Abstract
Bone metastases are virtually incurable resulting in significant disease morbidity, reduced quality of life, and mortality. Bone provides a unique microenvironment whose local interactions with tumor cells offer novel targets for therapeutic interventions. Increased understanding of the pathogenesis of bone disease has led to the discovery and clinical utility of bone-targeted agents other than bisphosphonates and denosumab, currently the standard of care in this setting.
In this chapter, we present the recent advances in molecular-targeted therapies focusing on therapies that inhibit bone resorption and/or stimulate bone formation and novel antitumor agents that exert significant effects on skeletal metastases, nowadays available in clinical practice or in phase of development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Denosumab
- Receptor Activator Of Nuclear Factor kappa-B Ligand (RANKL)
- Cabozantinib
- Zoledronic Acid
- Enzalutamide
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
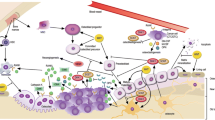
1 Biological Background: The Bone Niche
Bone, particularly trabecular bone, is one of the most preferential metastatic target sites for malignancies such as breast, prostate, and lung cancers. Bone metastases are associated with a reduced quality of life and an increased risk of complications arising from bone weakness or deregulated calcium homeostasis. These complications (such as pathological fractures, spinal cord compression, or radiation, or surgery to the bone) are collectively defined as skeletal-related events (SREs). Additionally, the patient with metastatic bone disease frequently experiences significant pain that may be difficult to treat.
Depending on their radiographic appearance, bone metastases can be predominantly osteolytic, involving bone destruction, or osteoblastic characterized by large amounts of newly deposed woven bone. The lesion phenotype reflects the local interaction between tumor cells and the bone remodeling system [1,2,3].
Cross talk between tumor and bone cells, both through direct cell-cell contact and through soluble factors, is considered critical for the development and progression of bone metastases. Although tumor cells secrete proteolytic enzymes and can directly destroy bone matrix in vitro, the main mediators of bone destruction within a metastatic lesion are the osteoclasts (OCLs) [4]. Osteolysis activity causes the release of growth factors, stored in the bone matrix, into tumor microenvironment. These factors stimulate the growth of tumor cells and alter their phenotype, thus promoting a vicious cycle of metastasis and bone pathology. Physical factors within the bone microenvironment, including low oxygen levels, acid pH, and high extracellular calcium concentrations, may also enhance tumor growth [5]. Furthermore, there is evidence that osteolytic lesions are linked not only with increased OCL activity but also with impaired osteoblast (OBL) differentiation, activity [6, 7], and apoptosis [8]. OBL metastases are characterized by a higher OBL proliferation and bone matrix deposition associated with an increased OCL activity [9, 10]. The net result is a raise of OBL proliferation and differentiation that increases the deposition of abnormal, woven bone.
Anatomically, the bone areas most frequently colonized by disseminated tumor cells (DTCs) are the axial skeleton, including the spine, ribs, and pelvic bones. Bone stromal cells, such as osteoblasts, osteoclasts, mesenchymal stem/stromal cells (MSCs), endothelial cells, macrophages, neutrophils, lymphocytes, and hematopoietic stem/progenitor cells (HSPCs), have been shown to either expedite or impede the progression of cancer cell metastases [11, 12]. Furthermore, a series of trophic factors, cytokines, and chemokines serve as bone stroma-derived mediators that play critical roles in building the specialized bone metastatic niche. Of these known regulators, CX-chemokine ligand 12 (CXCL12), integrins, osteopontin (OPN), vascular cell adhesion molecule-1 (VCAM-1), transforming growth factor beta (TGF-β), Jagged 1, and the receptor activator of nuclear factor kappa-B ligand (RANKL) display the greatest influence in specifying the metastatic niche. Taken together, these bone marrow (BM) niche cells and factors constitute a finely organized network that promotes DTC homing, seeding, hibernation, and proliferation while facilitating the progressive breakdown of normal hematopoiesis and osteogenesis [5, 13, 14]. These tumor-stroma interactions could lead to the development of effective therapeutic agents, such as osteoclast-targeting bisphosphonates and the monoclonal antibody, which inhibits activation of the receptor activator of nuclear factor kappa-B ligand (RANKL) denosumab for controlling cancer-induced bone complications [15].
In the last two decades, the bisphosphonates and denosumab, a monoclonal antibody that inhibits activation of the receptor activator of nuclear factor kappa-B ligand (RANKL), have become established as a valuable additional approach to the range of current treatments. Multiple randomized controlled trials have clearly demonstrated that they are effective in reducing skeletal morbidity from metastatic cancer [16]. Moreover, radiopharmaceuticals are other interesting agents targeting bone metastases able to improve overall survival in patients with prostate cancer bone metastases. Finally, several molecules that are already approved as anticancer agents (such as antiandrogens, mTOR inhibitors, and c-Met inhibitors) are now in clinical evaluation for their potential beneficial effects on bone metabolism.
2 Bisphosphonates
Bisphosphonates are well established as successful agents for the management of osteoporosis as well as bone metastases in patients with solid cancer and multiple myeloma [17].
