Abstract
Implantation is a finely coordinated, species-specific orchestration of events between the nascent embryo and a receptive endometrium, culminating in the opportunity for new life. Implantation represents a stepwise progression of dynamic events that enjoins the embryonic proliferation and intrusion with endometrial differentiation to avoid excessive invasion, optimizing survival for both mother and offspring. The response to the embryo’s need to establish nutrition and waste management is achieved through eloquent endometrial accommodation that supplies the growing embryo with uterine milk from glandular secretions and later vascular integration through cytotrophoblast modification of those same vessels. This complex dance, as it progresses, provides ample opportunity for failure along the way. Indeed, success occurs in only a minority of pregnancies and occurs at a relatively low efficiency, particularly in Homo sapiens. Nonetheless, this process of acceptance of the fetal allograph and implantation is one of the most fascinating events in human biology. This chapter serves as an introduction to this book and provides an overview of that process.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Pregnancy has been one of life’s great mysteries and captivated both the scientific and artistic realms (Fig. 1.1). The endometrium is where life begins, and a receptive endometrium lies at the crossroads of menstruation and pregnancy. In a perfect world, the peak of receptivity of the endometrial lining is achieved synchronously with the arrival into the uterine cavity of a healthy blastocyst that can then adhere, attach and invade, and grow protected until parturition. However, for human reproduction in particular, it is not always a perfect world; for every successful pregnancy, there are many fertilized eggs that either implant and fail as clinical or subclinical pregnancies or never are able to interact with the endometrium, resulting in infertility. Issues involving embryo quality and chromosome number and diseases that can impair normal endometrial receptivity have the potential to alter the outcome of pregnancy in devastating ways. The endometrium is a specialized, almost immortal, tissue that regenerates again and again with the sole purpose of continuing the survival of our species. In this chapter, we will review a global overview of normal implantation and what is known about the signaling components of embryo and endometrial interactions. Our current understanding of implantation also provides a better appreciation for why pregnancies fail.
Timing of Implantation
Normal implantation occurs in the mid-secretory phase of the menstrual cycle and requires synchronous development of the endometrium, oocyte, and subsequent embryo. Events leading to a successful pregnancy begin several months ahead of time with recruitment of the cohort of oocytes that will mature and ovulate some months hence. In the month of implantation, at the time of menstruation, progesterone levels fall with the demise of the corpus luteum. It has been suggested that menstruation is more than simply a decline in ovarian steroids [1]; there is evidence that menstruation is an active and complex process with purposeful blockade of progesterone action through induction of inflammatory mediators leading to progesterone resistance [2]. With an abrupt and active loss of progesterone support coupled with the concomitant rise in ovarian estrogen, the upper layers of the endometrial (the functionalis layer) are sloughed but rapidly repaired and reconstituted without scarring, from underlying stroma and epithelial fragments [3]. This remarkable process of renewal can occur up to 400 times in a woman’s lifetime.
The cessation of bleeding and repair of the endometrial lining is an estrogen-dependent process and occurs as the negative hypothalamic and pituitary feedback is released after the fall in progesterone. In response to rising follicle-stimulating hormone (FSH) , a cohort of ovarian follicles begins to develop, releasing increasing amounts of estradiol into the circulation. In response to rising estrogen concentrations, the endometrium produces more estrogen receptors (ER), allowing proliferation and thickening, ultimately achieving a trilaminar appearing layer by ultrasound by the time ovulation occurs. In natural cycles, the dominant follicle is selected as that follicle has adequate FSH receptors to grow despite falling gonadotropin levels in the mid-proliferative phase of the menstrual cycle.
In response to positive feedback to rising estrogen, an ovulatory LH peak from the pituitary triggers release of the mature oocyte(s). As shown in Fig. 1.2, the release of the egg results in collapse and consolidation of the vacated follicular cyst, with subsequent development of the corpus luteum and a rise in serum progesterone. Progesterone is essential for the success of a pregnancy. Unlike estrogen that stimulates endometrial cell proliferation, progesterone transforms the thickened endometrium into a secretory structure and induces a host of factors essential for embryonic survival, attachment, and invasion. Meanwhile, the released oocyte is picked up by the fimbria and transported along the fallopian tube where it is fertilized by waiting sperm. The newly formed embryo undergoes progressive development from zygote to 8-cell embryo to blastocyst during its transit down the fallopian tube culminating in its discharge into the uterine cavity. By the time it arrives, under optimal conditions, the endometrium has developed into a receptive surface with the appropriate glandular secretions, adhesion moieties, and vascular changes required to support a pregnancy.
Stages of implantation correspond to and are largely driven by ovarian steroids from the developing follicle and subsequent corpus luteum that forms. Development and progression of the embryo is synchronously timed to endometrial development, such that both embryo and endometrium become receptive toward each other at the proper time
Implantation is a complex network of events happening synchronously in the embryo and endometrium that culminates with the envelopment of the blastocyst within a decidualized endometrial stroma. The stages of early implantation have been divided into three phases: apposition, attachment, and invasion. When implantation occurs, this process is rapid, with apposition, attachment, and invasion happening within hours rather than days.
In one of the early morphologic studies on the timing of implantation, hysterectomy specimens were obtained from volunteers who agreed to try and become pregnant prior to surgery to remove the uterus. In this remarkable and usual study, 34 embryos were found within luteal phase hysterectomy specimens. Based on the time of hysterectomy relative to the last menstrual period, 8 embryos were found free-floating within the uterine cavity, while the remaining 26 embryos were in various stages of implantation and had begun to complete the invasion process [4, 5]. Based on these findings, the timing of implantation appeared to occur around cycle day 19 to 20 of an idealized menstrual cycle. The timing of implantation based on assisted reproductive technology cycles has tended to agree with these results [6,7,8]. In vitro studies have tried to record these events as well, with mixed success [9, 10].
