Abstract
Due to complicated structure and composition of high-phosphorus iron ore , pure substances were used to simulate the chemical composition of high-phosphorus iron ore to study the reaction of fluorapatite at different temperatures and effect of additives . The gasification process of P during carbothermic reduction process was investigated using a quadrupole mass spectrometer gas analysis. It is found fluorapatite was reduced to phosphorus which diffused into iron and gas phase. From the gas analysis, it was observed that PO and P gas species were vaporized around 800 °C. As the reduction temperature increased, the diffusion of phosphorus into iron was enhanced. The presence of Ca(OH)2 as additives not only promoted the reduction of iron oxide, but also decreased the degree of fluorapatite reaction to inhibit P into iron.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
With the increasingly tension of iron ore resources, heavily dependent on imported iron ore has been restricting the development of steel industry in China. More than 7.45 billion tons high-phosphorus iron ore is mainly distributed in Hunan, Hubei, Sichuan, and Yunnan Provinces of China [1, 2]. Although the grade of total iron of high-phosphorus iron ore is high, up to about 50%, but the P content reaches up to 0.3–1.8 wt%. P in the form of fluorapatite is existed with other minerals in high-phosphorus iron ore . However, due to the high temperature of the blast furnace, liquid iron absorb almost all P, thus the high-phosphorus iron ore is not suitable for the blast furnace process [3, 4].
Direct reduction process is a new method with a huge potential in the efficient utilization of high-phosphorus iron ore [5, 6]. There are some studies on the dephosphorization in direct reduction of high-phosphorus iron ore . Matinde [7, 8] used the pre-reduction and screening combined with mechanical crushing or air jet milling to process high-phosphorus iron ore . CHA [9] employed carbothermic reduction to investigate the effect of temperature, C/O molar ratio, and CaO addition on the distribution behavior of phosphorus and metallization of iron ore in the reduction process. Yu [10] treated the high-phosphorus oolitic hematite using coal-based direct reduction followed by magnetic separation, where Ca(OH)2 was used as additives . Yin et al. [11]. proposed a reduction process combined with microwave and magnetic separation. However, the complex nature of high-phosphorus iron ore chemical composition and the complicated interactive relationships between gangue phases caused great difficulties for reduction mechanism of iron ore and phosphorus mineral.
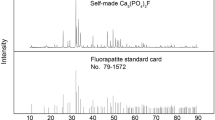
Our research group is engaged in the basic research of high-phosphorus iron ore using direct reduction process. Recently it was found that C and P showed the same surface distribution in the iron phase, and the carburization and melting process of iron phase promoted each other, liquid iron had a strong ability of phosphorus absorption. It was proposed that the direct reduction temperature should be below 1148 °C to limit the P absorption of iron [12]. Based on the previous studies [13] and successful preparation of fluorapatite , the pure substances were used to simulate the chemical composition of high phosphorus iron ore in order to improve the production efficiency of high-phosphorus iron ore . During the process, direct reduction temperature was increased progressively to observe the reaction of fluorapatite and the P absorption of iron phase. Then Ca(OH)2 was used to investigate the effect of additives on the reaction of fluorapatite . The gasification process of P during carbothermic reduction process was investigated using a quadrupole mass spectrometer gas analysis. In this study, the reduction process of high-phosphorus iron ore is discussed from both micro- and macro-level. A variety of analytical methods e.g., XRD, SEM-EDS were used to characterize the reduction process. This study will therefore provide the theoretical basis for dephosphorization.
Experimental
Material
The high-phosphorus iron ore used in this study was collected from western Hubei Province, China. The chemical composition and X-ray diffraction (XRD) pattern of the sample are shown in Table 1 and Fig. 1. In the first part of experiment, the reaction of phosphorus mineral at different temperatures was investigated without additives . In the second part of experiment Ca(OH)2 was added to investigate the effect of additives at a constant temperature. Pure Fe2O3, SiO2, Al2O3, and synthetic fluorapatite [14] were used to simulate high-phosphorus iron ore . High purity graphite was used as reducing agent. The mass ratio of raw materials can be calculated through the chemical composition of high-phosphorus iron ore in Table 1. As shown in Table 2, in order to highlight the reaction of fluorapatite and other gangue minerals, the content of Fe2O3 was reduced and the effect of substances with a weight fraction of less than 1 wt% on the fluorapatite reaction is ignored in this study.