Bisphosphonates are analogues of pyrophosphate with a strong affinity for divalent metal ions, such as calcium ions, and for the skeleton. Indeed bisphosphonates are incorporated into the bone matrix by binding to exposed hydroxyapatite crystals that provide a barrier to osteoclast-mediated bone resorption and have direct inhibitory effects on osteoblasts. In particular, bisphosphonates are embedded in bone at active remodeling sites, released in the acidic environment of the resorption lacunae under active osteoclasts and are taken up by them. There are two classes of bisphosphonates, nonnitrogen-containing and nitrogen-containing bisphosphonates (N-BPs). The nitrogen-containing biphosphonates (alendronic, ibandronic, pamidronic, risedronic, and zoledronic acid) are more potent osteoclast inhibitors than nonnitrogen-containing bisphosphonates (e.g., clodronic, etidronic, and tiludronic acid) [18]. Moreover, nitrogen-containing bisphosphonates inhibit farnesyl pyrophosphatase, an enzyme responsible for the prenylation of GTPases that are essential for osteoclast function, structural integrity, and the prevention of apoptosis [18,19,20]. The inhibition of farnesyl pyrophosphatase also results in the accumulation of isopentenyl diphosphate that is incorporated into a cytotoxic nucleotide metabolite, ApppI [19]. Therefore, bisphosphonates affect osteoclast differentiation and maturation and thereby act as potent inhibitors of bone resorption. Preclinical evidence demonstrated that bisphosphonates do not affect only the bone microenvironment but have also a direct effect on macrophages, gamma delta T cells, osteoblasts, and cancer cells showing antitumor and/or antiangiogenic effects [21].
Strong evidence supports the role of bisphosphonates in the treatment of advanced breast cancer. A Cochrane Collaboration systematic review and meta-analysis of nine studies, which included 2806 patients, demonstrated that bisphosphonates decreased the SRE rate by 15% compared with placebo in women with breast cancer who had bone metastasis [22]. All bisphosphonates were effective (clodronic, pamidronic, ibandronic, and zoledronic acid) and reduced SREs by 20–40%, depending on the agent [20, 23,24,25,26,27,28,29]. The Cochrane Collaboration meta-analysis did not show an overall survival benefit for the use of bisphosphonates in women with breast cancer and bone metastasis. In addition, the review did not show consistent improvement in global quality of life or improvement in bone pain associated with bisphosphonate therapy. In a large randomized controlled trial that included more than 1000 patients, the effectiveness of zoledronic acid was compared with that of denosumab. This study showed the superiority of denosumab in delaying the time-to-first SRE and time-to-subsequent SREs [30]. However, overall survival, disease progression, and rate of adverse events were similar between the groups. Only a very modest improvement in health-related quality of life was noted, favoring the use of denosumab [31]. The National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO), and the European Society of Medical Oncology (ESMO) are consistent in recommending either zoledronic acid or denosumab [32,33,34].
Currently zoledronic acid is also used in men with bone metastatic prostate cancer that has progressed after initial hormone therapy. In this setting zoledronic acid reduced the frequency of SREs, prolonged median time-to-develop SREs, and decreased pain and analgesic scores [35, 36]. Moreover, zoledronic acid efficacy in preventing bone fractures was demonstrated in patients with high grade and/or locally advanced, nonmetastatic prostate adenocarcinoma receiving luteinizing hormone-releasing hormone (LHRH) agonist and radiotherapy (RT) [37].
Currently, the key question is: what is the role of zoledronic acid in hormone-sensitive prostate cancer? In the STAMPEDE trial, the addition of zoledronic acid to docetaxel did not improve survival outcomes or delay the SRE incidence [38]. In the CALGB/ALLIANCE 90202 study comparing early treatment in hormone-sensitive prostate cancer versus delayed treatment in castration-resistant prostate cancer (CRPC), no difference in SRE-free survival and no change in survival outcomes were noted. Thus, zoledronic acid did not improve SRE in hormone-sensitive disease (median time-to-first SRE was 31.9 months in the zoledronic acid group and 29.8 months in the placebo group) but showed, as previously described, benefit in SRE in castration-resistant disease [39].
3 Denosumab
The development and approval of denosumab, a fully monoclonal antibody against RANKL, have heralded a new era in the treatment of bone diseases by providing a potent, targeted, and reversible inhibitor of bone resorption.
The RANKL/RANK/OPG are members of the TNF and TNF-receptor superfamily and act as essential mediators of OCL formation, function, and survival. In particular, RANKL in normal process is secreted by OBLs and binds to its receptor RANK, expressed by OCL precursors and mature OCLs stimulating bone resorption activity; at contrast osteoprotegerin (OPG), the decoy receptor for RANKL, prevents OCL activation [40]. Moreover, RANKL acts as a key paracrine effector for the mitogenic action of progesterone in mouse mammary epithelium and modulating estrogen-dependent expansion and regenerative potential of mammary stem cells [41, 42], mechanisms known to be important for mammary tumorigenesis. Murine in vivo models showed RANKL as a potent chemoattractant in tumors and supports the pro-migratory activity of RANK-expressing breast and prostate cancer cell lines; moreover in an in vivo melanoma model of bone metastases, the inhibition of RANKL results in a reduction of bone lesions and tumor burden [43]. RANKL is also expressed in some cancer cells, while in other case, cell-to-cell contact of tumor cells with OBLs enhances its expression; this contextually promotes the entry of cancer cells into the vicious cycle where the interaction with RANK-expressing OCLs stimulate their activation [43].
