Abstract
Most of the assisted reproductive technologies (ARTs) overcome the selection of spermatozoa occurring within the female genital tract in mammals. This selection ensures the fertilization with spermatozoa containing chromatin of high integrity for supporting the ulterior embryo development. Consequently the use of nonselected spermatozoa porting fragmented DNA has been related to developmental and postnatal effects in animal models. The present chapter provides an overview on the implications of using sperm porting fragmented DNA in the reproductive outcome focusing in the knowledge acquired through experimentation in animal models. This is highly relevant given that intracytoplasmic sperm injection (ICSI) has been widely used in fertility treatments in humans. However, experimentation in mouse has demonstrated the risk of using DNA-fragmented spermatozoa in ICSI reducing the embryo and fetal performance as well as provoking long-term deleterious effects in the offspring at adulthood. In addition, the use of storage techniques for spermatozoa such as freezing-thawing and vitrification has been shown to increase the risk of fertilizing with fragmented DNA. Together with mammalian studies, the use of other nonmammalian animal models like fish could help in understanding the consequences of these treatments and to develop new strategies for sperm evaluation and selection before applying ARTs.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Assisted reproductive technologies (ARTs)
- Sperm DNA fragmentation
- Intracytoplasmic sperm injection (ICSI)
- Sperm selection
- Sperm cryopreservation
- DNA repairmen in fish oocyte
1 Sperm Selection in the Female Genital Tract and DNA Fragmentation
Mammalian spermatozoa must overcome a number of obstacles along the female genital tract before reaching the fertilization site at the ampulla. The vaginal pH, the resistance by cervical mucus to sperm migration, the narrowness of the uterotubal junction, the tortuosity of the oviductal lumen, the response of the immune system, etc. are physio-anatomical conditions of the female genital tract that configure a stringent selection mechanism for those spermatozoa with certain features [1, 2]. In all mammalian species studied to date, among many millions of spermatozoa ejaculated only tens to hundreds reach the ampulla [3,4,5,6], where the fertilization occurs. Presumably, this is a select group of spermatozoa with higher fertilization capability and better characteristics for supporting embryo development. However, little is known about this sperm subpopulation and its relative effectiveness as well as about which are the characteristics that are selected in vivo (for a review see Sakkas et al. [2]). In fact, if there is a mechanism for sperm selection that has evolved in mammalians through Darwinian forces, the genetic material ported in this sperm subpopulation should be of high integrity for ensuring a successful embryo development and a correct transmission of the genetic information. Unfortunately, only few studies have addressed directly or indirectly this hypothesis.
In the vagina, the first selective barrier encountered by spermatozoa is the viscosity of the cervical mucus. This secretion has been pointed to positively select motile spermatozoa exhibiting specific kinetics and normal motility to pass across the cervix to the uterus. Whereas the selective function of the cervical mucus has never been satisfactorily proven in vivo, the ability of spermatozoa to migrate within it has been correlated to sperm quality and selection [7], especially regarding sperm kinetics [8]. In a study conducted in mouse, Hourcade et al. [9] illustrated that the spermatozoa in the uterus show a higher level of fragmented DNA compared to spermatozoa retrieved from the epididymis, going against the selective function of the cervical mucus. It has been suggested that the fragmentation of the DNA might be provoked by the immune responses occurring in the cervix and uterus in response to the sperm migration [10] or because of the presence of nucleases in the seminal fluid affecting the spermatozoa in the uterus [11, 12]. Hourcade et al. [9] also demonstrated that there is a strong positive selection in the uterotubal junction for spermatozoa carrying low fragmented DNA. Thus, from all the highly DNA-damaged spermatozoa found in the uterus, this selective checkpoint allows only the sperm subpopulation containing DNA of high integrity to enter the oviduct. The selective function of the uterotubal junction in mouse has been also pointed by Nakanishi et al. [13], showing that from chimeric mice porting a sperm subpopulation lacking functional testis-specific putative chaperone, only the spermatozoa with the wild-type phenotype entered the oviduct. Furthermore, it has been postulated that the involvement of the sperm reservoir, present at this location, is important in aiding in the selection of spermatozoa able to interact with the epithelium [14].
Once in the oviduct, it is currently accepted that the spermatozoa must be actively guided in order to reach the fertilization site. To date, two sperm tropism mechanisms (sperm thermotaxis and rheotaxis), operating both as long-range guidance mechanisms within the oviduct, and a third one (chemotaxis) for guiding the spermatozoa in the proximity of the oocyte at the fertilization site have been proposed [15]. Thermotaxis has been described for human, mouse [16], and rabbit spermatozoa [17], whereas rheotaxis has been found in human and mouse spermatozoa [18] and chemotaxis in a large variety of species mammals [19]. The tropism shown as a response of the spermatozoa to the stimuli in vitro together with the existence of well-defined molecular mechanisms in the spermatozoa for each of them [16, 18, 20] points to their functioning in vivo. This hypothesis is reinforced by the use of mouse strains to which the receptors for thermotaxis was knocked out affecting the sperm migration in a temperature gradient. In addition the stimuli for each of the tropism (temperature gradient, oviductal fluid flow, and chemoattractants) have been found to exist in the oviduct [15]. Thus, the ability of the spermatozoa to direct their swimming direction in response to these guidance stimuli could provide a sort of selective mechanisms that could be also linked to the genomic integrity of the spermatozoa. This is a very interesting hypothesis that however no one has ever approached.
