Abstract
In contrast to the global trend of mangrove decline, New Zealand mangroves are rapidly expanding, facilitated by elevated sediment inputs in coastal waters as a consequence of large-scale land use changes following European settlement. New Zealand mangroves are at the southern limit of the global mangrove extent, which limits the tree height of Avicennia marina var. australasica, the only mangrove species present. Mangroves in New Zealand thrive in the sheltered environments of infilling drowned river valleys with abundant supply of fine terrigenous sediments, showing various stages of mangrove succession and expansion dynamics. Bio-physical interactions and carbon dynamics in these expanding temperate mangrove systems show similarities to, but also differ from those in tropical mangrove forests, for instance due to the limited height and complexity of the mangrove communities. Likewise, ecosystem services provided by New Zealand mangroves deviate from those offered by tropical mangroves. In particular, the association of mangrove expansion with the accumulation of (the increased supply of) fine sediments and the consequent change of estuarine ecosystems, has provoked a negative perception of mangrove expansion and subsequently led to mangrove clearance. Over recent decades, a body of knowledge has been developed regarding the planning and decision making relating to mangrove removal, yet there are still effects that are unknown, for example with respect to the post-clearance recovery of the original sandflat ecosystems. In this chapter we discuss the dynamics of New Zealand’s expanding mangroves from a range of viewpoints, with the aim of elucidating the possible contributions of expanding mangroves to coastal ecosystem services, now and in the future. This chapter also reviews current policies and practice regarding mangrove removal in New Zealand and addresses the (un)known effects of mangrove clearance. These combined insights may contribute to the development of integrated coastal management strategies that recognise the full potential of expanding mangrove ecosystems.
The original version of this chapter was revised. An erratum to this chapter can be found at https://doi.org/10.1007/978-3-319-73016-5_32
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Temperate mangroves
- Avicennia marina
- Mangrove expansion
- Bio-physical interactions
- Carbon dynamics
- Ecosystem services
- Mangrove removal
- Mangrove management
1 Introduction
Mangroves are located throughout estuaries around the shores of northern New Zealand and are part of the island’s native vegetation. The presence of mangroves in New Zealand has been dated back to 19 million years BP by association with Miocene deposits (Sutherland 2003). Pollen records show that the mangrove species Avicennia marina has been present from around 14,000 years BP (Pocknall et al. 1989), long before New Zealand was discovered.
The early Māori settlers valued the mangroves (mānawa in Māori) for their provisioning function for food and resources. Māori traditionally gathered kaimoana—fish and shellfish—in the mangroves and on adjacent mudflats (Crisp et al. 1990). To date, the Māori belief in the interrelationship of all parts of the environment resonates in the concept of kaitiakitanga—guardianship of the environment—which also applies to estuaries, mangroves included. The fundamental idea behind this concept is that all benefits are reciprocal and that an ecosystem that is sustained and cared for can provide benefits back to humans (Harmsworth and Awatere 2013).
The approach to ecosystem management changed with the arrival of the European settlers, with widespread land use changes such as those associated with agricultural development and construction works initially resulting in a direct loss of mangrove habitat (Crisp et al. 1990; Dingwall 1984). However, following rapid clear felling of the land and consequent erosion, sedimentation in estuaries increased and tidal flats started expanding rapidly, accommodating widespread mangrove expansion since the mid-twentieth century (Lovelock et al. 2007; Lundquist et al. 2014b; Morrisey et al. 2007). These newly established mangroves generally occupy muddy intertidal flats that have expanded into estuaries that were previously dominated by coarser sandy sediments (Ellis et al. 2004; Lovelock et al. 2007; Stokes et al. 2010).
More recently, the rapid expansion of mangroves and the ‘muddification’ of estuaries has raised concerns about the loss of diversity of estuarine habitats and a concomitant decline of ecosystem functioning (Harty 2009; Thrush et al. 2004), in addition to the more tangible consequences related to recreational, navigational and amenity values of the estuaries (Lundquist et al. 2014b). Therefore, mangrove removal is a common response to mangrove expansion in New Zealand, with little consideration of the ecosystem services that may be impacted, either positively or negatively, due to mangrove removal (Lundquist et al. 2014b; Morrisey et al. 2010; Stokes and Harris 2015).
The New Zealand practice of mangrove removal contrasts strongly with international efforts to restore and expand mangrove area with the goal of enhancing coastal safety by (re-)establishing the attenuating and stabilizing effects of mangroves (Friess et al. 2016a; Lewis 2005; Spalding et al. 2014; UNEP 2014). Moreover, in the face of expected sea-level rise , accelerated sedimentation in mangrove forests could provide coastal zones with a natural resilience that traditional engineered structures are lacking (Alongi 2008; Barbier 2014; Borsje et al. 2011; Bouma et al. 2014; Stagg et al. 2016; Temmerman et al. 2013). Another benefit of mangrove expansion is the potential for carbon burial, combating increasing carbon emissions that are aggravating the severity and impacts of climate change (Bouillon et al. 2008; Bulmer et al. 2016b; Donato et al. 2011; Friess et al. 2016b; Twilley et al. 1992).
In this chapter we address lessons learned from the expanding mangroves in New Zealand, focussing on their (changing) biophysical, ecological and carbon dynamics, as well as local management practices coping with mangrove expansion. By doing so, we hope to provide a useful view on the dynamics and management of coastal areas where mangroves are expanding, either naturally (as in New Zealand) or as a result of successful mangrove restoration schemes.
We start with an introduction to the mangroves of New Zealand, their characteristic geophysical settings and their expansion dynamics (section “Mangroves in New Zealand”), followed by an appraisal of the biophysical interactions in these expanding mangroves based on recent work in contrasting mangrove systems (section “Biophysical Dynamics of Expanding Mangroves”). The resulting morphological development and ecological succession of New Zealand mangroves is discussed from a forest succession perspective, identifying different pathways for the succession of prograding and stable mangroves (section “Succession of Expanding Mangrove Systems”). Both the morphological development and the forest succession are closely interlinked with the carbon dynamics of expanding mangrove systems, to be addressed in section “Carbon Dynamics of Expanding Mangroves”. Moving on to the management of expanding mangroves, we first identify the ecosystem services and dis-services related to the expanding mangroves in New Zealand (section “Ecosystem (Dis-)services of Expanding Mangroves”). Perceptions of the consequences associated with mangrove expansion are the main driver of mangrove removals in New Zealand. Historically, only minimal information on effects of mangrove removal was available to inform policy planning and decision making regarding mangrove removal, but recent research has led to more informed mangrove management practices, which will be discussed in section “Mangrove Removal in New Zealand”. Analysis of recovery trajectories of multiple decades of mangrove removal practices in New Zealand allows us to compare the ecosystem services provided by both intact and cleared mangrove sites. However, not all consequences of mangrove removal for the biophysical properties and functioning of these intertidal habitats are known and these knowledge gaps will be addressed in section “The Known and Unknown Effects of Mangrove Removal”. We conclude with an outlook on the future development of and the (dis-)services provided by New Zealand mangroves and how these could be managed effectively and sustainably.
2 Mangroves in New Zealand
2.1 Mangrove Distribution
Although mangroves predominantly occur in the tropics, they also can extend into temperate climate regions. Mangroves flourish in areas where mean monthly air temperatures are above 20 °C, with a seasonal range of up to 10 °C (Chapman 1977). Beyond these areas, the poleward extent of mangroves is often limited by the incidence of ground frost and generally coincides with the winter position of the 20 °C seawater isotherm (Duke et al. 1998). The mangroves on the North Island of New Zealand, together with those in southern Australia and eastern South America, are exceptions to the above water temperature limitation. These mangrove communities, at the extremities of the global mangrove distribution, are likely relict populations of a greater poleward mangrove distribution during a warmer period in the past, and may also be facilitated by extensions of irregular ocean currents, warming coastal waters and transporting propagules from the north (Chapman 1976; Duke et al. 1998; Macnae 1966).
Both mangrove area and mangrove diversity generally decline with increasing latitude (Ellison 2002; Giri et al. 2011). Temperate mangroves (sensu Morrisey et al. 2010) typically comprise a few species only, as opposed to greater species diversity in mangroves at lower latitudes. Restricted species diversity may limit the range of habitats that are suitable for mangroves at their latitudinal extremes, depending on species’ tolerance of abiotic factors such as temperature, day length, aridity, water and soil salinity, tidal range and hydrodynamic exposure (Chapman 1977; Duke 1990; Morrisey et al. 2010; Tomlinson 1986; Watson 1928). According to recent estimates, less than 1% of the total global mangrove cover of ~13.8 million ha is found at latitudes greater than 30° (Giri et al. 2011).
New Zealand’s mangrove distribution covers about 26,050 ha (Spalding et al. 2010). Mangroves are found from 34°27′S in the far north of the North Island to 38°05′S at Kawhia Harbour on the west coast and 38°03′S at Ohiwa Harbour on the east coast (Fig. 2.1; Crisp et al. 1990; de Lange and de Lange 1994). Planted mangroves have been observed to establish and survive as far south as 41°13′S on the Hutt River, at the southern end of the North Island (de Lange and de Lange 1994). Frequency and duration of frost events, causing tissue damage in mangroves (Duke 1990; Saenger and Snedaker 1993), form a primary constraint on mangrove expansion at the latitudinal limits of their distribution in New Zealand (Lundquist et al. 2014b). Additional abiotic factors inhibiting the southward expansion of New Zealand mangroves are the prevailing currents and the lack of suitable habitats within the dispersal range of the propagules (de Lange and de Lange 1994). Propagules of the native mangrove remain viable for a few days only, which is too short for wind-induced drift currents to transport them past the rocky shores directly south of the southernmost limit of the present mangrove distribution (de Lange and de Lange 1994; Duke et al. 1998).
Mangroves in New Zealand are found from the tip of the North Island, down to Kawhia Harbour on the west coast and Ohiwa Harbour on the east coast (mangrove distribution data: Crisp et al. 1990; Spalding et al. 2010). Names of locations are included for mangrove areas that are referred to throughout this chapter
2.2 Geomorphological Setting of New Zealand Mangroves
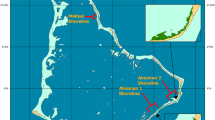
Similar to most mangroves around the world, New Zealand mangroves thrive in sheltered coastal environments with a terrigenous sediment supply (Augustinus 1995; Chapman 1977; Thom 1982; Woodroffe 1992). Whereas tropical mangroves also occur in river deltas and at muddy open coasts with extensive intertidal flats, New Zealand mangroves predominantly occur in estuaries, tidal basins, lagoons, tidal creeks and river mouths, as shown in Fig. 2.1 (Chapman 1976; Morrisey et al. 2010; Spalding et al. 2010). The largest mangrove areas are observed in estuaries with large terrigenous sediment supplies such as Rangaunu Harbour (2416 ha) and the Firth of Thames (1100 ha; locations indicated in Fig. 2.1 Lundquist et al. 2014b; Morrisey et al. 2010).
Estuaries in New Zealand are ancestral river valleys and bays that were flooded by rising sea levels during the last post-glacial marine transgression (Hume 2003; Roy et al. 2001). Since the sea level stabilized, around 6500 years BP, the drowned river valleys and bays started infilling. The rate of estuarine infilling depends on the supply of sediment from the river catchment discharging in the estuary and the tidal prism flushing the estuary (Hume and Herdendorf 1992). At the same time, coastal processes caused the formation of sandy barriers and spits across the entrances of some of these estuaries, reducing tidal ranges and currents inside the estuaries (Hume 2003; Roy et al. 2001). The infilling of drowned river valley estuaries, which has accelerated since European settlement in New Zealand (see section “Mangrove Expansion in New Zealand’s Anthropocene”), created intertidal flats that were suitable for mangrove establishment.
2.3 Mānawa: The New Zealand Mangrove
The mangrove genus Avicennia tolerates a wide range of environmental conditions such as air and water temperatures, frequency and severity of frosts, day lengths and salinity (Chapman 1976; Duke 1990; Krauss et al. 2008; Stuart et al. 2007). Hence, Avicennia spp. cover the largest latitudinal range of all mangroves, from 32°N on Bermuda to 38°S in Australia and New Zealand (Duke et al. 1998; Krauss et al. 2008; Spalding et al. 2010; Tomlinson 1986). These distributional limits are hypothesized to coincide with a trend towards zero reproductive success, due to the limited summer period available for growth, flowering and fruiting (Duke 1990).
New Zealand mangroves consist of a single species: Avicennia marina var. australasica (Fig. 2.2), previously named Avicennia marina var. resinifera (Chapman 1976; Duke 1991; Tomlinson 1986). The same species is also found in south-eastern Australia, New Caledonia and Lord Howe Island (Duke 1991). Avicennia marina is also commonly known as the ‘grey mangrove’ due to the grey shade of its bark. Avicennia spp. have pneumatophores (i.e. pencil roots) that emerge from the bed for aeration on waterlogged soils. In New Zealand, these pneumatophores have heights generally ranging between 5 and 25 cm, and reaching up to several decimetres on the deeper banks of creeks and in runnels through the mangroves (pers. obs. by the authors).
Avicennia marina var. australasica in New Zealand: (a) a stunted (or dwarfed) growth form of 0.5–1 m of height in Tauranga Harbour, (b) a dense growth form of trees of 2–3 m of height in the Firth of Thames, and (c) a tall growth form of trees of up to about 7 m of height in the inner Whitianga Harbour (Photo credits: C. Lundquist and E. Horstman)
Greater temperature stresses towards the latitudinal limits of mangrove distribution are associated with smaller tree heights and reduced net primary productivity (Bouillon et al. 2008; Saenger and Snedaker 1993). Low winter temperatures and frost have been reported to cause a direct loss of stem height due to die back of twigs (Lovelock et al. 2007) and impact on terminal buds, limiting the growth of mangrove tree crowns so that only lower branches can continue growing (Chapman 1976). Such periodic damage due to low temperatures can cause a stunted or dwarfed growth form, typically featuring a well-developed trunk and a wide, spreading canopy of limited height (Fig. 2.2a); (Chapman 1976; Crisp et al. 1990; de Lange and de Lange 1994; Saintilan et al. 2009). Nevertheless, stunted trees also occur in areas with taller trees, and hence this growth form is not uniquely caused by temperature stresses. De Lange and de Lange (1994) observed that, in New Zealand, stunted growth forms mostly occur on substrates with <50% mud (<20 μm), whereas taller trees can be found on substrates with higher mud contents under the same climatic conditions (Fig. 2.2b, c).
Avicennia marina var. australasica can grow to heights of up to about 10 m (Chapman 1976; Duke 1991) and New Zealand mangroves in the far north approach this height. Although mean tree heights generally reduce with latitude, other controlling factors such as abovementioned substrate type, but also soil drainage and tidal inundation can locally affect tree heights. Hence, stunted trees are also observed in the far north of New Zealand, whereas taller tree growth is observed closer to the southern limits of mangrove distribution as well, with 7–8 m tall trees in e.g. the Auckland region and in the Whangarei and Whitianga Harbours (Chapman 1976; Crisp et al. 1990; pers. obs. by the authors). In well-established mangroves, the taller trees are often observed at the mangrove fringe or close to a creek and tree heights decline in the landward direction (Chapman 1976; Lovelock et al. 2007; Morrisey et al. 2007). In rapidly expanding mangroves, tree age progressively declines towards the forest fringe, which coincides with a decline in height near the seaward fringe where seedlings have only recently established (Morrisey et al. 2007). These tree height gradients become non-uniform on creek-dissected mudflats (May 1999) and in wide stands of mangroves that have extended in a non-linear fashion over time, such as the extensive mangroves at the southern fringe of the Firth of Thames (Lovelock et al. 2010; Swales et al. 2015).