Recently evidences suggest an important role for RANKL/RANK in the immune system including in lymph node development, lymphocyte differentiation, dendritic cell survival, and T-cell activation and tolerance induction [44,45,46].
Denosumab was developed for the treatment of osteoporosis, cancer treatment-induced bone loss, bone metastases, and other skeletal pathologies mediated by OCLs. Denosumab showed superiority to zoledronic acid in delaying time-to-first SRE and time-to-first-and-subsequent SRE in bone metastatic breast cancer patients, as previously described [30]. In a castration-resistant prostate cancer patient population presenting bone metastases, the median time-to-first on-study SRE for the denosumab arm was significantly prolonged (21 months) compared to the zoledronic acid ones (17 months) with no improvements in the overall survival or progression of disease [47]. Another trial enrolled 1776 patients with myeloma-induced osteolysis and solid tumors other than breast and prostate cancers [48]. The results showed a median time-to-first on-study SRE of 21 months in the denosumab group and 16 months in the arm receiving zoledronic acid demonstrating a non-inferiority for denosumab versus zoledronic acid but neither a superiority after adjustment for multiple comparisons nor an advantage in the overall survival of denosumab over zoledronic acid.
Nevertheless, a post hoc analysis of these three phase III trials in patients with breast cancer [30], prostate cancer [47], or other solid tumors [48] (excluding multiple myeloma patients) showed that denosumab was superior to zoledronic acid in preventing SREs in patients with bone metastases, regardless of ECOG performance status, bone metastasis number, baseline visceral metastasis presence/absence, and urine N-telopeptide (uNTX) level [49].
In another phase III trial, 1432 men with nonmetastatic castration-resistant prostate cancer were randomly assigned to denosumab or placebo. Denosumab increased the time-to-development of first bone metastasis by a median of 4.2 months compared with placebo, in a population of men deemed to be at a high risk for the development of metastatic disease. No difference in the overall survival (OS) was noted [50].
4 Antiandrogen Agents
Recent advances demonstrated that androgen-based pathways continue to have a clinically significant role in the progression of castrate-resistant prostate cancer (CRPC). In addition to androgen production by the adrenal gland and the testis, several enzymes involved in the synthesis of testosterone and dihydrotestosterone, including cytochrome P450 17 alpha hydroxysteroid dehydrogenase (CYP17), are highly expressed in tumor tissue [51].
Persistent androgen signaling is a validated therapeutic target in metastatic CRPC (mCRPC). Preclinical and clinical findings confirm that transition from endocrine-dependent to intracrine androgen signaling progression is a milestone in the lethal progression of prostate cancer and resistance to standard androgen deprivation therapy [52, 53]. Moreover, over the course of mCRPC progression, androgen receptor (AR) changes ensue. These include overexpression, mutation, alternative splicing, posttranslational modifications, or interactions with other pathways (nonclassical AR signaling) [54, 55].
Randomized trials that led to regulatory approval of CYP17 inhibitor, abiraterone acetate, and antiandrogen, enzalutamide, in mCRPC have also demonstrated that these drugs decreased the time-to-first SRE onset and radiological skeletal progression [56,57,58,59,60].
4.1 Abiraterone
Abiraterone acetate is an orally administered selective androgen biosynthesis inhibitor derived from the structure of pregnenolone. It potently and irreversibly inhibits both the hydroxylase and lyase activity of CYP17A with approximately 10–30-fold greater potency than ketoconazole [61] resulting in virtually undetectable serum and intratumoral androgen production in the adrenals, testes, and prostate cancer cells [62, 63]. Because adrenal inhibition of CYP17A results in blockade of glucocorticoid as well as adrenal androgen synthesis, abiraterone is co-administered with prednisone to ameliorate the secondary rise in adrenocorticotropic hormone (ACTH) that can lead to excess mineralocorticoid synthesis [64].
In phase III studies in mCRPC patients, it was demonstrated that abiraterone treatment is associated not only with a significant survival advantage in both chemotherapy-treated [64] and chemotherapy-naive patients [65] but also with a better pain control from skeletal metastases, a delay in time-to-develop SREs and in radiological skeletal progression in chemotherapy-treated patients. In this group, 25% of patients developed a skeletal event in 9.9 months when treated with abiraterone and 4.9 months with placebo, and the time-to-first SRE was 25.0 months with abiraterone compared to 20.3 months with placebo [64]. The benefits of abiraterone on metastatic bone disease may be not only secondary to a systemic control of the disease due to a direct antitumor effect but also due to a specific effect on bone microenvironment. Indeed, recently direct bone anabolic and an anti-resorptive effect of abiraterone both in vitro and in mCRPC patients was found. In particular, abiraterone was found to be able to specifically modulate OCLs and OBLs leading to direct anabolic and anti-resorptive effects both in the presence and absence of steroids, suggesting a noncanonical mechanism of action that seems to be, at least in part, androgen-independent [65].