Implantation begins with the hatching of the embryos out of its zona pellucida about 1–3 days after the morula enters the uterine cavity (Fig. 1.2). Apposition of the hatched blastocyst to the uterine epithelium usually occurs 2–4 days after the morula has entered the cavity. By this point, the blastocyst, as it is now called, has differentiated into an inner cell mass (ICM) that subsequently forms the embryo and the trophectoderm which will give rise to the placenta. Importantly, hatching from its protective shell exposes a variety of adhesive molecules expressed on the outer surface of the embryo, complementing those on the endometrial epithelium and later the decidualized stroma. Penetration of the embryo through the uterine epithelium and basal lamina occurs quickly, allowing in the invasion of cytotrophoblast inside the uterine vasculature [11]. This clogging of the arterioles of the maternal endometrium reduces hemostatic pressure on the implanting blastocyst but also means that blood supply to the embryo is limited until the end of the first trimester [12].
The Endometrium
The endometrium is composed of a mucosal layer within the myometrial layers of the uterus. The female reproductive tract is derived from the urogenital ridge, which arises from paired mesodermal (paramesonephric) tubes that form from the longitudinal invaginations of the coelomic epithelium [13]. The early uterus is lined by a simple cuboidal epithelium that subsequently becomes columnar and pseudostratified. Beneath this epithelial layer is a dense mesenchymal layer that becomes the endometrial stroma as well as the surrounding myometrium. What later will become the glandular epithelium invaginates from buds arising in the luminal epithelium, growing into the underlying stroma.
By mid-gestation, the uterus has the appearance of the adult organ. After delivery, with the fall in maternal steroids, the endometrium may have an initial menstruation event, but then regresses to an inactive state, where it will remain until puberty and the rise in ovarian steroid secretions. With the initiation of cyclic menstrual cycles, the endometrium will undergo repetitive stages of development in response to follicular estrogen followed by ovulatory progesterone. These changes are predicated on the timely induction of cognate steroid receptors for both estrogen and progesterone, which orchestrate the genomic activation of thousands of endometrial genes. In the event that pregnancy does not occur, the endometrium breaks down and then is rapidly rebuilt until pregnancy is established. This process of menstruation, proliferation, and regeneration occurs without scarring and speaks to the seeming immortality of the endometrium, a structure that continues to proliferate throughout the woman’s life without deterioration.
Endometrial Receptivity
Anyone who studies the endometrial cycles and implantation, in particular, recognizes that the endometrium is non-receptive through much most of its cyclic changes. Our understanding of endometrial function comes largely from early animal studies on implantation [14,15,16,17]. Over 65 years ago, Noyes and colleagues described the histological changes that the endometrium undergoes during its cyclic development from menses to menses [18]. Within this 28-day menstrual cycle, receptivity toward the embryo only occurs for 3–5 days. The endometrium is unique as one of the few tissues into which an embryo will not attach and grow, except for a narrow period of uterine receptivity [19, 20]. This putative “window” of implantation, as first suggested by Finn [16], has been demonstrated in both animal models [17, 21] and in humans [6, 7].
As interest in the role of the endometrium in blastocyst attachment and invasion has increased, a great deal of research has been done to identify biomarkers of a receptive endometrium. Significant progress has been made in this field although the majority of potential biomarkers require further randomized studies in order to test their validity and clinical usefulness. The ideal biomarker is accurate, reproducible, and sensitive but should be able to be obtained by noninvasive means [22]. Potential sources of noninvasive biomarkers may include urine, saliva, vaginal fluid, cervical mucus, vaginal epithelial smears, blood, ultrasound, and basal body temperature measurement [23, 24].
Histologic Dating
The traditional “gold standard” for comparison of methods assessing the quality of luteal function remains histologic dating described by Noyes et al. in 1950 [18]. Use of this method leads to the description of the luteal phase defect (LPD) in which infertility and early pregnancy loss were thought to occur as a consequence of delayed endometrial maturation secondary to inadequate corpus luteum progesterone (P) production [25]. Since the 1950s, the clinical usefulness of histologic dating has been challenged off and on, due to methodological flaws noted in the original study as well as high inter-and intra-observer variation of histologic interpretation [26, 27]. The major flaw identified in the Noyes study is all of the endometrial samples were obtained from women with infertility, not from normally cycling parous controls. A subsequent prospective, randomized observational study reexamined histologic dating criteria in 130 regularly cycling, fertile women [26]. This landmark study concluded that endometrial dating does not have the accuracy or precision necessary to diagnose a luteal phase defect or guide clinical management of infertility. Furthermore, a prospective study of 847 subjects compared the endometrial biopsies of fertile and infertile patients. The pathologists, blinded to fertility status and menstrual day of biopsy, were not able to reliably discriminate between the two patient populations, and the authors recommended against the use of histologic dating in the routine evaluation of infertility [28].
Adhesion Molecules
At implantation, the trophectoderm of the embryo and the endometrial luminal epithelium acquire mutual adhesiveness [29]. Changes in cellular motility occur in response to adhesion and intracellular signaling. Embryos begin to exhibit a high rate of protrusion formation [30]. Sutherland captured the intrusive behavior of mouse embryos, with trophoblast cells probing out until finding a cleavage plane to intrude into the uterine wall [31]. The basis for this adhesion and signaling is complex and has been reviewed elsewhere [32,33,34] but represents an unsettled area of research as to the primary or most important adhesion molecule for embryo implantation.
