the above circulatory disturbances can affect end-organ perfusion and function in sepsis. They are accompanied by changes at a cellular level, which are only partly understood, likely driven by inflammatory mediators, as well as changes to the microcirculation.
Given that many clinicians use blood lactate concentrations and oliguria to guide fluid resuscitation, these two areas warrant specific discussion.
First, increased blood lactate concentrations that occur with sepsis are generally not due to cellular hypoxia from hypoperfusion [11], although this may sometimes be the case if there is significant intravascular volume depletion. Serum lactate concentration may be better viewed as a nonspecific indicator of cellular or metabolic ‘stress’ [12]. This is not to downplay its importance as a marker of illness severity, with increases strongly associated with mortality. However, attempts to increase the cardiac output using fluids or inotropes, simply in response to an elevated serum lactate concentration in sepsis, may not be effective in improving patient outcomes [13, 14].
Second, animal models of sepsis-induced renal dysfunction in the setting of a hyperdynamic circulation suggest that renal blood flow is actually increased rather than decreased – with oliguria and acute kidney injury developing in parallel with increased renal blood flow [15,16,17]. It is therefore hypothesized that redistribution of blood flow within the renal microvasculature, with efferent arteriolar vasodilatation, might explain the associated reduction in observed glomerular filtration rate [18, 19]. Accordingly, fluid boluses for oliguria, if given to augment renal blood flow, which may already be enhanced during sepsis, are logically unlikely to benefit and may well cause harm. Such an approach risks fluid accumulation and may explain the association between favourable outcomes and restrictive fluid regimens observed in several studies. For example, the recent Scandinavian CLASSIC (Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care) trial reported that worsening of acute kidney injury occurred less frequently in septic patients who received a conservative approach to fluid management after their initial resuscitation (with fluid boluses only if there were signs of severe hypoperfusion), when compared to ongoing fluid boluses as long as the patient appeared to continue to respond to filling (odds ratio for worsening acute kidney injury 0.46, 0.23–0.92, p = 0.03) [20]. The signal from this feasibility study, which included 151 patients, requires further investigation.
3 The Fluid Bolus
Rapid administration of intravenous fluid with the aim of improving circulatory disturbances constitutes the ‘fluid bolus’, described around the birth of modern critical care by Max Harry Weil more than 50 years ago. Its effect can be judged; he wrote, ‘by objective changes in circulation, such as blood pressure, mental alertness, urine flow, peripheral venous filling, and appearance and texture of the skin’ [21]. Intravenous fluid resuscitation dates even further back—to the 1830s—when the life-restoring forces of a fluid bolus were eloquently described during the cholera epidemic [22]. Hence, its origins are from hypovolemic shock.
The rationale underlying the administration of a fluid bolus is to achieve an increase in end-organ perfusion rapidly and thereby minimize the duration of end-organ hypoperfusion. The 2016 Surviving Sepsis Campaign guidelines strongly recommend that ‘in the initial resuscitation of sepsis-induced hypoperfusion, at least 30 mL/kg of IV crystalloid fluid be given within the first 3 h’, though they acknowledge that the quality of evidence supporting this is low [23]. Interesting, a retrospective analysis of 49,331 patients receiving mandated emergency care for sepsis found that time to completion of a first bolus of intravenous fluids was unrelated to mortality (Fig. 8.1) (odds ratio 1.01, 95% CI 0.99–1.02), a result that requires prospective evaluation [24]. It is our opinion that administration of lesser volumes, particularly in those patients with significant coexisting illnesses such as congestive heart failure and chronic renal disease, may be prudent. In all patients, frequent reassessment of the haemodynamic status after initial resuscitation is recommended [23].