4.2 Enzalutamide
Another promising oral AR inhibitor that targets multiple steps in the AR signaling pathway is enzalutamide. In the randomized phase III AFFIRM study, significant improvements in survival versus placebo were observed when enzalutamide was used as a treatment for patients with mCRPC following prior treatment with docetaxel. Additional benefits included significant delay in time-to-first SREs and improvement in several measures of pain and health-related quality of life [60]. Furthermore, in the phase III PREVAIL study evaluating enzalutamide versus placebo in patients with mCRPC, who had not received chemotherapy, the antiandrogen significantly decreased the risk of radiographic progression and death. There were also significant improvements in all secondary and prespecified exploratory endpoints, including delayed initiation of chemotherapy and a high percentage of patients with objective response compared with placebo [66]. Moreover, median time-to-first skeletal-related event was longer in the enzalutamide group than in the placebo group. Finally, treatment with enzalutamide was associated with a reduction in the risk of a first skeletal-related event, which was not dependent on bisphosphonate or denosumab use at baseline [67]. Ongoing and planned trials will help further define the optimal use of both abiraterone acetate and enzalutamide in the treatment of metastatic prostate cancer.
5 mTOR Inhibitors
Preclinical analyses show that mTOR pathway is involved in bone remodeling [68,69,70,71,72,73,74]. These effects are likely exerted via signal transduction by cytokines through the mTOR pathway, which decreases osteoclast apoptosis and promotes osteoclast survival [69, 70]. One cytokine pathway influenced by mTOR that is critical for osteoclast growth and differentiation is the RANK/osteoprotegerin pathway [69, 70, 75]. Notably, downregulating mTOR via suppression of mTOR phosphorylation in the ST2 bone marrow-derived stromal cell line led to upregulation of osteoprotegerin [72].
Other factors that reflect osteoclast activity may also be influenced by mTOR inhibition including cathepsin K, the main osteoclast-derived protease responsible for digesting collagen type I in the bone [71]. Cathepsin K mRNA expression and protein levels in human osteoclasts decreased substantially after treatment with everolimus, an inhibitor of mTOR signaling [71]. Moreover, a study in bone marrow cells of cultured rabbit demonstrated that treatment with the mTOR inhibitor rapamycin decreased production of CTX, a bone resorption marker [69]. Finally, inhibiting mTOR in mice decreased osteoclast maturation and increased osteoclast apoptosis [69], suggesting that blocking the mTOR pathway may lead to a protective effect on the bone.
The phase III study BOLERO-2 showed a statistically significant benefit in progression-free survival (PFS) adding everolimus to nonsteroidal aromatase inhibitor therapy in postmenopausal women with estrogen receptor-positive breast cancer progressing despite nonsteroidal aromatase inhibitor therapy [74]. Moreover an exploratory analyses in this trial evaluating the effect of everolimus on bone marker levels and bone disease progression showed a significant decrease of bone marker level at 6 months and 12 months from baseline and a reduction in bone disease progression in the combination arm (everolimus plus exemestane) [75]. As demonstrated by Gnant et al. [75], differences in the incidence of bone disease progression became evident between the treatment arms by week 12, with a lower cumulative incidence rate of bone disease progression for the combination arm (3.5%) versus the exemestane-only arm (6.6%) in the overall population. Bone disease progression remained nearly twofold lower in the combination arm versus the exemestane-only arm through week 30 (8.1% vs 15.0%, respectively), and similar trends continued beyond 30 weeks [75]. The influence of bisphosphonate use on bone marker level changes was also examined in both treatment arms. At 12 weeks, bone marker levels were lower in the combination arm versus the exemestane-only arm and differences in changes from baseline to week 12 between treatment arms at this timepoint were larger in patients who received baseline bisphosphonates versus those who did not [75].
In a double-blind, placebo-controlled, phase II, randomized discontinuation study (RADAR) in breast cancer patients with HER2-negative breast cancer patients with bone metastases only, patients were randomized to everolimus-continuation or placebo, after being stable on 8 weeks of everolimus. Time to progression in patients with everolimus-continuation was 37.0 versus 12.6 weeks (95% CI 7.1–17.9) with placebo suggesting that patients with bone metastases only may retrieve long-term benefit from everolimus if they do not progress within 8 weeks of treatment [76].
Finally, in an ongoing phase II study, symptomatic skeletal event-free survival (SSE-FS) are evaluated in metastatic breast cancer patients treated with radium-223 dichloride in combination with exemestane and everolimus versus placebo in combination with exemestane and everolimus (NCT02258451).
These evidences from phase III clinical trial suggest that mTOR inhibition in combination with exemestane may have a beneficial effect on bone health in patients with bone metastases, reducing the incidence of bone metastases morbidity and mortality.
6 Radiopharmaceutical
Radiopharmaceuticals are other interesting agents targeting bone metastases; several studies showed how beta-emitting radiopharmaceuticals allow bone pain relief in mCRPC patients due to their similarity to calcium, emitting radiation when they are taken up at the site of osteoblastic activity. Strontium-89 and samarium-153 were the first radiopharmaceuticals approved for bone metastases pain relief in patients with mCRPC [77, 78]. Although these radiopharmaceuticals are useful tool for pain palliations, no study showed impact on the overall survival, but only one randomized control trial showed that strontium-89 after six cycles of docetaxel improved clinical progression-free survival (CPFS) despite frequent hematological adverse events [79], limiting their use only in symptomatic patients with multiple bone sites.
