Abstract
The interactions between avian brood parasites and their hosts provide tractable model systems to study coevolutionary processes under natural conditions. Here, I review evidence of reciprocal adaptations and counter-adaptations in brood parasites and hosts that are deployed prior to the parasite depositing its egg in the host nest: the ‘frontline’ of the arms race. Unlike interactions at latter stages of the nesting cycle, frontline interactions primarily concern adult brood parasites and adult hosts, offering opportunities to study how exchanges between these species influence adult phenotypes. Placing emphasis on recent advances, I discuss how frontline interactions have shaped the life histories, behaviours, morphologies and physiologies of adult brood parasites and adult hosts. Similar to latter stages of the nesting cycle, frontline interactions comprise diverse adaptations and counter-adaptations that appear to be a product of coevolution and are important for determining the outcome of the exchanges between these species. Further investigation of these interactions is essential for categorizing the diversity of adaptations and counter-adaptations at this stage of the nesting cycle and expanding our understanding of how adaptations and counter-adaptations at all stages of the nesting cycle evolve in relation to one another.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Humans have demonstrated a deep and lasting interest in the natural habits of the avian brood parasites. Their unmistakeable calls are recognized as synonymous with seasonal change throughout Europe, Asia and Australia (Lai 1998; Davies 2000; Gray and Fraser 2013); one acts as foraging partner with humans in sub-Saharan Africa (Spottiswoode et al. 2016); and references to their parasitic breeding habits are evident throughout the world’s folklores, languages and mythologies (Lai 1998; Davies 2000; Gray and Fraser 2013; Møller et al. 2017). Throughout history myriad explanations have been proposed to rationalize why these species never tend to their offspring (Davies 2000). Of these, natural selection prevailed (Darwin 1859), and these systems have since become models for studying the ecological and evolutionary ramifications of species interactions under natural conditions (Rothstein 1990; Davies 2000; Feeney et al. 2014).
Research is currently being conducted on parasite–host systems that include representatives from each of the seven brood parasite lineages (Sorenson and Payne 2005) and on species pairs that inhabit every continent except for Antarctica (where none occur, Davies 2000). We know that the cost of hosting a parasite varies across species pairs (Kilner 2005) and that the benefits of hosting a parasite can outweigh the costs under some circumstances (Canestrari et al. 2014). The ecological consequences of these interactions can be globally conspicuous, predicting broad patterns of trait diversity such as small clutch sizes (Hauber 2003) and cooperative breeding (Feeney et al. 2013) in hosts, as well as hawk mimicry (Gluckman and Mundy 2013) and plumage polymorphisms (Thorogood and Davies 2013) in parasitic cuckoos. The depth of coevolution between these species is also becoming clear, with data now demonstrating that coevolved adaptations and counter-adaptations are evident at all stages of the host’s nesting cycle (reviewed in Feeney et al. 2014; Soler 2014)
In this chapter, I will discuss the interactions between avian brood parasites and hosts that occur prior to the parasite laying its egg in the host’s nest: the ‘frontline’ of the arms race (Welbergen and Davies 2009). These exchanges are of utmost importance, as a parasite’s reproductive output hinges on its ability to successfully deposit eggs in host nests, and successful deterrence of adult parasites offers the first opportunity for hosts to defend their nests. Unlike reciprocal adaptations and counter-adaptations at latter stages of the nesting cycle, frontline interactions primarily concern adult brood parasites and hosts, offering opportunities for selection to shape adult phenotypes of the involved species as well as their natural histories. Following a recent review of this topic (Feeney et al. 2012), I will discuss evidence of how coevolution has affected the life histories, behaviours, morphologies and physiologies of brood parasites and hosts. However, to minimize any unnecessary overlap, this chapter will place emphasis on new research as well as research that was not heavily discussed in Feeney et al. (2012). Discussion of traits will be conducted in a roughly chronological order.
2 Life Histories
2.1 Breeding Phenology
Parasitism can only occur if there is breeding synchrony between parasite and host. Despite being inherently difficult to demonstrate, evidence suggests that some host species, or populations of hosts within species, may have shifted their breeding phenologies to avoid breeding in synchrony with parasites. Brooker and Brooker (1989) were the first to compare breeding records of Australian cuckoos and their hosts and found numerous examples of hosts starting breeding before their cuckoos arrived or ending after they left, consistent with the hypothesis that hosts shift their breeding phenology to minimize overlap with cuckoos. Medina and Langmore (2016) built on this and found that early-laying yellow-rumped thornbills (Acanthiza chrysorrhoa) were parasitized significantly less than late breeders. Similar trends have been found in other host species (see Middleton 1977; Campomizzi et al. 2013 for similar evidence in hosts of the brown-headed cowbird (Molothrus ater); Table 17.1).
Research into the effects that climate change has had on bird breeding behaviours may offer insights into effectiveness and potential evolutionary consequences of this putative defence. Notably, migratory parasites are likely more vulnerable to shifts in host breeding phenology compared to their resident counterparts, as they are less able to track their host’s breeding schedule (Saino et al. 2009; Møller et al. 2011b; Péron et al. 2016). Those that are unable to effectively track host laying may be forced to switch hosts (Møller et al. 2011b; Péron et al. 2016) or face a reduction in their abundance (Saino et al. 2009). Host switching, in turn, may promote hybridization of closely related parasite species (Péron et al. 2016) or divergence into distinct ‘races’ (gentes) (Møller et al. 2011a) or species (Sorenson et al. 2003; Péron et al. 2016). Together, these studies suggest that shifts in host breeding phenology could provide an extremely effective defence against brood parasitism, especially in parasite–host systems that comprise migratory parasites and resident hosts, and could have dire evolutionary repercussions for the parasites.