Mangroves are distributed across the intertidal profile from approximately mean sea level (MSL) to the highest spring tide mark (Duke et al. 1998; Tomlinson 1986; Watson 1928). The lower elevation limit around MSL is associated with the inundation duration of the aerial roots under regular tidal conditions and physiological constraints of the mangroves with respect to this variable. Increased inundation frequency and wave activity at lower elevations inhibit the establishment and survival of mangrove seedlings (Balke et al. 2013, 2015; Lovelock et al. 2010). Mangroves can extend to elevations at the upper end of the tidal prism, that are only infrequently flooded by extreme spring tides and episodic storm surges, with the latter substantially (but temporarily) increasing the inundation duration of the mangroves (Swales et al. 2007).
Mangroves in New Zealand can be part of an extensive coastal ecosystem with multiple zones comprising different vegetation types that occupy different intertidal elevations, as schematized in Fig. 2.3 (Chapman 1976; Crisp et al. 1990; Graeme 2013; Wassilieff 2006). The lower parts of muddy or sandy intertidal flats are often colonized by seagrass ( Zostera muelleri ) communities, which can extend from the edge of the mangrove zone down into the shallow subtidal (Chapman 1976; Wassilieff 2006). At higher intertidal elevations, directly in front of the mangroves we generally observe a seaward extension of the mangrove root systems, forming a pneumatophore ‘carpet’ interspersed with (smaller) more isolated trees and seedlings, named the mangrove fringe. The upper end of the intertidal is the domain of saltmarshes and salt meadows. Saltmarsh vegetation consists of sea rush ( Juncus kraussii ) and oioi ( Leptocarpus similis ) that can form dense grasslands up to 1.5 m of height. Salt meadow vegetation is of lower height and consists of species such as saltwort ( Sarcocornia quinqueflora ) and remuremu ( Selliera radicans ) (Chapman 1976; Wassilieff 2006). The separation between saltmarsh and salt meadow is not very strict, systems can intermingle and pervade into the upper mangroves. At the landward end, intertidal vegetation gives way to coastal scrubs and other land uses.
A typical cross-shore profile of an intertidal area with mangroves in an estuary in northern New Zealand (After: Chapman 1976; Crisp et al. 1990; Swales et al. 2015; Wassilieff 2006): the lower intertidal is dominated by seagrass ( Zostera muelleri ); mangroves (Avicennia marina ) thrive in the regularly flooded area above mean sea level; the upper intertidal is home to salt meadows, comprising succulents and herbs such as saltwort ( Sarcocornia quinqueflora ) and remuremu ( Selliera radicans ), and salt marshes , covering rushes such as sea rush ( Juncus kraussii ) and oioi ( Leptocarpus similis ); and at the landward end, coastal scrubs such as flax ( Phormium tenax ) are prevalent
2.4 Mangrove Expansion in New Zealand’s Anthropocene
European settlement and subsequent developments initially caused a large-scale loss of mangrove habitat in New Zealand (Crisp et al. 1990; Lundquist et al. 2014b; Morrisey et al. 2007). Human activities such as land reclamations for ports, landfills, airports (e.g. Auckland International Airport), agriculture and industrial and urban development directly impacted the mangrove area. Additionally, construction of causeways, marinas and other engineering works has restricted tidal flows and/or elevated water levels in some estuaries, reducing suitable mangrove habitats (Crisp et al. 1990; Dingwall 1984; Lundquist et al. 2014b).
European settlement was followed by large-scale deforestation of river catchments since the mid-nineteenth century, intensifying land erosion and accelerating infilling of the drowned river valley estuaries (Burns and Ogden 1985; Lovelock et al. 2007; Lundquist et al. 2014b; Morrisey et al. 2010; Nichol et al. 2000; Swales et al. 2007). Sediment supply to the estuaries was enhanced by the typical topography of New Zealand catchments with steep slopes and limited spatial extents, and also by the erratic precipitation pattern with episodic rainstorms. Large supplies of relatively fine terrigenous sediments to the estuaries resulted in increased water turbidity and accelerated sediment accretion, shifting these estuaries to muddier intertidal systems (Morrisey et al. 2010; Nichol et al. 2000; Thrush et al. 2004). Consequently, sedimentation rates increased by an order of magnitude or more (Jones 2008; Nichol et al. 2000; Swales et al. 2009), as illustrated by the estuaries on the Coromandel Peninsula where accretion rates were found to be 50–80 times greater in the second half of the twentieth century than before European settlement (Jones 2008). Although the stabilization of land-use practices has reduced sediment supply from the catchments over the past century, sediment yields and consequent sediment accretion rates are still substantially higher than in pre-European times (Healy 2002; Jones 2008; Swales et al. 2002).
The accelerated estuarine infilling and vertical accretion of tidal flats, providing additional habitat suitable for mangroves, combined with larger nutrient inputs, climate warming and changes in relative sea level (due to accretion/subsidence) have facilitated the expansion of New Zealand mangroves during the past 50–70 years (Burns and Ogden 1985; Harty 2009; Lovelock et al. 2007; Morrisey et al. 2010; Swales et al. 2007, 2015, 2016; Young and Harvey 1996). These processes have resulted in rapid mangrove expansion in the majority of the estuaries on the Coromandel Peninsula (Jones 2008). In the Firth of Thames mangroves have expanded over a width of 1 km over the past 50 years (Lovelock et al. 2010; Swales et al. 2007, 2016). Historical analyses of aerial imagery of a limited number of Auckland estuaries suggested mangrove expansion rates of up to 20% per year, with an average of 4.1% per year, during the second half of the twentieth century (Swales et al. 2009). Updated analyses for all estuaries in the Auckland region suggest expansion rates of 3.4% per year for the 1940–2014 period. Since 1970, large differences in expansion rates between different mangrove growth forms have been reported, with average expansion rates of 1.1% and 20.7% per year for tall and dwarf mangroves, respectively (Suyadi, University of Auckland, pers. comm.).
The infilling of estuaries as they ‘age’ causes a shift in the relative proportion of different estuarine habitats, such as a decline of the sandflat area (see section “Ecosystem (Dis-)services of Expanding Mangroves” for related consequences to ecosystem services), in favour of intertidal mudflats and mangrove expansion (Morrisey et al. 2007; Roy et al. 2001; Saintilan 2004). The accelerated infilling of New Zealand estuaries accelerates the shift towards mangrove habitats, and is also associated with increased turbidity levels, which is further enhanced due to sediment resuspension by wave action in these shallow waters (Green et al. 1997; Green and Coco 2007; Roy et al. 2001). These changes come at the risk of reduced habitat diversity and a loss of ecosystem values (Saintilan 2004; Thrush et al. 2004). For example, mangrove cover in the Whangapoua Harbour (on the Coromandel Peninsula) has more than doubled between 1945 and 2006, while seagrass habitat in this estuary has halved over the same period (Jones 2008). Consequences of the rapid succession of New Zealand mangroves are further discussed in section “Ecosystem (Dis-)services of Expanding Mangroves”.
3 Biophysical Dynamics of Expanding Mangroves
3.1 Morphological Change in Expanding Mangroves
Increased sedimentation in New Zealand’s drowned river valley estuaries raises the intertidal flat heights and allows mangroves to establish. Sediment loads delivered to mangroves in these estuaries have a terrigenous origin, supplied by catchment run-off, and are primarily composed of fine sediments such as silts and clays (Griffiths and Glasby 1985; Healy 2002). Nutrient addition coupled to the terrigenous sediment input, especially from catchments dominated by agricultural land use, can also promote expansion, although this effect plays a secondary role relative to that of sediment supply (Lovelock et al. 2007; Martin 2007; Melville and Pulkownik 2006). Estimation of precise sediment budgets can be difficult, owing to the highly variable (both temporally and spatially) nature of sediment delivery (Bryce et al. 2003), with large pulses of sediment delivered to estuaries episodically during storm events (Fig. 2.6; Stern et al. 1986) and additional variation over seasonal timescales. Longer-term records (over the past century) of sediment accumulation can be determined using 210Pb geochronology (Nittrouer et al. 1979), and these rates have been shown to be strongly dependent on the sediment supply and geomorphic setting. Within New Zealand, short-term estimates (but integrating over seasonal timescales) of sediment accumulation vary widely and range up to 100 mm year−1 (Table 10 in Morrisey et al. 2010).
Following sediment delivery to the system, deposition processes vary between different types of mangrove systems. In more exposed-coast locations, deposition can be controlled by the larger-scale shelf and tidal forcing and baroclinic (river-influenced) flows (Wolanski et al. 2001). These processes often lead to the formation of the classic flat-fronted deltaic shape commonly found in tropical deltaic environments. In the quiescent upper reaches of estuaries, sediment resuspension and transport (asymmetry) are controlled by a balance between tidal currents, water depth, wind-driven currents and wind wave growth (Green et al. 1997; Green and Coco 2007; Hunt et al. 2015, 2017). Mangroves occupy the landward edges of intertidal flats and expand seaward from these estuarine fringes (see section “Succession of Expanding Mangrove Systems” for succession patterns). Deposition is also modulated by estuarine maturity as the tidal prism is smaller for (more mature) infilled estuaries (Friedrichs and Aubrey 1988). In mangrove systems with incised creeks, the presence of the creeks further controls flow routing at low water depths, with sediment delivered to the forest once creek banks are overtopped, and in particular with formation of sheet flow and direct sediment supply from the estuarine waters once water depths exceed the height of the root system (Horstman et al. 2015).
3.2 Biophysical Interactions
Mangrove trees introduce considerable complexity to the hydrodynamics within coastal areas. The presence of obstacles (tree trunks, branches, leaves and roots) enhances drag. This vegetative drag removes energy from the system and has been observed to induce large-scale changes in flow directions (Horstman et al. 2013; Kobashi and Mazda 2005; Mullarney et al. 2017b) and also dissipates swell and wind wave energy (Henderson et al. 2017; Horstman et al. 2014; Quartel et al. 2007; Vo-Luong and Massel 2008). Mangroves can also dissipate longer-period waves such as tsunamis (Alongi 2008; Danielsen et al. 2005; Wolanski 2007) or storm surges, provided the forest is of sufficient width (Mullarney and Henderson 2017), see Fig. 2.4. Thus, mangrove forests can act as an ‘eco-defence’, providing a protective barrier against wave impact and mitigating against coastal erosion, whilst simultaneously offering ecological co-benefits (see section “Ecosystem (Dis-)services of Expanding Mangroves” for these ecosystem services).
Attenuation of hydrodynamic forces in mangroves creates a favourable environment for further sediment deposition (Horstman et al. 2014, 2017; van Santen et al. 2007; Young and Harvey 1996) with spatial patterns of deposition within the forest being controlled by the small-scale interactions between the water and the vegetation elements, as illustrated in Fig. 2.5. In some areas, the enhanced vegetative drag (e.g. from water moving through the pneumatophores) removes energy from the system, creating zones of low flow which prevent sediment resuspension and promote deposition. However, in other cases, the vegetation elements have been shown to generate turbulence in the form of eddies and wakes at small length scales (corresponding to the widths of the obstacles or the spacing between them), which can lead to resuspension and local scour (Bouma et al. 2007; Mullarney et al. 2017a). The shift between these two modes of behaviour is still not well known. In tropical systems, intense turbulence (characterised by dissipation rates of turbulent kinetic energy of up to 0.1 W kg−1, values comparable to those observed near the bed in surf zones) has been observed to occur within pneumatophore canopies , with smaller values observed on the adjacent unvegetated mudflats. Even more intense turbulence was reported during large wave events or in regions with denser vegetation (Norris et al. 2017). In particular, the forest fringe between the unvegetated and vegetated regions, where relatively fast flows first interact with vegetation, is shown to be a particularly dynamic area. This intense turbulence at the fringe has potential to winnow fine sediments, which are then transported and deposited further into the forest, resulting in a gradient in grain size with distance into the forest (Fricke et al. 2017). Likewise, turbulence production and near-bed turbulence intensities have been observed to decrease with vegetation density (see insets in Fig. 2.5), creating a similar gradient in grain sizes of the deposits (Horstman et al. 2017, 2018; van Katwijk et al. 2010).
Schematic representing physical processes and biophysical interactions within a typical mangrove forest (insets after Horstman et al. 2018)
Results from tropical regions have raised the possibility that mangroves may act as ecosystem engineers, by growing at densities which promote maximal energy dissipation and sediment deposition, thus creating a feedback mechanism which allows for mangrove expansion (Henderson et al. 2017). Furthermore, there are many other small-scale processes, whose influence on deposition in vegetated regions has not, to date, been studied in detail. Examples include the effect of vegetation-generated turbulence on the formation and break up of aggregates of silt and clay particles (‘flocs’), which influences the settling velocity of particles (Wolanski 1995), as well as the effects of bioturbation, which changes bed roughness, and biostabilisation, which can increase cohesiveness e.g. through the stabilizing effect of mangrove roots or excretion of extra-cellular polymeric substances (EPS) by microorganisms (cf. Widdows et al. 2004).
The balances between erosion and deposition on the small-scale may be different in mangrove forests in New Zealand, relative to those found in tropical environments, owing to substantial differences in tree geometry. New Zealand mangrove trees are smaller (more bush-like, see section “Mānawa: The New Zealand Mangrove”) and pneumatophores are thinner, shorter and more flexible. Data obtained in the Whangapoua Harbour (for location see Fig. 2.1) revealed pneumatophores with basal diameters of around 7 mm and mean and maximum heights of 150 and 250 mm, respectively, compared to basal diameters of around 13 mm and heights of up to 820 mm for Sonneratia mangroves in the Mekong Delta in Vietnam (Mullarney et al. 2017b). However, similar to observations by Henderson et al. (2017) in tropical mangroves, enhanced deposition is observed in the fringing zone of mangroves in Whangapoua Harbour (Fig. 2.6), where the flow first interacts with the vegetation, facilitating an ongoing seaward expansion of these mangroves (further discussed in section “Succession of Expanding Mangrove Systems”).
(a) Sediment deposition measured in the Whangapoua Harbour (location indicated in Fig. 2.1) along, (b) a transect covering tidal flat, fringe and mangrove forest (Horstman et al. 2017). Deposition rates shown for regular conditions (11-15 April 2016) and just after a torrential rain storm (17-19 April 2016). (c, d) Sediment deposition rates were monitored with sediment traps (Ø 0.33 m) that were deployed in triplicates at the positions marked with ▲ in the cross-shore profile (Photo credits: E. Horstman)
Although preliminary observations suggest that turbulence generated within the smaller New Zealand pneumatophores is less intense (values of TKE dissipation rates from Whangapoua did not often exceed 10−4 W kg−1), the extent of the influence of the other tree elements is not yet clear. In some cases, for the smaller mangroves in New Zealand, the branches and leaves of the trees themselves are submerged during high tides, and therefore, once water levels reach the tree canopy, will likely enhance drag (as observed in similar mangroves in Japan and Vietnam by Chen et al. 2016; Mazda et al. 1997; Quartel et al. 2007) and possibly also generate additional turbulence. Whether these differences suggest that other factors such as sediment supply may play a stronger role in setting sediment deposition patterns than local turbulence conditions is an active area of research (see for example Horstman et al. 2016, 2017).