Structural changes have also been found to occur on the luminal epithelium throughout implantation and thought to play a pivotal role in attachment. Pinopodes are cell membrane prominences on the apical cell membrane of the endometrial luminal epithelium. First identified in 1958 [35], they have since been investigated as potential biomarkers for endometrial receptivity. While their timing of expression appears to coincide with the window of implantation (WOI) [36, 37], not all studies agree. Pinopodes have been studied extensively by electron microscopy [37,38,39], and their appearance appears to be cycle dependent and under the control of progesterone [40]. They are visible by light microscopy as well [41] and are decorated with both endometrial integrins as well as osteopontin (OPN), two candidate biomarkers of embryo/endometrial attachment (Fig. 1.3a) [42]. Bentin-Ley has captured human embryos attaching to cultured endometrium, in vitro, seeming to show a preference for areas containing pinopode structures (Fig. 1.3b; [43]). Aplin has used similar techniques with mouse and human embryos and demonstrated the increased expression of OPN and integrin ανβ3 at the site of attachment [44]. They also showed that decreased attachment occurred if either OPN or the integrin was artificially downregulated. Despite the association between pinopode expression and the WOI, the clinical usefulness of their expression has been criticized. Arguments against the use of pinopodes as a biomarker of endometrial receptivity include their brief time of expression, the subjective nature of scoring them, and subsequent studies which have failed to show their temporal expression within the WOI [45,46,47].
Integrins are transmembrane glycoproteins which function as cell adhesion molecules (CAMs). They are formed from alpha and beta subunits which function as cell surface ligands between the embryo and the endometrium. Several integrins have been found to be only expressed during the WOI suggesting a role a possible biomarker for endometrial receptivity [48,49,50]. In humans, low expression of certain integrins has been linked to infertility [51, 52]. Furthermore, multiple studies have described abnormally low or absent levels of integrins, particularly the ανβ3 integrin, in inflammatory disease states associated with implantation failure such as endometriosis, polycystic ovarian syndrome, and hydrosalpinges [52,53,54,55].
Extracellular matrix proteins such as fibronectin and laminin are secreted by the endometrium under progesterone control [56]. These proteins have been found to interact with integrins and likely play a role in limiting trophoblastic invasiveness [57,58,59]. Damsky et al. [58, 60] have shown cells at the maternal-fetal interface switch their integrin phenotype expression at least twice during trophoblastic invasion. Fisher went on to show the importance of the ανβ3 integrin, a fibronectin receptor, as part of the mimicry cytotrophoblast uses to masquerade as endothelial cells and invade maternal vascular during early implantation [61]. High maternal levels of fibronectin have been associated with fetal growth restriction, hypertensive disorders, and abnormal umbilical artery Doppler in the third trimester of pregnancy [59]. These findings not only shed light on the well-orchestrated events that must occur in normal implantation, but they also provide a foundation to study disease processes where trophoblastic invasion is either insufficient or excessive (i.e., placenta accreta, preeclampsia, choriocarcinoma).
Selectins are carbohydrate-binding proteins known to mediate interactions between leukocytes and endothelium in the vasculature [62]. These proteins help facilitate leukocyte capture by L-selectin expression on the endothelial surface allowing “rolling adhesion” to slow the leukocyte to an eventual stop at the appropriate location. Genbacev et al. found selectin expression was also present at the maternal-fetal interface increases during the window of receptivity where it may play a similar role [63]. Studies suggest that L-selectin expression was increased in both the uterine epithelial cells and on the trophoblast cells suggesting these adhesion interactions may help slow the embryo down as it approaches the site of implantation [64]. The loss of L-selectin has been shown to occur in women with infertility [65, 66] suggesting that this class of molecules remains a promising area of interest.
Mucin 1 (MUC-1) , a glycoprotein, is found at many secretory epithelial sites throughout the body where it forms a mucin coating. In the endometrium, its expression is increased during the luteal phase and WOI where it is produced and secreted by the luminal epithelium [67]. MUC-1 has displayed both adhesive and anti-adhesive properties in various studies [32, 68, 69] suggesting a complex balance in its role in implantation. In humans, MUC-1 was found at the implantation site, but not at the surface of pinopodes possibly to allow the blastocyst to preferentially bind to these specialized structures [70].
Growth Factors and Cytokines
Several growth factors including insulin-like growth factor (IGF), heparin-binding epidermal growth factor (HB-EGF), and vascular endothelial growth factor (VEGF) have been identified whose expression in the endometrium coincides with the window of implantation [51, 72,73,74,75].
The two subtypes of insulin-like growth factors, IGF-I and IGF-II, appear to both play a role in implantation and placentation. IGF expression appears to correlate with estrogen concentration with IGF-I expressed primarily during the proliferative phase and IGF-II expression seen in the secretory endometrium [51]. IGF-I has been implicated in a variety of functions including endometrial proliferation [51, 71], placental function [72], and enhancement of embryo development and quality [73, 74]. IGF-II expression is seen at both the maternal-fetal interface in early human pregnancy and by the trophoblastic cells in early intrauterine pregnancies. The spatial expression of IGF at the decidual-trophoblastic interface suggests these peptides may function as mediators of trophoblastic invasion; however, the mechanism of this action remains unknown [75].
Heparin-binding epidermal growth factor (HB-EGF) expression within the uterus has been shown in both human and mouse models to occur in a cycle-dependent manner with its maximal expression occurring at the window of implantation [76]. Furthermore, immunohistochemistry staining for HB-EGF on endometrial biopsies have shown the coexistence of pinopodes with HB-EGF expression [77, 78]. HB-EGF is also expressed in early pregnancy on both the villous and extravillous trophoblastic tissue suggesting a role implantation and trophoblastic invasion [79]. Studies have shown its expression is associated with increased rates of embryo hatching and development and can also promote trophoblastic growth in vitro [80, 81]. Thus, HB-EGF appears to function in communication between the early embryo and endometrium although further studies are needed to clarify its exact role in implantation.
Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis throughout the body. VEGF is produced by both the embryo and endometrium during implantation highlighting its potential role in angiogenesis and vasodilation at the implantation site [78]. Interestingly, VEGF expression is increased in preeclampsia [82]. It is hypothesized that this increase occurs as a result of inadequate angiogenesis at the placentation site wherein VEGF is upregulated in a compensatory fashion. VEGF has also been studied in assisted reproduction. Elevated levels of VEGF appear to be markers of follicular hypoxia and suboptimal embryo development [83]. Dorn et al. found that higher serum concentrations of VEGF on the day of oocyte retrieval were correlated with IVF outcome; however, the mechanism behind these findings has not yet been elucidated [84].
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) comprise a family of zinc-dependent extracellular matrix (ECM)-degrading endopeptidases. MMPs, secreted by the cytotrophoblast, appear to play a key role in matrix degradation during trophoblastic invasion [85]. They can be classified into four subfamilies based on their substrate specificity and structure: gelatinases, collagenases, stromelysins, and a subfamily containing MMP-14, MMP-15, MMP-16, and MMP-17 [85]. Animal models suggest MMP-2 and MMP-9 (members of the gelatinase subfamily) have the most important role in ECM degradation and trophoblastic invasion [86,87,88,89,90,91]. Similar to MMPs, ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) are also proteolytic enzymes that likely contribute to the invasive properties of the blastocyst [92]. In particular, ADAM-TS5 is highly expressed by day 7 embryos with decreased expression thereafter suggesting this enzyme may play a role in proteolytic processing during the peri-implantation phase [85, 92]. MMP and ADAM are modulated locally by tissue inhibitors of metalloproteinases (TIMP). TIMP binds to and inhibits the active forms of MMP and ADAM within the extracellular space [85]. It appears the co-localization of MMP, ADAM, and TIMP at the maternal-fetal interface promotes implantation while also regulating the limits of trophoblastic invasion.
HOX Genes
The homeobox (Hox) genes encode transcription factors which guide embryologic development but have also been shown to regulate gene expression within the endometrium during the menstrual cycle [93]. The DNA-binding domains of these transcription factors are highly conserved across divergent organisms suggesting communal ancestry and genetic importance [94]. There are 39 HOX genes arranged in four parallel clusters (termed A, B, C, and D) [95]. HOXA10 and HOXA11 are expressed by endometrial glands and stroma at varying levels throughout menstruation [96]. Both genes are upregulated by 17β-estradiol and progesterone which are maximally expressed during the mid-secretory phase at the time of implantation. The spatial and temporal expression of HOXA10 and HOXA11 within the endometrium suggests a role in endometrial development, implantation, and maintenance of pregnancy [97]. Hox genes are known to mediate the expression of endometrial receptivity markers such as LIF, pinopodes, and integrin ανβ3. Furthermore, diseases associated with subfertility such as polycystic ovarian syndrome (PCOS), hydrosalpinges, and endometriosis have also been associated with defects of HOX gene expression [98,99,100]. Unfortunately, much of the available research regarding HOX genes and endometrial receptivity involves mouse models. The possible role of gene therapy involving manipulation of HOX expression to enhance implantation is promising although further research is necessary.
Prostaglandins
An increase in endometrial vascular permeability has been proposed as an essential requirement for trophoblastic implantation and decidualization [15, 101]. Prostaglandins (PGs) have been identified as important mediators of this localized vascular response in addition to playing a critical role in the decidualization reaction in animal models [102,103,104,105,106,107,108].
PGs are produced from arachidonic acid through the cyclooxygenase (COX) pathway. The rate-limiting step in this conversion pathway is the enzyme COX which exists in two isoforms, COX-1 and COX-2. PG-H2 is the common precursor for all prostaglandins produced from this pathway [PG-E2, PG-F2, PG-D2, thromboxane A2 (TX-A2), and prostacyclin (PG-I2)]. Uncertainty exists regarding the exact site of PG production although it appears both the blastocyst and the endometrium are able to produce PGs. Endometrial prostaglandin production changes throughout the menstrual cycle with increased PG-F2 and PG-E2 concentrations seen during the mid-luteal phase during the WOI [109,110,111,112,113,114]. This cyclical rise in PG production suggests a possible role in implantation. Furthermore, multiple studies using animal models have shown administration of nonsteroidal anti-inflammatory agents, such as indomethacin, inhibits prostaglandin synthesis which leads to inhibition or delay of decidualization and implantation [105, 115,116,117,118]. Additional studies have shown administration of exogenous PGs can overcome the effects of indomethacin on implantation [119, 120]. Despite substantial evidence to support the role of PGs in implantation and decidualization, significant knowledge gaps exist regarding the specific types of PGs involved as well as their specific mode of action.
Cytokines
Cytokines are a group of proinflammatory signaling proteins that control the immune response. They have also been implicated in playing an important role in mammalian implantation [121,122,123] and have been characterized as biomarkers as a noninvasive test of endometrial receptivity [124]. Implantation is associated with elevated levels of proinflammatory markers including cytokines, prostaglandins, and leukocytes [125]. Clinical findings have shown improved implantation rates when the endometrium is mechanically disrupted prior to embryo transfer in patients with recurrent pregnancy loss further supporting the importance of a proinflammatory environment during embryo implantation [121]. Proinflammatory cytokines identified at the maternal-fetal interface in early pregnancy include interleukin-1 (IL-1), interleukin-6 (IL-6), leukemia inhibitory factor (LIF), and numerous others [126, 127]. IL-1 exists as two subtypes, IL-1-α and IL-1-β. Both forms of IL-1 are under progesterone control although the receptor antagonist is not [128]. Animal experiments have shown IL-1 knockout mice are able to implant successfully, whereas implantation is impaired when their receptor is blocked with IL-1 receptor antagonist (IL-1ra) [125]. It is hypothesized that administration of IL-1ra causes downregulation of endometrial integrins leading to implantation failure [125].