Radium-223 is an alpha emitter that differs from beta emitter agents since it delivers a highly localized radiation to bone surface, causing double-stranded DNA breaks that lead to cell death, giving less irradiation to healthy bone marrow than beta emitters [80]. In particular it is a calcium-mimetic molecule that forms complex with hydroxyapatite, which forms 50% of the bone matrix; their linking allows radium-223 to be incorporated to the bone matrix emitting alpha particle preserving the health of bone tissue and bone marrow and limiting distribution to soft tissue [81].
Radium-223 was recently approved by FDA in men with symptomatic mCRPC with bone and no visceral metastases, presenting a significant impact on the overall survival in patients who progress with docetaxel or unfit to docetaxel. The rationale of its beneficial use as a bone target comes from several phase I and II trials that show safety and tolerability of Alpharadin, radium-223 chloride in solution, in mCRPC patients, with significant effects on bone turnover markers such as bone alkaline phosphatase (bALP) and uNTX [82, 83]. These encouraging data allowed investigators to conduct a randomized open-label, multicenter phase III trial evaluating the impact on the overall survival of radium-223 in mCRPC patients with bone metastases previously treated with docetaxel or unfit to receive docetaxel. This phase III trial was early stopped after preplanned efficacy interim analysis, since OS was significantly improved in the radium-223 arms versus placebo control arm (median, 14.0 vs 11.2 months), respectively; updated analyses in all 921 patients, performed before crossover from placebo to radium-223, showed a similar survival advantage for radium-223 treatment (median, 14.9 vs 11.3 months) [84]. Moreover radium-223 showed efficacy in all secondary end points including time-to-first symptomatic skeletal events (median, 15.6 months vs 9.8 months, respectively).
Furthermore, a prespecified subgroup analysis from this trial showed that radium-223 is effective and well tolerated irrespective of previous docetaxel use [85]. Starting from these promising results, new trials are under investigation to better understand combination therapy with docetaxel and other new emergent therapies, such as abiraterone acetate, that will improve the overall survival in this subset of patients (NCT01106352 and NCT02097303). Furthermore, several studies are also under evaluation in order to better understand the potential role of Alpharadin in patients with other cancers that have the tendency to metastasize to the bone (e.g., lung cancer, NCT 02283749).
Finally, data exist to support the co-administration of radium-223 with bisphosphonates. Indeed in ALSYMPCA, 41% of patients were on bisphosphonates at registration, and there was a clear delay in symptomatic skeletal events (SSEs) in these patients (19.6 vs 10.2 months). Although only a hypothesis-generating subset analysis, this observation suggests a possible positive interaction between radium-223 and osteoclast-targeted agents [86].
7 Agents Targeting Dikkopf-1/WNT Pathway
Dikkopf-1 (DKK1) is an inhibitory signal belonging to the WNT pathway. It performs a critical role in the onset of osteolytic skeletal metastases. In this setting the inhibition of OBL activity has been linked to the production of this soluble protein by tumor cells. DKK1 produced by tumor cells (breast, prostate) induces osteolytic lesions in in vivo animal models and sustains the formation of osteolytic cancer metastases. In addition elevated DKK1 levels are observed in serum of patients with multiple myelomas and in women with breast cancer metastatic to bone. Compelling evidences in humans and mice show that WNT signaling pathway increases bone mass stimulating, at least in part, OBL proliferation and activity. In particular WNT signaling acts by upregulating OPG and downregulating OBL RANKL expression [87] suggesting a mechanism by which WNT indirectly regulates osteoclastogenesis. In this axis the role of DKK1 inhibits OBL activity by blocking the action of WNT proteins on these cells [15]. Several data report that DKK1 promotes the formation of osteolytic metastases and may facilitate the conversion of osteoblastic metastases into an osteolytic phenotype. Preclinical data suggest that DKK1-neutralizing antibodies restored the bone mineral density (BMD) of the implanted myelomatous bone, increased the number of osteocalcin-expressing OBLs, and reduced the number of multinucleated tartrate-resistant acid phosphatase (TRAP)-expressing OCLs. Furthermore, the anti-DKK1-treated mice showed reduced tumor burden [15].
Treatment with a DKK-1-neutralizing antibody, BHQ880, resulted in increased osteoblast numbers and trabecular bone and inhibition of multiple myeloma cells growth in murine MM models [88]. This led to the evaluation of BHQ880 in a number of clinical trials of which the complete results have yet to be reported (NCT00741377, NCT01302886, and NCT01337752). The phase Ib trial showed that BHQ880 in combination with zoledronic acid and anti-myeloma therapy was well tolerated and demonstrated potential clinical activity in patients with relapsed/refractory multiple myeloma [89].
8 Agents Targeting c-MET/HGF Pathway
The receptor tyrosine kinase MET and its ligand hepatocyte growth factor (HGF) signaling pathway promote stemness phenotype, tumor growth, invasion, and metastases in several malignancies. Prominent expression of MET has been observed in primary and metastatic prostate carcinomas; in particular, it has been demonstrated that bone metastases have higher levels of expression of MET oncogene compared with lymph node metastases or primary tumors [90, 91].