Experimental Procedure
Before the experiment, raw materials were mixed in accordance with simulated ratio as shown in Table 2. In the second part of experiment 5 wt% Ca(OH)2 was added accordingly. 1.5 g mixture was pressed into cylindrical pellets at 16 MPa with a diameter of 8 mm and a thickness of about 10 mm. The reduction temperature was in the range of 950–1200 °C under the protection of Ar with a flow rate of 2 L/min. When the temperature stabilized, the pellets were put into an Quartz tube, then, the crucible was pushed into the furnace for 30 min. A quadrupole mass spectrometer (QMS) was used to analyze gas species vaporized during the non-isothermal carbothermic reduction of pellets without additive and pellets added 5 wt% Ca(OH)2 under Ar gas conditions. Figure 2 shows the schematic representation of the experimental set up used in the gas analysis experiments. Pellets of different compositions were placed at the centre of the isothermal zone of the furnace. Then they were heated to 1200 °C under Ar atmosphere.
The phosphorus content of the pellets was measured by chemical analysis. The macroscopic analysis of pellets was investigated by X-ray diffraction (XRD). Microstructure of pellets and micro-composition analysis were observed using scanning electron microscope equipped with energy dispersive spectrometers (SEM-EDS). Dephosphorization ratio of pellets was calculated by the following formula:
Where P0min and m0min are the original P content and mass in the pellets. And P30min and m30min are the P content and mass in the pellets after roasting for 30 min.
Results and Discussion
Minerals Evolution of High-Phosphorus Iron Ore in Reduction Process
Macroscopic Morphology Changes and XRD Analysis of Pellets
Figure 3 shows macroscopic changes of direct reduction pellets at different temperatures. Some tiny holes were formed at the surface of pellets after reduction, caused by loss of O during reduction of iron oxide. At the reduction temperatures between 950 and 1050 °C, pellets remained compact and did not melt. When the reduction temperature reached at 1100 °C, pellets started to melt. This is because that a large amount of SiO2 and Al2O3 reacted with FeO to form fayalite and spinel, which have low-melting point, as Reaction (2) and (3). The formation of low-melting point substances promoted the melting of pellets, and the mass transferring kinetic conditions of gangue phase including SiO2, Al2O3, and fluorapatite were improved significantly. At the reduction temperature of 1150 °C, reduced metallic iron particles gathered and continued to grow, forming some small iron beads, and more iron beads were formed at the reduction temperature of 1200 °C.
Figure 4 shows XRD patterns of pellets reduced at different temperatures. At the reduction temperature of 950 °C, diffraction peaks of fluorapatite , metallic iron, Fe2SiO4, FeAl2O4, and graphite were obvious, which indicates that SiO2 and Al2O3 in gangue reacted with FeO to generate Fe2SiO4 and FeAl2O4. Compared the Gibbs free energy of Reaction (2) with Reaction (3) at 950 °C, it is clear that FeO predominantly reacted with Al2O3, and thus the diffraction peak of FeAl2O4 was more evident. At the reduction temperature of 1050 °C, unreacted Al2O3 contacted the Fe2SiO4 to form SiO2 and FeAl2O4 as shown in Reaction (4), thus the diffraction peak of Fe2SiO4 disappeared. When the reduction temperature was increased to 1100 °C, the diffraction peak of CaAl2Si2O8 appeared. Spinel was gradually reduced to form metallic iron and therefore some iron beads were generated. At the reduction temperature of 1200 °C, diffraction peaks of fluorapatite , FeAl2O4, and SiO2 disappeared while the intensity of CaAl2Si2O8 increased gradually until it was detectable in the XRD spectrum. It is likely that fluorapatite reacted with the slag. With increasing the interface tension between iron and slag, the separation of iron and slag became easier.
Microscopic Changes in Pellets and Phosphorus Content of Pellets
The reduction of pellets at different temperatures was described by XRD spectrum. However, it was difficult to make a detailed explanation about the reaction of fluorapatite . For a complete discussion, it was necessary to analyze the reaction of fluorapatite using SEM-EDS.