4 Succession of Expanding Mangrove Systems
The structure of any mangrove forest depends on its successional history that evolves in a dynamic equilibrium with the environmental gradients that occur in the coastal zone. Because New Zealand mangroves are monospecific (see section “Mānawa: The New Zealand Mangrove”), their successional history relates to growth forms within Avicennia rather than replacement of pioneer species with more mature forest. In addition, mangrove forests exclude understory species which could be a consequence of multiple factors: the high-stress environment making it difficult to cope with the added stress of reduced light (Janzen 1985), competition, or the ability of mangroves to change soil conditions so their own recruits survive better (Bosire et al. 2006). Environmental gradients in the coastal zone relate to resources, regulators and the inundation regime: resources are the factors which are consumed by growth such as nutrients; regulators are physical and biological parameters that cause stress such as salinity, accumulation of mud or predation; and the inundation regime controls the degree to which resources (sediment, nutrients etc.) are delivered and stresses, associated with tidal currents and wave action, are administered within the forest (Twilley and Rivera-Monroy 2009). Of all the mangrove species, Avicennia is known to be the most tolerant to regulating stresses imposed by muddy substrates and salinity (Twilley et al. 1999).
Factors limiting zonation and succession in mangroves are highly complex (Alongi 2009). Within New Zealand, environmental gradients vary greatly around the country due to variations in geomorphic setting, and so mangrove succession patterns are difficult to generalise. According to the original five-class classification by Thom (1982), most New Zealand mangroves can be classified as wave-dominated barrier lagoon type mangroves (typically with autochthonous sediment types), river-dominated type mangroves (typically with allochthonous sediments), or a combination of both. However, due to the rapid infilling of New Zealand lagoons with catchment-derived terrigenous sediments (see section “Mangrove Expansion in New Zealand’s Anthropocene”), sediments in these lagoons are more likely to be allochthonous than not. However, even when geomorphic conditions of estuarine mangrove environments are similar, the classic succession states of initial pioneering, rapid growth, maturity, old age and death (e.g. Fromard et al. 1998) do not always occur (Lugo 1997). Disturbance events (sometimes on large scale), restart the succession locally, and therefore succession patterns are sensitive to the external controls on the mangrove structure (Twilley et al. 1999).
Forest development in New Zealand depends on the ability of the fringing mangroves to colonise seaward, which in turn depends on seedling establishment (Lovelock et al. 2010), and on the inundation regime being appropriate for mangrove growth (Swales et al. 2016). It is thought that expansion occurs during ‘windows of opportunity’ that correspond to relatively quiescent conditions which allow seedlings to establish in stable sediment conditions without being uprooted (Balke et al. 2015). Following seaward expansion of the fringing mangroves, the condition of the landward forest is dependent on the provision of resources and stresses across the (extended) forest fringe (salinity, pH, sulphide levels, nutrients, waterlogging), both of which are controlled by the inundation regime (Lara et al. 2009). If growth is too intense at the fringe, growth and sustenance in the inward forest can suffer due to nutrient depletion by the fringe, but also because of dissipation of energy at the fringe, reducing the tidal range (and sediment and nutrient delivery mechanism) inward. Consequently, the trees are often locally taller at the fringe and diminish in height in the landward direction (section “Mānawa: The New Zealand Mangrove”).
Balke et al. (2015) and Lovelock et al. (2010) both discuss the role of waves on recruitment in mangroves in the Firth of Thames, which is exposed to a large fetch from the north. During La Niña years, the winds align with the orientation of the estuary, increasing the hydrodynamic exposure on the tidal flats and in the mangrove fringe, and seedlings are unable to establish. Conversely, during the protected south-westerlies of El Niño conditions, rapid establishment and expansion has been reported. Consequently, bands of dense trees can be detected as historic ‘waves of expansion’. Measurements collected in 2016 show the rapid increase in vegetation density across a very short distance in the Firth of Thames (Fig. 2.7b), with the accompanying flat-topped morphology (Fig. 2.7a) indicating a reduced supply of sediment to the back of the mangroves. The photos in Fig. 2.8a–d provide evidence for the rapid expansion of the mangrove fringe at this site, with very little change inside the dense forest (photo shown in Fig. 2.9b) over the last decade. As described in section “Biophysical Interactions”, these dense zones of vegetation at the fringe feed back into the hydrodynamics by dissipating energy and increasing sedimentation at the fringe (see also Fig. 2.6a), with supply to the back forest reduced so that the morphology develops a nearly level surface.
(a, d, g) Elevation profiles, (b, e, h) vegetation distribution, and (c, f, i) sediment characteristics along cross-shore transects in three mangrove sites: (a–c) Firth of Thames, (d–f) Whitianga and (g–i) Whangapoua (see Fig. 2.1 for site locations).
Changes in spatial distribution of the mangrove forest at the three mangrove sites from Fig. 2.7: (a–d) Firth of Thames, (e–h) Whitianga, and (i–l) Whangapoua. The printed scale is 100 m long, and north is upward. Images are extracted from GoogleEarth (map data: panels (a, b, f,g, h, l): Digital Globe; panels (c, d): CNS/Airbus 2017; and panels (e, i, j, k): Thames-Coromandel District Council)
Photos of the forest characteristics along the transects at the study sites from Fig. 2.7: (a–c) Firth of Thames, (d–f) Whitianga and (g–i) Whangapoua. Panels (a, d and g) are taken at the fringe, panels (b, e, and h) are in the middle of the forest and panels (c, f, and i) are toward the back of the mangroves (Photo credits: K. Bryan, E. Horstman, J. Mullarney and D. Sandwell)
Very few studies exist of forest development at other New Zealand mangrove sites. Yet, our observations suggest that developmental controls might be quite different. Figure 2.8 shows the vegetation dynamics of the Firth of Thames and of two mangrove forests on the Coromandel Peninsula on the northeast coast of New Zealand’s North Island over the past 16 years: one in a sandy back-barrier lagoon (Whangapoua Harbour), and one in a more muddy river-dominated estuary (Whitianga Harbour; see Fig. 2.1 for locations). The median sediment particle sizes for these sites are shown in Fig. 2.7. Imagery from GoogleEarth shows the mangroves in the Whangapoua Harbour are changing rapidly (Fig. 2.8i–l), whereas those in Whitianga have remained remarkably stable (Fig. 2.8e–h). Historical aerial photos from Whangapoua (not shown) show no mangroves were present in 1970, corroborating the rapid expansion of these mangroves, whereas mangrove stands in Whitianga were very similar to those observed today. Whitianga was also one of the estuaries visited by Captain Cook in November 1769, when he noted “low flat islands all cover’d with a sort of Mangrove trees” (Beaglehole 1955; p. 196). In the case of the expanding Whangapoua site, mangroves do not expand seaward in waves of establishment, like those in the Firth of Thames, but rather by establishment of seedlings in the gaps between widely dispersed single trees throughout the intertidal. Recruitment is therefore controlled by the conditions in the gaps (Clarke 2004), where light availability is better and growth is higher (Krauss et al. 2008), and predation might be reduced (Osborne and Smith 1990). The lack of expansion in the riverine site (Whitianga) may reflect the rapid changes in sediment load associated with the common flooding events that regularly inundate the site. Although the lower salinity environment favours enhanced mangrove growth (Krauss et al. 2008), sediment loads can cause fluctuations in the surface which may bury or uproot seedlings (Balke et al. 2013) and shrubs. Ten centimetres of episodic sediment deposition has been reported to kill Avicennia trees (Ellison 1999) and a sedimentation rate of 10 cm year−1 causes substantially reduced growth (Sidik et al. 2016). Certainly, the longevity of the Whitianga forest has allowed the development of mature stands composed of large trees (Fig. 2.9d).
The above three examples illustrate the large differences in growth form and forest development that can occur over a short geographical distance. The youngest of the three forests discussed here is the Whangapoua site, which is located in a sandy estuary with low sediment supply, and is characterized by short trees (Fig. 2.9g–i) and a linear intertidal profile (Fig. 2.7). Sediment and tidal energy can easily penetrate this sparse canopy to supply nutrients and sediments to the inner forest, accounting for the recruitment and morphology building inward. The Firth of Thames is a medium-aged site, where the sediment loads from the nearby rivers create conditions that are ideal for rapid seaward expansion. The relatively high surface elevation gains (see upper panels in Fig. 2.7 for inter-site comparison), mainly at the seaward fringe of the mangroves, compared to other Indo-Pacific countries (Lovelock et al. 2015), show the importance of sediment loading to expansion in New Zealand mangroves. Together with regulating stresses, the rapid seaward expansion of the intertidal flats accommodates fast expansion of the mangrove vegetation (Fig. 2.8a–d), resulting in a very dense canopy just behind the forest fringe (Fig. 2.9b). Finally, at the oldest forest, in the Whitianga Harbour, we hypothesize sediment loading is too great for rapid development of the fringe; in this case the sparse fringing canopy of very tall, old growth trees (Fig. 2.9d) allows sediments to penetrate inward to the point where the inundation regime is too small and larger mangroves die out to be replaced by inland species (Fig. 2.9f). In both the Firth of Thames and Whitianga, the established vegetation at the fringe is characterised by larger trees (Fig. 2.9a, d), which benefit from the unrestricted supply of water-borne resources and presumably larger trees are also better equipped to withstand the higher stresses due to waves and currents impacting on the mangrove fringe. The smaller shrubs, affected by a limited supply of resources across the forest fringe and more exposed to temperature stresses (section “Mānawa: The New Zealand Mangrove”) due to greater distance to the water edge, only become commonplace as the gaps are infilled further into the forest.
5 Carbon Dynamics of Expanding Mangroves
Mangrove ecosystems are thought to account for as much as 14% of carbon sequestration by the global ocean (Alongi 2014). Primary production in mangrove ecosystems typically decreases with increasing distance from the equator (Bouillon et al. 2008). However, even at the limits of mangrove distribution, primary production in New Zealand mangroves remains high, estimated at 4–10 tonnes C ha−1 year−1 based on litter fall rates of 3.24–8.1 tonnes dry weight ha−1 year−1 (Gladstone-Gallagher et al. 2014; Woodroffe 1982), biomass carbon concentrations (Bulmer et al. 2016a, b), and established relationships between litter fall and wood and root production (Bouillon et al. 2008). As mangroves in New Zealand are estimated to cover 26,050 ha (Morrisey et al. 2010), this rate equates to 104,000–260,000 tonnes of carbon produced each year within New Zealand mangrove ecosystems. High rates of primary production in combination with unique sediment conditions, such as anoxia which slows the breakdown of carbon (Fig. 2.10), result in carbon burial rates in New Zealand mangroves of 0.67 tonnes C ha−1 year−1 (Pérez et al. 2017). The accumulated carbon stocks within New Zealand mangrove ecosystems are estimated at 117 tonnes C ha−1, including above and belowground biomass, and carbon within the sediment up to 1 m below the surface (Bulmer et al. 2016b).
Carbon cycling in a mangrove ecosystem, after Bulmer et al. (2017b)
The high stocks of carbon within New Zealand mangrove sediments also result in high rates of sediment CO2 efflux (average of 168.5 ± 45.8 mmol m−2 day−1 (Bulmer et al. 2015)) relative to habitats containing lower organic carbon stocks, such as sandflat ecosystems where sediment CO2 effluxes are close to zero (Bulmer et al. 2017b). Sediment CO2 efflux from mangrove ecosystems (Fig. 2.10) is primarily derived from CO2 released through root/mycorrhizae respiration and microbial respiration associated with the decomposition of organic matter (Bouillon et al. 2008). Other pathways for carbon losses from mangrove ecosystems include the emission of other greenhouse gasses such as CH4, export of dissolved carbon from the sediment to the water column, and the export of particulate organic carbon from litter and other detritus (Bouillon et al. 2008).
Mangroves in New Zealand are primarily expanding over adjacent unvegetated mudflats, which often used to have a sandier substrate prior to the rapid infilling and muddification of the estuaries (see section “Mangrove Expansion in New Zealand’s Anthropocene”). Consequently, mangrove expansion in New Zealand is not only associated with enhanced carbon stocks, but also an increase in sediment CO2 efflux from the mangrove ecosystems compared to the original sandier ecosystems (Fig. 2.10). However, it is important to note that when carbon losses associated with the CO2 efflux are subtracted from carbon stocks stored within tree biomass and buried within the sediment, expanding mangrove ecosystems still provide a net gain in carbon stocks (Pérez et al. 2017). Taking account of these factors, expansion of New Zealand mangroves has been estimated to result in an increase in carbon stocks by 79 tonnes C ha−1 (Bulmer et al. 2016b). This gain in carbon stocks following mangrove expansion is not immediate, rather it is estimated to occur over decade to century time scales, based on carbon accrual rates in sediments of Avicennia marina forests of 0.67 tonnes C ha−1 year−1 (Pérez et al. 2017). As mangrove ecosystems in New Zealand have been estimated to be growing in extent by 1068 ha−1 year−1 (Morrisey et al. 2010), mangrove expansion is estimated to result in an enhanced storage of 716 tonnes carbon per annum.
6 Ecosystem (Dis-)services of Expanding Mangroves
The expansion of mangroves in New Zealand initiates change to the ecosystem services delivered by the mangroves and their adjacent estuarine and coastal habitats. The enhanced capacity for carbon storage was described in section “Carbon Dynamics of Expanding Mangroves”, and the coastal protection and erosion mitigation benefits of expanding mangroves were addressed in section “Biophysical Interactions”. In addition to improving coastal stability, the retention of fine sediments within the mangroves reduces the risk of detrimental levels of siltation across sensitive coastal habitats located in proximity to the mangroves (Fig. 2.11). Moreover, water flushing through the mangroves is ‘purified’, with nutrients and heavy metals being adsorbed to the sediments and becoming trapped in the mangrove ‘sink’ (Barbier et al. 2011; Ewel et al. 1998). In this section we discuss the aspects of (mostly) biological ecosystem functioning that may be positively or negatively impacted as a consequence of mangrove expansion.
Globally, mangroves are considered to be an important ecological habitat, through the provision of safe haven, habitat and food for juvenile stages of numerous fish, and a diversity of crustaceans and terrestrial species (Claudino et al. 2015; Gladstone-Gallagher et al. 2016; Nagelkerken et al. 2008). However, studies of mangrove productivity and related ecosystem services are predominantly conducted in tropical settings, or where extensive muddy coastlines or deltas support vast mangrove forests (Lee et al. 2014; Nagelkerken et al. 2008). The local climate and geomorphological setting (cf. section “Mangroves in New Zealand”) will influence the overall productivity and ecosystem services that a particular mangrove forest can provide (Ewel et al. 1998; Lee et al. 2014). New Zealand mangroves survive in a temperate climate, are often found in the upper reaches of estuaries and harbours, and are often relatively young, recently colonised forests (see section “Mangroves in New Zealand”). Therefore, the ecosystem services these forests provide may differ from those reported for mature tropical species.