Furthermore it is known that both HGF and MET are expressed by OBLs and OCLs, mediating cellular responses such as proliferation, migration, and differentiation. In OCLs, HGF and M-CSF signals, through tyrosine kinase receptors, lead to phosphorylation of common transducers and effectors such as Src, Grb2, and PI3-kinase. Additionally it has been demonstrated that HGF is able to support monocyte-OCL differentiation in the presence of RANKL as evidenced by the formation of numerous multinucleated TRAP and vitronectin receptor-positive cells which formed F-actin rings and which were capable of lacunar resorption [92]. On the other hand, HGF activates many signaling cascades in human mesenchymal stem cells, including rapid phosphorylation of ERK, p38, and AKT/PI3K, promoting OBL differentiation [93]. Moreover c-Met activation increases osteopontin (OPN) expression in human OBLs via the PI3K, Akt, c-Src, c-Jun, and AP-1 signaling pathway [94]. Interestingly, OCLs are found to synthesize and secrete biologically active HGF. These data strongly suggest the possibility of an autocrine regulation of the OCLs by HGF and a paracrine regulation of the OBLs by the HGF produced by the OCLs [95].
Cabozantinib (XL184) is an orally bioavailable tyrosine kinase inhibitor with potent activity against MET and VEGF receptor 2 (VEGFR2).
In a multicenter, phase II, nonrandomized expansion study of men with CRPC, bone metastases, and disease progression despite docetaxel treatment, cabozantinib was associated with improvements in bone scans, patient-reported pain and analgesic use, measurable disease, CTCs, and bone biomarkers. The randomization was stopped because of these improvements in bone response, and a group of 31 patients had been randomly assigned. In this group, there was a marked improvement in the primary end point of progression-free survival (PFS) in patients receiving cabozantinib compared with placebo (median, 23.9 vs 5.9 weeks, respectively) [96]. Anyway in a following phase III trial (COMET-1), cabozantinib did not meet its primary end point of demonstrating a statistically significant increase in the overall survival (OS) compared to prednisone. COMET-1 yielded a median overall survival (OS) for men treated with cabozantinib of 11 months, compared with 9.8 months for the prednisone arm, which was not statically significant [97]. However, cabozantinib was associated with an improvement in bone scan responses at week 12 (42% for cabozantinib vs 3% for prednisone), in progression-free survival (median of 5.5 months in cabozantinib group vs 2.8 months in prednisone group), and with a reduction of skeletal-related event (SRE) rates (14% among patients on cabozantinib and 21% in patients on prednisone) [97]. Recently, a phase III study (METEOR) showed that cabozantinib reduced the risk of disease progression or death compared to everolimus in patients with metastatic renal cell carcinoma (RCC) [98]. Furthermore, in a prespecified analysis in the subgroup of patients with metastatic bone disease treated with cabozantinib (23%), a marked prolongation of PFS was observed (7.4 months in the cabozantinib arm vs 2.7 months in the everolimus arm). Moreover, SRE, in men who showed previous events, was observed in 15 of 91 patients (16%) in the cabozantinib arm and in 31 of 90 patients (34%) in the everolimus arm [99, 100].
Our group has previously demonstrated that cabozantinib inhibits OCL functions “directly” and “indirectly,” reducing the RANKL/osteoprotegerin ratio in OBLs [101]. In particular, cabozantinib significantly inhibited OCL differentiation and bone resorption activity and downmodulated the expression of osteoclast marker genes in primary human OCLs. Differently, cabozantinib treatment had no effect on osteoblast viability or differentiation but increased osteoprotegerin mRNA and protein levels and downmodulated receptor activator of nuclear factor kappa-B ligand (RANKL) at both mRNA and protein levels.
Conclusions
Recent advances showed the important role played by adaptation of metastatic cells in the bone environment and the subsequent cross talk between tumor and host tissue, underlining their involvement in skeletal metastasis growth.
Despite the different approaches investigated to target this cross talk, up to now, only denosumab and bisphosphonates demonstrated to be a changing practice agent in delaying SRE. Moreover translational evidence seems to indicate some kind of efficacy of these compounds as direct anticancer agents. Anyway, currently, we are still far from fully understanding what really happens when disrupting the RANK/RANKL axis in the “real world,” and, at the same time, we do not know which patients could benefit from this approach over and above the effects of denosumab as an antiresorptive agent.
Radium-223 is the first radiopharmaceutical with an overall survival benefit approved for the palliation of pain in patients with prostate cancer bone metastases. The significant efficacy in a hard-to-treat setting such as CRPC makes this compound worth of further exploration either in prostate cancer (hormone-sensitive setting, combination with chemotherapy or androgen deprivation therapy) or bone metastases from other solid tumors.
Recent interesting evidences demonstrated that antiandrogen molecules such as abiraterone and enzalutamide may simultaneously target prostate cancer cells and bone microenvironment. This could significantly influence future therapeutic approaches evaluating the possibility to combine antiandrogen treatment with bone-modifying agents (bisphosphonates, denosumab) in order to achieve a better disease control and management of prostate cancer bone metastases.
One of the most promising pathways, which deserves to be investigated more in detail, is mTOR signaling. Indeed the mechanisms underlying the anabolic antiresorptive effects of mTOR inhibition remain unknown as well as the biological elucidation of potential synergism with other bone target therapies.