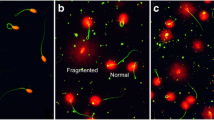
When spermatozoa encounter the oocyte, they have to penetrate the zona pellucida (ZP) . Thus, the ZP could function as a last selective barrier. Accordingly, two investigations on in vitro fertilization (IVF) have reported that mouse and human spermatozoa attached to the ZP exhibit lower level of DNA fragmentation compared to the nonattached spermatozoa [9, 21]. Furthermore, when the ZP-attached spermatozoa were used for ICSI, they failed to generate viable blastocysts [9]. Together these results indicate a fine-tuned selection process occurring during ZP penetration of spermatozoa able to support embryo development. This ZP-mediated selection seems to be linked to the source and type of sperm damage because experiments performed by Hourcade et al. [9] in mouse show that when the sperm DNA fragmentation was generated by ɣ[gamma]-radiation during spermatogenesis, there were a decrease in the production of blastocysts by IVF and a reduction in the percentage of implantations in vivo. Conversely, following the same experiment but generating the DNA fragmentation by heat shock, the blastocyst production by IVF and the percentage of implantation in vivo were similar to the control using undamaged spermatozoa [9]. This discrimination of the sperm damage is possibly related to the effect of the heat shock on different structures of the spermatozoa that then are negatively selected by the female genital tract. On the other hand, the ɣ[gamma]-radiation affects mainly the DNA leaving the rest of the sperm structures undamaged.
Since the female reproductive tract cannot get direct access to the sperm nucleus for assessing directly the DNA quality of the spermatozoa, the selection has to be based in other sperm features linked to the integrity of the genetic material. Consistently, also Hourcade et al. [9] showed that the subpopulation of mouse spermatozoa with the highest velocities separated in vitro contained lower level of fragmented DNA than the whole sperm population. Other studies have shown a negative correlation between various sperm quality parameters and DNA fragmentation levels in humans [22, 23] and in other animals such as turkey [24] and ram [25]. Kasimanickam et al. [26], employing heterospermic doses of bulls for the insemination of receptive cows, showed that the female genital tract selected the spermatozoa from those bulls reporting lower DNA fragmentation and higher plasma membrane integrity. As suggested by Holt and Fazeli [27], these results point to a connection between the status of the DNA integrity and externally exposed characteristics of the spermatozoa, for example, some plasma membrane components that could be “read” at the surface of the spermatozoa as a “passport” by the female genital tract. These authors gone even further in their hypothesis of the “cryptic female choice” suggesting a connection between the spermatozoa features and the genotype contained in its nucleus over which the female genital tract could select the spermatozoa containing specific sets of genes.
Sperm selection is a challenging field of research that still needs to address basic questions for a deeper understanding of the fundamental mechanism involved in the selection of the spermatozoa within the female genital tract. Animal experiments will certainly contribute to the discovery of the sperm characteristics that are selected, their linkage to the DNA integrity and to the reproductive outcome. This basic knowledge would be of great interest for designing procedures for the in vitro selection of spermatozoa that eventually could improve the outcomes of the currently poorly efficient assisted reproductive technologies (ARTs).
2 Long-Term Effects of Mouse Intracytoplasmic Sperm Injection with DNA-Fragmented Sperm on Health and Behavior of Adult Offspring
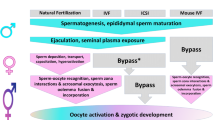
Nowadays 1% of babies born in the first world are conceived using assisted reproductive techniques (ART). Except for artificial insemination, ARTs bypass the sperm selection occurring within the female genital tract. This is especially relevant in case of the intracytoplasmic sperm injection (ICSI) and could explain the low efficiency of ART in general [28]. Nearly half of the male patients diagnosed as infertile show high levels of sperm DNA damage [29], and most patients subjected to fertility treatments show alterations in the sperm chromatin [30,31,32,33]. Moreover, low sperm counts have been related to higher presence of chromosomal aberrations in the spermatozoa. Azoospermic males show a higher frequency of numerical sex chromosome alterations such as XXY or XYY [34] and oligozoospermic have a higher frequency of translocations at autosomal chromosomes [35]. It has also been reported that mutations causing infertility could be transmitted to the male descendants, such as Y-chromosome deletions [36, 37]. Therefore, when applying ICSI, the probability of choosing a sperm with damaged chromatin, fragmented DNA, or any kind of genetic alteration should not be neglected. This is especially important considering that using ICSI, the DNA-fragmented spermatozoa (DFS) are able to fertilize oocyte resulting in pronucleus formation, chromatin decondensation, and embryos developing to blastocyst stage as was shown in mouse [38]. But human oocyte can partially repair low DNA fragmentation levels before cleavage leading to a viable embryo to blastocyst, the fertilization with spermatozoa containing highly fragmented DNA reduces pregnancy rates [39]. Furthermore, abnormal fetal karyotypes have been found in the offspring of spermatozoa containing aberrant DNA and processed by ICSI, resulting from numerical or structural sex chromosomal anomalies and autosomal anomalies both inherited and de novo [40]. It is known that all these aberrations in the DNA produce alterations on fertility and failures, affecting pregnancy rates and the health of the adult descendants. Since the first humans born from ICSI procedures are nearly 25 years old, the long-term effects in adulthood remain unknown. That is why it is important to study the possible consequences on the health of ICSI offspring with DNA-fragmented sperm through animal models.