If parasites and hosts do breed in synchrony, hosts may tactically adjust their laying dates to minimize their likelihood of parasitism or exhibit compensatory breeding behaviours to mitigate the cost of parasitism if it does occur. As parasites are constrained by their laying schedules, synchronous breeding in host communities reduces the likelihood of any individual being parasitized through a ‘swamping’ or ‘dilution’ effect (Clark and Robertson 1979; Martínez et al. 1996; Massoni and Reboreda 2001). Parasites can counter these traits with behaviours, such as the tactical destruction (i.e. ‘farming’) of host nests (e.g. Hoover and Robinson 2007; Swan et al. 2015), which forces renesting and offers an opportunity for subsequent parasitism (see Chap. 15; Table 17.2). If parasitism does occur, hosts may double-brood in an attempt to compensate for their lost reproductive success. Recently, Louder et al. (2015b) found that parasitism by brown-headed cowbirds prompts compensatory double-brooding in prothonotary warblers (Protonotaria citrea). They also found that double-brooding females are often parasitized during their subsequent breeding attempt, suggesting that cowbirds may be promoting and then exploiting compensatory breeding behaviours to maximize their own reproductive success (Table 17.2).
2.2 Nest Architecture and Placement
When hosts and parasites breed sympatrically, hosts can also lower their likelihood of parasitism with defensive nest architectures or by building their nests in areas that are less likely to be located by parasites. In an elegant series of studies, Soler et al. (1995a, 1998, 1999, 2001) demonstrated that deceptive nest architectures can be employed by hosts in response to brood parasitism. They showed that in magpies (Pica pica), nest size is a sexually selected trait that correlates with reproductive success (Soler et al. 2001), that great spotted cuckoos (Clamator glandarius) preferentially parasitize larger magpie nests (Soler et al. 1995a), and that magpies that breed in sympatry with cuckoos have smaller nests than those in allopatry (Soler et al. 1999), together suggesting that brood parasitism is influencing sexually selected traits in these populations.
Hosts can also choose nesting sites that lower their likelihood of being parasitized. Individual parasites vary in their nest-searching behaviours and patterns of host use and can vary these behaviours according to the abundance of suitable host nests (Table 17.2). Hosts can respond by building nests that counter typical parasitic searching strategies, such as building nests that are removed from vantage points (e.g. ‘perch proximity’ hypothesis, Øien et al. 1996; Hauber and Russo 2000) or by increasing their defences when nesting in higher-risk areas (Welbergen and Davies 2012). Alternatively, hosts may place nests in locations that are less desirable to potential parasites, such as near aggressive interspecifics (Clark and Robertson 1979). Whether these kinds of interspecific associations lower the likelihood of brood parasitism awaits experimental investigation.
Alternatively, hosts could build secretive nests (e.g. Burhans 1997; Clarke et al. 2001; Jelínek et al. 2014) or nests that obstruct parasite access to minimize their likelihood of parasitism (Rutila et al. 2002) (Table 17.1). Notably, cavity nests also appear to obstruct parasites from depositing their eggs, and their design may also inhibit the nestling parasite’s competitiveness after hatching (e.g. Rutila et al. 2002, also see Grim et al. 2011). This may explain why cavity nesters are so rarely hosts of the common cuckoo (Cuculus canorus) (Moksnes and Røskaft 1995).
2.3 Breeding Modes
A recent series of studies has demonstrated that host breeding mode can facilitate defences against brood parasitism (Table 17.1). While the behavioural mechanism that underpins parasite deterrence varies (e.g. decreasing the opportunity for parasitism, Canestrari et al. 2009; or facilitating host aggression, Feeney et al. 2013), they cumulatively suggest that more defence is better defence (however see Ursino et al. 2011). Recently, Feeney et al. (2013) found that brood parasitism and cooperative breeding evolve together; however, they were not able to determine the direction of the relationship.
3 Behaviour
3.1 Cognitive Adaptations
Considerable research has been and continues to be conducted into the cognition of brood parasites and hosts. In parasites, investigation of brain morphologies tends to show that they have relatively large hippocampal regions areas that are generally associated with spatial memory (see Chap. 11). A recent series of experimental and field-based studies on the brown-headed cowbird built on these findings and has demonstrated that females have a more accurate spatial memory than males (Guigueno et al. 2014), are able to assess host nest readiness to maximize laying synchrony (White et al. 2009) and that they can remember and target particular host individuals, within and between seasons, that have a history of successfully raising cowbird offspring (Louder et al. 2015a; also see Guigueno et al. 2015; Astié et al. 2015; de la Colina et al. 2016). Similarly, a variety of studies have demonstrated that parasites can strategically choose nests according to the perceived quality of the host parents or nest (Table 17.2) in addition to those that are perceived to be less likely to elicit defences (Avilés et al. 2006) or have a lower likelihood of being depredated (Soler et al. 2014a, b). Some also appear able to change their nest-searching strategy according to the availability of nests (e.g. Jelínek et al. 2014). Together, these studies suggest that cognitive adaptations have played an important role in facilitating parasitic life histories and that these traits may have been further shaped through interactions with hosts.