Due to the extremely new mechanisms of the action of cabozantinib, it will be interesting to design novel clinical trials in order to investigate the activity of cabozantinib on skeletal disease-related end points and its potential synergism with standard antiresorptive agents in patients with bone metastatic solid tumors.
In the future, a more comprehensive understanding of the bone metastatic niche will facilitate the development of novel therapeutic strategies for preventing or curing otherwise fatal bone complications.
References
Keller ET, Zhang J, Cooper CR, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20:333–49.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93.
Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57–62.
Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–80.
Kingsley LA, Fournier PG, Chirgwin JM, et al. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–17.
Mercer RR, Miyasaka C, Mastro AM. Metastatic breast cancer cells suppress OBL adhesion and differentiation. Clin Exp Metastasis. 2004;21:427–35.
Bu G, Lu W, Liu CC, et al. Breast cancer-derived Dickkopf1 inhibits OBL differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer. 2008;123:1034–42.
Mastro AM, Gay CV, Welch DR, et al. Breast cancer cells induce OBL apoptosis: a possible contributor to bone degradation. J Cell Biochem. 2004;91:265–76.
Hall CL, Bafico A, Dai J, et al. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–60.
Hall CL, Kang S, MacDougald OA, et al. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661–72.
Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006;25:521–9. https://doi.org/10.1007/s10555-006-9036-9.
Park SI, Soki FN, McCauley LK. Roles of bone marrow cells in skeletal metastases: no longer bystanders. Cancer Microenviron. 2011;4:237–46. https://doi.org/10.1007/s12307-011-0081-8.
Shen Y, Nilsson SK. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. 2012;19:250–5. https://doi.org/10.1097/MOH.0b013e328353c714.
Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. https://doi.org/10.1038/nrc2621.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–25.
Coleman RE, McCloskey EV. Bisphosphonates in oncology. Bone. 2011;49:71–6.
Roelofs AJ, Thompson K, Gordon S, et al. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–30s.
Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9.
Mönkkönen H, Auriola S, Lehenkari P, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437–45.
Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133–7.
Coleman R, Gnant M, Morgan G, et al. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059–67.
Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–21.
Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–90.
Body JJ, Diel IJ, Lichinitser MR, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol. 2003;14:1399–405.
Heras P, Kritikos K, Hatzopoulos A, Georgopoulou AP. Efficacy of ibandronate for the treatment of skeletal events in patients with metastatic breast cancer. Eur J Cancer Care (Engl). 2009;18:653–6.
Kristensen B, Ejlertsen B, Groenvold M, et al. Oral clodronate in breast cancer patients with bone metastases: a randomized study. J Intern Med. 1999;246:67–74.
Paterson AH, Powles TJ, Kanis JA, et al. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol. 1993;11:59–65.
Tubiana-Hulin M, Beuzeboc P, Mauriac L, et al. Double-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastases. Bull Cancer. 2001;88:701–7.
Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9.
Martin M, Bell R, Bourgeois H, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18:4841–9.
Van Poznak CH, Von Roenn JH, Temin S. American Society of Clinical Oncology clinical practice guideline update: recommendations on the role of bone-modifying agents in metastatic breast cancer. J Oncol Pract. 2011;7:117–21.
Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25(suppl 3):iii124–i37.
Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force report: bone health in cancer care. J Natl Compr Cancer Netw. 2013;11(suppl 3):S1–50. quiz S51
Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–68.
Saad F, Gleason DM, Murray R, et al. Long term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone refractory prostate carcinoma. J Natl Cancer Inst. 2004;96(11):879–82.
Kachnic LA, Pugh SL, Tai P, Smith M, Gore E, Shah AB, Martin AG, Kim HE, Nabid A, Lawton CA. RTOG 0518: randomized phase III trial to evaluate zoledronic acid for prevention of osteoporosis and associated fractures in prostate cancer patients. Prostate Cancer Prostatic Dis. 2013;16(4):382–6. https://doi.org/10.1038/pcan.2013.35. Epub 2013 Oct 1
James ND, Sydes MR, Clarke NW, Mason MD, STAMPEDE investigators, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
Smith MR, Halabi S, Ryan CJ, Hussain A, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143–50.
Liu XH, Kirschenbaum A, Yao S, et al. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–8.
Gonzalez-Suarez E, Jacob AP, Jones J, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–7.
Schramek D, Leibbrandt A, Sigl V, et al. OCL differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102.
Nguyen DX, Bos PD, Massagu J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84.
Loser K, Mehling A, Loeser S, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–9.
Akiyama T, Shimo Y, Yanai H, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–37.
Knoop KA, Kumar N, Butler BR, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–47.
Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22.
Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32.
Lipton A, Fizazi K, Stopeck AT, et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur J Cancer. 2016;53:75–83.
Smith MR, Saad F, Oudard S, Shore N, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31(30):3800–6.
Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54.
Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. J Endocrinol. 2012;215:221–37.
Liu LL, Xie N, Sun S, et al. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33:3140–50.
Waltering KK, Urbanucci A, Visakorpi T. Androgen receptor (AR) aberrations in castration-resistant prostate cancer. Mol Cell Endocrinol. 2012;360:38–43.
