Abstract
We have reviewed the most important results relating to particular egg characteristics responsible for recognition and subsequent rejection by hosts of brood parasites. Hosts remove a foreign egg after determining that it differs in one or more parameters. In turn, brood parasites have often evolved various mechanisms to confuse host defences and prevent egg recognition. The most conspicuous one is egg mimicry—imitation of the appearance of host eggs. We evaluate and discuss egg rejection experiments, particularly from a historical perspective, and the use of cameras in experiments. Further, we describe assessments of egg mimicry, and in particular we focus on the role played by particular characteristics in discrimination including egg colour, spottiness, chromatic versus achromatic cues, the role of UV spectra, the blunt egg pole, and the shape and volume of the parasitic egg. In addition, we discuss how research methodology and the application of experimental approaches to studying avian vision have affected studies on egg discrimination.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A successful act of brood parasitism has a disastrous effect on host fitness because it decreases host reproduction considerably (Payne and Payne 1998; Davies 2000). However, in the face of this selection pressure, many hosts have evolved egg discrimination, as an antiparasite defence mechanism (Davies and Brooke 1989). Strong evidence that egg recognition can function as a defence against brood parasitism is provided by studies showing that egg rejection rates correlate spatially (Soler et al. 1999a; Lindholm and Thomas 2000) or temporally (Brooke et al. 1998; Nakamura et al. 1998) with the likelihood of cuckoo parasitism.
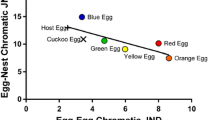
Egg discrimination is a two-stage process comprising perceptual (egg recognition) and operational (egg rejection) components (Hauber and Sherman 2001). With regard to perception, hosts can discern a foreign egg by at least two cognitive mechanisms (Moskát and Hauber 2007; Moskát et al. 2010), namely, (1) direct comparison (Rothstein 1974; Lahti and Lahti 2002) or (2) memory (Hauber et al. 2006; Moskát and Hauber 2007; see Chap. 24 for a discussion on how different mechanisms influence egg recognition and rejection). Many cues facilitating recognition of a foreign egg play an important role in the host recognition processes (see Fig. 22.1). After assessing the level of difference between foreign and its own eggs, the host takes into account other factors such as the costs associated with egg rejection and the risk of parasitism (Davies et al. 1996; Soler et al. 2012) and may then elect to remove the foreign egg. In turn, brood parasites have often evolved various tricks to confuse hosts and prevent egg recognition. The most conspicuous is egg mimicry—imitation of the appearance of host eggs—which evolves in response to selection pressure from host ejection of brood parasite eggs (see Chap. 20).
2 Egg Rejection Experiments
2.1 A Historical Perspective
After establishing that brood parasites lay eggs in host nests and that the egg sometimes disappears from the parasitized nests, naturalists turned their attention to answering questions about how the parasitic egg disappears and which factors caused its removal. Sealy and Underwood (2012) provide a detailed summary of observations by early naturalists who experimentally tested host responses towards real eggs originating from different species. Perhaps two names from a large number of experimenters should be highlighted: Swynnerton (1918)—who confirmed egg ejection—and Rensch (1925), who examined egg recognition by the hosts. A milestone in progress of the experimental approach is the series of papers published by Rothstein in the early 1970s (Rothstein 1974). These well and carefully designed studies based on experiments (using model eggs) provided both a baseline and inspiration for future studies. Early ones were Álvarez et al. (1976), Davies and Brooke (1988, 1989), Cruz and Wiley (1989), Higuchi (1989), Moksnes and Røskaft (1989), Soler (1990), and Sealy (1992), carried out in different areas studying different host–parasite systems. Since then, a numerous studies involving egg recognition experiments have been performed in 182 different host species (see Appendix in Soler 2014) based on different approaches using painted real, model or conspecific, or other natural parasitic eggs.
The next step involved the use of carefully designed colour manipulation of the both parasitic and host eggs: this approach has yielded new insights into underlying cognitive processes regarding discrimination of the parasitic egg (de la Colina et al. 2012). This has allowed the documentation of adaptive modulation of antiparasitic strategies through shifts in the acceptance threshold of hosts (Hauber et al. 2006) or the conditions under which hosts reduce discrimination of foreign eggs (Moskát et al. 2008a) and has demonstrated that rejection using a recognition template might be advantageous in populations with high rates of multiple parasitism (Moskát et al. 2010). A variety of other aspects of egg rejection behaviour have been tested experimentally, such as its repeatability (Honza et al. 2007a; Croston and Hauber 2014a), the costs of rejection (Martín-Vivaldi et al. 2002; Underwood and Sealy 2006a; Segura et al. 2016), responses to avian brood parasitism in sympatric and allopatric host populations (Soler and Møller 1990; Briskie et al. 1992; Soler et al. 1999b; Stokke et al. 2008; Vikan et al. 2010; Yang et al. 2014), methods of rejection of parasitic eggs (Sealy 1995; Soler et al. 2015), and timing of ejection (Požgayová et al. 2011).
Egg rejection experiments do not necessarily imply manipulation of egg colour alone. For example, tests of the “egg arrangement hypothesis” by Polačiková et al. (2013) and Hanley et al. (2015) and the evaluation of egg nest contrasts by Aidala et al. (2015) have brought new insights into additional cues driving parasitic egg rejection.