LIF, an IL-6-like cytokine, is expressed by the human endometrium in a cycle-dependent manner with highest expression seen during the window of implantation [129]. LIF was one of the first cytokines shown to be essential for implantation of embryos in mice [130, 131] and in humans [132]. LIF affects trophoblastic differentiation, shifting the embryo toward a more adhesive phenotype [129, 133]. Studies of uterine flushings and endometrial biopsies of women with unexplained infertility and recurrent pregnancy loss have shown decreased expression of LIF when compared to fertile controls suggesting its role in implantation and establishment of pregnancy [134, 135]. Unfortunately, a recent randomized controlled trial (n = 149) using recombinant human LIF in patients with recurrent implantation failure did not show an improvement in implantation or pregnancy rates when compared to the placebo group [136]. Thus, further research is needed to investigate the complex implantation process in order to develop possible treatments to improve reproductive outcomes.
Theories of Endometrial Receptivity Defects
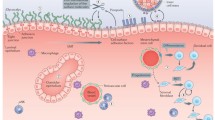
There remains much to understand about normal endometrial receptivity. The complexity of the process of implantation makes it also prone to dysfunction. Indeed, for every successful pregnancy culminating in live birth, there are a vast number of miscarriages, subclinical losses, and failed implantation events that preclude establishment of pregnancy [137]. We recently published a paradigm of endometrial receptivity defects that is focused on inflammation as the central defect [138]. As shown in Fig. 1.4, the activation of STAT3 by inflammatory cytokines, as seen in endometriosis, has been reported to recruit and stabilize hypoxia-induced factor 1-alpha (HIF1α) [139]. STAT3 also stabilizes a gene suppressor, BCL6 which is overexpressed in women with hydrosalpinges or endometriosis [140]. BCL6 appears to be a prime candidate as a cause of progesterone resistance along with SIRT1, which together have been shown to inhibit GLI1, which is involved in the progesterone-driven Indian Hedgehog pathway [141, 142]. Without progesterone working properly, progesterone-induced STAT5 [143] is not there to inhibit STAT3 [144]. Further, protein inhibitor of STAT3 (PIAS3) is also downregulated in inflammatory conditions such as endometriosis [145], which results in further chronic activation of STAT3. This favors estrogen action and proliferation and contributes to cyclooxygenase 2 (COX2), prostaglandin production, aromatase expression, angiogenesis, and inflammation. We believe this model helps explain why pregnancy can be difficult in the setting of inflammation and conditions such as endometriosis. Finally, the oncogene KRAS is elevated in endometriosis and thought to drive this elevation in SIRT1, contributing to progesterone resistance [142].
Model of endometrial dysfunction in the setting of inflammatory conditions including endometriosis. Inflammatory cytokines such as interferon gamma (INFg), tumor necrosis factor alpha (TNFa), and interleukin-1 and interleukin-17 (IL-1 and IL-17) stimulate downstream events including interleukin-6 (IL-6) that activates STAT3 and HIF-1a. Mechanisms to destabilize STAT3 are inhibited through BCL6 and progesterone resistance and by overexpression of protein inhibitor of stat3 (PIAS3). Together the activation of STAT3 and HIF1a promotes inflammation, angiogenesis, and proliferation. Further, in the setting of progesterone resistance, pregnancy and normal implantation does not easily occur (Used with permission from Fertility and Sterility [138]) (Used with permission of Elsevier Inc.)
Summary and Conclusions
An understanding of embryo implantation requires an extensive emersion into endocrinology, physiology, and cell biology. The concept of a window of implantation is a valid construct to frame the mechanisms of implantation and appreciate the temporal chain of events. Synchrony and cooperation between the embryo and endometrium appear critical to a successful pregnancy. Failure of implantation, while not covered by this introductory chapter, can be examined in the context of normal implantation and the molecular constraints required by synchrony and complex sequential events. There are multiple steps required for normal pregnancy to occur and conclude successfully. In the context of this book, many of those aspects will be discovered.
References
Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13:277–88.
Lessey BA, Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Semin Reprod Med. 2014;32:365–75.
Garry R, Hart R, Karthigasu KA, Burke C. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod. 2009;1:1–9.
Hertig AJ, Behrman SJ, Kistner RW. Implantation of the human ovum. In: Progress in infertility, vol. 1. Boston: Little, Brown, & Co.; 1975. p. 435.
Hertig AT, Rock J, Adams EC. A description of 34 human ova within the first 17 days of development. Am J Anat. 1956;98:435–93.
Navot D, Bergh P. Preparation of the human endometrium for implantation. Ann N Y Acad Sci. 1991;622:212–9.
Navot D, Bergh PA, Williams M, Garrisi GJ, Guzman I, Sandler B, Fox J, Schreiner-Engel P, Hofmann GE, Grunfeld L. An insight into early reproductive processes through the in vivo model of ovum donation. J Clin Endocrinol Metab. 1991;72:408–14.
Bergh PA, Navot D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992;58:537–42.
Bischof P, Aplin JD, Bentin-Ley U, Brannstrom M, Casslen B, Castrillo JL, Classen-Linke I, Critchley HO, Devoto L, D'Hooghe T, Horcajadas JA, Groothuis P, et al. Implantation of the human embryo: research lines and models. From the implantation research network ‘fruitful’. Gynecol Obstet Investig. 2006;62:206–16.
Bentin-Ley U, Sjîgren A, Nilsson L, Hamberger L, Larsen JF, Horn T. Presence of uterine pinopodes at the embryo-endometrial interface during human implantation in vitro. Hum Reprod. 1999;14:515–20.
Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8.
Burton GJ, Jauniaux E, Charnock-Jones DS. Human early placental development: potential roles of the endometrial glands. Placenta. 2007;28(Suppl A):S64–9.
Lessey BA, Young SL. The structure, function and evaluation of the female reproductive tract. In: Strauss JFI, Barbieri RL, editors. Reproductive endocrinology: physiology, pathology and clinical management, vol. 7. Philadelphia: Saunders Elsevier; 2012. p. 192–235.