Drake JM, Graham NA, Stoyanova T, et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci U S A. 2012;109:1643–64.
De Bono S, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46.
Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92.
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Rowlands MG, Barrie SE, Chan F, et al. Esters of 3-pyridylacetic acid that combine potent inhibition of 17 alpha-hydroxylase/C17,20-lyase (cytochrome P45017 alpha) with resistance to esterase hydrolysis. J Med Chem. 1995;38:4191–7.
O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25.
Barrie SE, Potter GA, Goddard PM, et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J Steroid Biochem Mol Biol. 1994;50:267–73.
Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–16.
Iuliani M, Pantano F, Buttigliero C, et al. Biological and clinical effects of abiraterone on anti-resorptive and anabolic activity in bone microenvironment. Oncotarget. 2015;6(14):12520–8.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509–21.
Moriceau G, Ory B, Mitrofan L, et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (everolimus): pivotal role of the prenylation process. Cancer Res. 2010;70:10329–39.
Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNF-alpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–77.
Bertoldo F, Silvestris F, Ibrahim T, et al. Targeting bone metastatic cancer: role of the mTOR pathway. Biochim Biophys Acta. 2014;1845(2):248–54.
Kneissel M, Luong-Nguyen NH, Baptist M, et al. Everolimus sup-presses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35:1144–56.
Mogi M, Kondo A. Down-regulation of mTOR leads to up-regulation of osteoprotegerin in bone marrow cells. Biochem Biophys Res Commun. 2009;384:82–6.
Ory B, Moriceau G, Redini F, Heymann D. mTOR inhibitors(rapamycin and its derivatives) and nitrogen containing bisphosphonates: bi-functional compounds for the treatment of bone tumours. Curr Med Chem. 2007;14:1381–7.
Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Gnant M, Baselga J, Rugo HS, et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J Natl Cancer Inst. 2013;105:654–63.
Maass N, Harbeck N, Mundhenke C, et al. Everolimus as treatment for breast cancer patients with bone metastases only: results of the phase II RADAR study. J Cancer Res Clin Oncol. 2013;139:2047–56.
Sartor O, Reid RH, Bushnell DL, et al. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109:637–43.
Silberstein EB. Dosage and response in radiopharmaceutical therapy of painful osseous metastases. J Nucl Med. 1996;37:249–52.
James ND, Pirrie S, Barton D, et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium-89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747). ASCO meeting abstract. J Clin Oncol. 2013;31(Suppl).
Allen BJ. Clinical trials of targeted alpha therapy for cancer. Rev Recent Clin Trials. 2008;3:185–91.
Henriksen G, Breistol K, Bruland OS, et al. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62:3120–5.
Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94.
Larsen RH, Saxtorph H, Skydsgaard M, et al. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and hematology. In Vivo. 2006;20:325–31.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Hoskin P, Sartor O, O'Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15:1397–406.
Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850–8.
Spencer GJ, Utting JC, Etheridge SL, et al. Wnt signalling in OBLs regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–96.
Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–9.
Iyer SP, Beck JT, Stewart AK, et al. A phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br J Haematol. 2014;167:366–75.
Zhang S, Zhau HE, Osunkoya AO, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer. 2010;9:9.
Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–7.
Adamopoulos IE, Xia Z, Lau YS, et al. Hepatocyte growth factor can substitute for M-CSF to support osteoclastogenesis. Biochem Biophys Res Commun. 2006;350:478–83.
Aenlle KK, Curtis KM, Roos BA, et al. Hepatocyte growth factor and p38 promote osteogenic differentiation of human mesenchymal stem cells. Mol Endocrinol. 2014;28:722–30.
Chen HT, Tsou HK, Chang CH, et al. Hepatocyte growth factor increases osteopontin expression in human OBLs through PI3K, Akt, c-Src, and AP-1 signaling pathway. PLoS One. 2012;7:e38378.
Grano M, Galimi F, Zambonin G, et al. Hepatocyte growth factor is a coupling factor for OCLs and OBLs in vitro. Proc Natl Acad Sci U S A. 1996;93:7644–8.
Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–9.
Smith MR, De Bono JS, Sternberg CN, et al. Final analysis of COMET-1: cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E). In: 2015 genitourinary cancers symposium.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Geczi L, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–23. https://doi.org/10.1056/NEJMoa1510016.
Santini D, Tonini G. Treatment of advanced renal-cell carcinoma. N Engl J Med. 2016;374:888–9. https://doi.org/10.1056/NEJMc1515613#SA2.
Motzer RJ, Escudier B, Choueiri TK. Treatment of advanced renal-cell carcinoma. N Engl J Med. 2016;374:889–90. https://doi.org/10.1056/NEJMc1515613.
Fioramonti M, Santini D, Iuliani M, et al. Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions. Oncotarget. 2017;8(12):20113–21. https://doi.org/10.18632/oncotarget.15390.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Santini, D. et al. (2019). Bone-Modifying Agents and Anticancer Agents with Bone Effects. In: Denaro, V., Di Martino, A., Piccioli, A. (eds) Management of Bone Metastases. Springer, Cham. https://doi.org/10.1007/978-3-319-73485-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-73485-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73484-2
Online ISBN: 978-3-319-73485-9
eBook Packages: MedicineMedicine (R0)