In addition, there have been many studies testing egg shape discrimination of the hosts of brood parasites using various non-egg-shaped objects, e.g. Ortega and Cruz (1988) and Moskát et al. (2003), as well as over a range of different-sized objects (Guigueno and Sealy 2009, 2012; Álvarez et al. 1976). Underwood and Sealy (2006b) concluded that rejection of odd-shaped objects most likely represents an expression of nest sanitation behaviour, where debris is removed from the nest. Egg shape recognition is predicted to be most advanced in birds which can differentiate between non-egg items in the nest and parasite or own eggs (Peer et al. 2007; Guigueno and Sealy 2009).
Igic et al. (2015) recently suggested a new method—3D printing technology—of producing experimental eggs which enables more precise manipulation of egg size and shape. Soler et al. (2015) also suggested a new method to reproduce the exact colour of both background and spot colours, in which a specialized company (Copingra Pinturas) used a laser scanner to produce paints for experimental eggs. In conclusion, there has been dramatic progress in experimental approaches over the last two decades in the use of egg rejection experiments, providing new insights into our understanding of the behaviour of hosts towards parasitic eggs.
2.2 Assessment of Egg Mimicry: From Human Vision to Model Avian Vision
Although in retrospect the method of the human scoring mimicry mostly from photographs using a three- to five-point scale (Moksnes and Røskaft 1993, 1995) seems crude, this method was successfully used in many studies on brood parasitism and certainly pushed the boundaries of our knowledge in this area. It must be noted that these studies did not take into consideration the ability of birds to detect UV wavelengths in the 300–400 nm range which is invisible to humans. In addition, eggs used in experimental studies were painted using acrylic paints, which have different spectral reflectances to natural eggshells—in particular not reflecting in the UV. Cherry and Bennett’s (2001) paper, by placing emphasis on the potential role of the UV spectrum in avian egg discrimination, inspired a new wave of studies incorporating visual perception in brood parasitism research. These findings led to increased interest in studying the role of UV signals using portable spectrophotometers enabling measurements in the field; and the importance for human invisible spectra was also tested experimentally using UV blocking chemicals (see Sect. 22.3.3). Subsequent studies incorporated measurement of the UV visible range in the cuckoo (Avilés and Møller 2004; Cherry et al. 2007a) and pallid cuckoo (Cacomantis pallidus) hosts (Starling et al. 2006), but these studies measured the difference in reflectance between cuckoo and host eggs, ignoring how avian sensory systems process this information (Vorobyev et al. 1998; Cuthill et al. 2000). One should expect that the efficacy of a cuckoo egg in terms of matching would be influenced by the colour of the cuckoo egg itself, in contrast to the colour of the host eggs, the environment in which matching is perceived by the host, and the perceptual abilities of the host (Endler 1990; Vorobyev et al. 1998). This approach integrates reflectance spectra of cuckoo and host eggs and light conditions in the nest vicinity with published information on photoreceptor sensitivities, photoreceptor noise, and the transmission properties of avian colour vision media (Hart et al. 2000) to calculate differences in matching in host colour space (Vorobyev et al. 1998). The model, developed for the tetrachromatic visual system of birds in its long form (Vorobyev et al. 1998), provides a way of calculating the ability of a bird to distinguish between different colours while accounting for visual pigments absorbance and oil droplet transmittance (Hart et al. 2000; Hart 2001). Avilés (2008) developed a discrimination model approach that simulates host retinal functioning; Cassey et al. (2008) used a photoreceptor noise-limited colour opponent model of host perceptual physiology to predict behavioural rates of experimental egg discrimination of cuckoo or artificial eggs. Spectrometric measures have obvious advantages over human visual estimates of egg colouration, but they have the disadvantage of not assessing the spatial attributes of egg colouration. Stoddard and Stevens (2010) therefore extended this approach and developed a new technique to evaluate spotting patterns in several hosts of the common cuckoo, using digital image analysis to measure a range of attributes including marking size, diversity in size, contrast, coverage, and dispersion. This technique uses Fourier analysis of granularity based on early-stage, low-level visual processes. A refinement of this technique which explicitly tries to quantify recognisability in a way that mimics how a bird’s brain works, rather than just extracting objective measures of pattern, was published recently (Stoddard et al. 2014).
2.3 Artificial Versus Real Eggs
Generally, researchers have used either real brood parasite eggs, conspecific host eggs, or similarly sized eggs of different host species. The usefulness of real eggs can be limited because the relative influences of egg shape, size, and colour on rejection decisions cannot be easily differentiated (Antonov et al. 2009). One great advantage of real eggs is that results more accurately reflect patterns of egg rejection particularly in species that are puncture ejectors. But apart from the fact that real brood parasite eggs are often not easily available, another obvious disadvantage is the fact that eggshell colouration may change quickly with time: Moreno et al. (2011), Navarro and Lahti (2014), and Hanley et al. (2016) have provided evidence that such changes impact brood parasite host eggshell colour mimicry during the incubation stage. So far published work has mostly neglected this fact, although it could be of significance. The use of natural eggs in experiments can also result in the destruction of viable eggs, which is ethically questionable, although if abandoned natural eggs are used (e.g. Cherry et al. 2007a), this is not problematic. A suitable alternative is the use of eggs from commercial breeds like Chinese quail (Coturnix chinensis) (Stokke et al. 2010) or non-fertilized eggs from exotic breeds like Bourke’s parrot, (Neophema bourkii) (Honza et al. 2007a).