Using epididymal mouse spermatozoa, Yamauchi et al. [41] demonstrated that sperm DNA damage induced by various treatments persists after ICSI without changes. Epididymal mouse spermatozoa were either frozen without cryoprotectant or treated with Triton X-100 together with dithiothreitol to induce DNA damage. Both treatment groups showed increased sperm DNA fragmentation when compared to untreated group used as control. After ICSI, chromosome analysis demonstrated paternal DNA damage in those oocytes injected with both sperm-treated groups, frozen-thawed, or Triton X-100 but not with fresh sperm. However, there were no differences in the incidence of abnormal paternal karyoplates prior and after DNA synthesis in all the examined groups. Fernández-Gonzalez et al. [38] analyzed the short- and long-term effects of ICSI using DFS in a mouse model. In their work, DNA fragmentation was produced by freezing and thawing epididymal spermatozoa retrieved from B6D2F1 males. In addition to the DNA damage, telomere loss was also observed. Oocytes were then injected with fresh- or frozen-thawed spermatozoa, and the resultant two-cell embryos were transferred to pseudopregnant CD1 females. The first notorious effect noticed was a delay of 2 h on the active demethylation of male pronucleus in those embryos produced by ICSI with DFS. Furthermore, when ICSI-DFS was performed, both the rate of preimplantation embryo development and litter size were reduced, and the transcription and methylation of epigenetically regulated genes were altered. In addition, adult animals produced by ICSI showed behavioral alterations as well as abnormal weight gain and anatomopathological alterations including solid tumors in the lungs and dermis and premature aging symptoms. Moreover, surviving rates of mice generated with ICSI-DFS were reduced dramatically compared with in vivo controls. This work concluded that depending on the level of DFS, oocytes may either repair fragmented DNA, producing blastocysts able to implant and produce live offspring, or partially repair DNA damage leading to short- and long-term alterations or completely fail on repairing DNA aberrations producing the death of the embryo.
3 Effects of Intracytoplasmic Sperm Injection Using DNA-Fragmented Spermatozoa on Embryo-Derived Embryonic Stem Cells and on Transgenerational Heritability of Epiallele in Mice
Embryonic stem cells (ESCs) are commonly used as a valuable model to analyze embryonic development. Thus, Moreira et al. [42] reported that mouse embryos produced by DFS-ICSI show a reduced efficiency for ESC derivation that was suggested to be related to the low quality of the DFI-ICSI-derived embryos. Consistently, these embryos show low implantation and development rates. In another study, Yamagata et al. [43] reported that 40% of DFI-ICSI-generated mouse embryos show abnormal chromosome segregation and chromosome fragmentation. Although these embryos developed to normal-looking blastocysts, almost all of them were lost shortly after implantation, and embryos with abnormal karyotype are less capable of generating ESC lines. Furthermore, alterations of the gene expression in the ESCs lines generated with DFS-ICSI embryos were found at early passages: abnormalities at the cellular level were associated with embryo performance and offspring health. The genetic alterations described in the ESC lines include alterations in DNA methylation and histone acetylation, on pluripotency, on epigenetic gene silencing, as well as on DNA damage and genes related to its reparation. However, these alterations were not maintained in the long-term culture [44]. Interestingly, males of the offspring produced by DFS-ICSI showed alterations in the testes, including low weight, reduced spermatogenesis, morphological abnormalities in the seminiferous tubules, and an increased number of apoptotic cells [44]. Sperm quantity, vaginal plug detection, and pregnancy rates after mating were also significantly lower in these animals, while the number of females showing resorptions was higher. Moreover, a significant decrease in pregnancy rates and an increase in the resorptions rate related to the age were reported. These results suggest a deleterious effect of the DNA damage when DFS-ICSI is used in the resultant embryos that affects the male germ line and could transmit genetic alterations toward following generations. Consistently, it has been reported that DFS-ICSI induces epigenetic modifications that are transmitted to the progeny. Axin1Fu allele is a locus very sensitive to epigenetic alterations which regulates embryonic axis formation in vertebrates. In mice the Axin1Fu phenotype consists of kinks in the tail, which are determined by the DNA methylation pattern. Modifications in this allele may persist across several generations because its methylation state in mature spermatozoa is identical to somatic cells, indicating that it is not epigenetically reprogrammed during gametogenesis [45]. Using spermatozoa retrieved from Axin1Fu/+ mice in ICSI revealed a higher proportion of pups in the second generation expressing the active kinky-tail epiallele, indicating that this procedure affected the postnatal expression of Axin1Fu and that this modification was inherited across generations [44].
The experiments in animal models conducted to date reveal that the analyses of sperm DNA damage are critical when ARTs are applied. This is especially relevant for ICSI because all the barriers of sperm selection operating along the female genital tract are being bypassed. In an era in which advanced forms of ART are frequently used in clinical treatments of fertility, it is essential to apply protocols and methodologies for preselecting the sperm samples or for the separation of spermatozoa carrying the genetic material integral in order to avoid deleterious effects in the offspring.