Data from a large retrospective database of 49,331 patients looking at time to treatment and mortality during mandated emergency care for sepsis. Time to completion of the initial bolus of intravenous fluid was unrelated to risk-adjusted in-hospital mortality. Ninety-five percent confidence intervals are displayed (OR 1.01, 95% CI 0.99–1.02) [24] (With permission, New England Journal of Medicine)
Conventional Guytonian physiology teaches that if a fluid bolus is to improve organ perfusion, it must increase the stressed volume of the circulation and thereby venous return and cardiac output [25]. If a fluid bolus is administered and patient is not a ‘responder’ (i.e., it does not produce a significant increase in cardiac output, which might arbitrarily be defined as >10–15% [26]), it may only produce tissue oedema, thereby exacerbating organ dysfunction. There are an increasing number of studies associating a positive fluid balance and increased mortality in sepsis [27, 28]. While this association may represent that higher severity of illness is associated with greater volumes of fluid to treat more substantial haemodynamic disturbances, an alternative explanation is that administration of less fluid—and, by extension, more vasopressor use to correct hypotension—may have merit.
A recent analysis of a large retrospective dataset of approximately 23,000 septic patients reported an association with greater mortality for those patients who received more than 5 L of fluid in the first 24 h, after adjusting for illness severity [29]. For lesser volumes, mortality appeared unaffected by volume.
In the recent FENICE study that described fluid challenge practices in 2213 patients in 46 countries, the median volume of fluid given was 500 mL (IQR 500–1000 mL), over a median time of 24 min (IQR 40–60 min) [30]. The most common indication for its administration was hypotension, present in 59% of patients [30]. Approximately half of the patients with a negative response to fluid received a further fluid bolus, the same proportion as in those who did respond. A relatively low proportion of responders, between 54.4 and 60.5% (depending on the volume and rate of administration), was also found in a recent large systematic review and meta-analysis of the fluid challenge technique [31]. This suggests that decision-making surrounding fluid boluses remains somewhat arbitrary, or at least not guided by classical teaching regarding fluid responsiveness. It has been suggested that decision-making may be driven by a clinical culture where there is a fear of not giving enough fluid, more than anything else [32].
Whether a fluid bolus is actually the best way to resuscitate a septic patient has been challenged by the observations from the landmark Fluid Expansion as Supportive Therapy (FEAST) Study [33]. This trial compared the use of fluid boluses with 5% albumin or 0.9% saline versus resuscitation without boluses, in African children with severe febrile illness and impaired perfusion. Of note, children with severe hypotension all received fluid boluses initially. These data revealed significantly greater mortality at 48 h in those patients assigned a fluid bolus: 10.6% and 10.5% mortality with albumin boluses and 0.9% saline boluses, respectively, compared with 7.3% with no bolus (p = 0.004). The difference in survival was apparent even after 4 h. Subsequent analysis suggested that the mechanism of death in the fluid bolus group was likely cardiovascular collapse, rather than neurological or respiratory events. This has led to the suggestion of cardiotoxicity or ischemia-reperfusion as the mechanism for these deaths [34, 35].
While these patients were generally aged between 1 and 3 years, in regions that had limited capacity for advanced supportive cardiorespiratory care that would be considered standard in many countries, and many were suffering from malaria and severe anaemia, these results are thought-provoking. To summarize, we are uncertain that the conventional approach to liberal fluid bolus therapy leads to optimal outcomes.
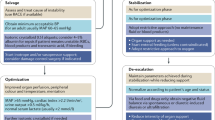
4 Indications for Fluid Administration
When considering whether to administer fluid to a patient with sepsis or septic shock, we believe that several factors may be relevant.
-
1.
A fluid bolus (i.e., administration of fluid without any prior predictive test of responsiveness), is appropriate initially if there is evidence of hypoperfusion (e.g., delayed capillary refill, mottling, oliguria) and/or hypotension (e.g., MAP < 60 mmHg).
-
2.
The stage of sepsis is an important consideration with regard to fluid administration. Note that an ongoing positive fluid balance beyond the early phase, particularly beyond 24 h, is associated with harm, rather than benefit [5, 6]. This remains only an association, however.
-
3.