Microscopic changes in pellets at the reduction temperature of 950–1050 °C are shown in Fig. 5a. In this temperature range, most of P and Ca were detected in the same region, which indicates that fluorapatite was not reduced and most P was assumed to be concentrated in the form of fluorapatite in the gangue phase. As the temperature increased, iron particles grew and gathered together. The majority of metallic iron did not overlap with P. A large amount of FeAl2O4 was formed on the surface of the Al2O3, and needle-like fayalite appeared, the diffraction peaks of which were also detected by XRD spectrum.
Figure 5b shows the microstructure of pellets reduced at 1100 °C. The distribution behavior of P and Ca was different, which indicates that some of the fluorapatite phase was reduced to P. Thus, a lot of fluorapatite was reduced at 1100°C. However, the transfer process of P was restricted and part of P diffused into iron phase in inhomogeneous distribution. Figure 5b shows the EDS quantitative analysis of the new phase, which was determined by atomic ratio to be CaAl2Si2O8. It was deduced that fluorapatite decomposed in the presence of SiO2 and reacted with fayalite and spinel to form CaAl2Si2O8 and phosphorus, according to following Reactions (5–6).
Microscopic changes in high-phosphorus iron ore pellets at the reduction temperature of 1150–1200 °C are shown in Fig. 5c, d. As the temperature increased, more fluorapatite were reduced and a large amount of P diffused into the iron phase. P in the iron phase gradually become homogeneous. As fluorapatite reacted with other gangue minerals, the stable Ca-Si-Al gangue phase was formed, and the diffraction peak of CaAl2Si2O8 was also detected by XRD spectrum.
The dephosphorization ratio of the roasted pellets is used to be a representative of the P changes in pellets as in Fig. 6. At the reduction temperature of 950–1050 °C, the dephosphorization ratio of the roasted pellets was about 8.56–11.97%, which is relatively low and increased moderately. At 1100 °C fluorapatite was reduced and P went into gaseous phase. As the temperature increased, more reduced P volatilized. The dephosphorization ratio of the roasted pellets increased quickly during 1100–1200 °C and reached about 25.96% at 1200 °C. That means 25.96% of the P removed from the pellets by gaseous phase.
Effect of Ca(OH)2 as Additives
Figure 7a shows XRD patterns of reduction pellets without additives . It can be seen from Fig. 7a that FeAl2O4 was generated by the reaction of Al2O3 and FeO. Hematite was reduced to metallic iron, but the diffraction peak of Fe was not evident in this case. Figure 7b shows XRD patterns of roasted pellets with 5 wt% Ca(OH)2. Diffraction peak of FeAl2O4 disappeared as CaO, dissociated from Ca(OH)2, can react with FeAl2O4 to replace the FeO as shown in Reaction (7). On the other hand, diffraction peak of Fe increased markedly with 5% Ca(OH)2 compared with pellets roasted without additives . Thus the reduction of iron oxide was promoted. Additionally the diffraction peak of fluorapatite increased obviously. This was attributed to the reaction between CaO and SiO2, which promoted the reduction of fluorapatite . And the depletion of SiO2 decreased the degree of fluorapatite reaction.
Figure 8 shows the microstructure of high-phosphorus iron ore pellets roasted with 5 wt% Ca(OH)2. It can be seen that the size of iron particles became coarser than that without additives . The area maps show that majority of Fe did not overlap with P. Al2O3 and SiO2 stayed complete. P and Ca were detected in the same region, which indicated that Ca(OH)2 decreased the degree of fluorapatite reaction.
Transport and Transformation of P
It can be seen from Fig. 9 that PO and P gas are generated at t about 800 °C without additive , reaching maximum respectively at 1000 and 1100 °C. This shows that in this process, the fluorapatite is reduced and the reduced P is volatilized in the form of PO and P gaseous, and part of the P enters liquid iron. However, with the addition of fluxes, the PO and P gas generation temperatures were significantly improved and did not reach the maximum. This is due to the addition of additives easy to react with silica, inhibition of fluorapatite and SiO2 and graphite reduction reaction. Figure 10 shows the microstructure of the reduced iron, as can be seen from Fig. 10, it was found by SEM-EDS that P be in the iron produced by pellets without additive ,but there is no P in the iron produced by pellets with 5 wt% Ca(OH)2. Compared with Fig. 10a, b, it can be seen that the additive effectively inhibits the absorption of P by the metal iron and reduces the phosphorus content of the pearl iron.