Artificial eggs are specifically useful in separating the relative influences of different phenotypic characteristics on rejection decisions (Álvarez et al. 1976; Rothstein 1982; Hauber et al. 2015; Roncalli et al. 2017 although see Lahti 2015). However, there are limitations to using artificial eggs, traditionally made of plasticine, plastic, wood, or plaster of Paris (Prather et al. 2007). For example, some researchers concede that rejection of hard-shelled eggs may underestimate rejection rate (Moksnes et al. 1991; Martín-Vivaldi et al. 2002; Prather et al. 2007), but see Honza and Moskát (2008) for opposing results. On the other hand, the softness of the material may overestimate ejection (Roncalli et al. 2017). The size of the host, which is manifest in its ability to grasp foreign eggs, plays a major role in this respect. Artificial material may also affect the mode of the responses towards such eggs, as unsuccessful attempts to puncture hard model eggs could increase the costs of rejection and/or provoke clutch desertion (Martín-Vivaldi et al. 2002).
In addition, shell thickness and strength of the parasitic egg (real or artificial) may also influence host reaction times (Antonov et al. 2008; Honza and Moskát 2008). Painting experimental or real eggs using acrylic paints, which have different spectral reflectances to natural eggshells (in particular not reflecting in the UV), is problematic as these eggs may be rejected based on features that were not experimentally manipulated or controlled (Prather et al. 2007). The advent of new acrylic paints reflecting in the UV opens new research possibilities (Šulc et al. 2016).
2.4 The Use of Cameras
The use of small cameras in well-planned experiments to minimize disturbance to breeding birds has recently become increasingly possible and should enable more detailed study of host responses towards parasitic eggs. Analyses of video recordings of experimentally parasitized nests in particular could contribute to a better understanding of cognitive processes in the context of egg discrimination.
Using cameras has enabled the discovery of birds aggressively pecking the experimental egg (Soler et al. 2002), indicating that egg recognition does not necessarily imply egg rejection. Further evidence of this was the study by Antonov et al. (2009), revealing that eastern olivaceous warblers (Hippolais pallida) frequently pecked real cuckoo eggs or experimental egg models but accepted almost half of them; the same behaviour exhibited by warbling vireos (Vireo gilvus) was recorded by Underwood and Sealy (2006a). Soler et al. (2012) suggested that pecking not followed by rejection should be considered as part of a stepwise discrimination process, in which accumulating motivation plays a key role in determining behavioural pathways shaping host response to parasitic eggs.
Furthermore, video recordings may be also useful in the detection of variation of the responses towards parasitic egg at the level of host populations, host species, or host individuals. These techniques have confirmed that in species where both sexes incubate and spend time in the nest, both males and females eject eggs (Soler et al. 2002; Požgayová et al. 2009), whereas unsurprisingly, in species when only females incubate, only females reject parasitic eggs (Požgayová et al. 2011). Furthermore, it has been demonstrated that increased egg mass provokes the acceptance of an experimental egg that has been previously recognized, because ejection of a heavy egg may imply higher rejection costs for hosts (Ruíz-Raya et al. 2015). So video recording has in particular allowed investigation of the relationship between egg recognition and egg ejection (Antonov et al. 2009; Soler et al. 2012, 2015, 2017; Ruíz-Raya et al. 2015), its timing (Antonov et al. 2008; Požgayová et al. 2011), and the consistency in egg rejection behaviour of hosts when parasitized repeatedly within one breeding attempt (Honza et al. 2007a).
In all these studies, hosts were typically confronted with an artificial, non-mimetic foreign egg placed into their nest. Because it is time-consuming, the majority of studies were conducted for only a short time after the host was “parasitized”. Therefore, despite the difficulties involved, it would be very interesting to study the behaviour and discriminative processes towards real parasitic eggs, to try and resolve the puzzle of why some hosts delay their egg rejection decisions (Požgayová et al. 2011) or, even more important, they decide to accept a previously recognized foreign egg (Soler et al. 2017).
3 The Role of Different Factors Affecting Discrimination
3.1 Egg Colour and Spottiness
Inspired by the early experiments of Rothstein (1982), researchers have attempted to clarify the importance of these cues in recognition. Egg colouration is clearly vitally important as even relatively small perceivable differences in eggshell colouration can result in substantial increase in host rejection rates (Honza et al. 2011; Hauber et al. 2015). Generally, experiments have shown that hosts are able to recognize parasitic eggs on the basis of colour and spotting (Table 22.1): the larger the difference in colour between parasitic egg and host eggs and the greater the difference in spottiness, the greater the probability of rejection. There are some exceptions: for example, Honza et al. (2007b) in a study of song thrushes (Turdus philomelos) revealed that some colours of the parasitic eggs classified by humans as non-mimetic were accepted by the hosts. Some hosts may have strong rejection biases towards specific colours. Hanley et al. (2017), working on two Turdid species, the blackbird (Turdus merula) and the American robin (Turdus migratorius), found that across a natural colour gradient, both species were more likely to accept blue-green eggs and reject brown eggs, regardless of the perceived difference between foreign eggs and their own. By contrast, their responses did not vary across an artificial (green to purple) gradient, suggesting that in Turdids, at least, egg recognition is specifically tuned to the natural gradient of eggshell colouration.
In addition, in some cases, the response to the same colour models is affected by the presence of a parasite (Moksnes and Røskaft 1989; Astie and Reboreda 2005), so colour is not the only cue used in discrimination (Segura et al. 2016). Rather interesting is the ability of South American host species which were tested with a white egg: almost all tested species exhibited fine-tuned recognition ability of white, which is an adaptation towards brood parasitism by shiny cowbirds (Table 22.1).