There is no marine or estuarine fauna that is unique to mangroves in New Zealand (Morrisey et al. 2010). However, the habitat still supports numerous invertebrate and vertebrate species (Alfaro 2006; Crisp et al. 1990), including a diversity of terrestrial species (Doyle and Hogg 2015). Similarities exist between temperate and tropical mangrove ecosystems in the broad functional groups that are represented. Most mangroves, in the tropics as well as at higher latitudes, host a range of algal and microbial communities. Epiphytic algae adhere to the mangrove roots, stems and sediments and provide nutrient dense food to grazing snails (Morrisey et al. 2010). Additionally, benthic macrofaunal communities in temperate mangroves consist of a diversity of burrowing infaunal polychaetes and oligochaetes, gastropods, small crustaceans and crabs (Morrisey et al. 2003).
A review of fish use of temperate mangrove systems suggests that fish are occasionally found in the mangroves, most commonly at the edge of mangrove habitats near deeper channels (Sheaves et al. 2016). Species found to visit mangrove habitats in New Zealand are abundant fish species such as mullet, pilchards, eel, triplefins and parore (Morrisey et al. 2010). Diet studies suggest most fish observed in New Zealand mangroves are feeding from the water column rather than deriving trophic benefits from mangrove ecosystems (Lowe 2013). Migration of larvae and juvenile fish in New Zealand mangroves is limited compared to tropical mangrove systems where multiple tree species provide a complexity of submerged root structures, some of which are deeply or even permanently inundated, allowing for easy fish migration. Many New Zealand mangrove forests, in comparison, are only submerged by the tide for a few hours each tidal cycle and with water depths generally less than 0.3 m (Lundquist et al. 2017). There is, however, site specific variability of tidal inundation depth and exposure throughout the mangrove forests in New Zealand. For example, some fringing mangroves positioned at lower tidal elevations (see Fig. 2.7) can be exposed to inundation depths of over a meter.
The rapid expansion of New Zealand mangroves is also associated with a decline of some coastal ecosystem services, particularly with respect to societal and cultural values associated with the use of coastal habitats. Much of the mangrove expansion has occurred in the past 50 years (see section “Mangrove Expansion in New Zealand’s Anthropocene”), within the living memory of New Zealanders who reside at and interact with the coast. As such, this notable change in the coastal landscape is often perceived to be detrimental to the ‘health’ of the estuary as it used to be (Harty 2009).
Rapid infilling and the associated mangrove expansion in New Zealand estuaries are at the basis of widespread concerns about the impacts on estuarine habitats (Fig. 2.12), such as a loss of seagrass meadows, a decline of wading and roosting grounds for birds, and a reduced variety and abundance of shellfish (Ellis et al. 2004; Harty 2009; Jones 2008; Lundquist et al. 2014b; Saintilan 2004). An increase in muddier, mangrove dominated habitat is typically associated with a change in macrofaunal communities, usually resulting in declines in bivalve abundance and increases in mud tolerant, opportunistic polychaete species (Ellis et al. 2004). The low bivalve abundance and high mud content of the surface sediment within mangrove ecosystems suggest that mangrove expansion will also be associated with reduced fluxes of inorganic nutrients between the sediment and the water column, and a decline in the ecosystem value of this service (Bulmer et al. 2017b). It is important to note that declines in shellfish beds would likely occur regardless of the presence of mangroves, driven by the increased mud content within estuarine habitats due to accelerated sedimentation (Robertson et al. 2015; Thrush et al. 2004).
The broader implications of the increase in muddy, mangrove dominated intertidal areas on estuarine ecosystem functions are difficult to predict. However, the increasing homogeneity of estuarine habitats has been associated with a reduction in ecosystem resilience and an increased likelihood of profound and unpredictable changes to ecosystem functioning (Thrush et al. 2004). A key functional difference between mangrove ecosystems and bare tidal flats, apart from their carbon storage capacity (see section “Carbon Dynamics of Expanding Mangroves”), is the increased potential for nutrient redistribution within mangrove habitat. Biogeochemical processes such as nutrient absorption by mangrove roots or litter fall (Bouillon et al. 2008; Gladstone-Gallagher et al. 2014) are independent of direct sediment-water column exchange. Mangrove ecosystem expansion is also likely to be associated with an increase in the contribution of mangrove derived carbon and nutrients within estuarine food-webs, due to the increased mangrove biomass and associated detritus. These additional pathways may build resilience back into the system which was lost due to an increase in the sediment mud fraction.
7 Mangrove Removal in New Zealand
7.1 Overview of Recent Mangrove Expansion
Mangrove expansion rates (reviewed in section “Mangrove Expansion in New Zealand’s Anthropocene”) vary both within and between estuaries. Historically, mangroves were more commonly distributed around creeks incising the upper intertidal flats, often in areas far away from residential areas (Lundquist et al. 2017). However, increased deposition associated with estuarine infilling, and reduced tidal flows due to infrastructure development (e.g. causeways and marinas), have resulted in expansion of intertidal flats. This allows mangroves to establish in locations where they do conflict with other values such as recreational and navigational access and ocean views (Fig. 2.12). Concomitant declines in other estuarine habitats (e.g seagrass meadows, shellfish beds) are caused by increases in sediment supply and deposition, and not, as commonly perceived by the public, by invasion of mangroves into these habitats (Thrush et al. 2004). Regardless, the perceived relationship between mangrove expansion and the decline of other intertidal estuarine habitats feeds the assumption that removal of mangroves will result in the restoration of other estuarine habitats and the values associated with these habitats (Harty 2009).
7.2 Mangrove Removal Policies in New Zealand
In response to mangrove expansion and perceived correlation with declines in estuarine ecosystem services , many community groups—estuary care groups—were established to support estuary restoration initiatives. These community groups were often focussing on mangrove removal only, with objectives of restoring historical ecological balances between mangroves and unvegetated sandflat habitats. Consequently, the majority of early mangrove removals (1990s and 2000s) were small community initiatives, using manual removal methods. The first mechanical removal consent was approved in 2010, using a wide-tracking mechanical digger to clear 100 ha of mangroves in the sub-estuaries of Tauranga Harbour (Lundquist et al. 2012). Motivations for mangrove removal include improving recreational and amenity values; restoring ecological habitat such as sandflats, seagrass meadows and shellfish beds; restoring access and navigation; maintenance and use of coastal infrastructure; improving the function of drainage systems; and flood protection.
Mangrove removal policies and consent processes differ between the four regional councils (regional authorities charged with managing natural and physical resources) in whose jurisdiction mangroves are found in northern New Zealand. Overarching national legislation guiding council mangrove removal policies includes the New Zealand Coastal Policy Statement (NZCPS) and the Resource Management Act (RMA). Policy 11 of the NZCPS includes provisions to “avoid significant adverse effects and avoid, remedy or mitigate other adverse effects of activities on indigenous ecosystems and habitats that are only found in the coastal environment and are particularly vulnerable to modification, including estuaries, lagoons, coastal wetlands , dunelands, intertidal zones , rocky reef systems, eelgrass and saltmarsh .” Further guidance is provided by section 12 of the RMA which states that “no person may, in the coastal marine area, destroy, damage or disturb the foreshore or seabed in a manner that has or is likely to have an adverse effect on the foreshore or seabed, or on plants or animals or their habitat, unless expressly allowed by a rule in a regional coastal plan or a resource consent.”
Most councils allow some provision for non-notified mangrove removal to support public safety, coastal access, and maintenance of infrastructure (e.g. clearing for drainage; preventing tree growth from blocking roads and motorways). Policies for the removal of mangrove seedlings, intended to limit further mangrove expansion, vary across councils; for example, Auckland Council allows manual removal of single stem, non-reproductive seedlings less than 60 cm in height, with formal council notification required if the total area cleared is greater than 30 m2. Removal of adult, reproductive mangrove trees typically does not comply with most regional coastal policies, therefore requiring application for resource consent which often results in contentious Environment Court proceedings to assess whether adverse impacts are likely to occur, and if the objectives of consents are likely to be achieved. Regular reviews occur of these regional coastal plans, with the latest Auckland regional plan being finalised in 2017. Initial proposed planning conditions for the Auckland region allowed mangrove removal, in response to community desire to remove mangroves; however, these permissive conditions were removed during plan revisions in response to public submissions and a committee hearings process, which reflected on mounting evidence supporting best practice methods and likelihood of success of mangrove removals.
7.3 Mangrove Removal Strategies
The two primary methods for mangrove clearance are manual methods typically using chainsaws, and mechanical clearance of aboveground biomass , most commonly using a digger with a shovel/rake attachment (Fig. 2.13). The use of mechanical diggers is associated with greater sediment disturbance than hand clearance, as sediment is compacted in mechanical tracks and the sediment column mixed due to digger/rake activity (Fig. 2.14), with anoxic sediment commonly present on the sediment surface after application (Lundquist et al. 2012, 2017). Cleared aboveground biomass is sometimes removed from the site, but mulching of aboveground biomass and leaving mulch in situ has also occurred. These approaches have been associated with adverse impacts as mulched biomass smothers the sediment and disrupts sediment chemistry, resulting in nutrient release and associated macroalgal blooms (Lundquist et al. 2012). More recent mechanical trials have shown reduced disturbance, by limiting the total area tracked by the digger (Fig. 2.14; Bulmer and Lundquist 2016). Below-ground biomass is generally left in situ, due to both expense and difficulties in removing it without creating substantial sediment disturbance (Alfaro 2010; Lundquist et al. 2012, 2014a; Stokes 2008). Seedling removal is the method of mangrove removals with the least impact, although regular seedling removal is required at most locations to keep them from being recolonised (Lundquist et al. 2017).
Impacts of mechanical mangrove clearance: (a) tracking disturbance from mechanical mangrove clearance at Tauranga (Photo credits: C. Lundquist), and (b) reduced impacts of mechanical clearance at Whangamata through minimisation of tracking to 10–15% of sediment surface (Photo credits: Waikato Regional Council)
7.4 Removal ‘Success’ Rates
The aim of many clearance operations is to restore sandflats that may have been historically present (Harty 2009). However, there is little scientific evidence to suggest that a loss of mud and return to a sandflat system will occur following mangrove clearance (Alfaro 2010; Lundquist et al. 2012, 2014a; Stokes 2008). The conclusions on the impacts of mangrove clearance are further limited due to the lack of baseline measurements prior to mangrove clearance in most published studies (Alfaro 2010). Most early mangrove removals in New Zealand anticipated an immediate return to sandflats, but had limited or no monitoring to demonstrate whether they were successful at achieving their objectives (Alfaro 2010). Furthermore, removals using new methodologies (e.g. mechanical mulching) often lacked adequate trials of these methods to determine if adverse impacts were likely to occur (Lundquist et al. 2012; Stokes et al. 2016). Nonetheless, two large scale removals have been subjected to long-term monitoring, either through associated government funded scientific research, in Tauranga Harbour (Lundquist et al. 2012), or through consent requirements, in Whangamata Harbour (Bulmer et al. 2017a). The Tauranga monitoring assessed removals at three sites in each of two sub-estuaries after removal of 110 ha of mangroves using mechanical mulching with in situ disposal. Monitoring over 5 years showed consistent responses of degraded macrofaunal communities, anoxic sediments, low rates of sediment erosion, and persistence of mulched aboveground biomass that was left in situ, and was further associated with extensive macroalgal blooms (Lundquist et al. 2012, Lundquist unpublished data). The large-scale mechanical removals consented in Whangamata used either burn piles or offsite disposal of aboveground vegetation biomass (Bulmer and Lundquist 2016). A trial and adaptive management process was included in the consent conditions (Bulmer et al. 2017a) to allow comparison and selection of mechanical (or non-mechanical) methods that provided the least risk of adverse impacts and the most rapid recovery trajectory toward sandflat conditions (Bulmer et al. 2017a).
One additional study analysed the status of ~40 mangrove removal sites on the east coast, from Whangarei to Tauranga (Lundquist et al. 2014a). This study showed that most mangrove removals occurred at sites that had already been impacted by changes in sediment characteristics prior to or beyond the observed mangrove expansion. Sites which had adjacent tidal flats with high mud content were unlikely to have sufficiently energetic hydrodynamic conditions to result in rapid erosion of fine sediments and associated decomposition of belowground mangrove biomass. Sandier, more exposed removal sites showed some change toward sandier unvegetated states and macrofaunal communities associated with these sandy sediments, though these sandier removal sites accounted for only a limited proportion of the total areas where mangroves had been removed (Lundquist et al. 2014a, Lundquist unpublished data).
Comparisons between removal methods suggest that mechanical methods are typically associated with larger immediate adverse impacts and longer timeframes before sediment erosion occurs. Comparison of sizes and shapes of removals suggest that removals of alongshore narrow strips of vegetation (<30 m in cross-shore width) on seaward sides of forests were most likely to trend toward sandflat characteristics (Bulmer and Lundquist 2016). Larger or shoreward removals were associated with degraded conditions except at the seaward edges. Cleared narrow strips perpendicular to the shore, a common strategy to create recreational access through mangrove forest, were rapidly recolonised by seeds from adjacent forest, and required regular seedling removal to maintain access (Lundquist et al. 2017).
Generally poor success rates of mangrove removals to date have influenced multiple Environment Court decisions on regulatory policies for mangrove removal, usually resulting in more stringent rather than permissive requirements for removals, and often requiring site-specific assessments for any consents for removing adult mangroves.
8 The Known and Unknown Effects of Mangrove Removal
8.1 Effects of Mangrove Clearance on Estuarine Morphodynamics
Morphodynamic changes following mangrove clearance are influenced by a range of environmental factors, including the hydrodynamic characteristics of the site, the size of the area cleared, and the pre-existing sediment conditions (further discussed in section “Effects of Mangrove Clearance on Sediment Composition”). A cleared estuarine site positioned in proximity to either a tidal channel or the ocean entrance is more likely to experience surface erosion after mangrove removal, as waves and tidal currents are no longer attenuated (see section “Biophysical Dynamics of Expanding Mangroves”). Strong tidal currents, particularly flood flows, may be responsible for rapid scour after mangrove clearance, or conversely, may introduce fresh terrestrial sediments to the intertidal. Engineering works such as the construction of causeways or revetments, whether undertaken prior or post mangrove clearance, may induce further morphodynamic change. These solid obstructions may result in reduced flushing potential or enhanced erosion by the reflection of incoming waves (Winterwerp et al. 2013).
The capacity of mangroves to trap sediments is well established (Furukawa and Wolanski 1996; Furukawa et al. 1997; Horstman et al. 2015; van Santen et al. 2007). The vertical elevation, or surface elevation, of a mangrove forest can increase by a combination of sediment accretion, root production (and decomposition) and groundwater influx (Krauss et al. 2014). The capacity of mangroves to adapt and survive in a changing environment is contingent upon the rates of change of both mangrove surface elevation and sea-level rise (Lovelock et al. 2015; Webb et al. 2013). However, with the felling of aboveground mangrove vegetation, both the sediment trapping capacity of the site and the production of root material are removed. The resulting combination of sediment flushing, sediment compaction and the structural collapse of the remaining root material will likely produce a significant deflation of the substrate and a loss of surface elevation. Only one study has addressed this process in New Zealand, where surface elevation was reported to fall up to 38 mm year−1 in the first 2 years following mangrove clearance (Stokes et al. 2010). Elsewhere, a tropical site in Belize was assessed 1 and 2 years following the clearing of Rhizophora mangroves that fringed a small island. Here, the rate of elevation loss was spatially variable, ranging from 2 to 20 mm after 2 years (Hayden and Granek 2015). Similar low rates of elevation loss have been reported where mangroves were destroyed in catastrophic hurricane conditions (Cahoon et al. 2003). In southern Australia, sediment compaction and changed groundwater conditions have been linked to a similar geomorphological change (Rogers et al. 2014).