4 Cryopreservation and Damage of the Sperm DNA
Cryopreservation of gametes has been widely used over the last century in human assisted reproduction, animal breeding, and conservation programs for endangered species. Although several protocols have been developed for both male and female gametes, sperm cryopreservation is the most extensively used technique. The limited volume of spermatozoa cytoplasm, together with their small size, makes sperm particularly suitable for cryopreservation. Moreover, some protocols, coupled to recently developed ARTs, permit long-term storage of freeze-dried sperm, even at room temperature, capable of producing live offspring [46]. However, cryopreservation is not completely effective in stopping sperm degradation because its quality declines with storage time [47]. The effects of DNA fragmentation after sperm cryopreservation are also a controversial issue as it adversely affects early embryonic development and results in reduced implantation rates and pregnancy outcomes [48]. Fluctuation of media pH [49], osmotic stress [50, 51] or the cryoprotectant used [52, 53] may increase the amount of reactive oxygen species (ROS) inside the cell or activate endonucleases that ultimately lead to breaks in the sperm DNA. Interestingly, cryopreservation seems to cause more DNA fragmentation to subfertile or infertile males [54], so perhaps inherent defects in the spermatozoa could enhance cryodamage. This also points to the need for evaluating the DNA integrity of the cryopreserved spermatozoa of this type of patients when subjected to fertility treatments involving ARTs and for the improvement of the sperm cryopreservation procedures.
Conventional cryopreservation (also known as slow-freezing) results in slowly lowering cell temperature until enzymatic reactions cannot take place within the cytoplasm. As intracellular milieu is an aqueous environment, ice crystals are formed inside the cells during freezing process, and this causes deleterious effects on sperm, like membrane damage, leading to decreased motility and viability. To avoid ice crystal formation, cryoprotectants are added to sperm prior to freezing in order to diminish water content of the cells. Cryoprotectants are usually small molecules with a high solubility in water at low temperatures. The presence of these small molecules in the freezing solution generates an osmotic pressure that forces intracellular water to leave the cell and, as a consequence, solutes concentrate in the cytoplasm. At the same time, cryoprotectants slowly diffuse through the plasma membrane and substitute intracellular water, impeding ice crystal formation diminishing osmotic shock after thawing due to high salt concentration in the cytoplasm. However, slow-freezing physically damages sperm to some extent in a variety of ways: decreased motility, alterations of the plasma membrane and mitochondrial activity, or degradation of acrosomes [55].
Besides the physical damages that ice crystals can cause on membranes, cryopreservation can also compromise sperm DNA integrity. Spermatozoa are highly specialized haploid cells in which DNA is tightly packaged in order to reduce cell size and to protect DNA from fragmentation. In many species, most of the nuclear histones are exchanged for protamines during spermiogenesis [56]. DNA forms loops that attach to the membrane and progressively compact around protamines forming toroids (donut-loop model, reviewed in Ward and Ward [57]). Although disulfide bridges between protamines reinforce the stability of the DNA [58], it can be attacked by endonucleases at toroid linker regions [59]. One interesting finding is that some endonucleases are actually packaged inside sperm heads [60]. After cryopreservation, these enzymes can be released from damaged spermatozoa and activated by cations present in the media [61]. Using mouse spermatozoa, Szczygiel and Ward [62] demonstrated that adding chelating factors to the media improves chromosome stability after freezing-thawing processes, even when sperm is intentionally damaged with detergents and DTT. However, some fragmentation persists, suggesting that other mechanisms also cause sperm DNA degradation. For example, an investigation performed with koala spermatozoa demonstrated that extreme osmotic changes during cryopreservation can also disturb the tertiary structure of the DNA so the chromatin relaxes and becomes more prone to DNA degradation [63].
Oxidative stress has also been shown to produce DNA fragmentation during cryopreservation. It is caused by an imbalance between the production of ROS and the ability of the sample to detoxify or to repair the damages in DNA [64]. ROS are very reactive free radicals and oxidizing subproducts of metabolism capable of reacting with the DNA and producing breaks in the double chain. Damaged spermatozoa produce a greater amount of ROS compared to normal ones [65], but both sperm and seminal plasma contain antioxidant systems that prevent genetic and cellular damage [66]. Unfortunately, cryopreservation can lead to an imbalance in the ratio between ROS and antioxidants promoting DNA fragmentation [67] and ejaculated sperm lack DNA repair mechanisms, so they are highly vulnerable to oxidative stress. Studies conducted in bulls and stallions have shown that ROS production increases immediately after thawing slow-frozen sperm samples [55, 68] inducing DNA fragmentation [69]. Oxidative stress also triggers apoptosis of damaged spermatozoa (reviewed in Said et al. [70]). Therefore, identification of apoptotic markers in individual spermatozoa could be used to determine the overall quality of sperm or even to preselect only spermatozoa suitable for ARTs.