Similar evidence of cognitive adaptations is evident in hosts. For example, it is becoming clear that at least some hosts recognize brood parasites as unique threats and produce alarm calls that denote parasites in order to elicit specific defences (Table 17.2). At least some can also learn to respond to the sight of a parasite (Davies and Welbergen 2009; Langmore et al. 2012; Feeney and Langmore 2013) and use personal and social information to adjust their behaviours in order to minimize their likelihood of being parasitized (Table 17.2). To date, these studies have tended to focus on the interactions between one host and its primary parasite. However, most brood parasite–host pairs coexist with other parasites and hosts (Davies 2000; Feeney et al. 2013), and while they may have preferred hosts, a variety of parasite species rarely use one host species exclusively (Davies 2000). Therefore, hosts species that exist in these environments may eavesdrop on one another to acquire information about the local risk of parasitism and/or cooperate with one another when defending their nests.
3.2 Stealth and Detection
By and large, parasites and hosts appear to increase their respective likelihoods of successfully parasitizing host nests, or dodging parasitism, by minimizing the likelihood of interacting with one another. Evidence suggests that hosts can lower the likelihood of parasites discovering their nests by decreasing the amount of time they spend around them (Banks and Martin 2001) and by producing complex versus simple vocalizations, which may be difficult for parasites to use as location cues (Garamszegi and Avilés 2005). Parasites also tend to be secretive around host nests (however, not always Gloag et al. 2013; Soler et al. 2014a, b). For example, a combination of monitoring, experimental, video and radio telemetry studies suggests the common cuckoo monitors nests from distant perches (Álvarez 1993; Øien et al. 1996), increases the amount of time spent monitoring a nest the day of host laying (Honza et al. 2002), lays eggs extremely quickly (Davies 2000) and adjusts nest-searching strategies according to the availability of host nests (Table 17.2). In turn, hosts use various sources of direct and indirect information to gauge the risk of being parasitized (Table 17.2). Notably, Thorogood and Davies (2016) recently demonstrated that reed warblers (Acrocephalus scirpaceus) use a combination of personal and social information to inform their deployment of defences at latter stages of the nesting cycle. Parasites can further minimize the likelihood of host recognition through a variety of morphological adaptations, which will be discussed in more detail below (see Sects. 17.4.1 and 17.4.2).
3.3 Aggression
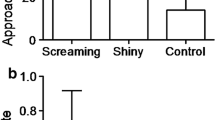
Hosts and parasites also use physical aggression to defend or gain access to the nest. While not universal, most host species aggressively mob brood parasites that approach their nests (Table 17.2). This behaviour can deter parasitism from occurring (Welbergen and Davies 2009; Feeney et al. 2013) and can even result in the death of the parasite (Gloag et al. 2013). In general, physical aggression appears to be an effective defence against brood parasitism (Welbergen and Davies 2009; Feeney et al. 2013; Fig. 17.1); however, at least some parasites appear able to withstand vicious mobbing while they lay their eggs (e.g. Neudorf and Sealy 1992; Gloag et al. 2013; Soler et al. 2014a, b) or may even incite mobbing in order to gain information about the location of a nest (e.g. Robertson and Norman 1976; Strausberger 1998, but see Gill et al. 1997). Species that do not exhibit evidence of mobbing tend to be small-bodied species that rush to and sit on their nest when the risk of parasitism is high in an attempt to block parasite access (e.g. Hobson and Sealy 1989; Medina and Langmore 2016; but see Gloag et al. 2013; Soler et al. 2014a, b for notable exceptions).
Adult parasites can also use aggressive and violent behaviours to increase the likelihood of their offspring surviving. These adaptations have been best studied in the cowbirds, which have been shown to puncture host eggs to assess nest suitability for parasitism (Massoni and Reboreda 1999), destroy host clutches in order to force renesting (Peer and Sealy 1999; Hoover and Robinson 2007; Dubina and Peer 2012; Swan et al. 2015) and destroy eggs during laying to minimize the competition faced by their chicks after hatching (e.g. Gloag et al. 2013; Fiorini et al. 2014; also see Spottiswoode and Colebrook-Robjent 2007 for a reference to similar behaviours in the greater honeyguide (Indicator indicator) and Soler and Martínez 2000 for references to similar behaviours in the great spotted cuckoo). Several parasites have also been suggested to monitor host nests following parasitism and destroy clutches that reject their eggs (e.g. ‘mafia’ behaviours, Soler et al. 1995b; Hoover and Robinson 2007). These kinds of manipulative behaviours appear to be more common in less virulent parasite species, where hosts do not always surrender their entire reproductive effort through hosting a parasite (but see Peer et al. 2013) and the parasite may therefore not be required to prioritize discretion near the nest (see Chap. 15).
4 Morphology
4.1 Camouflage
Parasites that are detected near host nests risk being vigorously attacked (Table 17.2) and forfeiting information about the host’s risk of parasitism, which may result in an increased likelihood of defences being deployed at latter stages of the nesting cycle (Davies and Brooke 1988; Peer and Sealy 2004; Langmore et al. 2009). Similarly, conspicuous hosts risk becoming easy targets (Banks and Martin 2001; Garamszegi and Avilés 2005). Selection should therefore favour cryptic phenotypes in adult brood parasites and hosts.