3.2 The Role of Chromatic Versus Achromatic Cues in Egg Discrimination
At present, we can accurately measure the reflectance of birds’ eggs and even simulate (on the basis of the sensitivity of bird cones) how reflected radiation is perceived by birds. From this information, the degree of similarity from the perspective of the host’s eye can be assessed to determine the level of colour mimicry of parasitic eggs in the nest of the host. The Vorobyev-Osorio model (Vorobyev and Osorio 1998) calculates chromatic and achromatic contrasts between two coloured objects in a visual space that depends on the number of receptor types of the signal receiver in JNDs (just noticeable differences).
Table 22.2 shows that chromatic contrast is more important in open-nesting hosts, whereas achromatic contrasts have been suggested to play a crucial role in egg discrimination of species nesting in dark nests where colour information is less important (Avilés et al. 2006; Langmore et al. 2009). This explains why achromatic contrasts do not appear to be important cues for the majority of both cuckoo and cowbird hosts.
3.3 Ultraviolet Reflectance
In the following studies in which egg appearance was measured using spectrophotometry, the importance of particular wavelengths for egg discrimination was documented in the spotless starling (Sturnus unicolor) (Avilés et al. 2006), great reed warbler (Acrocephalus arundinaceus) (Cherry et al. 2007b), song thrush (Honza et al. 2007b), blackcap (Polačiková et al. 2007), magpie (Pica pica) (Avilés et al. 2004; Soler et al. 2003), and several cowbird hosts (Underwood and Sealy 2008). This strongly suggests that UV vision is used in egg discrimination by birds.
Abernathy and Peer (2015) propose that hosts with brighter UV-reflecting eggs should be more likely to reject UV-blocked eggs than hosts with duller UV-reflecting eggs. Šulc et al. (2016) suggest that such signals may play a more important role when parasitic eggs are non-mimetic rather than mimetic when hosts can use additional cues, such as spotting pattern, to discriminate. Of the total 11 host species (Table 22.3) of hosts that have been tested using own or conspecific UV-blocked eggs, at least two species (blackcap; Honza and Polačiková 2008) and brown thrasher (Toxostoma rufum; Abernathy and Peer 2015) appear to use the UV range as the sole cue for discrimination.
3.4 Egg Pole
It is well known that maculated bird eggs have more spots at the blunt pole than at the sharp and that the overall surface area of eggshell around the blunt pole is evidently larger than around the sharp pole. Therefore, one should expect that blunt poles have the potential for greater amount of information content of eggshell signals. Polačiková et al. (2007) drew attention to this in the brood parasitism context, showing that colour characteristics of the blunt part of natural conspecific eggs experimentally added to blackcap nests may play a major role in the recognition of parasitic egg. Further studies by Polačiková et al. (2010), Polačiková and Grim (2010), and Zoelei et al. (2012) have revealed that host species rejected eggs manipulated at the blunt pole at significantly higher rates than eggs manipulated at sharp poles, indicating that they perceive critical recognition cues at the blunt pole. Polačiková and Grim (2010) regard the presence of egg recognition cues at the blunt egg pole as a general phenomenon in birds parasitized by interspecific parasites. To confirm this, it is necessary to study the effects of the appearance of the blunt egg pole of real parasitic eggs.
3.5 Egg Volume and Shape
Rigorous research studying the effects of the volume and shape of parasitic eggs with respect to rejection started in the early 1980s by Rothstein (1982) and has continued since then (Table 22.4). A variety of experimental methods have been used: conspecific eggs (Lahti and Lahti 2002; Underwood and Sealy 2006a), oversized eggs (Davies and Brooke 1988; Álvarez 2000), model eggs (Marchetti 2000; Antonov et al. 2006), and, in a single study, real parasitic eggs (Segura et al. 2016). With the exception of two studies (Marchetti 2000; Guigueno et al. 2014), results showed that egg size and/or shape were not generally used as a rejection cue.
Concluding Remarks and Future Directions
It is evident that the past decade has brought considerable progress in elucidating the particular cues that birds use during the process of discriminating parasitic eggs. Evaluation of egg colouration has improved considerably with the advent of spectrophotometric techniques allowing objective quantification of colour, including the UV-reflectant range that is invisible to humans. The use of video cameras has proven very useful in studying discriminatory processes, and further applications of this methodology should allow for better designed experiments related to discrimination.
We have a relatively good knowledge of the characteristics of parasitic eggs responsible for recognition, and subsequent rejection, in cuckoo and cowbird hosts. Our review suggests that some aspects of egg appearance could be more important for hosts than others. Individual cues could also interact with each other and play different roles in different circumstances, for example, UV signals could be more important in the recognition of non-mimetic eggs rather than mimetic. As the majority of published studies have focused on individual cues, we encourage the study of combined cues potentially responsible for rejection. This is because selection has shaped egg size, shape, colour, luminance, and patterns, and these cues together could contribute to egg detection and rejection behaviour. Future research should also explore different functions of eggshell components, e.g. shape, size, colour, and spottedness in egg discrimination in less well-known brood parasitic systems.
There is an apparent lack of knowledge on whether birds can recognize the shape of a natural parasitic egg. More studies are therefore needed with natural parasitic eggs to identify whether shape and volume are valid cues for egg recognition. In addition, further research is needed to clarify how hosts use the information content of the eggshell around the blunt pole across all brood parasitism systems, testing potential hosts in natural conditions.