McLaren A, Michie D. Studies on the transfer of fertilized mouse eggs to uterine foster-mothers. J Exp Biol. 1954;33:394.
Psychoyos A. Hormonal control of ovoimplantation. Vitams Horm. 1973;31:201–56.
Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39:195–206.
Hodgen GD. Surrogate embryo transfer combined with estrogen-progesterone therapy in monkeys: implantation, gestation, and delivery without ovaries. JAMA. 1983;250:2167–71.
Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25.
Fawcett DW. The development of mouse ova under the capsule of the kidney. Anat Rec. 1950;108:71.
Kirby DR. The development of mouse blastocysts transplanted to the scrotal and cryptorchid testis. J Anat. 1963;97:119.
Beier HM. Oviducal and uterine fluids. J Reprod Fertil. 1974;37:221–37.
Campbell KL, Rockett JC. Biomarkers of ovulation, endometrial receptivity, fertilisation, implantation and early pregnancy progression. Paediatr Perinat Epidemiol. 2006;20(Suppl 1):13–25.
May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17:637–53.
May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16:651–74.
Jones GS. Some newer aspects of management of infertility. JAMA. 1949;141:1123–9.
Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–43.
Practice Committee of the American Society for Reproductive M. Current clinical irrelevance of luteal phase deficiency: a committee opinion. Fertil Steril. 2015;103:e27–32.
Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP, Silva S, Vogel DL, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–72.
Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod. 1975;12:41.
Martin PM, Sutherland AE. Exogenous amino acids regulate trophectoderm differentiation in the mouse blastocyst through an mTOR-dependent pathway. Dev Biol. 2001;240:182–93.
Sutherland A. Mechanisms of implantation in the mouse: differentiation and functional importance of trophoblast giant cell behavior. Dev Biol. 2003;258:241–51.
Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–37.
Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25:461–75.
Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–67.
Nilsson O. Ultrastructure of mouse uterine surface epithelium under different estrogenic influences. 5. Continuous administration of estrogen. J Ultrastruct Res. 1959;2:342–51.
Psychoyos A, Mandon P. Study of the surface of the uterine epithelium by scanning electron microscope. Observations in the rat at the 4th and 5th day of pregnancy. C R Acad Sci Hebd Seances Acad Sci D. 1971;272:2723–5.
Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, Psychoyos A. Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod. 1995;10:1208–13.
Psychoyos A, Nikas G. Uterine pinopodes as markers of uterine receptivity. Assist Reprod Rev. 1994;4:26–32.
Nikas G. Cell-surface morphological events relevant to human implantation. Hum Reprod. 1999;14(Suppl 2):37–44.
Martel D, Monier MN, Roche D, Psychoyos A. Hormonal dependence of pinopode formation at the uterine luminal surface. Hum Reprod. 1991;6:597.
Develioglu OH, Nikas G, Hsiu JG, Toner JP, Jones HW Jr. Detection of endometrial pinopodes by light microscopy. Fertil Steril. 2000;74:767–70.
Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, Lessey BA. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab. 2001;86:4991–5000.
Bentin-Ley U, Horn T, Sjîgren A, Sorensen S, Larsen JF, Hamberger L. Ultrastructure of human blastocyst-endometrial interactions in vitro. J Reprod Fertil. 2000;120:337–50.
Kang YJ, Forbes K, Carver J, Aplin JD. The role of the osteopontin-integrin alphavbeta3 interaction at implantation: functional analysis using three different in vitro models. Hum Reprod. 2014;29:739–49.
Acosta AA, Elberger L, Borghi M, Calamera JC, Chemes H, Doncel GF, Kliman H, Lema B, Lustig L, Papier S. Endometrial dating and determination of the window of implantation in healthy fertile women. Fertil Steril. 2000;73:788–98.
Quinn CE, Casper RF. Pinopodes: a questionable role in endometrial receptivity. Hum Reprod Update. 2009;15:229–36.
Usadi RS, Murray MJ, Bagnell RC, Fritz MA, Kowalik AI, Meyer WR, Lessey BA. Temporal and morphologic characteristics of pinopod expression across the secretory phase of the endometrial cycle in normally cycling women with proven fertility. Fertil Steril. 2003;79:970–4.
Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994;62:497–506.
Reddy KV, Meherji PK. Integrin cell adhesion molecules in endometrium of fertile and infertile women throughout menstrual cycle. Indian J Exp Biol. 1999;37:323–31.
Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–46.
Hoozemans DA, Schats R, Lambalk CB, Homburg R, Hompes PG. Human embryo implantation: current knowledge and clinical implications in assisted reproductive technology. Reprod Biomed Online. 2004;9:692–715.
Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63:535–42.
Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–9.
Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, Lessey BA. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod. 1997;12:1393–8.
Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66:297–304.
Zhu HH, Huang JR, Mazela J, Elias J, Tseng L. Progestin stimulates the biosynthesis of fibronectin and accumulation of fibronectin mRNA in human endometrial stromal cells. Hum Reprod. 1992;7:141–6.
Giudice LC. Potential biochemical markers of uterine receptivity. Hum Reprod. 1999;14(Suppl 2):3–16.
Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–66.
Karsdorp VH, Dekker GA, Bast A, van Kamp GJ, Bouman AA, van Vugt JM, van Geijn HP. Maternal and fetal plasma concentrations of endothelin, lipidhydroperoxides, glutathione peroxidase and fibronectin in relation to abnormal umbilical artery velocimetry. Eur J Obstet Gynecol Reprod Biol. 1998;80:39–44.
Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–22.
Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate - a strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–51.
Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104.
Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yan ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–8.
Fazleabas AT, Kim JJ. Development. What makes an embryo stick? Science. 2003;299:355–6.
Foulk RA, Zdravkovic T, Genbacev O, Prakobphol A. Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. J Assist Reprod Genet. 2007;24:316–21.