Regardless of the driving process , the abovementioned observations imply that mangrove clearance results in a rising relative sea level, which then may impact on adjacent saltmarsh habitats or other land use functions (Rogers et al. 2014; Saintilan and Rogers 2013). Exposed shorelines are also likely to become more vulnerable to erosion, especially if the localised rise in relative sea level is exacerbated by predicted global sea-level rise (Hayden and Granek 2015).
8.2 Effects of Mangrove Clearance on Sediment Composition
The relationship between mangrove expansion and the higher mud content of intertidal areas is one of the reasons for mangrove removal in New Zealand (sections “Ecosystem (Dis-)services of Expanding Mangroves” and “Mangrove Removal in New Zealand”). Sediment conditions prior to clearance can be characterised by the sediment texture (i.e. grain size distribution), the relative contribution of dissolved and visible organic matter , the amount of structural and fine root material, the density of algae and sediment nutrient loads. Importantly, these sediment conditions may, or may not, change with depth. An aspect of ‘recovery’ after mangrove removal is the change in these sedimentological characteristics towards a sand-dominated system which is more representative of the conditions that typically preceded the phase of rapid estuarine infilling and mangrove expansion. Benthic faunal community composition is linked to the sediment mud fraction (Robertson et al. 2015), with most bivalve species sensitive to increased levels of silt and clay. As such, mud content is often reported as an index of recovery of benthic fauna as well. Other sediment metrics are typically measured alongside grain size: organic matter content (Fig. 2.15c) and chlorophyll a content typically show higher values in a mangrove habitat compared to adjoining bare sandflats (see data presented in Fig. 2.7). Organic content in particular is correlated with the mud content, and so temporal shifts tend to mirror textural changes (e.g. Grellier et al. 2017).
Post mangrove clearance sediment condition in the Waikaraka estuary (a sub-estuary within the Tauranga Harbour): (a, b) sediment cores collected 2 years after the clearing are still predominantly muddy; (c) the remaining root material from the core in panel (b) separated into structural roots and fine roots (left to right); and (d) the soft sediments of the cleared zone (Photo credits D. Stokes)
In locations where repeated monitoring has been a requirement for mangrove removal resource consents, changes in sediment composition are reported over 3 years or less. However, the comprehensive assessment of removal sites, undertaken by Lundquist et al. (2014a), reported up to decadal recovery timeframes. Generally, post-clearance mud contents were found to decrease more rapidly at sites that exhibited a higher sand content prior to mangrove clearance, associated with a greater exposure of such sites to tidal currents and wind waves. Conversely, more protected sites showed limited release of the sediment’s mud content, even after 5 years.
Stokes and Harris (2015) monitored a non-consented clearing in Whangamata Harbour at 3, 4 and 6 years post-removal, and described a considerable coarsening of the surface sediment between 3 and 4 years post-clearance. The delay in this textural change may be linked to the slow rate of root decomposition. It was speculated that the dense mangrove roots helped to hold the sediments until decomposition reached a point where the hydrodynamics of the site overrode the declining holding capacity of the root matrix. The timeframe sits within model predictions developed by Gladstone-Gallagher et al. (2014), showing that buried Avicennia marina var. australasica wood and pneumatophores decompose to half their original weight between 317 and 613 days. A broader examination of root biomass across ~40 mangrove removal sites indicated that belowground biomass may persist for as long as 16 years after mangrove removal (Lundquist et al. 2014a, Lundquist unpublished data). In situ decomposition experiments also reported up to decadal delays in decomposition of belowground biomass, associated with low rates of sediment erosion and surface elevation change at most mangrove removal sites in New Zealand (Gladstone-Gallagher et al. 2014; Lundquist et al. 2014a).
Although the rate at which silts and clays are removed from the sediment surface and redistributed is variable after mangrove removal, the abovementioned work demonstrates that generally some textural coarsening is observed after a number of years. It is possible, however, that any textural changes to the surface may mask persistence of muddy material at depth (Fig. 2.15). Quantitative assessments of sediment characteristics below the surface are rare, although these assessments should be undertaken as (i) benthic faunal composition may not shift toward a typical sandflat community if mud remains at depths similar to those where filter feeding bivalves dwell, and (ii) the desired firming up of the substrate may not be realised in the short term.
‘Sand armouring’ has been observed following mangrove clearance in Whangamata Harbour (Stokes and Harris 2015), where a 5 mm sand cap developed between two monitoring surveys at 3 and 6 years after clearance. The sand cap increased the erosion threshold of the substrate, reducing the sediment’s mobility under the typical hydrodynamic conditions of the estuary. This finding implies that continued ‘recovery’ would require stronger than normal tidal currents or wave forces for any further entrainment and redistribution of sediments to be realised. Sandy silts were observed beneath the surface sand lens, although the grain size coarsened to a silty sand within the upper 20–60 cm. The depth of mangrove muds may have been limited at this site due to the relative immaturity of the mangroves that were cleared. In comparison, a recent assessment of changing soil characteristics following mangrove clearance in Vietnam found that sediment properties evolved to a depth of 35 cm as a result of the clearing activity. The mud and sediment organic carbon content decreased by >65% in the 2 years following mangrove removal, with a variable mud content of <80% from the surface to around 30 cm depth, after which both the cleared and intact mangrove sediment cores presented similar mud content of >90% (Grellier et al. 2017).
Surface sediments may evolve to reflect sandflat conditions , but buried sediment may not mirror the textural conditions at the surface. Nevertheless, sediment core profiles are rarely included in the assessment of mangrove clearance impacts, despite the potential of these sediments at depth to limit faunal recolonization. Further investigation into the temporal changes of the sediments below the surface is required. A cleared site must be exposed to sufficiently strong tidal flows to facilitate the on-going removal and redistribution of muddy sediments. Consequently, recovery may be impeded by low-energy hydrodynamic conditions, and also by continued supplies of terrigenous muddy sediment, particularly where catchments and riparian zones are still poorly managed. Indeed, there may be some locations that will not realise substantial restoration. Monitoring changes in both surface and deeper sediments is therefore a requirement to avoid unjustified conclusions regarding the ‘success’ of the mangrove removal in terms of the return to a sandier system that could support greater abundance and diversity of intertidal macrofauna (Stokes et al. 2016).
8.3 Effects of Mangrove Clearance on Carbon Dynamics
It is estimated that mangrove deforestation generates as much as 10% of carbon emissions from deforestation globally, yet mangrove ecosystems account for only 0.7% of the deforested area (Donato et al. 2011). Not only does mangrove clearance result in a loss of mangrove primary production, estimated at 4–10 tonnes carbon per year for New Zealand (derived from Bouillon et al. 2008; Bulmer et al. 2016a, b; Gladstone-Gallagher et al. 2014; Woodroffe 1982), mangrove clearance also results in a loss of estuarine carbon stocks, estimated at 79 tonnes carbon per hectare of mangrove cleared (Bulmer et al. 2016b). Rather than a rapid loss of carbon immediately following mangrove clearance, this loss is expected to occur over a number of years to decades following clearance due to the slow decomposition of organic matter within New Zealand mangrove sediments (Bulmer et al. 2015, 2017b; Gladstone-Gallagher et al. 2014).
As the majority (>90%) of carbon stocks within mangrove ecosystems are stored belowground in the sediment (Bulmer et al. 2016b), one of the primary pathways for carbon export from mangrove ecosystems following mangrove clearance is via sediment CO2 efflux (Bouillon et al. 2008). The average rate of sediment CO2 efflux from mangrove clearance sites throughout New Zealand is 133.9 ± 37.2 mmol m−2 day−1, which is comparable to rates observed in cleared mangrove sites in the tropics (Bulmer et al. 2015). The rate of sediment CO2 efflux from cleared mangroves varies based on a number of factors, such as time since clearance (Grellier et al. 2017; Lovelock et al. 2011), the organic matter content of the sediment column, as well as clearance methodology (Bulmer et al. 2015, 2017b). For example, mechanical mulching and dispersal of aboveground biomass in situ has been associated with sediment CO2 efflux seven times higher than that of smaller hand clearances where aboveground biomass was removed from the site (Bulmer et al. 2015). Nonetheless, the loss of carbon stocks and carbon emissions associated with mangrove removal is not currently considered in consent applications for mangrove clearance.
8.4 Effects of Mangrove Clearance on Other Ecosystem Services
Many of the expected benefits of mangrove clearance rely on a transition (or return) to a previous state of the estuarine system when sandflats were still a common feature. This return should be resulting from the erosion of muddy sediments and erosion or decomposition of belowground biomass post mangrove clearance (see section “Effects of Mangrove Clearance on Sediment Composition”). If clearance sites do return to sandflat characteristics, the associated changes in macrofaunal community structure are likely to support an increase in macrofaunal diversity and abundance and associated ecosystem functioning (Thrush et al. 2004). Nevertheless, regardless of whether mangrove ecosystems are expanding or are being cleared, New Zealand faces a fundamental problem with regard to high levels of terrigenous fine sediment inputs and enhanced sedimentation impacting estuarine habitats and reducing many of the ecosystem services provided (Thrush et al. 2004). As increased mud content and loss of macrofauna is driving many of the declines in ecosystem functioning faced in New Zealand estuaries (Thrush et al. 2004), mangrove clearance appears unlikely to increase provisioning of these ecosystem services (see also section “Ecosystem (Dis-)services of Expanding Mangroves”). Rather, the infilling of estuaries that facilitates mangrove expansion and degrades other estuarine habitats, is a long lasting geomorphological process and clearance sites remain comparable to intact mangrove ecosystems many years after clearance (in regards to sediment conditions, macrofaunal communities and carbon and nutrient cycling; as discussed in section “Effects of Mangrove Clearance on Sediment Composition”). Holistic catchment management approaches that reduce sediment loads into estuaries should thus be an integral part of both mangrove management and restoration of estuarine ecosystems (Lundquist et al. 2017).
Community group perceptions of the effects of mangrove clearance on the restoration of ecosystem services are generally positive, reflecting restoration of ocean views through removal of vegetation, and in some cases, successful restoration of recreational access through clearing of channels through the mangroves that support boat or kayak access. However, these benefits should be balanced with losses of other ecosystem services that benefit humans, such as carbon sequestration (section “Effects of Mangrove Clearance on Carbon Dynamics”) and nutrient storage (Bulmer et al. 2017b), flood protection, and buffers against coastal erosion (section “Biophysical Interactions”). Removal of the latter function of mangroves has resulted in visible erosion of neighbouring saltmarsh vegetation at a number of mangrove removal sites (Fig. 2.16).
Initial concerns that mangrove removals might have negative downstream impacts (through sediment erosion and subsequent deposition) on neighbouring seagrass meadows and shellfish beds have not eventuated (Lundquist et al. 2012). However, mangrove removals have also not been associated with the return of previously abundant seagrass and shellfish beds, suggesting that further changes in catchment management and reductions in sediment loads are required to benefit these habitats, whose declines were unlikely to be caused by mangrove expansion. Further study is required to assess concerns that mangrove removals could have negative impacts on shorebirds that utilise mangrove habitats for roosting, foraging, or as protection from predators, as limited information collected at Whangamata suggested that there were no impacts of removals on shorebird populations (Wildlands 2014). To further avoid impacts on bird populations, consent conditions typically require mangrove removals to occur at times that are unlikely to disturb roosting or nesting seasons.
9 Synthesis: Expanding Mangroves and the Future
The complexity of the interlinked biophysical processes in mangroves, as discussed in section “Biophysical Dynamics of Expanding Mangroves”, means that the outlook for New Zealand mangroves with future changes in climate and physical forcings is not well known. Nonetheless, several key drivers of change can be identified: sea-level rise , change in weather patterns and alterations to sediment supply.
Sea-level rise can be difficult to assess in a geologically active area such as New Zealand, as estimations of (relative) vertical elevation changes need to include vertical landmass movements (Swales et al. 2016). Moreover, in other locations, responses of mangroves to sea-level rise have been shown to be highly site specific (Lovelock et al. 2015). Although inundation is increased under higher sea levels, which may imply drowning of mangrove forests (Gilman et al. 2006; Ward et al. 2016), a higher sea level also leads to a greater tidal prism, which can potentially support larger waves and stronger tidal currents with the ability to resuspend more sediment, enhancing inward sediment transport (Green and Coco 2007; Horstman et al. 2015). Additionally, mangrove forests can also accrete through below-ground biomass production (Kirwan and Megonigal 2013). Indeed, forests in regions with ample sediment supply have shown to possess the ability to accrete vertically to keep pace with sea-level rise (Krauss et al. 2014). A similar resilience could be expected for New Zealand mangroves, provided local sediment supplies into the estuaries remain high.
Mangrove root architecture and hence also sediment trapping ability has been found to depend on salinity (Krauss et al. 2014), and therefore changes in rainfall may lead to a changing mangrove distribution, particularly at their upstream extent on riverbanks. Changes in the frequency and intensity of storms may further enhance sediment delivery events (see section “Mangrove Expansion in New Zealand’s Anthropocene”), but conversely, may reduce ‘windows of opportunity’ in which mangroves can expand (see section “Succession of Expanding Mangrove Systems”). Moreover, an excess of sediment supply can lead to burial and death of mangroves (Sidik et al. 2016). Hydrodynamic changes can also affect sediment storage and supply in offshore shelf regions (Liu et al. 2017; Nittrouer et al. 2017), indirectly affecting sediment supply to the mangroves.
Lastly, human interventions provide further unknowns. Activities such as damming reduce sediment supply and may eventually lead to a sediment starved system lacking the resilience to cope with sea-level rise (Allison et al. 2017; Horstman et al. 2015; McBride et al. 2016; Syvitski et al. 2005; Willemsen et al. 2016). Construction of landward boundaries such as dikes and barriers limits tidal fluxes of water and sediments (Willemsen et al. 2016; Winterwerp et al. 2005) and also restricts landward movement of the forest, the latter causing coastal squeeze (Lovelock et al. 2015; Mills et al. 2016). Nevertheless, despite these uncertainties, it is thought that New Zealand’s temperate and sediment rich mangroves will be more resilient than tropical mangrove forests (Alongi 2008; Schaeffer-Novelli et al. 2002).
The capacity of New Zealand mangroves to mitigate erosion and to raise surface elevations provides these coastal ecosystems with an intrinsic resilience, potentially enabling them to combat land subsidence and (relative) sea-level rise (McBride et al. 2016). For example, in the Firth of Thames bed elevations inside the mangroves (Fig. 2.7a) have accreted to an elevation of more than a meter above the elevation of the land directly behind the stop bank that separates the mangroves from the subsiding Hauraki Plains (Horstman unpublished data). This mangrove forest has a width of more than a kilometre, and has been observed to reduce tidal water level fluctuations and storm surges, thereby reducing the intrusion of saline (ground)water and enhancing the protection provided by the stop bank. This forest is a typical example of a coastal ecosystem defence, where ecological engineering (by the mangroves) benefits the safety and quality of the coastal zone (Borsje et al. 2011; Bouma et al. 2014; Temmerman et al. 2013).