Brood parasites exhibit a suite of behaviours that minimize their likelihood of being detected while monitoring host nests (Øien et al. 1996; Honza et al. 2002), and the effectiveness of these behaviours could be increased through cryptic morphological phenotypes. Recent phylogenetic analyses have provided some support to the observation that brood parasites tend to have dull plumages, suggesting that these attributes may be a product of coevolution with hosts (Payne 1967; Krüger et al. 2007; Medina and Langmore 2015). Notably, Krüger et al. (2007) found that, in contrast to other cuckoos, the Clamator cuckoos had evolved showier plumages, which may reflect their more conspicuous behaviours around host nests (Macías-Sánchez et al. 2013; Soler et al. 2014a, b; but see Medina and Langmore 2015). Breast barring, which is associated with hawk mimicry in a variety of parasitic cuckoos (Gluckman and Mundy 2013), may also serve to facilitate camouflage while monitoring host nests from tree branches.
4.2 Deceptive Plumages
The common cuckoo has become a model for understanding the ecology and evolution of deceptive plumage phenotypes in avian brood parasites. This species exhibits two distinct plumage polymorphisms: a grey and rufous morph (Davies 2000). The grey morph bears a striking resemblance to Eurasian sparrowhawks (Accipiter spp.), while the rufous morph has been noted to resemble Eurasian kestrels (Falco tinnunculus) (Davies and Welbergen 2008; Thorogood and Davies 2012; Trnka et al. 2015). Numerous studies have demonstrated that both host and non-host species are cautious when presented with models of grey morph cuckoos (Table 17.3), confirming that this cuckoo has evolved mimicry to facilitate parasitism. By contrast, mixed and negative support for mimicry has been found regarding mimicry in the rufous morph (Honza et al. 2006; Trnka et al. 2015). These studies instead support findings that plumage polymorphisms can evolve as a further counter-adaptation against hosts transmitting knowledge of cuckoos throughout a population (Tables 17.2 and 17.3). This correlated evolution of mimicry and polymorphisms across a wide array of parasite species suggests that this series of coevolved adaptations and counter-adaptations is relatively common among parasite–host systems (Krüger et al. 2007; Thorogood and Davies 2013; Gluckman and Mundy 2013; Tanaka 2016).
Deceptive plumages also appear to exist in other brood parasite species (Table 17.3); however, they remain largely unstudied. Putative examples include drongo-cuckoos (Surniculus spp.) mimicking drongos (Dicrurus spp.) (Duckworth 1997), Wahlberg’s honeyguide (Prodotiscus regulus) mimicking small grey flycatchers (Muscicapidae) (Payne 1967) and cuckoo-finches (Anomalospiza imberbis) mimicking female Euplectes weavers (Feeney et al. 2015). These putative examples of plumage mimicry largely await formal examination (for an exception, see Feeney et al. 2015).
4.3 Weapons and Armoury
Despite numerous studies suggesting that violent interactions between brood parasites and hosts may be common at host nests (Table 17.2), almost no research has investigated whether these interactions have selected morphological weaponry and armoury. Adaptations such as thicker skin or denser bones may be present in a variety of brood parasites (Moksnes et al. 2000; Gloag et al. 2013; Soler et al. 2014a, b); however, they await investigation.
5 Physiology
Evidence of physiological adaptations resulting from brood parasite–host coevolution remains scarce. Investigation of these kinds of adaptations at this stage of the nesting is largely limited to fast parasite laying, internal egg incubation, and investigation of immune function and hormone profiles in parasites (Dufty et al. 1987; Davies and de Brooke 1988; Kattan 1997; Mermoz and Reboreda 2003; Birkhead et al. 2011; Hahn et al. 2013; Merrill et al. 2013). Building on this, a recent study by Jung et al. (2016) investigated the testosterone profiles of adult common and lesser cuckoos (C. poliocephalus) throughout the breeding season and found that they largely resembled those that would be expected in non-brood parasites (also see Dufty et al. 1987).
Concluding Remarks and Future Directions
Frontline interactions, similar to those at latter stages of the nesting cycle, provide tractable opportunities to study coevolutionary processes under natural conditions. Though, unlike interactions at the egg, chick and fledgling stages, frontline interactions primarily concern adult brood parasites and adult hosts, offering opportunities to investigate how selection imposed on hosts by brood parasites, and vice versa, shapes adult phenotypes of the involved species. Recent research has and continues to demonstrate the ecological and evolutionary repercussions of frontline coevolution between these species, such as their ability to explain broad patterns of trait diversity in hosts (Feeney et al. 2013) and parasites (Thorogood and Davies 2013; Gluckman and Mundy 2013). However, they also offer unique avenues for future research, such as further investigation of the causal links between brood parasitism and host breeding systems (Table 17.1), how interactions between adult brood parasites and hosts affect behaviours at latter stages of the nesting cycle (Table 17.2) and whether the cost of brood parasitism has selected cryptic vocal or plumage phenotypes in hosts (Table 17.3). Ultimately, like interactions at other stages of the nesting cycle, a deeper understanding of frontline interactions is key to realizing the ecological and evolutionary repercussions of these iconic relationships.