Future work should also focus on improving visual models by incorporating physiologically appropriate, individual specific cone densities/absorbance spectra, as well as nest site-specific egg, nest lining, and ambient light availability data. In the future, new techniques such as 3D printing should provide opportunities for more extensive experimentation on the potential biological or evolutionary significance of size and shape variation of foreign eggs in rejection decisions.
References
Abernathy VE, Peer BD (2015) Mechanisms of egg recognition in brown-headed cowird hosts: the role of ultraviolet reflectance. Anim Behav 109:73–79
Abernathy VE, Peer BD (2016) Reduced ultraviolet reflectance does not affect egg rejection by Northern cardinals (Cardinalis cardinalis). Wilson J Ornithol 128:334–342
Aidala Z, Croston R, Schwartz J, Tong L, Hauber ME (2015) The role of egg-nest contrast in the rejection of brood parasitic eggs. J Exp Biol 218:1126–1136
Álvarez F (1999) Attractive non-mimetic stimuli in cuckoo Cuculus canorus eggs. Ibis 141:142–144
Álvarez F (2000) Response to common cuckoo Cuculus canorus model egg size by a parasitized population of rufous bush chat Cercotrichas galactotes. Ibis 142:683–686
Álvarez F, Arias de Reyna L, Segura M (1976) Experimental brood parasitism of the magpie (Pica pica). Anim Behav 24:907–916
Antonov A, Stokke BG, Moksnes A, Røskaft E (2006) Egg rejection in marsh warblers (Acrocephalus palustris) heavily parasitized by common cuckoos (Cuculus canorus). Auk 123:419–430
Antonov A, Stokke BG, Moksnes A, Røskaft E (2008) Getting rid of the cuckoo Cuculus canorus egg: why do hosts delay rejection? Behav Ecol 19:100–107
Antonov A, Stokke BG, Moksnes A, Røskaft E (2009) Evidence for discrimination preceding failed rejection attempts in a small cuckoo host. Biol Lett 5:169–171
Astie AA, Reboreda JC (2005) Creamy – bellied thrush defenses against shiny cowbird brood parasitism. Condor 107:788–796
Avilés JM (2008) Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc R Soc B 275:2345–2352
Avilés JM, Møller AP (2004) Meadow pipit (Anthus pratensis) egg appearance in cuckoo (Cuculus canorus) sympatric and allopatric populations. Biol J Linn Soc 79:543–549
Avilés JM, Soler JJ, Soler M, Møller AP (2004) Rejection of parasitic eggs in relation to egg appearance in magpies. Anim Behav 67:951–958
Avilés JM, Soler JJ, Pérez-Contreras T (2006) Dark nests and egg colour in birds: a possible functional role of ultraviolet reflectance in egg detectability. Proc R Soc B 273:2821–2829
Avilés JM, Vikan JR, Fossoy F, Antonov A, Moksnes A, Røskaft E, Stokke BG (2010) Avian colour perception predicts behavioural responses to experimental brood parasitism in chaffinches. J Evol Biol 23:293–301
Briskie JV, Sealy SG, Hobson KA (1992) Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 46:334–340
Brooke M de L, Davies NB, Noble DG (1998) Rapid decline of host defences in response to reduced cuckoo parasitism: behavioural flexibility of reed warblers in a changing world. Proc R Soc B 265:1277–1282
Cassey P, Honza M, Grim T, Hauber ME (2008) The modelling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol Lett 4:515–517
Cherry MI, Bennett ATD (2001) Egg colour matching in an African cuckoo, as revealed by ultraviolet-visible reflectance spectrophotometry. Proc Biol Soc 268:565–571
Cherry MI, Bennett ATD, Moskát C (2007a) Do cuckoos choose nests of great reed warblers on the basis of host egg appearance? J Evol Biol 20:1218–1222
Cherry MI, Bennett ATD, Moskát C (2007b) Host intra-clutch variation, cuckoo egg matching and egg rejection by great reed warblers. Naturwissenschaften 94:441–447
Croston R, Hauber ME (2014a) High repeatability of egg rejection in response to experimental brood parasitism in the American robin (Turdus migratorius). Behaviour 151:703–718
Croston R, Hauber ME (2014b) Spectral tuning and perceptual differences do not explain the rejection of brood parasitic eggs by American robins (Turdus migratorius). Behav Ecol Sociobiol 68:351–362
Cruz A, Wiley JW (1989) The decline of an adaptation in the absence of a presumed selection pressure. Evolution 43:55–62
Cuthill IC, Partridge JC, Bennett ATD, Church SC, Hart NS, Hunt E (2000) Ultraviolet vision in birds. Adv Study Behav 29:159–214
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & AD Poyser, London
Davies NB, Brooke MD (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284
Davies NB, Brooke MD (1989) An experimental study of co-evolution between cuckoo, Cuculus canorus, and its hosts. 2. Host egg markings, chick discrimination and general discussion. J Anim Ecol 58:225–236
Davies NB, Brooke M de L, Kacelnik A (1996) Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc R Soc Lond B 263:925–931
De La Colina MA, Pompilio L, Hauber ME, Reboreda JC, Mahler B (2012) Different recognition cues reveal the decision rules used for egg rejection by hosts of a variably mimetic avian brood parasite. Anim Cogn 15:881–889