Conclusion
Based on the experiments of effect of temperature and additive on the reaction of fluorapatite , some conclusions can be drawn as follows.
-
(1)
At the temperature of 950–1050 °C, fluorapatite was not reduced and phosphorus was concentrated in the form of fluorapatite in the gangue phase. It was found that 1100 °C is the starting temperature for the reduction of fluorapatite contained in the high-phosphorus iron ore without additives . Fluorapatite was reduced to phosphorus which diffused into iron and gas phase at 1100 °C. As the temperature increased, the diffusion of phosphorus into iron was found to accelerate and phosphorus was reported to be distributed evenly.
-
(2)
Iron oxide reduced firstly in the carbothermal reduction of high phosphorus iron ore, then reacted with Al2O3 and SiO2 to form FeAl2O4 and Fe2SiO4 at low temperature. As the temperature increased, more metallic iron and the stable CaAl2Si2O8 in slag were reported to form.
-
(3)
Ca(OH)2 contained in pellets leads to the depletion of SiO2, which would not only promote the reduction of iron oxide, but also decrease the degree of fluorapatite reaction.
-
(4)
PO and P gas species were vaporized around 800 °C without additive and at 1000 and 1100 °C, the amount of PO and P produced was maximized, respectively.
References
Sun Y, Han Y, Gao P (2012) Exploitation situation and development trend of high phosphorus oolitic hematite. Metal Mine 3:1–5
Wei D (2010) Study on the ore properties of high phosphorus oolitic hematite in Western Hubei. Metal Mine 10:61–64
Zhang H (2013) Mineralogical properties and tendency on utilizing methods of oolitic hematite. China Metall 23(11):6–9
Guo HJ (2004) Metallurgical physical chemistry course. Metallurgy Industry Pres, Beijing, p 257
Gao W et al (2008) Present status of coal-based direct reduction and rotary hearth furnace processes. Min Metall 2:017
Wang G, Xue Q, Kong Lt (2013) Rotary hearth furnace iron nugget technology and its prospect in China. China Metall 23(12):6
Elias M, Mitsutaka H (2011) Dephosphorization treatment of high phosphorus iron ore by pre-reduction, mechanical crushing and screening methods. Int ISIJ 51(2): 220
Matinde E, Hino M (2011) Dephosphorization treatment of high phosphorus iron ore by pre-reduction, air jet milling and screening methods. Int ISIJ 51:544
Cha J-W, Kim D-Y, Jung S-M (2015) Distribution behavior of phosphorus and metallization of iron oxide in carbothermic reduction of high-phosphorus iron ore. Metall Mater Trans B 46(5):2165–2179
Yu W et al (2013) The function of Ca(OH)2 and Na2CO3 as additive on the reduction of high-phosphorus oolitic hematite-coal mixed pellets. Int ISIJ 53(3):427–433
Yin J et al (2012) Dephosphorization of iron ore bearing high phosphorous by carbothermic reduction assisted with microwave and magnetic separation. Int ISIJ 52(9):1579–1584
Cheng C et al (2015) Dynamic migration process and mechanism of phosphorus permeating into metallic iron with carburizing in coal-based direct reduction. Int ISIJ 55(12):2576–2581
Cheng C et al (2016) Phosphorus migration during direct reduction of coal composite high-phosphorus iron ore pellets. Metall Mater Trans B 47(1):154–163
Fabian R et al (1998) Preparation and chemical characterization of high purity fluorapatite. Talanta 46(6):1273–1277
Acknowledgements
Project was funded by the National Natural Science Foundation of China (51374024), Fundamental Research Funds for the Central Universities (FRF-TP-16-019A1) and China Postdoctoral Science Foundation(2016M600919).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Zhang, Y., Xue, Q., Wang, G., Wang, J. (2018). The Effect of Temperature and Additive on Transport and Transformation of P of High-Phosphorus Iron Ore During Carbothermic Reduction. In: Hwang, JY., et al. 9th International Symposium on High-Temperature Metallurgical Processing. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72138-5_82
Download citation
DOI: https://doi.org/10.1007/978-3-319-72138-5_82
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72137-8
Online ISBN: 978-3-319-72138-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)