References
Álvarez F (1993) Proximity of trees facilitates parasitism by cuckoos Cuculus canorus on rufous warblers Cercotrichas galactotes. Ibis 135:331–331
Álvarez F (1996) Model cuckoo Cuculus canorus eggs accepted by rufous bush chats Cercotrichas galactotes during the parasite’s absence from the breeding area. Ibis 138:340–342
Astié AA, Scardamaglia RC, Muzio RN, Reboreda JC (2015) Sex differences in retention after a visual or a spatial discrimination learning task in brood parasitic shiny cowbirds. Behav Process 119:99–104
Avilés JM, Stokke BG, Moksnes A et al (2006) Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J Evol Biol 19:1901–1910
Banks AJ, Martin TE (2001) Host activity and the risk of nest parasitism by brown-headed cowbirds. Behav Ecol 12:31–40
Birkhead TR, Hemmings N, Spottiswoode CN et al (2011) Internal incubation and early hatching in brood parasitic birds. Proc R Soc Lond [Biol] 278:1019–1024
Boves TJ, Sperry JH, Comolli K, Weatherhead PJ (2014) Brood parasitism of Black-capped Vireos: frontline and post-laying behavioral responses and effects on productivity. J Field Ornithol 85:364–378
Brooker MG, Brooker LC (1989) Cuckoo hosts in Australia. Aust Zool Rev 2:1–67
Brown M, Lawes MJ (2007) Colony size and nest density predict the likelihood of parasitism in the colonial southern red bishop Euplectes orix – diederik cuckoo Chrysococcyx caprius system. Ibis 149:321–327
Burhans DE (1997) Habitat and microhabitat features associated with cowbird parasitism in two forest edge cowbird hosts. Condor 99:866–872
Campobello D, Sealy SG (2011) Use of social over personal information enhances nest defense against avian brood parasitism. Behav Ecol 22:422–428
Campomizzi AJ, Mathewson HA, Morrison ML et al (2013) Understanding nest success and brood parasitism in the endangered black-capped vireo: comparisons with two sympatric songbirds. Wilson J Ornithol 125:709–719
Canestrari D, Marcos JM, Baglione V (2009) Cooperative breeding in carrion crows reduces the rate of brood parasitism by great spotted cuckoos. Anim Behav 77:1337–1344
Canestrari D, Bolopo D, Turlings TCJ et al (2014) From parasitism to mutualism: unexpected interactions between a cuckoo and its host. Science 343:1350–1352
Clark KL, Robertson RJ (1979) Spatial and temporal multi-species nesting aggregations in birds as anti-parasite and anti-predator defenses. Behav Ecol Sociobiol 5:359–371
Clarke AL, Oien IJ, Honza M et al (2001) Factors affecting reed warbler risk of brood parasitism by the common cuckoo. Auk 118:534
Darwin C (1859) On the origin of species. Murray, London
Davies NB (2000) Cuckoos, cowbirds and other cheats. T. & A.D. Poyer, London
Davies NB, Brooke M de L (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284
Davies NB, Welbergen JA (2008) Cuckoo-hawk mimicry? An experimental test. Proc R Soc Lond [Biol] 275:1817–1822
Davies NB, Welbergen JA (2009) Social transmission of a host defense against cuckoo parasitism. Science 324:1318–1320
Davies NB, Butchart SHM, Burke TA et al (2003) Reed warblers guard against cuckoos and cuckoldry. Anim Behav 65:285–295
de la Colina MA, Hauber ME, Strausberger BM et al (2016) Molecular tracking of individual host use in the shiny cowbird – a generalist brood parasite. Ecol Evol 6:4684–2696
Dubina KM, Peer BD (2012) Egg pecking and discrimination by female and male brown-headed cowbirds. J Ornithol 154:553–557
Duckworth JW (1997) Mobbing of a drongo cuckoo, Surniculus lugubris. Ibis 139:190–192
Dufty AM, Goldsmith AR, Wingfield JC (1987) Prolactin secretion in a brood parasite, the brown-headed cowbird, Molothrus ater. J Zool 212:669–675
Feeney WE, Langmore NE (2013) Social learning of a brood parasite by its host. Biol Lett 9:20130443
Feeney WE, Langmore NE (2015) Superb Fairy-wrens (Malurus cyaneus) increase vigilance near their nest with the perceived risk of brood parasitism. Auk 132:359–364
Feeney WE, Welbergen JA, Langmore NE (2012) The frontline of avian brood parasite–host coevolution. Anim Behav 84:3–12
Feeney WE, Medina I, Somveille M et al (2013) Brood parasitism and the evolution of cooperative breeding in birds. Science 342:1506–1508
Feeney WE, Welbergen JA, Langmore NE (2014) Advances in the study of coevolution between avian brood parasites and their hosts. Annu Rev Ecol Evol Syst 45:227–246
Feeney WE, Troscianko J, Langmore NE, Spottiswoode CN (2015) Evidence for aggressive mimicry in an adult brood parasitic bird, and generalized defences in its host. Proc R Soc Lond [Biol] 282:20150795
Fiorini VD, Gloag R, Kacelnik A, Reboreda JC (2014) Strategic egg destruction by brood-parasitic cowbirds? Anim Behav 93:229–235
Garamszegi L, Avilés J (2005) Brood parasitism by brown-headed cowbirds and the expression of sexual characters in their hosts. Oecologia 143:167–177