Endler JA (1990) On the measurement and classification of color in studies of animal color patterns. Biol J Linn Soc 41:315–352
Guigueno MF, Sealy SG (2009) Nest sanitation plays a role in egg burial by yellow warblers. Ethology 115:247–256
Guigueno MF, Sealy SG (2012) Nest sanitation in passerine birds: implications for egg rejection in hosts of brood parasites. J Ornithol 153:35–52
Guigueno MF, Sealy S, Westphal A (2014) Rejection of parasitic eggs in passerine hosts: size matters more for a non-ejecter. Auk 131:583–594
Hanley D, Samas P, Hauber M, Grim T (2015) Who moved my eggs? An experimental test of the egg arrangement hypothesis for the rejection of brood parasitic eggs. Anim Cogn 18:299–305
Hanley D, Šulc M, Brennan PLR, Hauber M, Grim T, Honza M (2016) Dynamic egg color mimicry. Ecol Evol 6:4192–4202
Hanley D, Grim T, Igic B, Samaš P, Lopez AV, Shawkey MD, Hauber ME (2017) Egg discrimination along a gradient of natural variation in eggshell coloration. Proc R Soc B 284:2016–2592
Hart NS (2001) The visual ecology of avian photoreceptors. Proc Retin Eye Res 20:675–703
Hart NS, Partridge JC, Cuthill IC, Bennet ATD (2000) Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula). J Comp Physiol A 186:375–387
Hauber ME, Sherman PW (2001) Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci 24:609–616
Hauber ME, Moskát C, Bán M (2006) Experimental shift in hosts´ acceptance threshold of inacurate-mimic brood parasite eggs. Biol Lett 2:177–189
Hauber ME, Tong LG, Bán M, Croston R, Grim T, Waterhouse GIN, Shawkey MD, Barron AB, Moskat C (2015) The value of artificial stimuli in behavioral research: making the case for egg rejection studies in avian brood parasitism. Ethology 121:521–528
Higuchi H (1989) Responses of the bush warbler Cettia diphone to artificial eggs of Cuculus canorus in Japan. Ibis 131:94–98
Honza M, Moskát C (2008) Egg rejection behaviour in the great reed warbler (Acrocephalus arundinaceus): the effect of egg type. J Ethol 26:38–395
Honza M, Polačiková L (2008) Experimental reduction of ultraviolet wavelengths reflected from parasitic eggs affects rejection behaviour in the blackcap Sylvia atricapilla. J Exp Biol 211:2519–2523
Honza M, Požgayová M, Procházka P, Tkadlec E (2007a) Consistency in egg rejection behaviour: responses to repeated brood parasitism in the blackcap (Sylvia atricapilla). Ethology 113:344–351
Honza M, Polačiková L, Procházka P (2007b) Ultraviolet and green parts of the colour spectrum affect egg rejection in the song thrush (Turdus philomelos). Biol J Linn Soc 92:269–276
Honza M, Procházka P, Morongová K, Čapek M, Jelínek V (2011) Do nest light conditions affect rejection of parasitic eggs? A test of the light environment hypothesis. Ethology 117:539–546
Igic B, Numez W, Voss HU, Croston R, Aidala Z, López AV, van Tatenhove A, Holford ME, Shawkey MD, Hauber ME (2015) Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. PeerJ 3:e965
Jackson WM (1993) Causes of couspecific nest parasitism in the northern masked weaver. Beh Ecol Soc 2:119–126
Lahti D (2015) The limits of artificial stimuli in behavioral research: the Umwelt gamble. Ethology 121:529–537
Lahti DC, Lahti AR (2002) How precise is egg discrimination in weaverbirds? Anim Behav 63:1135–1142
Langmore NE, Stevens M, Maurer G, Kilner RM (2009) Are dark cuckoo eggs cryptic in host nests? Anim Behav 78:461–468
Lawes MJ, Kirkman S (1996) Egg recognition and interspecific brood parasitism rates in red bishops (Aves: Ploceidae). Anim Behav 52:553–563
Li D, Ruan Y, Wang Y, Chang AK, Wan D, Zhang Z (2016) Egg-spot matching in common cuckoo parasitism of the oriental warbler: effects of host nest availability and egg rejection. Avian Res 7:21
Lindholm AK, Thomas RJ (2000) Differences between populations of reed warblers in defences against brood parasitism. Behaviour 137:25–42
Marchetti K (2000) Egg rejection in a passerine bird: size does matter. Anim Behav 59:877–883
Martín-Vivaldi M, Soler M, Møller AP (2002) Unrealistically high costs of rejecting artificial model eggs in cuckoo Cuculus canorus hosts. J Avian Biol 33:295–301
Mermoz ME, Reboreda JC (1994) Brood parasitism of the shiny cowbird, Molothrus bonariensis, on the brown-and-yellow marshbird, Pseudoleistes virescens. Condor 96:716–721
Moksnes A, Røskaft E (1989) Adaptations of meadow pipits to parasitism by the common cuckoo. Behav Ecol Sociobiol 24:25–30
Moksnes A, Røskaft E (1993) Rejection of cuckoo (Cuculus canorus) eggs by meadow pipits (Anthus pratensis). Behav Ecol 4:120–127
Moksnes A, Røskaft E (1995) Egg-morphs and host preferences in the common cuckoo (Cuculus canorus) – an analysis of cuckoo and host eggs from European museum collections. J Zool 236:625–648
Moksnes A, Røskaft E, Braa AT (1991) Rejection behaviour by common cuckoo hosts towards artificial brood parasite eggs. Auk 108:348–354