Dynamic predictors of fluid responsiveness (e.g. passive leg raising, pulse pressure variation) are more sensitive than static measures (e.g. central venous pressure), which are less reliable [26, 36,37,38,39,40].
-
4.
An acute physiological response to fluid does not guarantee that fluid administration will lead to improved patient-centred outcomes.
-
5.
While the optimal blood pressure is uncertain, the judicious use of vasopressors rather than persistence with large volumes of fluid should be considered.
-
6.
Oliguria and acute kidney injury may be an epiphenomenon and are not absolute indications for further fluid.
-
7.
Further evaluation (e.g., using echocardiogram) may have an important role when trying to understand mechanisms of shock in patients who are not responding well to initial treatment.
Predicting fluid responsiveness may be useful, particularly when deciding between ongoing fluid administration and introducing or increasing vasopressors. There are several methods to predict whether a patient will be fluid responsive. The most appropriate method used will depend on local availability, expertise, personal preference, and patient factors. Some of the more commonly employed methods are summarized in Table 8.1.
Studies incorporating fluid responsiveness as a guide to fluid resuscitation have been small and few in number, with conflicting results [41, 42]. As has been observed, such an approach may lead to cardiac output being ‘maximized’ rather than ‘optimized’, depending on the algorithm used [43]. In other words, continuing to administer fluids until a patient enters the flat part of the Frank-Starling curve may not be optimizing their haemodynamic state at all. Conversely, the potential benefit of incorporating fluid responsiveness into management would be that those unlikely to respond would be identified, thereby avoiding potentially deleterious fluid loading [41].
Moreover, when a fluid bolus is given, its actual effect may not be sustained. Knowledge about the duration of a fluid bolus’ effect appears limited: a recent systematic review of the fluid challenge found that in only 5 of 85 studies was the haemodynamic effect actually assessed beyond 10 min [44]. A study of 26 postoperative patients, post general and cardiothoracic surgery, who received a 250 mL fluid bolus of Hartmann’s solution over 5 min suggested that even in those patients who had an initial increase in cardiac output at 1 min as assessed by a lithium-dilution calibrated system (LiDCOplus), the haemodynamic effects had essentially dissipated after just 10 min [44]. Another study of 20 patients with predominantly septic shock who received 500 mL crystalloid bolus over 30 min, after the initial resuscitation phase (greater than 6 h of vasopressor use), similarly found that cardiac output had returned to baseline within 1 h in responders [45]. While these studies focus on physiological outcomes, one might extrapolate these observations to favour vasopressor use rather than further fluid boluses beyond the early resuscitation period.
5 Endpoints of Initial Fluid Resuscitation
The acute physiological endpoint chosen to guide fluid administration may be clinical (e.g., blood pressure, heart rate, cognition, urine output, capillary refill, and skin temperature) or an investigation (which varies in sophistication and invasiveness, from a serum lactate to estimating changes in cardiac output and tissue perfusion). Each endpoint has various strengths and limitations and only tells part of a complex circulatory picture. For example, as mentioned above, serum lactate is a good marker of severity of illness, but is nonspecific and unreliable as a marker of organ perfusion in sepsis.
An approach known as early goal-directed therapy (EGDT), where early resuscitation of septic patients was guided by targeting central venous oxygen saturations >70%, achieved through a combination of intravenous fluids, vasopressors, inotropes, and blood transfusion, has been shown in three large RCTs from the United States (PROCESS) [51], Australia/New Zealand (ARISE) [52] and the United Kingdom (PROMISE) [53] to not be superior to ‘usual care’, where treatment was guided by clinical assessment. Furthermore, EGDT led to more interventions and greater cost.
6 Choice of Fluid
An ideal fluid for the septic patient would be one that was inexpensive and readily available; did not accumulate, cause toxicity or metabolic derangements; and was associated with a sustained intravascular effect [54].