Gill SA, Sealy SG (2004) Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav Ecol Sociobiol 56:71–80
Gill SA, Neudorf DL, Sealy SG (1997) Host responses to cowbirds near the nest: cues for recognition. Anim Behav 53:1287–1293
Gill SA, Neudorf DLH, Sealy SG (2008) Do hosts discriminate between sexually dichromatic male and female brown-headed cowbirds? Ethology 114:548–556
Gloag R, Fiorini VD, Reboreda JC, Kacelnik A (2013) The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim Behav 86:1023–1029
Gluckman T-L, Mundy NI (2013) Cuckoos in raptors’ clothing: barred plumage illuminates a fundamental principle of Batesian mimicry. Anim Behav 86:1165–1181
Grant ND, Sealy SG (2002) Selection of red-winged blackbird (Agelaius phoeniceus) hosts by the brown-headed cowbird (Molothrus ater). Bird Behav 15:21–30
Gray J, Fraser I (2013) Australian bird names: a complete guide. Csiro Publishing, Collingwood
Grim T, Samaš P, Moskát C et al (2011) Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? J Anim Ecol 80:508–518
Guigueno MF, Snow DA, MacDougall-Shackleton SA, Sherry DF (2014) Female cowbirds have more accurate spatial memory than males. Biol Lett 10:20140026
Guigueno MF, MacDougall-Shackleton SA, Sherry DF (2015) Sex differences in spatial memory in brown-headed cowbirds: males outperform females on a touchscreen task. PLoS One 10:e0128302
Hahn DC, Summers SG, Genovese KJ et al (2013) Obligate brood parasites show more functionally effective innate immune responses: an eco-immunological hypothesis. Evol Biol 40:544–561
Hauber ME (2003) Interspecific brood parasitism and the evolution of host clutch sizes. Evol Ecol Res 5:559–570
Hauber ME, Russo SA (2000) Perch proximity correlates with higher rates of cowbird parasitism of ground nesting song sparrows. Wilson Bull 112:150–153
Hobson KA, Sealy SG (1989) Responses of yellow warblers to the threat of cowbird parasitism. Anim Behav 38:510–519
Honza M, Taborsky B, Taborsky M et al (2002) Behaviour of female common cuckoos, Cuculus canorus, in the vicinity of host nests before and during egg laying: a radiotelemetry study. Anim Behav 64:861–868
Honza M, Šicha V, Procházka P, Ležalová R (2006) Host nest defense against a color-dimorphic brood parasite: great reed warblers (Acrocephalus arundinaceus) versus common cuckoos (Cuculus canorus). J Ornithol 147:629–637
Hoover JP, Robinson SK (2007) Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proc Natl Acad Sci USA 104:4479–4483
Jelínek V, Procházka P, Požgayová M, Honza M (2014) Common cuckoos Cuculus canorus change their nest-searching strategy according to the number of available host nests. Ibis 156:189–197
Jung W-J, Kim M-S, Noh H-J et al (2016) Hormone profiles of obligate avian brood parasites during the breeding season. Ibis 158:371–379
Kattan GH (1997) Shiny cowbirds follow the ‘shotgun’ strategy of brood parasitism. Anim Behav 53:647–654
Kilner RM (2005) The evolution of virulence in brood parasites. Ornithol Sci 4:55–64
Krüger O, Davies NB, Sorenson MD (2007) The evolution of sexual dimorphism in parasitic cuckoos: sexual selection or coevolution? Proc R Soc Lond [Biol] 274:1553–1560
Lai CM (1998) Messenger of spring and morality: cuckoo lore in Chinese sources. J Am Orient Soc 118:530–542
Langmore NE, Kilner RM (2007) Breeding site and host selection by Horsfield’s bronze-cuckoos, Chalcites basalis. Anim Behav 74:995–1004
Langmore NE, Cockburn A, Russell AF, Kilner RM (2009) Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav Ecol 20:978–984
Langmore NE, Feeney WE, Crowe-Riddell J et al (2012) Learned recognition of brood parasitic cuckoos in the superb fairy-wren Malurus cyaneus. Behav Ecol 23:798–805
Louder MIM, Schelsky WM, Albores AN, Hoover JP (2015a) A generalist brood parasite modifies use of a host in response to reproductive success. Proc R Soc Lond B Biol Sci 282:20151615
Louder MIM, Schelsky WM, Benson TJ, Hoover JP (2015b) Brown-headed cowbirds exploit a host’s compensatory behavioral response to fecundity reduction. Behav Ecol 26:255–261
Macías-Sánchez E, Martínez JG, Avilés JM, Soler M (2013) Sexual differences in colour and size in the great spotted cuckoo Clamator glandarius. Ibis 155:605–610
Martínez JG, Soler M, Soler JJ (1996) The effect of magpie breeding density and synchrony on brood parasitism by great spotted cuckoos. Condor 98:272–278
Massoni V, Reboreda JC (1999) Egg puncture allows shiny cowbirds to assess host egg development and suitability for parasitism. Proc R Soc Lond [Biol] 266:1871–1874
Massoni V, Reboreda JC (2001) Number of close spatial and temporal neighbors decreases the probability of nest failure and shiny cowbird parasitism in colonial yellow-winged blackbirds. Condor 103:521–529