Regulating ecosystem services, such as the contribution of mangroves to coastal defence (section “Biophysical Dynamics of Expanding Mangroves”) and carbon sequestration (section “Carbon Dynamics of Expanding Mangroves”), together with other provisioning ecosystem services (as discussed in section “Ecosystem (Dis-)services of Expanding Mangroves”), are the motivation behind the global surge in mangrove restoration and management efforts (Bosire et al. 2008; Gilman and Ellison 2007; Hashim et al. 2010; Lewis 2005). New Zealand mangroves do not at present suffer from reduced sediment inputs or other physical stressors (apart from the temperature limitation) and therefore these systems provide a useful example illustrating the biophysical interactions and succession dynamics of expanding mangrove ecosystems, as discussed in sections “Biophysical Dynamics of Expanding Mangroves” and “Succession of Expanding Mangrove Systems”.
In New Zealand, however, mangrove expansion is associated with a negative connotation, owing to the reduced ecosystem value of temperate mangroves compared to their tropical equivalent, as well as the change of intertidal ecosystems and the loss of amenity values associated with mangrove expansion. The consequent tendency towards mangrove removal, following these negative impacts, has partly been due to un- or misinformed decision making (as discussed in section “Mangrove Removal in New Zealand”) and a general lack of knowledge concerning the consequences of mangrove removal. However, with a growing body of knowledge related to mangrove removal, opportunities arise for integrated coastal zone management strategies carefully weighing both the disadvantages and the opportunities related to mangrove expansion. Planning such comprehensive coastal management strategies could benefit from models that allow the simulation of morphological changes in the coastal zone in relation to the establishment and succession of mangroves, also including related impacts on the biophysical interactions in the mangroves and the consequences for coastal stability and safety. A range of such interdisciplinary models exists for salt marshes (e.g. Kirwan and Murray 2007; Temmerman et al. 2005; van de Koppel et al. 2012), but for mangroves the development of these models is still in its infancy (Bryan et al. 2017; Horstman et al. 2015; van Maanen et al. 2015). As shown throughout this chapter, these processes form the basis of an active area of research, which will contribute towards integrated coastal zone management strategies incorporating the services provided by mangroves and honouring the traditional belief in kaitiakitanga—that if an ecosystem is sustained and cared for, it can then provide benefits back to humanity.
References
Alfaro AC (2006) Benthic macro-invertebrate community composition within a mangrove/seagrass estuary in northern New Zealand. Estuar Coast Shelf Sci 66(1–2):97–110. https://doi.org/10.1016/j.ecss.2005.07.024
Alfaro AC (2010) Effects of mangrove removal on benthic communities and sediment characteristics at Mangawhai Harbour, northern New Zealand. ICES J Mar Sci 67(6):1087–1104. https://doi.org/10.1093/icesjms/fsq034
Allison MA, Nittrouer CA, Ogston AS, Mullarney JC, Nguyen TT (2017) Sedimentation and survival of the Mekong delta: a case study of decreased sediment supply and accelerating rates of relative sea level rise. Oceanography 30(3):98–109. https://doi.org/10.5670/oceanog.2017.317
Alongi DM (2008) Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76(1):1–13. https://doi.org/10.1016/j.ecss.2007.08.024
Alongi DM (2009) Paradigm shifts in mangrove biology. In: GME P, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands: an integrated ecosystem approach. Elsevier, Amsterdam, pp 615–640
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6(1):195–219. https://doi.org/10.1146/annurev-marine-010213-135020
Augustinus PGEF (1995) Geomorphology and sedimentology of mangroves. In: GME P (ed) Developments in sedimentology, vol 53. Elsevier, Amsterdam, pp 333–357
Balke T, Bouma TJ, Herman PMJ, Horstman EM, Sudtongkong C, Webb EL (2013) Cross-shore gradients of physical disturbance in mangroves: implications for seedling establishment. Biogeosciences 10(8):5411–5419. https://doi.org/10.5194/bg-10-5411-2013
Balke T, Swales A, Lovelock CE, Herman PMJ, Bouma TJ (2015) Limits to seaward expansion of mangroves: translating physical disturbance mechanisms into seedling survival gradients. J Exp Mar Biol Ecol 467:16–25. https://doi.org/10.1016/j.jembe.2015.02.015
Barbier EB (2014) A global strategy for protecting vulnerable coastal populations. Science 345(6202):1250–1251. https://doi.org/10.1126/science.1254629
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81(2):169–193. https://doi.org/10.1890/10-1510.1
Beaglehole JC (1955) The journals of Captain James cook on his voyages of discovery. Hakluyt Society at the University Press, Cambridge, UK
Borsje BW, van Wesenbeeck BK, Dekker F, Paalvast P, Bouma TJ, van Katwijk MM, de Vries MB (2011) How ecological engineering can serve in coastal protection. Ecol Eng 37(2):113–122. https://doi.org/10.1016/j.ecoleng.2010.11.027
Bosire JO, Dahdouh-Guebas F, Kairo JG, Wartel S, Kazungu J, Koedam N (2006) Success rates of recruited tree species and their contribution to the structural development of reforested mangrove stands. Mar Ecol Prog Ser 325:85–91. https://doi.org/10.3354/meps325085
Bosire JO, Dahdouh-Guebas F, Walton M, Crona BI, Lewis RR III, Field C, Kairo JG, Koedam N (2008) Functionality of restored mangroves: a review. Aquat Bot 89(2):251–259
Bouillon S, Borges AV, Castañeda-Moya E, Diele K, Dittmar T, Duke NC, Kristensen E, Lee SY, Marchand C, Middelburg JJ, Rivera-Monroy VH, Smith TJ III, Twilley RR (2008) Mangrove production and carbon sinks: a revision of global budget estimates. Glob Biogeochem Cycles 22(2):GB2013
Bouma TJ, Van Duren LA, Temmerman S, Claverie T, Blanco-Garcia A, Ysebaert T, Herman PMJ (2007) Spatial flow and sedimentation patterns within patches of epibenthic structures: combining field, flume and modelling experiments. Cont Shelf Res 27:1020–1045. https://doi.org/10.1016/j.csr.2005.12.019
Bouma TJ, van Belzen J, Balke T, Zhu Z, Airoldi L, Blight AJ, Davies AJ, Galvan C, Hawkins SJ, Hoggart SPG, Lara JL, Losada IJ, Maza M, Ondiviela B, Skov MW, Strain EM, Thompson RC, Yang S, Zanuttigh B, Zhang L, Herman PMJ (2014) Identifying knowledge gaps hampering application of intertidal habitats in coastal protection: opportunities & steps to take. Coast Eng 87:147–157. https://doi.org/10.1016/j.coastaleng.2013.11.014
Bryan KR, Nardin W, Mullarney JC, Fagherazzi S (2017) The role of cross-shore tidal dynamics in controlling intertidal sediment exchange in mangroves in Cù Lao Dung, Vietnam. Cont Shelf Res 147:128–143. https://doi.org/10.1016/j.csr.2017.06.014
Bryce S, Larcombe P, Ridd PV (2003) Hydrodynamic and geomorphological controls on suspended sediment transport in mangrove creek systems, a case study: Cocoa Creek, Townsville, Australia. Estuar Coast Shelf Sci 56:415–431. https://doi.org/10.1016/s0272-7714(02)00192-0
Bulmer RH, Lundquist CJ (2016) Whangamata mangrove removal monitoring: summary of 36 month post-removal sampling, NIWA Client Report, Waikato Regional Council. National Institute of Water & Atmospheric Research Ltd., Hamilton
Bulmer RH, Lundquist CJ, Schwendenmann L (2015) Sediment properties and CO2 efflux from intact and cleared temperate mangrove forests. Biogeosciences 12(20):6169–6180. https://doi.org/10.5194/bg-12-6169-2015
Bulmer RH, Schwendenmann L, Lundquist CJ (2016a) Allometric models for estimating aboveground biomass, carbon and nitrogen stocks in temperate Avicennia marina forests. Wetlands:1–8. https://doi.org/10.1007/s13157-016-0793-0
Bulmer RH, Schwendenmann L, Lundquist CJ (2016b) Carbon and nitrogen stocks and below-ground allometry in temperate mangroves. Front Mar Sci 3 (150). doi:https://doi.org/10.3389/fmars.2016.00150
Bulmer RH, Lewis M, O’Donnell E, Lundquist CJ (2017a) Assessing mangrove clearance methods to minimise adverse impacts and maximise the potential to achieve restoration objectives. N Z J Mar Freshw Res 51(1):110–126. https://doi.org/10.1080/00288330.2016.1260605
Bulmer RH, Schwendenmann L, Lohrer A, Lundquist CJ (2017) Sediment carbon and nutrient fluxes from cleared and intact temperate mangrove ecosystems and adjacent sandflats. Sci Total Environ 599–600:1874–1884. https://doi.org/10.1016/j.scitotenv.2017.05.139
Burns BR, Ogden J (1985) The demography of the temperate mangrove [Avicennia marina (Forsk.) Vierh.] at its southern limit in New Zealand. Aust J Ecol 10(2):125–133. https://doi.org/10.1111/j.1442-9993.1985.tb00874.x
Cahoon DR, Hensel P, Rybczyk J, McKee KL, Proffitt CE, Perez BC (2003) Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after Hurricane Mitch. J Ecol 91(6):1093–1105. https://doi.org/10.1046/j.1365-2745.2003.00841.x
Chapman VJ (1976) Mangrove vegetation. Cramer, Vaduz
Chapman VJ (1977) Introduction. In: Chapman VJ (ed) Ecosystems of the world: wet coastal ecosystems. Elsevier, Amsterdam, pp 1–29
Chen Y, Li Y, Cai T, Thompson C, Li Y (2016) A comparison of biohydrodynamic interaction within mangrove and saltmarsh boundaries. Earth Surf Process Landf 41(13):1967–1979. https://doi.org/10.1002/esp.3964
Clarke PJ (2004) Effects of experimental canopy gaps on mangrove recruitment: lack of habitat partitioning may explain stand dominance. J Ecol 92(2):203–213. https://doi.org/10.1111/j.0022-0477.2004.00861.x
Claudino MC, Pessanha ALM, Araújo FG, Garcia AM (2015) Trophic connectivity and basal food sources sustaining tropical aquatic consumers along a mangrove to ocean gradient. Estuar Coast Shelf Sci 167(Part A):45–55. https://doi.org/10.1016/j.ecss.2015.07.005
Crisp P, Daniel L, Tortell P (1990) Mangroves in New Zealand – trees in the tide. GP Books, Wellington
Danielsen F, Sørensen MK, Olwig MF, Selvam V, Parish F, Burgess ND, Hiraishi T, Karunagaran VM, Rasmussen MS, Hansen LB, Quarto A, Suryadiputra N (2005) The Asian tsunami: a protective role for coastal vegetation. Science 310(5748):643. https://doi.org/10.1126/science.1118387
de Lange WP, de Lange PJ (1994) An appraisal of factors controlling the latitudinal distribution of mangrove (Avicannia marina var. resinifera) in New Zealand. J Coast Res 10(3):539–548
Dingwall PR (1984) Overcoming problems in the management of New Zealand mangrove forests. In: Teas HJ (ed) Physiology and management of mangroves. Dr W. Junk Publishers, The Hague, pp 97–106. https://doi.org/10.1007/978-94-009-6572-0_13
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4(5):293–297. https://doi.org/10.1038/ngeo1123
Doyle EJ, Hogg ID (2015) Assessing the diversity of terrestrial invertebrates in the mangrove forests of the Firth of Thames, New Zealand. Genome 58(5):213–213
Duke NC (1990) Phenological trends with latitude in the mangrove tree avicennia marina. J Ecol 78(1):113–133. https://doi.org/10.2307/2261040
Duke NC (1991) A systematic revision of the mangrove genus Avicennia (Avicenniaceae) in Australasia. Aust Syst Bot 4:299–324
Duke NC, Ball MC, Ellison JC (1998) Factors influencing biodiversity and distributional gradients in mangroves. Glob Ecol Biogeogr Lett 7(1):27–47. https://doi.org/10.2307/2997695
Ellis J, Nicholls P, Craggs R, Hofstra D, Hewitt J (2004) Effects of terrigenous sedimentation on mangrove physiology and associated macrobenthic communities. Mar Ecol Prog Ser 270:71–82. https://doi.org/10.3354/meps270071
Ellison JC (1999) Impacts of sediment burial on mangroves. Mar Pollut Bull 37(8–12):420–426. https://doi.org/10.1016/S0025-326X(98)00122-2
Ellison AM (2002) Macroecology of mangroves: large-scale patterns and processes in tropical coastal forests. Trees 16(2):181–194. https://doi.org/10.1007/s00468-001-0133-7
Ewel K, Twilley R, Ong JIN (1998) Different kinds of mangrove forests provide different goods and services. Glob Ecol Biogeogr Lett 7(1):83–94. https://doi.org/10.1111/j.1466-8238.1998.00275.x
Fricke AT, Nittrouer CA, Ogston AS, Vo-Luong HP (2017) Asymmetric progradation of a coastal mangrove forest controlled by combined fluvial and marine influence, Cù Lao Dung, Vietnam. Cont Shelf Res 147:78–90. https://doi.org/10.1016/j.csr.2017.07.012
Friedrichs CT, Aubrey DG (1988) Non-linear tidal distortion in shallow well-mixed estuaries: a synthesis. Estuar Coast Shelf Sci 27(5):521–545. https://doi.org/10.1016/0272-7714(88)90082-0
Friess DA, Lee SY, Primavera JH (2016a) Turning the tide on mangrove loss. Mar Pollut Bull 109(2):673–675. https://doi.org/10.1016/j.marpolbul.2016.06.085
Friess DA, Richards DR, Phang VXH (2016b) Mangrove forests store high densities of carbon across the tropical urban landscape of Singapore. Urban Ecosyst 19(2):795–810. https://doi.org/10.1007/s11252-015-0511-3
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL, Cadamuro L (1998) Structure, above-ground biomass and dynamics of mangrove ecosystems: new data from French Guiana. Oecologia 115(1):39–53. https://doi.org/10.1007/s004420050489
Furukawa K, Wolanski E (1996) Sedimentation in mangrove forests. Mangrove Salt Marshes 1(1):3–10. https://doi.org/10.1023/a:1025973426404
Furukawa K, Wolanski E, Mueller H (1997) Currents and sediment transport in mangrove forests. Estuar Coast Shelf Sci 44(3):301–310
Gilman E, Ellison J (2007) Efficacy of alternative low-cost approaches to mangrove restoration, American Samoa. Estuar Coasts 30(4):641–651. https://doi.org/10.1007/bf02841961