Crystalloids are solutions containing freely permeable ions, whereas colloids are suspensions of molecules in solution. It is important to recognize that no particular type of fluid has been proven to improve patient-centred outcomes, although starch-containing colloids have been reported to worsen some important outcomes [55, 56]. The lack of a proven superior type of fluid may explain the wide variation in fluid prescription internationally [57].
6.1 Colloids
Semisynthetic colloids, such as starch and gelatins, were popular due to their decreased cost when compared to albumin. Two recent landmark RCTs have compared the use of starches to crystalloid in ICU patients. The 6S Trial compared hydroxyethyl starch (130/0.42, Tetraspan) to Ringer’s acetate in 798 patients admitted to the ICU with sepsis. This trial reported an increased risk of death and increased use of renal replacement therapy in patients who received starch [56]. The CHEST study compared hydroxyethyl starch (130/0.4, Voluven) with 0.9% NaCl in 6651 ICU patients. Of these, 1937 (29%) were septic. Increased renal injury and renal failure were reported in patients who received starch [55]. Gelatins, another semisynthetic colloid, may be similarly toxic [58]. We therefore would recommend avoidance of both starches and gelatins.
An alternative colloid is albumin. In 2004, a preplanned subgroup analysis of septic patients in Australian and New Zealand SAFE study (the Saline versus Albumin Fluid Evaluation [SAFE] study), which compared 4% albumin with 0.9% saline as fluid replacement in critically ill patients, suggested that the risk of death may be lower with albumin than saline in sepsis [59]. Within the limitation of a subgroup analysis, this observation is thought-provoking, with a subsequent meta-analysis suggesting an association between the use of albumin-containing solutions in sepsis and lower mortality [60].
More recently, the results of the ALBIOS (Albumin Italian Outcome Sepsis) trial, which included 1795 patients with sepsis, who were randomized to receive daily 20% albumin aiming for an albumin concentration of 30 g/L when compared to standard care, were not superior in terms of organ failure rates—as measured by SOFA scores—or in the mortality rate [61]. However, a post hoc analysis reported a reduction in mortality in the subgroup of patients with septic shock, which requires further evaluation. While albumin appears not to be harmful in sepsis, except in traumatic brain injury patients [62], it does not have any established benefit over crystalloid.
The idea that colloids might have a dramatic volume-sparing benefit in the critically ill (as could be assumed from studies in health) was not observed in the SAFE study of 4% albumin, nor the above two starch studies, where the observed ratio of colloid to crystalloid was 1: 1.3 [55, 56, 59]. Damage to the endothelial glycocalyx layer in sepsis plays a major role in increased membrane permeability, such that the increased intravascular half-life of colloid is largely lost [63].
6.2 Crystalloids
Balanced crystalloid solutions (e.g., Hartmann’s solution/Ringer’s lactate and Plasma-Lyte) intuitively have potential benefits when compared to 0.9% NaCl, particularly as their composition is usually representative of electrolyte concentrations in humans. Other problems associated with 0.9% NaCl, including a metabolic acidosis from the chloride load, and the potential for chloride-induced nephrotoxicity, are concerns, but more likely to be problematic when the administered volume is larger. A before-after study suggested that avoidance of chloride-rich fluids might lead to decreased rates of acute kidney injury and need for renal replacement therapy [64]. However, the subsequent ‘SPLIT’ (Effect of a Buffered Crystalloid Solution versus Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit) study failed to demonstrate any renoprotective effect from avoiding 0.9% NaCl, similar to the SALT (Balanced Crystalloids versus Saline in the Intensive Care Unit) study that also addressed this question [65, 66]. Further trials in this area are ongoing [67, 68]. Resuscitation of septic patients with hypertonic saline has an insufficient evidence basis, and as such it cannot be recommended.
The authors would currently support the use of a balanced crystalloid, or 4% (or 5%) albumin for filling in septic patients, and the avoidance of semisynthetic colloids (starch, gelatins). In the absence of any further evidence, 0.9% NaCl remains an acceptable and inexpensive alternative to balanced crystalloid solution, although it may be problematic if used in very large volumes. 4% albumin is a reasonable alternative where readily available, as long as the patient does not have a traumatic brain injury.