McLaren CM, Sealy SG (2003) Factors influencing susceptibility of host nests to brood parasitism. Ethol Ecol Evol 15:343–353
Medina I, Langmore NE (2015) Coevolution is linked with phenotypic diversification but not speciation in avian brood parasites. Proc R Soc Lond [Biol] 282:20152056
Medina I, Langmore NE (2016) Batten down the thatches: front-line defences in an apparently defenceless cuckoo host. Anim Behav 112:195–201
Mermoz ME, Reboreda JC (2003) Reproductive success of shiny cowbird (Molothrus bonariensis) parasitizing the larger brown-and-yellow marshbird (Pseudoleistes virescens) in Argentina. Auk 120:1128–1139
Merrill L, O’Loghlen AL, Wingfield JC, Rothstein SI (2013) Immune function in an avian brood parasite and its nonparasitic relative. Physiol Biochem Zool 86:61–72
Middleton ALA (1977) Effect of cowbird parasitism on American Goldfinch nesting. Auk 94:304–307
Moksnes A, Røskaft E (1995) Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J Zool 236:625–648
Moksnes A, Røskaft E, Hagen LG et al (2000) Common cuckoo Cuculus canorus and host behaviour at reed warbler Acrocephalus scirpaceus nests. Ibis 142:247–258
Møller AP, Antonov A, Stokke BG et al (2011a) Isolation by time and habitat and coexistence of distinct host races of the common cuckoo. J Evol Biol 24:676–684
Møller AP, Saino N, Adamík P et al (2011b) Rapid change in host use of the common cuckoo Cuculus canorus linked to climate change. Proc R Soc Lond [Biol] 278:733–738
Møller AP, Stokke BG, Samia DSM (2015) Hawk models, hawk mimics, and antipredator behavior of prey. Behav Ecol 26:1039–1044
Møller AP, Morelli F, Tryjanowski P (2017) Cuckoo folklore and human well-being: cuckoo calls predict how long farmers live. Ecol Indic 72:766–768
Moskát C, Honza M (2000) Effect of nest and nest site characteristics on the risk of cuckoo Cuculus canorus parasitism in the great reed warbler Acrocephalus arundinaceus. Ecography 23:335–341
Neudorf DL, Sealy SG (1992) Reactions of four passerine species to threats of predation and cowbird parasitism: enemy recognition or generalized responses. Behaviour 123:84–105
Øien IJ, Honza M, Moksnes A, Røskaft E (1996) The risk of parasitism in relation to the distance from reed warbler nests to cuckoo perches. J Anim Ecol 65:147–153
Payne RB (1967) Interspecific communication signals in parasitic birds. Am Nat 101:363–375
Peer BD, Sealy SG (1999) Parasitism and egg puncture behaviour by bronzed and brown-headed cowbirds in sympatry. Stud Avian Biol 18:235–240
Peer BD, Sealy SG (2004) Correlates of egg rejection in hosts of the brown-headed cowbird. Condor 106:580–599
Peer BD, Rivers JW, Rothstein SI (2013) Cowbirds, conservation, and coevolution: potential misconceptions and directions for future research. Chin Birds 4:15–30
Péron G, Altwegg R, Jamie GA, Spottiswoode CN (2016) Coupled range dynamics of brood parasites and their hosts responding to climate and vegetation changes. J Anim Ecol 85:1191–1199
Polačiková L, Procházka P, Cherry M, Honza M (2009) Choosing suitable hosts: common cuckoos Cuculus canorus parasitize great reed warblers Acrocephalus arundinaceus of high quality. Evol Ecol 23:879–891
Robertson RJ, Norman RF (1976) Behavioral defenses to brood parasitism by potential hosts of the brown-headed cowbird. Condor 78:166–173
Rothstein SI (1990) A model system for coevolution: avian brood parasitism. Annu Rev Ecol Evol Syst 21:481–508
Rutila J, Latja R, Koskela K (2002) The common cuckoo Cuculus canorus and its cavity nesting host, the redstart Phoenicurus phoenicurus: a peculiar cuckoo-host system? J Avian Biol 33:414–419
Saino N, Rubolini D, Lehikoinen E et al (2009) Climate change effects on migration phenology may mismatch brood parasitic cuckoos and their hosts. Biol Lett 5:539–541
Skjelseth S, Moksnes A, Røskaft E et al (2004) Parentage and host preference in the common cuckoo Cuculus canorus. J Avian Biol 35:21–24
Soler M (2014) Long-term coevolution between avian brood parasites and their hosts. Biol Rev Camb Philos Soc 89:688–704
Soler M, Martínez JG (2000) Is egg-damaging behaviour by great spotted cuckoos an accident or an adaptation? Behav Ecol 11:495–501
Soler JJ, Soler M, Moller AP, Martínez JG (1995a) Does the great spotted cuckoo choose magpie hosts according to their parenting ability? Behav Ecol Sociobiol 36:201–206
Soler M, Soler JJ, Martínez JG, Moller AP (1995b) Magpie host manipulation by great spotted cuckoos: evidence for an avian mafia? Evolution 49:770–775
Soler J, Møller A, Soler M (1998) Nest building, sexual selection and parental investment. Evol Ecol 12:427–441