Gilman EL, Ellison J, Jungblut V, van Lavieren H, Wilson L, Areki F, Brighouse G, Bungitak J, Henry M, Kilman M, Matthews E, Sauni I, Teariki-Ruatu N, Tukia S, Yuknavage K (2006) Adapting to Pacific Island mangrove responses to sea level rise and climate change. Clim Res 32(3):161–176. https://doi.org/10.3354/cr032161
Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, Masek J, Duke N (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Glob Ecol Biogeogr 20(1):154–159. https://doi.org/10.1111/j.1466-8238.2010.00584.x
Gladstone-Gallagher RV, Lundquist CJ, Pilditch CA (2014) Mangrove (Avicennia marina subsp. australasica) litter production and decomposition in a temperate estuary. N Z J Mar Freshw Res 48(1):24–37. https://doi.org/10.1080/00288330.2013.827124
Gladstone-Gallagher RV, Lohrer AM, Lundquist CJ, Pilditch CA (2016) Effects of detrital subsidies on soft-sediment ecosystem function are transient and source-dependent. PLoS One 11(5):e0154790. https://doi.org/10.1371/journal.pone.0154790
Graeme M (2013) Estuarine vegetation survey: Whangapoua Harbour. Waikato Regional Council, Hamilton
Green MO, Coco G (2007) Sediment transport on an estuarine intertidal flat: measurements and conceptual model of waves, rainfall and exchanges with a tidal creek. Estuar Coast Shelf Sci 72(4):553–569. https://doi.org/10.1016/j.ecss.2006.11.006
Green MO, Black KP, Amos CL (1997) Control of estuarine sediment dynamics by interactions between currents and waves at several scales. Mar Geol 144(1–3):97–116. https://doi.org/10.1016/S0025-3227(97)00065-0
Grellier S, Janeau J-L, Dang Hoai N, Nguyen Thi Kim C, Le Thi PQ, Pham Thi Thu T, Tran-Thi N-T, Marchand C (2017) Changes in soil characteristics and C dynamics after mangrove clearing (Vietnam). Sci Total Environ 593–594:654–663. https://doi.org/10.1016/j.scitotenv.2017.03.204
Griffiths GA, Glasby GP (1985) Input of river-derived sediment to the New Zealand continental shelf: I. Mass. Estuar Coast Shelf Sci 21(6):773–787. https://doi.org/10.1016/0272-7714(85)90072-1
Harmsworth GR, Awatere S (2013) Indigenous Māori knowledge and perspectives of ecosystems. In: Dymond JR (ed) Ecosystem services in New Zealand – conditions and trends. Manaaki Whenua Press, Lincoln, pp 274–286
Harty C (2009) Mangrove planning and management in New Zealand and South East Australia – a reflection on approaches. Ocean Coast Manag 52(5):278–286. https://doi.org/10.1016/j.ocecoaman.2009.03.001
Hashim R, Kamali B, Tamin NM, Zakaria R (2010) An integrated approach to coastal rehabilitation: mangrove restoration in Sungai Haji Dorani, Malaysia. Estuar Coast Shelf Sci 86(1):118–124
Hayden HL, Granek EF (2015) Coastal sediment elevation change following anthropogenic mangrove clearing. Estuar Coast Shelf Sci 165:70–74. https://doi.org/10.1016/j.ecss.2015.09.004
Healy TR (2002) Muddy coasts of mid-latitude oceanic islands on an active plate margin – New Zealand. In: Healy TR, Wang J, Healy J-A (eds) Muddy coasts of the world: processes, deposits and function. Elsevier, Amsterdam, pp 347–374. https://doi.org/10.1016/S1568-2692(02)80088-2
Henderson SM, Norris BK, Mullarney JC, Bryan KR (2017) Wavefrequency flows within a near-bed vegetation canopy. Cont Shelf Res 147:91–101. https://doi.org/10.1016/j.csr.2017.06.003
Horstman EM, Dohmen-Janssen CM, Hulscher SJMH (2013) Flow routing in mangrove forests: a field study in Trang province, Thailand. Cont Shelf Res 71:52–67. https://doi.org/10.1016/j.csr.2013.10.002
Horstman EM, Dohmen-Janssen CM, Narra PMF, Van den Berg NJF, Siemerink M, Hulscher SJMH (2014) Wave attenuation in mangroves: a quantitative approach to field observations. Coast Eng 94:47–62. https://doi.org/10.1016/j.coastaleng.2014.08.005
Horstman EM, Dohmen-Janssen CM, Bouma TJ, Hulscher SJMH (2015) Tidal-scale flow routing and sedimentation in mangrove forests: combining field data and numerical modelling. Geomorphology 228:244–262. https://doi.org/10.1016/j.geomorph.2014.08.011
Horstman EM, Bryan KR, Mullarney JC, Pilditch CA (2016) Model versus nature: hydrodynamics in mangrove pneumatophores. In: Lynett P (ed) Proceedings of 35th International Conference on Coastal Engineering, vol 35. Coastal Engineering Research Council, Antalya. https://doi.org/10.9753/icce.v35.management.19
Horstman EM, Mullarney JC, Bryan KR, Sandwell DR (2017) Deposition gradients across mangrove fringes. In: Aagaard T, Deigaard R, Fuhrman D (eds) Coastal dynamics 2017. Helsingør, Denmark
Horstman EM, Bryan KR, Mullarney JC, Pilditch CA, Eager CA (2018) Are flow-vegetation interactions well represented by mimics? A case study of mangrove pneumatophores. Adv Water Resour 111:360–371. https://doi.org/10.1016/j.advwatres.2017.11.018
Hume TM (2003) Estuaries and tidal inlets. In: Goff JR, Nichol SL, Rouse HL (eds) The New Zealand coast: Te Tai O Aotearoa. Dunmore Press, Palmerston North, pp 191–213
Hume TM, Herdendorf CE (1992) Factors controlling tidal inlet characteristics on low drift coasts. J Coast Res 8(2):355–375
Hunt S, Bryan KR, Mullarney JC (2015) The influence of wind and waves on the existence of stable intertidal morphology in meso-tidal estuaries. Geomorphology 228:158–174. https://doi.org/10.1016/j.geomorph.2014.09.001
Hunt S, Bryan KR, Mullarney JC (2017) The effect of wind waves on spring-neap variations in sediment transport in two meso-tidal estuarine basins with contrasting fetch. Geomorphology 280:76–88. https://doi.org/10.1016/j.geomorph.2016.12.007
Janzen DH (1985) Mangroves: where’s the understory? J Trop Ecol 1(1):89–92. https://doi.org/10.1017/S0266467400000122
Jones H (2008) Coastal sedimentation: what we know and the information gaps. Waikato Regional Council, Hamilton
Kirwan ML, Megonigal JP (2013) Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504(7478):53–60. https://doi.org/10.1038/nature12856
Kirwan ML, Murray AB (2007) A coupled geomorphic and ecological model of tidal marsh evolution. Proc Natl Acad Sci U S A 104(15):6118–6122. https://doi.org/10.1073/pnas.0700958104
Kobashi D, Mazda Y (2005) Tidal flow in riverine-type mangroves. Wetl Ecol Manag 13(6):615–619. https://doi.org/10.1007/s11273-004-3481-4
Krauss KW, Lovelock CE, McKee KL, López-Hoffman L, Ewe SML, Sousa WP (2008) Environmental drivers in mangrove establishment and early development: a review. Aquat Bot 89(2):105–127. https://doi.org/10.1016/j.aquabot.2007.12.014
Krauss KW, McKee KL, Lovelock CE, Cahoon DR, Saintilan N, Reef R, Chen L (2014) How mangrove forests adjust to rising sea level. New Phytol 202(1):19–34. https://doi.org/10.1111/nph.12605
Lara RJ, Szlafsztein CF, Cohen MCL, Oxmann J, Schmitt BB, Filho PWMS (2009) Geomorphology and sedimentology of mangroves and salt marshes: the formation of geobotanical units. In: GME P, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands: an integrated ecosystem approach. Elsevier, Amsterdam, pp 593–613
Lee SY, Primavera JH, Dahdouh-Guebas F, McKee K, Bosire JO, Cannicci S, Diele K, Fromard F, Koedam N, Marchand C, Mendelssohn I, Mukherjee N, Record S (2014) Ecological role and services of tropical mangrove ecosystems: a reassessment. Glob Ecol Biogeogr 23(7):726–743. https://doi.org/10.1111/geb.12155
Lewis RR (2005) Ecological engineering for successful management and restoration of mangrove forests. Ecol Eng 24(4):403–418. https://doi.org/10.1016/j.ecoleng.2004.10.003
Liu JP, DeMaster DJ, Nittrouer CA, Eidam EF, Nguyen TT (2017) A seismic study of the Mekong subaqueous delta: proximal versus distal accumulation. Oceanography 30(3). https://doi.org/10.5670/oceanog.2017.315
Lovelock CE, Feller IC, Ellis J, Schwarz AM, Hancock N, Nichols P, Sorrell B (2007) Mangrove growth in New Zealand estuaries: the role of nutrient enrichment at sites with contrasting rates of sedimentation. Oecologia 153(3):633–641. https://doi.org/10.1007/s00442-007-0750-y
Lovelock CE, Sorrell BK, Hancock N, Hua Q, Swales A (2010) Mangrove forest and soil development on a rapidly accreting shore in New Zealand. Ecosystems 13(3):437–451. https://doi.org/10.1007/s10021-010-9329-2
Lovelock CE, Ruess RW, Feller IC (2011) CO2 efflux from cleared mangrove peat. PLoS ONE 6(6):e21279
Lovelock CE, Cahoon DR, Friess DA, Guntenspergen GR, Krauss KW, Reef R, Rogers K, Saunders ML, Sidik F, Swales A, Saintilan N, Thuyen LX, Triet T (2015) The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526(7574):559–563. https://doi.org/10.1038/nature15538
Lowe M (2013) Factors affecting the habitat usage of estuarine juvenile fish in northern New Zealand. The University of Auckland, Aucland
Lugo AE (1997) Old-growth mangrove forests in the United States. Conserv Biol 11(1):11–20. https://doi.org/10.1046/j.1523-1739.1997.96012.x
Lundquist CJ, Hailes S, Cartner K, Carter K, Gibbs M (2012) Physical and ecological impacts associated with mangrove removals using in situ mechanical mulching in Tauranga Harbour. NIWA Technical Report No.137. NIWA Technical Report No137
Lundquist CJ, Hailes SF, Carter KR, Burgess TC (2014a) Ecological status of mangrove removal sites in the Auckland region. National Institute of Water & Atmospheric Research Ltd., Hamilton
Lundquist CJ, Morrisey DJ, Gladstone-Gallagher RV, Swales A (2014b) Managing mangrove habitat expansion in New Zealand. In: Mangrove ecosystems of Asia: status, challenges and management strategies. p 415–438. doi:10.1007/978-1-4614-8582-7_19
Lundquist CJ, Carter K, Hailes S, Bulmer RH (2017) Guidelines for managing mangrove expansion in New Zealand. National Institute of Water & Atmospheric Research Ltd., Hamilton
Macnae W (1966) Mangroves in eastern and southern Australia. Aust J Bot 14:67–104
Martin KC (2007) Interactive effects of salinity and nutrients on mangrove physiology: implications for mangrove forest structure and function. Australian National University, Canberra
May JD (1999) Spatial variation in litter production by the mangrove Avicennia marina var. australasica in Rangaunu harbour, Northland, New Zealand. N Z J Mar Freshw Res 33(2):163–172. https://doi.org/10.1080/00288330.1999.9516866
Mazda Y, Magi M, Kogo M, Hong PN (1997) Mangroves as a coastal protection from waves in the Tong King delta, Vietnam. Mangrove Salt Marshes 1(2):127–135. https://doi.org/10.1023/a:1009928003700
McBride G, Reeve G, Pritchard M, Lundquist C, Daigneault A, Bell R, Blackett P, Swales A, Wadhwa S, Tait A, Zammit C (2016) The Firth of Thames and Lower Waihou River. Synthesis report RA2, coastal case study. Climate Changes, Impacts and Implications (CCII) for New Zealand to 2100. National Institute of Water & Atmospheric Research Ltd., Hamilton
Melville F, Pulkownik A (2006) Investigation of mangrove macroalgae as bioindicators of estuarine contamination. Mar Pollut Bull 52(10):1260–1269. https://doi.org/10.1016/j.marpolbul.2006.02.021
Mills M, Leon JX, Saunders MI, Bell J, Liu Y, O'Mara J, Lovelock CE, Mumby PJ, Phinn S, Possingham HP, Tulloch VJD, Mutafoglu K, Morrison T, Callaghan DP, Baldock T, Klein CJ, Hoegh-Guldberg O (2016) Reconciling development and conservation under coastal squeeze from rising sea level. Conserv Lett 9(5):361–368. https://doi.org/10.1111/conl.12213
Morrisey DJ, Beard CM, Morrison MA, Craggs R, Lowe M (2007) The New Zealand mangrove: review of the current state of knowledge. Auckland Regional Council, Auckland
Morrisey DJ, Skilleter GA, Ellis JI, Burns BR, Kemp CE, Burt K (2003) Differences in benthic fauna and sediment among mangrove (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuar Coast Shelf Sci 56(3–4):581–592. https://doi.org/10.1016/S0272-7714(02)00208-1
Morrisey DJ, Swales A, Dittmann S, Morrison MA, Lovelock CE, Beard CM (2010) The ecology and management of temperate mangroves. In: Gibson RN, RJA A, JDM G (eds) Oceanography and marine biology – an annual review, Oceanography and Marine Biology – An Annual Review, vol 48. CRC Press, New York, pp 43–160. https://doi.org/10.1201/EBK1439821169-c2
Mullarney JC, Henderson SM (2018) The effect of marine vegetation on shorelines. In: Pan-Chang V, Kaihatu J (eds) Advances in coastal hydraulics. World Scientific Publishing Ltd (in press)
Mullarney JC, Henderson SM, Norris BK, Bryan KR, Fricke AT, Sandwell DR, Culling DP (2017a) A question of scale: how turbulence around aerial roots shapes the seabed morphology in mangrove forests of the Mekong Delta. Oceanography 30(3):20–33. https://doi.org/10.5670/oceanog.2017.312
Mullarney JC, Henderson SM, Reyns JAH, Norris BK, Bryan KR (2017) Spatially varying drag within a wave-exposed mangrove forest and on the adjacent tidal flat. Cont Shelf Res 147:102–113. https://doi.org/10.1016/j.csr.2017.06.19
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89(2):155–185. https://doi.org/10.1016/j.aquabot.2007.12.007
Nichol SL, Augustinus PC, Gregory MR, Creese R, Horrocks M (2000) Geomorphic and sedimentary evidence of human impact on the New Zealand coastal landscape. Phys Geogr 21(2):109–132. https://doi.org/10.1080/02723646.2000.10642702
Nittrouer CA, Sternberg RW, Carpenter R, Bennett JT (1979) The use of Pb-210 geochronology as a sedimentological tool: application to the Washington continental shelf. Mar Geol 31(3):297–316. https://doi.org/10.1016/0025-3227(79)90039-2
Nittrouer CA, DeMaster DJ, Eidam EF, Nguyen TT, Liu JP, Ogston AS, Phung PV (2017) The Mekong continental shelf: primary sink for deltaic sediment particles and their passengers. Oceanography 30(3):60–70. https://doi.org/10.5670/oceanog.2017.314
Norris BK, Mullarney JC, Bryan KR, Henderson SM (2017) The effect of pneumatophore density on turbulence: a field study in a Sonneratia-dominated mangrove forest, Vietnam. Cont Shelf Res 147:114–127. https://doi.org/10.1016/j.csr.2017.06.002