In the last decades, some new cryopreservation techniques, like vitrification or freeze-drying (FD), have been developed in order to overcome the potential deleterious effects of cryopreservation. Vitrification consists of ultra-quick freezing using liquid nitrogen resuspended in an aqueous solution with high concentrations of cryoprotectants [71]. Cooling is so quick that cryoprotectants effectively prevent ice crystal nucleation, and the solution becomes viscous and solidifies into a glassy state without forming ice. Despite this apparent advantage, vitrification seems to damage human sperm as much as the conventional slow-freezing protocol [72, 73]. In mouflon spermatozoa, for example, vitrification has been reported to generate damage at a greater extent than freezing-thawing [74]. As an alternative to cryoprotectants, it has been suggested that some components present in the seminal fluid could exert cryoprotective characteristics in boar [75], bull [76], and dog [77] and act like an important factor for pregnancy success (reviewed in Schjenken and Robertson [78]). Actually, artificial solutions have been developed based on seminal fluid composition, and they are already achieving good results in terms of sperm motility in human [79]. On the other hand, FD or lyophilization is a method in which frozen material is dried by sublimation of ice [46]. Due to lack of water molecules, enzymatic reactions cannot take place even though sperm is stored at 4° or transported at room temperature. This feature makes FD really attractive for long-term storage of sperm, as liquid nitrogen is not needed. Freeze-dried sperm can then be rehydrated by adding pure water to the original volume of the sample, and then it can be diluted with a suitable physiological saline buffer. The main drawback of FD is that membranes are destroyed during the process, so sperm is dead after rehydration. However, sperm heads retain their fertilizing capacity if they are used for ICSI procedure, and sperm DNA integrity seem to be less compromised compared to conventional freezing as was demonstrated in mouse [46] and later in humans [80].
Cryopreservative techniques have improved greatly in the last decades in terms of designing procedures that are easy to perform and increasing the time that the samples can be stored. However, DNA fragmentation caused by cryopreservation and inherent to the quality of the sperm sample can result in poor-quality spermatozoa and ultimately in a low pregnancy success. Animal experimentation can contribute to the basic knowledge about the cryobiology of spermatozoa and help improve this important methodology for reproductive medicine.
5 Sperm DNA Damage and Repair in Fish: A Useful Model
From the shape of the cell to the highly compacted status of its chromatin, the architecture of the sperm cell has been designed through natural selection for an effective transportation and protection of the paternal genetic information. However, once delivered spermatozoa encounter a hostile environment through which they migrate. Thus, spermatozoa are exposed to different agents that could provoke damage to their DNA with effects the reproductive outcomes. As mentioned earlier, it has been demonstrated in mouse that despite the strong sperm selection occurring within the female genital tract, spermatozoa porting damaged DNA are able to fertilize, potentially affecting the embryo production [9]. In addition, the use of artificial reproductive techniques overcomes this selective process suggesting that the study of the paternal contribution to the embryo development deserves more attention.
Studying the paternal effects on mammalian embryo development in detail has the significant restriction of an internal location of the embryo, rendering difficult the monitoring of in vivo development. Thus, for the following reasons, external fertilizers are excellent models for studying embryo development. First, the external location of the embryo allows real-time monitoring of the development. Second, embryos are more resistant to manipulation facilitating the in vivo study of developmental processes. Third, a high number of embryos can be obtained from each mating minimizing the variability related to the individual. Fourth, for studying the paternal effect on embryo development, the weaker sperm selection in contrast to mammals allows for easy fertilization with damaged or altered spermatozoa [81, 82].
Pérez-Cerezales et al. [82] were the first to show unequivocally the ability of trout spermatozoa porting damaged DNA to fertilize the egg. In this work, the authors reported a direct relationship between the level of fragmented DNA and the percentage of abortions during development. Furthermore, they demonstrated for first time in a fish specie the ability of the egg to repair the sperm DNA by the base scission repair (BER) pathway. Due to the limited capacity of the spermatozoa to repair DNA [83, 84], the repairmen of the paternal DNA relies on the oocyte after fertilization occurring in the zygote and in the first developmental stages [85]. Consequently, like in mammals, the fish oocyte contains the elements of the BER pathway for repairing simple-strand breaks of the DNA [86] as well as the homologous end-joining (HR) and nonhomologous end-joining (NHEJ) pathways for repairing double-stand breaks [85, 87]. However, these systems are limited in that they can only repair a certain level of damaged DNA. In trout, the zygote can repair around 10% of the damaged DNA by the BER [82], a similar percentage to that reported in mice by Ahmadi and Ng [88]. In addition, these repair systems can introduce errors in the DNA sequence and provoke mutations with consequences of different magnitude potentially affecting offspring performance [38].
In an earlier work, Pérez-Cerezales et al. [89] similarly demonstrated in trout that fertilization with damaged DNA spermatozoa provokes genetic alterations in offspring survival after hatching. These authors found overexpression of genes related to growth and development in the larvae obtained using spermatozoa with cryodamaged DNA. Moreover, whereas cryodamage provoked a reduction in the telomere length of the spermatozoa, the resultant embryos showed higher telomere length. To explain these surprising findings, the authors also found an overexpression of the telomerase reverse transcriptase (TERT) , a subunit of the telomerase which function is to increase the telomere length in the larvae [90]. In accordance with these results, Fernández-Díez et al. [91], using microarrays from BER repaired trout embryos, reported that 810 genes were differentially expressed after hatching. Their results point to long-term effects of fertilizing with DNA-damaged spermatozoa due to an impaired DNA damage signalization and repair in the oocyte possibly introducing punctual mutations. These results are in agreement with the ones reported by Fernández-González et al. [38], showing the negative effects of DNA-fragmented spermatozoa used for ICSI in the pre-implantational development, implantation rates and embryo development to term as well as provoking abnormal behavior, diverse anatomopathologies, and higher incidence of cancer in the adulthood in a mouse model.