Soler JJ, Martínez JG, Soler M, Møller AP (1999) Host sexual selection and cuckoo parasitism: an analysis of nest size in sympatric and allopatric magpie Pica pica populations parasitized by the great spotted cuckoo Clamator glandarius. Proc R Soc Lond [Biol] 266:1765–1771
Soler JJ, de Neve L, Martínez JG, Soler M (2001) Nest size affects clutch size and the start of incubation in magpies: an experimental study. Behav Ecol 12:301–307
Soler M, Martín-Vivaldi M, Fernández-Morante J (2012) Conditional response by hosts to parasitic eggs: the extreme case of the rufous-tailed scrub robin. Anim Behav 84:421–426
Soler JJ, Avilés JM, Martin-Gálvez D et al (2014a) Eavesdropping cuckoos: further insights on great spotted cuckoo preference by magpie nests and egg colour. Oecologia 175:105–115
Soler M, Pérez-Contreras T, de Neve L (2014b) Great spotted cuckoos frequently lay their eggs while their magpie host is incubating. Ethology 120:965–972
Sorenson MD, Payne RB (2005) A molecular genetic analysis of cuckoo phylogeny. In: Payne RB (ed) The cuckoos. Oxford University Press, Oxford, pp 68–94
Sorenson MD, Sefc KM, Payne RB (2003) Speciation by host switch in brood parasitic indigobirds. Nature 424:928–931
Spottiswoode CN, Colebrook-Robjent JFR (2007) Egg puncturing by the brood parasitic Greater Honeyguide and potential host counteradaptations. Behav Ecol 18:792–799
Spottiswoode CN, Begg KS, Begg CM (2016) Reciprocal signaling in honeyguide-human mutualism. Science 353:387–389
Strausberger BM (1998) Evident nest-searching behavior of female brown-headed cowbirds while attended by males. Wilson Bull 110:133–136
Strausberger BM, Ashley MV (2005) Host use strategies of individual female brown-headed cowbirds Molothrus ater in a diverse avian community. J Avian Biol 36:313–321
Swan DC, Zanette LY, Clinchy M (2015) Brood parasites manipulate their hosts: experimental evidence for the farming hypothesis. Anim Behav 105:29–35
Tanaka KD (2016) Polymorphism in avian brood parasitism: a coevolutionary perspective. Ornithol Sci 15:133–140
Thorogood R, Davies NB (2012) Cuckoos combat socially transmitted defenses of reed warbler hosts with a plumage polymorphism. Science 337:578–580
Thorogood R, Davies NB (2013) Hawk mimicry and the evolution of polymorphic cuckoos. Chin Birds 4:39–50
Thorogood R, Davies NB (2016) Combining personal with social information facilitates host defences and explains why cuckoos should be secretive. Sci Rep 6:19872
Trnka A, Prokop P (2011) Polygynous great reed warblers Acrocephalus arundinaceus suffer more cuckoo Cuculus canorus parasitism than monogamous pairs. J Avian Biol 42:192–195
Trnka A, Prokop P (2012) The effectiveness of hawk mimicry in protecting cuckoos from aggressive hosts. Anim Behav 83:263–268
Trnka A, Trnka M, Grim T (2015) Do rufous common cuckoo females indeed mimic a predator? An experimental test. Biol J Linn Soc 116:134–143
Ursino CA, Mársico MCD, Sued M et al (2011) Brood parasitism disproportionately increases nest provisioning and helper recruitment in a cooperatively breeding bird. Behav Ecol Sociobiol 65:2279–2286
Uyehara JC, Narins PM (1995) Nest defense by willow flycatchers to brood-parasitic intruders. Condor 97:361–368
Welbergen JA, Davies NB (2008) Reed warblers discriminate cuckoos from sparrowhawks with graded alarm signals that attract mates and neighbours. Anim Behav 76:811–822
Welbergen JA, Davies NB (2009) Strategic variation in mobbing as a front line of defense against brood parasitism. Curr Biol 19:235–240
Welbergen JA, Davies NB (2012) Direct and indirect assessment of parasitism risk by a cuckoo host. Behav Ecol 23:783–789
White DJ, Ho L, Freed-Brown G (2009) Counting chicks before they hatch female cowbirds can time readiness of a host nest for parasitism. Psychol Sci 20:1140–1145
Wiley JW (2012) Anti-brood parasite strategies of naïve populations of nesting birds in Puerto Rico. J Caribbean Ornithol 25:41–63
Woolfenden BE, Gibbs HL, Sealy SG, McMaster DG (2003) Host use and fecundity of individual female brown-headed cowbirds. Anim Behav 66:95–106
Acknowledgements
WEF would like to thank M. Soler for an invitation to contribute a chapter to this book, as well as B.D. Peer, M. Soler, S.G. Sealy and T. Ryan for helpful comments on the draft. WEF is funded by the Hermon Slade Foundation and the University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Feeney, W.E. (2017). Evidence of Adaptations and Counter-Adaptations Before the Parasite Lays Its Egg: The Frontline of the Arms Race. In: Soler, M. (eds) Avian Brood Parasitism. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-73138-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-73138-4_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73137-7
Online ISBN: 978-3-319-73138-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)