Conclusions
Fluid administration is a frequent intervention in septic patients, with increasing evidence that it may considerably influence the outcome. Considerations should include the patient’s cumulative fluid balance, fluid responsiveness and the early use of a vasopressor to avoid excessive fluid administration beyond the initial resuscitation phase.
While there is not compelling evidence for one crystalloid over another, there is the potential that balanced crystalloids may be associated with less harm, particularly if a significant amount of fluid is given. Semisynthetic colloids (starches and gelatins) should be avoided, while 4% albumin appears safe in the absence of traumatic brain injury.
Further data are needed to determine whether fluid administered as a bolus is harmful in the adult critical care setting, to explore the optimal balance between fluids and vasopressors in the supportive phase of septic shock, and to understand whether certain crystalloids lead to better patient-centred outcomes.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14(11):759–67.
Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, et al. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21(3):708–19.
Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17.
Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251.
de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015;30(1):97–101.
Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92(1):37–46.
Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Crit Care Clin. 2000;16(2):251–87.
Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15(5):392–7.
Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36(6):1701–6.
Garcia-Alvarez M, Marik P, Bellomo R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol. 2014;2(4):339–47.
Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18(5):503.
Bakker J, de Backer D, Hernandez G. Lactate-guided resuscitation saves lives: we are not sure. Intensive Care Med. 2016;42(3):472–4.
Monnet X, Delaney A, Barnato A. Lactate-guided resuscitation saves lives: no. Intensive Care Med. 2016;42(3):470–1.
Zafrani L, Payen D, Azoulay E, Ince C. The microcirculation of the septic kidney. Semin Nephrol. 2015;35(1):75–84.
Martensson J, Bellomo R. Sepsis-induced acute kidney injury. Crit Care Clin. 2015;31(4):649–60.
Maiden MJ, Otto S, Brealey JK, Finnis ME, Chapman MJ, Kuchel TR, et al. Structure and function of the kidney in septic shock. A prospective controlled experimental study. Am J Respir Crit Care Med. 2016;194(6):692–700.
Lipcsey M, Bellomo R. Septic acute kidney injury: hemodynamic syndrome, inflammatory disorder, or both? Crit Care. 2011;15(6):1008.
Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11.
Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettila V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42(11):1695–705.
Weil MH, Shubin H, Rosoff L. Fluid repletion in circulatory shock: central venous pressure and other practical guides. JAMA. 1965;192:668–74.
Latta T. Dr Latta’s cases of venous injections in cholera. Lancet. 1832;18(460):370–3.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–44.
Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock-part I: physiology. Crit Care Med. 2013;41(1):255–62.
Carsetti A, Cecconi M, Rhodes A. Fluid bolus therapy: monitoring and predicting fluid responsiveness. Curr Opin Crit Care. 2015;21(5):388–94.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65.
Sadaka F, Juarez M, Naydenov S, O'Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med. 2014;29(4):213–7.
Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625–32.
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41(9):1529–37.
Toscani L, Aya HD, Antonakaki D, Bastoni D, Watson X, Arulkumaran N, et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. 2017;21(1):207.
Perner A, Vieillard-Baron A, Bakker J. Fluid resuscitation in ICU patients: quo vadis? Intensive Care Med. 2015;41(9):1667–9.
Maitland K, Babiker A, Kiguli S, Molyneux E, Group FT. The FEAST trial of fluid bolus in African children with severe infection. Lancet. 2012;379(9816):613. author reply −4
Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68.
Myburgh J, Finfer S. Causes of death after fluid bolus resuscitation: new insights from FEAST. BMC Med. 2013;11:67.
Magder S. Fluid status and fluid responsiveness. Curr Opin Crit Care. 2010;16(4):289–96.
Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–8.