6 Conclusions
Altogether, studies in fish, mouse, and other animals have demonstrated the importance and implications of sperm DNA damage in reproductive outcomes and offspring performance. Understanding the origin of the sperm DNA damage, the mechanisms and dynamics for its reparation, the effects on embryo development, as well as the long-term effects on the offspring are questions that are being explored and need to get more attention by the scientific community, especially in the context of ARTs in the clinical treatment of human fertility.
References
Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37.
Sakkas D, Ramalingam M, Garrido N, Barratt CL. Sperm selection in natural conception: what can we learn from mother nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21:711–26.
Timothy T, Koyanagi F, Yanagimachi R. Distribution and number of spermatozoa in the oviduct of the golden after natural mating and artificial insemination & hamster. Biol Reprod. 1987;37:225–34.
Williams M, Hill CJ, Scudamore I, Dunphy B, Cooke ID, Barratt CL. Sperm numbers and distribution within the human fallopian tube around ovulation. Hum Reprod. 1993;8:2019–26.
Hino T, Muro Y, Tamura-Nakano M, Okabe M, Tateno H, Yanagimachi R. The behavior and acrosomal status of mouse spermatozoa in vitro, and within the oviduct during fertilization after natural mating. Biol Reprod. 2016;95:50.
Muro Y, Hasuwa H, Isotani A, Miyata H, Yamagata K, Ikawa M, Yanagimachi R, Okabe M. Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biol Reprod. 2016;94:80.
Ola B, Afnan M, Papaioannou S, Sharif K, Björndahl L, Coomarasamy A. Accuracy of sperm–cervical mucus penetration tests in evaluating sperm motility in semen: a systematic quantitative review. Hum Reprod. 2003;18:1037–46.
Aitken RJ, Bowie H, Buckingham D, Harkiss D, Richardson DW, West KM. Sperm penetration into a hyaluronic acid polymer as a means of monitoring functional competence. J Androl. 1992;13:44–54.
Hourcade JD, Pérez-Crespo M, Fernández-González R, Pintado B, Gutiérrez-Adán A. Selection against spermatozoa with fragmented DNA after postovulatory mating depends on the type of damage. Reprod Biol Endocrinol. 2010;8:9.
Schuberth HJ, Taylor U, Zerbe H, Waberski D, Hunter R, Rath D. Immunological responses to semen in the female genital tract. Theriogenology. 2008;70:1174–81.
Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002;66:1061–7.
Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21.
Nakanishi T, Isotani A, Yamaguchi R, Ikawa M, Baba T, Suarez SS, Okabe M. Selective passage through the uterotubal junction of sperm from a mixed population produced by chimeras of calmegin-knockout and wild-type male mice. Biol Reprod. 2004;71:959–65.
Talevi R, Gualtieri R. Molecules involved in sperm-oviduct adhesion and release. Theriogenology. 2010;73:796–801.
Perez-Cerezales S, Boryshpolets S, Eisenbach M. Behavioral mechanisms of mammalian sperm guidance. Asian J Androl. 2015;17:628–32.
Pérez-Cerezales S, Boryshpolets S, Afanzar O, Brandis A, Nevo R, Kiss V, Eisenbach M. Involvement of opsins in mammalian sperm thermotaxis. Sci Rep. 2015;5:16146.
Bahat A, Tur-Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat Med. 2003;9:149–50.
Miki K, Clapham DE. Rheotaxis guides mammalian sperm. Curr Biol. 2013;23:443–52.
Sugiyama H, Chandler DE. Sperm guidance to the egg finds calcium at the helm. Protoplasma. 2014;251:461–75.
Alvarez L, Friedrich BM, Gompper G, Kaupp UB. The computational sperm cell. Trends Cell Biol. 2014;24:198–207.
Liu DY, Baker HWG. Human sperm bound to the zona pellucida have normal nuclear chromatin as assessed by acridine orange fluorescence. Hum Reprod. 2007;22:1597–602.
Ramos L, Wetzels AM. Low rates of DNA fragmentation in selected motile human spermatozoa assessed by the TUNEL assay. Hum Reprod. 2001;16:1703–7.
Huang CC, Lin DP, Tsao HM, Cheng T-C, Liu CH, Lee MS. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril. 2005;84:130–40.
Kotłowska M, Dietrich G, Wojtczak M, Karol H, Ciereszko A. Effects of liquid storage on amidase activity, DNA fragmentation and motility of turkey spermatozoa. Theriogenology. 2000;67:276–86.
Kasimanickam R, Pelzer KD, Kasimanickam V, Swecker WS, Thatcher CD. Association of classical semen parameters, sperm DNA fragmentation index, lipid peroxidation and antioxidant enzymatic activity of semen in ram-lambs. Theriogenology. 1980;65:1407–21.
Kasimanickam R, Nebel RL, Peeler ID, Silvia WL, Wolf KT, McAllister AJ, Cassell BG. Breed differences in competitive indices of Holstein and Jersey bulls and their association with sperm DNA fragmentation index and plasma membrane integrity. Theriogenology. 1997;66:1307–15.
Holt WV, Fazeli A. Do sperm possess a molecular passport? Mechanistic insights into sperm selection in the female reproductive tract. Mol Hum Reprod. 2015;21:491–501.
Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod Biomed Online. 2005;10:247–88.
Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69:528–32.
Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–6.
Enciso M, Muriel L, Fernández JL, Goyanes V, Segrelles E, Marcos M, Montejo JM, Ardoy M, Pacheco A, Gosálvez J. Infertile men with varicocele show a high relative proportion of sperm cells with intense nuclear damage level, evidenced by the sperm chromatin dispersion test. J Androl. 2006;27:106–11.