Margarit L, Gonzalez D, Lewis PD, Hopkins L, Davies C, Conlan RS, Joels L, White JO. L-selectin ligands in human endometrium: comparison of fertile and infertile subjects. Hum Reprod. 2009;24:2767–77.
Hey NA, Graham RA, Seif MW, Aplin JD. The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab. 1994;78:337–42.
Hey NA, Aplin JD. Sialyl-Lewis x and Sialyl-Lewis a are associated with MUC1 in human endometrium. Glycoconj J. 1996;13:769–79.
Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77.
Lessey BA. Two pathways of progesterone action in the human endometrium: implications for implantation and contraception. Steroids. 2003;68:809–15.
Humbel RE. Insulin-like growth factors I and II. Eur J Biochem. 1990;190:445–62.
Murata K, Maruo T, Matsuo H, Mochizuki M. insulin-like growth factor-I (IGF-I) as a local regulator of proliferation and differentiation of villous trophoblasts in early pregnancy. Nihon Sanka Fujinka Gakkai Zasshi. 1994;46:87–94.
Oner J, Oner H. Immunolocalization of insulin-like growth factor I (IGF-I) during preimplantation in rat uterus. Growth Hormon IGF Res. 2007;17:271–8.
Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol. 1998;12:399–406.
Van Sinderen M, Menkhorst E, Winship A, Cuman C, Dimitriadis E. Preimplantation human blastocyst-endometrial interactions: the role of inflammatory mediators. Am J Reprod Immunol. 2013;69:427–40.
Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–55.
Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8:765–9.
Smith SK. Angiogenesis and implantation. Hum Reprod. 2000;15(Suppl 6):59–66.
Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–63.
Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–83.
Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–52.
Baker PN, Krasnow J, Roberts JM, Yeo KT. Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet Gynecol. 1995;86:815–21.
Barroso G, Barrionuevo M, Rao P, Graham L, Danforth D, Huey S, Abuhamad A, Oehninger S. Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid levels correlate negatively with embryo quality in IVF patients. Fertil Steril. 1999;72:1024–6.
Dorn C, Reinsberg J, Kupka M, van der Ven H, Schild RL. Leptin, VEGF, IGF-1, and IGFBP-3 concentrations in serum and follicular fluid of women undergoing in vitro fertilization. Arch Gynecol Obstet. 2003;268:187–93.
Minas V, Loutradis D, Makrigiannakis A. Factors controlling blastocyst implantation. Reprod Biomed Online. 2005;10:205–16.
Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–36.
Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev Genet. 1997;21:44–54.
Maia-Filho VO, Rocha AM, Ferreira FP, Bonetti TC, Serafini P, Motta EL. Matrix metalloproteinases 2 and 9 and e-cadherin expression in the endometrium during the implantation window of infertile women before in vitro fertilization treatment. Reprod Sci. 2015;22:416–22.
Rechtman MP, Zhang J, Salamonsen LA. Effect of inhibition of matrix metalloproteinases on endometrial decidualization and implantation in mated rats. J Reprod Fertil. 1999;117:169–77.
Riley SC, Webb CJ, Leask R, McCaig FM, Howe DC. Involvement of matrix metalloproteinases 2 and 9, tissue inhibitor of metalloproteinases and apoptosis in tissue remodelling in the sheep placenta. J Reprod Fertil. 2000;118:19–27.
Salamonsen LA, Nagase H, Woolley DE. Matrix metalloproteinases and their tissue inhibitors at the ovine trophoblast-uterine interface. J Reprod Fertil Suppl. 1995;49:29–37.
Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–63.
Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–45.
Kappen C, Schughart K, Ruddle FH. Early evolutionary origin of major homeodomain sequence classes. Genomics. 1993;18:54–70.
Schughart K, Kappen C, Ruddle FH. Mammalian homeobox-containing genes: genome organization, structure, expression and evolution. Br J Cancer Suppl. 1988;9:9–13.
Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–84.
Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84:1129–35.
Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:238–43.
Daftary GS, Taylor HS. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril. 2002;78:577–80.
Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31.
Psychoyos A, Nikas G, Gravanis A. The role of prostaglandins in blastocyst implantation. Hum Reprod. 1995;10(Suppl 2):30–42.
Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16:107–22.
Gupta A, Huet YM, Dey SK. Evidence for prostaglandins and leukotrienes as mediators of phase I of estrogen action in implantation in the mouse. Endocrinology. 1989;124:546–8.
Johnson DC, Dey SK. Role of histamine in implantation: dexamethasone inhibits estradiol-induced implantation in the rat. Biol Reprod. 1980;22:1136–41.
Kennedy TG. Evidence for a role for prostaglandins in the initiation of blastocyst implantation in the rat. Biol Reprod. 1977;16:286–91.
Lau IF, Saksena SK, Chang MC. Pregnancy blockade by indomethacin, an inhibitor of prostaglandin synthesis: its reversal by prostaglandins and progesterone in mice. Prostaglandins. 1973;4:795–803.
Malathy PV, Cheng HC, Dey SK. Production of leukotrienes and prostaglandins in the rat uterus during peri-implantation period. Prostaglandins. 1986;32:605–14.
Tawfik OW, Sagrillo C, Johnson DC, Dey SK. Decidualization in the rat: role of leukotrienes and prostaglandins. Prostaglandins Leukot Med. 1987;29:221–7.
Brumsted JR, Chapitis J, Deaton JL, Riddick DH, Gibson M. Prostaglandin F2 alpha synthesis and metabolism by luteal phase endometrium in vitro. Fertil Steril. 1989;52:769–73.
Ishihara O, Tsutsumi O, Mizuno M, Kinoshita K, Satoh K. Metabolism of arachidonic acid and synthesis of prostanoids in human endometrium and decidua. Prostaglandins Leukot Med. 1986;24:93–102.