Mesquida J, Gruartmoner G, Ferrer R. Passive leg raising for assessment of volume responsiveness: a review. Curr Opin Crit Care. 2017;23(3):237–43.
Vignon P, Repesse X, Begot E, Leger J, Jacob C, Bouferrache K, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017;195(8):1022–32.
Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249–54.
Richard JC, Bayle F, Bourdin G, Leray V, Debord S, Delannoy B, et al. Preload dependence indices to titrate volume expansion during septic shock: a randomized controlled trial. Crit Care. 2015;19:5.
See KC, Mukhopadhyay A, Lau SC, Tan SM, Lim TK, Phua J. Shock in the first 24 h of intensive care unit stay: observational study of protocol-based fluid management. Shock. 2015;43(5):456–62.
Saugel B, Vincent JL, Wagner JY. Personalized hemodynamic management. Curr Opin Crit Care. 2017;23(4):334–41.
Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44(5):880–91.
Nunes TS, Ladeira RT, Bafi AT, de Azevedo LC, Machado FR, Freitas FG. Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care. 2014;4:25.
Geisen M, Rhodes A, Cecconi M. Less-invasive approaches to perioperative haemodynamic optimization. Curr Opin Crit Care. 2012;18(4):377–84.
Boyd JH, Sirounis D. Assessment of adequacy of volume resuscitation. Curr Opin Crit Care. 2016;22(5):424–7.
Biais M, de Courson H, Lanchon R, Pereira B, Bardonneau G, Griton M, et al. Mini-fluid challenge of 100 ml of crystalloid predicts fluid responsiveness in the operating room. Anesthesiology. 2017;127:450–6.
Guinot PG, Bernard E, Deleporte K, Petiot S, Dupont H, Lorne E. Mini-fluid challenge can predict arterial pressure response to volume expansion in spontaneously breathing patients under spinal anaesthesia. Anaesth Crit Care Pain Med. 2015;34(6):333–7.
Marik PE. Fluid therapy in 2015 and beyond: the mini-fluid challenge and mini-fluid bolus approach. Br J Anaesth. 2015;115(3):347–9.
Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93.
Investigators A, Group ACT, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506.
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11.
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(25):2462–3.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34.
Hammond NE, Taylor C, Finfer S, Machado FR, An Y, Billot L, et al. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: an international cross-sectional study. PLoS One. 2017;12(5):e0176292.
Pisano A, Landoni G, Bellomo R. The risk of infusing gelatin? Die-hard misconceptions and forgotten (or ignored) truths. Minerva Anestesiol. 2016;82(10):1107–14.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56.
Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39(2):386–91.
Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–21.
Myburgh J, Investigators SS, Australian, New Zealand Intensive Care Society Clinical Trials G, Australian Red Cross Blood S, George Institute for International H, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874–84.
Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–91.
Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–72.
Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314(16):1701–10.
Semler MW, Wanderer JP, Ehrenfeld JM, Stollings JL, Self WH, Siew ED, et al. Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med. 2017;195(10):1362–72.
Zampieri FG, Azevedo LCP, Correa TD, Falavigna M, Machado FR, Assuncao MSC, et al. Study protocol for the balanced solution versus saline in intensive care study (BaSICS): a factorial randomised trial. Crit Care Resusc. 2017;19(2):175–82.
Semler MW, Self WH, Wang L, Byrne DW, Wanderer JP, Ehrenfeld JM, et al. Balanced crystalloids versus saline in the intensive care unit: study protocol for a cluster-randomized, multiple-crossover trial. Trials. 2017;18(1):129.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Anstey, J.R., Deane, A.M., Bellomo, R. (2018). Fluids in Sepsis. In: Wiersinga, W., Seymour, C. (eds) Handbook of Sepsis. Springer, Cham. https://doi.org/10.1007/978-3-319-73506-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-73506-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73505-4
Online ISBN: 978-3-319-73506-1
eBook Packages: MedicineMedicine (R0)