Zini A, Libman J. Sperm DNA damage: importance in the era of assisted reproduction. Curr Opin Urol. 2006;16:428–34.
Ozmen B, Caglar GS, Koster F, Schopper B, Diedrich K, Al-Hasani S. Relationship between sperm DNA damage, induced acrosome reaction and viability in ICSI patients. Reprod Biomed Online. 2007;15:208–14.
Yoshida A, Nakahori Y, Kuroki Y, Miura K, Shirai M. An azoospermic male with an unbalanced autosomal-Y translocation. Jpn J Hum Genet. 1997;42:451–5.
Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, Van Steirteghem A, Liebaers I. Cytogenetics of infertile men. Hum Reprod. 1996;11(Suppl 4):1–24. 6
Kent-First MG, Kol S, Muallem A, Blazer S, Itskovitz-Eldor J. Infertility in intracytoplasmic-sperm-injection-derived sons. Lancet. 1996;348:332.
Silber SJ, Repping S. Transmission of male infertility to future generations: lessons from the Y chromosome. Hum Reprod Update. 2002;8:217–29.
Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, Bilbao A, Bermejo-Alvarez P, de Dios HJ, de Fonseca FR, Gutiérrez-Adán A. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–72.
Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, Guérin JF. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–8.
Devroey P, Van Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28.
Yamauchi Y, Riel JM, Ward MA. Paternal DNA damage resulting from various sperm treatments persists after fertilization and is similar before and after DNA replication. J Androl. 2012;33:229–38.
Moreira PN, Pérez-Crespo M, Ramírez MA, Pozueta J, Montoliu L, Gutiérrez-Adán A. Effect of transgene concentration, flanking matrix attachment regions, and RecA-coating on the efficiency of mouse transgenesis mediated by intracytoplasmic sperm injection. Biol Reprod. 2007;76:336–43.
Yamagata K, Suetsugu R, Wakayama T. Assessment of chromosomal integrity using a novel live-cell imaging technique in mouse embryos produced by intracytoplasmic sperm injection. Hum Reprod. 2009;24:2490–9.
Ramos-Ibeas P, Calle A, Fernández-González R, Laguna-Barraza R, Pericuesta E, Calero A, Ramírez MA, Gutiérrez-Adán A. Intracytoplasmic sperm injection using DNA Fragmented sperm in mice negatively affects embryo-derived embryonic stem cells, reduces the fertility of male offspring and induces heritable changes in epialleles. PLoS One. 2014;9:e95625.
Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100:2538–43.
Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol. 1998;16:639–41.
Fraser L, Strzeżek J, Kordan W. Post-thaw sperm characteristics following long-term storage of boar semen in liquid nitrogen. Anim Reprod Sci. 2014;147:119–27.
Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, Hotaling J, Carrel DT. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402–12.
Kaneko T, Whittingham DG, Overstreet JW, Yanagimachi R. Tolerance of the mouse sperm nuclei to freeze-drying depends on their disulfide status. Biol Reprod. 2003;69:1859–62.
Yildiz C, Law N, Ottaviani P, Jarvi K, McKerlie C. Comparison of sperm quality and DNA integrity in mouse sperm exposed to various cooling velocities and osmotic stress. Theriogenology. 2010;74:1420–30.
McCarthy MJ, Baumber J, Kass PH, Meyers SA. Osmotic stress induces oxidative cell damage to rhesus macaque spermatozoa. Biol Reprod. 2010;82:644–51.
Yildiz C, Ottaviani P, Law N, Ayearst R, Liu L, McKerlie C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction. 2007;133:585–95.
Taşdemir U, Büyükleblebici S, Tuncer PB, Coşkun E, Ozgürtaş T, Aydın FN, Büyükleblebici O, Gürcan IS. Effects of various cryoprotectants on bull sperm quality, DNA integrity and oxidative stress parameters. Cryobiology. 2013;66:38–42.
Donnelly ET, Steele EK, McClure N, Lewis SE. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod. 2001;16:1191–9.
Gürler H, Malama E, Heppelmann M, Calisici O, Leiding C, Kastelic JP, Bollwein H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology. 2016;86:562–71.
Balhorn R. A model for the structure of chromatin in mammalian sperm. J Cell Biol. 1982;93:298–305.
Ward MA, Ward WS. A model for the function of sperm DNA degradation. Reprod Fertil Dev. 2004;16:547–54.
Evenson DP, Jost LK, Varner DD. Relationship between sperm nuclear protamine free -SH status and susceptibility to DNA denaturation. J Reprod Fertil Suppl. 2000:14(3):199–204.
Sotolongo B, Lino E, Ward WS. Ability of hamster spermatozoa to digest their own DNA. Biol Reprod. 2003;69:2029–35.
Maione B, Pittoggi C, Achene L, Lorenzini R, Spadafora C. Activation of endogenous nucleases in mature sperm cells upon interaction with exogenous DNA. DNA Cell Biol. 1997;16:1087–97.
Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R. Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa. Proc Natl Acad Sci U S A. 2001;98:13501–6.
Szczygiel MA, Ward WS. Combination of dithiothreitol and detergent treatment of spermatozoa causes paternal chromosomal damage. Biol Reprod. 2002;67:1532–7.