Maathuis JB, Kelly RW. Concentrations of prostaglandins F2alpha and E2 in the endometrium throughout the human menstrual cycle, after the administration of clomiphene or an oestrogen-progestogen pill and in early pregnancy. J Endocrinol. 1978;77:361–71.
Salamonsen LA, Findlay JK. Regulation of endometrial prostaglandins during the menstrual cycle and in early pregnancy. Reprod Fertil Dev. 1990;2:443–57.
Singh EJ, Baccarini I, Zuspan FP. Levels of prostaglandins F-2alpha and E-2 in human endometrium during the menstrual cycle. Am J Obstet Gynecol. 1975;121:1003–6.
van der Weiden RM, Helmerhorst FM, Keirse MJ. Influence of prostaglandins and platelet activating factor on implantation. Hum Reprod. 1991;6:436–42.
Evans CA, Kennedy TG. The importance of prostaglandin synthesis for the initiation of blastocyst implantation in the hamster. J Reprod Fertil. 1978;54:255–61.
Hoos PC, Hoffman LH. Effect of histamine receptor antagonists and indomethacin on implantation in the rabbit. Biol Reprod. 1983;29:833–40.
Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134:635–43.
Lundkvist O, Nilsson BO. Ultrastructural studies of the temporal relationship between loss of zona pellucida and appearance of blastocyst-induced stromal changes during normal pregnancy in rats. Anat Embryol. 1984;170:45–9.
Kennedy TG, Doktorcik PE. Effects of analogues of prostaglandin E2 and F2 alpha on the decidual cell reaction in the rat. Prostaglandins. 1988;35:207–19.
Oettel M, Koch M, Kurischko A, Schubert K. Direct evidence for the involvement of prostaglandin F2 alpha in the first step of estrone-induced blastocyst implantation in the spayed rat. Steroids. 1979;33:1–8.
Granot I, Gnainsky Y, Dekel N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction. 2012;144:661–8.
Kelly RW, King AE, Critchley HO. Cytokine control in human endometrium. Reproduction. 2001;121:3–19.
Ross JW, Malayer JR, Ritchey JW, Geisert RD. Characterization of the interleukin-1beta system during porcine trophoblastic elongation and early placental attachment. Biol Reprod. 2003;69:1251–9.
Boomsma CM, Kavelaars A, Eijkemans MJ, Amarouchi K, Teklenburg G, Gutknecht D, Fauser BJ, Heijnen CJ, Macklon NS. Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod Biomed Online. 2009;18:85–94.
Simon C, Valbuena D, Krussel J, Bernal A, Murphy CR, Shaw T, Pellicer A, Polan ML. Interleukin-1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium. Fertil Steril. 1998;70:896–906.
Blitek A, Morawska E, Ziecik AJ. Regulation of expression and role of leukemia inhibitory factor and interleukin-6 in the uterus of early pregnant pigs. Theriogenology. 2012;78:951–64.
Modric T, Kowalski AA, Green ML, Simmen RC, Simmen FA. Pregnancy-dependent expression of leukaemia inhibitory factor (LIF), LIF receptor-beta and interleukin-6 (IL-6) messenger ribonucleic acids in the porcine female reproductive tract. Placenta. 2000;21:345–53.
Simon C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1 beta in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1993;77:549–55.
Lass A, Weiser W, Munafo A, Loumaye E. Leukemia inhibitory factor in human reproduction. Fertil Steril. 2001;76:1091–6.
Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. ProcNatlAcad SciUS A. 1991;88:11408–12.
Stewart CL. The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals. Ann N Y Acad Sci. 1994;734:157.
Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93:3115–20.
Nachtigall MJ, Kliman HJ, Feinberg RF, Olive DL, Engin O, Arici A. The effect of leukemia inhibitory factor (LIF) on trophoblast differentiation: a potential role in human implantation. J Clin Endocrinol Metab. 1996;81:801–6.
Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39:137–43.
Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod. 1997;12:569–74.
Brinsden PR, Alam V, de Moustier B, Engrand P. Recombinant human leukemia inhibitory factor does not improve implantation and pregnancy outcomes after assisted reproductive techniques in women with recurrent unexplained implantation failure. Fertil Steril. 2009;91:1445–7.
Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update. 2002;8:333–43.
Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105:873–84.
Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–78.
Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, Schammel DP, Young SL. Endometrial BCL6 overexpression in Eutopic endometrium of women with endometriosis. Reprod Sci. 2016;23:1234–41.
Tiberi L, Bonnefont J, van den Ameele J, Le Bon SD, Herpoel A, Bilheu A, Baron BW, Vanderhaeghen P. A BCL6/BCOR/SIRT1 complex triggers neurogenesis and suppresses medulloblastoma by repressing sonic hedgehog signaling. Cancer Cell. 2014;26:797–812.
Yoo JY, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C, Schammel DP, Young SL, Jeong JW, Lessey BAKRAS. Activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep. 2017;7:6765.
Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55:795–810.
Walker SR, Nelson EA, Yeh JE, Pinello L, Yuan GC, Frank DA. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol Cell Biol. 2013;33:2879–90.
Yoo JY, Jeong JW, Fazleabas AT, Tayade C, Young SL, Lessey BA. Protein inhibitor of activated STAT3 (PIAS3) is down-regulated in Eutopic endometrium of women with endometriosis. Biol Reprod. 2016;95(1):11.
Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, Kim MK. Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin alphanubeta3) expression in peri-implantation mouse embryos. J Assist Reprod Genet. 2006;23:111–9.
Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Fox, C., Lessey, B.A. (2018). Signaling Between Embryo and Endometrium: Normal Implantation. In: Franasiak, J., Scott Jr., R. (eds) Recurrent Implantation Failure. Springer, Cham. https://doi.org/10.1007/978-3-319-71967-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-71967-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71966-5
Online ISBN: 978-3-319-71967-2
eBook Packages: MedicineMedicine (R0)