Moreno J, Lobato E, Morales J (2011) Eggshell blue-green colouration fades immediately after oviposition: a cautionary note about measuring natural egg. Ornis Fenn 88:51–56
Moskát C, Hauber M (2007) Conflict between egg recognition and egg rejection decisions in common cuckoo Cuculus canorus hosts. Anim Cogn 10:377–386
Moskát C, Szekely T, Kisbenedek T, Karcza Z, Bártol I (2003) The importance of nest cleaning in egg rejection behaviour of great reed warblers Acrocephalus arundinaceus. J Avian Biol 34:16–19
Moskát C, Avilés JM, Bán M, Hargitai R, Zölei A (2008a) Experimental support for the use of egg uniformity in parasite discrimination by cuckoo hosts. Behav Ecol Sociobiol 62:1885–1890
Moskát C, Szekely T, Cuthill IC, Kisbenedek T (2008b) Hosts’ responses to parasitic eggs: which cues elicit hosts’ egg discrimination? Ethology 114:186–194
Moskát C, Bán M, Szekely T, Komdeur J, Lucassen RWG, van Boheemen LA, Hauber ME (2010) Discordancy or template-based recognition? Dissecting the cognitive basis of the ejection of foreign eggs in hosts of avian brood parasites. J Exp Biol 213:1976–1983
Moskát C, Zoelei A, Bán M, Elek Z, Tong L, Geltsch N, Hauber ME (2014) How to spot a stranger's egg? A mimicry-specific discordancy effect in the recognition of parasitic eggs. Ethology 120:616–626
Nakamura H, Kubota S, Suzuki R (1998) Co-evolution between the common cuckoo and its major hosts in Japan. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts. Oxford University Press, Oxford, pp 94–112
Navarro JY, Lahti DC (2014) Light dulls and darkens bird eggs. PLoS One 9:e116112
Ortega CP, Cruz A (1988) Mechanisms of egg acceptance by marsh-dwelling blackbirds. Condor 90:349–358
Payne R, Payne L (1998) Brood parasitism by cowbirds: risks and effects on reproductive success and survival in indigo buntings. Behav Ecol 9:64–73
Peer BD, Rothstein SK, Delaney SK, Fleischer RC (2007) Defence behaviour against brood parasitism is deeply rooted in mainland and island scrub-jays. Anim Behav 73:55–63
Polačiková L, Grim T (2010) Blunt egg pole holds cues for alien egg discrimination: experimental evidence. J Avian Biol 41:111–116
Polačiková L, Honza M, Procházka P, Topercer J, Stokke BG (2007) Colour characteristics of the blunt egg pole: cues for recognition of parasitic eggs as revealed by reflectance spectrophotometry. Anim Behav 74:419–427
Polačiková L, Stokke BG, Procházka P, Honza M, Moksnes A, Røskaft E (2010) The role of blunt egg pole characteristics for recognition of eggs in the song thrush (Turdus philomelos). Behaviour 147:465–478
Polačiková L, Takasu F, Stokke BG, Moksnes A, Røskaft E, Cassey P, Hauber ME, Grim T (2013) Egg arrangement in avian clutches covaries with the rejection of foreign eggs. Anim Cogn 16:819–828
Požgayová M, Procházka P, Honza M (2009) Sex-specific defence behaviour against brood parasitism in a host with female-only incubation. Behav Process 81:34–38
Požgayová M, Procházka P, Polačiková L, Honza M (2011) Closer clutch inspection – quicker egg ejection: timing of host responses toward parasitic eggs. Behav Ecol 22:46–51
Prather JW, Cruz A, Weaver PF, Wiley JW (2007) Effects of experimental egg composition on rejection by village weavers (Ploceus cucullatus). Wilson J Ornithol 119:703–711
Rensch B (1925) Verhalten von Singvӧgeln bei Aenderung des Geleges. Ornithol Monatschr 33:169–173
Roncalli G, Ibanéz-Álamo JD, Soler M (2017) Size and material of model parasitic eggs affect the rejection response of Western Bonelli’s warbler Phylloscopus bonelli. Ibis 159:113–123
Rothstein SI (1974) Mechanisms of avian egg recognition: possible learned and innate factors. Auk 91:796–807
Rothstein SI (1982) Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 11:229–239
Ruíz-Raya F, Soler M, Sanchez-Perez LLI, Ibanez-Alamo JD (2015) Could a factor that does not affect egg recognition influence the decision of rejection? PLoS One 10:e0135624
Sackmann P, Reboreda JC (2003) A comparative study of shiny cowbird parasitism of two large hosts, the chalk-browed mockingbird and the rufous-bellied thrush. Condor 105:728–736
Sealy SG (1992) Removal of yellow warbler eggs in association with cowbird parasitism. Condor 94:40–54
Sealy SG (1995) Burial of cowbird eggs by parasitized yellow warblers: an empirical and experimental study. Anim Behav 49:877–889
Sealy SG, Underwood TJ (2012) Egg discrimination by hosts and obligate brood parasites: a historical perspective and new synthesis. Chin Birds 3:274–294
Segura LN, Di Sallo FG, Mahler B, Reboreda JC (2016) Red-crested cardinals use color and width as cues to reject shiny cowbird eggs. Auk 133:308–315
Soler M (1990) Relationship between the great spotted cuckoo Clamator glandarius and its corvid hosts in a recently colonized area. Ornis Scand 21:212–223
Soler M (2014) Long-term coevolution between avian brood parasites and their host. Biol Rev 89:688–704
Soler M, Møller AP (1990) Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature 343:748–750