Johnston SD, Satake N, Zee Y, López-Fernández C, Holt WV, Gosálvez J. Osmotic stress and cryoinjury of koala sperm: an integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction. 2012;143:787–97.
Amidi F, Pazhohan A, Shabani Nashtaei M, Khodarahmian M, Nekoonam S. The role of antioxidants in sperm freezing: a review. Cell Tissue Bank. 2016;17:745–56.
Sikka SC. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci. 1996;1:e78–86.
Peeker R, Abramsson L, Marklund SL. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol Hum Reprod. 1997;3:1061–6.
Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 2010;2010:686137.
Ortega Ferrusola C, González Fernández L, Macías García B, Salazar-Sandoval C, Morillo Rodríguez A, Rodríguez Martinez H, Tapia JA, Peña FJ. Effect of cryopreservation on nitric oxide production by stallion spermatozoa. Biol Reprod. 2009;81:1106–11.
Baumber J, Ball BA, Linfor JJ, Meyers SA. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J Androl. 2003;24:621–8.
Said TM, Gaglani A, Agarwal A. Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 2010;21:456–62.
Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature. 1985;313:573–5.
Ali Mohamed MS. Slow cryopreservation is not superior to vitrification in human spermatozoa; an experimental controlled study. Iran J Reprod Med. 2015;13:633–44.
Valcarce DG, Cartón-García F, Riesco MF, Herráez MP, Robles V. Analysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo development. Andrology. 2013;1:723–30.
Pradiee J, Esteso MC, Castaño C, Toledano-Díaz A, Lopez-Sebastián A, Guerra R, Santiago-Moreno J. Conventional slow freezing cryopreserves mouflon spermatozoa better than vitrification. Andrologia. 2017;49(3):e12629.
Fernández-Gago R, Álvarez-Rodríguez M, Alonso ME, González JR, Alegre B, Domínguez JC, Martínez-Pastor F. Thawing boar semen in the presence of seminal plasma improves motility, modifies subpopulation patterns and reduces chromatin alterations. Reprod Fertil Dev. 2017;29(8):1576–1584.
Patel M, Gandotra VK, Cheema RS, Bansal AK, Kumar A. Seminal plasma heparin binding proteins improve semen quality by reducing oxidative stress during cryopreservation of cattle bull semen. Asian-Australasian J Anim Sci. 2016;29:1247–55.
Treulen F, Sánchez R, Risopatrón J. Effects of seminal fluid fractions on plasma and acrosome membrane integrity and mitochondrial membrane potential determined by flow cytometry in chilled canine spermatozoa. Reprod Domest Anim. 2012;47:1043–8.
Schjenken JE, Robertson SA. Seminal fluid signalling in the female reproductive tract: implications for reproductive success and offspring health. Adv Exp Med Biol. 2015;868:127–58.
Agha-Rahimi A, Khalili MA, Nottola SA, Miglietta S, Moradi A. Cryoprotectant-free vitrification of human spermatozoa in new artificial seminal fluid. Andrology. 2016;4(6):1037–1044.
Gianaroli L, Magli MC, Stanghellini I, Crippa A, Crivello AM, Pescatori ES, Ferraretti AP. DNA integrity is maintained after freeze-drying of human spermatozoa. Fertil Steril. 2012;97:1067–73. e1
Holt WV, Van Look KJ. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction. 2004;127:527–35.
Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez MP. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction. 2010;139:989–97.
Reinardy HC, Syrett JR, Jeffree RA, Henry TB, Jha AN. Cobalt-induced genotoxicity in male zebrafish (Danio rerio), with implications for reproduction and expression of DNA repair genes. Aquat Toxicol. 2013;126:224–30.
Smith TB, Dun MD, Smith ND, Curry BJ, Connaughton HS, Aitken RJ. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J Cell Sci. 2013;126:1488–97.
Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17:1922–37.
Mitra S, Boldogh I, Izumi T, Hazra TK. Complexities of the DNA base excision repair pathway for repair of oxidative DNA damage. Environ Mol Mutagen. 2001;38:180–90.
Bladen CL, Lam WK, Dynan WS, Kozlowski DJ. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 2005;33:3002–10.
Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704.
Pérez-Cerezales S, Gutiérrez-Adán A, Martínez-Páramo S, Beirão J, Herráez MP. Altered gene transcription and telomere length in trout embryo and larvae obtained with DNA cryodamaged sperm. Theriogenology. 2011;76:1234–45.
Ocalewicz K, Babiak I, Dobosz S, Nowaczyk J, Goryczko K. The stability of telomereless chromosome fragments in adult androgenetic rainbow trout. J Exp Biol. 2004;207:2229–36.
Fernández-Díez C, González-Rojo S, Montfort J, Le Cam A, Bobe J, Robles V, Pérez-Cerezales S, Herráez MP. Inhibition of zygotic DNA repair: transcriptome analysis of the offspring in trout (Oncorhynchus mykiss). Reproduction. 2015;149:101–11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Perez-Cerezales, S. et al. (2018). Experimental Studies on Sperm DNA Fragmentation and Reproductive Outcomes. In: Zini, A., Agarwal, A. (eds) A Clinician's Guide to Sperm DNA and Chromatin Damage. Springer, Cham. https://doi.org/10.1007/978-3-319-71815-6_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-71815-6_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71814-9
Online ISBN: 978-3-319-71815-6
eBook Packages: MedicineMedicine (R0)