Osborne K, Smith TJ (1990) Differential predation on mangrove propagules in open and closed canopy forest habitats. Vegetatio 89(1):1–6. https://doi.org/10.1007/bf00134429
Pérez A, Machado W, Gutierrez D, Stokes D, Sanders L, Smoak JM, Santos I, Sanders CJ (2017) Changes in soil organic carbon accumulation driven by mangrove expansion and deforestation in a New Zealand estuary. Estuar Coast Shelf Sci. https://doi.org/10.1016/j.ecss.2017.05.009
Pocknall DT, Gregory MR, Greig DA (1989) Palynology of core 80/20 and its implications for understanding Holocene sea level changes in the Firth of Thames, New Zealand. J R Soc N Z 19(2):171–179. https://doi.org/10.1080/03036758.1989.10426446
Quartel S, Kroon A, Augustinus PGEF, Van Santen P, Tri NH (2007) Wave attenuation in coastal mangroves in the Red River Delta, Vietnam. J Asian Earth Sci 29(4):576–584. https://doi.org/10.1016/j.jseaes.2006.05.008
Robertson BP, Gardner JPA, Savage C (2015) Macrobenthic–mud relations strengthen the foundation for benthic index development: a case study from shallow, temperate New Zealand estuaries. Ecol Indic 58:161–174. https://doi.org/10.1016/j.ecolind.2015.05.039
Rogers K, Saintilan N, Copeland C (2014) Managed retreat of saline coastal wetlands: challenges and opportunities identified from the Hunter River Estuary, Australia. Estuar Coasts 37(1):67–78. https://doi.org/10.1007/s12237-013-9664-6
Roy PS, Williams RJ, Jones AR, Yassini I, Gibbs PJ, Coates B, West RJ, Scanes PR, Hudson JP, Nichol S (2001) Structure and function of South-east Australian estuaries. Estuar Coast Shelf Sci 53(3):351–384. https://doi.org/10.1006/ecss.2001.0796
Saenger P, Snedaker SC (1993) Pantropical trends in mangrove above-ground biomass and annual litterfall. Oecologia 96(3):293–299. https://doi.org/10.1007/bf00317496
Saintilan N (2004) Relationships between estuarine geomorphology, wetland extent and fish landings in New South Wales estuaries. Estuar Coast Shelf Sci 61(4):591–601. https://doi.org/10.1016/j.ecss.2004.07.002
Saintilan N, Rogers K (2013) The significance and vulnerability of Australian saltmarshes: implications for management in a changing climate. Mar Freshw Res 64(1):66–79. https://doi.org/10.1071/MF12212
Saintilan N, Rogers K, McKee KL (2009) Salt marsh-mangrove interactions in Australasia and the Americas. In: Perillo GME, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands an integrated ecosystem approach. Elsevier, Amsterdam, pp 855–884
Schaeffer-Novelli Y, Cintron-Molero G, Soares MLG (2002) Chapter nine angroves as indicators of sea level change in the muddy coasts of the world. Proc Marine Sci 4:245–262. https://doi.org/10.1016/S1568-2692(02)80083-3
Sheaves M, Johnston R, Baker R (2016) Use of mangroves by fish: new insights from in-forest videos. Mar Ecol Prog Ser 549:167–182. https://doi.org/10.3354/meps11690
Sidik F, Neil D, Lovelock CE (2016) Effect of high sedimentation rates on surface sediment dynamics and mangrove growth in the Porong River, Indonesia. Mar Pollut Bull 107(1):355–363. https://doi.org/10.1016/j.marpolbul.2016.02.048
Spalding M, Kainuma M, Collins L (2010) World atlas of mangroves (version 1.1), A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. Earthscan, London. http://data.unep-wcmc.org/datasets/5
Spalding MD, Ruffo S, Lacambra C, Meliane I, Hale LZ, Shepard CC, Beck MW (2014) The role of ecosystems in coastal protection: adapting to climate change and coastal hazards. Ocean Coast Manag 90:50–57. https://doi.org/10.1016/j.ocecoaman.2013.09.007
Stagg CL, Krauss KW, Cahoon DR, Cormier N, Conner WH, Swarzenski CM (2016) Processes contributing to resilience of coastal wetlands to sea-level rise. Ecosystems 19(8):1445–1459. https://doi.org/10.1007/s10021-016-0015-x
Stern MK, Day JW, Teague KG (1986) Seasonality of materials transport through a coastal freshwater marsh: riverine versus tidal forcing. Estuaries 9(4):301–308. https://doi.org/10.2307/1352102
Stokes DJ (2008) Assessment of physical changes after mangrove removal: Whangamata 2008. Report prepared for Environment Waikato TR 2009/13. Hamilton, New Zealand
Stokes DJ, Harris RJ (2015) Sediment properties and surface erodibility following a large-scale mangrove (Avicennia marina) removal. Cont Shelf Res 107:1–10. https://doi.org/10.1016/j.csr.2015.07.011
Stokes DJ, Healy TR, Cooke PJ (2010) Expansion dynamics of monospecific, temperate mangroves and sedimentation in two embayments of a barrier-enclosed lagoon, Tauranga Harbour, New Zealand. J Coast Res 26(1):113–122. https://doi.org/10.2112/08-1043.1
Stokes DJ, Bulmer RH, Lundquist CJ (2016) Addressing the mismatch between restoration objectives and monitoring needs to support mangrove management. Ocean Coast Manag 134:69–78. https://doi.org/10.1016/j.ocecoaman.2016.09.024
Stuart SA, Choat B, Holbrook NM, Ball MC (2007) The role of freezing in setting the latitudinal limits of mangrove forests. New Phytol 173:576–583. https://doi.org/10.1111/j.1469-8137.2006.01938.x
Sutherland JI (2003) Miocene petrified wood and associated borings and termite faecal pellets from Hukatere Peninsula, Kaipara Harbour, North Auckland, New Zealand. J R Soc N Z 33(1):395–414. https://doi.org/10.1080/03014223.2003.9517736
Swales A, Williamson RB, van Dam LF, Stroud MJ, McGlone MS (2002) Reconstruction of urban stormwater contamination of an estuary using catchment history and sediment profile dating. Estuaries 25(1):43–56
Swales A, Bentley S, Lovelock C, Bell R (2007) Sediment processes and mangrove-habitat expansion on a rapidly-prograding muddy coast, New Zealand. In: Coastal Sediments ‘07: Proceedings of the Sixth International Conference on Coastal Engineering and Science of Coastal Sediment Processes, New Orleans. American Society of Civil Engineers, p 1441–1454. doi:10.1061/40926(239)111
Swales A, Bell RG, Gorman R, Oldman JW, Altenberger A, Hart C, Claydon L, Wadwha S, Ovenden R (2009) Potential future changes in mangrove-habitat in Auckland’s east-coast estuaries. Auckland Regional Council, Auckland
Swales A, Bentley SJ, Lovelock CE (2015) Mangrove-forest evolution in a sediment-rich estuarine system: opportunists or agents of geomorphic change? Earth Surf Process Landf 40(12):1672–1687. https://doi.org/10.1002/esp.3759
Swales A, Denys P, Pickett VI, Lovelock CE (2016) Evaluating deep subsidence in a rapidly-accreting mangrove forest using GPS monitoring of surface-elevation benchmarks and sedimentary records. Mar Geol 380:205–218. https://doi.org/10.1016/j.margeo.2016.04.015
Syvitski JPM, Vörösmarty CJ, Kettner AJ, Green P (2005) Impact of humans on the flux of terrestrial sediment to the global coastal ocean. Science 308(5720):376–380. https://doi.org/10.1126/science.1109454
Temmerman S, Bouma TJ, Govers G, Wang ZB, De Vries MB, Herman PMJ (2005) Impact of vegetation on flow routing and sedimentation patterns: three-dimensional modeling for a tidal marsh. J Geophys Res Earth Surf 110:F04019. https://doi.org/10.1029/2005jf000301
Temmerman S, Meire P, Bouma TJ, Herman PMJ, Ysebaert T, De Vriend HJ (2013) Ecosystem-based coastal defence in the face of global change. Nature 504(7478):79–83. https://doi.org/10.1038/nature12859
Thom BG (1982) Mangrove ecology: a geomorphological perspective. In: Clough BF (ed) Mangrove ecosystems in Australia; structure, function and management. Australian National University Press, Canberra, pp 3–17
Thrush SF, Hewitt JE, Cummings VJ, Ellis JI, Hatton C, Lohrer A, Norkko A (2004) Muddy waters: elevating sediment input to coastal and estuarine habitats. Front Ecol Environ 2(6):299–306. https://doi.org/10.1890/1540-9295(2004)002[0299:MWESIT]2.0.CO;2
Tomlinson PB (1986) The botany of mangroves, Cambridge tropical biology series. Cambridge University Press, Cambridge, UK
Twilley RR, Rivera-Monroy VH (2009) Ecogeomorphic models of nutrient biogeochemistry for mangrove wetlands. In: GME P, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands: an integrated ecosystem approac. Elsevier, Amsterdam, pp 641–683
Twilley RR, Chen RH, Hargis T (1992) Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut 64(1):265–288. https://doi.org/10.1007/bf00477106
Twilley RR, Rivera-Monroy VH, Chen R, Botero L (1999) Adapting an ecological mangrove model to simulate trajectories in restoration ecology. Mar Pollut Bull 37(8–12):404–419. https://doi.org/10.1016/S0025-326X(99)00137-X
UNEP (2014) The importance of mangroves to people: a call to action. United Nations Environment Programme World Conservation Monitoring Centre, Cambridge, UK
van de Koppel J, Bouma TJ, Herman PMJ (2012) The influence of local-and landscape-scale processes on spatial self-organization in estuarine ecosystems. J Exp Biol 215(6):962–967
van Katwijk MM, Bos AR, Hermus DCR, Suykerbuyk W (2010) Sediment modification by seagrass beds: muddification and sandification induced by plant cover and environmental conditions. Estuar Coast Shelf Sci 89(2):175–181. https://doi.org/10.1016/j.ecss.2010.06.008
van Maanen B, Coco G, Bryan KR (2015) On the ecogeomorphological feedbacks that control tidal channel network evolution in a sandy mangrove setting. Proc Royal Soc Lond A: Math Phys Eng Sci 471(2180):1–24. https://doi.org/10.1098/rspa.2015.0115
van Santen P, Augustinus PGEF, Janssen-Stelder BM, Quartel S, Tri NH (2007) Sedimentation in an estuarine mangrove system. J Asian Earth Sci 29(4):566–575. https://doi.org/10.1016/j.jseaes.2006.05.011
Vo-Luong HP, Massel SR (2008) Energy dissipation in non-uniform mangrove forests of arbitrary depth. J Mar Syst 74:603–622. https://doi.org/10.1016/j.jmarsys.2008.05.004
Ward RD, Friess DA, Day RH, MacKenzie RA (2016) Impacts of climate change on mangrove ecosystems: a region by region overview. Ecosyst Health Sustain 2(4):e01211. https://doi.org/10.1002/ehs2.1211
Wassilieff M (2006) Estuaries – plants of the estuary. http://www.TeAra.govt.nz/en/interactive/4623/vegetation-profile. Accessed 1 May 2017
Watson JG (1928) Mangrove forests of the Malay Peninsula. Malayan forest records No. 6. Federated Malay States Government, Kuala Lumpur
Webb EL, Friess DA, Krauss KW, Cahoon DR, Guntenspergen GR, Phelps J (2013) A global standard for monitoring coastal wetland vulnerability to accelerated sea-level rise. Nat Clim Chang 3(5):458–465. https://doi.org/10.1038/nclimate1756
Widdows J, Blauw A, Heip CHR, Herman PMJ, Lucas CH, Middelburg JJ, Schmidt S, Brinsley MD, Twisk F, Verbeek H (2004) Role of physical and biological processes in sediment dynamics of a tidal flat in Westerschelde Estuary, SW Netherlands. Mar Ecol Prog Ser 274:41–56. https://doi.org/10.3354/meps274041
Wildlands (2014) Monitoring of banded rail and other avifauna before and after mangrove clearance at Whangamata Harbour – Annual report (Mar 2013–2014)
Willemsen PWJM, Horstman EM, Borsje BW, Friess DA, Dohmen-Janssen CM (2016) Sensitivity of the sediment trapping capacity of an estuarine mangrove forest. Geomorphology 273:189–201. https://doi.org/10.1016/j.geomorph.2016.07.038
Winterwerp JC, Borst WG, de Vries MB (2005) Pilot study on the erosion and rehabilitation of a mangrove mud coast. J Coast Res 21(2):223–230. https://doi.org/10.2112/03-832a.1
Winterwerp JC, Erftemeijer PLA, Suryadiputra N, Eijk P, Zhang L (2013) Defining eco-morphodynamic requirements for rehabilitating eroding mangrove-mud coasts. Wetlands 33(3):515–526. https://doi.org/10.1007/s13157-013-0409-x
Wolanski E (1995) Transport of sediment in mangrove swamps. Hydrobiologia 295(1–3):31–42
Wolanski E (2007) Tidal wetlands. In: Wolanski E (ed) Estuarine ecohydrology. Elsevier, Amsterdam, pp 71–89. https://doi.org/10.1016/B978-044453066-0.50005-2
Wolanski E, Moore K, Spagnol S, D’Adamo N, Pattiaratchi C (2001) Rapid, human-induced siltation of the macro-tidal ord river estuary, Western Australia. Estuar Coast Shelf Sci 53(5):717–732. https://doi.org/10.1006/ecss.2001.0799
Woodroffe CD (1982) Litter production and decomposition in the New Zealand mangrove, Avicennia marina var. resinifera. N Z J Mar Freshw Res 16(2):179–188. https://doi.org/10.1080/00288330.1982.9515961
Woodroffe CD (1992) Mangrove sediments and geomorphology. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, DC, pp 7–41
Young BM, Harvey EL (1996) A spatial analysis of the relationship between mangrove (Avicennia marina var. australasica) physiognomy and sediment accretion in the Hauraki Plains, New Zealand. Estuar Coast Shelf Sci 42(2):231–246. https://doi.org/10.1006/ecss.1996.0017
Acknowledgements
Contributions by EMH, KRB and JCM and collection of the field data presented in sections “Biophysical Dynamics of Expanding Mangroves” and “Succession of Expanding Mangrove Systems” were supported by the Royal Society of New Zealand’s Marsden Fund (grant number 14-UOW-011). Contributions by CJL and RHB were supported by MBIE Programme C01X1002, and NIWA Freshwater Programme core funding. Contributions by DJS were supported by Waikato Regional Council and Southern Cross University MERC grants.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Horstman, E.M., Lundquist, C.J., Bryan, K.R., Bulmer, R.H., Mullarney, J.C., Stokes, D.J. (2018). The Dynamics of Expanding Mangroves in New Zealand. In: Makowski, C., Finkl, C. (eds) Threats to Mangrove Forests. Coastal Research Library, vol 25. Springer, Cham. https://doi.org/10.1007/978-3-319-73016-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-73016-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73015-8
Online ISBN: 978-3-319-73016-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)