Soler JJ, Martinez JG, Soler M, Møller AP (1999a) Host sexual selection and cuckoo parasitism: an analysis of nest size in sympatric and allopatric magpie Pica pica populations parasitized by the great spotted cuckoo Clamator glandarius. Proc R Soc B 266:1765–1771
Soler JJ, Martinez JG, Soler M, Møller AP (1999b) Genetic and geographic variation in rejection behavior of cuckoo eggs by European magpie populations: an experimental test of rejecter-gene flow. Evolution 53:947–956
Soler JJ, Soler M, Møller AP (2000) Host recognition of parasite eggs and the physical appearance of host eggs: the magpie and its brood parasite the great spotted cuckoo. Etología 8:9–16
Soler M, Martín-Vivaldi M, Pérez-Contreras T (2002) Identification of the sex responsible recognition and the method of ejection of parasitic eggs in some potential common cuckoo hosts. Ethology 108:1093–1101
Soler JJ, Avilés JM, Soler M, Møller AP (2003) Evolution of host egg mimicry in a brood parasite, the great spotted cuckoo. Biol J Linn Soc 79:551–563
Soler M, Fernandez-Morante J, Espinosa F, Martín-Vivaldi M (2012) Pecking but accepting the parasitic eggs may not reflect ejection failure: the role of motivation. Ethology 118:662–672
Soler M, Ruiz-Raya F, Roncalli G, Ibáñez-Álamo JD (2015) Nest desertion cannot be considered an egg-rejection mechanism in a medium-sized host: an experimental study with the common blackbird Turdus merula. J Avian Biol 46:369–377
Soler M, Ruiz-Raya F, Roncalli G, Ibanez-Alamo JD (2017) Relationships between egg-recognition and egg-ejection in a grasp-ejector species. PLoS One 12:e0166283
Spottiswoode C (2013) A brood parasite selects for its own egg traits. Biol Lett 9:20130573
Spottiswoode C, Stevens M (2010) Visual modelling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc Natl Acad Sci U S A 107:8672–8676
Starling M, Heinsohn R, Cockburn A, Langmore NE (2006) Cryptic gentes revealed in pallid cuckoos Cuculus pallidus using reflectance spectrophotometry. Proc R Soc B 273:1929–1934
Stoddard MC, Stevens M (2010) Pattern mimicry of host eggs by the common cuckoo, as seen through a bird’s eye. Proc R Soc B 277:1387–1393
Stoddard MC, Kilner RM, Town C (2014) Pattern recognition algorithm reveals how birds evolve individual egg pattern. Nat Commun 5:4117
Stokke BG, Hafstad I, Rudolfsen G, Moksnes A, Møller AP, Røskaft E, Soler M (2008) Predictors of resistance to brood parasitism within and among reed warbler populations. Behav Ecol 19:612–620
Stokke BG, Polačiková L, Dyrcz A, Hafstad I, Moksnes E, Røskaft E (2010) Responses of reed warblers Acrocephalus scirpaceus to non-mimetic eggs of different sizes in a nest parasitism experiment. Acta Ornithol 45:98–104
Šulc M, Procházka P, Čapek M, Honza M (2016) Birds use eggshell UV reflectance when recognizing non-mimetic parasitic eggs. Behav Ecol 27:677–684
Swynnerton CFM (1918) Rejections by birds of eggs unlike their own: with remarks on some of the cuckoo problems. Ibis Ser 10 6:127–154
Underwood TJ, Sealy SG (2006a) Influence of shape on egg discrimination in American robins and gray catbirds. Ethology 112:164–173
Underwood TJ, Sealy S (2006b) Parameters of brown-headed cowbird Molothrus ater egg discrimination in warbling vireos Vireo gilvus. J Avian Biol 37:457–466
Underwood TJ, Sealy SG (2008) UV reflectance of eggs of brown-headed cowbirds (Molothrus ater) and accepter and rejecter hosts. J Ornithol 149:313–321
Vikan JR, Stokke BG, Rutila J, Huhta E, Moksnes A, Røskaft E (2010) Evolution of defences against cuckoo (Cuculus canorus) parasitism in bramblings (Fringilla montifringilla): a comparison of four populations in Fennoscandia. Evol Ecol 24:1141–1157
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B 265:351–358
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Yang CC, Liu Y, Zeng LJ, Liang W (2014) Egg color variation, but not egg rejection behavior, changes in a cuckoo host breeding in the absence of brood parasitism. Ecol Evol 4:2239–2246
Zoelei A, Hauber M, Geltsch N (2012) Asymmetrical signal content of egg shape as predictor of egg rejection by great reed warblers, hosts of the common cuckoo. Behaviour 149:391–406
Acknowledgements
We are grateful to Manuel Soler, Daniel Hanley, and Phil Cassey for their helpful comments and suggestions. This work was partly supported by the Grant Agency of the Czech Republic (grant no. 17-12262S to MH) and the National Research Foundation of South Africa (grant 96257 to MIC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Honza, M., Cherry, M.I. (2017). Egg Characteristics Affecting Egg Rejection. In: Soler, M. (eds) Avian Brood Parasitism. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-73138-4_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-73138-4_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73137-7
Online ISBN: 978-3-319-73138-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)